Abstract
Background
and purpose: Radiotherapy (RT) is an effective treatment for most malignant chest tumors. However, radiation-induced myocardial fibrosis (RIMF) is a serious side effect of RT. Currently, due to the mechanism of RIMF has not been fully elucidated, there is a lack of effective therapeutic approach. In this study, we aimed to investigate the role and possible mechanisms of bone marrow mesenchymal stem cells (BMSCs) in the therapy of RIMF.
Materials and methods
Twenty-four New Zealand white rabbits were allotted into four groups (n = 6). Rabbits in the Control group received neither irradiation nor treatment. A single dose of 20 Gy heart X-irradiation was applied to the RT group, RT + PBS group and RT + BMSCs group. Rabbits in the RT + PBS group and RT + BMSCs group were injected with 200 μL PBS or 2 × 106 cells via pericardium puncture 24 h following irradiation, respectively. Echocardiography was used to test the cardiac function; Then the heart samples were collected, and processed for histopathological, Western blot and immunohistochemistry investigations.
Results
It was observed that BMSCs have therapeutic effect on RIMF. Compared with the Control group, inflammatory mediators, oxidative stress and apoptosis were significantly increased, meanwhile, cardiac function was remarkably decreased in the RT group and RT + PBS group. However, in the BMSCs group, BMSCs significantly improved cardiac function, decreased inflammatory mediators, oxidative stress and apoptosis. Furthermore, BMSCs remarkably reduced the expression level of TGF-β1 and the phosphorylated-Smad2/3.
Conclusions
In conclusion, our research indicates BMSCs have the potential to alleviate RIMF through TGF-β1/Smad2/3 and would be a new therapeutic approach for patients with myocardial fibrosis.
Keywords: RIMF, BMSCs, Cardiac function, TGF-β1, Smad2/3
Highlights
-
•
BMSCs transplantation improves cardiac function.
-
•
BMSCs transplantation reduces inflammatory response and oxidative stress.
-
•
BMSCs transplantation alleviates radiation-induced myocardial fibrosis.
1. Introduction
Radiotherapy (RT) is an essential treatment for most thoracic tumors, including mediastinal lymphoma, esophageal cancer, and breast cancer. Meanwhile, radiation-induced heart disease (RIHD) is a common and severe complication of RT [1]. A previous study showed cardiac toxicity after RT is the major cause of noncancer-related mortality [2]. Manifestations of RIHD include pericarditis, microvascular damage, myocardial fibrosis, coronary artery disease, and valvular heart disease [3]. RIMF, the advanced stage of RIHD, is a slow and progressive process that usually occurs years after radiotherapy. Currently, due to the mechanism of RIMF has not been fully elucidated, there is a lack of effective therapeutic approach.
Over the last 20 years, abundant adult stem cells have already been adopted in the basic research and clinic practice. Among the various stem cell types, BMSCs are the most widely studied, because of its easy accessibility, low immunogenicity, self-renewal, and differentiation potential [4]. Several researches have showed that MSCs are capable of differentiating into endothelial cells to participate in angiogenesis by regulating various cytokines (such as TGF-β, VEGF, and ICAM) [5,6]. In vivo studies have shown that BMSCs transplantation significantly improved cardiac function, reduced inflammation and myocardial fibrosis, and recruited DNA repair proteins after irradiation [7]. BMSCs transplantation also mitigated radiation-induced oxidative stress and artery inflammation [8]. Additionally, a study demonstrated the MSCs could restore the disrupted intestinal function by RT through inhibiting inflammation, promoting neovascularization, and maintaining epithelial homeostasis [9].
Transforming growth factor–β1 (TGF-β1) is a typical profibrotic cell growth factor, involved in regulating cell growth and differentiation, promoting cell proliferation and inhibiting inflammation [10], which plays a vital role in RIMF [11]. Smad2/3 is the downstream signaling molecules of the TGF-β1 signaling pathway, which has been found participated in the process of fibrosis, and also plays an essential role in the synthesis of extracellular matrix components [12,13]. Studies have shown that inhibition of the TGF-β1/Samd2/3 pathway may alleviate myocardial fibrosis [14,15].
Therefore, in order to clarify the role of BMSCs in the therapy of RIMF and the possible mechanisms, we completed this in vivo experiment.
2. Materials and methods
2.1. Animal experiments
Twenty-four female/male New Zealand white rabbits (10–12 weeks, 2–2.5 kg) were purchased from the Animal Experimental Centre of Yangzhou University. The rabbits were randomly evenly allotted into four groups. 1) Control group: The rabbits received neither irradiation nor treatment. 2) RT group: The rabbits received irradiation but not treatment. 3) RT + PBS group: The rabbits received irradiation and PBS treatment. 4) RT + BMSCs group: The rabbits received irradiation and BMSCs treatment. The irradiation protocol was performed with some modifications based on the previous description [16]. The rabbit hearts were exposed to X-ray irradiation with a medical linear accelerator (Varian Clinac IX, USA), operating a beam energy of 6-MV with a dose rate of 2 Gy/min, using a single fraction of 20 Gy, setting source-skin distance at 2 cm and radiation field at 1 cm × 1 cm. The rabbit hearts were localized by a simulator before irradiation. The rabbits were killed with a lethal dose of pentobarbital three months after irradiation. All animal experiments and procedures were approved by Yangzhou University Ethics Committee.
2.2. Isolation, culture and identification of BMSCs
BMSCs were extracted from 2-months-old New Zealand white rabbits. A total of 2 ml bone marrow aspirates were taken with a heparinized syringe from the lateral tibial tubercle. Density gradient centrifugation and adherent method were used to isolate BMSCs, as previously described [17]. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in an incubator at 5% CO2 at 37 °C.BMSCs from the third passage were used for the following experiments.
BMSCs were identified by flow cytometry (FCM) with CD29, CD34 and CD44 antibodies (Abcam Inc., Cambridge, MA, USA). BMSCs from passage three were harvested by centrifugation at 1000 rpm for 5 min at 4 °C, and washed with pre-cooled PBS. Cells were incubated with antibodies (1:50) at 4 °C for 30 min in the dark. After incubation, the cells were detected using a FACSCalibur II flow cytometer (BD Biosciences) and analyzed by Flow Jo software (Flow Jo, LLC, USA).
2.3. Adipogenic/osteogenic differentiation
For adipogenic differentiation, the 3rd-passage BMSCs were cultured for 2 weeks in DMEM supplemented with 10% fetal bovine serum, 0.5 mM isobutyl methyl xanthine, 10 μM insulin, 1 μM dexamethasone and 200 μM indomethacin (Sigma–Aldrich). Oil Red O (Sigma–Aldrich) staining was used to indentify lipid droplets. For osteogenic differentiation, the 3rd-passage BMSCs were cultured for 3 weeks in DMEM with 10% fetal bovine serum supplemented with 100 nM dexamethasone, 0.05 mM ascorbic acid and 10 mM β-glycerol phosphate (Sigma–Aldrich). Alizarin Red S (Sigma–Aldrich) staining was performed to detect deposited calcium phosphates.
2.4. BMSCs transplantation
The BMSCs transplantation was performed 24 h after RT. All adherent BMSCs were digested with trypsin and prepared as a cell suspension. Then, the cells were centrifuged and gently resuspended in sterile phosphate buffer solution (PBS). A percutaneous subxiphoid puncture approach was used to enter the pericardial space, as previously described [18]. In total, 2 × 106 cells in 200 μL sterile PBS were injected into each rabbit.
2.5. Echocardiography
Rabbits were immobilized but not anesthetized in the supine position on a table. The chest area was shaved with an electric razor. Echocardiograms were performed using the ultrasonic equipment (GE VividE9) with an M5 Sprobe (1.7–3.3 MHz). First, B-mode long-axis image was obtained by positioning the probe parallel to the long axis of the left ventricular (LV). Next, the probe was rotated at 90° to obtain a M-mode short-axis image of the LV. Echocardiographic images were recorded at least two cardiac cycles. These measurements included ejection fraction (EF), fractional shortening (FS), left ventricular internal diameter at end-diastole (LVIDd) and left ventricular internal diameter at end-systole (LVIDs).
2.6. Electrocardiography
All rabbits were immobilized but not anesthetized in the supine position on a table. Four limb electrodes needle were inserted under the skin of the right upper limb, right lower limb, left upper limb, and left lower limb. Additionally, six (V1–V6) precordial electrodes needle were connected in the chest. After 5 min of acclimation, the electrocardiographic waves were recorded in an ECG recorder (COMEN CM1200B, Shenzhen, China).
2.8. ELISA assay
The frozen plasma samples were used to measure the plasma levels of TNF-α, BNP, and cTnI. ELISA kit (Solaibao Biological Technology Co., Ltd., Beijing, China). The assay was performed according to the manufacturer's instructions.
2.9. Measurement of SOD
Total SOD activity was measured in serum using an SOD assay kit based on the manufacturer's instructions (Jiancheng Biotech Ltd., Nanjing, China). Briefly, the nitroblue tetrazolium (NBT)/enzyme working solutions was added to the serum samples for in incubation at 37 °C for 30 min. The absorbance at 560 nm was measured using a Tecan Spark 20M microplate reader.
2.10. Histological analysis
After the rabbits were sacrificed, the heart samples were fixed in 4% paraformaldehyde solution for 24 h, dehydrated, and embedded in paraffin. Then, 5 μm thick slices were cut, and stained with hematoxylin-eosin (HE) and Masson. Heart pathological changes were observed using light microscopy. Three different visual fields were randomly selected in each slice. Collagen deposition areas were analyzed by Image Pro Plus 6.0 (IPP6.0).
2.11. TUNEL staining
The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was used to ascertain cellular death. The TUNEL assay was performed using a TUNEL assay kit (Roche, Shanghai, China) according to the manufacturer's instructions. Staining was observed using light microscopy; TUNEL-positive nuclei were expressed as a percentage of the total nuclei per field.
2.12. Immunohistochemistry
Immunohistochemical staining was performed according to a previously described protocol [19]. In brief, the heart tissues were sectioned at 4 μm and onto glass slides. Then, the sections were quenched with 3% hydrogen peroxide, digested with compound digestive juice, and blocked with 5% bovine serum albumin. Then, tissues were incubated with the primary antibody anti-TGFβ1 (cat. no. AF5449; 1:200; Affinity Biosciences) at 4 °C overnight. The sections were incubated with the horseradish peroxidase-conjugated secondary antibody (cat. No. ab6721; 1:500; Abcam Biotech., Cambridge, MA, USA) for 1 h at room temperature. Three randomly selected areas in each slice were used to evaluate the images. Relative expression was compared using average optical density (IOD/area), as measured using IPP6.0 software.
2.13. Western blotting
Western blotting was performed as reported previously [20]. Briefly, the protein was extracted from heart tissues using RIPA lysis buffer with protease/phosphatase inhibitor (Thermo Fisher), then, the protein was separated on 10% SDS-PAGE and transferred to Immun-Blot polyvinylidene difluoride membranes, membranes were blocked with 5% BSA for 2 h and then incubated with the anti-collagen I (cat. no. A21059; 1:2000; ABclonal), anti-collagen III (cat. no. ab7778; 1:2000; Abcam), anti-gapdh (cat. no. ab9485; 1:2000; Abcam), anti-cleaved-caspase 3 (cat. no. ab2302; 1:2000; Abcam), anti-α-tubulin (cat. no. AF5449; 1:2000; Affinity Biosciences), anti-Smad2/3 (cat. no. AF6367; 1:2000; Affinity Biosciences), and anti-pSmad2/3 (cat. no. AF3367; 1:2000; Affinity Biosciences) overnight at 4 °C. Subsequently, membranes were washed with TBST and incubated with the HRP- labeled secondary antibody (cat. no. S0001; 1:3000; Affinity Biosciences) for 2 h. The corresponding band was revealed using enhanced chemiluminescence reagents (Thermo Fisher Scientific). Autoradiographs were quantified by densitometry (NIH Image J).
2.14. Statistical analysis
Results are presented as means ± standard deviation in GraphPad Prism6.0 (GraphPad Software Inc., San Diego, USA). All dates were derived from at least three independent experiments. Student's t-test was used for comparison between the two groups, and one-way ANOVA was used for comparison between multiple groups. Differences were considered significant when p < 0.05.
3. Results
3.1. Culture and identification of BMSCs
The isolated BMSCs exhibited fibroblast-like-shaped after 12 days of culture (Supplementary Fig. S1A). After 2 weeks of adipogenic induction, lipid droplet aggregation was observed by Oil Red staining, confirming adipogenic differentiation (Supplementary Fig. S1B). After 3 weeks of osteogenic induction, Osteogenic differentiation was confirmed by deposition of calcium by Alizarin Red staining (Supplementary Fig. S1C). Meanwhile, Flow cytometric analysis showed that third generation cells expressed of CD29 and CD44 (94.9% and 92.4% respectively), with little evidence of the expression of CD45 (0.23%) (Supplementary Fig. S1D).
3.2. BMSCs transplantation improves the cardiac function of RIMF rabbits
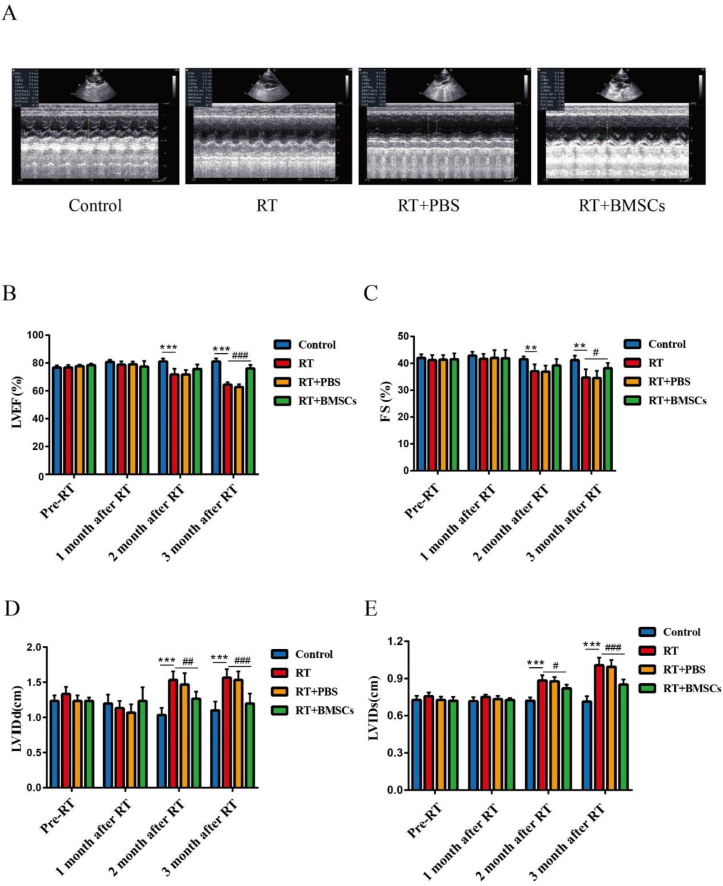
The cardiac function was measured before radiotherapy and at the 1, 2, and 3 months after irradiation (Fig. 1A). The results showed that compared to the Control group, the LVEF and FS of rabbits in the RT group were significantly reduced, while that of rabbits in the RT + BMSCs group were slightly reduced; There was a significant difference between the RT group and RT + BMSCs group (Fig. 1B,C). In addition, For LVIDd and LVIDs, there were a considerable difference between the RT + BMSCs group and the RT group (Fig. 1D,E).
Fig. 1.
BMSCs improve cardiac function. The cardiac function was measured after irradiation. A. Images of heart ultrasound at 3 months after irradiation; B. LVEF: left ventricular ejection fraction; C. FS: fractional shortening; D. LVIDd: left ventricular internal diameter at end-diastole. E. LVIDs: left ventricular internal diameter at end-systole; Values are expressed as mean ± SD (n = 6 rabbits); ∗∗P < 0.01 and ∗∗∗P < 0.001vs Control; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs RT.
3.3. BMSCs transplantation attenuates the myocardial injury
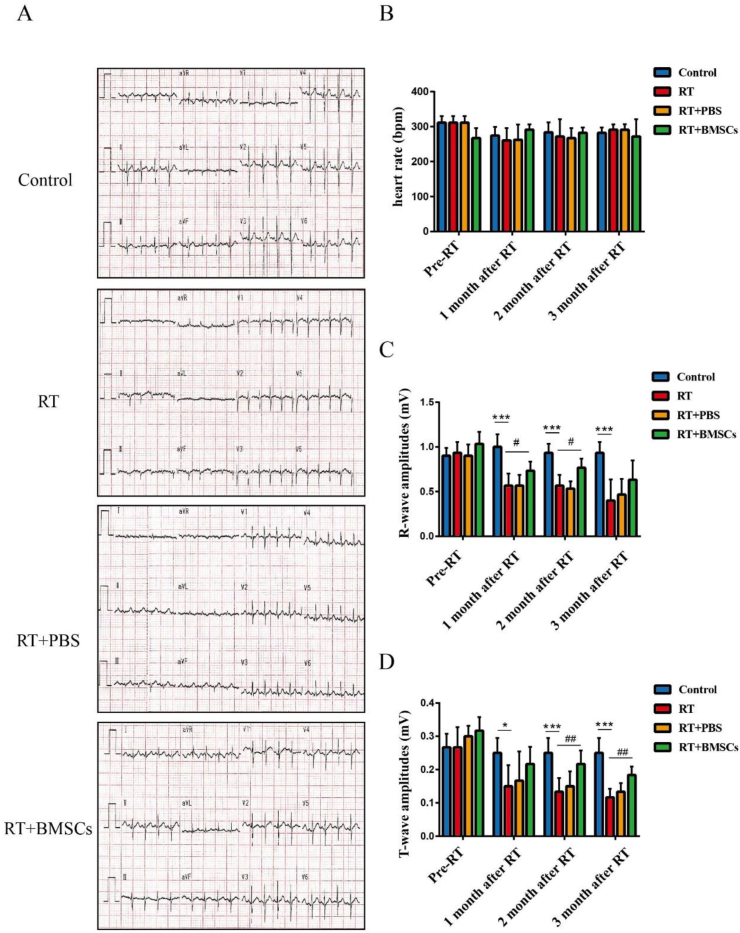
Electrocardiogram measurements were performed before radiotherapy and at the 1, 2, and 3 months after irradiation (Fig. 2A). The results showed that there was no significant difference in the heart rate in each group before and after radiotherapy (Fig. 2B). A significant decrease in R-wave and T-wave was evidenced in the RT group in comparison to the control group; The RT + BMSCs groups have significantly improved R-wave and T-wave than the RT group (Fig. 2C,D).
Fig. 2.
BMSCs reduce myocardial damage. The electrocardiography was measured after irradiation. A. Images of electrocardiography at 3 months after irradiation; B. heart rate (bpm); C. R-wave amplitudes (mV); D. T-wave amplitudes (mV). Values are expressed as mean ± SD (n = 6 rabbits); ∗p < 0.05 and ∗∗∗P < 0.001 vs Control; #P < 0.05 and ##P < 0.01 vs RT.
3.4. BMSCs transplantation alleviates functional heart parameters
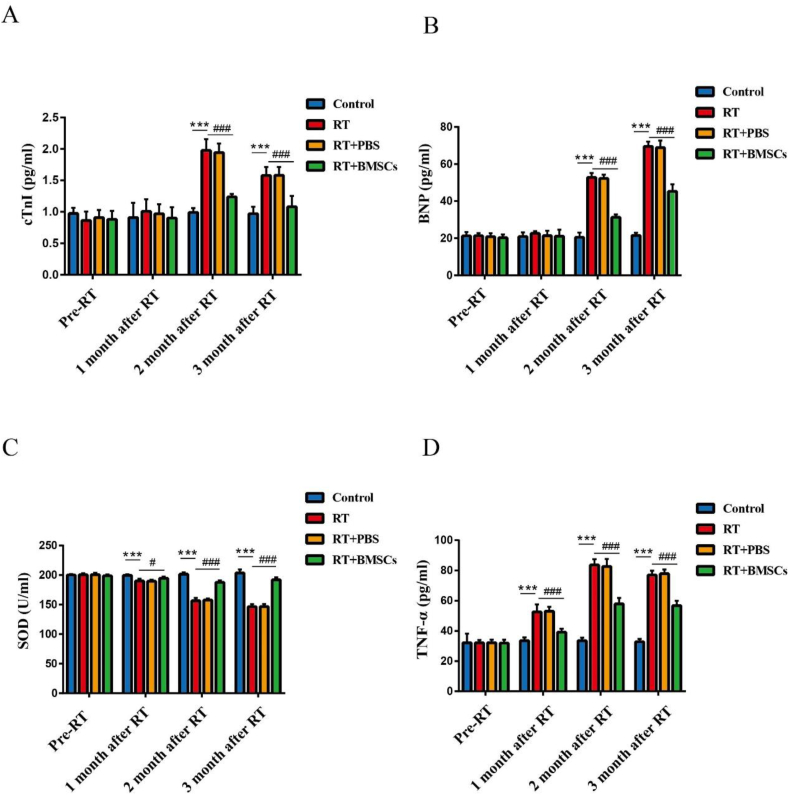
The functional heart parameters were detected before radiotherapy and at the 1, 2, and 3 months after irradiation. The cTnI and BNP in the RT group showed a significant increase compared to the control group at the 2 and 3 months after irradiation; The RT + BMSCs group has shown a decrease in cTnI and BNP than the RT group (Fig. 3A,B). Further, the levels of antioxidant critical regulator SOD were remarkably decreased in the RT group; The SOD levels were significantly elevated in the RT + BMSCs group compared to the RT group (Fig. 3C). Also, the levels of TNF-α were significantly increased in the RT group; The TNF-α levels were significantly decreased in the RT + BMSCs group in comparison with the RT group (Fig. 3D).
Fig. 3.
BMSCs inhibit radiation-induced oxidative stress and inflammation and improve functional heart parameters. The cTnI, BNP, SOD and TNF-α were measured after irradiation. A. cTnI; B. BNP; C. SOD; D. TNF-α. Values are expressed as mean ± SD (n = 6 rabbits); ∗∗∗P < 0.001 vs Control; #P < 0.05 and ###P < 0.001 vs RT.
3.5. BMSCs transplantation mitigates cardiac morphological changes after irradiation
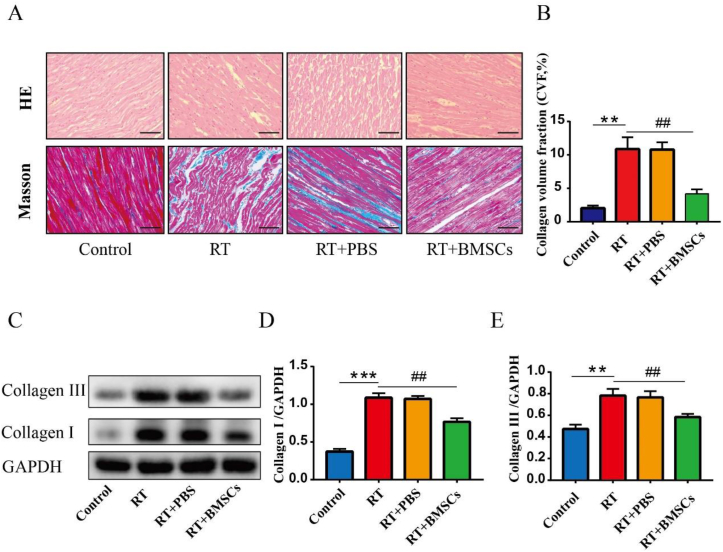
H&E staining revealed morphological changes in rabbit hearts at 3 months after irradiation. The control group showed normal cardiac myocytes. On the other hand, the RT group showed hyperemia and inflammation in the cardiac tissues; The RT + BMSCs group demonstrated slight hyperemia of cardiac myocytes (Fig. 4A). Masson's staining revealed increased collagen deposition in the heart in the RT group compared with the control group; The RT + BMSCs group exhibited less collagen compared to the RT group (Fig. 4B). Western Blot showed that the protein expression of Collagen I and Collagen III was a significant increase in the RT group compared with the control group; Compared with the RT group, the Collagen I and Collagen III was significantly down-regulated in the RT + BMSCs groups (Fig. 4C–E).
Fig. 4.
BMSCs reduce inflammation and myocardial fibrosis. Histopathology was performed on hematoxylin-eosin and Masson sections. A. HE sections and Masson sections; B. CVF: collagen volume fraction. C. Western blotting analysis of Collagen I and Collagen III in each group. D. Densitometric analysis of western blotting for Collagen I. E. Densitometric analysis of western blotting for Collagen III. Values are expressed as mean ± SD (n = 6 rabbits); Original magnification ×200; Scale bar = 100 μm ∗∗P < 0.01 and ∗∗∗P < 0.001 vs Control; ##P < 0.01 vs RT.
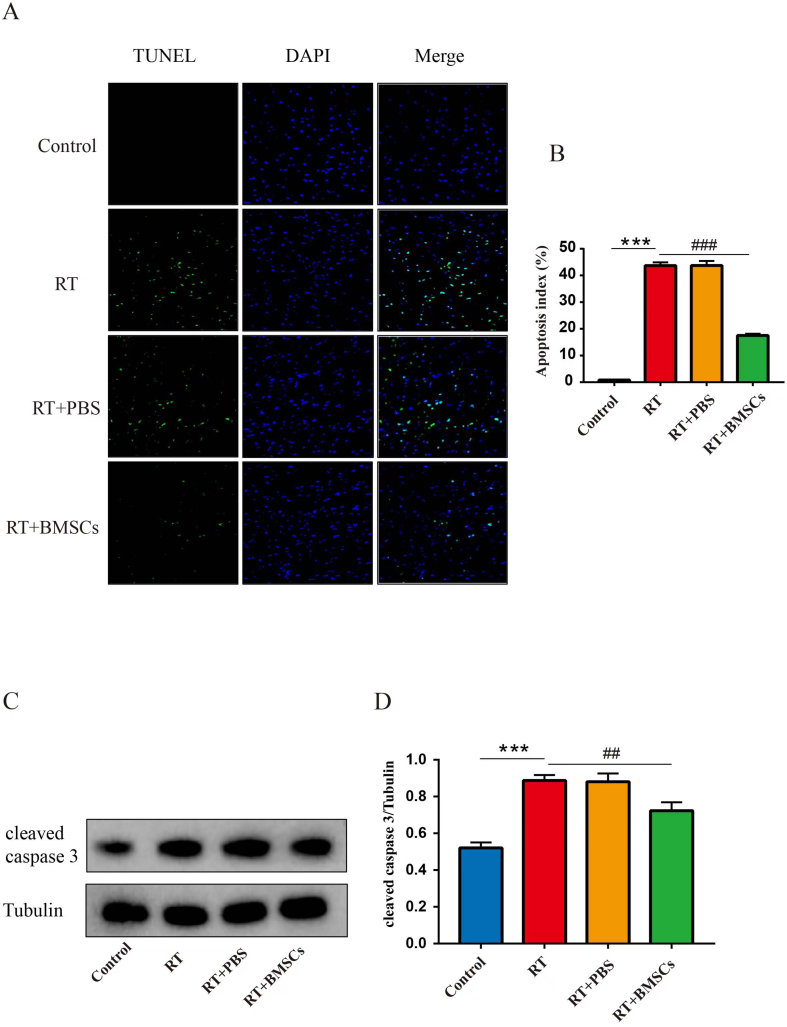
3.6. BMSCs transplantation inhibits apoptosis in cardiac tissue after irradiation
TUNEL staining indicated that TUNEL-positive cells were more frequently observed in the RT group compared to the control group. Compared with the RT group, TUNEL-positive cells were significantly decreased in the RT + BMSCs groups (Fig. 5A,B); Western blot results indicated that the protein expression of cleaved caspase 3 was a significant increase in the RT group as compared with the control group; The protein level of cleaved caspase 3 was significantly decreased in the RT + BMSCs group in comparison with the RT group.
Fig. 5.
BMSCs inhibit apoptosis. Apoptosis of cardiac tissue in each group. A. Representative images of apoptotic cells stained green with TUNEL in cardiac tissue. Total nuclei were stained blue with DAPI. B. Quantitative analysis of apoptotic rate. TUNEL-positive cells (%) are expressed as (number of apoptotic myocytes/total myocytes) ×100%. C. Western blotting analysis of cleaved caspase 3 in each group. D. Densitometric analysis of western blotting for cleaved caspase 3. Values are expressed as mean ± SD (n = 6 rabbits); Original magnification ×200; Scale bar = 100 μm ∗∗∗P < 0.001 vs Control; ##P < 0.01 and vs ###P < 0.001 vs RT.
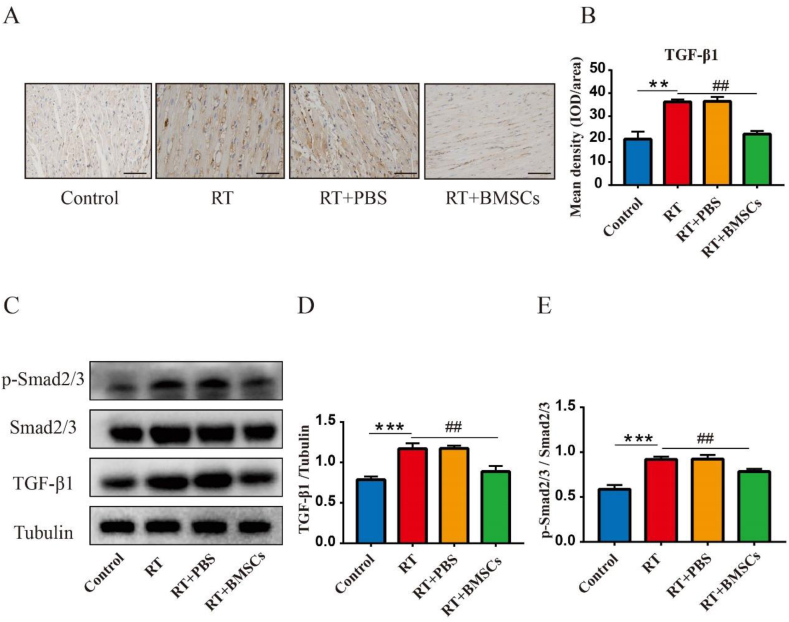
3.7. BMSCs transplantation reduces the protein levels of TGF-β1, Smad2/3, and p-Smad2/3 in the heart
Immunohistochemical staining (IHC) shows that TGF-β1 was highly expressed in the RT group compared to the control group; Compared with the RT group, the TGF-β1was significantly down-regulated in the RT + BMSCs groups (Fig. 6A,B). Western blot results indicated that the protein expression of TGF-β1, Smad2/3 and p-Smad2/3 was a significant increase in the RT group as compared with the control group; Compared with the RT group, the TGF-β1, Smad2/3 and p-Smad2/3 was significantly down-regulated in the RT + BMSCs groups (Fig. 6C–E).
Fig. 6.
BMSCs reduce the expression of TGF-β1, Smad2/3 and p-Smad2/3. Immunohistochemical staining was performed to test the expression of TGF-β1. The level of TGF-β1, Smad2/3 and p-Smad2/3 was checked by Western blot. A: The expression and distribution of TGF-β1; B: IOD of TGF-β1 expression in the heart. C. Western blotting analysis of TGF-β1, Smad2/3 and p-Smad2/3 in each group. D. Densitometric analysis of western blotting for TGF-β1; E. Densitometric analysis of western blotting for Smad2/3 and p-Smad2/3. Values are expressed as mean ± SD (n = 6 rabbits); Original magnification ×200; Scale bar = 100 μm. IOD: integrated optical density. ∗∗P < 0.01 and ∗∗∗P < 0.001 vs Control; ##P < 0.01 vs RT.
4. Discussion
Unavoidable heart expouse would increase the risk of RIHD during radiotherapy. Our study showed that BMSCs could repair myocardial injury, improve cardiac function, reduce oxidative stress, inflammation and apoptosis. Moreover, BMSCs mitigated collagen deposition and decreased the expression of TGF-β1, Smad2/3, and p-Smad2/3 after irradiation, suggesting that BMSCs may play its role via the TGF-β1/Smad2/3 pathway.
In the first few minutes of irradiation, endothelial cell swelling induces early acute inflammatory reactions, such as neutrophil infiltration, macrophage and monocyte activation, which promote the release of cytokines (such as TNF-α, IL-6, and IL-8) [21]. Then, the PDGF, TGF-β, and CTGF were released, leading to chronic inflammation [22]. Consistent with those, in our study, we observed that within three months after radiotherapy, the level of TNF-α experienced a continuous process of increasing, maintaining and decreasing. It has also been reported that BMSCs inhibited TNF-α production and exerted an anti-inflammatory effect in lung injury [23]. Furthermore, Forte A et al. found that MSCs inhibited inflammatory responses to promote endothelial reparation [24]. Consistent with those, in our present research, we found that BMSCs diminished the expression of TNF-α after irradiation, which suggested that BMSCs had an effect on reducing radiation-induced inflammation.
Oxidative stress and inflammation are both important risk factors for cardiovascular disease [25]. It is well known that irradiation can induce mitochondrial dysfunction, resulting in increased ROS production. Inflammatory cytokines also can induce ROS production [26]. In addition, radiation can cause an abnormal decrease in endogenous antioxidants (including SOD, GPX, and CAT), resulting in persistent ROS generation. Wei L et al. have showed that MSCs could enhance the activity of antioxidant enzymes after RT [27]. In our study, we observed that BMSC significantly increased the accumulation of SOD from 1 month to 3 months after radiation. These results indicated the antioxidant effect of BMSCs, which was in line with Shen Y et al., they found that transplantation of BMSCs prevented radiation-induced artery injury through suppressing oxidative stress [8].
The current data suggested that cTnI and BNP were increased, and ECG showed that R-wave and T-wave were decreased after radiation. These are sensitive indicators of cardiac injury. Moreover, Gao S et al. demonstrated that after irradiation of the heart, apoptosis occurred [28], which consisted with our findings. BMSCs transplantation mitigates these adverse outcomes, mainly due to BMSCs reducing the inflammatory response, oxidative stress and significantly downregulating the expression levels of cleaved caspase 3 and repairing myocardial damage.
RIMF is a dynamic and complex process, which is initiated and aggravated by pro-inflammatory, pro-fibrotic cytokines and oxidative stress, ultimately resulting in reduced ventricular elasticity and dilatation, leading to decreased ejection fraction, heart failure, and even sudden cardiac death. TGF-β1 is the most closely related to the development of tissue fibrosis, and has a variety of functions, such as regulating cell growth and proliferation, and inhibiting inflammation. Smad2/3 is the major effector of profibrotic TGF-β1 signaling, which has been found involved in the process of fibrosis [29]. Therefore, suppression of the TGF-β1/Smad2/3 signaling pathway may be effective method to prevent RIMF. Expectedly, our study found the expressions level of TGF-β1, Smad2/3, and p-Smad2/3 was all significantly increased after radiation, accompanied by increased collagen depositing in heart tissue. However, BMSCs transplantation significantly reduced the expressions of TGF-β1, Smad2/3, and p-Smad2/3, partially relieving the RIMF. Which was consistent with previous studies that human bone marrow mesenchymal stem cells (hBMSCs) administration partially prevented the aortic fibrosis and remodeling by suppressing the expression of TGF-β [8]. BMSCs improved myocardial fibrosis and inflammation in a RIHD rat model [7].
It is generally believed that MSCs have powerful tissue repair capacity due to their paracrine effects. In our following experiments, we will further explore specific factors secreted by BMSCs, which have anti myocardial fibrosis effect. Furthermore, because the investigation was limited by its small sample size and possible bias, further and more profound studies are needed to characterize the exact mechanism of action of this possible clinical treatment.
5. Conclusion
In the present study, we observed that BMSCs transplantation ameliorates RIMF, and the result showed that the underlying mechanism is partially related to the suppression of radiation-induced oxidative stress, inflammation and apoptosis, upregulation of antioxidant enzymes SOD as well as downregulation of TGF-β1 and Smad2/3. Therefore, BMSCs may be a promising therapeutic approach to treat RIMF.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
All animal experiments and procedures were approved by Yangzhou University Ethics Committee.
Authors contributions
JG and HL performed the experiments, analyzed the data, prepared figures, authored or reviewed drafts of the paper. WW, XS, YC and JL performed material preparation, performed the experiments. BL and XG conceived and designed the experiments. All authors reviewed the manuscript and agreed to be accountable for the content of the work.
Funding
This study was supported by the Science and Technology Department of Jiangsu Province (funding code BL2013022). National Natural Science Foundation of China (grant no. 81370305).
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2023.04.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Wang H., Wei J., Zheng Q., Meng L., Xin Y., Yin X., et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15(10):2128–2138. doi: 10.7150/ijbs.35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palaskas N., Patel A., Yusuf S.W. Radiation and cardiovascular disease. Ann Transl Med. 2019;7(Suppl 8):S371. doi: 10.21037/atm.2019.08.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnellan E., Phelan D., McCarthy C.P., Collier P., Desai M., Griffin B. Radiation-induced heart disease: a practical guide to diagnosis and management. Cleve Clin J Med. 2016;83(12):914–922. doi: 10.3949/ccjm.83a.15104. [DOI] [PubMed] [Google Scholar]
- 4.Naji A., Eitoku M., Favier B., Deschaseaux F., Rouas-Freiss N., Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–3348. doi: 10.1007/s00018-019-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamawaki-Ogata A., Fu X., Hashizume R., Fujimoto K.L., Araki Y., Oshima H., et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells in formed aortic aneurysms of a mouse model. Eur J Cardio Thorac Surg. 2014;45(5):e156–e165. doi: 10.1093/ejcts/ezu018. [DOI] [PubMed] [Google Scholar]
- 6.Dong H.J., Shang C.Z., Li G., Niu Q., Luo Y.C., Yang Y., et al. The distribution of transplanted umbilical cord mesenchymal stem cells in large blood vessel of experimental design with traumatic brain injury. J Craniofac Surg. 2017;28(6):1615–1619. doi: 10.1097/SCS.0000000000003563. [DOI] [PubMed] [Google Scholar]
- 7.Gao S., Zhao Z., Wu R., Zeng Y., Zhang Z., Miao J., et al. Bone marrow mesenchymal stem cell transplantation improves radiation-induced heart injury through DNA damage repair in rat model. Radiat Environ Biophys. 2017;56(1):63–77. doi: 10.1007/s00411-016-0675-0. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y., Jiang X., Meng L., Xia C., Zhang L., Xin Y. Transplantation of bone marrow mesenchymal stem cells prevents radiation-induced artery injury by suppressing oxidative stress and inflammation. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/5942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang P., Qu Y., Liu Y., Cui S., Zhu D., Wang H., et al. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell Death Dis. 2013;4:e685. doi: 10.1038/cddis.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeglinski M.R., Hnatowich M., Jassal D.S., Dixon I.M. SnoN as a novel negative regulator of TGF-beta/Smad signaling: a target for tailoring organ fibrosis. Am J Physiol Heart Circ Physiol. 2015;308(2):H75–H82. doi: 10.1152/ajpheart.00453.2014. [DOI] [PubMed] [Google Scholar]
- 11.Mori H., Yoshioka H., Mori K., Ahmadi T., Okumura T., Saida Y., et al. Radiation-induced liver injury showing low intensity on T2-weighted images noted in Budd-Chiari syndrome. Radiat Med. 2002;20(2):69–76. [PubMed] [Google Scholar]
- 12.Roberts A.B., Tian F., Byfield S.D., Stuelten C., Ooshima A., Saika S., et al. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17(1–2):19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Wang W., Huang X.R., Canlas E., Oka K., Truong L.D., Deng C., et al. Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circ Res. 2006;98(8):1032–1039. doi: 10.1161/01.RES.0000218782.52610.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X., Long L., Cheng Y., Chu J., Shen Z., Liu L., et al. Qingda granule attenuates cardiac fibrosis via suppression of the TGF-beta1/Smad2/3 signaling pathway in vitro and in vivo. Biomed Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111318. [DOI] [PubMed] [Google Scholar]
- 15.Shu J., Hu L., Wu Y., Chen L., Huang K., Wang Z., et al. Daidzein suppresses TGF-beta1-induced cardiac fibroblast activation via the TGF-beta1/SMAD2/3 signaling pathway. Eur J Pharmacol. 2022;919 doi: 10.1016/j.ejphar.2022.174805. [DOI] [PubMed] [Google Scholar]
- 16.Tewart J.R., Fujardo L.F., Cohn K.E., Page V. Experimental radiation-induced heart disease in rabbits. Radiology. 1968;91(4):814–817. doi: 10.1148/91.4.814. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Sosa E., Scanavacca M., D'Avila A., Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7(6):531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 19.Aoki T., Nishimura M., Matsuoka T., Yamamoto K., Furuyashiki T., Kataoka H., et al. PGE (2)-EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-kappaB. Br J Pharmacol. 2011;163(6):1237–1249. doi: 10.1111/j.1476-5381.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangali S., Bhat A., Jadhav K., Kalra J., Sriram D., Vamsi K.V.V., et al. Upregulation of PKR pathway mediates glucolipotoxicity induced diabetic cardiomyopathy in vivo in wistar rats and in vitro in cultured cardiomyocytes. Biochem Pharmacol. 2020;177 doi: 10.1016/j.bcp.2020.113948. [DOI] [PubMed] [Google Scholar]
- 21.Taunk N.K., Haffty B.G., Kostis J.B., Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. doi: 10.3389/fonc.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarnold J., Brotons M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz L.A., Dutreil M., Fattman C., Pandey A.C., Torres G., Go K., et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forte A., Finicelli M., Mattia M., Berrino L., Rossi F., De Feo M., et al. Mesenchymal stem cells effectively reduce surgically induced stenosis in rat carotids. J Cell Physiol. 2008;217(3):789–799. doi: 10.1002/jcp.21559. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S., Gambhir J.K., Kalra O., Gautam A., Shukla K., Mehndiratta M., et al. Association of biomarkers of inflammation and oxidative stress with the risk of chronic kidney disease in Type 2 diabetes mellitus in North Indian population. J Diabet Complicat. 2013;27(6):548–552. doi: 10.1016/j.jdiacomp.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Koh K.K., Oh P.C., Quon M.J. Does reversal of oxidative stress and inflammation provide vascular protection? Cardiovasc Res. 2009;81(4):649–659. doi: 10.1093/cvr/cvn354. [DOI] [PubMed] [Google Scholar]
- 27.Wei L., Zhang J., Yang Z.L., You H. Extracellular superoxide dismutase increased the therapeutic potential of human mesenchymal stromal cells in radiation pulmonary fibrosis. Cytotherapy. 2017;19(5):586–602. doi: 10.1016/j.jcyt.2017.02.359. [DOI] [PubMed] [Google Scholar]
- 28.Gao S., Wu R., Zeng Y. Up-regulation of peroxisome proliferator-activated receptor gamma in radiation-induced heart injury in rats. Radiat Environ Biophys. 2012;51(1):53–59. doi: 10.1007/s00411-011-0390-9. [DOI] [PubMed] [Google Scholar]
- 29.Hu H.H., Chen D.Q., Wang Y.N., Feng Y.L., Cao G., Vaziri N.D., et al. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.