Abstract
Seedless grapes are increasingly popular throughout the world, and the development of seedless varieties is a major breeding goal. In this study, we demonstrate an essential role for the grapevine MADS-box gene VvMADS28 in morphogenesis of the ovule. We found that VvMADS28 mRNA accumulated in the ovules of a seeded cultivar, ‘Red Globe’, throughout the course of ovule and seed development, especially within the integument/seed coat. In contrast, in the seedless cultivar ‘Thompson Seedless’, VvMADS28 was expressed only weakly in ovules, and this was associated with increased levels of histone H3 lysine 27 trimethylation (H3K27me3) within the VvMADS28 promoter region. RNAi-mediated transient suppression of VvMADS28 expression in ‘Red Globe’ led to reduced seed size associated with inhibition of episperm and endosperm cell development. Heterologous overexpression of VvMADS28 in transgenic tomatoes interfered with sepal development and resulted in smaller fruit but did not obviously affect seed size. Assays in yeast cells showed that VvMADS28 is subject to regulation by the transcription factor VvERF98, and that VvMADS28 could interact with the Type I/ Mβ MADS-domain protein VvMADS5. Moreover, through DNA-affinity purification-sequencing (DAP-seq), we found that VvMADS28 protein specifically binds to the promoter of the grapevine WUSCHEL (VvWUS) gene, suggesting that maintenance of the VvMADS28–VvMADS5 dimer and VvWUS expression homeostasis influences seed development. Taken together, our results provide insight into regulatory mechanisms of ovule and seed development associated with VvMADS28.
Introduction
Table grapes are a nutritious fruit popular with consumers throughout the world. Recently, seedless table grapes have become a focus of marketing, and breeding for seedlessness has become an important goal. In angiosperms, a mature seed comprises the embryo, the endosperm, and the seed coat. The embryo and endosperm originate from the fertilized egg cell and central cell, respectively, while the seed coat develops from the sporophytic integuments [1]. Seedlessness can arise though arrest of embryo development resulting from a variety of conditions, including abnormalities in the micropyle, integument, endosperm or embryo sac, and poor pollination or fertilization [2]. In Arabidopsis thaliana (arabidopsis) and rice, several signaling pathways have been shown to control ovule development by influencing the growth of maternal tissues. These include or involve the ubiquitin–proteasome pathway, phytohormone perception, G-protein signaling, and transcriptional regulators [3]. For example, the HAIKU (IKU) pathway controls endosperm development in response to abscisic acid and brassinosteroid signaling, and cytokinin acts downstream of the IKU pathway to control seed growth [4, 5]. Interestingly, some studies have shown that the regulators of seed development are conserved among plant species. For example, three homologous genes from diverse plants have been shown to have similar function: arabidopsis (STK) [6], grapevine (VvAGL11) [7], and rice (OsMADS13) [8]. Therefore, knowledge of mechanisms of seed development in a variety of plants should facilitate understanding of ovule development in grapevine.
The MADS-box gene class has been greatly expanded in higher plants and encodes transcription factors recruited for various roles in development. The studies of MADS-box gene function in plants first focused on floral organ development [9]. MADS-domain proteins interact as homodimers or heterodimers, which expand functional diversity. Recently, several grapevine MADS-box genes involved in seed formation have been discovered. The VviAGL11 gene plays a key role, and an R197L mutation within the VviAGL11 open reading frame is associated with the seedless fruit trait [10]; in VviAGL11-R197L genotypes, the lignification of endopleura in seeds cannot be initiated, eventually leading to arrested seed development [7]. Functional analyses of additional MADS-box genes, VvAGAMOUS2 (VvAG2), VvSEPALLATA3 (VvSEP3), VvMADS39, and VvMADS45, have shown that these four genes also influence seed development [11–13].
VvMADS28 is a homolog of arabidopsis FRUITFULL (FUL). FUL is required for the development of the fruit valves after fertilization, and transgenic overexpression of FUL leads to indehiscent fruit [14]. In ful loss of function mutants, siliques are shaped abnormally and the dehiscence zone is not specified [15]. Tomato (Solanum lycopersicum) TDR4/FUL1 and MBP7/FUL2, which have high sequence similarity to arabidopsis FUL and appear to predominantly regulate cellular differentiation and fruit ripening [16], can heterodimerize with the MADS-domain ripening regulator RIN [17, 18]. In addition, a FUL homolog in bilberry (Vaccinium myrtillus) was found to regulate anthocyanin biosynthesis and color development during ripening of the berry [19]. These results indicate that the FUL regulatory networks involved in the development of both dry and fleshy fruits may be similar, although the outcomes are morphologically very different. The fruit is a specialized structure that provides a suitable environment for seed maturation and a mechanism for dispersal. In ful-1 arabidopsis, the lack of coordinated growth of the fruit tissues crowds the seeds and limits seed growth [20]. Fertilization-generated signals may be recognized in the integuments and lead to seed coat differentiation, including activation of MADS-box genes with seed coat-promotive roles. The expression of tomato TDR4 genes in ovules also supports this [21].
In this study, we focused on the function of the VvMADS28 gene in ovule development. Expression studies show a defined spatial and temporal expression pattern for VvMADS28, suggesting an important role in the establishment of seed identity. Combined with analysis of VvMADS28 transgenic lines and interactions between proteins, our results suggest a potential function and molecular mechanism of VvMADS28 in seed development in grapevine.
Results
Spatio-temporal expression of VvMADS28 in grapevine
To gain insight into the potential developmental function of VvMADS28 in grapevine, we first assessed the expression of VvMADS28 mRNA in various structures from mature ‘Red Globe’ and ‘Thompson Seedless’ plants. In these two cultivars, VvMADS28 was found to be expressed in all the analyzed structures, suggesting that it may have a general developmental function (Fig. 1a). For both cultivars, the strongest expression was seen in the inflorescence, flower, and fruit, whereas the weakest expression was seen in the root and leaf. We also assessed expression within the four major floral organs and found that VvMADS28 was expressed in all of them, with highest expression in the sepal. Finally, we examined expression in ovules at progressive stages of development, from 20 to 45 days after flowering (DAF). During this period, overall expression generally decreased in both cultivars. However, for ‘Red Globe’, strongest expression was seen at 30 DAF, and expression was stronger in ‘Red Globe’ than in ‘Thompson Seedless’ at 30 DAF and later (Fig. 1a). The observed stronger expression in the seeded variety during this period is consistent with a role for VvMADS28 in seed development.
Figure 1.
Expression analysis of VvMADS28 and subcellular localization. a Expression pattern of VvMADS28 in grapevine structures, floral organs, and ovule at progressive developmental stages in ‘Red Globe’ and ‘Thompson Seedless’. Values are means ± standard deviation of three biological replicates; *P < .05 (one-way ANOVA). bIn situ hybridization of VvMADS28 in ‘Red Globe’ and ‘Thompson Seedless’ during ovule developmental. c Subcellular localization of VvMADS28-GFP protein expressed in arabidopsis protoplasts. Scale bars = 75 μm.
Moreover, in situ hybridization was carried out to document the spatial expression pattern of VvMADS28 within the developing ovule. In both cultivars, VvMADS28 mRNA was detected in developing ovules at 15 DAF (Fig. 1b). For ‘Red Globe’, relatively strong expression was seen in the integument at both 30 and 35 DAF. In contrast, almost no expression was observed in ovules of ‘Thompson Seedless’ after 25 DAF. These expression data are similar to those derived by RT–PCR (Fig. 1a) and suggest that VvMADS28 has a role in formation of the integument.
Gene function is determined not only by the spatio-temporal expression pattern, but also by the subcellular localization of the gene product. To examine the subcellular localization of VvMADS28, we expressed a green fluorescent protein (GFP)-tagged VvMADS28 gene in arabidopsis protoplasts and found that the fluorescent protein product localized to the nucleus, as anticipated for a transcriptional regulator (Fig. 1c).
Repression of VvMADS28 in ‘Thompson Seedless’ ovules is associated with histone 3 Lys-27 trimethylation
In multicellular organisms, histone 3 Lys-27 trimethylation (H3K27me3) serves a conserved role in transcriptional repression of key developmental genes. It has been reported that some ovule development genes in arabidopsis, such as STK, INO, STM, LEC, and FUS3, are modified by H3K27me3 [22, 23]. In ‘Pinot Noir’, H3K27me3 contributes to seed formation by inhibiting the biosynthesis of salicylic acid, thus precluding programmed cell death [24]. Our observation that VvMADS28 was differentially expressed between the ovules of ‘Red Globe’ and ‘Thompson Seedless’ suggested that expression might be influenced by H3K27me3 within its promoter. Therefore, chromatin immunoprecipitation (ChIP)–qPCR assays were performed to assess H3K27me3 enrichment for three promoter regions: R1 (−2000/−1854), R2 (−866/−749), and R3 (−382/−249) (Fig. 2a). The results of Western blotting showed that the anti-H3K27me3 antibody and chromatin were of sufficiently high quality to be used for further study (Fig. 2b). RT–PCR revealed that both R1 and R3 showed a significant H3K27me3 enrichment in ‘Thompson Seedless’ compared with ‘Red Globe’ (Fig. 2c). This finding is consistent with the conserved role of H3K27me3 in gene repression, and with our observation that VvMADS28 was relatively silenced in ‘Thompson Seedless’.
Figure 2.
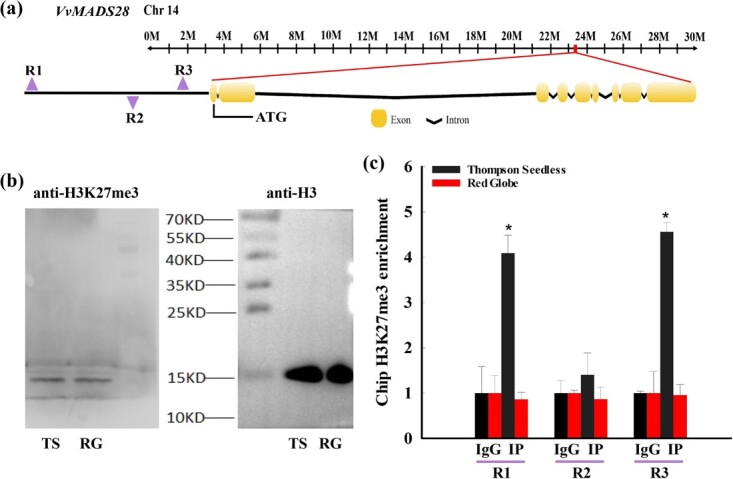
Analysis of H3K27me3 within the VvMADS28 promoter region in ovules from ‘Thompson Seedless’ and ‘Red Globe’. a Diagram of the regions used for ChIP–qPCR assays. b Immunoblot analysis of overall levels of H3K27me3 and H3 in ‘Thompson Seedless’ and ‘Red Globe’. c H3K27me3 enrichment of the three VvMADS28 promoter regions in ovules of ‘Thompson Seedless’ and ‘Red Globe’ as analyzed by ChIP–qPCR. ChIP values were normalized to their respective inputs. Error bars indicate ± standard deviation from three technical replicates. Statistical significance is denoted by an asterisk.
Phenotypic observation of transgenic tomato plants overexpressing VvMADS28
We then expressed VvMADS28 in transgenic tomato to evaluate the effects on fruit and seed development. VvMADS28-overexpression (OE-MADS28) plants did not show any obvious defects in growth, development, or physiology compared with the non-transgenic (NT) control plants during vegetative growth (Fig. 3a). However, floral sepals were greatly elongated, enclosing the floral buds at anthesis (Supplementary Data Fig. S1a–d) and elongating further during early fruit development (Supplementary Data Fig. S1e and f). Moreover, fruit of OE-MADS28 plants were smaller and contained fewer seeds (Fig. 3b and c). To quantify these phenotypes, average sepal length, fruit diameter, seed number, and seed length were determined from three independent OE-MADS28 lines (Table 1). Sepal length for the three OE-MADS28 lines was ~15.01 mm, while that for the NT was ~10.41 mm. The average fruit diameter for the OE-MADS28 lines was ~15.10 mm, whereas that for the NT was ~18.51 mm. Moreover, the average number of seeds per fruit for the OE-MADS28 lines ranged from 15 to 20, whereas that for NT was ~28. We did not observe any obvious difference in seed size between OE-MADS28 and NT plants.
Figure 3.

Functional analysis of transgenic tomato plants overexpressing VvMADS28.a Phenotype of non-transgenic (NT) control plants and two VvMADS28 overexpression lines. b Morphology of sepals and fruit from NT and VvMADS28 overexpression line OE-5. c Cut fruit showing seed number for NT (top) and overexpression line OE MADS28. Scale bars are 1 cm in each panel.
Table 1.
Statistical analysis of related traits in the VvMADS28 overexpression lines.
| Genotype | Sepal length | Fruit diameter | Average seeds | Seed length |
| (mm) | (mm) | per fruit | (mm) | |
| NT | 10.41 ± 0.12 | 18.51 ± 0.15 | 28 ± 1.35 | 5.12 ± 0.23 |
| OE28-5 | 15.01 ± 0.11 | 15.68 ± 0.32 | 20 ± 1.02 | 4.88 ± 0.09 |
| OE28-13 | 14.38 ± 0.06 | 14.55 ± 0.09 | 18 ± 2.36 | 5.04 ± 0.18 |
| OE28-15 | 15.65 ± 0.31 | 14.95 ± 0.27 | 15 ± 1.68 | 5.18 ± 0.13 |
Values are expressed as mean ± standard deviation. The number of samples used for each set of data was 5.
Characterization of seed development in VvMADS28-RNAi fruit
Furthermore, we used Agrobacterium-mediated transient transformation to introduce a VvMADS28-RNAi construction into developing fruit of ‘Red Globe’ (Fig. 4a). qPCR showed that VvMADS28 transcript accumulation was reduced in the ovules of the transgenic lines (VvMADS28-RNAi-2# and VvMADS28-RNAi-5#) compared with the NT, wild-type controls. To determine the specificity of the RNAi, we evaluated expression of VvMADS38 and VvMADS2, which are the two genes in the grapevine genome most closely related to VvMADS28, in VvMADS28-RNAi lines, and found that expression of neither gene was impacted (Fig. 4b). These results indicated that the observed phenotype was due to loss of VvMADS28 function. We found that, in VvMADS28-RNAi lines, the average seed mass was about half that of the wild-type (Fig. 4c), and the fruit and seeds from them were significantly reduced in size (Fig. 4d, e and 4i, j, respectively). As shown in Fig. 4f–h, the endosperm cells of the wild-type seeds were arranged in a compact and regular pattern, whereas those of VvMADS28-RNAi seeds were shriveled and irregularly arranged and failed to fill the embryo sac (Fig. 4k–n). The integument and endopleura cells of wild-type seeds were relatively thick and compact (Fig. 4h), but episperm cells of VvMADS28-RNAi lines were irregularly arranged and loosely packed (Fig. 4k–n). These results suggest that VvMADS28 has a role in promoting normal seed formation.
Figure 4.
Transformation of grapevine and phenotype of VvMADS28-RNAi fruit and seed. aAgrobacterium-mediated transient transformation. b Expression of VvMADS28 and closely related genes in wild-type and two VvMADS28-RNAi lines. Error bars indicate ± standard deviation (n = 3). Statistical significance is denoted by an asterisk. c Average seed mass of wild-type and VvMADS28-RNAi lines. d, e Fruit and seed from wild-type. f Whole seed from wild-type. g, h Enlarged partial images of (f). i, j Fruit and seed from VvMADS28-RNAi line. k, m Seed from VvMADS28-RNAi plant. l, n Partial enlarged images of (k) and (m), respectively. END, endosperm; INT, integument; ET, endotesta.
VvERF98, encoding an ethylene-response factor transcription factor, promotes VvMADS28 expression in the seed
To identify potential upstream regulators of VvMADS28, we carried out a yeast one-hybrid screen, using an ovule cDNA library in combination with an ~2 kb VvMADS28 promoter region. This resulted in the identification of multiple cDNA clones corresponding to VvERF98 (gene ID 100241203), encoding an ethylene-response factor (ERF). VvERF98 was expressed in the two cultivars during ovule development in a pattern similar to that of VvMADS28 (Supplementary Data Fig. S2a). As shown in Fig. 5a, when a plasmid expressing VvERF98 was transformed into the yeast one-hybrid (Y1H) strain, cells harboring the VvMADS28 promoters grew well with aureobasidin A, whereas cells co-transformed with the empty pGADT7 vector did not. These results suggested that VvERF98 binds to the promoter of VvMADS28. Moreover, a dual-luciferase (LUC) reporter assay in Nicotiana benthamiana leaves showed that the relative intensity of LUC was markedly increased with co-transformation of pVvMADS28-LUC with 62SK-VvERF98, compared with that of the control transformed with pVvMADS28-LUC alone (Fig. 5b). To gain additional support, a VvERF98-GUS overexpression vector was introduced into grapevine seeds (Fig. 5c). GUS staining indicated that Agrobacterium solution had penetrated into the grape seeds. Expression of VvMADS28 was markedly increased in the seeds of VvERF98-GUS plants (Fig. 5d). These results showed that VvMADS28 can be positively regulated by VvERF98.
Figure 5.
Analysis of upstream binding genes and activity of the VvMADS28 promoter. a Analysis of the interaction between VvERF98 and the VvMADS28 promoter using a Y1H system. b Relative LUC activity measured in N. benthamiana leaves using a dual-LUC reporter assay. Error bars indicate ± standard deviation (n = 6). Statistical significance is denoted by an asterisk. c Diagram of the VvERF98 overexpression vector and transient overexpression in grape seeds. d, eVvMADS28 and VvERF98 expression in the seeds of wild-type and VvERF98-overexpressing lines after treatment with water, sucrose, and ethylene.
The identification of VvERF98 as an ERF suggests that VvMADS28 expression may be responsive to ethylene signaling. To test this, we examined the transcriptional response of VvERF98 and VvMADS28 to ethephon. As can be seen in Fig. 5d and e, both VvERF98 and VvMADS28 were more highly upregulated in the seed after treatment with 10 mM ethephon compared with control plants treated with water or sucrose alone. Collectively, these results indicate that VvERF98 acts as an upstream transcriptional regulator of VvMADS28 in seed development and is responsive to ethylene signaling.
DNA affinity purification sequencing analysis
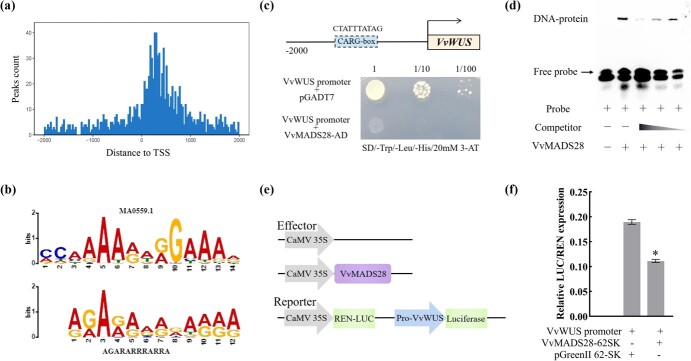
To identify potential targets of VvMADS28 in the grapevine genome, we performed a DNA affinity purification sequencing (DAP-seq) assay, using a tagged version of VvMADS28 as bait. The results from two biological replicates showed that the average unique mapped ratio of reads produced by VvMADS28-bound DNA in the grapevine reference genome was 41.51%, and the average mapped ratio of reads was 90.40% (Supplementary Data Table S2). Moreover, as shown in Supplementary Data Fig. S3a and b, VvMADS28 binding peaks were distributed across all chromosomes. We identified 760 intergenic peaks (located in the region beyond 2 kb upstream of the gene initiation transcription site) as well as 726 promoter peaks (located within 2 kb upstream of the gene transcription start site). A total of 238 peaks were identified in both replicates and were designated as high-confidence VvMADS28 binding sites. These were mainly located near transcription start sites (Fig. 6a), and motif enrichment analysis identified the CArG sequence CC(A/T)6/8GG as significantly enriched (Fig. 6b). KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis of potential VvMADS28 target genes revealed over-representation for biological metabolism, the biosynthesis pathway, and the MAPK signaling pathway (Supplementary Data Fig. S3c).
Figure 6.
DAP-seq analysis and potential targets of VvMADS28 in grapevine. a Distribution of VvMADS28 binding sites in 2-kb flanking sequences around genic peak summits. b Significantly enriched motif sequence of VvMADS28 binding sites specifically detected by DAP-seq. c Analysis of the interaction between VvMADS28 and the VvWUS promoter using a Y1H system. d EMSA of interaction between recombinant VvMADS28 protein and the CArG region of the VvWUS promoter. e Dual-reporter and effector plasmids utilized in the dual-LUC reporter test. f Relative LUC/REN expression in N. benthamiana leaves. Co-expression of VvMADS28 decreased the expression of the LUC reporter gene driven by the VvWUS promoter. Data are from three biological replicates, with error bars indicating the standard deviation. Statistical significance is denoted by an asterisk.
Potential targets of VvMADS28 in grapevine
Several of the high-confidence VvMADS28 binding sites were proximal to genes homologous to ovule development genes identified in arabidopsis, including WUSCHEL-related homeobox 1 (WUS, VIT_217s0000g02460), HAIKU (IKU, VIT_202s0012g01280), DA (VIT_213s0019g02430), and INNER NO OUTER (INO, VIT_201s0127g00330). The promoter sequences of all these four genes contained at least one CArG [CC(A/T)6/8GG] site. To assess potential interaction between VvMADS28 protein and DNA sequences within these four promoters, we carried out Y1H experiments. We found that VvMADS28 interacted only with the promoter of the grapevine WUS homologue VvWUS (Fig. 6c). To further confirm that VvMADS28 can specifically bind to CArG (CTATTTATAG) site(s), we carried out an electrophoretic mobility shift assay (EMSA). As shown in Fig. 6d, recombinant His-tagged VvMADS28 showed specific interaction with the biotin-labeled fragment of the VvWUS promoter. Furthermore, dual-LUC assays in tobacco leaves showed that the potential interaction of VvMADS28 with the promoter of VvWUS led to a nearly 1.5-fold decrease in the LUC/REN ratio (Fig. 6e and f). These results suggest that VvMADS28 inhibited the expression of grapevine VvWUS by binding to the CArG (CTATTTATAG) site in the VvWUS promoter.
VvMADS28 physically interacts with VvMADS5
Given the developmental complexity of the ovule, we anticipated that VvMADS28 cooperates with numerous additional transcription factors. We used a yeast two-hybrid (Y2H) approach to discover proteins that physically interact with VvMADS28. Because expression of full-length VvMADS28 containing the MIKC region was independently able to activate the reporter gene, we engineered a non-activating VvMADS28 sequence by successive deletions of its carboxyl-terminal sequence. The longest VvMADS28 sequence lacking activation potential was used as the bait. Screening of a grapevine ovule cDNA library resulted in the identification of VvMADS5 (VIT_00010218001). Further Y2H experiments in yeast confirmed interaction of VvMADS28 and VvMADS5 (Fig. 7b). To gain further support, we used a co-immunoprecipitation (Co-IP) assay, with a VvMADS28-HA fusion protein in combination with a VvMADS5-Flag recombinant protein, transiently co-expressed in N. benthamiana. The result showed that VvMADS28 and VvMADS5 were efficiently co-immunoprecipitated (Fig. 7c). Furthermore, a split-LUC complementation assay showed that co-expression of VvMADS28-NLUC and VvMADS5-CLUC in N. benthamiana leaves generated a clear signal of interaction (Fig. 7d).
Figure 7.
Interaction between VvMADS28 and VvMADS5. a Activation potential of VvMADS28 and respective deletions in yeast. b–d Y2H analysis (b), co-immunoprecipitation (c), and split-LUC (d) assays were performed to evaluate the interaction between VvMADS28 and VvMADS5.
Functional characterization of VvERF98, VvMADS5, and VvWUS in arabidopsis
To obtain additional evidence to support the biological relevance of the interactions among VvERF98, VvMADS5, and VvWUS for seed development, we conducted preliminary functional analyses of these three genes in arabidopsis. For each gene, stable transgenic lines were engineered constitutively expressing the coding sequence under control of the 35S CaMV promoter. In comparison with non-transgenic plants, OE-VvMADS5 plants had larger leaves and siliques, and seeds showed increased size and weight (Fig. 8 and Supplementary Data Fig. S4). These results indicate that VvMADS5 plays an important role in ovule and organ development. Similarly, seeds of OE-VvERF98 plants were larger than those of control plants (Fig. 8). This suggests that VvERF98 is involved in ovule development. In contrast, OE-VvWUS plants produced twisted leaves and tufted branches, and the siliques and seeds were smaller than those of the controls (Fig. 8 and Supplementary Data Fig. S4). In addition, it is worth mentioning that gene expression patterns provide clues to this functional differentiation; VvWUS mRNA was relative highly accumulated in the ovule of ‘Thompson Seedless’; its expression pattern was the opposite of VvMADS5 and VvERF98 (Supplementary Data Fig. S2a–c). These results also suggest that the function of VvWUS may be different from that of VvMADS5 and VvERF98.
Figure 8.
Phenotypic analysis of non-transgenic wild-type (WT) arabidopsis and transgenic arabidopsis constitutively expressing VvERF98, VvMADS5, and VvWUS. a Inflorescence stalks. b Siliques. c Opened siliques. d Seeds. Scale bars are 1 cm in (a), 2 mm in (b), and 1 mm in (c) and (d). e–g Quantification of silique length (e), projective area (f), and seed weight (g). Error bars in (e–g) indicate means ± standard deviation relative to the respective WT values, set as 100%. *P < .05 (one-way ANOVA).
Discussion
Duplication of MADS-box genes is a common phenomenon during plant evolution, and not only gives rise to functionally redundant genes, but also permits changes in expression pattern or protein function, leading to diversification of developmental processes [25, 26]. Phylogenetic analysis shows that VvMADS28 belongs to the AP1/FUL subfamily (Supplementary Data Fig. S5), and therefore is a class A gene according to the ABC model. The general function of AP1 in arabidopsis is to help direct formation of sepals and petals. Interestingly, we found that VvMADS28 was highly expressed in flowers, sepals, petals, and fruit, analogous to the expression pattern of its arabidopsis counterparts. In addition, the tomato genome contains a VvMADS28 homolog, designated FUL1 (also known as TDR4), which is primarily involved in fruit ripening and shares a similar expression pattern to VvMADS28, indicating that homologous genes from different species may perform a conserved function.
We found that VvMADS28 was relatively highly expressed in floral organs as well as in the ovule during later development stages of ‘Red Globe’, indicating that VvMADS28 not only is involved in flower morphogenesis but also participates in development of the integument. ChIP–qPCR assays indicated that the enrichment of the H3K27me3 repressive marker in the promoter region of VvMADS28 was much higher in ‘Thompson Seedless’ than in ‘Red Globe’, potentially explaining the differential expression of VvMADS28 in the ovules of these two cultivars. On the other hand, the bigger seeds produced by overexpression of VvERF98 (Fig. 8) as well as the differential expression pattern of VvERF98 in ‘Thompson Seedless’ and ‘Red Globe’ (Supplementary Data Fig. S2a) indicate that VvERF98 plays a guiding role in the expression and phenotypic regulation of VvMADS28. However, this inference warrants further verification in stable transgenic grapevine lines.
When ectopically expressed in tomato to high levels, VvMADS28 promoted sepal growth, but repressed fruit growth. Likewise, overexpression of the homologous SlFUL in tomato led to smaller fruit, due to reduced division and expansion of pericarp cells, suggesting that VvMADS28 may affect fruit development [27]. However, no obvious effect of SlFUL overexpression on sepal morphology was noted. In our study, seed size was not greatly affected by ectopic expression of VvMADS28, but seed numbers were reduced. This could result from limitation of maternal resources. On the other hand, our results showed that inhibition of episperm and endosperm cell development correlated with reduced nuclear proliferation and reduced seed size in VvMADS28-RNAi transgenic lines. This is consistent with the reduction in seed mass observed in arabidopsis ful mutants [20]. Interestingly, the expression of two other genes from AP1/FUL group, VvMADS38 and VvMADS2, was not impacted in VvMADS28-RNAi lines. Moreover, the expression patterns of VvMADS38 and VvMADS2 during ovule development were obviously different from VvMADS28 (Supplementary Data Fig. S2d and e), suggesting that the different AP1/FUL genes have evolved by acquiring temporal-specific expression patterns and may perform different functions.
The MADS-box superfamily can be divided into Type I and Type II sequences [28]. Type II genes, including AP1, FUL, and VvMADS28, have been investigated extensively; however, there is more limited information about potential function(s) of Type I genes [29]. In grapevine, previous studies indicated that Type I genes are widely expressed in roots, stems, leaves, flowers, tendrils, fruits, and ovules [30], suggesting general and important functions. In general, Type II proteins interact with other Type II proteins, while interactions between Type I and II proteins are rarely reported [31]. We found that VvMADS28 can interact with a Type I protein of the Mβ clade, VvMADS5. VvMADS5 is closely related to the arabidopsis Type I MADS domain protein AGL62. AGL62 was shown to be a key regulator of endosperm cellularization, which correlates with the extent of nuclear proliferation and may influence seed size [32]. In this study, heterologous overexpression of VvMADS5 in arabidopsis produced larger seeds and organs (Fig. 8 and Supplementary Data Fig. S4b and c). Therefore, we can reasonably anticipate it has a role in ovule development analogous to that of AGL62. Correspondingly, the expression of VvMADS5 was also significantly reduced in VvMADS28-RNAi lines, associated with abnormal ovule development (Supplementary Data Fig. S2g). This was associated with reduced nuclear proliferation, similar to the phenotype conferred by loss of function of AGL62 in arabidopsis. Decreased expression of VvMADS5 led to precocious cellularization, and in VvMADS28-RNAi transgenic lines development of the embryo and embryo sac was inhibited, resulting in the collapse of the endosperm and fewer and smaller seeds. These results suggest that VvMADS5 and VvMADS28 are important for maintaining normal ovule development.
DAP-seq analysis can provide a simple access to understanding transcriptional regulators. MADS-box proteins have been found to generate a relatively small DAP-seq dataset, compared with bZIP or NAC proteins [33]. Our DAP-seq analysis of VvMADS28 generated specific peaks and binding-site motifs (CArG) consistent with previous reports [34], indicating that our results are reliable. Meanwhile, four candidate genes related to ovule development were selected from the DAP-seq results. HAIKU (IKU) is a key gene regulating endosperm development in arabidopsis. It has been reported that iku1/iku2/miniseed3 triple mutants show precocious endosperm cellularization that leads to premature developmental arrest. The HAIKU pathway regulates seed size in collaboration with auxin and cytokinin [3]; DA1 is a receptor gene that affects integument/seed coat development by controlling cell proliferation. The arabidopsis da1-1 line produced larger organs, indicating that DA1 inhibited the development of seeds [35]; INO encodes a YABBY transcription factor; ino mutants show developmental defects in the integument, and this phenotype can be rescued by transgenic expression of VvINO [36]; WUS encodes a homeodomain protein, and was originally identified as a central regulatory gene in shoot and flower meristem development [37]. Moreover, WUS activity is required to initiate the transformation from chalaza to integument and is involved in the regulation of ovule development [38]. We performed Y1H assays to evaluate binding of VvMADS28 with promoter regions from each of these four genes, and only the interaction with VvWUS was identified (Supplementary Data Fig. S6). It has been noted that some DNA-binding proteins require other co-factors or interacting proteins for binding to their cis-element targets [39, 40]. That we did not observe Y1H interaction of VvMADS28 with the remaining three genes identified by DAP-seq may be due to the requirement for specific transcriptional co-regulators not found in yeast cells, or plant-specific post-translational modification of VvMADS28.
Furthermore, we analyzed the expression pattern of VvWUS during seed development. VvWUS mRNA was relatively highly expressed in the ovules of ‘Thompson Seedless’, and in an opposite pattern to that of VvMADS28 (Supplementary Data Fig. S2c). Interestingly, expression of VvWUS was increased in VvMADS28-RNAi seeds. In the seeds of plants overexpressing VvERF98, VvMADS28 was obviously upregulated; however, VvWUS mRNA remained constant (Supplementary Data Fig. S2f). We suspect that VvWUS activity is dependent on VvMADS28 expression, and upregulation of VvWUS was only observed when the VvMADS28 gene was inactive. A previous report suggests that MADS activities balance ovule identity activity by regulating WUS [41]. Therefore, the presence of WUS signaling in ovules might reflect a short-range conserved signaling module, which is employed in ovules to coordinate the development of neighboring cell groups [38]. In addition, we found that overexpression of VvWUS in arabidopsis resulted in aberrant development, including distorted leaves and smaller seeds (Supplementary Data Fig. S4c and Fig. 8). Similarly, it has been reported that overexpression of WUS in tobacco causes severe leaf curl and reduced seed germination. These results suggest that strict regulation of WUS expression may play an important role in maintaining normal development. In view of the gene expression pattern and the negative regulatory relationship between VvMADS28 and VvWUS, we hypothesize that VvMADS28 expression in the ovule of ‘Red Globe’ limits VvWUS expression to maintain normal ovule development. The low expression of VvMADS28 in ‘Thompson Seedless’ associated with histone methylation may result in derepression of VvWUS and inhibition of seed development. Although this hypothesis is grounded in our observation of the molecular regulatory relationship between VvMADS28 and VvWUS, it still needs further testing. MADS-box and WUS transcriptional regulators are anticipated to have many additional roles in the developing ovule, and regulation of these processes is an area of active investigation.
In conclusion, we put forward the model shown in Fig. 9. The differential enrichment of histone methylation is associated with the differential expression of VvMADS28 in the ovules of these two cultivars. H3K27me3 within the VvMADS28 promoter in ‘Thompson Seedless’ inhibits the expression of VvMADS28. In addition, the upstream transcription factor VvERF98 promotes the expression of VvMADS28. Meanwhile, VvMADS28 interacts with the Type I MADS protein VvMADS5, and VvMADS28 protein specifically binds to the VvWUS promoter. The maintenance of VvMADS28-VvMADS5 dimer and VvWUS expression homeostasis may affect seed development.
Figure 9.

A model for the regulatory network of seed development involving VvMADS28. VvMADS28 is positively regulated by VvERF98. The relatively low expression level of VvMADS28 in ‘Thompson Seedless’ is associated with increased H3K27me3 repressive marks within its promoter region. VvMADS28 protein specifically binds to the VvWUS promoter and VvMADS28 interacts with VvMADS5 to repress VvWUS. The maintenance of VvMADS28 and VvWUS expression homeostasis may affect seed development.
Materials and methods
Plant material
Plant structures were dissected from one seeded (‘Red Globe’) and one seedless (‘Thompson Seedless’) cultivar maintained in the Grape Germplasm Resources of Northwest A&F University, Yangling, China (34°20′N, 108°24′E). ‘Micro-Tom’, a control tomato, and transgenic tomato were grown in a containment greenhouse at temperatures between 23 and 25°C [42].
Transient suppression of VvMADS28 in grape berries and heterologous overexpression of VvMADS28, VvMADS5, VvERF98, and VvWUS in model plant
The plasmid pHellsgate2 was utilized to construct the RNA silencing vector for VvMADS28 based on gateway recombination technology (Invitrogen). When grapevine plants were flowering, infiltration was performed by submerging an inflorescence into the Agrobacterium suspension and applying a vacuum for 10 minutes. The same plants were subjected to transformation once a week for 4 weeks.
The overexpression vector pCAMBIA2300-flag-VvMADS28 was created and introduced into Agrobacterium tumefaciens strain GV3101 as elaborated previously [13]. Tomato (‘Micro-Tom’) plants were subjected to transformation by the cotyledon disk method [11, 43].
For heterologous expression in transgenic arabidopsis, VvMADS5/VvERF98/VvWUS coding sequences were engineered into the pCAMBIA2300-GFP vector. Transformation of arabidopsis used the floral dip method [44]. Seeds were collected after the transformed plants matured and screened on nutrient agar medium containing 50 mg/l kanamycin. T3 plants were used for characterization.
Morphological observation and measurements of arabidopsis seeds
The images of siliques and seeds were obtained using a stereo microscope. Seed area was determined with ImageJ software according to the method described earlier [45]. Seed weight was measured with 600 seeds using a stereo microscope (Leica MZ10F).
Real-time quantitative polymerase chain reaction
Plant RNA extraction was performed as described previously [13]. First-strand cDNA was synthesized using the Evo M-MLV RT Reagent Kit (Accurate Biotechnology Co., Ltd, Hunan, China). Quantitative RT–PCR was conducted on a StepOne™ instrument in 20-μl reaction volumes. Grapevine EF1-α (GenBank accession number EC931777) and ACTIN (EC969944) served as internal standards. The 2-ΔΔCT method was used to determine expression levels using data from three biological replications [46]. Sequences of oligonucleotide primers can be found in Supplementary Data Table S1.
In situ hybridization
Ovules were preserved and embedded in paraffin at various stages of development. Sections (6–8 μm) were cut using a microtome (Leica, Wetzlar, Germany). Digoxigenin (DIG)-labeled probes were synthesized by in vitro transcription from VvMADS28 cDNA using sequence-specific primers and T7 or SP6 RNA polymerase, with a DIG RNA Labeling Kit (Roche, Basel, Switzerland). The sections were subjected to hybridization as described elsewhere [24, 47]. Hybridization signals were detected by colorimetry with an anti-DIG-AP antibody. Sections were photographed under a light microscope (Nikon Eclipse Ci).
Subcellular localization of VvMADS28
VvMADS28 cDNA was inserted into the pHBT-GFP-NOS green fluorescence expression vector to create VvMADS28-pHBT-GFP-NOS. The recombinant expression plasmid was introduced into arabidopsis protoplasts using a previously described method [48, 49]. Green fluorescence was scanned using a confocal laser scanning microscope (Leica TCS-SP8 SR). The nucleus was marked with m-Cherry red fluorescent protein.
Yeast two-hybrid assay
The VvMADS28 cDNA was inserted into the pGBKT7 plasmid to yield VvMADS28-BD, which was introduced into the yeast strain Y2H Gold. A cDNA library was prepared from ‘Thompson Seedless’ ovules at various developmental stages as an outservice (TaKaRa Bio). The cDNA library was mated with Y2H-VvMADS28-BD as described in the Matchmaker user manual, and colonies were selected on synthetic dropout medium. To directly evaluate protein–protein interactions, VvMADS5-AD and VvMADS28-BD were then co-transformed into Y2H Gold and colonies were screened on dropout medium. A more detailed description can be found in the Matchmaker™ Gold Yeast Two-Hybrid System (TaKaRa Bio) user manual.
Yeast one-hybrid assay
The pAbAi system was used to verify the interaction between VvERF98 and the promoter of VvMADS28, using the protocols specified in the Matchmaker™ Gold Yeast One-Hybrid Library Screening System (Clontech) user manual. The pHIS2 system was used to verify the interaction between VvMADS28 and the promoter of VvWUS. Briefly, a promoter segment of VvWUS was inserted into the pHIS2 reporter vector. VvMADS28 was inserted into the pGADT7 vector. The recombinant plasmids were then co-transformed into Y187. The non-modified pGADT7 plasmid was used as a negative control. Other steps were carried out as specified by the manufacturer (TaKaRa Bio).
Split luciferase assay
The VvMADS5 coding sequence was cloned into the pCB1300-CLUC vector to create pCB1300-CLUC-VvMADS5. The full-length VvMADS28 coding sequence without the stop codon was recombined into pCB1300-NLUC to generate pCB1300-NLUC-VvMADS28. The resulting plasmids were introduced into A. tumefaciens strain GV3101, and the mixed bacterial solution was transiently transformed into N. benthamiana leaves. At 48 hours after infiltration, LUC activity was detected using a charge-coupled device (CCD) camera [49].
Dual-luciferase assay
Approximately 2-kb promoter segments of VvMADS28 and VvWUS were recombined into the transient gene expression vector pGreenII 0800-LUC. VvERF98 and VvMADS28 were inserted into pGreenII-62SK under control of the CaMV 35S promoter. The plasmids were transformed into Agrobacterium GV3101 pSoup cells and infiltrated into N. benthamiana leaves. After 48 hours, REN and LUC activities were quantified using the Dual-Luciferase Reporter Assay System Kit (Beyotime, China). Six measurements were conducted per assay. DNA–protein interaction was quantified by calculating the LUC/REN ratio.
Co-immunoprecipitation assays
VvMADS28-HA and VvMADS5-Flag were created by cloning the VvMADS28 and VvMADS5 coding sequences without stop codons into the pEarleyGate202-Flag and pEarleyGate201-HA vectors, respectively. Total proteins were then obtained from the infiltrated N. benthamiana leaves using a previously reported method [50]. Extracts were incubated with 4 μl of Flag antibody for 2 hours at 4°C. After addition of G-Sepharose, the samples were incubated for an additional 12 hours. Beads were then washed three times for 10 minutes each time. Protein samples were resolved by SDS–PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (0.45 μm). After transfer, membranes were inoculated with specific antibodies (anti-HA and anti-Flag), and membranes were subjected to luminescence imaging (Alliance Q9 Advanced).
Chromatin immunoprecipitation
ChIP experiments were conducted following an improvised version of a previously reported protocol [51, 52]. Immunoprecipitation of chromatin from ‘Red Globe’ or ‘Thompson Seedless’ ovules utilized an anti-H3K27me3 mouse monoclonal antibody (1:1000, Abcam ab6002), anti-H3 antibody (positive control), and anti-IgG. Three aliquots for incubation without antibodies were used as input. After purification and recovery, immunoprecipitated DNAs were used as templates. Gene-specific primers are listed in Supplementary Data Table S1. Three technical measurements were conducted per sample. CT values obtained from DNA immunoprecipitated using IgG only as template were used to determine the level and significance of H3K27me3 enrichment.
Electrophoretic mobility shift assay
For production of recombinant, His-tagged VvMADS28 protein, the VvMADS28 cDNA was engineered into the pET-32a vector, introduced into Escherichia coli BL21 cells, and induced at 16°C for 12 hours. Protein was recovered and purified using a His-Tagged protein purification kit. Double-stranded, 5′-end biotin-labeled oligonucleotides containing 3× tandem repeats of the putative binding sites were created by a commercial service. Identical, non-labeled oligonucleotides were employed as competitor probes. The LightShift Chemiluminescent EMSA Kit (ThermoFisher) was used for the EMSA according to the manufacturer’s instructions.
DNA affinity purification sequencing analysis
DAP-seq was performed as an outservice by Bluescape Scientific, Hebei, China. Genomic DNA (gDNA) was extracted from leaves, and a DAP library was constructed after fragmentation of the gDNA. Recombinant VvMADS28 protein was obtained by engineering the VvMADS28 cDNA into the pGEX 4 T expression vector, followed by expression and purification following the manufacturer’s specifications (Pierce™ Glutathione Magnetic Agarose Beads, Thermo Fisher). VvMADS28 protein and the gDNA library were incubated in vitro and DNA bound to VvMADS28 was isolated as described elsewhere [53, 54]. DNA obtained after affinity purification and elution was subjected to paired-end sequencing on an Illumina HiSeq platform. Quality-filtered reads were aligned to a Grapevine genome sequence (https://urgi.versailles.inra.fr/Species/Vitis/Annotations) by Bowtie2 [55]. Conserved motifs within peak regions were identified using MEME [56].
Supplementary Material
Contributor Information
Songlin Zhang, State Key Laboratory of Crop Stress Biology in Arid Areas, College of Horticulture, Northwest A&F University, Yangling, Shaanxi 712100, China; Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest China, Ministry of Agriculture, Northwest A&F University, Yangling, Shaanxi 712100, China.
Li Wang, State Key Laboratory of Crop Stress Biology in Arid Areas, College of Horticulture, Northwest A&F University, Yangling, Shaanxi 712100, China; College of Horticulture, Hebei Agricultural University, Baoding 071000, China.
Jin Yao, State Key Laboratory of Crop Stress Biology in Arid Areas, College of Horticulture, Northwest A&F University, Yangling, Shaanxi 712100, China; Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest China, Ministry of Agriculture, Northwest A&F University, Yangling, Shaanxi 712100, China.
Na Wu, State Key Laboratory of Crop Stress Biology in Arid Areas, College of Horticulture, Northwest A&F University, Yangling, Shaanxi 712100, China; Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest China, Ministry of Agriculture, Northwest A&F University, Yangling, Shaanxi 712100, China.
Bilal Ahmad, State Key Laboratory of Crop Stress Biology in Arid Areas, College of Horticulture, Northwest A&F University, Yangling, Shaanxi 712100, China; Agriculture Genomics Institute, Chinese Academy of Agricultural Sciences, Shenzhen 518000, China.
Steve van Nocker, Department of Horticulture, Michigan State University, East Lansing, MI 48823, USA.
Jiuyun Wu, Turpan Research Institute of Agricultural Sciences, Xinjiang Academy of Agricultural Sciences, Turpan 838000, Xinjiang, China.
Riziwangguli Abudureheman, Turpan Research Institute of Agricultural Sciences, Xinjiang Academy of Agricultural Sciences, Turpan 838000, Xinjiang, China.
Zhi Li, State Key Laboratory of Crop Stress Biology in Arid Areas, College of Horticulture, Northwest A&F University, Yangling, Shaanxi 712100, China; Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest China, Ministry of Agriculture, Northwest A&F University, Yangling, Shaanxi 712100, China.
Xiping Wang, State Key Laboratory of Crop Stress Biology in Arid Areas, College of Horticulture, Northwest A&F University, Yangling, Shaanxi 712100, China; Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest China, Ministry of Agriculture, Northwest A&F University, Yangling, Shaanxi 712100, China; Turpan Research Institute of Agricultural Sciences, Xinjiang Academy of Agricultural Sciences, Turpan 838000, Xinjiang, China.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1603234), the Program for Innovative Research Team of Grape Germplasm Resources and Breeding (2013KCT-25), the Xinjiang Uygur Autonomous Region Tianchi Talent – Special Expert Project, and the Natural Science Youth Foundation of Hebei, China (C2021204146).
Author contributions
S.Z. and X.W. designed the study. S.Z., L.W., and J.Y. carried out the majority of the experiments. N.W., B.A., J.W., and R.A. assisted with the analysis of the results. Z.L., X.W. and L.W. provided guidance on the study. S.Z. and S.V.N. wrote the manuscript. All the authors approved the ultimate manuscript.
Date availability
All relevant data are included in the article and its supporting materials.
Conflict of interest
There are no competing interests in this paper, and the authors do not have any possible conflict of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
References
- 1. Colombo L, Battaglia R, Kater MM. Arabidopsis ovule development and its evolutionary conservation. Trends Plant Sci. 2008;13:444–50. [DOI] [PubMed] [Google Scholar]
- 2. Garcia D, Fitz Gerald JN, Berger F. Maternal control of integument cell elongation and zygotic control of endosperm growth are coordinated to determine seed size in Arabidopsis. Plant Cell. 2005;17:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li N, Xu R, Li Y. Molecular networks of seed size control in plants. Annu Rev Plant Biol. 2019;70:435–63. [DOI] [PubMed] [Google Scholar]
- 4. Garcia D, Saingery V, Chambrier Pet al. Arabidopsis haiku mutants reveal new controls of seed size by endosperm. Plant Physiol. 2003;131:1661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Nie X, Tan JLHet al. Integration of epigenetic and genetic controls of seed size by cytokinin in Arabidopsis. Proc Natl Acad Sci USA. 2013;110:15479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinyopich A, Ditta GS, Savidge Bet al. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003;424:85–8. [DOI] [PubMed] [Google Scholar]
- 7. Malabarba J, Buffon V, Mariath JEAet al. The MADS-box gene Agamous-like 11 is essential for seed morphogenesis in grapevine. J Exp Bot. 2017;68:1493–506. [DOI] [PubMed] [Google Scholar]
- 8. Dreni L, Jacchia S, Fornara Fet al. The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J. 2007;52:690–9. [DOI] [PubMed] [Google Scholar]
- 9. Sommer H, Beltrán JP, Huijser Pet al. Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J. 1990;9:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Royo C, Torres-Pérez R, Mauri Net al. The major origin of seedless grapes is associated with a missense mutation in the MADS-box gene VviAGL11. Plant Physiol. 2018;177:1234–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun XM, Zhang S, Li Xet al. A MADS-box transcription factor from grapevine, VvMADS45, influences seed development. Plant Cell Tissue Organ Cult. 2020;141:105–18. [Google Scholar]
- 12. Wang Y, Liu Z, Wu Jet al. MADS-box protein complex VvAG2, VvSEP3 and VvAGL11 regulates the formation of ovules in Vitis vinifera L. cv. 'Xiangfei'. Genes. 2021;12:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang S, Yao J, Wang Let al. Role of grapevine SEPALLATA-related MADS-box gene VvMADS39 in flower and ovule development. Plant J. 2022;111:1565–79. [DOI] [PubMed] [Google Scholar]
- 14. Ferrandiz C, Liljegren SJ, Yanofsky MF. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science. 2000;289:436–8. [DOI] [PubMed] [Google Scholar]
- 15. Ferrandiz C, Pelaz S, Yanofsky MF. Control of carpel and fruit development in arabidopsis. Annu Rev Biochem. 1999;68:321–54. [DOI] [PubMed] [Google Scholar]
- 16. Hileman LC, Sundstrom JF, Litt Aet al. Molecular and phylogenetic analyses of the MADS-box gene family in tomato. Mol Biol Evol. 2006;23:2245–58. [DOI] [PubMed] [Google Scholar]
- 17. Vrebalov J, Ruezinsky D, Padmanabhan Vet al. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (Rin) locus. Science. 2002;296:343–6. [DOI] [PubMed] [Google Scholar]
- 18. Bemer M, Karlova R, Ballester ARet al. The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell. 2012;24:4437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaakola L, Poole M, Jones MOet al. A SQUAMOSA MADS box gene involved in the regulation of anthocyanin accumulation in bilberry fruits. Plant Physiol. 2010;153:1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gu Q, Ferrándiz C, Yanofsky MFet al. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development. 1998;125:1509–17. [DOI] [PubMed] [Google Scholar]
- 21. Busi MV, Bustamante C, D'Angelo Cet al. MADS-box genes expressed during tomato seed and fruit development. Plant Mol Biol. 2003;52:801–15. [DOI] [PubMed] [Google Scholar]
- 22. Simonini S, Roig-Villanova I, Gregis Vet al. BASIC PENTACYSTEINE proteins mediate MADS domain complex binding to the DNA for tissue-specific expression of target genes in Arabidopsis. Plant Cell. 2012;24:4163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu J, Mohamed D, Dowhanik Set al. Spatiotemporal restriction of FUSCA3 expression by class I BPCs promotes ovule development and coordinates embryo and endosperm growth. Plant Cell. 2020;32:1886–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li ZQ, Jiao Y, Zhang Cet al. VvHDZ28 positively regulate salicylic acid biosynthesis during seed abortion in Thompson seedless. Plant Biotechnol J. 2021;19:1824–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferrario S, Immink RG, Angenent GC. Conservation and diversity in flower land. Curr Opin Plant Biol. 2004;7:84–91. [DOI] [PubMed] [Google Scholar]
- 26. Smyth D. A reverse trend – MADS functions revealed. Trends Plant Sci. 2000;5:315–7. [DOI] [PubMed] [Google Scholar]
- 27. Wang SF, Lu G, Hou Zet al. Members of the tomato FRUITFULL MADS-box family regulate style abscission and fruit ripening. J Exp Bot. 2014;65:3005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Folter S, Angenent GC. Trans meets cis in MADS science. Trends Plant Sci. 2006;11:224–31. [DOI] [PubMed] [Google Scholar]
- 29. Parenicova, de Folter, Kieffer L, S, Met al. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell. 2003;15:1538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Hu X, Jiao Cet al. Transcriptome analyses of seed development in grape hybrids reveals a possible mechanism influencing seed size. BMC Genomics. 2016;17:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Folter S, Immink RGH, Kieffer Met al. Comprehensive interaction map of the Arabidopsis MADS box transcription factors. Plant Cell. 2005;17:1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang I-H, Steffen JG, Portereiko MFet al. The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. Plant Cell. 2008;20:635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Malley RC, et al. Cistrome and epicistrome features shape the regulatory DNA landscape (Erratum for Cell 2016;165:1280). Cell. 2016;166:1598–8. [DOI] [PubMed] [Google Scholar]
- 34. Petrella R, Caselli F, Roig-Villanova Iet al. BPC transcription factors and a Polycomb group protein confine the expression of the ovule identity gene SEEDSTICK in Arabidopsis. Plant J. 2020;102:582–99. [DOI] [PubMed] [Google Scholar]
- 35. Du L, et al. The ubiquitin receptor DA1 regulates seed and organ size by modulating the stability of the ubiquitin-specific protease UBP15/SOD2 in Arabidopsis. Plant Cell. 2014;26:665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rienzo V, Imanifard Z, Mascio Iet al. Functional conservation of the grapevine candidate gene INNER NO OUTER for ovule development and seed formation. Hortic Res. 2021;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yadav RK, Perales M, Gruel Jet al. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev. 2011;25:2025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gross-Hardt R, Lenhard M, Laux T. WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes Dev. 2002;16:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li C, Qiao Z, Qi Wet al. Genome-wide characterization of cis-acting DNA targets reveals the transcriptional regulatory framework of Opaque2 in maize. Plant Cell. 2015;27:532–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhan J, Li G, Ryu CHet al. Opaque-2 regulates a complex gene network associated with cell differentiation and storage functions of maize endosperm. Plant Cell. 2018;30:2425–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brambilla V, Battaglia R, Colombo Met al. Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. Plant Cell. 2007;19:2544–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yao J, Zhang S, Wu Net al. KNOX transcription factor VvHB63 affects grape seed development by interacting with protein VvHB06. Plant Sci. 2023;330:111665. [DOI] [PubMed] [Google Scholar]
- 43. Li Z, Chen C, Zou Det al. Ethylene accelerates grape ripening via increasing VvERF75-induced ethylene synthesis and chlorophyll degradation. Fruit Res. 2023;3:1–9. [Google Scholar]
- 44. Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43. [DOI] [PubMed] [Google Scholar]
- 45. Fang W, Wang Z, Cui Ret al. Maternal control of seed size by EOD3/CYP78A6 in Arabidopsis thaliana. Plant J. 2012;70:929–39. [DOI] [PubMed] [Google Scholar]
- 46. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 47. Meyerowitz EM. In situ hybridization to RNA in plant tissue. Plant Mol Biol Rep. 1987;5:242–50. [Google Scholar]
- 48. Hu Y, Cheng Y, Yu Xet al. Overexpression of two CDPKs from wild Chinese grapevine enhances powdery mildew resistance in Vitis vinifera and Arabidopsis. New Phytol. 2021;230:2029–46. [DOI] [PubMed] [Google Scholar]
- 49. Lang Z, Lei M, Wang Xet al. The methyl-CpG-binding protein MBD7 facilitates active DNA demethylation to limit DNA hyper-methylation and transcriptional gene silencing. Mol Cell. 2015;57:971–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mu Y, Zou M, Sun Xet al. BASIC PENTACYSTEINE proteins repress ABSCISIC ACID INSENSITIVE4 expression via direct recruitment of the Polycomb-repressive complex 2 in Arabidopsis root development. Plant Cell Physiol. 2017;58:607–21. [DOI] [PubMed] [Google Scholar]
- 51. Mizzotti C, Ezquer I, Paolo Det al. SEEDSTICK is a master regulator of development and metabolism in the Arabidopsis seed coat. PLoS Genet. 2014;10:e1004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xie YP, Chen P, Yan Yet al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol. 2018;218:201–18. [DOI] [PubMed] [Google Scholar]
- 53. Chen P, Yan M, Li Let al. The apple DNA-binding one zinc-finger protein MdDof54 promotes drought resistance. Hortic Res. 2020;7:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao J, Shen Z, Zhang Yet al. Populus euphratica WRKY1 binds the promoter of H+-ATPase gene to enhance gene expression and salt tolerance. J Exp Bot. 2020;71:1527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Machanick P, Bailey TL. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27:1696–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.