Abstract
In response to viral infection, mammalian cells activate several innate immune pathways to antagonize viral gene expression. Upon recognition of viral double-stranded RNA, protein kinase R (PKR) phosphorylates eIF2α on serine 51. This inhibits canonical translation initiation, which broadly antagonizes viral protein synthesis. It also promotes the assembly of cytoplasmic ribonucleoprotein complexes termed stress granules (SGs). SGs are widely thought to promote cell survival and antiviral signalling. However, co-activation of the OAS/RNase L antiviral pathway inhibits the assembly of SGs and promotes the assembly of an alternative ribonucleoprotein complex termed an RNase L-dependent body (RLB). The formation of RLBs has been observed in response to double-stranded RNA, dengue virus infection, or SARS-CoV-2 infection. Herein, we review the distinct biogenesis pathways and properties of SGs and RLBs, and we provide perspective on their potential functions during the antiviral response.
Graphical Abstract

RNase L-mediated mRNA decay inhibits stress granule assembly and promotes the assembly of RNase L-dependent bodies.
1. Introduction
Double-stranded RNA (dsRNA) is a viral-associated molecular pattern recognized by pattern recognition receptors (PRRs) (Mogensen, 2009). Upon detection of cytoplasmic dsRNA, mammalian cells initiate pathways that antagonize viral gene expression and replication. These include the protein kinase R (PKR) pathway, the oligo(A) synthetase-ribonuclease L (OAS-RNase L) pathway, and the RIG-I-like receptor-mitochondrial antiviral-signalling (RLR-MAVS) pathway (Figure. 1). Because these antiviral pathways are independently activated, specific viruses can differentially activate and/or inhibit these specific antiviral pathways. Moreover, the activation of specific antiviral pathways can be heterogenous with respect to individual cells (Dalet et al., 2017; Burke et al., 2019). The specificity of innate immune responses with respect to cell-types and viruses, the heterogeneity of these responses between individual cells, and the cross-regulation between these antiviral pathways are emerging concepts in the field.
Figure 1. Select antiviral pathways.
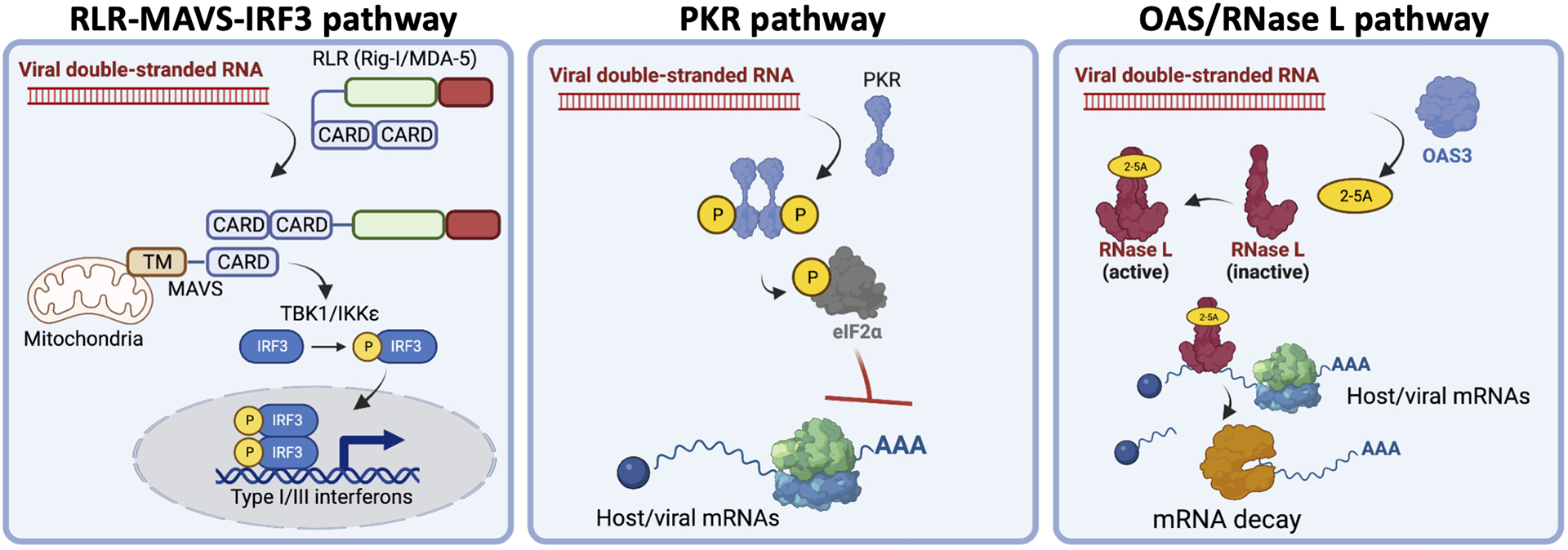
Diagrams showing the key activity of the RLR-MAVS, PKR, and OAS/RNase L pathways in response to viral double-stranded RNA.
The RLR-MAVS pathway triggers transcriptional induction of antiviral genes responsible for limiting viral replication capacity, priming antiviral defenses in non-infected cells to limit viral dissemination, and promoting cell-mediated innate and adaptive immune responses. Binding of viral double-stranded RNA by the RIG-I-like receptors (RLRs), RIG-I or MDA-5, in the cytoplasm activates the MAVS signaling complex, which resides on the outer membrane of mitochondria, as wells as peroxisomes and endoplasmic reticulum (Yoneyama et al., 2004; Kawai et al., 2005; Seth et al., 2005; Hornung et al., 2006; Pichlmair et al., 2006; Kato et al., 2006; Kumar et al., 2006). Activation of the MAVS signaling complex leads to de-repression of nuclear factor-κB (NF-κB) and phosphorylation of interferon regulatory factor 3/7 (IRF3/7). NF-κB and phospho-IRF3 translocate to the nucleus to induce the expression of antiviral proteins and cytokines (i.e., type I interferons, PRRs, and IFIT1) (Nguyen et al., 1997).
Protein kinase R (PKR) broadly limits viral protein synthesis. Upon binding of viral dsRNA via its N-terminal RNA-binding domain, PKR forms homodimers (Figure 1). Dimerization results in autophosphorylation of Thr446 (p-PKR) within the activation segment of its catalytic domain, which fully activates PKR catalytic activity (Nanduri et al., 2000, Ung et al., 2001, Zhang et al., 2001). Activated p-PKR phosphorylates eIF2α on serine 51 (p-eIF2α), which inhibits canonical AUG-dependent translation initiation while promoting expression of select host mRNA transcripts with upstream open reading frames (uORFs) (Wek et al., 2006; Dalet et al., 2015).
The OAS/RNase L antiviral pathway accelerates RNA decay to limit viral replication capacity (Figure 1) (see Silverman, 2007; Chakrabarti et al., 2011 for in-depth reviews). Upon binding to dsRNA, oligo(A) synthetases (OASs) synthesizes 2–5-oligo(A) (Slattery et all., 1979; Li et al., 2016). 2–5-oligo(A) promotes homodimerization of RNase L, which activates its endoribonuclease activity on single-stranded RNA regions of viral RNAs and cellular tRNAs, rRNAs, and mRNAs (Floyd-Smith et al., 1981; Wreschner et al., 1981; Silverman et al., 1983; Wreschner et al., 1981; Li et al., 1998, Andersen et al., 2009, Chakrabarti et al., 2011, Brennan-Laun et al., 2014, Donovan et al., 2017; Burke et al., 2019; Rath et al., 2019).
The repression of translation by PKR promotes the formation of stress granules (SGs), which are membrane-less organelles composed of non-translating mRNAs and RNA-binding proteins that form during diverse cellular stresses (see Buchan, 2014 and Protter and Parker, 2016 for in-depth reviews). PKR-mediated SGs are widely thought to promote RLR-MAVS signaling (Onamoto et al., 2012, Yoo et al., 2014, and Reineke and Lloyd, 2015). However, RNase L-mediated mRNA decay inhibits the assembly of SGs in a dominant manner, while promoting the assembly of unique SG-like granules, termed RNase L-dependent bodies (RLBs) (Burke et al., 2019; Burke et al., 2020). Herein, we review the distinct biogenesis pathways and composition of SGs and RLBs and provide perspective on the role of RNase L in regulating these cytoplasmic ribonucleoprotein complexes during the antiviral response.
2. RNase L-mediated regulation of cytoplasmic ribonucleoprotein granules.
The regulation of cytoplasmic ribonucleoprotein (RNP) complexes by RNase L was uncovered by screening for antiviral proteins that modulate stress granules via immunofluorescence for G3BP1, an SG-associated RNA-binding protein. The key observation was that lipofection of poly(I:C) (a viral dsRNA mimic) in A549 and U-2 OS cell lines resulted in small, punctate G3BP1 structures (Burke et al., 2019). However, in RNase L-knockout cell lines, poly(I:C) induced G3BP1 assemblies that resembled canonical stress granules (larger and more disordered morphology) typically observed during other stresses such as sodium arsenite treatment (Figure 2A). These data suggested that RNase L limits the size of SGs assembled in response to dsRNA. However, as discussed below, these RNase L-dependent “mini” SGs are in fact a distinct ribonucleoprotein complex, termed RNase L-dependent body (RLB). Importantly, RLBs have also been observed in response to dengue virus or SARS-CoV-2 infection (Burke et al., 2021b; Burke et al., 2022) (Figure 2B,C), both of which activate the OAS/RNase L pathway (Whelan et al., 2019; Li et al. 2021). Thus, RNase L-mediated inhibition of SGs and promotion of RLBs represents a new branch of the OAS/RNase L innate immune pathway that occurs during pathogenic viral infections.
Figure 2. RNase L inhibits stress granules and promotes RNase L-dependent bodies.
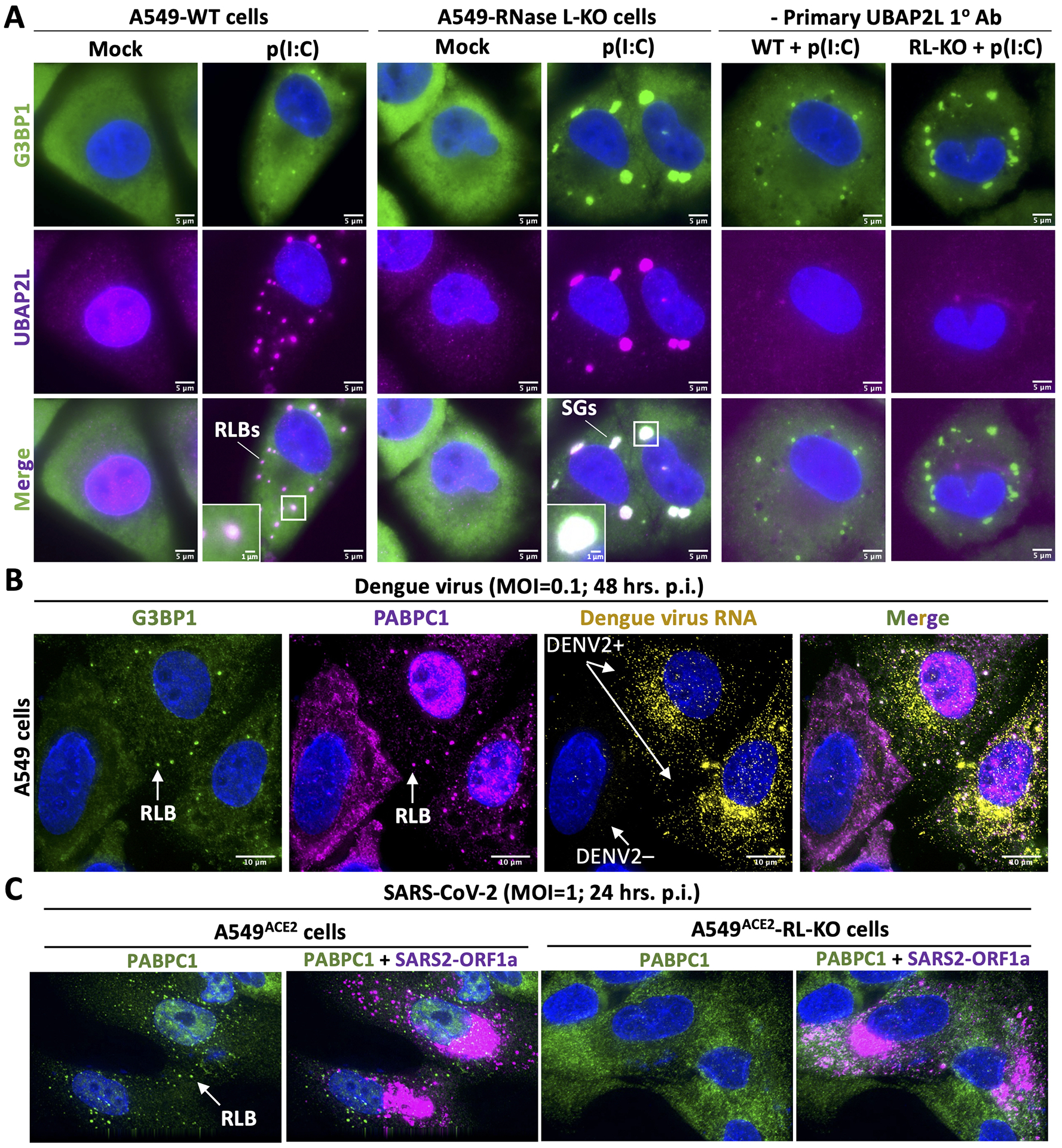
(A) Immunofluorescence assay for G3BP1 (Abcam: ab56574) and UBAP2L (Abcam: ab70319), which are both SG markers, in parental (WT) or RNase L-KO A549 cells (Burke et al., 2019) (12-well format) 4 hours post-lipofection with lipofectamine 2000 (Thermo Fisher Scientific: 11668019) and 500 ng of poly(I:C) (Invivogen:tlrl-pic), a viral double-stranded RNA mimic. As we previously reported (Burke et al., 2019; Burke et al., 2020), lipofection of poly(I:C) in A549-WT cells results in small punctate foci that enrich G3BP1. These structures are dependent on RNase L catalytic activity, and thus are termed RNase L-dependent bodies. These data demonstrate that UBAP2L strongly enriches in RLBs, which was previously unknown. In contrast to WT cells, lipofection of poly(I:C) in RNase L-KO cells leads to large (canonical) stress granules that highly enrich both G3BP1 and UBAP2L. See Burke et al., 2019 for detailed methodology. (B) As previously reported (Burke et. al, 2022), immunofluorescence assay for the G3BP1 and PABPC1 (Abcam: ab21060) in A549 cells infected with dengue virus serotype 2 16681 strain. In cells infected with dengue virus (DENV+), G3BP1 and PABP form small punctate foci consistent with RLB morphology. PABPC1 also concentrates in the nucleus in dengue viruses infected cells, which is a marker of RNase L activation (Burke et al., 2019). Figure adapted from Burke et. al, 2022. (C) As previously reported (Burke et. al, 2021b), immunofluorescence assay for PABPC1 and smFISH for SARS-CoV-2 ORF1a 24 hours post-infection with SARS-CoV-2 in A549ACE2 or A549ACE2-RNase L-KO cells. Notably, RLBs form in SARS-CoV-2-infected WT cells, whereas SARS-CoV-2-infected RNase L-KO cells do not generate PABPC1-positive foci. Figure adapted from Burke et al., 2021b.
For future studies, it is important to consider why previous studies primarily observed canonical SGs, as opposed to RLBs, in response to dsRNA or viral infection. First, G3BP1 foci may have been assumed to be SGs, when in fact they were RLBs. Therefore, the presence of G3BP1 foci does not necessarily make them SGs. Second, many studies were performed in cell lines that have a weak RNase L response, such as MEFs (Banerjee et al., 2014), and thus SGs are primarily observed. Third, many of the viruses used to study stress granules inhibit activation of the OAS/RNase L pathway, such as influenza A virus (Min and Krug, 2006). Fourth, the role of RNase L was not directly examined in previous studies in which viral infection attenuated canonical SGs. Lastly, inhibition of canonical SGs were previously reported during viral infection, including rotavirus and chikungunya virus (Montero et al., 2008; Fros et al., 2012; Dhillon and Rao, 2018). Whether RNase L contributes to the formation of these non-canonical SGs remains unaddressed. Thus, future experiments should consider the activity of specific innate immune pathways in cell lines and the specific virus being examined. Moreover, distinction between a viral protein directly modulating SG assembly, as opposed to indirectly modulating SG assembly by promoting viral replication that triggers differential innate immune pathway activation/inactivation, should be considered.
2.1. Distinct biogenesis pathways of RLBs and SGs
Stress granules induced by viral infection predominately form via PKR-mediated translation repression (Figure 3A). Upon binding viral double-stranded RNA, PKR phosphorylates eIF2α on serine 51. We note that additional cellular stresses triggered by viral infection can activate additional eIF2α kinases (HRI, PERK, GCN2) (see Liu et al., 2020 for review). Phosphorylation of eIF2α reduces the guanine nucleotide exchange activity of eIF2B, resulting in inhibition of translation initiation (Wek et al., 2006). As translating ribosomes run off initiation-stalled mRNAs, the mRNAs and RNA-binding proteins (RBPs) condense into stress granule cores. SG cores nucleate shell formation, which in turn facilitates merger of SG cores into mature SGs (Wheeler et al., 2016).
Figure 3. Distinct biogenesis pathways of SGs and RLBs.
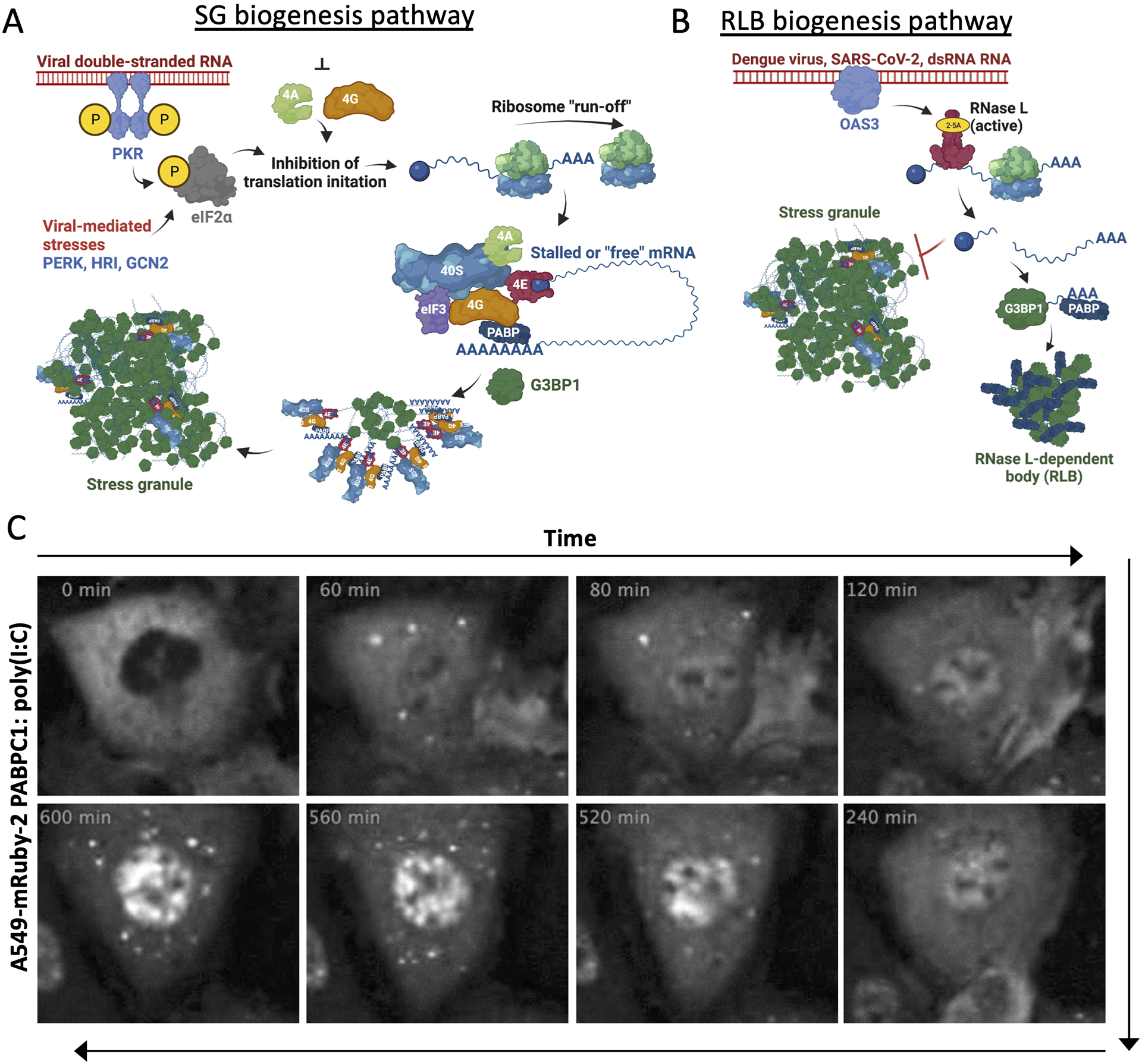
(A) Biogenesis pathway of SGs in response to viral dsRNA. (B) Biogenesis pathway of RLBs in response to viral dsRNA. (C) Live-cell imaging adapted from Burke et al., 2020. Panels show A549 cell expressing mRuby-2-PABPC1 following poly(I:C) lipofection at indicated times. RLB foci assemble at 60 minutes, disassemble by 120 minutes, but then re-assemble at 520 minutes.
Both non-translating mRNAs and specific RNA-binding proteins are required for stress granule assembly, including G3BP1 and G3BP2 paralogs, Caprin1, TIA1, and UBAP2L (Tourrière et al., 2003; Wheeler et al., 2016; Kedersha et al., 2016; Van Treeck et al., 2018; Cirillo et al., 2020). These RNA-binding proteins are modeled to promote RNA condensation by increasing the valency of RNA-RNA, RNA-protein, and protein-protein interactions necessary to drive RNA condensation via a summation of interactions model (Tauber et al., 2020; VanTreeck and Parker, 2018; Matheny et al., 2021). Because of the central role of mRNAs in SG assembly, RNase L inhibits the assembly of SGs via degradation of mRNAs required for SG assembly (Burke et al., 2020) (Figure 3B). Consistent with this, when RNase L is artificially localized to the nucleus, RNase L activation perturbs nuclear RNA granules, including Cajal bodies, nuclear speckles, and the nucleolus (Decker et al., 2022).
Several aspects of RLB biogenesis contrast with SG formation (Burke et al., 2020). First, RLBs do not require PKR because they can form in PKR knockout cells. Moreover, they do not require phosphorylation of eIF2α because they form in cells that solely express the eIF2α-S51A allele. RLBs do not require ribosomes to run-off of mRNAs, as long as RNase L is activated, since they form in the presence of cycloheximide, which traps ribosomes on mRNAs. RLBs can assemble independently of G3BP1/2 because they form in G3BP1/2 knockout cell lines. RLBs form faster than SGs in response to dsRNA, though this may reflect the differential rate of activation between OAS/RNase L and PKR. However, RLBs do not typically form from pre-assembled SGs, further indicating that their biogenesis is independent of SG assembly.
The only known factor required for RLB assembly is RNase L catalytic activity. Because RLBs are not observed in cells that have not yet initiated RNase L-mediated mRNA decay in response to dsRNA, this suggests that RLBs may either form as a consequence of mRNA decay or that RNA decay signals for their formation (Figure 3B). Interestingly, two observations suggest that RLB assembly is specific to RNase L-mediated mRNA decay. First, SARS-CoV-2 nsp1 protein initiates widespread decay of cellular mRNAs in RNase L-KO cells (Burke et al., 2021b), yet RLBs (PABP-positive foci) do not form in SARS-CoV-2-infected RNase L-KO cells (Burke et al., 2021b) (Figure 2C). Similarly, RLBs (PABP-positive foci) have not been reported to form in response to cellular mRNA decay initiated by either Kaposi’s sarcoma-associated herpesvirus (KSHV) or murine gammaherpesvirus 68 (MHV68) SOX and muSOX proteins, respectively (Kumar and Glaunsinger, 2010; Gilbertson et al., 2018). Two possibilities explain this: i) Nsp1, SOX and muSOX have a different mechanism by which they degrade cellular mRNAs, ii) RLBs are not simply a consequence of cellular mRNA decay, but instead their assembly is initiated via a signaling pathway dependent on RNase L activation. Nevertheless, these observations indicate that RNase L-mediated cleavage of either translationally stalled or translating mRNAs results, directly or indirectly, in condensation of RNAs (or RNA fragments) and RNA-binding proteins into RLBs (Figure 3B).
Another difference between SG and RLBs is their dynamics. SGs induced by dsRNA in A549 cells generally only remain assembled for ~2 hours before the GADD34 eIF2α phosphatase, which is both transcriptionally induced in an IRF3-dependent manner and preferentially translated during stress, promotes their disassembly via translation re-initiation (Dalet et al., 2017; burke et al., 2020). Following disassembly in individual cells, dsRNA induced SGs do not commonly re-assemble (Burke et al., 2020). It is unknown why dsRNA-induced SGs do not re-assemble after disassembly. One possibility is that elevated levels of GADD34 increase the threshold of PKR activity required to accumulate p-eIF2α. Another possibility is that dsRNA induces RNA helicases and/or RNA-binding proteins that interfere with SG assembly (Tauber et al., 2020). In contrast to SGs, RLBs typically remain assembled for long periods of time (>8 hours) (Burke et al., 2020). While this may suggest RLBs form might form as a consequence of RNase L-mediated mRNA decay, and thus never disassemble, RLBs can undergo periods of disassembly followed by re-assembly (Burke et al., 2020) (Figure 3C). Whether RLB disassembly reflects fluctuating RNase L activity or RNase L-dependent signaling is unknown. Future studies will address RLB maintenance and disassembly.
2.2. Composition and physical properties of RLBs and SGs.
SGs and RLBs are similar, yet unique, in their protein composition. Mass spectrometry of purified SGs demonstrated that stress granules enrich numerous RNA-binding proteins, RNA helicases, heat shock proteins, and components of the CCT complex (Jain et al., 2016). Similar analyses of RLBs revealed considerable overlap between the SG and RLB proteome (Burke et al., 2020). This was confirmed by immunofluorescence for SG-associated proteins, which revealed that G3BP1, PABPC1, Caprin-1, and ataxin-2 localize to RLBs (Figure 4). RLBs also contain UBAP2L, which was previously not examined (Figure 2A). However, RLBs lack common SG-associated proteins such as TIA-1, FAM120A, pumilio 1, and FMRP (Burke et al., 2020). Mass spectrometry of RLBs indicate that they contain RBPs that do not localize to SGs (Figure 4), though further validation of these proteins localizing to RLBs needs to be addressed.
Figure 4. RNA-binding proteins that localize to SG and/or RLBs.
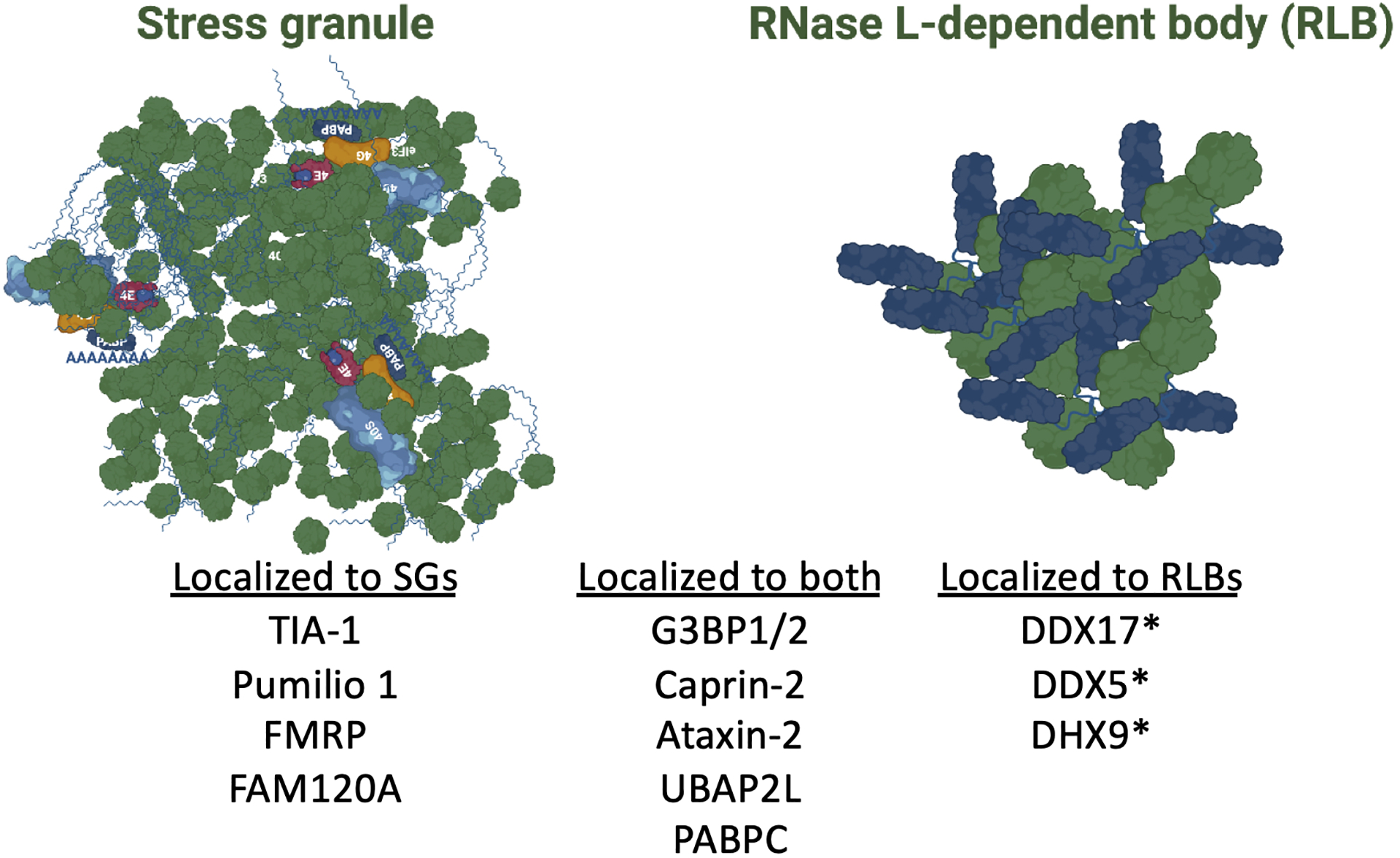
The proteins that have been confirmed to localize specifically to either SGs or RLBs or that localize to both SGs and RLBs. The asterisks indicate proteins reported to localize to RLBs based on mass spectrometry, but have not been verified by immunofluorescence.
Both SGs and RLBs are ribonucleoprotein complexes since they stain for poly(A)+ RNA based on FISH analyses. Consistent with this, both SGs and RLBs concentrate poly(A)-binding protein (PABP). SGs contain ~9% of cellular mRNAs, with long and/or inefficiently translated mRNAs predominately enriching in SGs (Khong et al., 2017). While RNA interactions with SGs are dynamic, RNAs may become integrated into a more stable scaffold-like structure once docked with SGs (Moon et al., 2019). RLBs do not appear to contain intact mRNAs that enrich in SGs based on smFISH analyses (Burke et al., 2020). Thus, it is unclear if RLBs contain a different repertoire of mRNAs or if RLB-associated RNAs are mRNA fragments containing poly(A) tails. Identification of the RNAs in RLBs will be useful for resolving their biogenesis pathway and defining their function.
Both SGs and RLBs exhibit liquid-like properties, as they can merge and readily exchange proteins with the cytoplasm. Moreover, both SGs and RLBs can interact with processing bodies (P-bodies), a distinct ribonucleoprotein complex condensate enriched with RNA decay machinery (Sheth and Parker, 2003). Notably, RLBs stably interact with P-bodies (Burke et al., 2020), whereas SG interactions with p-bodies are more transient (discussed below). Live-cell imaging of RLBs demonstrated that they are highly mobile in comparison to SGs (Burke et al., 2020), but whether their mobility is via diffusion or is directed in an ATP-dependent manner has not been examined.
2.3. Potential functions of RLBs and SGs
Stress granules (SGs) promote cell survival in response to cellular stress (Arimoto et al., 2008; Buchan and Parker, 2009; Park et al., 2020). However, a prevailing model for the function of stress granules assembled in response to viral infection is that they promote antiviral signaling (Figure 5A). This is because several viruses generally attempt to inhibit proteins involved in the stress granule biogenesis pathway (e.g., PKR or G3BP) and/or modulate stress granule composition. For example, SARS-CoV-2 nucleocapsid protein binds and inhibits G3BP1 (Zheng et al., 2021), and poliovirus 3C cleaves G3BP1 to inhibit SGs (White et al., 2007). Moreover, many viruses inhibit PKR (See Lloyd, 2013 for an in-depth review).
Figure 5. Hypothetical models for the functions of SGs, RNase L-mediated inhibition of SGs, and RLBs.
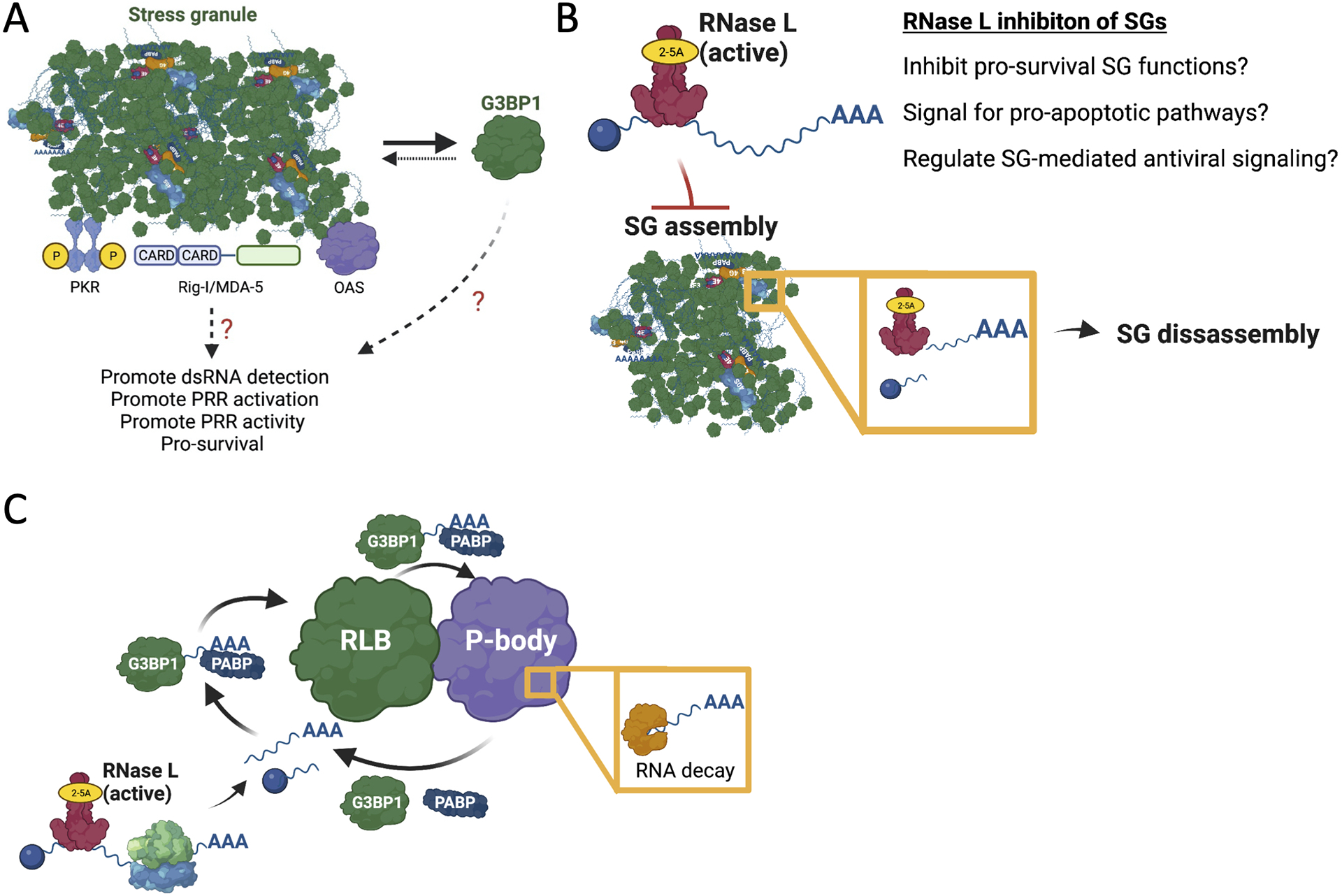
(A) Stress granules have been shown to concentrate PRRs, which is modeled to promote their activation and/or activity. However, direct evidence that stress granules, as opposed to the constituents of stress granules, are responsible for this is unclear. This is because most SG-associated RNA-binding proteins, such as G3BP1, are primarily localized to the cytoplasm and can directly modulate antiviral signaling and/or viral gene expression. (B) RNase L inhibits both the assembly of SGs and promotes the disassembly of SGs. This could inhibit the pro-survival functions of SGs, promote the pro-apoptotic functions of RNase L, or could inhibit SG-mediated antiviral signaling. (C) Hypothetical model for RLB function. RLBs enrich for RNase L-cleaved RNAs and associated RBPs. The close association of RLBs with P-bodies allows for transfer of RNA fragments into P-bodies, which enrich for RNA decay machinery. This promotes rapid decay of mRNAs and releases RBPs for exchange with the cytoplasm and/or RLBs.
The prevailing model by which stress granules function during viral infection is promotion of PRR antiviral signaling. In this model, SGs have been suggested to concentrate viral RNA and PRRs to increase their interaction and/or promote the RLR-MAVS and PKR signaling (Figure 5A). This is based on two primary observations. First, RNAs encoded by specific viruses (e.g., influenza A virus) and PRRs (PKR) and RLRs (RIG-I) localize to stress granules (Onomoto et al., 2012, Yoo et al., 2014). This suggests that RLRs and PRRs interact with viral RNA in SGs. Second, depletion of proteins required for stress granule assembly (PKR and G3BP1) have been reported to reduce antiviral signaling (Onomoto et al., 2012, Yoo et al., 2014).
However, there are several recent observations that weaken the foundation of this model. i) PKR is not required for type I interferon induction, and depletion of PKR increases interferon beta synthesis (Dalet et al., 2017; Burke et al., 2019; Burke et al., 2021a). ii) In addition to abolishing SGs, depletion of PKR increases host and viral translation and abolishes additional PKR-dependent signaling cascades. This could directly alter viral replication capacity, and in turn the antiviral response, independently of SGs. iii) G3BP1 can promote PRR activation and/or antagonize viral replication independently of SGs (Bidet et al., 2014; Galan et al., 2017; Kim et al., 2019; Yang et al., 2019), and most G3BP1 proteins (82%) do not localize to SGs during stress (Wheeler at al., 2017) (Figure 5A). Thus, depletion of G3BP1/2 could alter antiviral signaling independently of SGs. iv) Similar to G3BP1 and other stress-granule associated RBPs, it is likely that only a small percentage of each PRR localizes to SGs, thus limiting the potential effect of SG association on PRR activation/signaling. v) Recent studies indicate that dsRNA-binding PRRs, including PKR, do not localize to SGs (Corbet et al., 2022; Zappa et al., 2022). vi) dsRNA does not concentrate in SGs, but instead concentrates in double-stranded RNA-induced foci (dRIFs) (Corbet et al., 2022; Zappa et al., 2022). Thus, future studies will need to further address these contradictory findings to determine if SGs indeed promote antiviral signaling by concentrating viral dsRNA and PRRs.
RLBs have no known function. Gene ontology analyses of their proteome indicates that RLBs play a role in mRNA metabolism and processing, protein targeting to the endoplasmic reticulum, and/or SRP-dependent co-translational protein targeting to membrane (Burke et al., 2020). RLBs closely and stably associate with P-bodies, suggesting that these two ribonucleoprotein complexes could exchange some proteins and/or RNAs. Notably, RLBs contain much more poly(A)+ RNA in comparison to P-bodies, and PABP is dynamic in RLBs by fluorescence recovery after photobleaching (FRAP) (Burke et al., 2020). Since RNase L promotes mRNA decay, one possibility is that RLBs promote the transport of RNAs to P-bodies where the RNAs then undergo de-tailing and decay, thus releasing PABP and allowing for exchange of PABP-associated RNAs (Figure 5C). In contrast, SGs might store RNAs as previously suggested (Decker and Parker, 2012). Consistent with this idea, PABP stably associates with SGs during dsRNA stress in RNase L KO cells (Burke et al., 2020), and SGs and P-bodies remain separate and only transiently interact (Kedersha et al., 2005; Burke et al., 2020). It is unknown if RLBs contain antiviral proteins or promote antiviral signaling, and it is unlikely that RLBs directly promote OAS/RNase L activation since they form after initiation of RNase L-mediated mRNA decay (Burke et al., 2019; Burke et al., 2020). However, whether RLBs promote downstream pathways of the RNase L response has not been addressed.
3. Concluding remarks
The inhibition of PKR-mediated stress granules by RNase L is an example of one wing of the innate immune response regulating another. Activation of RNase L can both prevent SG assembly and can disassemble pre-formed SGs. Because SGs are implicated in cell survival and PRR signaling, inhibition of SGs by RNase L may serve as a cellular switch for pro-apoptotic cellular responses or for downregulation of specific antiviral programs promoted by SGs.
While RNase L typically inhibits PKR-mediated SGs in response to dsRNA or viral infection (Burke et al., 2019; Burke et al., 2020; Burke et al., 2021b; Burke et al., 2022), transfection of oligo 2–5A into cells promotes PKR-dependent stress granules (Manivannan et al., 2020), consistent with reports that RNase L promotes PKR activation by degrading circular RNAs that inhibit PKR (Liu et al., 2019). Notably, transfection of 2–5A into cells (Manivannan et al., 2020) results in low RNase L activity in comparison to dsRNA transfection based on ribosomal RNA cleavage activity (Burke et al., 2019). Thus, low levels of RNase L activity that are below the threshold to initiate mass mRNA decay may promote stress granule formation by generating immunostimulatory RNAs that promote PKR activation or by degrading circular RNAs that inhibit PKR activation. Thus, the effect of RNase L on SGs could be differential with respect to RNase L activity level, and this could be relevant to viral infections during which RNase L activity remains low and/or changes throughout the infection.
The precise function of SGs and SG-associated RNA-binding proteins during the antiviral response remain unclear, and understanding their function is complicated by virus-specific manipulation of SGs and differential activation and/or inhibition of innate immune pathways. Moreover, components of stress granules, such as G3BP1, exhibit antiviral activity independently of SGs (Bidet et al., 2014; Galan et al., 2017; Kim et al., 2019; Yang et al., 2019), confounding SG-dependent and SG-independent antiviral activity of these proteins. Developing new methodologies to separate the function of SG from the function of their RNA-binding proteins will be imperative for resolving SG functions and the impact of RNase L-mediated regulation of SGs.
RLBs have served as a useful marker for identifying cells that have activated RNase L in response to dsRNA, dengue virus infection, or SARS-CoV-2 infection (Burke et al., 2019; Burke et al., 2021b; Burke et al., 2022) (Figure 2B,C). However, their function remains unknown. Although antiviral proteins do not typically localize to RLBs (e.g., PKR) (Corbet et al., 2022a), current efforts are underway to determine whether additional antiviral factors localize to RLBs and whether RLBs play a role in antiviral signaling. To assess the specific function of RLBs during the OAS/RNase L response, it will be necessary to perturb RLB assembly during RNase L-mediated RNA decay. This will require further resolving the RLB biogenesis pathway.
In addition to RLBs, several unique cytoplasmic ribonucleoprotein complexes have recently been identified during the antiviral response (reviewed in Corbet et al., 2022b). Paracrine granules (PGs) are SG-like foci generated from virus-free supernatant from infected cells and exhibit a unique protein/RNA composition in comparison to SGs (Brocard et al., 2020; Iadevaia et al., 2022). PGs are linked to antiviral activity, yet their defined function remains unclear. Double-stranded RNA-induced foci (dRIF) form in response to dsRNA or viral infection (Zappa et al., 2021; Corbet et al., 2022a). dRIFs contain dsRNA and numerous dsRNA-binding proteins, including PKR, ADAR1, PACT, but do not appear to contain RLRs (Rig-I and MDA-5) (Corbet et al., 2022a). dRIFs may function as an autoregulatory mechanism of dsRNA signaling molecules, whereby they promote signaling at low concentrations of dsRNA by promoting PRR-dsRNA interactions but inhibit signaling at higher concentrations via sequestration of dsRNA and dsRNA-binding proteins (Corbet et al., 2022a). Lastly, G3BP1 has been shown to promote cGAS complex assemblies in response to dsDNA (Lui et al., 2019), and inflammasome complex activation in response to dsRNA (Shen et al, 2021).
Combined, these recent findings demonstrate that RNA and RNA-binding proteins promote numerous and diverse cytoplasmic ribonucleoprotein assemblies during the antiviral response. It will be paramount to understand how these antiviral ribonucleoprotein complexes assemble to further investigate their role during the innate immune response to viral infection.
Acknowledgements
We thank Dr. Roy Parker for research support and valuable comments regarding this manuscript. Biorender software was used to generate schematic figures.
Funding information
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number F32AI145112 and start-up funds from the University of Florida Scripps Biomedical Research Institute (J.M.B).
Footnotes
Conflict of interest: The authors declare no conflict of interests.
References
- Andersen JB, Mazan-Mamczarz K, Zhan M, Gorospe M, & Hassel BA (2009). Ribosomal protein mRNAs are primary targets of regulation in RNase-L-induced senescence. RNA biology, 6(3), 305–315. 10.4161/rna.6.3.8526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, & Takekawa M (2008). Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nature cell biology, 10(11), 1324–1332. 10.1038/ncb1791 [DOI] [PubMed] [Google Scholar]
- Banerjee S, Chakrabarti A, Jha BK, Weiss SR, & Silverman RH (2014). Cell-type-specific effects of RNase L on viral induction of beta interferon. mBio, 5(2), e00856–14. 10.1128/mBio.00856-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet K, Dadlani D, & Garcia-Blanco MA (2014). G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS pathogens, 10(7), e1004242. 10.1371/journal.ppat.1004242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan-Laun SE, Li XL, Ezelle HJ, Venkataraman T, Blackshear PJ, Wilson GM, & Hassel BA (2014). RNase L attenuates mitogen-stimulated gene expression via transcriptional and post-transcriptional mechanisms to limit the proliferative response. The Journal of biological chemistry, 289(48), 33629–33643. 10.1074/jbc.M114.589556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard M, Iadevaia V, Klein P, Hall B, Lewis G, Lu J, Burke J, Willcocks MM, Parker R, Goodfellow IG, Ruggieri A, & Locker N (2020). Norovirus infection results in eIF2α independent host translation shut-off and remodels the G3BP1 interactome evading stress granule formation. PLoS pathogens, 16(1), e1008250. 10.1371/journal.ppat.1008250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR (2014). mRNP granules. Assembly, function, and connections with disease. RNA biology, 11(8), 1019–1030. 10.4161/15476286.2014.972208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, & Parker R (2009). Eukaryotic stress granules: the ins and outs of translation. Molecular cell, 36(6), 932–941. 10.1016/j.molcel.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Gilchrist AR, Sawyer SL, & Parker R (2021). RNase L limits host and viral protein synthesis via inhibition of mRNA export. Science advances, 7(23), eabh2479. 10.1126/sciadv.abh2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Lester ET, Tauber D, & Parker R (2020). RNase L promotes the formation of unique ribonucleoprotein granules distinct from stress granules. The Journal of biological chemistry, 295(6), 1426–1438. 10.1074/jbc.RA119.011638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Moon SL, Matheny T, & Parker R (2019). RNase L Reprograms Translation by Widespread mRNA Turnover Escaped by Antiviral mRNAs. Molecular cell, 75(6), 1203–1217.e5. 10.1016/j.molcel.2019.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, St Clair LA, Perera R, & Parker R (2021). SARS-CoV-2 infection triggers widespread host mRNA decay leading to an mRNA export block. RNA (New York, N.Y.), 27(11), 1318–1329. 10.1261/rna.078923.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Ripin N, Ferretti MB, St Clair LA, Worden-Sapper ER, Salgado F, Sawyer SL, Perera R, Lynch KW, Parker R RNase L activation in the cytoplasm induces aberrant processing of mRNAs in the nucleus. PLOS Pathogens 18(11): e1010930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Jha BK, & Silverman RH (2011). New insights into the role of RNase L in innate immunity. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 31(1), 49–57. 10.1089/jir.2010.0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo L, Cieren A, Barbieri S, Khong A, Schwager F, Parker R, & Gotta M (2020). UBAP2L Forms Distinct Cores that Act in Nucleating Stress Granules Upstream of G3BP1. Current biology : CB, 30(4), 698–707.e6. 10.1016/j.cub.2019.12.020 [DOI] [PubMed] [Google Scholar]
- Corbet GA, Burke JM, Bublitz GR, Parker R dsRNA-induced condensation of antiviral proteins modulates PKR activity. Proceedings of the National Academy of Sciences of the United States of America, 119 (33) e2204235119. 10.1073/pnas.2204235119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet GA, Burke JM, & Parker R (2022). Nucleic acid-protein condensates in innate immune signaling. The EMBO journal, e111870. Advance online publication. 10.15252/embj.2022111870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalet A, Argüello RJ, Combes A, Spinelli L, Jaeger S, Fallet M, Vu Manh TP, Mendes A, Perego J, Reverendo M, Camosseto V, Dalod M, Weil T, Santos MA, Gatti E, & Pierre P (2017). Protein synthesis inhibition and GADD34 control IFN-β heterogeneous expression in response to dsRNA. The EMBO journal, 36(6), 761–782. 10.15252/embj.201695000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalet A, Gatti E, & Pierre P (2015). Integration of PKR-dependent translation inhibition with innate immunity is required for a coordinated anti-viral response. FEBS letters, 589(14), 1539–1545. 10.1016/j.febslet.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Decker CJ, & Parker R (2012). P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harbor perspectives in biology, 4(9), a012286. 10.1101/cshperspect.a012286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Burke JM, Mulvaney PK, & Parker R (2022). RNA is required for the integrity of multiple nuclear and cytoplasmic membrane-less RNP granules. The EMBO journal, 41(9), e110137. 10.15252/embj.2021110137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon P, & Rao CD (2018). Rotavirus Induces Formation of Remodeled Stress Granules and P Bodies and Their Sequestration in Viroplasms To Promote Progeny Virus Production. Journal of virology, 92(24), e01363–18. 10.1128/JVI.01363-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J, Rath S, Kolet-Mandrikov D, & Korennykh A (2017). Rapid RNase L-driven arrest of protein synthesis in the dsRNA response without degradation of translation machinery. RNA (New York, N.Y.), 23(11), 1660–1671. 10.1261/rna.062000.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd-Smith G, Slattery E, & Lengyel P (1981). Interferon action: RNA cleavage pattern of a (2’−5’)oligoadenylate--dependent endonuclease. Science (New York, N.Y.), 212(4498), 1030–1032. 10.1126/science.6165080 [DOI] [PubMed] [Google Scholar]
- Fros JJ, Domeradzka NE, Baggen J, Geertsema C, Flipse J, Vlak JM, & Pijlman GP (2012). Chikungunya virus nsP3 blocks stress granule assembly by recruitment of G3BP into cytoplasmic foci. Journal of virology, 86(19), 10873–10879. 10.1128/JVI.01506-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan A, Lozano G, Piñeiro D, & Martinez-Salas E (2017). G3BP1 interacts directly with the FMDV IRES and negatively regulates translation. The FEBS journal, 284(19), 3202–3217. 10.1111/febs.14184 [DOI] [PubMed] [Google Scholar]
- Gilbertson S, Federspiel JD, Hartenian E, Cristea IM, & Glaunsinger B (2018). Changes in mRNA abundance drive shuttling of RNA binding proteins, linking cytoplasmic RNA degradation to transcription. eLife, 7, e37663. 10.7554/eLife.37663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, & Hartmann G (2006). 5’-Triphosphate RNA is the ligand for RIG-I. Science (New York, N.Y.), 314(5801), 994–997. 10.1126/science.1132505 [DOI] [PubMed] [Google Scholar]
- Iadevaia V, Burke JM, Eke L, Moller-Levet C, Parker R, & Locker N (2022). Novel stress granule-like structures are induced via a paracrine mechanism during viral infection. Journal of cell science, 135(4), jcs259194. 10.1242/jcs.259194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, & Parker R (2016). ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell, 164(3), 487–498. 10.1016/j.cell.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, & Akira S (2006). Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature, 441(7089), 101–105. 10.1038/nature04734 [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, & Akira S (2005). IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nature immunology, 6(10), 981–988. 10.1038/ni1243 [DOI] [PubMed] [Google Scholar]
- Kedersha N, Panas MD, Achorn CA, Lyons S, Tisdale S, Hickman T, Thomas M, Lieberman J, McInerney GM, Ivanov P, & Anderson P (2016). G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. The Journal of cell biology, 212(7), 845–860. 10.1083/jcb.201508028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, & Anderson P (2005). Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. The Journal of cell biology, 169(6), 871–884. 10.1083/jcb.200502088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, & Parker R (2017). The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Molecular cell, 68(4), 808–820.e5. 10.1016/j.molcel.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Sze L, Liu C, & Lam KP (2019). The stress granule protein G3BP1 binds viral dsRNA and RIG-I to enhance interferon-β response. The Journal of biological chemistry, 294(16), 6430–6438. 10.1074/jbc.RA118.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GR, & Glaunsinger BA (2010). Nuclear import of cytoplasmic poly(A) binding protein restricts gene expression via hyperadenylation and nuclear retention of mRNA. Molecular and cellular biology, 30(21), 4996–5008. 10.1128/MCB.00600-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, Yamamoto M, Uematsu S, Ishii KJ, Takeuchi O, & Akira S (2006). Essential role of IPS-1 in innate immune responses against RNA viruses. The Journal of experimental medicine, 203(7), 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Blackford JA, & Hassel BA (1998). RNase L mediates the antiviral effect of interferon through a selective reduction in viral RNA during encephalomyocarditis virus infection. Journal of virology, 72(4), 2752–2759. 10.1128/JVI.72.4.2752-2759.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Banerjee S, Wang Y, Goldstein SA, Dong B, Gaughan C, Silverman RH, & Weiss SR (2016). Activation of RNase L is dependent on OAS3 expression during infection with diverse human viruses. Proceedings of the National Academy of Sciences of the United States of America, 113(8), 2241–2246. 10.1073/pnas.1519657113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Renner DM, Comar CE, Whelan JN, Reyes HM, Cardenas-Diaz FL, Truitt R, Tan LH, Dong B, Alysandratos KD, Huang J, Palmer JN, Adappa ND, Kohanski MA, Kotton DN, Silverman RH, Yang W, Morrisey EE, Cohen NA, & Weiss SR (2021). SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America, 118(16), e2022643118. 10.1073/pnas.2022643118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CX, Li X, Nan F, Jiang S, Gao X, Guo SK, Xue W, Cui Y, Dong K, Ding H, Qu B, Zhou Z, Shen N, Yang L, & Chen LL (2019). Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity. Cell, 177(4), 865–880.e21. 10.1016/j.cell.2019.03.046 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang M, Cheng A, Yang Q, Wu Y, Jia R, Liu M, Zhu D, Chen S, Zhang S, Zhao XX, Huang J, Mao S, Ou X, Gao Q, Wang Y, Xu Z, Chen Z, Zhu L, Luo Q, … Chen X (2020). The role of host eIF2α in viral infection. Virology journal, 17(1), 112. 10.1186/s12985-020-01362-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZS, Cai H, Xue W, Wang M, Xia T, Li WJ, Xing JQ, Zhao M, Huang YJ, Chen S, Wu SM, Wang X, Liu X, Pang X, Zhang ZY, Li T, Dai J, Dong F, Xia Q, Li AL, … Li T (2019). G3BP1 promotes DNA binding and activation of cGAS. Nature immunology, 20(1), 18–28. 10.1038/s41590-018-0262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd RE (2013). Regulation of stress granules and P-bodies during RNA virus infection. Wiley interdisciplinary reviews. RNA, 4(3), 317–331. 10.1002/wrna.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivannan P, Siddiqui MA, & Malathi K (2020). RNase L Amplifies Interferon Signaling by Inducing Protein Kinase R-Mediated Antiviral Stress Granules. Journal of virology, 94(13), e00205–20. 10.1128/JVI.00205-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny T, Van Treeck B, Huynh TN, & Parker R (2021). RNA partitioning into stress granules is based on the summation of multiple interactions. RNA (New York, N.Y.), 27(2), 174–189. 10.1261/rna.078204.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JY, & Krug RM (2006). The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: Inhibiting the 2’−5’ oligo (A) synthetase/RNase L pathway. Proceedings of the National Academy of Sciences of the United States of America, 103(18), 7100–7105. 10.1073/pnas.0602184103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen TH (2009). Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical microbiology reviews, 22(2), 240–273. 10.1128/CMR.00046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero H, Rojas M, Arias CF, & López S (2008). Rotavirus infection induces the phosphorylation of eIF2alpha but prevents the formation of stress granules. Journal of virology, 82(3), 1496–1504. 10.1128/JVI.01779-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri S, Rahman F, Williams BR, & Qin J (2000). A dynamically tuned double-stranded RNA binding mechanism for the activation of antiviral kinase PKR. The EMBO journal, 19(20), 5567–5574. 10.1093/emboj/19.20.5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Hiscott J, & Pitha PM (1997). The growing family of interferon regulatory factors. Cytokine & growth factor reviews, 8(4), 293–312. 10.1016/s1359-6101(97)00019-1 [DOI] [PubMed] [Google Scholar]
- Onomoto K, Jogi M, Yoo JS, Narita R, Morimoto S, Takemura A, Sambhara S, Kawaguchi A, Osari S, Nagata K, Matsumiya T, Namiki H, Yoneyama M, & Fujita T (2012). Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PloS one, 7(8), e43031. 10.1371/journal.pone.0043031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Choi DW, Cho SW, Han J, Yang S, & Choi CY (2020). Stress Granule Formation Attenuates RACK1-Mediated Apoptotic Cell Death Induced by Morusin. International journal of molecular sciences, 21(15), 5360. 10.3390/ijms21155360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, & Reis e Sousa C (2006). RIG-I-mediated antiviral responses to single-stranded RNA bearing 5’-phosphates. Science (New York, N.Y.), 314(5801), 997–1001. 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Protter D, & Parker R (2016). Principles and Properties of Stress Granules. Trends in cell biology, 26(9), 668–679. 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S, Prangley E, Donovan J, Demarest K, Wingreen NS, Meir Y, & Korennykh A (2019). Concerted 2–5A-Mediated mRNA Decay and Transcription Reprogram Protein Synthesis in the dsRNA Response. Molecular cell, 75(6), 1218–1228.e6. 10.1016/j.molcel.2019.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke LC, & Lloyd RE (2015). The stress granule protein G3BP1 recruits protein kinase R to promote multiple innate immune antiviral responses. Journal of virology, 89(5), 2575–2589. 10.1128/JVI.02791-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, & Chen ZJ (2005). Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell, 122(5), 669–682. 10.1016/j.cell.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Shen C, Li R, Negro R, Cheng J, Vora SM, Fu TM, Wang A, He K, Andreeva L, Gao P, Tian Z, Flavell RA, Zhu S, & Wu H (2021). Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell, 184(23), 5759–5774.e20. 10.1016/j.cell.2021.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U, & Parker R (2003). Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science (New York, N.Y.), 300(5620), 805–808. 10.1126/science.1082320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH (2007). Viral encounters with 2’,5’-oligoadenylate synthetase and RNase L during the interferon antiviral response. Journal of virology, 81(23), 12720–12729. 10.1128/JVI.01471-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH, Skehel JJ, James TC, Wreschner DH, & Kerr IM (1983). rRNA cleavage as an index of ppp(A2’p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. Journal of virology, 46(3), 1051–1055. 10.1128/JVI.46.3.1051-1055.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery E, Ghosh N, Samanta H, & Lengyel P (1979). Interferon, double-stranded RNA, and RNA degradation: activation of an endonuclease by (2’−5’)An. Proceedings of the National Academy of Sciences of the United States of America, 76(10), 4778–4782. 10.1073/pnas.76.10.4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber D, Tauber G, Khong A, Van Treeck B, Pelletier J, & Parker R (2020). Modulation of RNA Condensation by the DEAD-Box Protein eIF4A. Cell, 180(3), 411–426.e16. 10.1016/j.cell.2019.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourrière H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, & Tazi J (2003). The RasGAP-associated endoribonuclease G3BP assembles stress granules. The Journal of cell biology, 160(6), 823–831. 10.1083/jcb.200212128 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ung TL, Cao C, Lu J, Ozato K, & Dever TE (2001). Heterologous dimerization domains functionally substitute for the double-stranded RNA binding domains of the kinase PKR. The EMBO journal, 20(14), 3728–3737. 10.1093/emboj/20.14.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Treeck B, & Parker R (2018). Emerging Roles for Intermolecular RNA-RNA Interactions in RNP Assemblies. Cell, 174(4), 791–802. 10.1016/j.cell.2018.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Treeck B, Protter D, Matheny T, Khong A, Link CD, & Parker R (2018). RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proceedings of the National Academy of Sciences of the United States of America, 115(11), 2734–2739. 10.1073/pnas.1800038115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JR, Matheny T, Jain S, Abrisch R, & Parker R (2016). Distinct stages in stress granule assembly and disassembly. eLife, 5, e18413. 10.7554/eLife.18413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JR, Jain S, Khong A, & Parker R (2017). Isolation of yeast and mammalian stress granule cores. Methods (San Diego, Calif.), 126, 12–17. 10.1016/j.ymeth.2017.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan JN, Li Y, Silverman RH, & Weiss SR (2019). Zika Virus Production Is Resistant to RNase L Antiviral Activity. Journal of virology, 93(16), e00313–19. 10.1128/JVI.00313-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Cardenas AM, Marissen WE, & Lloyd RE (2007). Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell host & microbe, 2(5), 295–305. 10.1016/j.chom.2007.08.006 [DOI] [PubMed] [Google Scholar]
- Wreschner DH, James TC, Silverman RH, & Kerr IM (1981). Ribosomal RNA cleavage, nuclease activation and 2–5A(ppp(A2’p)nA) in interferon-treated cells. Nucleic acids research, 9(7), 1571–1581. 10.1093/nar/9.7.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner DH, McCauley JW, Skehel JJ, & Kerr IM (1981). Interferon action--sequence specificity of the ppp(A2’p)nA-dependent ribonuclease. Nature, 289(5796), 414–417. 10.1038/289414a0 [DOI] [PubMed] [Google Scholar]
- Yang W, Ru Y, Ren J, Bai J, Wei J, Fu S, Liu X, Li D, & Zheng H (2019). G3BP1 inhibits RNA virus replication by positively regulating RIG-I-mediated cellular antiviral response. Cell death & disease, 10(12), 946. 10.1038/s41419-019-2178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, & Fujita T (2004). The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature immunology, 5(7), 730–737. 10.1038/ni1087 [DOI] [PubMed] [Google Scholar]
- Yoo JS, Takahasi K, Ng CS, Ouda R, Onomoto K, Yoneyama M, Lai JC, Lattmann S, Nagamine Y, Matsui T, Iwabuchi K, Kato H, & Fujita T (2014). DHX36 enhances RIG-I signaling by facilitating PKR-mediated antiviral stress granule formation. PLoS pathogens, 10(3), e1004012. 10.1371/journal.ppat.1004012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa F, Muniozguren NL, Wilson MZ, Costello MS, Ponce-Rojas JC, & Acosta-Alvear D (2022). Signaling by the integrated stress response kinase PKR is fine-tuned by dynamic clustering. The Journal of cell biology, 221(7), e202111100. 10.1083/jcb.202111100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Romano PR, Nagamura-Inoue T, Tian B, Dever TE, Mathews MB, Ozato K, & Hinnebusch AG (2001). Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. The Journal of biological chemistry, 276(27), 24946–24958. 10.1074/jbc.M102108200 [DOI] [PubMed] [Google Scholar]
- Zheng ZQ, Wang SY, Xu ZS, Fu YZ, & Wang YY (2021). SARS-CoV-2 nucleocapsid protein impairs stress granule formation to promote viral replication. Cell discovery, 7(1), 38. 10.1038/s41421-021-00275-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


