Abstract
Here, we examined the effects of crossbreeding and sex on growth performance, slaughter performance, and meat quality in Xingguo gray (XG) goose, using transcriptomic and metabolomic techniques. The experiment was conducted using 400 goslings (1-day old) of 2 genotypes: the XG breed and its ternary hybrids [F2 geese; (XG Goose♂ × Yangzhou Goose♀)♀ × Shitou Goose♂]. The goslings were divided into 4 groups: female XG, male XG, female F2 geese, and male F2 geese, and growth parameters were examined at 70 d of age, using 30 birds from each group. Following slaughter, samples of breast and thigh muscles were collected from each group for chemical, metabolome, and transcriptome analyses. Growth rate, live body and slaughter weights, meat chemical composition, and muscle fiber diameter were affected by crossbreeding and sex. Crossbreeding significantly improved the dressing percentage, semieviscerated rate, eviscerated yield, and abdominal fat yield of XG geese. To clarify the potential regulatory network affected by crossbreeding and sex, we used RNA-seq and nontargeted metabolomics to detect changes in male and female goose breast muscle. The transcriptome results showed that there were 534, 323, 297, and 492 differently expressed genes (DEGs) among the 4 comparison groups (XG-Female vs. F2-Female, XG-Male vs. F2-Male, F2-Male vs. F2-Female, and XG-Male vs. XG-Female, respectively) that were mainly related to muscle growth and development and fatty acid metabolism pathways. A total of 141 significantly differentially accumulated metabolites (DAMs) were enriched in serine and threonine, propionate, and pyruvate metabolism. Finally, we comprehensively analyzed the metabolome and transcriptome data and found that many DEGs and DAMs played crucial roles in lipid metabolism and muscle growth and development. In summary, crossbreeding can improve XG goose production performance and affect breast muscle gene expression and metabolites in both female and male geese.
Key words: crossbreeding, goose, meat quality, metabolomics, transcriptome
INTRODUCTION
Goose meat is increasingly becoming popular among consumers owing to its nutritional qualities, such as high protein and unsaturated fatty acid contents and low fat content (Gendaszewska-Darmach et al., 2012; Okruszek et al., 2013; Razmaitė et al., 2022). The Xingguo gray (XG) goose, which originates from eastern China and is distributed in south-central Jiangxi, is known for its good meat quality and high intramuscular fat (IMF) content (Liu et al., 2021). However, it has lower reproductive performance and growth rates than commercial crossbreeds, resulting in high production costs and lesser suitability for commercial production. A previous study showed that crossbreeds perform better than purebreeds with regard to most important traits, including body weight (BW) and production performance (Wolf and Knizetova, 1994; Padhi, 2010). These production metrics as well as carcass characteristics are also influenced by the sex of the goose. Recent research has found significant differences in muscle and carcass fat content between male and female geese (Lisiak et al., 2021). Such differences, including slaughter weight, were also confirmed in a study on the effect of sex on slaughter characteristics of heavy Czech and Eskildsen Schwer hybrid geese (Uhlirova et al., 2018), and similar results were observed in male and female Lindowskaya geese (Akbaş et al., 2020).
The Shitou (ST) goose is usually used as a sire breed because of its high feed conversion ratio (FCR) (Zhao et al., 2019), while the Yangzhou (YZ) goose is usually used as a dam breed because of its high annual egg production (Shi et al., 2007). Hence, introducing ternary crosses between YZ and ST geese and XG geese is a successful technique to swiftly enhance the production performance of Xingguo gray geese.
In the past, research on carcass traits and meat quality were mainly focused on fatty acid and amino acid contents, meat yield, and meat texture and microstructure (Geldenhuys et al., 2013; Uhlirova et al., 2018; Gumulka and Poltowicz, 2020; Kokoszynski et al., 2022). However, recent advances in molecular technology, such as multiomics, have facilitated the study of molecular mechanisms of complex traits. Gene expression could be considered an intermediate phenotype between genotypes and observable characteristics (Hubner et al., 2005) and contributes to phenotypic heterosis (Stupar et al., 2008). RNA-Seq analysis can be used to compare the mRNA levels of specific genes in breast muscle tissues between sex and breeds. Moreover, metabolome analysis can reveal real-time dynamic changes in metabolites in meat in response to post-transcriptional regulation, revealing key metabolites and metabolic pathways related to changes in meat quality (Wen et al., 2020). These methods have proven to be effective for understanding meat quality traits (Jung et al., 2022; Li et al., 2022; Zhan et al., 2022). An integrated metabolome and transcriptome analysis can reveal differences in meat quality based on gene actions and metabolites in F2 crosses and XG geese.
To date, few studies have investigated the effects of crossbreeding using an integrated transcriptome and metabolome analysis. Here, we examined the effects of crossbreeding and sex on growth performance, carcass traits, meat quality in XG geese and XG × ST × YZ hybrids. It is anticipated that the findings of this study will serve as a theoretical basis for the selection and breeding of geese.
MATERIALS AND METHODS
Ethics Statement
All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Experimental Animals by the Ministry of Agriculture of China, and all protocols were approved by the Animal Ethics Committee of the Institute of Animal Husbandry and Veterinary, Jiangxi Academy of Agricultural Science (JXAAS 2020-0025).
Experimental Design and Growth Performance
The experiment was conducted at the XG Goose Reservation Farm (Ganzhou, Jiangxi Province, China). A total of 400 one-day-old (100 male and 100 female of each genotype) XG ternary hybrids [(male XG × YZ female parent) × ST male; F2 goose] were grown under the same conditions of natural light and temperature, with free access to water and feed. For the first 7 d of rearing, 24-h lighting was used (4–5 W/m2) and temperature was maintained in a range of 28°C to 32°C. Relative air humidity was 60 to 70%. From the 2nd to the 10th week of age, geese were kept outside in partially roofed pens that were divided by wire mesh and covered with netted beds. Each flock was reared separately.
Animal Handling and Sample Collection
The BWs of the geese were measured every 10 d, and the average daily feed intake (ADFI) and feed/gain ratio (FGR) were calculated during this period. BW of each goose was recorded after fasting overnight. Sixty geese (30 females and 30 males) from each group were selected based on the average BW of birds in the pens and killed by cervical dislocation at the slaughter facility of the Jiangxi Poultry Breeding Engineering Laboratory. After bleeding, plucking, and carcass weight measurement, the carcass was dissected and tissue samples were collected. The left breast muscle was divided into 2 parts. One part was fixed with 4% paraformaldehyde for histological analysis, and the other was frozen in liquid nitrogen and stored at −80°C for transcriptome and metabolome analyses. The geese were then eviscerated and the semieviscerated carcass, eviscerated carcass, breast muscle, thigh muscle, and abdominal fat were weighed. The percentage weight of carcass, semieviscerated carcass, and eviscerated carcass was calculated relative to the live BW, whereas that of breast muscle, thigh muscle, and abdominal fat was calculated relative to the eviscerated carcass weight.
Meat Quality
The protein, fat, and moisture of roasted goose meat were determined using AOAC methods (Nalbandov, 1963). Samples of the breast and thigh muscles were separated from tendons and muscle membranes, cut into pieces, ground into a paste with a high-speed universal crusher (FW100, Taisite Ltd., Tianjin, China), and placed into sample cups. The moisture content (%) was calculated by weight loss after oven drying of samples (3 g) at 102°C for 12 h (to constant weight) in a Memmert laboratory dryer (UN 75, Schwabach, Germany). Crude protein content (%) was determined by the Kjeldahl method with an automatic Kjeldahl nitrogen analyzer (SKD-200, Shanghai Peiou Analysis Instruments Co., Ltd., Shanghai, China). Fat content (%) was measured by the Soxhlet method with petroleum ether extraction using a Hanon Automatic Soxhlet Extractor (SZF-06A, Shanghai Lichen Instruments Technology Co., Ltd., Shanghai, China). Measurements were performed on samples from 6 geese in the same group and repeated 3 times.
Histological Analysis
Breast muscle was obtained from the same position in each goose. The samples for histological comparison were dehydrated and embedded using an automatic closed tissue dehydrator (Donatello; Diapath, Martinengo, Italy). A JB-P5 tissue-embedding machine (Wuhan Junjie Electronics Co., Ltd., Wuhan, China) was used for paraffin embedding at 70°C. The paraffin blocks were cut into 4-μm-thick sections along the horizontal axis using an RM 2016 microtome (Leica Instruments Ltd., Wetzlar, Germany) and stained with hematoxylin and eosin according to standard protocols. The breast muscle was examined using a light microscope (Axio Imager A2; Zeiss, Oberkochen, Germany).
Fiber diameter, cross-sectional area, and fiber density were calculated using an image analysis system (Image-Pro Plus; Media Cybernetics, Rockville, MD). The diameter of the muscle fiber was measured using a micrometer in a fluorescence imaging microscope (Olympus, Tokyo, Japan), and the measurement was performed under a field of view of 10 × 40. Five visual fields were randomly selected to measure the diameter of the muscle fibers, and 20 muscle fibers were measured in each visual field. A total of 100 muscle fibers were randomly measured in this experiment, and the average value was taken as the diameter of the muscle fiber. To calculate muscle fiber density, 10 visual fields were randomly selected, the number of muscle fibers in each visual field (approximately 0.07 mm2) was counted under a 10 × 40 high-power field of view, and the average value was converted into the number of muscle fibers in 1 mm2.
Oil Red O Staining
Breast tissues were embedded in OCT and cut into 10-μm frozen sections in accordance with standard procedures. The sections were stained with Oil Red O to detect lipids in the breast tissues. Subsequently, the sections were dried, fixed in 4% PFA, dehydrated with absolute propylene glycol, stained in prewarmed Oil Red O solution (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), treated with 85% propylene glycol solution for differentiation, washed, and counterstained with hematoxylin solution. The sections were photographed with a Nikon digital camera DS-U3 (Nikon, Tokyo, Japan). The lipid droplet area was measured using Image-Pro Plus. The relative percentage of lipid droplet area was calculated according to the following equation: Relative lipid droplet area (%) = lipid droplet area/tissue area × 100%.
Determination of Antioxidant Activities
Muscle tissue (0.5 g) was mixed with 4.50 mL saline, then homogenized in an ice water bath by a JXFSTPRP-I-02 fast homogenizer (Shanghai Jingxin Co., Ltd., Shanghai, China) until no particles were visible in the homogenate solution (approximately 60 s). The prepared homogenized solution was centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant (10% stock solution of muscle) was stored at −20°C for further analysis. The tissue samples were evaluated for total antioxidant capacity (T-AOC, cat. No. A015-2-1), superoxide dismutase activity (SOD, cat. No. A001-1), catalase (CAT, cat. No. A007-1-2), and glutathione peroxidase activity (GSH-Px, cat. No. A005-1-2), as described in a previous study (Luo et al., 2022b). The triglyceride (TG) content was determined using the kits produced by the Nanjing Institute of Biological Engineering, and absorbance was measured at 450 nm with a microplate reader (Elx808, Bio-Tek, Winooski, VT). Total protein (TP, cat. No. A045-3-2) was determined by the BCA method with a BCA kit (Vazyme, Nanjing, China). The TG content was adjusted according to the amount of protein in the sample, following the manufacturer's instructions.
RNA Extraction and Library Construction
Total RNA was isolated using a TRIzol total RNA extraction kit (TIANGEN, Cat.DP424), which yielded >2 μg of total RNA per sample. RNA quality was examined using 0.8% agarose gel electrophoresis and spectrophotometry. High-quality RNA with 260/280 absorbance ratio of 1.8 to 2.2 was used for Illumina HiSeq library construction (Illumina, San Diego, CA), according to the manufacturer's instructions. Oligo-dT primers were used to amplify mRNA to obtain cDNA (APExBIO, cat. No. K1159) and to amplify cDNA for the synthesis of the second chain of cDNA. Then, cDNA products were purified using an AMPure XP system (Beckman Coulter, Beverly, MA). Double-strand cDNA was subjected to end repair. Adenosine was added to the end and ligated to adapters. After library construction, the library fragments were enriched by PCR amplification and selected according to a fragment size of 350 to 550 bp. The quality of the library was assessed using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA), and the library was sequenced using an Illumina NovaSeq 6000 sequencing platform (paired end 150) to generate raw reads.
RNA-seq Data Analysis
Raw paired-end fastq reads were filtered using TrimGalore to discard adapters and low-quality bases using the Cutadapt tool (Javorka et al., 2019). Clean reads were aligned against the Anser cygnoides (Swan goose) genome using HISAT2 (Kim et al., 2015), followed by reference genome-guided transcriptome assembly and gene expression quantification using StringTie (Pertea et al., 2015). Differentially expressed genes (DEGs) were identified using DEseq2 (for samples with replications) or edgeR (for samples with no replication) with a cut-off value of log2|fold change| >1 and P-adjust <0.05 (Love et al., 2014). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analyses of DEGs were performed using ClusterProfiler (Robinson et al., 2010), and terms with P < 0.05 were considered significant. Gene set enrichment analysis (GSEA) was performed using the clusterProfiler package (version 3.4.4) (Yu et al., 2012).
Validation of DEGs by Real-Time PCR
Real-time PCR was performed to validate the expression levels of DEGs. Six genes were selected for validation using specific primers designed using Primer Premier 5.0 software (Table 1). The RNA samples were used for both qPCR and RNA-seq. Reverse transcription of mRNA to cDNA was performed using HiScript QRT SuperMix for qPCR (+gDNA wiper) (R123-01; Vazyme), according to the manufacturer's instructions. qPCR was conducted on the Archimed Quantitative PCR Detection System using the PerfectStart Green qPCR SuperMix Kit (AQ601; Transgen Biotech). The relative expression levels of the genes were normalized to that of GAPDH (internal control) and calculated using the 2−∆∆Ct method (Livak and Schmittgen, 2001).
Table 1.
Primer sequences for RT-PCR.
| Gene name | Nucleotide sequences (5′→3′) | Tm (°C) | Product size (bp) |
|---|---|---|---|
| GAPDH | CGTTGCGTTATGTTGTGAAA CCGACGGAAAGAACTTGGTA |
60 | 280 |
| ALDOA | GTGCCAGGGCAAGTACACCC GACAGGAGTGTCACACGGCG |
60 | 165 |
| GPX3 | GGCGGAGGAATTCGGGAACC CCGCATGTAGGCGACGATGT |
60 | 172 |
| METTL21C | CCCGGGGCACTGGCTTTATC AAGCCTGTTCCAGCGCCAAT |
60 | 98 |
| TRIM63 | CACCCCATGTGCAAGGAGCA CTTCGCAGTCCTTGTGGGCA |
60 | 112 |
| FABP3 | AGGGCTATCAGGAGGCACCC GAGAGAGAGCGAAACCGCCG |
60 | 146 |
| GATM | GCTACGATGGGCCTCAGTGC GTGTTCTGGGAGGCCGTAGC |
60 | 156 |
Comprehensive 2-Dimensional Gas Chromatography/Time-of-Flight Mass Spectrometry (GC × GC/TOFMS) Analysis
Identification and quantification of metabolites was performed using an Agilent 7890A GC device (Agilent) and a Pegasus 4D TOF/MS mass spectrometer (Leco Corporation, St. Joseph, MI). An Agilent DB-WAX column (30 m × 250 μm × 0.25 μm) was used for all analysis. The temperature program was as follows: 40°C for 3 min; 5°C/min to 250°C; 250°C for 5 min; helium (99.9999%) 1.0 mL/min. Injections were made in splitless mode. A 2-dimensional column was used (DB-17 MS; Agilent), with dimensions of 2 m × 100 μm × 0.10 μm, and an initial column temperature of 255°C. The offset temperature of the secondary GC oven was set at +5°C relative to the temperature of the primary GC oven. Remaining settings were as follows: modulation period: 6.0 s; interface temperature: 270°C; ion source: 250°C; electron impact: 70 eV; detector voltage: 1,680 V; acquisition rate: 50 spectra/s; scan range: m/z 33 to 500; mass spectral library: NIST.
GC-TOFMS analysis of 2 μL extracts was conducted. Volatile components were detected based on the data processing system of MassLynx V4.1 Chemical Workstation, Nist2008, and WILYL standard spectrum libraries, and spectrum analyses from the related literature. Quantitative analysis was conducted using the area normalization method, and the relative percentage contents of volatile components were obtained.
Data Analysis
Carcass traits, meat quality parameters, muscle characteristics, and antioxidant capacity were analyzed using an independent-sample t test in SPSS 16.0 software. Differences in mean values were considered statistically significant at P < 0.05. Pearson's correlation test was performed to determine the relationships between important DEG and DMs. Metabolites with variable importance in projection (VIP >1.0, P < 0.05) were screened.
RESULTS
Growth Performance
The effects of crossbreeding on the growth performance of the geese are shown in Table 2. The XG goose is an indigenous breed with an average BW of 3,400 to 3,800 g; however, the F2 goose had a larger body size and a fast growth rate, and its market weight at 70 d of age was in the range of 3,700 to 4,300 g. Regardless of sex, the initial BW of F2 goose was slightly higher than that of XG goose. However, F2 goose had a lower FCR, higher relative growth rate (RGR), and a 10% higher average final BW compared with XG goose, indicating a better growth performance.
Table 2.
Comparison of growth performance among the XG and F2 goose.
| XG goose |
F2 goose |
||||||
|---|---|---|---|---|---|---|---|
| Age (d) | BW (g) | RGR (%) | FCR | BW (g) | RGR (%) | FCR | P value |
| 0 | 90.11 ± 4.82 | 100.53 ± 8.62 | 1.14 | 0.865 | |||
| 10 | 345.26 ± 27.73 | 283.1 | 2.73 | 400.22 ± 50.3 | 298.1 | 1.33 | 0.322 |
| 20 | 932.00 ± 111.46a | 169.9 | 1.24 | 1125 ± 125.21b | 181.1 | 3.28 | 0.045 |
| 30 | 1593.50 ± 181.72a | 70.98 | 3.04 | 1695.55 ± 210.75b | 50.72 | 3.12 | 0.048 |
| 40 | 2321.31 ± 250.00a | 45.67 | 3.49 | 2754.41 ± 240.68b | 62.45 | 4.30 | 0.031 |
| 50 | 3068.57 ± 319.10a | 32.19 | 4.18 | 3410.62 ± 403.15b | 23.82 | 4.80 | 0.025 |
| 60 | 3478.99 ± 420.37a | 13.37 | 4.23 | 3789.45 ± 501.19b | 11.11 | 3.75 | 0.000 |
| 70 | 3661.54 ± 442.26a | 5.25 | 4.63 | 4120.20 ± 80.23b | 8.73 | 3.11 | 0.001 |
Abbreviations: BW, body weight; FCR, feed conversion rate; F2 goose, ternary hybrid goose; RGR, relative growth rate; XG goose, Xingguo gray goose.
Values with different superscript letters (a, b) within the same row per fixed effect (age of slaughter) differ significantly (P < 0.05); ns, not significant.
Slaughter Performance
The effects of sex and crossbreeding on slaughter performance at 70 d are shown in Table 3. The live BW, slaughter weight, dressing percentage, semieviscerated yield, and eviscerated yield of F2 goose were significantly higher (P < 0.05) than those of XG goose. There were no significant differences in pectoral and leg muscle weights between geese of different sexes. Notably, male geese had higher (P < 0.05) live BW and slaughter weight than female geese in both groups.
Table 3.
Comparison of slaughter performance between the XG and F2 goose.
| XG goose |
F2 goose |
P value |
|||||
|---|---|---|---|---|---|---|---|
| Items | Male (n = 15) | Female (n = 15) | Male (n = 15) | Female (n = 15) | Breed (B) | Sex (S) | B*S |
| Live body weight (kg) | 4.06 ± 0.28A | 3.26 ± 0.30B | 4.44 ± 0.32C | 3.82 ± 0.47D | 0.000 | 0.000 | 0.360 |
| Slaughter weight (kg) | 3.46 ± 0.23A | 2.78 ± 0.12B | 3.84 ± 0.33C | 3.23 ± 0.87D | 0.000 | 0.000 | 0.655 |
| Dressing-out percentage (%) | 85.06 ± 1.87a | 85.18 ± 1.17a | 86.44 ± 1.95b | 87.08 ± 3.60b | 0.031 | 0.602 | 0.721 |
| Semieviscerated yield (%) | 78.06 ± 1.93a | 76.57 ± 2.41a | 78.79 ± 2.11b | 80.14 ± 2.92b | 0.006 | 0.929 | 0.063 |
| Eviscerated yield (%) | 68.84 ± 1.43a | 67.83 ± 0.03a | 70.61 ± 2.12b | 71.37 ± 0.01b | 0.004 | 0.662 | 0.174 |
| Breast yield (%) | 12.17 ± 0.56 | 12.72 ± 1.25 | 12.73 ± 1.47 | 12.28 ± 1.21 | 0.738 | 0.058 | 0.340 |
| Thigh yield (%) | 13.35 ± 1.52 | 13.41 ± 0.88 | 14.15 ± 1.66 | 13.23 ± 0.68 | 0.464 | 0.310 | 0.256 |
| Abdominal fat yield (%) | 2.77 ± 0.65a | 3.55 ± 1.21a | 2.61 ± 0.87b | 2.51 ± 1.22b | 0.047 | 0.245 | 0.140 |
Abbreviations: F2 goose, ternary hybrid goose; XG goose, Xingguo gray goose.
Rows marked with different superscript letters differ significantly between groups.
Rows marked with different superscript letters differ extremely significantly between groups. ns, not significant.
Chemical Composition
Moisture, protein, and IMF contents were analyzed to compare the chemical composition of goose meat among the different groups (Table 4). All determined indices differed significantly among the groups. The breast and thigh muscles of XG goose had significantly lower (P < 0.01) moisture content and significantly higher (P < 0.05) IMF and protein contents than those of F2 goose. The crude protein and crude fat contents of the breast muscle of male goose were higher (P < 0.05) than those of female goose, whereas the moisture content of the breast muscle of male goose was lower (P < 0.05) than that of female goose. The crude protein content of the thigh muscle of male goose was higher (P < 0.05) than that of female goose.
Table 4.
Chemical composition in breast and thigh muscle from XG and F2 geese.
| XG goose |
F2 goose |
P value |
||||||
|---|---|---|---|---|---|---|---|---|
| Items | Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) | Breed (B) | Sex (S) | B*S | |
| Breast | Moisture | 71.90 ± 0.50A | 72.30 ± 0.34B | 73.90 ± 0.31C | 74.70 ± 0.23D | 0.000 | 0.005 | 0.293 |
| CP | 18.93 ± 0.20A | 18.77 ± 0.23B | 18.10 ± 0.13C | 17.37 ± 0.30D | 0.000 | 0.000 | 0.294 | |
| CF | 2.40 ± 0.12A | 2.22 ± 0.10B | 2.21 ± 0.12B | 1.62 ± 0.13C | 0.000 | 0.000 | 0.200 | |
| Thigh | Moisture | 71.52 ± 0.61A | 71.83 ± 0.33A | 73.50 ± 0.32B | 74.31 ± 0.10B | 0.000 | 0.062 | 0.262 |
| CP | 19.47 ± 0.25A | 18.890 ± 0.214B | 18.55 ± 0.16C | 17.78 ± 0.372D | 0.000 | 0.000 | 0.473 | |
| CF | 2.80 ± 0.21A | 2.72 ± 0.12A | 2.70 ± 0.13A | 2.43 ± 0.11B | 0.001 | 0.002 | 0.086 | |
Abbreviations: CF, crude fat; CP, crude protein; F2 goose, ternary hybrid goose; XG goose, Xingguo gray goose.
Rows marked with different superscript letters differ extremely significantly between groups. ns, not significant.
Muscle Characteristics
Oil Red O staining was used to observe the concentration of lipid droplets in pectoral muscle sections of the XG and F2 goose. The distribution area of lipid droplets in the breast muscle of XG goose was significantly higher (P < 0.01) than that of F2 goose (Table 5 and Figure 1). The muscle fiber characteristics of the breast muscle were not significantly affected by crossbreeding (Table 6 and Figure 2). However, sex had a significant influence on the characteristics of the breast muscle, with the muscle fiber diameter and muscle cross-sectional area significantly higher (P < 0.05) in male than in female goose.
Table 5.
Comparison of lipid droplets in the breast muscles of XG and F2 goose.
| Items | XG goose |
F2 goose |
P value |
||||
|---|---|---|---|---|---|---|---|
| Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) | Breed (B) | Sex (S) | B*S | |
| Lipid drops area | 17.03 ± 6.32A | 16.32 ± 7.12A | 9.55 ± 4.23B | 8.68 ± 3.84B | 0.000 | 0.147 | 0.632 |
Abbreviations: F2 goose, ternary hybrid goose; XG goose, Xingguo gray goose.
Rows marked with different superscript letters differ extremely significantly between groups. ns, not significant.
Figure 1.
Oil red O stained of pectoral muscle of Xingguo gray (XG) goose and its ternary hybrid (F2 goose); Scale bars, 100 µm; magnification: 200×.
Table 6.
Muscle fiber characteristics of breast muscle in XG and F2 geese.
| XG goose |
F2 goose |
P value |
|||||
|---|---|---|---|---|---|---|---|
| Items | Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) | Breed (B) | Sex (S) | B*S |
| Muscle fiber diameter (mm) | 0.025 ± 0.006A | 0.021 ± 0.006B | 0.028 ± 0.004A | 0.023 ± 0.004B | 0.021 | 0.012 | 0.664 |
| Muscle fiber density (n/mm², × 103) | 1.386 ± 0.460 | 1.423 ± 0.345 | 1.369 ± 0.489 | 1.461 ± 0.462 | 0.911 | 0.471 | 0.767 |
| Cross-sectional area (mm², × 10−3) | 0.615 ± 0.200 | 0.494 ± 0.202 | 0.708 ± 0.279 | 0.587 ± 0.224 | 0.045 | 0.010 | 1 |
Abbreviations: F2 goose, ternary hybrid goose; XG goose, Xingguo gray goose.
Rows marked with different letters differ extremely significantly between groups. ns, not significant.
Figure 2.
Hematoxylin and eosin (H&E)-stained breast muscle tissue sections of Xingguo gray (XG) goose and its ternary hybrid (F2 goose); Scale bars, 50 µm; magnification: 400×.
Biochemical Indices
The effects of sex and crossbreeding on the antioxidative capacity of goose muscle are shown in Table 7. Regardless of sex, the TG, GSH-Px, and T-AOC levels of breast and thigh muscles from XG goose were higher (P < 0.05) compared with those of F2 goose. The sex significantly affected the TG, GSH-Px, and SOD levels of XG goose and F2 goose. The breast and thigh meat from the male goose had a higher (P < 0.05) TG, GSH-Px, and SOD levels. The breast and thigh meat from the male XG goose had a higher (P < 0.05) T-AOC level, and breast meat from the male F2 goose had a higher (P < 0.05) T-AOC level. However, the CAT activities of breast and thigh muscles were not significantly affected (P > 0.05) by sex or crossbreeding.
Table 7.
Biochemical indices of muscle in XG and F2 geese.
| XG goose |
F2 goose |
P value |
||||||
|---|---|---|---|---|---|---|---|---|
| Items | Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) | Breed (B) | Sex (S) | B*S | |
| Breast | TG | 4.55 ± 0.45a | 4.17 ± 0.59b | 3.96 ± 0.83c | 3.82 ± 0.30d | 0.012 | 0.038 | 0.678 |
| T-AOC | 24.99 ± 1.46a | 21.22 ± 0.84b | 19.28 ± 1.32 | 20.61 ± 1.64 | 0.001 | 0.097 | 0.003 | |
| CAT | 166.83 ± 11.38 | 150.97 ± 6.99 | 160.52 ± 4.63 | 160.52 ± 13.23 | 0.743 | 0.127 | 0.127 | |
| GSH-Px | 327.96 ± 13.17a | 304.13 ± 20.58b | 267.4 ± 14.01c | 236.63 ± 21.49d | 0.000 | 0.011 | 0.738 | |
| SOD | 331.37 ± 55.38a | 312.57 ± 48.37b | 254.74 ± 26.43c | 236.22 ± 32.32d | 0.004 | 0.042 | 0.995 | |
| Thigh | TG | 5.16 ± 0.44a | 4.79 ± 0.64b | 4.61 ± 0.82b | 4.25 ± 0.28c | 0.008 | 0.037 | 0.970 |
| T-AOC | 25.81 ± 1.85a | 22.4 ± 2.64b | 19.47 ± 1.10c | 22.07 ± 2.03b | 0.006 | 0.687 | 0.010 | |
| CAT | 168.55 ± 7.42 | 152.88 ± 6.15 | 160.14 ± 5.46 | 161.1 ± 13.89 | 0.983 | 0.124 | 0.086 | |
| GSH-Px | 339.52 ± 18.63a | 301.99 ± 22.1b | 277.85 ± 25.76c | 245.47 ± 14.34d | 0.000 | 0.008 | 0.236 | |
| SOD | 331.64 ± 59.93a | 253.6 ± 31.73b | 315.27 ± 34.87a | 246.93 ± 18.64b | 0.568 | 0.003 | 0.809 | |
Abbreviations: F2 goose: ternary hybrid goose; XG goose, Xingguo gray goose.
Rows marked with different superscript letters differ significantly between groups.
RNA Sequencing of Breast Muscle Tissue and Data Analysis
Differential expression analysis identified 492, 297, 323, and 534 DEGs in the 4 comparison groups: XG-M vs. XG-F, F2-M vs. F2-F, XG-M vs. F2-M, and XG-F vs. F2-F, respectively. Overall, 294 upregulated and 198 downregulated DEGs were identified in the XG-M vs. XG-F group; 149 upregulated and 148 downregulated DEGs in the F2-M vs. F2-F group; 190 upregulated and 133 downregulated DEGs in the XG-M vs. F2-M group; and 281 upregulated and 253 downregulated DEGs in the XG-F vs. F2-F group. The top 10 up- and downregulated genes are shown in Figure 3.
Figure 3.
Heatmap of top 20 differentially expressed genes in breast muscle tissue from XG and its ternary hybrid (F2). Different colors were used to represent downregulated DEGs (blue), upregulated DEGs (red). The color scale indicates fold changes (log10) in gene expression. (A) XG-F vs. F2-F, (B) XG-M vs. F2-M, (C) F2-M vs. F2-F, and (D) XG-M vs. XG-F groups. M: male; F: female.
KEGG pathway analysis of DEGs in the 4 comparison groups was performed, and the top 10 significantly enriched pathways in each group are shown in Figure 4. The important enriched pathways include glycine, serine, and threonine metabolism, the calcium signaling pathway, oxidative phosphorylation, and pyruvate metabolism.
Figure 4.
Top 20 significantly enriched KEGG pathways by differentially expressed genes (DEGs). (A) XG-F vs. F2-F, (B) XG-M vs. F2-M, (C) F2-M vs. F2-F, and (D) XG-M vs. XG-F groups. M: male; F: female.
Validation DEGs by qRT-PCR
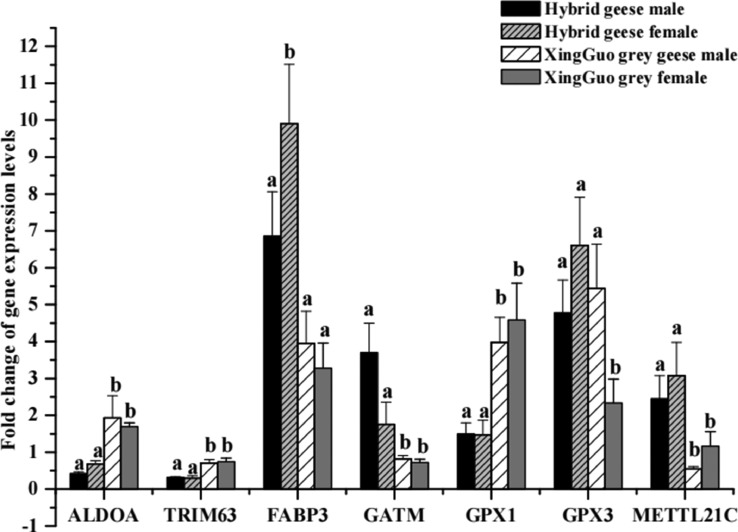
To verify the reliability of the transcriptome data, DEGs related to growth performance and muscle development were selected for qRT-PCR analysis. The qRT-PCR data were consistent with the RNA-seq data, confirming the validity of the transcriptome data (Figure 5).
Figure 5.
Validation of transcriptome data using quantitative real-time-PCR. Gene expression level was calculated using the 2−ΔΔCt method, and different letters indicate significant difference at P < 0.05.
Differentially Accumulated Metabolites (DAMs) in Breast Muscle From XG and F2 Geese
Annotated flavor compounds in breast muscle samples from XG and F2 goose were detected by GC × GC-TOFMS. A total of 38, 43, 28, and 32 DAMs were detected in the XG-F vs. F2-F, XG-M vs. F2-M, XG-F vs. XG-M, and F2-F vs. F2-M groups, respectively (Figure 6). Some of the most significant metabolites were short-chain fatty acids (SCFAs), including pentanoic acid, 3-methyl-, ethyl ester, propanoic acid, 2-methoxy-, vinyl butyrate, and cyanoacetic acid. KEGG pathway enrichment analysis was performed to elucidate the biological functions of the metabolites. However, several metabolites were not enriched in the KEGG pathway. The candidate biomarkers were enriched across different metabolic pathways, including serine and threonine metabolism (2-ketobutyric acid), propionate metabolism (2-ketobutyric acid), glycolysis/gluconeogenesis (L-glutamic acid), and pyruvate metabolism (lactic acid).
Figure 6.
Heatmap of top 20 differentially accumulated metabolites (DAMs) in breast muscle samples from Xingguo gray (XG) goose and its ternary hybrid (F2). (A) DAMs in XG-F vs. F2-F, (B) XG-M vs. F2-M, (C) XG-M vs. XG-F, and (D) F2-M vs. F2-F groups. M: male; F: female. The heatmaps are drawn according to the metabolomics data. Columns and rows in the heatmap represent samples and metabolites, respectively.
Integrated Analysis of the Breast Muscle Transcriptome and Metabolome Data
Based on Pearson's correlation analysis, a clustering correlation heatmap was generated to illustrate the relationships between DAMs and DEGs (Figure 7). 2-Ketobutyric acid was significantly positively correlated with GATM and was negatively correlated with ALAS1 and LOC106036048. L-lactic acid was significantly positively correlated with PGM2, GATM, GAMT, P2RX5, LOC106030553, LOC106039326, and LOC106039360 (CARNMT1) and negatively correlated with ALDOA, LOC106049487 (GPX1), EGF, and PGAM1. L-glutamic acid was significantly positively correlated with EGF and LOC106049487 (GPX1), and negatively correlated with P2RX5.
Figure 7.
Heatmaps showing the correlations between differentially accumulated metabolites (DAMs) and differentially expressed genes (DEGs). (A) Correlations between DAMs and DEGs in XG-F vs. F2-F; (B) XG-M vs. F2-M; (C) XG-M vs. XG-F groups. Each row in the figure represents 1 DAM and each column represents 1 DEG. The colors red and blue represent positive and negative correlations, respectively, and the depth of the color indicates correlation strength. * 0.01 < P < 0.05; **P < 0.01.
DISCUSSION
In the present study, we examined the effect of crossbreeding and sex on growth performance and meat quality of XG goose. Crossbreeding (XG × ST × YZ) significantly (P < 0.05) improved the BW and growth rate of geese. Additionally, early growth rate and final BW were significantly higher in F2 goose than in native XG goose, indicating that F2 goose had better growth performance than XG goose. This conforms to previous reports that crossbreeding can improve the growth performance of indigenous breeds (Heo et al., 2015; Sungkhapreecha et al., 2022). Furthermore, the effects of sex and crossbreeding on slaughter performance at 70 d were examined. F2 goose had significantly higher (P < 0.01) live BW, slaughter weight, dressing-out percentage, semieviscerated yield, and eviscerated yield than XG goose, indicating that F2 goose had relatively higher meat yield and was therefore preferable for intensive meat production. Similarly, previous studies have shown that crossbreeding significantly increased dressing-out percentage and slaughter and carcass weights in Czech goose, and that these effects were genotype- and sex-dependent (Uhlirova et al., 2018, 2019).
The chemical composition of goose meat is mainly affected by age and sex. IMF is often recognized as a key quality factor because of its positive correlation with tenderness, juiciness, and flavor (Madeira et al., 2013). Interestingly, geese in the XG group had higher breast and thigh muscle fat contents than those in the F2 group, which may increase the flavor of the meat.
Poultry breast meat is highly susceptible to oxidation owing to its relatively high unsaturated fatty acid content, leading to a higher risk of rancidity in muscle tissues in stored and processed meat products (Pecjak et al., 2022). The body is equipped with an antioxidant system, which scavenges free radicals; however, low antioxidant capacity promotes oxidative stress-induced lipid peroxidation and tissue damage (Akinyemi and Adewole, 2022). T-AOC, SOD, GSH-PX, CAT, and MDA are important indicators of the antioxidant capacity of the body, and T-AOC represents the antioxidant defense capacity of the muscle (Dou et al., 2022; Luo et al., 2022b). SOD is an important antioxidant enzyme in animals and its activity indirectly reflects the ability of the body to prevent muscle lipid oxidation, which in meats used for human consumption affects quality and shelf life (Wang et al., 2017; Chen et al., 2019). GSH-PX protects cell membranes from damage by specifically catalyzing the reduction of peroxides through reduced glutathione content (Muhlisin et al., 2016; Luo et al., 2022b). In the present study, crossbreeding decreased the GSH-Px activity and T-AOC of the breast and thigh muscles of geese, which was consistent with previous findings (Surai et al., 2019; Mahmoudi et al., 2022).
Muscle fiber characteristics strongly influence meat quality because skeletal muscle mainly consists of muscle fibers, which can be characterized by morphological traits, contractile properties, and metabolic properties (Lee et al., 2010). In the present study, the muscle fiber characteristics of the breast muscle were not affected by crossbreeding. However, sex significantly affected the characteristics of the breast muscle, with male goose having significantly higher muscle fiber diameter and cross-sectional area than female goose. This was consistent with previous findings (Kucharska-Gaca et al., 2022) and indicates that meat from female goose may be more tender than that from male goose.
Furthermore, transcriptome and metabolome analyses were performed to elucidate the potential mechanisms of muscle development in XG and hybrid goose. Correlation analysis was performed to investigate the relationship between changes in gene expression in response to crossbreeding or sex and changes and the metabolic profile of goose meat. The analysis of DEGs and DAMs at various stages of muscle development revealed significant changes in energy metabolism.
METTL21C acts as a skeletal muscle-specific lysine methyltransferase that promotes myoblast differentiation and inhibits muscle atrophy (Wiederstein et al., 2018). Recent studies have found that METTL21C promotes the development and differentiation of skeletal muscle in poultry (Huang et al., 2014; Wang et al., 2022). In the present study, METTL21C was upregulated in the breast muscle of F2 goose, and its expression in the breast muscle was positively correlated with final live BW, breast muscle weight, and muscle fiber diameter. Based on these findings, we hypothesize that METTL21C is a vital growth-promoting gene in geese. TRIM63 is essential for controlling muscle atrophy (Taillandier and Polge, 2019), and its upregulation is often associated with the loss of muscle mass, especially in the presence of muscle or nutrient deficiencies (Ayuso et al., 2015). In the present study, TRIM63 expression in the breast muscle was negatively related to final live weight, breast muscle weight, and muscle fiber diameter, indicating that the TRIM63 gene may act as a negative regulator of muscle growth and development.
IMF is a mixture of several lipids, including TG, phospholipid (PLIP), and total cholesterol (TCHO), which play an important role in determining the flavor, tenderness, and water-holding capacity of meat (Luo et al., 2022a). Some of these gene products may participate in lipid metabolism and the conversion of carotenoids. For example, the expression of the PPARα gene was significantly reduced in the breast muscle of male XG goose. PPARα regulates lipid transport and metabolism through the peroxisome fatty acid β-oxidation pathway (Aoyama et al., 1998). It has been found that PPARα plays a key role in fat accumulation and binding (Abdelmegeed et al., 2011). Therefore, we hypothesize that PPARα is involved in regulating abdominal fat deposition.
ALDOA is a glycolysis enzyme that has been shown to be negatively correlated with brightness (L*) of flesh colors and may be used as a marker for different meat regions or colors (Wiederstein et al., 2018). ALDOA has been reported to be associated with tenderness and IMF content (Kim et al., 2019). The GPX family is a group of important antioxidant enzymes in the animal body, and glutathione peroxidase 1 (GPX1) is highly sensitive to oxidative stress in the body. Several studies have shown that GPX1 overexpression can protect cells from peroxide-induced damage and hydrogen peroxide-induced apoptosis (Lubos et al., 2007). The GPX1 gene has been reported to be involved in IMF deposition by regulating pyruvate and citric acid metabolism (Luo et al., 2022a). In the present study, a positive correlation was observed between ALDOA, GPX1, and fat content and TG content, and a negative correlation was observed between ALDOA and GPX1 and L-lactic acid content in muscle tissues. L-lactic acid, an intermediate product of pyruvate metabolism in animals, plays an important role in glycolysis and gluconeogenesis of the muscle, and its content is the main factor affecting the pH value of meat (Gonzalez-Rivas et al., 2020). Moreover, previous studies have indicated that sodium pyruvate supplementation enhances fat loss by increasing the activity of enzymes related to lipid catabolism and hormone levels, and promotes muscle growth by promoting protein deposition (Chen et al., 2011; Zhao et al., 2017). Those results showed that IMF deposition in geese was negatively associated with pyruvate and lactic acid metabolism.
Along with excessive fat deposition, poor meat quality has attracted increasing consumer concern, and protein synthesis is an important determinant of meat quality and yield in poultry. Glycine amidinotransferase (GATM) is the rate-limiting enzyme in creatine production, which plays an important role in muscle energy metabolism and defense against diet-induced obesity (Sandell et al., 2003; Kazak et al., 2017; Li et al., 2018). Mutation or knockout of GATM can affect creatine biosynthesis, leading to developmental delays (Battini et al., 2002; Nouioua et al., 2013; Snow, 2013; Stockebrand et al., 2016). Creatine is one of the most important nitrogen-containing compounds involved in protein and energy metabolism (Navratil et al., 2010). Creatine supplementation has been shown to increase muscle mass and protein synthesis in primary myotubes (Vandenberghe et al., 1997; Young et al., 2007). In the present study, GATM expression was positively correlated with final live BW and breast muscle weight, and metabolomic analysis showed that the accumulation of 2-ketobutyric acid and lactic acid was positively related with GATM expression. 2-Ketobutyric acid is an intermediate product of cysteine and methionine metabolism in animals, and is related to protein deposition and improves the growth performance of animals (Lesner et al., 2020). In ovo methionine injection has been reported to increase the relative weight of the pectoral muscle as well as Myf-5 and MSTN expression in the breast muscle of geese at hatching (Dang et al., 2022a,b). Moreover, L-methionine was more abundant in a low IMF content group (L_IMF) than in a high IMF content group (H_IMF) in sheep (Zhang et al., 2022). The results of the present study indicate that GATM gene can increase breast muscle yield and goose muscle growth.
In summary, we elucidated the effect and mechanisms of crossbreeding and sex on carcass characteristics and meat quality in XG goose. Sex and crossbreeding affected carcass characteristics, muscle fiber diameter, and muscle biochemical indices in this goose breed. The meat of both XG and F2 goose exhibited good physical and chemical properties; however, F2 goose had larger carcass weight. Transcriptome analysis revealed that METTL21C is a vital growth-promoting gene and that TRIM63 may act as a negative regulator of muscle growth and development. An integrated transcriptome and metabolome analysis revealed that ALDOA and GPX1 promoted IMF accumulation by positively regulating pyruvate and lactic acid synthesis, and that 2-ketobutyric acid and L-lactic acid metabolism pathways may be key pathways associated with goose muscle development. These findings provide novel insights and data supporting the molecular regulatory network of IMF and muscle growth and development.
ACKNOWLEDGMENTS
This study was supported by the NSFC Regional Science Foundation Project [grant number 3210786], Jiangxi Province Waterfowl Industry Technology System [grant number JXARS-09], National Waterfowl Industry Technology System [grant number CARS-42], and the Key Research and Development Program of Jiangxi Province [grant number 20192BBF60028].
Author Contributions: J. N. designed the experiments and wrote the paper; L. J. and W. H. analyzed the data; X. L., H. Q., and F. F. conducted the experiments; J. F. and Q. P. finalized the manuscript.
DISCLOSURES
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. The study design was approved by the appropriate ethics review board. We have read and understood your journal's policies, and we believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare.
REFERENCES
- Abdelmegeed M.A., Yoo S.H., Henderson L.E., Gonzalez F.J., Woodcroft K.J., Song B.J. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J. Nutr. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbas A.A., Sari M., Bugdayci K.E., Saatci M. The effect of sex and slaughter age on growth, slaughter, and carcass characteristics in Lindovskaya geese reared under breeder conditions. Turk. J. Vet. Anim. Sci. 2020;44:1087–1092. [Google Scholar]
- Akinyemi F., Adewole D. Effects of brown seaweed products on growth performance, plasma biochemistry, immune response, and antioxidant capacity of broiler chickens challenged with heat stress. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T., Peters J.M., Iritani N., Nakajima T., Furihata K., Hashimoto T., Gonzalez F. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Ayuso M., Fernández A., Núñez Y., Benitez R., Isabel B., Barragán C., Fernández A.I., Rey A.I., Medrano J.F., Cánovas Á., González-Bulnes A., López-Bote C., Ovilo C. Comparative analysis of muscle transcriptome between pig genotypes identifies genes and regulatory mechanisms associated to growth, fatness and metabolism. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battini R., Leuzzi V., Carducci C., Tosetti M., Bianchi M.C., Item C.B., Stöckler-Ipsiroglu S., Cioni G. Creatine depletion in a new case with AGAT deficiency: clinical and genetic study in a large pedigree. Mol. Genet. Metab. 2002;77:326–331. doi: 10.1016/s1096-7192(02)00175-0. [DOI] [PubMed] [Google Scholar]
- Chen J., Wang M., Kong Y., Ma H., Zou S. Comparison of the novel compounds creatine and pyruvateon lipid and protein metabolism in broiler chickens. Animals. 2011;5:1082–1089. doi: 10.1017/S1751731111000085. [DOI] [PubMed] [Google Scholar]
- Chen R., Wen C., Cheng Y., Chen Y., Zhuang S., Zhou Y. Effects of dietary supplementation with betaine on muscle growth, muscle amino acid contents and meat quality in Cherry Valley ducks. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019;103:1050–1059. doi: 10.1111/jpn.13083. [DOI] [PubMed] [Google Scholar]
- Dang D.X., Zhou H., Lou Y., Li D. Effects of in ovo feeding of methionine and/or disaccharide on post-hatching breast development, glycogen reserves, nutrients absorption parameters, and jejunum antioxidant indices in geese. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.944063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang D.X., Zhou H., Lou Y., Li D. Effects of methionine and/or disaccharide injected in the amnion of geese on post-hatching pectoral muscle and small intestine development, glycogen reserves, jejunum morphology, and digestive enzymes activities. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou L., Liu C., Yang Z., Su R., Chen X., Hou Y., Hu G., Yao D., Zhao L., Su L., Jin Y. Effects of oxidative stability variation on lamb meat quality and flavor during postmortem aging. J. Food Sci. 2022;87:2578–2594. doi: 10.1111/1750-3841.16138. [DOI] [PubMed] [Google Scholar]
- Geldenhuys G., Hoffman L.C., Muller N. The effect of season, sex, and portion on the carcass characteristics, pH, color, and proximate composition of Egyptian Goose (Alopochen aegyptiacus) meat. Poult. Sci. 2013;92:3283–3291. doi: 10.3382/ps.2013-03443. [DOI] [PubMed] [Google Scholar]
- Gendaszewska-Darmach E., Laska E., Rytczak P., Okruszek A. The chemical synthesis of metabolically stabilized 2-OMe-LPA analogues and preliminary studies of their inhibitory activity toward autotaxin. Bioorg. Med. Chem. Lett. 2012;22:2698–2700. doi: 10.1016/j.bmcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rivas P.A., Chauhan S.S., Ha M., Fegan N., Dunshea F.R., Warner R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: a review. Meat Sci. 2020;162 doi: 10.1016/j.meatsci.2019.108025. [DOI] [PubMed] [Google Scholar]
- Gumulka M., Poltowicz K. Comparison of carcass traits and meat quality of intensively reared geese from a Polish genetic resource flock to those of commercial hybrids. Poult. Sci. 2020;99:839–847. doi: 10.1016/j.psj.2019.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo K.N., Hong E.C., Kim C.D., Kim H.K., Lee M.J., Choo H.J., Choi H.C., Mushtaq M.M.H., Parvin R., Kim J.H. Growth performance, carcass yield, and quality and chemical traits of meat from commercial korean native ducks with 2-way crossbreeding. Asian-Australas. J. Anim. Sci. 2015;28:382–390. doi: 10.5713/ajas.13.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Hsu Y.H., Mo C., Abreu E., Kiel D.P., Bonewald L.F., Brotto M., Karasik D. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-kappaB signaling pathway. J. Bone Miner. Res. 2014;29:1531–1540. doi: 10.1002/jbmr.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner N., Wallace C.A., Zimdahl H., Petretto E., Schulz H., Maciver F., Mueller M., Hummel O., Monti J., Zidek V., Musilova A., Kren V., Causton H., Game L., Born G., Schmidt S., Müller A., Cook S.A., Kurtz T.W., Whittaker J., Pravenec M., Aitman T.J. Integrated transcriptional profiling and linkage analysis for identification of genes underlying disease. Nat. Genet. 2005;37:243–253. doi: 10.1038/ng1522. [DOI] [PubMed] [Google Scholar]
- Javorka P., Raxwal V.K., Najvarek J., Riha K. artMAP: a user-friendly tool for mapping ethyl methanesulfonate-induced mutations in Arabidopsis. Plant Direct. 2019;3:e00146. doi: 10.1002/pld3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D.Y., Lee D., Lee H.J., Kim H.J., Jung J.H., Jang A., Jo C. Comparison of chicken breast quality characteristics and metabolites due to different rearing environments and refrigerated storage. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L., Chouchani E.T., Lu G.Z., Jedrychowski M.P., Bare C.J., Mina A.I., Kumari M., Zhang S., Vuckovic I., Laznik-Bogoslavski D., Dzeja P., Banks A.S., Rosen E.D., Spiegelman B.M. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 2017;26:660–671. doi: 10.1016/j.cmet.2017.08.009. .e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.D., Jeong J.Y., Yang H.S., Hur S.J. Differential abundance of proteome associated with intramuscular variation of meat quality in porcine longissimus thoracis et lumborum muscle. Meat Sci. 2019;149:85–95. doi: 10.1016/j.meatsci.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszynski D., Biesiada-Drzazga B., Zochowska-Kujawska J., Kotowicz M., Sobczak M., Saleh M., Fik M., Arpasova H., Hrncar C., Kostenko S. Effect of genotype and sex on carcase composition, physicochemical properties, texture and microstructure of meat from geese after four reproductive seasons. Br. Poult. Sci. 2022;63:519–527. doi: 10.1080/00071668.2022.2030051. [DOI] [PubMed] [Google Scholar]
- Kucharska-Gaca J., Adamski M., Biesek J. Effect of parent flock age on hatching, growth rate, and features of both sexes goose carcasses. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Joo S.T., Ryu Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010;86:166–170. doi: 10.1016/j.meatsci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- Lesner N.P., Gokhale A.S., Kota K., DeBerardinis R.J., Mishra P. α-Ketobutyrate links alterations in cystine metabolism to glucose oxidation in mtDNA mutant cells. Metab. Eng. 2020;60:157–167. doi: 10.1016/j.ymben.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Xu Y., Lin Y. Transcriptome analyses reveal genes of alternative splicing associated with muscle development in chickens. Gene. 2018;676:146–155. doi: 10.1016/j.gene.2018.07.027. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang D., Yin L., Li Z., Yu C., Du H., Jiang X., Yang C., Liu Y. Integration analysis of metabolome and transcriptome profiles revealed the age-dependent dynamic change in chicken meat. Food Res. Int. 2022;156 doi: 10.1016/j.foodres.2022.111171. [DOI] [PubMed] [Google Scholar]
- Lisiak D., Janiszewski P., Grzeskowiak E., Borzuta K., Lisiak B., Samardakiewicz L., Schwarz T., Powalowski K., Andres K. Research on the effects of gender and feeding geese oats and hybrid rye on their slaughter traits and meat quality. Animals (Basel) 2021;11 doi: 10.3390/ani11030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhu C., Song W., Xu W., Tao Z., Zhang S., Li H. Genomic characteristics of four different geese populations in China. Anim. Genet. 2021;52:228–231. doi: 10.1111/age.13035. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubos E., Loscalzo J., Handy D.E. Homocysteine and glutathione peroxidase-1. Antioxid. Redox Signal. 2007;9:1923–1940. doi: 10.1089/ars.2007.1771. [DOI] [PubMed] [Google Scholar]
- Luo N., Shu J., Yuan X., Jin Y., Cui H., Zhao G., Wen J. Differential regulation of intramuscular fat and abdominal fat deposition in chickens. BMC Genome. 2022;23:308. doi: 10.1186/s12864-022-08538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Wei R., Teng Y., Ning R., Bai L., Lu C., Deng D., Abdulai M., Li L., Liu H., Hu S., Wei S., Kang B., Xu H., Han C. Influence of different types of sugar on overfeeding performance-part of meat quality. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira M.S., Costa P., Alfaia C.M., Lopes P.A., Bessa R.J.B., Lemos J.P., Prates J.A. The increased intramuscular fat promoted by dietary lysine restriction in lean but not in fatty pig genotypes improves pork sensory attributes. J. Anim. Sci. 2013;91:3177–3187. doi: 10.2527/jas.2012-5424. [DOI] [PubMed] [Google Scholar]
- Mahmoudi S., Mahmoudi N., Benamirouche K., Estévez M., Mustapha M.A., Bougoutaia K., Djoudi N.E.H.B. Effect of feeding carob (Ceratonia siliqua L.) pulp powder to broiler chicken on growth performance, intestinal microbiota, carcass traits, and meat quality. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlisin D.T.U., Lee J.H., Choi J.H., Lee S.K. Antioxidant enzyme activity, iron content and lipid oxidation of raw and cooked meat of Korean native chickens and other poultry. Asian-Australas. J. Anim. Sci. 2016;29:695–701. doi: 10.5713/ajas.15.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandov A.V. 2nd ed. University of Illinois Press; Urbana, IL: 1963. Advances in Neuroendocrinology. [Google Scholar]
- Navrátil T., Kohlíková E., Petr M., Pelclova D., Heyrovský M., Přistoupilová K. Supplemented creatine induces changes in human metabolism of thiocompounds and one- and two-carbon units. Physiol. Res. 2010;59:431–442. doi: 10.33549/physiolres.931588. [DOI] [PubMed] [Google Scholar]
- Nouioua S., Cheillan D., Zaouidi S., Salomons G.S., Amedjout N., Kessaci F., Boulahdour N., Hamadouche T., Tazir M. Creatine deficiency syndrome. A treatable myopathy due to arginine-glycine amidinotransferase (AGAT) deficiency. Neuromuscul. Disord. 2013;23:670–674. doi: 10.1016/j.nmd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Okruszek A., Wołoszyn J., Haraf G., Orkusz A., Wereńska M. Chemical composition and amino acid profiles of goose muscles from native Polish breeds. Poult. Sci. 2013;92:1127–1133. doi: 10.3382/ps.2012-02486. [DOI] [PubMed] [Google Scholar]
- Padhi M.K. Production benefits of the crossbreeding of indigenous and non-indigenous ducks-growing and laying period body weight and production performance. Trop. Anim. Health Prod. 2010;42:1395–1403. doi: 10.1007/s11250-010-9597-z. [DOI] [PubMed] [Google Scholar]
- Pečjak M., Leskovec J., Levart A., Salobir J., Rezar V. Effects of dietary vitamin E, vitamin C, selenium and their combination on carcass characteristics, oxidative stability and breast meat quality of broiler chickens exposed to cyclic heat stress. Animals (Basel) 2022;12:1789. doi: 10.3390/ani12141789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M., Pertea G.M., Antonescu C.M., Chang T.C., Mendell J.T., Salzberg S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razmaitė V., Šiukščius A., Šveistienė R., Jatkauskienė V. Present conservation status and carcass and meat characteristics of Lithuanian Vistines goose breed. Animals (Basel) 2022;12:159. doi: 10.3390/ani12020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L.L., Guan X.J., Ingram R., Tilghman S.M. Gatm, a creatine synthesis enzyme, is imprinted in mouse placenta. Proc. Natl. Acad. Sci. U S A. 2003;100:4622–4627. doi: 10.1073/pnas.0230424100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.R., Wang Z.Y., Yang H.M., Zhang Y.Y. Nitrogen requirement for maintenance in Yangzhou goslings. Br. Poult. Sci. 2007;48:205–209. doi: 10.1080/00071660701227519. [DOI] [PubMed] [Google Scholar]
- Snow R.J. AGAT knockout mice provide an opportunity to titrate tissue creatine content. J. Physiol. 2013;591:393. doi: 10.1113/jphysiol.2012.247924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockebrand M., Nejad A.S., Neu A., Kharbanda K.K., Sauter K., Schillemeit S., Isbrandt D., Choe C.U. Transcriptomic and metabolic analyses reveal salvage pathways in creatine-deficient AGAT(-/-) mice. Amino Acids. 2016;48:2025–2039. doi: 10.1007/s00726-016-2202-7. [DOI] [PubMed] [Google Scholar]
- Stupar R.M., Gardiner J.M., Oldre A.G., Haun W.J., Chandler V.L., Springer N.M. Gene expression analyses in maize inbreds and hybrids with varying levels of heterosis. BMC Plant Biol. 2008;8:33. doi: 10.1186/1471-2229-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungkhapreecha P., Chankitisakul V., Duangjinda M., Boonkum W. Combining abilities, heterosis, growth performance, and carcass characteristics in a diallel cross from black-bone chickens and Thai native chickens. Animals (Basel) 2022;12:1602. doi: 10.3390/ani12131602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants (Basel) 2019;8 doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillandier D., Polge C. Skeletal muscle atrogenes: from rodent models to human pathologies. Biochimie. 2019;166:251–269. doi: 10.1016/j.biochi.2019.07.014. [DOI] [PubMed] [Google Scholar]
- Uhlířová L., Tůmová E., Chodová D., Vlčková J., Ketta M., Volek Z., Skřivanová V. The effect of age, genotype and sex on carcass traits, meat quality and sensory attributes of geese. Asian-Australas. J. Anim. Sci. 2018;31:421–428. doi: 10.5713/ajas.17.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlířová L., Tůmová E., Chodová D., Volek Z., Machander V. Fatty acid composition of goose meat depending on genotype and sex. Asian-Australas. J. Anim. Sci. 2019;32:137–143. doi: 10.5713/ajas.17.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe K., Goris M., Van Hecke P., Van Leemputte M., Vangerven L., Hespel P. Long-term creatine intake is beneficial to muscle performance during resistance training. J. Appl. Physiol. (1985) 1997;83:2055–2063. doi: 10.1152/jappl.1997.83.6.2055. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang L., Li J., Cong J., Gao F., Zhou G. Effects of dietary marigold extract supplementation on growth performance, pigmentation, antioxidant capacity and meat quality in broiler chickens. Asian-Australas. J. Anim. Sci. 2017;30:71–77. doi: 10.5713/ajas.16.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhao J., Wang L., Zhang T., Zeng W., Lu H. METTL21C mediates lysine trimethylation of IGF2BP1 to regulate chicken myoblast proliferation. Br. Poult. Sci. 2022;64:74–80. doi: 10.1080/00071668.2022.2121639. [DOI] [PubMed] [Google Scholar]
- Wen D., Liu Y., Yu Q. Metabolomic approach to measuring quality of chilled chicken meat during storage. Poult. Sci. 2020;99:2543–2554. doi: 10.1016/j.psj.2019.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederstein J.L., Nolte H., Günther S., Piller T., Baraldo M., Kostin S., Bloch W., Schindler N., Sandri M., Blaauw B., Braun T., Hölper S., Krüger M. Skeletal muscle-specific methyltransferase METTL21C trimethylates p97 and regulates autophagy-associated protein breakdown. Cell Rep. 2018;23:1342–1356. doi: 10.1016/j.celrep.2018.03.136. [DOI] [PubMed] [Google Scholar]
- Wolf J., Knizetova H. Crossbreeding effects for body weight and carcase traits in Pekin duck. Br. Poult. Sci. 1994;35:33–45. doi: 10.1080/00071669408417668. [DOI] [PubMed] [Google Scholar]
- Young J.F., Bertram H.C., Theil P.K., Petersen A.G.D., Poulsen K.A., Rasmussen M., Malmendal A., Nielsen N.C., Vestergaard M., Oksbjerg N. In vitro and in vivo studies of creatine monohydrate supplementation to Duroc and Landrace pigs. Meat Sci. 2007;76:342–351. doi: 10.1016/j.meatsci.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Yu G., Wang L., Han Y., He Q. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H., Xiong Y., Wang Z., Dong W., Zhou Q., Xie S., Li X., Zhao S., Ma Y. Integrative analysis of transcriptomic and metabolomic profiles reveal the complex molecular regulatory network of meat quality in Enshi black pigs. Meat Sci. 2022;183 doi: 10.1016/j.meatsci.2021.108642. [DOI] [PubMed] [Google Scholar]
- Zhang X., Liu C., Kong Y., Li F., Yue X. Effects of intramuscular fat on meat quality and its regulation mechanism in Tan sheep. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.908355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Chen J., Zhang X., Xu Z., Lin Z., Li H., Lin W., Xie Q. Genome-wide association analysis reveals key genes responsible for egg production of lion head goose. Front. Genet. 2019;10:1391. doi: 10.3389/fgene.2019.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M.M., Gao T., Zhang L., Li J.L., Lv P.A., Yu L.L., Gao F., Zhou G.H. In ovo feeding of creatine pyruvate alters energy reserves, satellite cell mitotic activity and myogenic gene expression of breast muscle in embryos and neonatal broilers. Poult. Sci. 2017;96:3314–3323. doi: 10.3382/ps/pex150. [DOI] [PubMed] [Google Scholar]