Abstract
Purpose
To determine the interreader agreement for reticular pseudodrusen (RPD) assessment on combined infrared reflectance (IR) and OCT imaging in the early stages of age-related macular degeneration across a range of different criteria to define their presence.
Design
Interreader agreement study.
Participants
Twelve readers from 6 reading centers.
Methods
All readers evaluated 100 eyes from individuals with bilateral large drusen for the following: (1) the presence of RPD across a range of different criteria and (2) the number of Stage 2 or 3 RPD lesions (from 0 to ≥ 5 lesions) on an entire OCT volume scan and on a selected OCT B-scan. Supportive information was available from the corresponding IR image.
Main Outcome Measures
Interreader agreement, as assessed by Gwet’s first-order agreement coefficient (AC1).
Results
When evaluating an entire OCT volume scan, there was substantial interreader agreement for the presence of any RPD, any or ≥ 5 Stage 2 or 3 lesions, and ≥ 5 definite lesions on en face IR images corresponding to Stage 2 or 3 lesions (AC1 = 0.60–0.72). On selected OCT B-scans, there was also moderate-to-substantial agreement for the presence of any RPD, any or ≥ 5 Stage 2 or 3 lesions (AC1 = 0.58–0.65) and increasing levels of agreement with increasing RPD stage (AC1 = 0.08, 0.56, 0.78, and 0.99 for the presence of any Stage 1, 2, 3, and 4 lesions, respectively). There was substantial agreement regarding the number of Stage 2 or 3 lesions on an entire OCT volume scan (AC1 = 0.68), but only fair agreement for this evaluation on selected B-scans (AC1 = 0.30).
Conclusions
There was generally substantial or near-substantial—but not near-perfect—agreement for assessing the presence of RPD on entire OCT volume scans or selected B-scans across a range of differing RPD criteria. These findings underscore how interreader variability would likely contribute to the variability of findings related to the clinical associations of RPD. The low levels of agreement for assessing RPD number on OCT B-scans underscore the likely challenges of quantifying RPD extent with manual grading.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found after the references.
Keywords: Age-related macular degeneration, Drusen, OCT, Reticular pseudodrusen, Subretinal drusenoid deposits
Reticular pseudodrusen (RPD), also called subretinal drusenoid deposits, have been increasingly recognized as a critical morphologic feature in age-related macular degeneration (AMD).1, 2, 3 There is preclinical and clinical evidence that the presence of RPD indicates a dysfunctional outer retina and retinal pigment epithelium (RPE).1 Numerous studies have reported that when RPD are seen in the nonlate AMD fellow eyes of individuals with unilateral neovascular AMD, there is an increased risk of late AMD development in the fellow eye.4, 5, 6, 7 Their presence is also associated with significantly impaired dark adaptation8, 9, 10, 11, 12 and reduced scotopic visual sensitivity.13, 14, 15 Importantly, we also observed in a post hoc analysis that RPD was a significant treatment effect modifier in a randomized trial of a subthreshold nanosecond laser in individuals with bilateral large drusen, where those with coexistent RPD had worse treatment outcomes when compared with treated eyes without RPD.16,17 Despite the importance of RPD, the underlying pathogenic mechanisms that contribute to its development have not yet been clearly established.
As we move forward to understand the mechanisms driving the development of these deposits and their role in vision loss, it is crucial to accurately identify eyes with or without RPD. To date, however, there have been different criteria by which RPD have been defined as being present within an eye. For example, these criteria include different combinations of imaging modalities, such as color fundus photography, infrared reflectance (IR) and fundus autofluorescence (FAF) imaging on confocal scanning laser ophthalmoscopy, and OCT. On OCT alone, an imaging modality that can determine the subretinal localization of RPD above the RPE,18 there are various differing descriptions of the morphologic features that characterize RPD. These differences include the criteria by which RPD are judged to be present, such as whether only subretinal hyperreflective lesions sufficient to alter the contour of (Stage 2), or break through (Stage 3), the overlying ellipsoid zone (EZ) should be considered, or if the presence of any diffuse hyperreflective material between the RPE and the EZ (Stage 1) is sufficient.3 There have also been differences in the number of lesions required to establish that RPD are present in an eye, from requiring only 1 lesion,19 to requiring ≥ 10 lesions,20 and whether there is a requirement that the lesions form a cluster, or orderly array.1 These variations in how the presence of RPD in an eye is defined can result in marked differences in their observed prevalence,1 and could potentially account for variations in their observed clinical associations across different studies, such as their association with disease progression.1
Currently, there is a paucity of data on interreader agreement for grading the presence of RPD on OCT, especially over a wide range of different potential criteria. Two studies have reported on intergrader agreement; 1 reported near-perfect agreement for grading the presence of RPD on OCT imaging (κ = 0.96) when conducted by 2 experienced ophthalmologists from the same center who evaluated 220 eyes from 114 individuals with newly diagnosed neovascular AMD.21 In this study, the presence of RPD on OCT imaging was defined by the presence of “≥ 5 hyperreflective mounds or triangular lesions above the RPE in > 1 B-scan” on OCT imaging. Another study also reported that there was near-perfect agreement for grading RPD presence on OCT imaging (κ = 0.86) of 35 eyes with nonlate AMD from individuals with unilateral neovascular AMD in the fellow eye. The presence of RPD on OCT imaging in that study was defined by evidence of “discrete accumulations… often occurring as sharp peaks,” although the number of required lesions was not mentioned, and the number of graders who performed this assessment was not indicated, nor was it mentioned whether graders were from the same center.22
The level of interreader agreement for the evaluation of RPD on OCT across a larger number of readers (especially across different centers), and whether it varies based on differing criteria for defining RPD presence, remains to be determined. We thus assessed the interreader agreement of 12 readers across 6 established reading centers who evaluated RPD across a range of different criteria, both when evaluating entire OCT volume scans and selected B-scans.
Methods
Six established reading centers were invited to participate in this technical evaluation study. These reading centers included the Cologne Imaging Reading Center and Laboratory, Doheny Imaging Reading Center, Duke Reading Center, GRADE Reading Center, Utah Retinal Reading Center, and Wisconsin Reading Center. The medical directors at each of these reading centers assigned 2 experienced readers to participate in this study.
Grading Data Set
The grading data set included OCT volume scans acquired using the Heidelberg Spectralis HRA+OCT device (Heidelberg Engineering), obtained from the central 20° × 20° region and consisting of 49 B-scans, each with 1024 A-scans and 25 frames averaged. A 30° × 30° IR image from a confocal scanning laser ophthalmoscope was simultaneously acquired during OCT imaging. All combined OCT and IR images were required to be correctly centered on the fovea and free of imaging artifacts (e.g., clipping or blink artifacts). This grading data set included 100 eyes from 70 participants with bilateral large drusen (> 125 μm in diameter) examined at baseline as part of the Laser Intervention in the Early stages of AMD (LEAD) randomized-controlled trial (clinicaltrials.gov identifier, NCT01790802).16 Institutional review board approval was obtained for each site that were part of the LEAD study, the study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and with the tenets of the Declaration of Helsinki, and all participants provided written informed consent. The data set consisted of 50 eyes with and 50 eyes without definite RPD based on the grading performed as part of the LEAD randomized trial (from which these images were collected), where definite RPD was defined as ≥ 5 definite lesions on > 1 B-scan on the OCT volume scan. Reticular pseudodrusen grading in the LEAD study was undertaken by a senior grader and a senior medical retina clinician (R.H.G.). The included cases spanned the full spectrum of the presence and extent of RPD, including eyes with questionable RPD, those with < 5 definite RPD lesions, and those with ≥ 5 RPD lesions on only 1 B-scan. One representative B-scan from each eye was also selected for the evaluation of RPD. The selection of cases and B-scans were performed by 1 of the authors (Z.W.). The readers were all masked to the distribution of the cases selected and the previous RPD grading that was performed as part of the LEAD study.
Grading Task
The grading task consisted of evaluating each OCT volume scan and selected B-scan for the presence and number of RPD, and specifically for the presence of RPD lesions of different stages, as previously described.18,23 Stage 1 lesions were defined as “diffuse deposition of granular hyperreflective material between the RPE and EZ,” Stage 2 lesions as “mounds of accumulated material sufficient to alter the contour of the [EZ],” and Stage 3 lesions as “material [that] was thicker, adopted a conical appearance, and broke through the [EZ].”18 Stage 4 lesions were defined as lesions showing “fading of the material because of reabsorption and migration within the inner retinal layers.”23
Readers were first asked to assess the presence of the following on the entire OCT volume scan in each of the 100 cases: (1) any RPD lesions, (2) number of definite RPD lesions at Stage 2 or 3 (from 0 to ≥ 5), and (3) a cluster (or an orderly array of lesions) of ≥ 5 definite RPD lesions present on IR imaging that corresponded to the colocated area of Stage 2 or 3 lesions on the OCT volume scan24; the IR images were used because they have a higher transverse resolution for visualizing the spatial distribution of the RPD lesions than the OCT volume scan protocol used in this study. The readers were also asked to determine the presence of the following on the 1 selected OCT B-scan in each of the 100 cases: (1) any RPD, (2) Stage 1 lesions, (3) Stage 2 lesions, (4) Stage 3 lesions, (5) Stage 4 lesions, and (6) number of definite RPD lesions at Stage 2 or 3 (from 0 to ≥ 5). The assessment of the quantity of RPD lesions in the entire OCT volume scan and selected B-scans was limited to the number of Stage 2 or 3 lesions present, as the diffuse depositions that characterize Stage 1 lesions did not always have distinct limits laterally (i.e., horizontally along a B-scan) that were conducive for quantification.
The directors of the reading centers selected experienced graders for this study. However, the readers were not provided with any specific, additional formal training for RPD grading (above that which they would have previously received in their role in the reading center) but were provided with examples from previous publications18,23,25 and references to previous studies describing RPD.18,23, 24, 25 Readers were asked to consider the presence of RPD as being definite if their certainty was ≥ 90%, questionable for features with a 50% to 90% certainty, and absent when there was < 50% certainty. Readers were also asked to consider the number of Stage 2 or 3 RPD lesions present with ≥ 90% certainty.
Statistical Analyses
Grading responses for the presence of RPD were binarized into the following 2 categories: (1) absent or questionable and (2) definitely present. The interreader agreement for the presence and number of RPD was determined based on Gwet’s first-order agreement coefficient (AC1), which is a chance-corrected measure of agreement that remains robust across variations in the prevalence of the trait assessed26; the AC1 values can range from −1 to 1. The AC1 was examined instead of the widely-used κ coefficient because estimates of agreement for the latter can be affected by the prevalence of the trait assessed. For example, low κ values can be present despite high levels of agreement when there is either very high or low prevalence of the trait.27 The magnitude of agreement based on the AC1 values was interpreted as follows: poor agreement (< 0.00), slight agreement (0.00–0.20), fair agreement (0.21–0.40), moderate agreement (0.41–0.60), substantial agreement (0.61–0.80), and near-perfect agreement (> 0.80).28 The prevalence of the readings that identified RPD (i.e., the proportion that identified RPD from the 1200 responses from 12 readers for the 100 cases) or the number of Stage 2 or 3 lesions present in OCT volume scans or the selected OCT B-scans across the range of different criteria were also reported.
Results
The prevalence and interreader agreement of readings for RPD across a range of criteria are summarized in Table 1.
Table 1.
Prevalence and Interreader Agreement of Gradings that Identified RPD Based on Different Criteria when Evaluated on the Entire OCT Volume Scan or on a Selected B-Scan among the Cases (n = 100) Evaluated by 12 Readers
| Prevalence of Readings (%)∗ | Agreement Rate (%) | Gwet’s AC1 | |
|---|---|---|---|
| Presence on the Entire OCT Volume Scan | |||
| Any RPD lesion(s) | 84 | 80 | 0.72 |
| Any Stage 2 or 3 RPD lesion(s) | 83 | 79 | 0.71 |
| ≥ 5 Stage 2 or 3 RPD lesion(s) | 76 | 76 | 0.62 |
| Cluster of ≥ 5 definite RPD lesions on IR corresponding to Stage 2 or 3 lesions on OCT | 42 | 79 | 0.60 |
| Presence on Selected OCT B-scans | |||
| Any RPD lesion(s) | 80 | 77 | 0.65 |
| Any Stage 1 RPD lesion(s) | 51 | 54 | 0.08 |
| Any Stage 2 RPD lesion(s) | 74 | 73 | 0.56 |
| Any Stage 3 RPD lesion(s) | 28 | 87 | 0.79 |
| Any Stage 4 RPD lesion(s) | 1 | 99 | 0.99 |
| Any Stage 2 or 3 RPD lesion(s) | 77 | 76 | 0.63 |
| ≥ 5 Stage 2 or 3 RPD lesions | 32 | 76 | 0.58 |
AC1 = first-order coefficient; IR = infrared reflectance; RPD = reticular pseudodrusen.
Proportion of the 1200 readings across 12 readers of the 100 cases considering RPD to be present based on the criteria evaluated.
Interreader Agreement for Presence of RPD on the Entire OCT Volume Scan
When evaluated based on the entire OCT volume scan, there was substantial interreader agreement for the presence of any RPD at any stage or number (AC1 = 0.72) and for the presence of any Stage 2 or 3 lesions (AC1 = 0.71; Table 1). There was also substantial agreement for the presence of ≥ 5 Stage 2 or 3 lesions to be present in the entire OCT volume scan (AC1 = 0.62) and when requiring the presence of these ≥ 5 Stage 2 or 3 lesions to also correspond to a cluster of definite lesions seen on IR imaging (AC1 = 0.60; Table 1).
Note that apart from interreader agreement, there was a large difference in the prevalence of readings that considered ≥ 5 Stage 2 and 3 RPD lesions that did or did not correspond with a cluster of definite lesions on IR imaging (42% and 76%, respectively; Table 1).
Interreader Agreement for Presence of RPD on Selected OCT B-Scans
When evaluated on selected OCT B-scans, there was also substantial interreader agreement for the presence of any stage or number of RPD (AC1 = 0.65) and for the presence of any Stage 2 or 3 lesions present (AC1 = 0.63; Table 1). However, there was slight agreement (AC1 = 0.08), moderate agreement (AC1 = 0.56), substantial agreement (AC1 = 0.79), and near-perfect agreement (AC1 = 0.99) for the presence of any Stage 1, 2, 3, and 4 lesions, respectively (although only 1% of the readings identified Stage 4 lesions; Table 1).
Interreader Agreement for the Number of RPD Lesions
There was substantial agreement (AC1 = 0.68) in the readings of the number of Stage 2 or 3 RPD lesions (from 0 to ≥ 5 lesions) at the OCT volume scan level, but only fair agreement (AC1 = 0.30) when selected B-scans were evaluated (Table 2).
Table 2.
Prevalence and Overall Interreader Agreement of Readings for the Number of Stage 2 or 3 RPD Lesions Present Evaluated on the Entire OCT Volume Scan, or on a Selected B-Scan, among the Cases (n = 100) Evaluated by 12 Readers.
| Presence on OCT Volume Scan |
Presence of Selected OCT B-scan |
|||||
|---|---|---|---|---|---|---|
| Prevalence of Readings (%)∗ | Agreement Rate (%) | Gwet’s AC1 | Prevalence of Readings (%)∗ | Agreement Rate (%) | Gwet’s AC1 | |
| 0 lesions | 18 | 71 | 0.68 | 23 | 41 | 0.30 |
| 1 lesion | 1 | 7 | ||||
| 2 lesions | 1 | 11 | ||||
| 3 lesions | 2 | 15 | ||||
| 4 lesions | 3 | 11 | ||||
| ≥ 5 lesions | 76 | 32 | ||||
AC1 = first-order coefficient; RPD = reticular pseudodrusen.
Proportion of the 1200 readings across 12 readers of the 100 cases considering RPD to be present based on the criteria evaluated.
Examples of Excellent and Poor Interreader Agreement
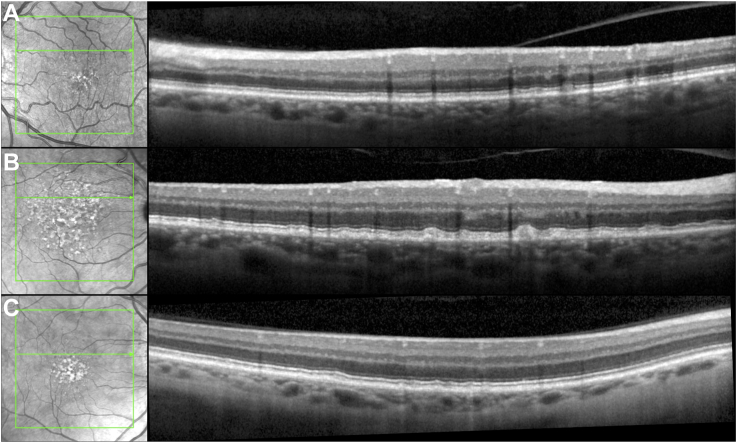
Examples showing perfect agreement among all 12 readers in the evaluation of the number of Stage 2 or 3 RPD lesions on selected OCT B-scans are shown in Figure 1, whereas examples showing poor agreement are shown in Figure 2.
Figure 1.
Examples of perfect agreement among the 12 readers for the number of Stage 2 or 3 reticular pseudodruse (RPD) lesions present, where (A) ≥ 5 lesions, (B) 3 lesions, and (C) no RPD were present in these OCT B-scans. Note that the lesions present in (C) are located below the retinal pigment epithelium and are not RPD.
Figure 2.
Examples of poor agreement on the number of Stage 2 or 3 reticular pseudodrusen on OCT B-scans among the 12 readers for the number (0 to ≥ 5 lesions) of Stage 2 or 3 lesions. A, There were 1, 2, 3, 3, 2, and 1 readers who identified 0, 1, 2, 3, 4, or ≥ 5 lesions, respectively (resulting in 12% agreement between all possible pairwise readings). B, There were 3, 1, 1, 1, 3, and 3 readers who identified 0, 1, 2, 3, 4, or ≥ 5 lesions, respectively (resulting in 14% agreement between all possible pairwise readings). C, There were 4, 2, 1, 0, 2, and 3 readers who identified 0, 1, 2, 3, 4, or ≥ 5 lesions, respectively (resulting in 17% agreement between all possible pairwise readings).
Discussion
This technical evaluation study involving readers from 6 different reading centers demonstrated that there was generally substantial or near-substantial, but not near-perfect, interreader agreement regarding the presence of RPD across different criteria of RPD on an entire OCT volume scan or on specific B-scans. It also revealed that there was only slight agreement for the presence of Stage 1 RPD on an OCT B-scan and only fair agreement for the specific number of Stage 2 or 3 lesions on an OCT B-scan. These findings underscore how interreader variability in grading the presence of RPD could contribute to the variability in findings across studies related to the clinical associations of RPD. This study also observed that the prevalence of RPD can vary substantially based on the criteria used, where, for example, the prevalence almost halves when the presence of ≥ 5 Stage 2 or 3 lesions were required to correspond to a cluster of lesions seen on IR imaging, compared with when this distinctive en face pattern was not required to be present.
The findings of this study demonstrate that the interreader agreement for assessing the presence of RPD may be lower than that suggested by previous studies. For example, the near-perfect agreement (κ = 0.96) on the presence of “≥ 5 hyperreflective mounds or triangular lesions” (likely representing Stage 2 or 3 lesions) based on the grading by 2 ophthalmologists from the same center from a previous study21 was substantially higher than observed for a similar criteria in this study based on assessments by 12 readers from 6 well-established and experienced reading centers (κ = 0.35, or AC1 = 0.62). Similarly, the near-perfect agreement (κ = 0.86) for the presence of an unspecified number of “discrete accumulations… often occurring as sharp peaks” (also likely representing Stage 2 and 3 lesions) from an unspecified number of graders in a previous study22 was also higher than observed in this study (κ = 0.26 for any Stage 2 or 3 lesions, or κ = 0.35 for ≥ 5 Stage 2 or 3 lesions, or AC1 = 0.71 and 0.62, respectively). In this study, we observed that there was almost always a higher level of within-center compared with between-center interreader agreement across the different criteria evaluated for the presence of RPD (data presented in Table S3, available at www.ophthalmologyscience.org), and this may partly account for the higher level of agreement observed for the above previous studies compared with those in this study. For example, although the overall interreader agreement across all centers for assessing the presence of a cluster of ≥ 5 Stage 2 or 3 lesions was substantial (AC1 = 0.60), the within-center interreader agreement was near-perfect (AC1 ≥ 0.80) for 3 reading centers, but only moderate to substantial (AC1 = 0.54 to 0.60) for the other 3 reading centers. Assessments across multiple reading centers, as performed in this study, are thus needed to provide robust and generalizable estimates of interreader agreement on the presence and number of RPDs.
This study also showed that there was a similar, substantial level of interreader agreement on the presence of RPD in an OCT volume scan across the 4 different criteria evaluated in this study, which varied based on the stage and number of lesions and evidence of clustering required. However, the prevalence of the readings that identified RPD in an OCT volume scan could differ markedly based on the criteria used. For example, 76% of the readings considered ≥ 5 Stage 2 or 3 RPD lesions to be present, but only 42% of the readings met this criterion as well as the additional requirement for evidence that they form a cluster on IR imaging. A difference in prevalence of the readings based on the number of Stage 2 or 3 lesions required to be present was also observed, but this difference was much smaller (76% and 83% when defined based on the presence of ≥ 5 and ≥ 1 lesions, respectively). These differences might reflect how there may be many more eyes detected as having ≥ 1 individual RPD lesion than eyes where a distinctive cluster of these lesions is present. As such, these findings underscore how variations in the criteria used to define the presence of RPD at the eye level could have a significant impact on its prevalence. Still unknown, however, is the clinical significance of these different criteria, such as their relationship with the risk of progression to vision loss,4, 5, 6, 7 or their association with impaired visual function.8, 9, 10, 11, 12, 13, 14, 15
The findings of this study also showed that there was only slight agreement on the presence of Stage 1 RPD lesions on an OCT B-scan (AC1 = 0.08), but moderate agreement on the presence of Stage 2 lesions (AC1 = 0.56) and substantial agreement for Stage 3 lesions (AC1 = 0.79). This increasing level of agreement with the increasing RPD stage was not unexpected given that the lesions generally form a more distinctive shape as the stage increases from Stages 1 to 3. Furthermore, it is often difficult to distinguish Stage 1 lesions from the normal interdigitation zone, especially because this band is often less visible in eyes with the early stages of AMD compared with those without AMD.29 As such, it is not surprising that there was poor agreement for the presence of Stage 1 lesions. However, the interreader agreement for the presence of RPD on individual OCT B-scans was similar whether the criteria excluded or included Stage 1 lesions (AC1 = 0.63 and 0.65, respectively). This was likely because most OCT B-scans had both Stage 1 lesions and Stage 2 or 3 lesions, evident by the finding that the prevalence of RPD at a B-scan level was similar when the criteria excluded or included stage 1 lesions (77% and 80%, respectively). We also observed near-perfect agreement for the presence of Stage 4 lesions on selected OCT B-scans, but there were only 8 (1%) out of 1200 readings (i.e., 100 cases by 12 readers) that identified Stage 4 lesions (across 6 different cases, with 5 out of the 8 readings coming from 1 grader), meaning that there were insufficient cases in our cohort to robustly assess the interreader agreement for this feature.
This study also showed that there was only fair interreader agreement for the number of Stage 2 or 3 lesions on selected OCT B-scans (between 0 to ≥ 5 lesions; AC1 = 0.30), which was lower than the agreement for ≥ 1 or ≥ 5 lesions (AC1 = 0.63 and 0.58, respectively). This level of interreader agreement for the number of Stage 2 or 3 lesions on selected OCT B-scans was lower than that of the entire OCT volume scan (AC1 = 0.68), likely because there was a substantially larger proportion of readings at the category ceiling (≥ 5 lesions) for the latter (76%) compared with the former (32%).
The findings of this study, that there was substantial or near-substantial agreement among 12 readers across 6 reading centers on the presence of RPD, and not near-perfect agreement, indicate that grading variability likely contributes to the heterogeneity of findings across different clinical studies seeking to understand the clinical significance and associations of these distinct deposits.1, 2, 3 The finding that there was only fair agreement when evaluating the number of Stage 2 or 3 lesions present on OCT B-scans further indicates that the manual quantification of the extent of RPD would likely be quite variable. This level of interreader agreement would probably worsen if the quantification of RPD extent included Stage 1 lesions, given that evaluation of its presence showed the lowest level of agreement in this study compared with lesions of other stages. The development of automated algorithms for segmenting RPD on OCT imaging, such as with artificial intelligence-based methods like deep learning, could potentially provide a consistent and more feasible means for quantifying their extent. These automated techniques, which will inevitably be developed using variable ground truth, will then need to be evaluated against a clinically meaningful reference standard. This could be achieved, for example, through evaluating their performance for predicting visual sensitivity13,15,30 or progression to late AMD,4, 5, 6, 7 which could then establish their validity and utility.
Finally, this study observed that the prevalence of readings considering RPD to be present based on having ≥ 5 Stage 2 or 3 lesions (76%) decreased markedly when further requiring these lesions to also correspond to a cluster of definite lesions on IR imaging (42%). This finding underscores how the prevalence of RPD can vary markedly based on the criteria used. This marked variation in prevalence of RPD based on the criteria used was also seen in our recent report, showing how the prevalence of RPD more than halved when RPD were required to be present on all of a suite of imaging modalities that included color fundus photographs, IR, FAF, and OCT imaging (as defined by the presence of ≥ 5 lesions on ≥ 1 B-scans), as compared with only requiring their presence of OCT imaging and confirmation on IR or FAF imaging.1 The requirement for the correspondence of the lesions seen on OCT imaging with an en face imaging modality like IR used in this study may allow RPD to be more reliably distinguished from other drusen phenotypes (such as cuticular drusen31,32), which can be difficult to differentiate on OCT alone.
Strengths of this study include the number of readers and reading centers evaluated in this study, the number of cases assessed, and the detailed assessment of the different RPD stages. However, limitations of this study include the relatively small number of parameters and criteria assessed, which were part of the study design to maximize the ease and feasibility of the grading task. For instance, this study did not include a separate assessment of the number of lesions at each stage, and this study limited the assessment of the number of Stages 2 and 3 RPD to categories between 0 and ≥ 5 lesions. It also excluded an evaluation of the en face spatial extent of RPD present. This study was also limited to the assessment of RPD on OCT imaging in the macular region (i.e., in the central 20° × 20° region, rather than over a larger field), except for 1 evaluation of their correspondence with lesions seen on IR imaging (that was simultaneously captured during OCT imaging). As such, this study is unable to report on the interreader agreement of different multimodal imaging-based definitions of RPD (such as when including other en face imaging modalities, such as FAF, for the confirmation of lesions seen on OCT imaging), which we and others have used to define the presence of RPD in an eye.16,33, 34, 35, 36, 37 Such an additional imaging modality may be useful if the definition of RPD requires a corresponding cluster on an en face imaging modality.
Before the findings of this study can be generalized, it should be noted that these findings were derived from an assessment of OCT imaging using only 1 scan protocol from 1 device that had relatively high-resolution and quality B-scans (lateral resolution 5.6 μm per pixel, with 25 frames averaged per B-scan for speckle noise reduction), and that the assessments were only performed in AMD eyes with large drusen (without including cases with early or late AMD, or without AMD). As such, the generalizability of these findings to other scan types (especially those with a lower resolution) and to a population with a broader spectrum of AMD severity remains to be determined.
In conclusion, this study demonstrated that 12 readers across 6 reading centers showed substantial or near-substantial—and not near-perfect—agreement for grading the presence of RPD on an OCT volume scan or selected B-scans across a range of different criteria. However, the evaluation of the presence of Stage 1 lesions and the quantity of Stage 2 and 3 lesions at the B-scan level showed low levels of interreader agreement. This study also observed how RPD prevalence at the eye level can vary markedly based on whether the presence of ≥ 5 Stage 2 or 3 lesions was or was not required to correspond with a cluster of definite lesions on IR imaging, underscoring the impact of varying criteria of its presence. These findings highlight how interreader variability would likely contribute to the variability of findings in studies seeking to understand the clinical associations of RPD and underscore the challenges when attempting to quantify the extent of RPD in an eye.
Manuscript no. XOPS-D-22-00225R1.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Dr SriniVas R. Sadda, an editorial board member of this journal, was recused from the peer-review process of this article and had no access to information regarding its peer review.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors made the following disclosures:
R.H.G.: Personal fees – Roche/Gentech, Bayer, Novartis, Apellis.
S.S.V.: Financial support – AlphaRET, Apellis, Bayer, Bioeq/Formycon, Carl Zeiss Meditec, Katairo, Kubota Vision, Novartis, Pixium, Roche/Genentech, SparingVision; Consultant – Apellis, Bioeq/Formycon, Galimedix, Novartis, Oxurion, Roche/Genentech; Nonfinancial support – Carl Zeiss Meditec and Heidelberg Engineering; Cofounder – STZ GRADE Reading Center.
G.J.J.: Consultant – Roche/Genentech, Annexon, Gemini Therapeutics.
F.G.H.: Grants – Acucela, Allergan, Apellis, Bayer, Bioeq/Formycon, Roche/Genentech, Geuder, Heidelberg Engineering, ivericBio, Pixium Vision, Novartis, Zeiss; Personal fees – Alexion, Boehringer-Ingelheim, Grayburg Vision, LinBioscience, Stealth BioTherapeutics, Aerie, Oxurion; cofounder of STZ GRADE Reading Center.
S.R.S.: Personal fees – Amgen, Apellis, Pfizer, Abbvie/Allergan, Roche/Genentech, Novartis, Regeneron, 4DMT, Oxurion, Gyroscope, Iveric, Jannsen, Nanoscope, Heidelberg, Optos, Notal, Biogen, Boerhinger Ingelheim, Bayer, Carl Zeiss Meditec, Nidek, Topcon, and CenterVue; Research instruments – Heidelberg, Carl Zeiss, Meditec, Nidek, Topcon, Optos, CenterVue.
S.L.: Personal fees – Apellis, Abbvie/Allergan, Bayer, Biogen, Novartis, Heidelberg Engineering, Carl Zeiss Meditec; Financial support – Novartis.
M.S.: Nonfinancial support – Heidelberg Engineering, Optos, Zeiss, CenterVue.
The other authors have no proprietary or commercial interest in any materials discussed in this article.
Supported by National Health & Medical Research Council of Australia (grant no.: GNT1181010 [R.H.G. and Z.W.], #1194667 [R.H.G.], and #2008382 [Z.W.]), a grant from the Macular Disease Foundation Australia (Z.W. and R.H.G.), in part by an unrestricted grant from Research to Prevent Blindness Inc to the University of Wisconsin—Madison Department of Ophthalmology and Visual Sciences (B.B.) and to the Department of Ophthalmology & Visual Sciences, University of Utah (S.S.V.), and by the BONFOR GEROK Program, Faculty of Medicine, University of Bonn, Grant No O-137.0030 (M.S.). CERA receives operational infrastructure support from the Victorian Government. The sponsor or funding organization played no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study.
Institutional review board approval was obtained for each site that were part of the LEAD study, and the study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and with tenets of the Declaration of Helsinki, and all participants provided written informed consent.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Wu, Schmitz-Valckenberg, Guymer.
Data collection: Wu, Bonse, Brown, Choong, Clifton, Corradetti, Corvi, Dieu, Dooling, Pak, Saßmannshausen, Skalak, Thiele, Guymer.
Analysis and interpretation: Wu, Schmitz-Valckenberg, Blodi, Holz, Jaffe, Liakopoulos, Sadda, Guymer.
Obtained funding: Wu, Guymer
Overall responsibility: Wu, Schmitz-Valckenberg, Blodi, Holz, Jaffe, Liakopoulos, Sadda, Bonse, Brown, Choong, Clifton, Corradetti, Corvi, Dieu, Dooling, Pak, Saßmannshausen, Skalak, Thiele, Guymer.
Supplementary Data
References
- 1.Wu Z., Fletcher E.L., Kumar H., et al. Reticular pseudodrusen: a critical phenotype in age-related macular degeneration. Prog Retin Eye Res. 2022;88 doi: 10.1016/j.preteyeres.2021.101017. [DOI] [PubMed] [Google Scholar]
- 2.Spaide R.F., Ooto S., Curcio C.A. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol. 2018;63:782–815. doi: 10.1016/j.survophthal.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Sivaprasad S., Bird A., Nitiahpapand R., et al. Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv Ophthalmol. 2016;61:521–537. doi: 10.1016/j.survophthal.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q., Shaffer J., Ying G.S. Pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration: a meta-analysis. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0149030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finger R.P., Wu Z., Luu C.D., et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121:1252–1256. doi: 10.1016/j.ophtha.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Q., Daniel E., Maguire M.G., et al. Pseudodrusen and incidence of late age-related macular degeneration in fellow eyes in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1530–1540. doi: 10.1016/j.ophtha.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nassisi M., Lei J., Abdelfattah N.S., et al. OCT risk factors for development of late age-related macular degeneration in the fellow eyes of patients enrolled in the HARBOR study. Ophthalmology. 2019;126:1667–1674. doi: 10.1016/j.ophtha.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Flamendorf J., Agrón E., Wong W.T., et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122:2053–2062. doi: 10.1016/j.ophtha.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser R.G., Tan R., Ayton L.N., et al. Assessment of retinotopic rod photoreceptor function using a dark-adapted chromatic perimeter in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57:5436–5442. doi: 10.1167/iovs.16-19295. [DOI] [PubMed] [Google Scholar]
- 10.Tan R., Guymer R.H., Luu C.D. Subretinal drusenoid deposits and the loss of rod function in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:4154–4161. doi: 10.1167/iovs.18-23970. [DOI] [PubMed] [Google Scholar]
- 11.Luu C.D., Tan R., Caruso E., et al. Topographic rod recovery profiles after a prolonged dark adaptation in subjects with reticular pseudodrusen. Ophthalmol Retina. 2018;2:1206–1217. doi: 10.1016/j.oret.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Flynn O.J., Cukras C.A., Jeffrey B.G. Characterization of rod function phenotypes across a range of age-related macular degeneration severities and subretinal drusenoid deposits. Invest Ophthalmol Vis Sci. 2018;59:2411–2421. doi: 10.1167/iovs.17-22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinberg J.S., Saßmannshausen M., Fleckenstein M., et al. Correlation of partial outer retinal thickness with scotopic and mesopic fundus-controlled perimetry in patients with reticular drusen. Am J Ophthalmol. 2016;168:52–61. doi: 10.1016/j.ajo.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Sassmannshausen M., Pfau M., Thiele S., et al. Longitudinal analysis of structural and functional changes in presence of reticular pseudodrusen associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020;61:19. doi: 10.1167/iovs.61.10.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corvi F., Pellegrini M., Belotti M., et al. Scotopic and fast mesopic microperimetry in eyes with drusen and reticular pseudodrusen. Retina. 2019;39:2378–2383. doi: 10.1097/IAE.0000000000002335. [DOI] [PubMed] [Google Scholar]
- 16.Guymer R.H., Wu Z., Hodgson L.A.B., et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology. 2019;126:829–838. doi: 10.1016/j.ophtha.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Guymer R.H., Chen F.K., Hodgson L.A.B., et al. Subthreshold nanosecond laser in age-related macular degeneration: observational extension study of the LEAD clinical trial. Ophthalmol Retina. 2021;5:1196–1203. doi: 10.1016/j.oret.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Zweifel S.A., Spaide R.F., Curcio C.A., et al. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Zarubina A.V., Neely D.C., Clark M.E., et al. The prevalence of subretinal drusenoid deposits in older persons with and without age-related macular degeneration by multimodal imaging. Ophthalmology. 2016;123:1090–1100. doi: 10.1016/j.ophtha.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung C.M.G., Gan A., Yanagi Y., et al. Association between choroidal thickness and drusen subtypes in age-related macular degeneration. Ophthalmol Retina. 2018;2:1196–1205. doi: 10.1016/j.oret.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Ueda-Arakawa N., Ooto S., Tsujikawa A., et al. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina. 2013;33:490–497. doi: 10.1097/IAE.0b013e318276e0ae. [DOI] [PubMed] [Google Scholar]
- 22.Hogg R.E., Silva R., Staurenghi G., et al. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology. 2014;121:1748–1755. doi: 10.1016/j.ophtha.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Querques G., Canouï-Poitrine F., Coscas F., et al. Analysis of progression of reticular pseudodrusen by spectral domain–optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:1264–1270. doi: 10.1167/iovs.11-9063. [DOI] [PubMed] [Google Scholar]
- 24.Wu Z., Ayton L.N., Luu C.D., et al. Reticular pseudodrusen in intermediate age-related macular degeneration: prevalence, detection, clinical, environmental, and genetics associations. Invest Ophthalmol Vis Sci. 2016;57:1310–1316. doi: 10.1167/iovs.15-18682. [DOI] [PubMed] [Google Scholar]
- 25.Spaide R.F. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33:1800–1808. doi: 10.1097/IAE.0b013e31829c3765. [DOI] [PubMed] [Google Scholar]
- 26.Gwet K.L. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29–48. doi: 10.1348/000711006X126600. [DOI] [PubMed] [Google Scholar]
- 27.Feinstein A.R., Cicchetti D.V. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43:543–549. doi: 10.1016/0895-4356(90)90158-l. [DOI] [PubMed] [Google Scholar]
- 28.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 29.Sevilla M.B., McGwin G., Jr., Lad E.M., et al. Relative retinal morphology and function in aging and early to intermediate age-related macular degeneration subjects. Am J Ophthalmol. 2016;165:65–77. doi: 10.1016/j.ajo.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar H., Guymer R.H., Hodgson L.A.B., et al. Exploring reticular pseudodrusen extent and impact on mesopic visual sensitivity in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2022;63:14. doi: 10.1167/iovs.63.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goh K.L., Chen F.K., Balaratnasingam C., et al. Cuticular drusen in age-related macular degeneration: association with progression and impact on visual sensitivity. Ophthalmology. 2022;129:653–660. doi: 10.1016/j.ophtha.2022.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Balaratnasingam C., Cherepanoff S., Dolz-Marco R., et al. Cuticular drusen: clinical phenotypes and natural history defined using multimodal imaging. Ophthalmology. 2018;125:100–118. doi: 10.1016/j.ophtha.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Yoneyama S., Sakurada Y., Mabuchi F., et al. Genetic and clinical factors associated with reticular pseudodrusen in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2014;252:1435–1441. doi: 10.1007/s00417-014-2601-y. [DOI] [PubMed] [Google Scholar]
- 34.De Bats F.M.D., Mathis T.M.D., Mauget-Faysse M.M.D., et al. Prevalence of reticular pseudodrusen in age-related macular degeneration using multimodal imaging. Retina. 2016;36:46–52. doi: 10.1097/IAE.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 35.Lynch A.M., Wagner B.D., Palestine A.G., et al. Plasma biomarkers of reticular pseudodrusen and the risk of progression to advanced age-related macular degeneration. Transl Vis Sci Technol. 2020;9:12. doi: 10.1167/tvst.9.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X., Wang X., Sadda S.R., Zhang Y. Subtype-differentiated impacts of subretinal drusenoid deposits on photoreceptors revealed by adaptive optics scanning laser ophthalmoscopy. Graefes Arch Clin Exp Ophthalmol. 2020;258:1931–1940. doi: 10.1007/s00417-020-04774-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz-Valckenberg S., Alten F., Steinberg J.S., et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:5009–5015. doi: 10.1167/iovs.11-7235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.