Abstract
Neuroinflammatory events prior to the diagnosis of schizophrenia may play a role in transition to illness. To date only one in-vivo study has investigated this association between peripheral proinflammatory cytokines and brain markers of inflammation (e.g., mitochondrial 18 kDa translocator protein, TSPO) in schizophrenia, but none in its putative prodrome.
In this study, we primarily aimed to (1) test study group (clinical high-risk (CHR) and healthy controls) differences in peripheral inflammatory markers and test for any associations with symptom measures, (2) investigate the interaction between brain TSPO levels (dorsolateral prefrontal cortex (DLPFC) and hippocampus) and peripheral inflammatory clusters (entire cohort and (CHR) group independently) within a relatively large group of individuals at CHR for psychosis (N = 38) and healthy controls (N = 20). Participants underwent structural brain magnetic resonance imaging (MRI) and TSPO [18F]FEPPA positron emission tomography (PET) scans. Serum samples were assessed for peripheral inflammatory markers (i.e., CRP and interleukins). For exploratory analysis, we aimed to examine cluster differences for symptom measures and identify independent peripheral predictors of brain TSPO expression.
Here, we report increased IL-8 levels that are positively correlated with prodromal general symptom severity and showed trend-level association with apathy in CHR. We identified distinct inflammatory clusters characterized by inflammatory markers (IL-1 β, IL-2, IFN-γ) that were comparable between entire cohort and CHR. TSPO levels did not differ between inflammatory clusters (entire cohort or CHR). Finally, we show that CRP, IL-1 β, TNF-α, and IFN-γ levels were the independent peripheral predictors of brain TSPO expression.
Thus, alterations in brain TSPO expression in response to inflammatory processes are not evident in CHR. Taken together, clustering by inflammatory status is a promising strategy to characterize the interaction between brain TSPO and peripheral markers of inflammation.
Keywords: Clinical high-risk, Peripheral inflammation, Neuroinflammation, MRI, PET, TSPO
Highlights
-
•
Serum IL-8 levels are elevated in individuals at CHR for psychosis.
-
•
Positive association between elevated IL-8 and prodromal general symptom severity.
-
•
Inflammatory clusters (IL-1β, IL-2, IFN-γ) are identified (entire cohort and CHR).
-
•
TSPO levels did not differ between inflammatory clusters (entire cohort or CHR).
-
•
CRP, IL-1β, TNF-α and IFN-γ levels are the independent predictors of brain TSPO.
1. Introduction
Several lines of evidence from epidemiological, preclinical and genetic studies suggest that abnormal immune response contribute to the pathophysiology of schizophrenia. Brain immune cells, in particular microglia and immunoproteins (e.g., cytokines and C-reactive protein, CRP), play an important role in neuroinflammatory responses both in the brain and periphery (Barron et al., 2017). In response to brain insults, microglial cells become activated, and at the molecular level increase the expression of a mitochondrial protein, translocator protein 18 kDa (TSPO). Currently, positron emission tomography (PET) using radioligands that target TSPO is the most reliable and valid method to quantify TSPO in vivo in brain (Hafizi et al., 2017a). Although previous studies using the prototypical radiotracer for TSPO, [11C]PK11195, reported higher TSPO expression in schizophrenia (Doorduin et al., 2009; van Berckel et al., 2008), the majority of recent studies using second-generation radiotracers (e.g., [11C]DPA-713, [11C]PBR28, and [18F]FEPPA) and validated outcome measures found reduced (Collste et al., 2017) or no significant difference (Hafizi et al., 2017a, 2018a; Coughlin et al., 2016) in brain TSPO expression in first-episode psychosis and individuals at clinical high risk for psychosis (CHR) (Hafizi et al., 2017b) as compared to healthy controls. Moreover, a recent meta-analysis (Plavén-Sigray et al., 2021) reported a significant decrease in TSPO expression in schizophrenia.
CRP and cytokines are commonly used as markers of inflammatory status. Based on their function, cytokines can be categorized as either pro-inflammatory such as Interleukin (IL) 1 beta (IL-1β), IL-6 and IL-12, or anti-inflammatory such as IL-2 and IL-10. A meta-analysis (Miller et al., 2011) combining data from 40 studies identified increases in peripheral pro-inflammatory and decreases in anti-inflammatory cytokine proteins in schizophrenia. The dynamic balance between these two categories of cytokines contributes to proper immune response.
CRP is an acute phase protein which is produced and released by liver cells in response to inflammation. Several studies have reported elevated levels of CRP in schizophrenia (Miller et al., 2014; Fernandes et al., 2016; North et al., 2021). Supporting this, a longitudinal study has reported an association between adolescent CRP level and consequent schizophrenia diagnosis by age 27 (odds ratio = 1.25) (Metcalf et al., 2017). Studies on cytokines levels in blood and cerebrospinal fluid (CSF) revealed elevated expressions of IL-1β, IL-6, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-12 and transforming growth factor beta (TGF-β) in blood and IL-1β in CSF of first-episode psychosis patients (Miller et al., 2011). A population-based longitudinal study reported an association between childhood IL-6 levels and risk of psychosis in adulthood (Khandaker et al., 2014). Similarly, pro-inflammatory cytokine levels (IL-6) were elevated in CHR, and associated with severity of attenuated psychotic symptoms (Stojanovic et al., 2014). More recently, plasma-based markers of inflammation were found to be predictors of conversion to a full psychotic break in CHR (Perkins et al., 2015). Several cross-sectional and post-mortem studies have also found large increases in cortical and subcortical cytokine levels in about half of those with chronic schizophrenia (Miller et al., 2011; North et al., 2021; van Kesteren et al., 2017; Boerrigter et al., 2017; Fillman et al., 2013, 2016); however, a post-mortem meta-analysis combining 41 studies reported no consistent diagnostic group effect on cytokine levels in schizophrenia samples (van Kesteren et al., 2017). Importantly, an inflammatory stratification approach was not applied in most studies which hinders their interpretation. Interestingly, previously reported studies in schizophrenia (Boerrigter et al., 2017) revealed that a subgroup of about ∼40% of people with schizophrenia had a pattern of relatively elevated cytokine expression compared to 20% in the peripheral blood (Fillman et al., 2016) and 10% in the post-mortem prefrontal cortex tissue (Fillman et al., 2013) in healthy controls. However, to our knowledge, similar inflammatory subsets have never been studied in individuals at risk for psychosis such as the well characterized CHR state (Hafizi et al., 2017b).
To date, only one in vivo study (Coughlin et al., 2016) has investigated the association between markers of inflammation in the brain (TSPO) and peripheral tissue (plasma) in schizophrenia, and reported no relationship, similar to the post-mortem brain when not stratified by inflammatory status. In the present study, we measured peripheral inflammatory protein levels together with TSPO expression in the brain (with [18F] FEPPA, or fluorine F 18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide using full kinetic modelling in a High-Resolution Research Tomograph (HRRT)) in a relatively large sample of mostly unmedicated CHR and healthy controls. We primarily aimed to (1) test study group (CHR and Healthy controls) differences in peripheral inflammatory markers and test for any associations with symptom and cognition measures, (2) identify clusters in the entire cohort and CHR group alone based on their peripheral inflammatory (including IL-2 and IL-10) profile, (3) examine cluster differences in the dorsolateral prefrontal cortex and hippocampal TSPO levels (Kreisl et al., 2013; Busse et al., 2012). Further, in the exploratory analyses, we aimed to (1) examine cluster differences for symptom severity and cognition and (2) identify the independent peripheral predictors of brain TSPO expression in the combined cohort. We expected to identify a subset of people with elevated inflammatory state (larger in the CHR cohort). We hypothesised that this inflammatory subset would be significantly associated with [18F]FEPPA total volume of distribution (VT) binding and have worse symptomatology and lower cognition. Finally, we expected to identify peripheral inflammatory markers that may be predictive of TSPO VT.
2. Materials and methods
2.1. Participants
Fifty-nine participants including 39 CHR and 20 healthy controls were included in this study. Most of the individuals in the CHR group were antipsychotic-naive (n = 33). Two healthy controls and one CHR whose PET images were of insufficient quality were excluded from the PET analyses, and another healthy control was excluded due to low-affinity binder (LAB) genotype which cannot be reliably quantified with [18F] FEPPA. While CRP levels were available for all participants, the cytokine levels were only available for 38 CHR (Age, 20.95 ± 2.78 (Mean ± Standard deviation (SD)) and 20 healthy controls (Age, 21.30 ± 2.06 (Mean ± SD) (see Table 1)). The study was performed under a repository protocol that allowed a re-analysis of previously acquired data approved by the Centre for Addiction and Mental Health Research Ethics Board and now approved under Clinical and Translational Sciences (CaTS) BioBank by Research Ethics Board (REB) of the Centre intégré universitaire de santé et de services sociaux (CIUSSS) de l'Ouest-de-l'Île-de-Montréal – Mental Health and Neuroscience subcommittee. Participants were recruited at the Centre for Addiction and Mental Health (CAMH) from January 1, 2015, to October 20, 2018 (Ontario, Canada). The study was performed in accordance with Good Clinical Practice guidelines, regulatory requirements, and the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was initially obtained from all participants at the beginning of screening after a full explanation of anticipated study procedures. The majority of the participants (healthy controls, n = 20 and CHR, n = 35) in this study were also part of our previous studies (Hafizi et al., 2017b, 2018a, 2018b).
Table 1.
Demographics and characteristics of the participants.
| Healthycontrols (n = 20) | Clinical high-risk (n = 38) | t-Test or χ2 Test value | p value | ||
|---|---|---|---|---|---|
| Age (years), Mean ± SD | 21.30 ± 2.06 | 20.95 ± 2.78 | t = 0.50 | p = 0.62 | |
| Sex | Male, n | 7 | 21 | χ2 = 2.16 | p = 0.14 |
| Female, n | 13 | 17 | |||
| BMI (kg/m2), Mean ± SD | 23.25 ± 4.79 | 23.63 ± 4.98 | t = - 0.28 | p = 0.78 | |
| TSPO rs6971 Genotype a | HAB, n | 14 | 24 | χ2 = 0.63 | p = 0.43 |
| MAB, n | 5 | 14 | |||
| PET Parameters, Mean ± SD a | Specific activity (mCi/μmol) | 1996.10 ± 1908.94 | 1678.42 ± 1305.83 | t = 0.73 | p = 0.47 |
| Mass injected (μg) | 1.70 ± 1.35 | 1.86 ± 1.49 | t = −0.39 | p = 0.70 | |
| Amount injected (mCi) | 4.96 ± 0.34 | 5.10 ± 0.26 | t = −1.68 | p = 0.10 | |
| Tobacco use | Non-users, n | 20 | 31 | χ2 = 4.19 | p = 0.04 |
| Users, n | 0 | 7 | |||
| Cannabis useb | Non-users, n | 20 | 34 | χ2 = 2.26 | p = 0.13 |
| Users, n | 0 | 4 | |||
Abbreviations: BMI – body mass index; χ2 – Chi-squared statistic; HAB – high-affinity binder; μg – microgram; μmol – micromole; mCi – millicurie; MAB – mixed-affinity binder; PET - positron emission tomography; p value - significance; SD – Standard deviation; TSPO - Translocator protein 18 kDa.
Two healthy controls and one CHR were excluded from the positron emission tomography (PET) analyses due to unreliable PET data, and another healthy control was excluded due to low-affinity binder (LAB) genotype which cannot be quantified with [18F]FEPPA.
4 CHR individuals had a positive urine drug screen for cannabis.
To be eligible, CHR individuals had to meet the following criteria: fulfilment of diagnostic criteria for prodromal syndrome as per the Criteria of Prodromal Syndromes (COPS) (Miller et al., 2002) with no current axis I disorders such as depression which has been identified to be associated with microglial activation (Setiawan et al., 2015), as determined with the Structured Clinical Interview for DSM-IV-TR (SCID) (First and Gibbon, 2004). Healthy controls did not have any history of psychiatric illness, psychoactive drug use, and/or first-degree relative with a major mental illness. Participants were excluded for any of the following: clinically significant medical illness, inflammatory condition that warranted anti-inflammatory medication use and/or chronic anti-inflammatory medication use, elevated body temperature indicating possible infection/inflammatory status, pregnancy or current breastfeeding, presence of metal implants precluding MRI scan. In CHR, clinical status and severity of symptoms (e.g., psychosis-risk symptoms) were assessed with the Structured Interview for Psychosis-Risk Syndromes (SIPS), Scale of Psychosis-Risk Symptoms (SOPS) (Miller et al., 2002), Calgary Depression Scale (CDS) (Addington et al., 2014), Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995), and Apathy Evaluation Scale (AES) (Marin et al., 1991). Neurocognitive performance was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., 1998).
2.2. PET and structural MRI data acquisition and analysis
PET and MRI data acquisition have been described in detail elsewhere (Hafizi et al., 2018a; Kenk et al., 2015) and are summarized below. A proton density-weighted (PD) brain MRI scan was obtained for each subject using a 3 T MR-750 scanner (General Electric Medical Systems). All [18F]FEPPA PET scans were performed using a high-resolution neuro-PET camera system (HRRT, Siemens Molecular Imaging, Knoxville, TN, USA) for 125 min following an intravenous bolus injection of 187.11 ± 10.83 MBq MBq of [18F]FEPPA. Arterial blood samples were collected automatically using an automatic blood sampling system (Model PBS-101, Veenstra Instrument, Joure, Netherland) for the first 22.5 min minutes after radioligand injection at a rate of 2.5 mL/minute and manually at −5, 2.5, 7, 12, 15, 20, 30, 45, 60, 90, and 120 min to measure radioactivity in blood and determine the relative proportion of radiolabelled metabolites. Dispersion-and metabolite-corrected plasma input function was generated as previously described (Rusjan et al., 2011).
2.3. PET image processing and calculation of total volume of distribution (VT)
Time-activity curves were extracted for the dorsolateral prefrontal cortex (DLPFC) and hippocampus using validated in-house imaging pipeline ROMI (Rusjan et al., 2006). DLPFC and hippocampus were chosen as the prioritized regions of interest (ROI) based on the previous data (Kreisl et al., 2013; Busse et al., 2012). The ROI was delineated using individual PD MRI. Kinetic parameters of [18F]FEPPA were derived from the time-activity curves using the two-tissue compartment model (2-TCM) and plasma input function to obtain the VT for each region of interest, which has been validated for [18F]FEPPA quantification and described elsewhere (Kenk et al., 2015; Rusjan et al., 2011).
2.4. TSPO rs6971 polymorphism genotyping
Participants were genotyped for their TSPO rs6971 polymorphism and categorized as high-(HAB), mixed- (MAB), or LAB, as described elsewhere (Mizrahi et al., 2012).
2.5. Serum acquisition and measurement of high sensitive-CRP (HsCRP) and cytokines
The serum samples obtained on the day of the PET scan from early non-fasting (period since last caloric intake, 0–2.9 h) participants were isolated from whole-blood specimens collected in BD Vacutainer K2EDTA tubes. The samples were centrifuged at 3000 r/min for 15 minminutes at 4 °C and was then transferred to a fresh polypropylene microtube (1.5 mL capacity) which was stored at −80 °C. No samples were thawed more than once before analysis. Cytokine levels in serum were measured with the MILLIPLEX Multi-Analyte Profiling Human Cytokine/Chemokine Assay employing Luminex technology according to manufacturer's protocol. The cytokines included in the assay were IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12, TNF-α and IFN-γ. HsCRP was measured in serum using a high-sensitivity ELISA according to the manufacturer's instructions (IBL-international, Hamburg, Germany). We quantitatively determined the steady state level of the circulating inflammatory (both pro- and anti-inflammatory) cytokines from serum using the MILLIPLEX Multi-Analyte Profiling (MAP) Human Cytokine/Chemokine Kit for 96-well assay (Millipore) run on a Luminex platform (MXH8060-2). 25 μl of neat serum from each participant was serially diluted and was diluted 1:4 using reconstituted stock standards supplied by the manufacturer. A standard curve range of 3.2–10,000 pg/mL was used to calculate average values generated using Millipore Analyst Software. A low and a high concentration quality control (QC) supplied by the manufacturer for each analyte was used to calibrate and quantify the analyte concentrations. For quality assurance, each sample was run twice, and the mean derived for each sample was used as the index value. Additionally, two kit-supplied quality controls were run on each plate in duplicate and confirmed to fall within the expected range (see S.1 for detailed description).
2.6. Statistical analyses
We used chi-square (χ2) tests and independent sample t-tests to evaluate differences in categorical variables (e.g., sex and TSPO rs6971) and in continuous variables (e.g., age), respectively between CHR and healthy controls. Initially, we tested for study group by cytokine interaction i.e., the effect of group which varies between cytokines using random effects mixed model analysis, adjusted for body mass index (BMI) with cytokines (interleukins and CRP) as a repeated within-subject fixed factor. Further, interleukin and CRP levels were compared using the Mann–Whitney U test and their mean ranks were presented. The results of the analyses are reported including the extreme outliers (defined as inflammatory marker values outside 3rd quartile + 3*interquartile and 1st quartile – 3*interquartile ranges or as determined using box-plot analysis) given their potential importance in understanding the divergence of study groups based on the actual measured peripheral inflammatory marker profile (see supplementary S.2 for the analysis excluding these outliers and S.3 for the analyses including only pro-inflammatory markers (excluding IL-2 and IL-10)). The extreme outliers observed study group-wise include CRP (HC, n = 1; CHR, n = 6), IFN-γ (HC, n = 1), IL-12 (CHR, n = 2) and IL-2 (HC, n = 1). We also explored the associations between the differentially expressed peripheral inflammatory biomarker(s) and clinical symptom scales (SOPS and CDS (CHR), cognition and other behavioural measures such as anhedonia, apathy) in the respective study group by using Pearson partial correlations, controlling for BMI.
Further, the inflammatory cytokine values were tested for normality (Shapiro-Wilk) and homogeneity of variances (Levene's test). Given the low-skewed distribution, all inflammatory cytokine values were log transformed to approximately conform to normality. Any extreme outlier values for cytokines (IL-12: HC, n = 1) following log transformation were excluded from the following cluster and exploratory analyses.
To identify inflammatory clusters based on peripheral inflammatory marker levels, a recursive two-step cluster analysis was performed on the entire cohort and then on the CHR cohort alone as previously described (Boerrigter et al., 2017; Fillman et al., 2016). The clustering for the entire cohort was performed in 57 individuals (19 HC and 38 CHR) (excluding extreme outlier, IL-12: HC, n = 1). Briefly, the clustering algorithm was run once with all predictive factors, in this case, all nine inflammatory markers (CRP, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12, TNF-α and IFN-γ). The model developed was examined for silhouette measure of cohesion and separation and the contribution of each variable to the model. The overall model quality was required to be > 0.5, with predictors of least importance removed until all predictors had significant contribution to the model (≥0.5 on a scale of 0–1.0) (Bacher et al., 2004). Following the clustering of the entire cohort, [18F]FEPPA VT differences were tested using random effects mixed model analysis in prioritized ROIs (DLPFC and hippocampus), controlling for TSPO rs6971 polymorphism and BMI. Non-significant interactions were removed from the model. Further, inflammatory cluster by subscale interactions i.e., effect of cluster which varies between subtests in attenuated psychosis symptom (SOPS positive, negative, disorganization, general) and cognition (RBANS immediate memory, visuospatial ability, language, attention, delayed memory) subscales were explored using random effects mixed model analysis with SOPS subscales and RBANS subtests as repeated within-subject fixed factor, respectively adjusted for BMI. Univariate analysis of variance was used for assessing inflammatory cluster differences in SOPS total symptom severity scale (CHR), RBANS total scale and other behavioural measures (depression (CHR), anhedonia, apathy) as exploratory analyses. Same procedures were followed for the CHR group (see Fig. 1 for the schematic illustration of the analyses).
Fig. 1.
Schematic CONSORT diagram as per analysis plan
Fig. 1. The schematic CONSORT diagram illustrates the analyses included in the study.
Abbreviations: AES - Apathy Evaluation Scale; CDS - Calgary Depression Scale; CHR – Clinical High-risk; HC – Healthy control; IL-12 – Interleukin-12; N – Sample size; PET – Positron emission tomography; RBANS - Repeatable Battery for the Assessment of Neuropsychological Status; SOPS - Scale of Psychosis-Risk Symptoms; SHAPS Snaith-Hamilton Pleasure Scale; TSPO - Translocator protein 18 kDa.
Finally, we investigated peripheral markers that predict [18F]FEPPA VT in the brain. We used a backward random effects mixed model analysis to find the independent predictors of [18F]FEPPA VT in the whole sample by including inflammatory serum proteins as covariates, controlling for TSPO rs6971 polymorphism, clinical group and BMI. Brain regions (DLPFC and hippocampus) were entered as within-subject factors. We started by including all the variables and dropping the variable with the least significance manually, until all the variables in the model were significant.
Statistical analyses were performed using SPSS (SPSS, Chicago, IL, USA). The significance level was set at p < 0.05 two-tailed.
3. Results
3.1. Demographic and clinical characteristics
Demographic and clinical characteristics of the participants are presented in Table 1. There were no significant group differences in age, sex, TSPO rs6971 genotype, PET parameters (specific activity, mass injected, and amount injected), and cannabis use. There was a significant group effect for tobacco use.
3.2. Study group differences in serum inflammatory markers and association with symptom measures
3.2.1. Group differences in peripheral serum inflammatory marker levels
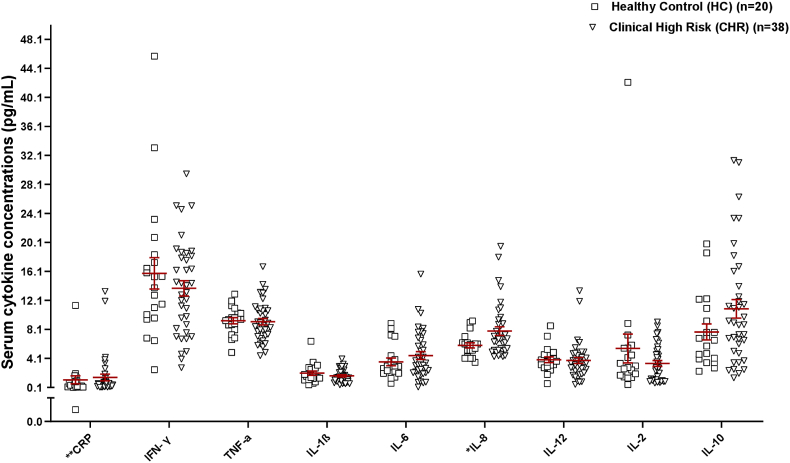
We found a statistically significant study group-by-cytokine interaction (F(17,107.69) = 53.46, p < 0.001), controlling for BMI (F(1,56.77) = 0.63, p = 0.43). We found that IL-8 levels in the CHR group (mean rank = 33.14) were significantly higher than the healthy control group (mean rank = 22.58) (U = 241.500, p = 0.02 (see supplementary S.4.1 for mean ranks) with pairwise comparisons surviving corrections for multiple testing (Fig. 2).
Fig. 2.
Serum inflammatory marker concentrations between study groups
Fig. 2. Peripheral inflammatory markers, IL-8 showed a significant increase by 33.6% in CHR compared to healthy controls. Data plotted as mean ± SEM; IL-8, *p = 0.035 surviving Bonferroni corrections for all peripheral measures tested. **CRP values are in μg/mL.
Abbreviations: CRP - C-reactive protein; IFNγ – Interferon gamma; IL – Interleukin; IL-1β – Interleukin-1 beta; mL – milliliter; pg - picograms; TNF-α – Tumor necrosis factor-alpha.
3.2.2. Positive association between IL-8 levels and General symptom severity and Apathy in CHR
Within the CHR group, increased IL-8 levels were positively correlated with SOPS general symptom score (r = 0.43, p = 0.008, Fig. 3A), surviving corrections for number of SOPS subscales. IL-8 levels also showed a trend for positive association with apathy (r = 0.32, p = 0.059, Fig. 3B) (see supplementary S.4.2 for other scales tested) in individuals at CHR for psychosis.
Fig. 3.
Association between IL-8 levels and prodromal General symptom severity and Apathy in CHR.
Fig. 3. Relationship between IL-8 and A) General symptom severity score and B) Apathy score as measured by Scale of Psychosis-Risk Symptoms (SOPS) General symptom subscale and Apathy Evaluation Scale (AES), respectively, in CHR individuals, adjusted for BMI.
Abbreviations: BMI – body mass index; CHR – clinical high-risk; IL-8 – Interleukin-8; mL – milliliter; pg - picograms.
3.3. Identification of inflammatory clusters
3.3.1. Inflammatory clusters in the entire cohort
Prior to cluster analysis, one extreme outlier for inflammatory marker, IL-12 (HC, n = 1) following log-transformation was noted. Analysis of the data excluding this outlier yielded three clusters in the entire cohort characterized by inflammatory markers IL-1β, IL-2 and IFN-γ. The clusters had an overall model quality (Silhouette measure) of 0.5 and all three variables (IL-1β, IL-2 and IFN-γ in order of their contribution) contributed significantly to the model (≥ 0.5 on a scale from 0.1 to -1.0). The clusters were labelled ‘low cytokine’ (total n = 16; 13 CHR and 3 healthy controls), ‘intermediate cytokine’ (total n = 21; 12 CHR and 9 healthy controls) and elevated cytokine’ (total n = 20; 13 CHR and 7 healthy controls) clusters (Fig. 4A).
Fig. 4.
Inflammatory clusters based on peripheral inflammatory marker levels
Fig. 4. Inflammatory clusters of (A) entire cohort characterized by low, intermediate, and elevated inflammatory marker levels and (B) CHR group characterized by low and elevated inflammatory marker levels in serum are represented.
3.3.2. Inflammatory clusters in the CHR group
Two clusters characterized by inflammatory markers IL-2, IL-1β and IFN-γ were identified. The resulting two clusters had an overall model quality (Silhouette measure) of 0.5 and were comparable to the clusters identified in the whole cohort except for the order of their contribution (IL-2, IL-1β and IFN-γ in order of their contribution) to the model (≥ 0.5 on a scale from 0.1 to -1.0). The clusters were labelled ‘elevated cytokine’ (total n = 24) and ‘low cytokine’ (total n = 14) (Fig. 4B).
3.4. Inflammatory cluster differences in brain TSPO levels
3.4.1. TSPO levels were not different between inflammatory clusters in the entire cohort
We found no significant differences in [18F]FEPPA VT between inflammatory clusters (main cluster effect: F(2,53) = 2.07, p = 0.14; ROI effect: F(1,53) = 5.16, p = 0.03, controlling for TSPO rs6971 polymorphism and BMI effect (F(1,53) = 7.33, p = 0.009) in the entire cohort. [18F]FEPPA VT levels were relatively same among the different inflammatory clusters namely, low (mean: 9.74; 95% CI: 8.15 to 11.32), intermediate (mean: 8.62; 95% CI: 7.36 to 9.88) and elevated (mean: 10.50; 95% CI: 9.17 to 11.83) cytokine clusters (Fig. 5A).
Fig. 5.
[18F]FEPPA (VT) in the prioritized ROIs among inflammatory clusters in the entire cohort and CHR group
Fig. 5. The scatter dot plot graph reflects [18F]FEPPA regional binding (VT) in prioritized ROIs (i.e., DLPFC and hippocampus) among the three inflammatory clusters (low, intermediate and elevated cytokine cluster) in the entire cohort (A) and two inflammatory clusters (low and elevated cytokine cluster) in the CHR group (B). Horizontal bar indicates group mean adjusted for TSPO rs6971 genotype and BMI using the estimated marginal means of each region.
Abbreviations: CHR - Clinical high risk; DLPFC - dorsolateral prefrontal cortex; [18F]FEPPA - fluorine F 18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide; mL/cm3 – milliliter/cubic centimetre; VT - total volume of distribution.
In the exploratory analysis, we found no main effect of inflammatory clusters on RBANS total score (F(2,51) = 1.39, p = 0.26) or RBANS subtest scores (immediate memory, visuospatial ability, language, attention, delayed memory) (F(2,60.71) = 1.35, p = 0.27). We also did not find any significant inflammatory clusters-by-RBANS subscales interaction (F(8,95.79) = 1.78, p = 0.09), adjusted for RBANS subscales and BMI effect. There were also no significant differences in clinical symptom measures (i.e., apathy (F(2,50) = 0.95, p = 0.39), anhedonia (F(2,51) = 1.73, p = 0.19) among inflammatory clusters, controlling for BMI.
3.4.2. TSPO levels were not different between inflammatory clusters in the CHR group
There were no significant differences in [18F]FEPPA VT between inflammatory CHR clusters (main cluster effect: F(1,37) = 0.003, p = 0.95; ROI effect: F(1,37) = 7.24, p = 0.01, controlling for TSPO rs6971 polymorphism and BMI effect (F(1,37) = 2.00, p = 0.17) (Fig. 5B).
In the exploratory analysis, no difference was observed between inflammatory CHR clusters in attenuated psychosis symptoms (SOPS total symptoms severity score (F(1,35) = 0.56, p = 0.46) or individual symptom dimensions (positive, negative, disorganization, general): F(1,50.13) = 0.32, p = 0.58) or found any significant inflammatory group-by-SOPS-subscale interaction (F(3,66.81) = 0.49, p = 0.69). We also found no statistically significant main effect of inflammatory clusters on RBANS total score (F(1,35) = 3.76, p = 0.06) or RBANS subscale scores (F(1,42.92) = 3.49, p = 0.07). We also did not find any significant inflammatory clusters-by-RBANS subscale interaction (F(4,73.68) = 1.85, p = 0.13), adjusted for RBANS subscales and BMI effect. There were also no significant differences in other clinical symptom measures (i.e., depression (F(1,35) = 0.10, p = 0.76), apathy (F(1,34) = 0.02, p = 0.89) and anhedonia (F(1,35) = 0.18, p = 0.67)) among inflammatory clusters, controlling for BMI.
3.5. CRP, IL-1β, TNF-α and IFN-γ are the independent predictors of brain TSPO expression
CRP, IL-1β, TNF-α and IFN-γ levels were significantly associated independently with [18F]FEPPA VT in the combined cohort (CRP: F(1, 53) = 28.48, p < 0.001; IL-1β: F(1, 53) = 12.51, p = 0.001; TNF-α: F(1, 53) = 7.73, p = 0.008; IFN-γ: F(1, 53) = 4.39, p = 0.04) controlling for TSPO rs6971 polymorphism, clinical group, ROI and BMI effect (BMI effect: F(1, 53) = 0.62, p = 0.43). We found no significant group interactions in the model (Group*CRP: F(1, 53) = 1.64, p = 0.21; Group* IL-1β: F(1, 53) = 2.69, p = 0.11; Group* TNF-α: F(1, 53) = 0.81, p = 0.37; Group*IFN-γ: F(1, 53) = 1.17, p = 0.29).
4. Discussion
Here, we report increased IL-8 levels that show a significant positive association with prodromal general symptom severity and a trend-level association with apathy in individuals at CHR for psychosis. We identified three distinct clusters with varying grades of inflammation (low, intermediate, and elevated) characterized by markers IL-1β, IL-2 and IFN-γ in the combined cohort and two clusters (low and elevated) in the CHR group. Brain TSPO levels were not different between the inflammatory clusters (entire cohort or CHR group). Finally, we show that CRP, IL-1β, TNF-α and IFN-γ levels are the independent peripheral predictors of TSPO expression in the brain.
IL-8, a potent chemokine was found to be elevated by 33.6% in CHR individuals when compared to healthy controls. In contrast, previous studies in CHR and Ultra-high risk (UHR) individuals found unaltered peripheral IL-8 levels (Park and Miller, 2020; Misiak et al., 2021). However, meta-analyses in first episode psychosis antipsychotic-naive (Miller et al., 2011; Çakici et al., 2020) and acute relapsed chronic patients (Goldsmith et al., 2016; Wang and Miller, 2018) repeatedly showed elevated peripheral and CSF (Gallego et al., 2018) IL-8 levels similar to our findings (but see (Pillinger et al., 2019)). Additionally, previous data suggests absence of elevated IL-8 levels in medicated patients (Frydecka et al., 2018). In our sample, of the 38 CHR individuals, only 5 of them were on low-dose antipsychotic treatment and others were antipsychotic-naive which may explain the findings. However, another meta-analysis non-stratified by treatment or patient cohort found IL-8 levels to be unaffected in antipsychotic treated patients (Romeo et al., 2018).
Research on chemokine alterations in schizophrenia is limited (Frydecka et al., 2018) in comparison to other non-chemokine interleukins (Goldsmith et al., 2016; Capuzzi et al., 2017) however, an interesting study on pregnant women reported a correlation between increased serum IL-8 levels and psychosis-risk in the adult offspring (Brown et al., 2004). Additionally, in our CHR sample, serum IL-8 levels also showed a positive correlation with general symptom severity and a trend-level association with apathy. These outcomes are consistent with our previous findings in CHR (Da Silva et al., 2018) demonstrating a positive relationship between lactate levels and prodromal negative symptoms. Similar results were also observed in a study investigating diagnostic markers in CHR (North American Prodrome Longitudinal Study (NAPLS)). The study showed that the blood levels of several interleukins including IL-8 were positively correlated with the severity of positive symptoms (i.e., delusional ideas), poor attention, and dysphoric mood (characteristic of general symptom severity) (Perkins et al., 2015). Previous studies in schizophrenia also reported elevated IL-8 levels in patients with aggravated negative symptoms (Enache et al., 2021) and clinical global impression (CGI) severity (Dahan et al., 2018) similar to positive correlation with apathy observed in our CHR sample.
We identified three distinct clusters (IL-1β, IL-2 and IFN-γ) with differentially expressed inflammation levels (low, intermediate and elevated) in the entire cohort which were comparable to the inflammatory cluster composition in the CHR group (IL-2, IL-1β and IFN-γ) characterizing the two clusters (low and elevated) identified. Elevations in the individual peripheral inflammatory markers identified in our inflammatory subsets (characterized by IL-1β, IL-2 and IFN-γ) have been reported in patients with psychosis extensively (Dawidowski et al., 2021). Whether this represents an inflammatory process defined by individual or subgroups of markers is still unclear. Our study takes the first step in identifying inflammatory subgroups within mostly unmedicated CHR and assesses their association with microglial activation. Previously, a study in psychosis population (Lizano et al., 2021) found that elevated levels of TNFα, CRP, VEGF and IL-6 were associated with changes in regional brain volume in multiple brain regions including hippocampus. However, in our study sample, brain TSPO levels were not differentially expressed between inflammatory clusters (entire cohort or CHR). This finding is consistent with six previous PET studies of TSPO expression in psychosis using second-generation radioligands and validated two-tissue compartment model for TSPO, including CHR (Hafizi et al., 2017b), first-episode psychosis (Coughlin et al., 2016; Hafizi et al., 2017a; Hafizi et al., 2018a) or chronic schizophrenia (Kenk et al., 2015; Takano et al., 2010) that reported no significant differences in microglial activation between study groups.
Previous studies have shown that knockdown of TSPO influenced aggravated inflammatory response to lipopolysaccharide (LPS), a potent neuroimmune stimulus while overexpression of TSPO caused reduced cytokine production (Wang et al., 2016; Bae et al., 2014) implying a negative regulatory mechanism (Bae et al., 2014; Ma et al., 2016). In our study sample, almost all the parameters are normal which may explain the negative results. Although our inflammatory stratification approach, identified inflammatory clusters with varying grades of inflammation, an aggravated inflammatory response with marked elevations in peripheral cytokines were not evident in our study groups. In contrast, schizophrenia patients have previously shown elevated levels of inflammatory cytokines in peripheral and central tissues in association with lowered TSPO expression in the middle frontal gyrus (Coughlin et al., 2016; Notter et al., 2018a). However, this does not preclude that altered TSPO signalling could be the result of other underlying factors. These include systemic changes through interplay between various other cell types involved in neuroimmune modulation (Notter et al., 2018a) or a consequence of the oxidative stress during inflammation (Notter et al., 2018b), cellular metabolism changes and/or mitochondrial dysfunction (Gut, 2015; Batoko et al., 2015; Banati et al., 2014), all of which have been previously implicated in schizophrenia (Steullet et al., 2016).
Although, we do not present data in a confirmatory cohort as it is not customary with PET studies, longitudinal clinical follow-up revealed that 5 of the 39 individuals at CHR in this study (∼13%) converted to psychosis. Although this was a cross-sectional study and conclusions were limited by small sample size, we found no significant differences in the levels of central or peripheral inflammatory markers (data not shown) between individuals at CHR who converted to psychosis and non-converters. More comprehensive indices such as proteomic prediction models may be better suited to predict transition to psychosis in CHR individuals over single inflammatory markers (Mongan et al., 2021). Together, our findings showing no evidence of altered brain TSPO levels among the inflammatory clusters (entire cohort or CHR) suggests that the negative relationship observed between TSPO expression and low-grade inflammation in schizophrenia is not evident in the putative prodromal state. Nevertheless, inflammatory stratification as depicted in the current study as opposed to most previous psychosis studies that focused on a singular marker at a time is crucial to understand the entangled interplay between multiple inflammatory signalling systems (central and periphery) in the body and across the psychosis spectrum.
Our exploratory analyses did not find any significant relationship between inflammatory clusters and symptom severity or cognition (entire cohort or CHR). This is in line with a previous study (Karanikas et al., 2017) which did not observe any relationship between clinical symptom severity and cytokine levels in first-episode psychosis. However, two previous studies (Stojanovic et al., 2014; Zeni-Graiff et al., 2016) reported an association between peripheral cytokines’ levels and severity of symptoms in line with the NAPLS evidence in CHR (Perkins et al., 2015).
On the contrary, CRP, IL-1β, TNF-α and IFN-γ levels in serum were found to independently predict TSPO in the brain (combined cohort). We found that the variance inflation factor (VIF) using regression analysis predicted only a moderate correlation among the significant predictors of brain TSPO (CRP: VIF = 1.01; IL-1β: VIF = 2.05; TNF-α: VIF = 1.00; IFN-γ: VIF = 2.06). Hence, the modelling takes care of the collinearity between the inflammatory proteins included in the model. Thus, the listed peripheral inflammatory markers are indeed reliable estimates of brain TSPO expression.
Coughlin and colleagues (Coughlin et al., 2016) reported an elevation of IL-6 levels in plasma of schizophrenia, however, found no association with TSPO. In contrast, LPS challenge produced significant TSPO elevation in the whole brain indicating a neuroimmune response associated with microglial activation in healthy adult brains (Woodcock et al., 2021). Interestingly, a longitudinal PET study in healthy male volunteers with repeated LPS challenges, found an initial increase and then a decrease in TSPO binding following subsequent LPS challenge (Nettis et al., 2020), suggestive of cerebral immunotolerance. Importantly, to date in vivo PET studies including patients with mood disorders found elevated brain TSPO levels compared with healthy subjects but no significant correlation between peripheral and central inflammation (Schubert et al., 2021). Recently, Attwells and colleagues (Attwells et al., 2020) reported that the natural logarithm of serum PGE2/CRP and TNFα/CRP to be highly predictive of TSPO in mood or anxiety disorders.
Of interest, our inflammatory clusters (entire cohort or CHR) did not include CRP levels. This discrepancy between CRP results and other pro-inflammatory markers could be due to methodological differences. A high sensitivity ELISA was used to measure peripheral CRP levels in our study, while a multiplex assay with the high sensitivity T-cell panel was used to measure other interleukins which is considered more accurate than most cytokine ELISAs, especially at low concentrations. Additionally, IL-8 levels which was differentially expressed between study groups did not influence the cluster separation. The precise mechanism underlying this heterogeneity and the relevance of interplay among inflammatory markers (individual or combined) characterising low-grade inflammation in schizophrenia (Notter et al., 2018a) and its putative prodrome remains to be determined.
There are limitations in the interpretation of our findings. First, several types of cells in the brain including microglia, astrocytes, neurons, and vascular endothelial cells express TSPO (Notter et al., 2018a; Lavisse et al., 2012). However, these other cell types are also involved in the immune response, and thus the overall conclusion of this study remains unaffected. Second, while our sample sizes are relatively large for PET studies, the study may have been underpowered to test relationships between brain TSPO and peripheral biomarkers. However, all our main findings survived corrections for multiple comparisons. Also, based on our previous studies (Hafizi et al., 2017b; Da Silva et al., 2019) with similar sample sizes, we should have been well powered to test TSPO levels between inflammatory clusters. Third, while several studies have reported cytokine profiles in the peripheral blood of schizophrenia patients, only a few studies have compared cytokine profiles in CSF and plasma. One such study found comparatively elevated IL-6 CSF levels in patients with schizophrenia and major depressive disorder when compared to their serum levels (Sasayama et al., 2013). CSF measures may be of more clinical relevance given their proximity to brain tissue reflecting inflammatory abnormalities in brain tissue more closely compared to peripheral blood. To date, only one study in schizophrenia compared plasma and CSF measures and investigated their relationship with brain TSPO, however, found no relationship with brain TSPO but found increased CSF IL-6 levels that significantly correlated with their plasma levels (Coughlin et al., 2016). To our knowledge, no previous study has reported CSF levels in CHR. Lastly, the present study provides cytokine levels for only one snapshot in time; longitudinal studies are warranted to study cytokine dysregulations in the disease course.
5. Conclusion
Our well-characterized examination of inflammatory (including IL-2 and IL-10) clusters showed no evidence of altered brain TSPO levels among inflammatory clusters, highlighting the complexity of brain/periphery immune interactions. In conclusion, alterations in brain TSPO expression in response to low-grade inflammatory processes are not evident in CHR, but elevations in individual markers (IL-8) may relate to the severity of prodromal symptoms, particularly general symptoms.
Funding
This work was partially supported by the National Institutes of Health (NIH) R01 grant MH100043 to Dr. Mizrahi. This work was done as part of the research undertaken thanks in part to funding from the Canada First Research Excellence Fund, awarded to the Healthy Brains for Healthy Lives initiative at McGill University. Dr. Mizrahi is a member of SAB for a single project (unrelated to this work) with Boehringer-Ingelheim.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the staff of the Focus on Youth Psychosis Prevention (FYPP) clinic and Research Imaging Centre. We would also like to thank the participants and their families for their cooperation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100636.
Contributor Information
Kankana Nisha Aji, Email: kankana.aji@mail.utoronto.ca.
Sina Hafizi, Email: sina.hafizi@gmail.com.
Tania Da Silva, Email: t.dasilva@mail.utoronto.ca.
Michael Kiang, Email: michael.kiang@camh.ca.
Pablo M. Rusjan, Email: pablo.rusjan@mcgill.ca.
Cynthia Shannon Weickert, Email: weickerc@upstate.edu.
Romina Mizrahi, Email: romina.mizrahi@mcgill.ca.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Addington J., Shah H., Liu L., Addington D. Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophr. Res. 2014;153(1–3):64–67. doi: 10.1016/j.schres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwells S., Setiawan E., Wilson A.A., Rusjan P.M., Miler L., Xu C., et al. Replicating predictive serum correlates of greater translocator protein distribution volume in brain. Neuropsychopharmacol. Off Publ. Am. Coll. Neuropsychopharmacol. 2020;45(6):925–931. doi: 10.1038/s41386-019-0561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher J., Wenzig K., Vogler M. 2004. SPSS TwoStep Cluster – a First Evaluation. Work and discussion paper. [Google Scholar]
- Bae K.R., Shim H.J., Balu D., Kim S.R., Yu S.W. Translocator protein 18 kDa negatively regulates inflammation in microglia. J. Neuroimmune. Pharmacol. Off. J. Soc. NeuroImmune. Pharmacol. 2014;9(3):424–437. doi: 10.1007/s11481-014-9540-6. [DOI] [PubMed] [Google Scholar]
- Banati R.B., Middleton R.J., Chan R., Hatty C.R., Kam W.W.Y., Quin C., et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 2014;5:5452. doi: 10.1038/ncomms6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron H., Hafizi S., Andreazza A.C., Mizrahi R. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int. J. Mol. Sci. 2017;18(3):651. doi: 10.3390/ijms18030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko H., Veljanovski V., Jurkiewicz P. Enigmatic Translocator protein (TSPO) and cellular stress regulation. Trends Biochem. Sci. 2015;40(9):497–503. doi: 10.1016/j.tibs.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Boerrigter D., Weickert T.W., Lenroot R., O'Donnell M., Galletly C., Liu D., et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J. Neuroinflammation. 2017;14(1):188. doi: 10.1186/s12974-017-0962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S., Hooton J., Schaefer C.A., Zhang H., Petkova E., Babulas V., et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatr. 2004;161(5):889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Busse S., Busse M., Schiltz K., Bielau H., Gos T., Brisch R., et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations. Brain Behav. Immun. 2012;26(8):1273–1279. doi: 10.1016/j.bbi.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Çakici N., Sutterland A., Penninx B., Dalm V.A.S.H., de Haan L., Beveren J. Altered peripheral blood compounds in drug-naive first-episode patients with either schizophrenia or major depressive disorder: a meta-analysis. Brain Behav. Immun. 2020;88:547–558. doi: 10.1016/j.bbi.2020.04.039. [DOI] [PubMed] [Google Scholar]
- Capuzzi E., Bartoli F., Crocamo C., Clerici M., Carrà G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: a meta-analysis. Neurosci. Biobehav. Rev. 2017;77:122–128. doi: 10.1016/j.neubiorev.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Collste K., Plavén-Sigray P., Fatouros-Bergman H., Victorsson P., Schain M., Forsberg A., et al. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol. Psychiatr. 2017;22(6):850–856. doi: 10.1038/mp.2016.247. [DOI] [PubMed] [Google Scholar]
- Coughlin J.M., Wang Y., Ambinder E.B., Ward R.E., Minn I., Vranesic M., et al. In vivo Markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Transl. Psychiatry. 2016;6(4):1–8. doi: 10.1038/tp.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva T., Wu A., Laksono I., Prce I., Maheandiran M., Kiang M., et al. Mitochondrial function in individuals at clinical high risk for psychosis. Sci. Rep. 2018;8:6216. doi: 10.1038/s41598-018-24355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva T., Hafizi S., Rusjan P.M., Houle S., Wilson A.A., Prce I., et al. GABA levels and TSPO expression in people at clinical high risk for psychosis and healthy volunteers: a PET-MRS study. J. Psychiat. Neurosci. JPN. 2019;44(2):111–119. doi: 10.1503/jpn.170201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan S., Bragazzi N.L., Yogev A., Bar-Gad M., Barak V., Amital H., et al. The relationship between serum cytokine levels and degree of psychosis in patients with schizophrenia. Psychiatr. Res. 2018;268:467–472. doi: 10.1016/j.psychres.2018.07.041. [DOI] [PubMed] [Google Scholar]
- Dawidowski B., Górniak A., Podwalski P., Lebiecka Z., Misiak B., Samochowiec J. The role of cytokines in the pathogenesis of schizophrenia. J. Clin. Med. 2021;10(17):3849. doi: 10.3390/jcm10173849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J., de Vries E.F.J., Willemsen A.T.M., de Groot J.C., Dierckx R.A., Klein H.C. Neuroinflammation in schizophrenia-related psychosis: a PET study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2009;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Enache D., Nikkheslat N., Fathalla D., Morgan B.P., Lewis S., Drake R., et al. Peripheral immune markers and antipsychotic non-response in psychosis. Schizophr. Res. 2021;230:1–8. doi: 10.1016/j.schres.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes B.S., Steiner J., Bernstein H.G., Dodd S., Pasco J.A., Dean O.M., et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol. Psychiatr. 2016;21(4):554–564. doi: 10.1038/mp.2015.87. [DOI] [PubMed] [Google Scholar]
- Fillman S.G., Cloonan N., Catts V.S., Miller L.C., Wong J., McCrossin T., et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol. Psychiatr. 2013;18(2):206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Fillman S.G., Weickert T.W., Lenroot R.K., Catts S.V., Bruggemann J.M., Catts V.S., et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca's area volume. Mol. Psychiatr. 2016;21(8):1090–1098. doi: 10.1038/mp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Gibbon M. vol. 2. John Wiley & Sons, Inc.; Hoboken, NJ, US: 2004. The structured clinical Interview for DSM-IV Axis I disorders (SCID-I) and the structured clinical Interview for DSM-IV Axis II disorders (SCID-II) pp. 134–143. (Comprehensive Handbook of Psychological Assessment). Personality assessment. [Google Scholar]
- Frydecka D., Krzystek-Korpacka M., Lubeiro A., Stramecki F., Stańczykiewicz B., Beszłej J.A., et al. Profiling inflammatory signatures of schizophrenia: a cross-sectional and meta-analysis study. Brain Behav. Immun. 2018;71:28–36. doi: 10.1016/j.bbi.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Gallego J.A., Blanco E.A., Husain-Krautter S., Madeline Fagen E., Moreno-Merino P., Del Ojo-Jiménez J.A., et al. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: new data and an updated meta-analysis. Schizophr. Res. 2018;202:64–71. doi: 10.1016/j.schres.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatr. 2016;21(12):1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut P. Targeting mitochondrial energy metabolism with TSPO ligands. Biochem. Soc. Trans. 2015;43(4):537–542. doi: 10.1042/BST20150019. [DOI] [PubMed] [Google Scholar]
- Hafizi S., Tseng H.H., Rao N., Selvanathan T., Kenk M., Bazinet R.P., et al. Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F]FEPPA. Am. J. Psychiatr. 2017;174(2):118–124. doi: 10.1176/appi.ajp.2016.16020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S., Da Silva T., Gerritsen C., Kiang M., Bagby R.M., Prce I., et al. Imaging microglial activation in individuals at clinical high risk for psychosis: an in vivo PET study with [18F]FEPPA. Neuropsychopharmacology. 2017;42(13):2474–2481. doi: 10.1038/npp.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S., Guma E., Koppel A., Da Silva T., Kiang M., Houle S., et al. TSPO expression and brain structure in the psychosis spectrum. Brain Behav. Immun. 2018;74:79–85. doi: 10.1016/j.bbi.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S., Da Silva T., Meyer J.H., Kiang M., Houle S., Remington G., et al. Interaction between TSPO—a neuroimmune marker—and redox status in clinical high risk for psychosis: a PET–MRS study. Neuropsychopharmacology. 2018;43(8):1700–1705. doi: 10.1038/s41386-018-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas E., Manganaris S., Ntouros E., Floros G., Antoniadis D., Garyfallos G. Cytokines, cortisol and IGF-1 in first episode psychosis and ultra high risk males. Evidence for TNF-α, IFN-γ, ΤNF-β, IL-4 deviation. Asian J. Psychiat. 2017;26:99–103. doi: 10.1016/j.ajp.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Kenk M., Selvanathan T., Rao N., Suridjan I., Rusjan P., Remington G., et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr. Bull. 2015;41(1):85–93. doi: 10.1093/schbul/sbu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatr. 2014;71(10):1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl W.C., Jenko K.J., Hines C.S., Lyoo C.H., Corona W., Morse C.L., et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J. Cerebr. Blood Flow Metabol. 2013;33(1):53–58. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavisse S., Guillermier M., Hérard A.S., Petit F., Delahaye M., Van Camp N., et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J. Neurosci. Off J. Soc. Neurosci. 2012;32(32):10809–10818. doi: 10.1523/JNEUROSCI.1487-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizano P., Lutz O., Xu Y., Rubin L.H., Paskowitz L., Lee A.M., et al. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol. Psychiatr. 2021;26(7):3430–3443. doi: 10.1038/s41380-020-00914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Zhang H., Liu N., Wang P qi, Guo W zhi, Fu Q., et al. TSPO ligand PK11195 alleviates neuroinflammation and beta-amyloid generation induced by systemic LPS administration. Brain Res. Bull. 2016;121:192–200. doi: 10.1016/j.brainresbull.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Marin R.S., Biedrzycki R.C., Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatr. Res. 1991;38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Metcalf S.A., Jones P.B., Nordstrom T., Timonen M., Mäki P., Miettunen J., et al. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: a prospective birth cohort study. Brain Behav. Immun. 2017;59:253–259. doi: 10.1016/j.bbi.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T.J., McGlashan T.H., Rosen J.L., Somjee L., Markovich P.J., Stein K., et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am. J. Psychiatr. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatr. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.J., Culpepper N., Rapaport M.H. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin. Schizophrenia Relat. Psychoses. 2014;7(4):223–230. doi: 10.3371/CSRP.MICU.020813. [DOI] [PubMed] [Google Scholar]
- Misiak B., Bartoli F., Carrà G., Stańczykiewicz B., Gładka A., Frydecka D., et al. Immune-inflammatory markers and psychosis risk: a systematic review and meta-analysis. Psychoneuroendocrinology. 2021;127 doi: 10.1016/j.psyneuen.2021.105200. [DOI] [PubMed] [Google Scholar]
- Mizrahi R., Rusjan P.M., Kennedy J., Pollock B., Mulsant B., Suridjan I., et al. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J. Cerebr. Blood Flow Metabol. 2012;32(6):968–972. doi: 10.1038/jcbfm.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongan D., Föcking M., Healy C., Susai S.R., Heurich M., Wynne K., et al. Development of proteomic prediction models for transition to psychotic disorder in the clinical high-risk state and psychotic experiences in adolescence. JAMA Psychiatr. 2021;78(1):77–90. doi: 10.1001/jamapsychiatry.2020.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettis M.A., Veronese M., Nikkheslat N., Mariani N., Lombardo G., Sforzini L., et al. PET imaging shows no changes in TSPO brain density after IFN-α immune challenge in healthy human volunteers. Transl. Psychiatry. 2020;10(1):89. doi: 10.1038/s41398-020-0768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North H.F., Bruggemann J., Cropley V., Swaminathan V., Sundram S., Lenroot R., et al. Increased peripheral inflammation in schizophrenia is associated with worse cognitive performance and related cortical thickness reductions. Eur. Arch. Psychiatr. Clin. Neurosci. 2021;271(4):595–607. doi: 10.1007/s00406-021-01237-z. [DOI] [PubMed] [Google Scholar]
- Notter T., Coughlin J.M., Gschwind T., Weber-Stadlbauer U., Wang Y., Kassiou M., et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol. Psychiatr. 2018;23(2):323–334. doi: 10.1038/mp.2016.248. [DOI] [PubMed] [Google Scholar]
- Notter T., Coughlin J.M., Sawa A., Meyer U. Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry. Mol. Psychiatr. 2018;23(1):36–47. doi: 10.1038/mp.2017.232. [DOI] [PubMed] [Google Scholar]
- Park S., Miller B.J. Meta-analysis of cytokine and C-reactive protein levels in high-risk psychosis. Schizophr. Res. 2020;226:5–12. doi: 10.1016/j.schres.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Perkins D.O., Jeffries C.D., Addington J., Bearden C.E., Cadenhead K.S., Cannon T.D., et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr. Bull. 2015;41(2):419–428. doi: 10.1093/schbul/sbu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger T., Osimo E.F., Brugger S., Mondelli V., McCutcheon R.A., Howes O.D. A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophr. Bull. 2019;45(5):1120–1133. doi: 10.1093/schbul/sby160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavén-Sigray P., Matheson G.J., Coughlin J.M., Hafizi S., Laurikainen H., Ottoy J., et al. Meta-analysis of the glial marker TSPO in psychosis revisited: reconciling inconclusive findings of patient–control differences. Biol. Psychiatr. 2021;89(3):e5–e8. doi: 10.1016/j.biopsych.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C., Tierney M., Mohr E., Chase T., Randolph C., Tierney M.C., Mohr E., Chase T.N. The repeatable Battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J clin exp neuropsychol 20: 310-319. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Romeo B., Brunet-Lecomte M., Martelli C., Benyamina A. Kinetics of cytokine levels during antipsychotic treatment in schizophrenia: a meta-analysis. Int. J. Neuropsychopharmacol. 2018;21(9):828–836. doi: 10.1093/ijnp/pyy062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusjan P., Mamo D., Ginovart N., Hussey D., Vitcu I., Yasuno F., et al. An automated method for the extraction of regional data from PET images. Psychiatr. Res. 2006;147(1):79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Rusjan P.M., Wilson A.A., Bloomfield P.M., Vitcu I., Meyer J.H., Houle S., et al. Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J. Cerebr. Blood Flow Metabol. 2011;31(8):1807–1816. doi: 10.1038/jcbfm.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D., Hattori K., Wakabayashi C., Teraishi T., Hori H., Ota M., et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J. Psychiatr. Res. 2013;47(3):401–406. doi: 10.1016/j.jpsychires.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Schubert J.J., Veronese M., Fryer T.D., Manavaki R., Kitzbichler M.G., Nettis M.A., et al. A modest increase in 11C-PK11195-Positron emission tomography TSPO binding in depression is not associated with serum C-reactive protein or body mass index. Biol. Psychiat. Cogn. Neurosci. Neuroimag. 2021;6(7):716–724. doi: 10.1016/j.bpsc.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G., et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatr. 2015;72(3):268. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith R.P., Hamilton M., Morley S., Humayan A., Hargreaves D., Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br. J. Psychiat. J. Ment. Sci. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Steullet P., Cabungcal J.H., Monin A., Dwir D., O'Donnell P., Cuenod M., et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr. Res. 2016;176(1):41–51. doi: 10.1016/j.schres.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic A., Martorell L., Montalvo I., Ortega L., Monseny R., Vilella E., et al. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. 2014;41:23–32. doi: 10.1016/j.psyneuen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Takano A., Arakawa R., Ito H., Tateno A., Takahashi H., Matsumoto R., et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int. J. Neuropsychopharmacol. 2010;13(7):943–950. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- van Berckel B.N., Bossong M.G., Boellaard R., Kloet R., Schuitemaker A., Caspers E., et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol. Psychiatr. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- van Kesteren C.F.M.G., Gremmels H., de Witte L.D., Hol E.M., Van Gool A.R., Falkai P.G., et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl. Psychiatry. 2017;7(3) doi: 10.1038/tp.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.K., Miller B.J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 2018;44(1):75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhang L., Zhang X., Xue R., Li L., Zhao W., et al. Lentiviral-mediated overexpression of the 18 kDa translocator protein (TSPO) in the hippocampal dentate gyrus ameliorates LPS-induced cognitive impairment in mice. Front. Pharmacol. 2016;7:384. doi: 10.3389/fphar.2016.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock E.A., Hillmer A.T., Sandiego C.M., Maruff P., Carson R.E., Cosgrove K.P., et al. Acute neuroimmune stimulation impairs verbal memory in adults: a PET brain imaging study. Brain Behav. Immun. 2021;91:784–787. doi: 10.1016/j.bbi.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni-Graiff M., Rizzo L.B., Mansur R.B., Maurya P.K., Sethi S., Cunha G.R., et al. Peripheral immuno-inflammatory abnormalities in ultra-high risk of developing psychosis. Schizophr. Res. 2016;176(2–3):191–195. doi: 10.1016/j.schres.2016.06.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.