Abstract
Diabetic wound is a great threat to patient's health and lives. The refractory diabetic wound shows spatial inflammation patterns, in which the early-wound pattern depicts a deprived acute inflammatory response, and the long-term non-healing wound pattern delineates an excessive and persistent inflammation due to the delayed immune cell infiltration in a positive feedback loop. In this work, we give points to some strategies to normalize the dysregulated immune process based on the spatial inflammation pattern differences in diabetic wound healing. First of all, inhibiting inflammatory response to avoid subsequent persistent and excessive immune infiltration for the early diabetic wound is proposed. However, diabetic wounds are unperceptive trauma that makes patients miss the best treatment time. Therefore, we also introduce two strategies for the long-term non-healing diabetic wound. One strategy is about changing chronic wounds to acute ones, which aims to rejuvenate M1 macrophages in diabetic wounds and make spontaneous M2 polarization possible. To activate the controllable proinflammatory response, western medicine delivers proinflammatory molecules while traditional Chinese medicine develops “wound-pus promoting granulation tissue growth theory”. Another strategy to solve long-term non-healing wounds is seeking switches that target M1/M2 transition directly. These investigations draw a map that delineates strategies for enhancing diabetic wound healing from the perspective of spatial inflammation patterns systematically.
Keywords: Diabetic wound, Spatial inflammation pattern, M1/M2 transition, Biomaterials
Highlights
-
•
Diabetic wounds are hard-to-heal that need smart strategies to expedite the healing process.
-
•
Diabetic wound healing shows spatial inflammation patterns in different timeline.
-
•
We give points to strategies for enhancing diabetic wound healing based on spatial inflammation patterns.
-
•
These strategies give inspirations to advance precision medicine for diabetic wounds.
1. Introduction
From the World Health Organization(WHO), it is reported that the number of people with diabetes is surging from all over the world, especially in middle-income countries [1]. Diabetic foot ulcer(DFU) is a rather common comorbidity of diabetes [2]. The lifetime risk of developing DFU for diabetics is up to 25% [3]. Among old adults, more presence of senescent cells has been hypothesized to contribute to pathophysiological conditions, which is the main factor for higher DFU morbidity [4]. With a growing and aging population in the world, the status of diabetic wounds will be more challenging for the healthcare system [5].
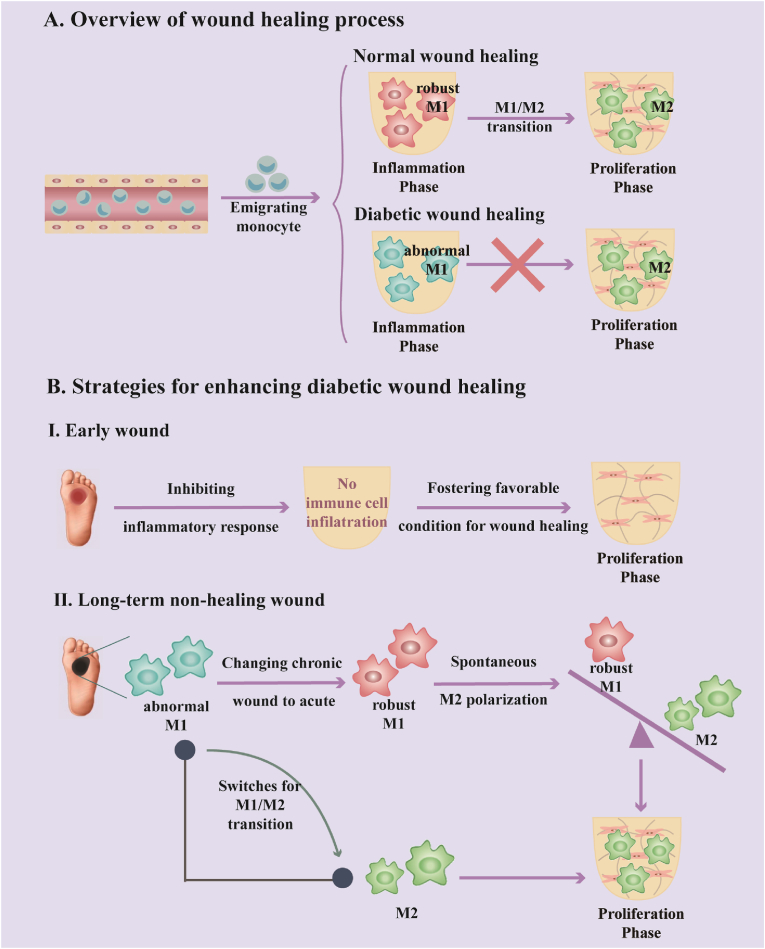
Wound healing is a sophisticated and highly coordinated process including four overlapping phases: hemostasis phase (0-several hours after injury), inflammatory phase (1–3 days), proliferation phase (4–21 days), and remodeling phase (21days-1year) [6,7]. However, diabetic wounds are difficult to heal due to pronounced immune imbalance, resulting in stagnating in the inflammatory phase (pro-inflammatory state) rather than entering the subsequent proliferation phase (pro-regenerative state) (Fig. 1A) [8,9]. The physiological conditions in diabetic wound healing have dynamic spatial inflammation patterns, which make a distinction between early injuries to long-term non-healing wounds. The early-wound pattern shows an absence of inflammatory storm while the long-term non-healing wound pattern displays excessive and persistent inflammation owing to the M1 macrophage overflow. In the early-wound pattern, both impaired peripheral neuropathy and unstabilization of mast cells blunt the ability to develop an acute pro-inflammatory response [10]. The suppression of boosting an acute pro-inflammatory response leads to decreased soluble mediators that function as endogenous danger signals to recruit neutrophils and macrophages to diabetic wound sites [11,12]. Even though immune cell infiltration is delayed and impaired in the early stage of diabetic wounds, the persistence of belated neutrophils and macrophages comes behind, and M1 macrophages gradually become the dominant role that participates in the inflammation pattern changes [[12], [13], [14]]. Chemocytokines or cytokines secreted by M1 macrophages in the diabetic wound environment attract monocytes infiltration from the blood circulation system and guide recruited monocytes to convert into M1 phenotype, which leads to a positive feedback loop for M1 accumulation and excessive inflammation in chronic wounds [[15], [16], [17]]. This strong polarization into M1 macrophage maintains a high level of M1/M2 ratio, resulting in the development of long-term non-healing wound pattern [18].
Fig. 1.
(A) The causative reason for the non-healing diabetic wound is immune dysfunction in the inflammatory phase, which makes the diabetic wound healing process fail to enter the proliferation phase. (B) To enhance the diabetic wound healing process, inhibiting inflammatory response to avoid subsequent immune cell infiltration and fostering a supportive physiological condition for wound repairing is feasible for the early-wound pattern. When an excessive and persistent inflammation pattern is established in long-term non-healing wound, one strategy is about changing chronic wounds to acute, which aims to normalize abnormal M1 macrophage and make its polarization spontaneously, another strategy is about employing switches for M1/M2 transition. All of these strategies lead to the proliferation phase.
Hence, we collectively and critically give points to some strategies for enhancing diabetic wound healing from the perspective of spatial inflammation patterns in early wounds and long-term non-healing wounds (Fig. 1B). Inhibiting proinflammatory response is introduced upon early wounds, which avoids the subsequent excessive and persistent inflammation pattern and fosters supportive condition for wound healing. Furthermore, strategies focusing on changing chronic wounds to acute ones and seeking switches on M1/M2 transition are overviewed, aiming for long-term non-healing wounds with a developed excessive and persistent inflammation pattern.
2. Normal wound healing process
Wound healing is a well-orchestrated and highly precise process consisting of four phases: hemostasis, inflammation, proliferation, and remodeling. The process is very fragile, which requires proceeding in a precise and regulated manner. Dysregulations like interruption or prolongation in each phase can lead to postponed wound healing or non-healing chronic wound [19,20]. To get inspiration in breaking bottlenecks for treating non-healing chronic wounds, a deeper understanding of the mechanisms for normal wound healing stages is of great importance.
Hemostasis is the first stage of wound healing and an emergency response after skin injury which causes bleeding to stop. It is achieved within the first minute of wound injury [21]. Platelets are disc-shaped small anuclear cell fragments that play a vital role in hemostasis by gathering and attaching to the site of injury [22,23]. Besides their traditional function in hemostasis, platelets also demonstrate the capacity to communicate with immune cells. Degranulation of platelets results in the release of inflammatory compounds such as IL-1α, IL-1β, IL-6, IL-8, and TNF-α. Especially, IL-8 is a potent chemokine for neutrophil recruitment. Therefore, the inflammatory phase is initiated [24].
The inflammatory phase can roughly be divided into two stages, with neutrophils recruitment in an early stage, monocytes appearance, and polarization in a late stage [25]. Neutrophils are the first wave of defense immune cells that migrate to the wound site and release inflammatory mediators [[26], [27], [28]]. However, neutrophils are short-lived cells. Once their physiological mission has been completed, neutrophils undergo spontaneous apoptosis, leading to the discharge of lysophosphatidylcholine that attracts monocytes via G2A receptors [29]. Besides, there are also some other factors that contribute to early acute inflammation. Cutaneous neurons can release neuropeptides, which interplay between the nervous and immune systems by upregulating levels of pro-inflammatory mediators such as TNF-α, IL-1β, and IL-6 in different kinds of skin cells [30,31]. Mast cells are tissue-resident immune cells whose cytoplasm contains a great number of granules that are reservoirs for preformed immunomodulatory compounds consisting of TNF, IL-4, histamine, and so on. When mast cells are appropriately activated to degranulate, these granule constituents are released to the surroundings in response [32]. The second wave of emigrating immune cells are monocytes, the appearance of which signifies the later stage of inflammation [26,27]. Upon arrival, these recruited circulating monocytes differentiate into M1 macrophages rapidly via the stimulation of IFN-γ and LPS, releasing pro-inflammatory cytokines to amplify the inflammatory effect [33,34]. Next, the M1 to M2 polarization is of critical significance for the transition from the inflammation phase to the proliferation phase. M2 macrophages secrete anti-inflammatory cytokines (like IL-10, IL-12) and growth factors (like TGF-β) that accelerate wound healing and turn off the damage-causing pro-inflammatory behaviors of the M1 macrophages [35].
Afterward, the proliferation phase of wound healing proceeds which encompasses epithelialization, angiogenesis, and collagen deposition [[36], [37], [38]]. The remodeling phase is the last stage in wound healing, during which collagen fibers reorganize from type Ⅲ to type Ⅰ by matrix metalloproteinase, resulting in the formation of scar tissue [39].
3. Pathological conditions in diabetic wound healing
Diabetes is a systemic metabolic disorder with chronic hyperglycemia due to insulin resistance. High glucose toxicity is associated with end-organ dysfunctions incorporating nerves, retina, kidney, heart, and blood vessels [40]. The specific pathological conditions in diabetic patients also lead to the impairment of wound healing.
Diabetes is always implicated in peripheral neuropathy [41]. Distal and sensory predominant nerve fiber degeneration is noteworthy in diabetic patients [42]. While neuropeptides are immunomodulators released from peripheral terminals of primary afferent sensory neurons in response to injury, their expression is reduced in the case of diabetic wounds [43,44]. Diminished neuropeptide level in diabetic wounds attenuates the acute inflammatory response since neuropeptides play a crucial role in triggering initial inflammation by recruiting leukocytes [45]. Besides, mast cells experience more degranulation in unwounded diabetic skin but less degranulation in the diabetic wound environment. This unstabilization of mast cells in unwounded skin and premature degranulation deadens the initiation of an acute immune response. It has been proven that pretreating mast cells with degranulation inhibitor disodium cromoglycate improved the diabetic wound healing process [46]. To sum up, both decreased neuropeptide release and mast cell degranulation in pathological diabetic early-wound pattern deprive the ability to mount an acute response to counteract injury.
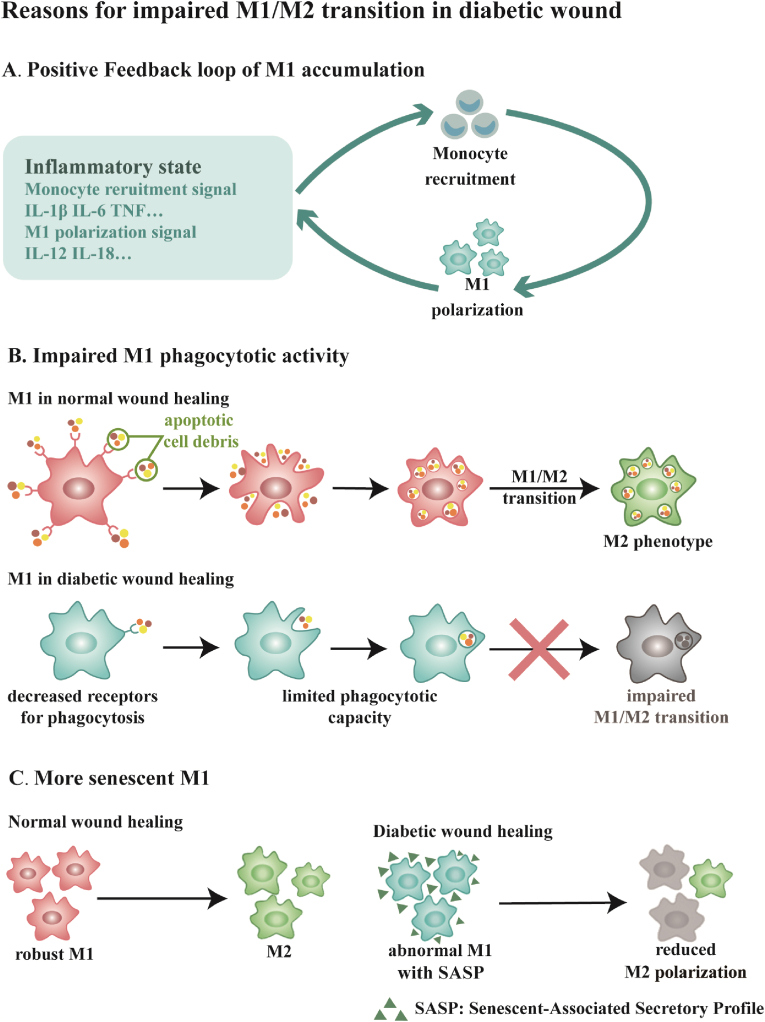
Although the early-wound pattern is characterized by failures to mount an acute immune response, with time it evolves into an excessive and persistent inflammation pattern [47,48]. Diabetic wounds are distinguished from acute wounds by a multitude of neutrophils and macrophages in the stalled inflammation phase during wound healing [49]. Pro-inflammatory M1 macrophages are dominant in the diabetic wound bed with few or no anti-inflammatory M2 macrophages existing [50]. M1 macrophages secrete proinflammatory cytokines involving IL-1β, IL-6, IL-12, IL-18, IL-23, and TNF that retain an inflammatory state. Among these cytokines, IL-1β, IL-6, and TNF attract monocytes circulating in the blood system to the inflammatory site. And then, the expression of IFN-γ is induced by IL-12 and IL-18, which converts monocytes to the M1 macrophage phenotype in a positive feedback loop (Fig. 2A) [[15], [16], [17],51]. Therefore, a strong tendency for M1 macrophage polarization and accumulation brings on M1/M2 imbalance, stagnating diabetic wound healing process in the inflammatory phase instead of moving on to the proliferation phase.
Fig. 2.
Scheme of reasons for impaired M1/M2 transition in diabetic wound healing, it includes (A) a positive feedback loop of M1 accumulation, (B) impaired M1 phagocytotic activity, and (C) more senescent M1. (A) Diabetic wounds are usually stuck in an excessive inflammatory state with IL-1β, IL-6, TNF, IL-12, IL-18, etc. Among these inflammatory molecules, IL-1β, IL-6, and TNF are signals that recruit monocytes to the wounded region. Subsequently, the recruited monocytes polarize to the M1 phenotype with the guide of IL-12 and IL-18 in the wound bed and secrete monocyte recruiting signals. (B) M1 in normal wound healing is robust with a powerful capacity of clearing apoptotic cell debris and the process of phagocytosis aids the occurrence of M1/M2 transition. M1 in diabetic wound healing is abnormal with decreased receptors for phagocytosis, which leads to limited phagocytotic capacity and reduced M2 polarization. (C) Compared to the spontaneous occurrence of M1/M2 transition in normal wound healing, M1 macrophges are abnormal with more senescent phenotypes, accompanied by shrinking plasticity that hinders M1/M2 transition. Senescent-Associated Secrectory Profile (SASP) is a significant feature of senescent cells.
M1 macrophages in diabetic wounds are abnormal. Normally, M1 macrophages are robust in the acute inflammation process. They have a powerful phagocytotic capacity to clear apoptotic cells, which is known as efferocytosis. What's more, efferocytosis is involved in switching macrophages from pro-inflammatory M1 phenotype to anti-inflammatory M2 phenotype. Nevertheless, constant high glucose levels in diabetes hinder phagocytotic activities of M1 macrophages by downregulating the expression of CD36 and Class B Scavenger type Ⅰ receptors that are crucial for phagocytosis. This impaired phagocytotic behavior is correlated with preventing M1/M2 phenotype turnover in diabetic wound healing (Fig. 2B) [[52], [53], [54], [55]]. In addition to defective phagocytosis, macrophages are prone to convert into senescent phenotype with the expression of a senescence-associated secretory profile (SASP) when they are activated in the diabetic wound environment. The senescent macrophage phenotype presents shrinking plasticity making M1/M2 transition harder (Fig. 2C) [56].
Moreover, abnormal M1 macrophages and impaired M1/M2 transition are also associated with deficient angiogenesis, epithelialization, and matrix deposition in diabetic wound healing. Since anti-inflammatory M2 phenotype is more supportive to angiogenesis as an important source of vascular endothelial growth factor (VEGF), the impaired M1/M2 transition in diabetic wound healing is linked to angiogenesis deficiency [[57], [58], [59]]. While M2 macrophages contribute to initiating re-epithelialization by producing epidermal growth factor (EGF) and keratinocyte growth factor (KGF), M1 macrophages hinder keratinocyte migration with over-expressed TNF-α [60,61]. Meanwhile, M1 macrophages produce a large number of proinflammatory cytokines to prompt overabundant metalloproteinases from fibroblasts in diabetic wound healing, which result in the breakdown of collagen fibers [62]. By contrast, IL-10 released from M2 macrophages gives rise to collagen deposition in the correct wound healing cascade [63]. Overall, M1/M2 transition is closely related to cellular or non-cellular components in diabetic wound healing.
4. Strategies for enhancing diabetic wound healing
As discussed above, we summarise some strategies here aiming to enhance diabetic wound healing. At the early time of wound injury, inhibiting inflammation response to avoid the evolvement of excessive and persistent inflammation pattern from an inadequate acute inflammation pattern supports diabetic wound healing. However, wound injury is hard to be perceived attributed to sensory neuropathy in diabetes [64]. As a result, patients often suffer from an excessive and persistent inflammation pattern when they hospitalize and miss the best time for treatment. When a persistent and excessive inflammatory pattern is settled, there are another two paths leading to the proliferation phase. One path is changing chronic wounds to acute, restoring and normalizing the capacity of boosting an acute inflammation response. Through this strategy, abnormal M1 macrophages rejuvenate into robust ones that are capable of polarizing to M2 phenotypes spontaneously and smoothly. Another path is seeking switches for M1/M2 transition that directly function on macrophages. In the following text, we will discuss these strategies in detail (Fig. 1B).
4.1. Inhibiting proinflammatory response
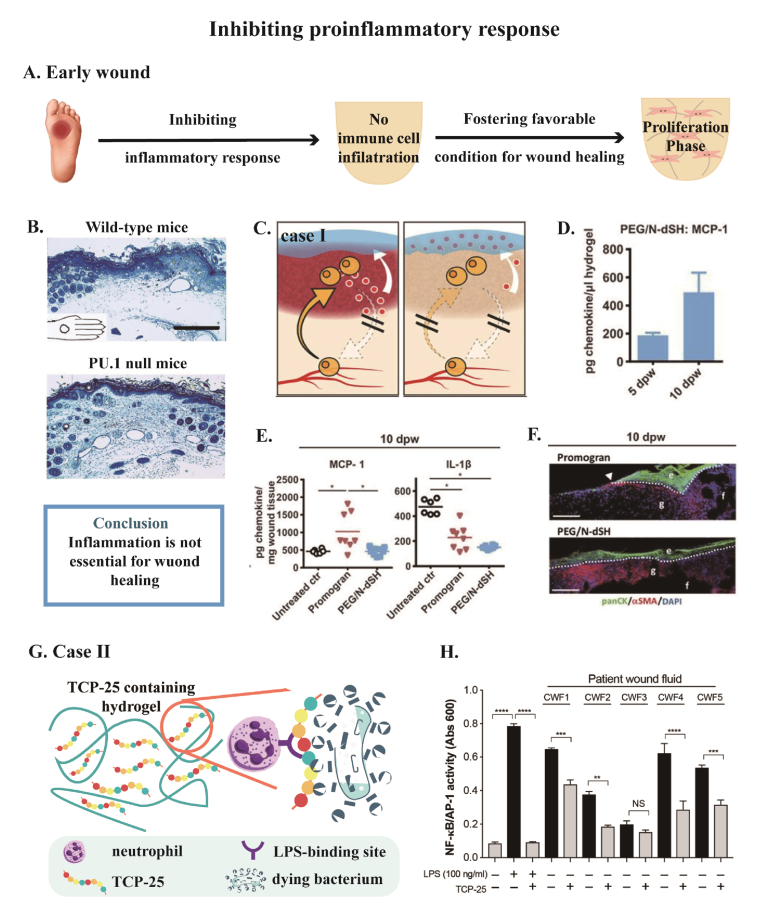
Although timely and appropriate inflammation is important for wound healing, evidence has shown inflammation is not essential for wound healing. For example, Martin et al. conducted wound healing studies in PU.1 null mice, whose neutrophils and macrophages were genetically depleted, were able to heal at a statistically similar time course to wild-type lineages, but they were scarless as in the fetal skin wound healing (Fig. 3B) [65]. Dovi et al. have shown that neutrophil-depleted diabetic wound closure was accelerated by nearly 50% with control animals [66].
Fig. 3.
(A) Inhibiting proinflammatory response at the early diabetic wound to avoid subsequent persistent and excessive immune process fosters a favorable condition for wound healing, thus wound healing process enters the proliferation phase. (B) Full-thickness excisional wound with a diameter of 2 mm is made in wide-type mice and PU.1 null mice. Macrophages and neutrophils are depleted genetically in PU.1 null mice. Wild-type mice and PU.1 null mice show similar time course of wound repair at 7 days post-wounding. Especially, tissue repair in PU.1 mice is more effective and appears scar-free. (C–F) and (G–H) are two cases about designing biomaterials for inhibiting proinflammatory response. (C) Resident immune cells establish a chemokine gradient that attracts neutrophils and monocytes from circulating system. A hydrogel that captures chemoattractant (MCP-1 and IL-β) is designed for cutting off the signal for neutrophil and monocyte infiltration. (D–F) Full-thickness injury in db/db mice treated with promogran (FDA-approved hydrogel-based wound dressing) or PEG/N-dSH (the chemoattractant-capturing hydrogel). (D) Concentration of MCP-1 in PEG/N-dSH hydrogel increases from 5 day to 10 day post-wounding (dpw). (E) Concentrations of MCP-1 and IL-1β in wound bed at 10 dpw suggest alleviated inflammatory response during wound healing. e, epidermis; f, fat tissue; g, granulation tissue. Arrowhead points to epithelial tip. (F) Immunofluorescence staining of newly formed tissue indicates faster reepithelialization with the treatment of PEG/N-dSH hydrogel. (G) The dual-action TCP-25 peptide contained in the hydrogel, not only binds to neutrophils, making neutrophils inaccessible to bacteria, but also has the ability to kill the bacteria itself. (H) In the presence of TCP-25 containing hydrogel, LPS-induced inflammatory response is reduced in chronic wound fluid from five patients. Scale bar: 100 μm for (B), 200 μm for (F). Reprinted with permission from Ref.65. Copyright 2003 Elsevier. Reprinted with permission from Ref.67. Copyright 2017 The American Association for the Advancement of Science. Reprinted with permission from Ref.68. Copyright 2020 The American Association for the Advancement of Science.
Cause the wound healing process is not dependent on neutrophils and macrophages, inhibiting inflammation response at the early time of wound injury avoids subsequent persistent and excessive inflammation, fostering a favorable condition for wound healing (Fig. 3A). Franz et al. designed a modular hydrogel based on end-functionalized star-shaped polyethylene glycol (starPEG) and derivatives of the glycosaminoglycan (GAG) heparin to capture inflammatory chemokines (MCP-1, IL-8) released by tissue-resident immune cells in wound fluids. Seeing that MCP-1 and IL-8 established a chemoattractant gradient at the wound site which facilitated the invasion of blood-derived immune cells, binding inflammatory chemokines with GAG heparin blocked the signal for blood-derived immune cells infiltration and safeguarded the wound healing process against the positive feedback loop of M1 accumulation (Fig. 3C–F) [67]. Puthia et al. prepared a TCP-25 peptide containing hydrogel. TCP-25, a thrombin-derived peptide, inhibits neutrophils to react to LPS by combining the LPS-binding hydrophobic pocket of CD14. In parallel with the inactivation of LPS, TCP-25 is antibacterial so it is prospective in the application of susceptible chronic wound environments (Fig. 3G). It has been proved that TCP-25 lessened LPS-induced inflammatory response to chronic wound fluid obtained from five patients (Fig. 3H) [68].
Mechanically, attempts to inhibit proinflammatory response in diabetic wound healing mimic the fetal-skin wound healing process, which is characterized by minimal to no inflammation and accelerated re-epithelialization [69]. Given that redundant growth factors brought by immune cells cause pathological matrix deposition and scar formation, the absence of immune cells fosters supportive physiological condition for fetal-skin wound healing [[70], [71], [72]]. Meanwhile, it has been proved that exogenous growth factors activate fibroblasts and induce scar formation in fetal skin wound healing [73,74]. In addition, since there is no influx of macrophages, fibroblasts work as an alternative to engulf and clear cell debris and thus contributes to favorable regenerative condition [75].
4.2. Changing chronic wounds to acute
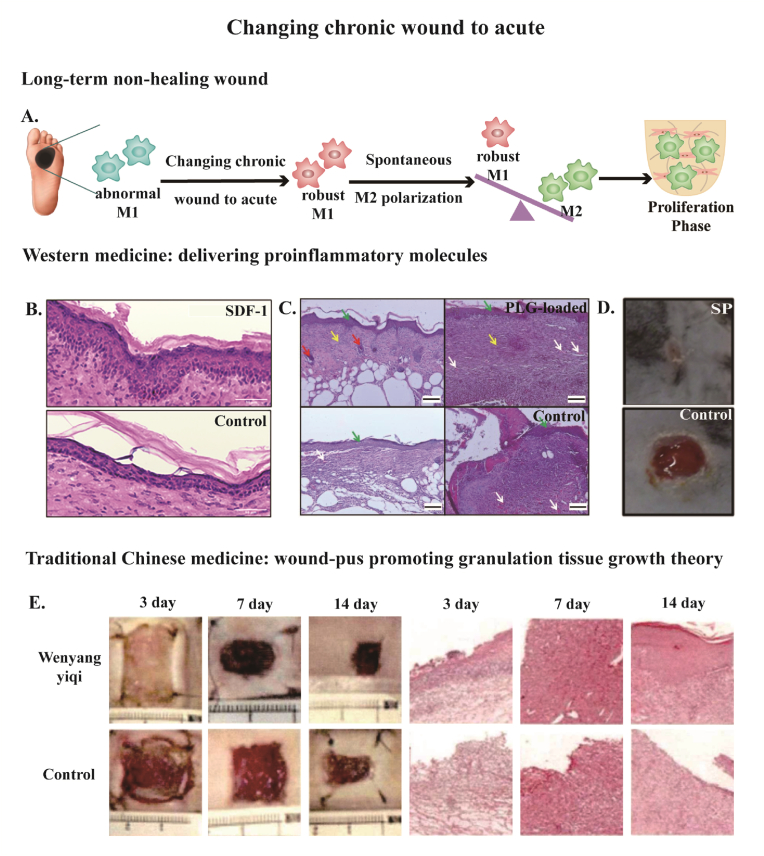
Owing to unnoticeable diabetic wounds, patients are always suffering from long-term non-healing wounds featuring excessive and persistent inflammation pattern when they are hospitalized. Thus, the treatment of diabetic wounds needs some other strategies and changing chronic wounds to acute is one of the resolutions. The essence of changing chronic wounds to acute is restoring abnormal M1 macrophages in chronic wounds and recovering acute inflammation response with robust M1 macrophages which can polarize to M2 phenotype smoothly, making the wound healing process move towards the proliferation phase (Fig. 4A).
Fig. 4.
(A) Scheme of changing chronic wound to acute in the long-term non-healing wound, which aims to normalize M1 macrophages and make the spontaneous M1/M2 transition. (B–D) Delivering proinflammatory molecules such as (B) SDF-1, (C)PLG, and (D) SP in western medicine strategy rescued the non-healing diabetic wound. (B) Samples at day 28 post-wounding. Scale bar: 50 μm. (C) Samples at day 14 post-wounding. Green arrow pointed to epithelialization, white arrow pointed to mild angiogenesis, red arrow pointed to hair follicle, yellow arrow pointed to connective tissue. Scale bar: 100 μm. (D) Samples at day 10 post-wounding. (E) A full-thickness injury measuring 1.8 cm*1.8 cm was created in diabetic mice and treated with a traditional Chinese medicine wound dressing, Wenyangyiqi formula. The H&E staining (100 magnifications) showed more immune cell infiltration at day 3 and day 7 treated with Wenyangyiqi formula. Few newly vessels, collagen fibers, and fibroblasts appeared at day 7 treated with Wenyagyiqi formula. In addition to alleviated inflammation, mature epithelial layer were observed at day 14 treated with Wenyangyiqi formula. Reprinted with permission from Ref.83. Copyright 2021 American Chemical Society. Reprinted with permission from Ref.88. Copyright 2022 MDPI. Reprinted with permission from Ref.45. Copyright 2015 Elsevier. Reprinted with permission from Ref.96. Copyright 2021 CNKI.
To normalize acute inflammation response, western medicine and traditional Chinese medicine have different therapies. Western medicine delivers pro-inflammatory molecules that are essential for proceeding with the wound healing process [76]. Traditional Chinese medicine has developed a theory of “wound-pus promoting granulation tissue growth theory” for thousands of years [77].
Among western medicine strategies, pro-inflammatory molecules such as stromal-derived factor (SDF-1), plasminogen (PLG), and substance P (SP) are good candidates for boosting acute inflammation and rescuing non-healing diabetic wound healing (Fig. 4B–D).
SDF-1 is a pro-inflammatory cytokine and a member that belongs to the CXC chemokine subfamily, which is also called CXCL12 [78,79]. Downregulation of SDF-1 level is one of the factors that brings on impaired diabetic wound healing [80]. Liechty et al. has proved that inhibition of SDF-1 further impeded diabetic wound healing but overexpression of SDF-1 reserved this abnormal process [81]. Tabata et al. has designed a dual-release gelatin hydrogel with a rapid release pattern of SDF-1 and a controlled release pattern of a macrophage recruitment agent. After the dual-release gelatin hydrogel was implanted into the diabetic wound site, macrophages recruited to the hydrogel expressed more pro-inflammatory cytokines (IL-6 and TNF-α) at day 1, and more anti-inflammatory cytokines (IL-10) at day 3. Finally, diabetic wounds treated with this dual-release hydrogel healed at a faster rate [82]. Fisher et al. delivered Liposal SDF-1α in nanocomposite hydrogel as a methodology for treating diabetic wounds. With this methodology, dominant macrophages with an anti-inflammatory phenotype were observed, along with an improved wound closure rate (Fig. 4B) [83].
PLG is a pivotal pro-inflammatory mediator that binds to immune cells migrating to damaged tissues [84]. Previous study has indicated that PLG-deficient mice had an impaired wound healing process [85]. It not only triggers but also plays an imperative role in terminating the inflammatory phase at the right time [86]. PLG rejuvenates macrophages by augmenting their phagocytosis, which is important for spontaneous M1/M2 turnover [87]. Losi et al. fabricated a PLG-loaded fibrin scaffold as a drug delivery system to treat diabetic wounds. The in-vitro release pattern demonstrated a burst of release within 3 days and then slowed down, reaching a plateau at 7 days. A pronounced effect of reduced open wound area was observed in full-thickness diabetic wounds applied with the PLG-loaded fibrin scaffold at 14 days (Fig. 4C) [88].
SP is a tachykinin neuropeptide family member that is made up of 11 amino acids [89]. The expression of SP is downregulated in diabetic wounds, which is connected with an absence of acute inflammatory response [90]. Veves et al. has shown that delivery of exogenous SP potentiated acute inflammatory response to diabetic skin injury with a peaked M1/M2 ratio at day 3, but the persistent inflammatory was solved with an obvious drop in M1/M2 ratio at day 10 (Fig. 4D) [45].
In traditional Chinese medicine, “wound-pus promoting granulation tissue growth theory” is a unique and curative strategy that has been summarized by practice for thousands of years [91]. It is about traditional Chinese herbal wound dressing that stimulates deathly pale diabetic wounds to suppurate with weeping pus, which suggests a change from chronic wounds to acute and removal of necrotic tissues [92]. When the evil rot has gone, wound exudates provide a nutritious and moist microenvironment that promotes healing under the medicine-wound interaction mechanism [93,94]. With an observable and elevated level of VEGF and TGF-β, the appearance of granulation comes after [95].
The underlying mechanism of “wound-pus promoting granulation tissue growth theory” is regulating the dysfunctioned immune response in diabetic wound healing progress. Zhang et al. treated diabetic wounds with Wenyang Yiqi formula, a typical ointment for “simmering wound-pus and promoting granulation growth”. Compared with the non-treating group, Wenyang Yiqi formula improved local inflammatory response with raised immune cell infiltration at day 3 and day 7 after medication, but the inflammation was alleviated at day 14 which indicated a controlled pro-inflammatory response (Fig. 4E) [96]. In addition, more robust macrophages with improved phagocytotic behavior are closely related to M1/M2 polarization in wound-pus [97]. Previous study has also shown the practice of “wound-pus promoting granulation tissue growth theory” lowered the M1/M2 ratio at 7 days after treatment [98].
4.3. Switches for M1/M2 transition
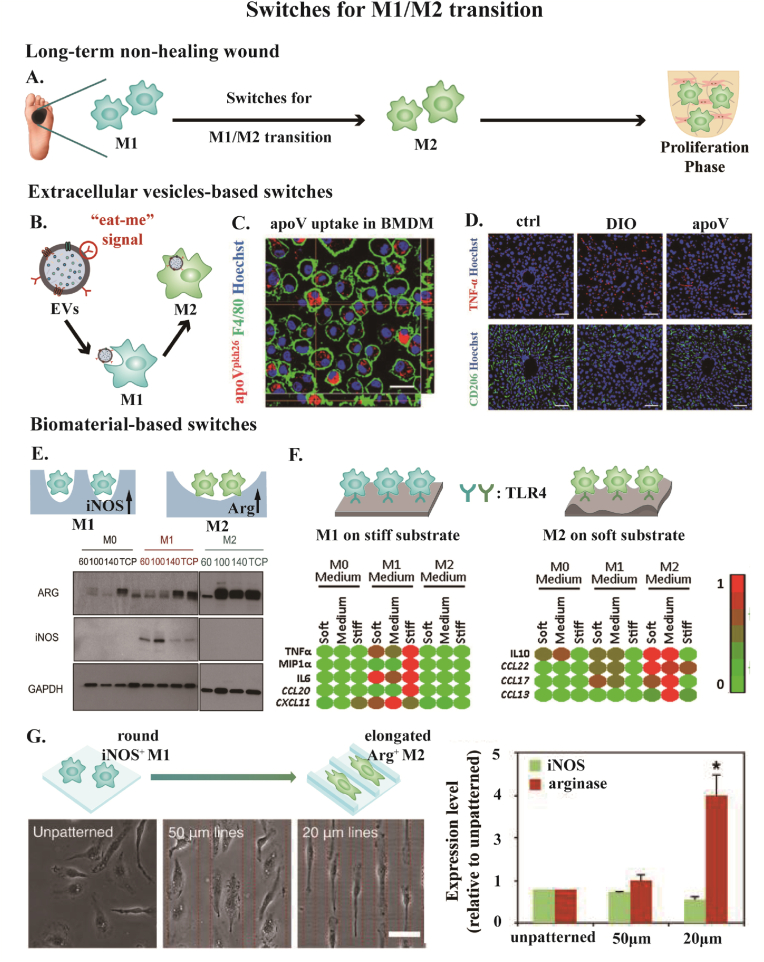
Normal wounds proceed with M1/M2 transition around 3 days after wounding [14]. M1 macrophages are classically activated, known as pro-inflammatory phenotype, whereas M2 macrophages are alternatively activated, known as anti-inflammatory phenotype that serves as a resolution of inflammation [99]. However, the M1/M2 transition is hindered in diabetic wounds with altered macrophage function [100]. Therefore, seeking switches guiding macrophages to shift from M1 to M2 phenotype is hopeful to save the dysregulated immune process (Fig. 5A). Switches for M1/M2 transition can be Extracellular Vesicles (EVs), biomaterial-based physical signals, and so on.
Fig. 5.
(A) Scheme of switches for M1/M2 transition to enhance diabetic wound healing. (B–D) Extracellular vesicles (EVs)-based switches. (B) Calreticulin exposed on the surface of EVs functions as “eat me” signal. The uptake of EVs guides the reprogramming of M1 macropahge to M2 phenotype. (C) The uptake of apoptotic vesicles (apoV, a member belonging to EVs family) by bone marrow-derived macrophage (BMDM). Scale bar: 20 μm (D) The treatment of apoV reprogrammed M1 to M2 phenotype in diabetic mice. Ctrl, control mice. DIO, diet-induced obesity mice. apoV, DIO mice treated with apoV. Scale bar: 50 μm. (E–H) Biomaterial-base switches for M1/M2 transition including (E) pores, (F) stiffness, and (G) surface topography. (E) Electrospun with varying concentrations of polydioxanone (60,100,140 mg/ml), higher concentration generated larger fiber with increased pore size. Macrophages prime to express more M1 marker iNOS in smaller pores and more M2 marker Arg in larger pores. (F) Macrophages prime pro-inflammatory phenotype on stiffer substrate and anti-inflammatory phenotype on softer substrate. Macrophages acquired the mechanical information of biomaterials by TLR4. M0 medium: with no induction factor. M1 medium: IFN + LPS induction. M2 medium: IL4+IL13 induction. TNF, MIP1α, IL-6, CCL20, and CXCL11 are pro-inflammatory expressions. IL10, CCL22, CCL17, and CCL13 are anti-inflammatory expressions. (G) Macrophages prime rounded M1 phenotype with the expression of iNOS on unpatterned surface and elongated M2 phenotype with the expression of Arg on microgrooved surface. Scale bar: 50 μm. Reprinted with permission from Ref.106. Copyright 2021 John Wiley and Sons. Reprinted with permission from Ref.111. Copyright 2013 Elsevier. Reprinted with permission from Ref.117. Copyright 2019 Elsevier. Reprinted with permission from Ref.123. Copyright 2013 NAS.
EVs are bilayered lipid membrane vesicles produced by nearly all types of cells into the extracellular space, which consists of three main subtypes: apoptotic bodies, exosomes, and microvesicles [101,102]. EVs exert biological functions as important messengers that transfer bioactive molecules involving proteins, lipids, and miRNA toward recipient cells [103,104]. The interaction via EVs and macrophages also modulates the phenotype switching process [105]. Jin et al. has conducted a series of studies to prove that efferocytosis of EVs by macrophage effectively improved the M2 polarization. It has been found that calreticulin exposed on the surface of apoptotic bodies derived from mesenchymal stem cells acted as an “eat-me” signal to guide macrophage efferocytosis. The uptake of apoptotic bodies reprogrammed macrophages at a translational level towards an anti-inflammatory phenotype (Fig. 5B–D) [106]. In another study, T cell-derived apoptotic bodies targeted macrophages in the inflammatory region due to the specific engulfment conducted by the presence of phosphatidylserine on the surface. This specific engulfment ameliorated the M1/M2 transition [107]. Yang et al. has shown melatonin-stimulated MSC-derived exosomes were appropriate options for diabetic wound healing because they increased the ratio of macrophages that polarized from M1 to M2 phenotype [108].
Utilizing biomaterial-based physical signals as switches for macrophage polarization has become an exciting topic. Factors including pores, substrate stiffness, and surface topography of biomaterials all have a significant impact on modulating macrophage behavior (Fig. 5E–G).
Increasing evidence has confirmed macrophages are prone to perform a pro-inflammatory phenotype with small pores and an anti-inflammatory phenotype with large pores. The underlying mechanism is small pores are physical confinements that suppress macrophage phagocytosis, which further affects macrophage polarization by regulating a STAT-related mRNA transcription pathway [109,110]. Bowlin et al. fabricated Polydioxanone scaffolds with different pore sizes by adjusting polymer concentration. With increasing pore size, bone marrow-derived macrophages expressed more M2 marker Arg1 and decreased M1 marker iNOS (Fig. 5E) [111]. Du et al. investigated the effects of varying pores with the same stiffness by decoupling the control of pores and stiffness. The result has demonstrated that macrophages tended to be M1 phenotype and M2 phenotype in small pores (30 μm) and large pores (80 μm) respectively [112]. Chen et al. fabricated genipin cross-linked collagen/chitosan (Col-Ch) scaffolds with pore sizes ranging 160 and 360 μm. Macrophages seeded on the scaffold with large pores expressed more M2-related cytokines on day 7. Taking conditional medium (CM) derived from macrophage-scaffold cultures to incubate human umbilical vein endothelial cells, it has been shown that CMs obtained from large pore-seeded macrophages demonstrated greater capacity to enhance new vessel formation [113]. Since impaired angiogenesis is one of the primary contributors to poor diabetic wounding, M1/M2 transition mediated by pore-size is a potential methodology for not only making the inflammation phase proceed to the proliferation phase but also creating a pro-angiogenesis condition for regeneration.
Macrophages are sensitive to substrate stiffness, which directs macrophage polarization state and function. In particular, macrophages in diabetic hyperglycemia conditions are more mechanosensitive [114]. The signaling pathways engaging in stiffness-regulated inflammation include Toll-like receptor 4 (TLR4), a specific surface receptor on macrophages, and Transient Receptor Potential Vanilloid 4 (TRPV4), a mechanosensitive ion channel [115,116]. With a stiffer substrate, macrophages have a stronger inclination to pro-inflammatory phenotype. Brien et al. cultured THP-1 derived macrophages on collagen-coated polyacrylamide gels with varying stiffness. Macrophages exhibited the pro-inflammatory phenotype with defective phagocytosis when cultured on stiff gels (323 kPa), and the anti-inflammatory phenotype with powerful phagocytosis when cultured on medium gels (88 kPa) and soft gels (11 kPa) (Fig. 5F) [117]. Wang et al. engineered methacrylate-gelatin (GelMA) hydrogels with varying compression modulus serving as soft, medium, and stiff matrices. With increased stiffness, macrophages exhibited more dispersed F-actin cytoskeleton filaments and tended to polarize towards the M1 phenotype with more iNOS expression. Conversely, macrophages in softer matrix primed to M2 phenotype with upregulated Arg-1 expression for inflammation resolution [118]. Ma et al.designed a novel biomaterial with NIR-triggered dropping stiffness. This stiffness-degradation strategy elicited macrophages to transfer from pro-inflammatory state to anti-inflammatory state [119]. According to the considerable studies focusing on the biological effects of stiffness on macrophages, stiffness-mediated macrophage polarization is reproducible and explicit, which provides new insights into advancing biomaterials for potential administration of diabetic wound healing in the near future.
Surface topography is a depiction of surface orientation and roughness, which features a succession of peaks and valleys [120]. Surface topography confers biomaterials with bioactive functions such as regulating macrophage phenotype as biophysical switches. Considering macrophage polarization is strongly linked to cytoskeletal rearrangement, growing studies have been deep into it and implicated that surface topography induces macrophage elongation to prime an anti-inflammatory phenotype [121]. Liu et al. designed a polycaprolactone film that transformed from flat to microgroove under near-infrared (NIR) irradiation. Accompanied by a change of surface topography under NIR irradiation, the morphology of macrophages transformed from round to elongated, along with an M1/M2 transition [122]. Sanders et al. controlled the shape of macrophages directly by micropatterns. It was found that elongation of morphology contributed M2 phenotype without the stimulation of cytokines (Fig. 5G) [123]. Snedeker et al. engineered aligned and randomly distributed polycaprolactone nanofiber surfaces to investigate fiber organization-driven macrophage polarization. Macrophages cultured on aligned and random fibrous surfaces demonstrated more elongated and more spreading morphology, respectively Quantification qPCR confirmed that macrophages expressed higher level of CD206 (M2 marker) and lower level of CD197 (M1 marker) on surfaces with aligned distributed fiber, which is in accordance with morphology differences resulted by surface topography [124]. In addition, roughness, as one of the governing variables of surface topography, also has the power to regulate the M1 to M2 transition, in which macrophages prime M2 phenotype on the rough surface rather than the smooth one [125]. Lee et al. designed heparin-doped polypyrrole substrates of different surface roughness, with Ra values ranging from 5.5 to 17.6 nm. The study provided empirical evidence that macrophages cultured on rougher substrate exhibited an inflammation profile with lower iNOS/Arg1 ratio, which indicated a higher proportion of pro-healing M2 phenotype [126].
5. Conclusion and perspectives
Diabetic wounds are difficult to heal because of pathological conditions in diabetes. The ability to mount an inflammatory response is deprived in early-wound inflammation pattern but an excessive and persistent inflammatory pattern is evolving along with time. M1 macrophages accumulate in a positive feedback loop and this strong inclination of M1 polarization makes long-term excessive inflammation infiltration. Besides, M1 macrophages in chronic diabetic wound environments are abnormal, with defective phagocytosis and increased senescent phenotype that impair the M1/M2 transition.
In this review, we summarise some strategies to rescue the impaired diabetic wound healing process from the perspective of spatial inflammation patterns. To avoid the evolvement of excessive and persistent inflammation pattern in long-term non-healing wounds from the deficient acute inflammation pattern in early wounds, inhibiting pro-inflammatory response is introduced upon early injuries. But diabetic wounds are hard to be perceived due to the neuropathy, so if patients have missed the best time for treatment and wounds have been stalled in the inflammatory phase, it needs new approaches. To restore abnormal macrophages in diabetic wounds and normalize acute inflammatory response, western medicine delivers pro-inflammatory molecules while traditional Chinese medicine proposes the theory of “wound-pus promoting granulation tissue growth”. Both western and traditional Chinese medicine aim to activate a controllable pro-inflammatory response. The last strategy is modulating macrophage polarization through biophysical cues from biomaterials. In future studies, more efforts need to be put into exploring new strategies that satisfy the clinical necessities precisely and turning theories into practice. All in all, drawing a timeline based on spatial inflammation patterns gives inspirations to enhance diabetic wound healing for precision medicine.
Last but not least, there is still a long way to develop synergistic treatment mode for improving non-healing diabetic wounds. Current therapy usually functions on a single target, ignoring the interactions among all pathological factors. For example, some wound dressings provide natural ECM and deliver growth factors for wound healing, but it doesn't suppress the activity of metalloproteinases, so the therapeutic effect is limited [127,128]. Some studies scavenge ROS to alleviate oxidative stress in diabetic wounds but the continuous generation of ROS induced by bacteria infection and hyperglycemia is not taken into consideration [[129], [130], [131]]. Even though some studies aim to deliver multiple drugs to ensure combined pro-regenerative functions, the drug-releasing profiles fail to follow the real-time wound healing cascade. In this scenario, constructing a spatiotemporal controlled release system is potential to meet the requirements of elaborate wound healing process [132]. All in all, developing new treatments that can function on pathogenic molecules and cells with multiple targets and get involved in the wound healing cascade is of great significance.
Ethics approval
This is a review article, so animal experiment has not been conducted.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors have no conflicts to disclose.
Acknowledgements
Support from the National Natural Science Foundation of China (31922041, 11932012, 32171341), National key research and development program (2021YFB3800800), the 111 project (B14018), the Science and Technology Innovation Project and Excellent Academic Leader Project of Shanghai Science and Technology Committee (21S31901500, 21XD1421100) are acknowledged.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.WHO Diabetes 2022. https://www.who.int/news-room/fact-sheets/detail/diabetes
- 2.Ouyang W., Jia Y., Jin L. Risk factors of diabetic foot ulcer in patients with type 2 diabetes: a retrospective cohort study. Am. J. Tourism Res. 2021;13 8:9554–9561. [PMC free article] [PubMed] [Google Scholar]
- 3.Fridman R., Rafat P., Van Gils C.C., Horn D., Vayser D., Lambert J.C., Jr. Treatment of hard-to-heal diabetic foot ulcers with a hepatic-derived wound matrix. Wounds : a compendium of clinical research and practice. 2020;32:244–252. [PubMed] [Google Scholar]
- 4.Thanapaul R., Shvedova M., Shin G.H., Roh D.S. An insight into aging, senescence, and their impacts on wound healing. Advances in geriatric medicine and research. 2021;3 doi: 10.20900/agmr20210017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould L.J., Fulton A.T. Wound healing in older adults. R. I. Med. J. 2013;99(2016):34–36. [PubMed] [Google Scholar]
- 6.Pinto A., Cerqueira M., Bañobre-López M., Pastrana L., Sillankorva S. Bacteriophages for chronic wound treatment: from traditional to novel delivery systems. Viruses. 2020;12:235. doi: 10.3390/v12020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landén N.X., Li D., Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell. Mol. Life Sci. 2016;73:3861–3885. doi: 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M., Hou Q., Zhong L., Zhao Y., Fu X. Macrophage related chronic inflammation in non-healing wounds. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.681710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J., Zhou H., Gerhard E.M., Zhang S., Parra Rodríguez F.I., Pan T., Yang H., Lin Y., Yang J., Cheng H. Smart bioadhesives for wound healing and closure. Bioact. Mater. 2023;19:360–375. doi: 10.1016/j.bioactmat.2022.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matoori S., Veves A., Mooney D.J. Advanced bandages for diabetic wound healing. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abe4839. [DOI] [PubMed] [Google Scholar]
- 11.Hannoodee S., Nasuruddin D.N. StatPearls, StatPearls Publishing Copyright ©. StatPearls Publishing LLC; Treasure Island (FL): 2022. Acute inflammatory response. 2022. [PubMed] [Google Scholar]
- 12.Sawaya A.P., Stone R.C., Brooks S.R., Pastar I., Jozic I., Hasneen K., O'Neill K., Mehdizadeh S., Head C.R., Strbo N., Morasso M.I., Tomic-Canic M. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat. Commun. 2020;11:4678. doi: 10.1038/s41467-020-18276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood S., Jayaraman V., Huelsmann E.J., Bonish B., Burgad D., Sivaramakrishnan G., Qin S., DiPietro L.A., Zloza A., Zhang C., Shafikhani S.H. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louiselle A.E., Niemiec S.M., Zgheib C., Liechty K.W. Macrophage polarization and diabetic wound healing. Transl. Res. : J. Lab. Clin. Med. 2021;236:109–116. doi: 10.1016/j.trsl.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Mirza R.E., Fang M.M., Ennis W.J., Koh T.J. Blocking interleukin-42 induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–2587. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goren I., Müller E., Schiefelbein D., Christen U., Pfeilschifter J., Mühl H., Frank S. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J. Invest. Dermatol. 2007;127:2259–2267. doi: 10.1038/sj.jid.5700842. [DOI] [PubMed] [Google Scholar]
- 17.Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00491. 491-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehak L., Giurato L., Meloni M., Panunzi A., Manti G.M., Uccioli L. The immune-centric revolution in the diabetic foot: monocytes and lymphocytes role in wound healing and tissue regeneration-A narrative review. J. Clin. Med. 2022;11:889. doi: 10.3390/jcm11030889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel S., Srivastava S., Rawat Singh M., Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108615. [DOI] [PubMed] [Google Scholar]
- 20.Lv F., Wang J., Xu P., Han Y., Ma H., Xu H., Chen S., Chang J., Ke Q., Liu M., Yi Z., Wu C. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017;60:128–143. doi: 10.1016/j.actbio.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Smith S.A., Travers R.J., Morrissey J.H. How it all starts: initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015;50:326–336. doi: 10.3109/10409238.2015.1050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerris E.W.J., Hoptay C., Calderon T., Freishtat R.J. Platelets and platelet extracellular vesicles in hemostasis and sepsis. J. Invest. Med. 2020;68:813. doi: 10.1136/jim-2019-001195. [DOI] [PubMed] [Google Scholar]
- 23.Locatelli L., Colciago A., Castiglioni S., Maier J.A. Platelets in wound healing: what happens in space? Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.716184. 716184-716184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis S., Lin E.J., Tartar D. Immunology of wound healing. Current dermatology reports. 2018;7:350–358. doi: 10.1007/s13671-018-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinke J.M., Sorg H. Wound repair and regeneration. European surgical research. Europaische chirurgische Forschung. Recherches chirurgicales europeennes. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 26.Kim M.H., Liu W., Borjesson D.L., Curry F.R., Miller L.S., Cheung A.L., Liu F.T., Isseroff R.R., Simon S.I. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J. Invest. Dermatol. 2008;128:1812–1820. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soehnlein O., Zernecke A., Eriksson E.E., Rothfuchs A.G., Pham C.T., Herwald H., Bidzhekov K., Rottenberg M.E., Weber C., Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00113. 113-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soehnlein O., Lindbom L., Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 30.Wei T., Guo T.-Z., Li W.-W., Hou S., Kingery W.S., Clark J.D. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. J. Neuroinflammation. 2012;9 doi: 10.1186/1742-2094-9-181. 181-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luger T.A., Lotti T. Neuropeptides: role in inflammatory skin diseases. J. Eur. Acad. Dermatol. Venereol. : JEADV. 1998;10:207–211. [PubMed] [Google Scholar]
- 32.Wernersson S., Pejler G. Mast cell secretory granules: armed for battle. Nat. Rev. Immunol. 2014;14:478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 33.Barman P.K., Koh T.J. Macrophage dysregulation and impaired skin wound healing in diabetes. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00528. 528-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie C., Liu C., Wu B., Lin Y., Ma T., Xiong H., Wang Q., Li Z., Ma C., Tu Z. Effects of IRF1 and IFN-β interaction on the M1 polarization of macrophages and its antitumor function. Int. J. Mol. Med. 2016;38:148–160. doi: 10.3892/ijmm.2016.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotwal G.J., Chien S. Macrophage differentiation in normal and accelerated wound healing. Results Probl. Cell Differ. 2017;62:353–364. doi: 10.1007/978-3-319-54090-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown B.N., Badylak S.F. In: Principles of Tissue Engineering. fourth ed. Lanza R., Langer R., Vacanti J., editors. Academic Press; Boston: 2014. Chapter 25 - the role of the host immune response in tissue engineering and regenerative medicine; pp. 497–509. [Google Scholar]
- 37.Kim J., Shin Y.-K., Kim K.-Y. Promotion of keratinocyte proliferation by tracheloside through ERK1/2 stimulation. Evid. base Compl. Alternative Med. 2018;2018 doi: 10.1155/2018/4580627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoukens G. In: Advanced Textiles for Wound Care. second ed. Rajendran S., editor. Woodhead Publishing; 2019. 5 - bioactive dressings to promote wound healing; pp. 135–167. [Google Scholar]
- 39.Guillamat-Prats R. The role of MSC in wound healing, scarring and regeneration. Cells. 2021;10 doi: 10.3390/cells10071729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsalamandris S., Antonopoulos A.S., Oikonomou E., Papamikroulis G.A., Vogiatzi G., Papaioannou S., Deftereos S., Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. European cardiology. 2019;14:50–59. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hicks C.W., Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr. Diabetes Rep. 2019;19 doi: 10.1007/s11892-019-1212-8. 86-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagihashi S., Mizukami H., Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Investig. 2011;2:18–32. doi: 10.1111/j.2040-1124.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi Y.-H., Moh S., Kim K.W. In: Textbook of Aging Skin. Farage M.A., Miller K.W., Maibach H.I., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. The role of neuropeptides in skin wound healing; pp. 1–14. [Google Scholar]
- 44.Theocharidis G., Veves A. Autonomic nerve dysfunction and impaired diabetic wound healing: the role of neuropeptides. Auton. Neurosci. 2020;223 doi: 10.1016/j.autneu.2019.102610. 102610-102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leal E.C., Carvalho E., Tellechea A., Kafanas A., Tecilazich F., Kearney C., Kuchibhotla S., Auster M.E., Kokkotou E., Mooney D.J., LoGerfo F.W., Pradhan-Nabzdyk L., Veves A. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 2015;185:1638–1648. doi: 10.1016/j.ajpath.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tellechea A., Leal E.C., Kafanas A., Auster M.E., Kuchibhotla S., Ostrovsky Y., Tecilazich F., Baltzis D., Zheng Y., Carvalho E., Zabolotny J.M., Weng Z., Petra A., Patel A., Panagiotidou S., Pradhan-Nabzdyk L., Theoharides T.C., Veves A. Mast cells regulate wound healing in diabetes. Diabetes. 2016;65:2006–2019. doi: 10.2337/db15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pradhan L., Nabzdyk C., Andersen N.D., LoGerfo F.W., Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expet Rev. Mol. Med. 2009;11 doi: 10.1017/S1462399409000945. e2-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y., Quan Y., Liu Y., Liu K., Li H., Jiang Z., Zhang T., Lei H., Radek K.A., Li D., Wang Z., Lu J., Wang W., Ji S., Xia Z., Lai Y. Hyperglycaemia inhibits REG3A expression to exacerbate TLR3-mediated skin inflammation in diabetes. Nat. Commun. 2016;7 doi: 10.1038/ncomms13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgess J.L., Wyant W.A., Abdo Abujamra B., Kirsner R.S., Jozic I. Diabetic wound-healing science. Medicina (Kaunas) 2021;57:1072. doi: 10.3390/medicina57101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louiselle A.E., Niemiec S.M., Zgheib C., Liechty K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021;236:109–116. doi: 10.1016/j.trsl.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Carmi Y., Voronov E., Dotan S., Lahat N., Rahat M.A., Fogel M., Huszar M., White M.R., Dinarello C.A., Apte R.N. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J. Immunol. 2009;183:4705–4714. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- 52.Aitcheson S.M., Frentiu F.D., Hurn S.E., Edwards K., Murray R.Z. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules. 2021;26:4917. doi: 10.3390/molecules26164917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morey M., O'Gaora P., Pandit A., Hélary C. Hyperglycemia acts in synergy with hypoxia to maintain the pro-inflammatory phenotype of macrophages. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khanna S., Biswas S., Shang Y., Collard E., Azad A., Kauh C., Bhasker V., Gordillo G.M., Sen C.K., Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009539. e9539-e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong X., Lee H.N., Kim S.H., Park S.A., Kim W., Cha Y.N., Surh Y.J. Myc-nick promotes efferocytosis through M2 macrophage polarization during resolution of inflammation. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 2018;32:5312–5325. doi: 10.1096/fj.201800223R. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson H.N., Clowes C., Banyard K.L., Matteuci P., Mace K.A., Hardman M.J. Elevated local senescence in diabetic wound healing is linked to pathological repair via CXCR2. J. Invest. Dermatol. 2019;139:1171–1181. doi: 10.1016/j.jid.2019.01.005. e6. [DOI] [PubMed] [Google Scholar]
- 57.Jetten N., Verbruggen S., Gijbels M.J., Post M.J., De Winther M.P.J., Donners M.M.P.C. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 58.Hassanshahi A., Moradzad M., Ghalamkari S., Fadaei M., Cowin A.J., Hassanshahi M. Macrophage-mediated inflammation in skin wound healing. Cells. 2022;11 doi: 10.3390/cells11192953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okonkwo U.A., DiPietro L.A. Diabetes and wound angiogenesis. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellis S., Lin E.J., Tartar D. Immunology of wound healing. Current dermatology reports. 2018;7:350–358. doi: 10.1007/s13671-018-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang S.M., Wu C.S., Chiu M.H., Wu C.H., Chang Y.T., Chen G.S., Lan C.E. High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-α: an important mechanism to delay the diabetic wound healing. J. Dermatol. Sci. 2019;96:159–167. doi: 10.1016/j.jdermsci.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Abas M., El Masry M., Elgharably H. In: Wound Healing, Tissue Repair, and Regeneration in Diabetes. Bagchi D., Das A., Roy S., editors. Academic Press; 2020. Chapter 19 - collagen in diabetic wound healing; pp. 393–401. [Google Scholar]
- 63.Joorabloo A., Liu T. Recent advances in nanomedicines for regulation of macrophages in wound healing. J. Nanobiotechnol. 2022;20:407. doi: 10.1186/s12951-022-01616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou X., Guo Y., Yang K., Liu P., Wang J. The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114662. [DOI] [PubMed] [Google Scholar]
- 65.Martin P., D'Souza D., Martin J., Grose R., Cooper L., Maki R., McKercher S.R. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr. Biol. : CB. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 66.Dovi J.V., He L.K., DiPietro L.A. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 2003;73:448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 67.Lohmann N., Schirmer L., Atallah P., Wandel E., Ferrer R.A., Werner C., Simon J.C., Franz S., Freudenberg U. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aai9044. [DOI] [PubMed] [Google Scholar]
- 68.Puthia M., Butrym M., Petrlova J., Strömdahl A.C., Andersson M., Kjellström S., Schmidtchen A. A dual-action peptide-containing hydrogel targets wound infection and inflammation. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aax6601. [DOI] [PubMed] [Google Scholar]
- 69.Wilgus T.A. Regenerative healing in fetal skin: a review of the literature. Ostomy/Wound Manag. 2007;53:16–31. quiz 32-3. [PubMed] [Google Scholar]
- 70.Wilgus T.A. Vascular endothelial growth factor and cutaneous scarring. Adv. Wound Care. 2019;8:671–678. doi: 10.1089/wound.2018.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tripathi S., Soni K., Agrawal P., Gour V., Mondal R., Soni V. Hypertrophic scars and keloids: a review and current treatment modalities. Biomedical Dermatology. 2020;4:11. [Google Scholar]
- 72.Lech M., Anders H.J. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 73.Lin R.Y., Sullivan K.M., Argenta P.A., Meuli M., Lorenz H.P., Adzick N.S. Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann. Surg. 1995;222:146–154. doi: 10.1097/00000658-199508000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilgus T.A., Ferreira A.M., Oberyszyn T.M., Bergdall V.K., DiPietro L.A. Regulation of scar formation by vascular endothelial growth factor. Lab. Invest. 2008;88:579–590. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin P., Dickson M.C., Millan F.A., Akhurst R.J. Rapid induction and clearance of TGFβ1 is an early response to wounding in the mouse embryo. Dev. Genet. 1993;14:225–238. doi: 10.1002/dvg.1020140309. [DOI] [PubMed] [Google Scholar]
- 76.Julier Z., Park A.J., Briquez P.S., Martino M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 77.Cheng Jiajun G.Y., Tang Lili, Feng Wenzhe. A randomized controlled trial of external treatment of traditional Chinese medicine to promote wound healing under the theory of simmering pus and growing meat: a systematic evaluation. Journal of Yunnan University of Traditional Chinese Medicine. 2019;42:26–32. [Google Scholar]
- 78.Salcedo R., Oppenheim J.J. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation. 2003;10:359–370. doi: 10.1038/sj.mn.7800200. [DOI] [PubMed] [Google Scholar]
- 79.Ogłodek E.A., Szota A.M., Just M.J., Moś D.M., Araszkiewicz A. The MCP-1, CCL-5 and SDF-1 chemokines as pro-inflammatory markers in generalized anxiety disorder and personality disorders. Pharmacol. Rep. : PR. 2015;67:85–89. doi: 10.1016/j.pharep.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Gallagher K.A., Liu Z.J., Xiao M., Chen H., Goldstein L.J., Buerk D.G., Nedeau A., Thom S.R., Velazquez O.C. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J. Clin. Investig. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bermudez D.M., Xu J., Herdrich B.J., Radu A., Mitchell M.E., Liechty K.W. Inhibition of stromal cell-derived factor-1α further impairs diabetic wound healing. J. Vasc. Surg. 2011;53:774–784. doi: 10.1016/j.jvs.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim Y.H., Tabata Y. Enhancement of wound closure by modifying dual release patterns of stromal-derived cell factor-1 and a macrophage recruitment agent from gelatin hydrogels. Journal of tissue engineering and regenerative medicine. 2017;11:2999–3013. doi: 10.1002/term.2202. [DOI] [PubMed] [Google Scholar]
- 83.Yu J.R., Varrey P., Liang B.J., Huang H.C., Fisher J.P. Liposomal SDF-1 alpha delivery in nanocomposite hydrogels promotes macrophage phenotype changes and skin tissue regeneration. ACS Biomater. Sci. Eng. 2021;7:5230–5241. doi: 10.1021/acsbiomaterials.1c01140. [DOI] [PubMed] [Google Scholar]
- 84.Shen Y., Guo Y., Mikus P., Sulniute R., Wilczynska M., Ny T., Li J. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood. 2012;119:5879–5887. doi: 10.1182/blood-2012-01-407825. [DOI] [PubMed] [Google Scholar]
- 85.Chan J.C.Y., Duszczyszyn D.A., Castellino F.J., Ploplis V.A. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am. J. Pathol. 2001;159:1681–1688. doi: 10.1016/S0002-9440(10)63015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fallah M., Viklund E., Bäckman A., Brodén J., Lundskog B., Johansson M., Blomquist M., Wilczynska M., Ny T. Plasminogen is a master regulator and a potential drug candidate for the healing of radiation wounds. Cell Death Dis. 2020;11 doi: 10.1038/s41419-020-2397-0. 201-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das R., Ganapathy S., Settle M., Plow E.F. Plasminogen promotes macrophage phagocytosis in mice. Blood. 2014;124:679–688. doi: 10.1182/blood-2014-01-549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al Kayal T., Buscemi M., Cavallo A., Foffa I., Soldani G., Losi P. Plasminogen-loaded fibrin scaffold as drug delivery system for wound healing applications. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graefe S., Mohiuddin S.S. StatPearls, StatPearls Publishing Copyright © 2022. StatPearls Publishing LLC; Treasure Island (FL): 2022. Biochemistry, substance P. [PubMed] [Google Scholar]
- 90.Pradhan L., Cai X., Wu S., Andersen N.D., Martin M., Malek J., Guthrie P., Veves A., LoGerfo F.W. Gene expression of pro-inflammatory cytokines and neuropeptides in diabetic wound healing. J. Surg. Res. 2011;167:336–342. doi: 10.1016/j.jss.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian X. Effect of Weiermai Ointment on Diabetic Foot Ulcer (Deficiency of Qi and Blood) by "simmering Pus and Growing Meat" Theory. Heilongjiang University of Chinese Medicine; 2021. [Google Scholar]
- 92.Sun H. 2022. The Clinical Observation on the Treatment of Chronic and Hard-To-Heal Wounds with the Method of "simmered Pus and Long Meat" to Guide the Dressing Change of Puffball. [Google Scholar]
- 93.Bao Yiru S.X., Lu Xuya, Zhang Chaohui, Zhu Chaojun. Discussion on the mechanism of medicine-wound interaction under the guidance of wound-pus promoting granulation tissue growth theory. Acta Chinese Medicine. 2022;37:516–519. [Google Scholar]
- 94.Jiang Yu-feng F.X.-b. Improving the healing microenvironment is the core concept in wound treatment for both traditional Chinese and Western medicine. Journal of Traumatic Surgery. 2019;21:241–243. [Google Scholar]
- 95.Zhu Chao-jun Z.Z.-h., Zhang Yang. Effect of weinongzhangrou method on expression of VEGF, Notch1 and TGFβ1 in granulation tissue of rats with chronic ulcer and its histopathology. Chinese Journal of Surgery of Integrated. 2020;26:8–14. [Google Scholar]
- 96.Wu L. Beijing University of Chinese medicine; CNKI: 2021. 基于Wnt/β-Catenin通路对回阳生肌膏治疗糖尿病大鼠阴证创面的作用机制研究. [Google Scholar]
- 97.Liu Xian-zhou Z.Z.-h., Zhu Zhao-jun. Discussion on simmer pus and cuddle pus promoting the regeneration. Academic Journal of Shanghai University of Traditional Chinese Medicine. 2017;31:7–8+23. [Google Scholar]
- 98.Chen Jia L.Q.-w., Xu-yang H.A.N., Liu Xin, Liu Yan, Ma Hui-ke, He Xiu-juan, Li Ping. vol. 37. 2021. pp. 1055–1106. (Effect of Huiyang-Shengji Decoction on Wound Healing of Chronic Skin Ulcer and Phenotypic Transformation of Mononuclear Phagocytes in Diabetic Mice). [Google Scholar]
- 99.Kivimäki K., Leppänen T., Hämäläinen M., Vuolteenaho K., Moilanen E. Pinosylvin shifts macrophage polarization to support resolution of inflammation. Molecules. 2021;26:2772. doi: 10.3390/molecules26092772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ganesh G.V., Ramkumar K.M. Macrophage mediation in normal and diabetic wound healing responses. Inflamm. Res. 2020;69:347–363. doi: 10.1007/s00011-020-01328-y. [DOI] [PubMed] [Google Scholar]
- 101.Doyle L.M., Wang M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bartel S., Deshane J., Wilkinson T., Gabrielsson S. Extracellular vesicles as mediators of cellular cross talk in the lung microenvironment. Front. Med. 2020;7 doi: 10.3389/fmed.2020.00326. 326-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stremersch S., De Smedt S.C., Raemdonck K. Therapeutic and diagnostic applications of extracellular vesicles. J. Contr. Release : official journal of the Controlled Release Society. 2016;244:167–183. doi: 10.1016/j.jconrel.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 104.Diaz-Garrido N., Cordero C., Olivo-Martinez Y., Badia J., Baldomà L. Cell-to-Cell communication by host-released extracellular vesicles in the gut: implications in health and disease. Int. J. Mol. Sci. 2021;22:2213. doi: 10.3390/ijms22042213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu Q., Lyon C.J., Fletcher J.K., Tang W., Wan M., Hu T.Y. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm. Sin. B. 2021;11:1493–1512. doi: 10.1016/j.apsb.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zheng C., Sui B., Zhang X., Hu J., Chen J., Liu J., Wu D., Ye Q., Xiang L., Qiu X., Liu S., Deng Z., Zhou J., Liu S., Shi S., Jin Y. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dou G., Tian R., Liu X., Yuan P., Ye Q., Liu J., Liu S., Zhou J., Deng Z., Chen X., Liu S., Jin Y. Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aba2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu W., Yu M., Xie D., Wang L., Ye C., Zhu Q., Liu F., Yang L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020;11:259. doi: 10.1186/s13287-020-01756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou H., Xue Y., Dong L., Wang C. Biomaterial-based physical regulation of macrophage behaviour. J. Mater. Chem. B. 2021;9:3608–3621. doi: 10.1039/d1tb00107h. [DOI] [PubMed] [Google Scholar]
- 110.Liu Z.Z., Xu N.Y., Wang M.L., Tang R.Z., Liu X.Q. Physical confinement in alginate cryogels determines macrophage polarization to a M2 phenotype by regulating a STAT-related mRNA transcription pathway. Biomater. Sci. 2022;10:2315–2327. doi: 10.1039/d1bm01719e. [DOI] [PubMed] [Google Scholar]
- 111.Garg K., Pullen N.A., Oskeritzian C.A., Ryan J.J., Bowlin G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials. 2013;34:4439–4451. doi: 10.1016/j.biomaterials.2013.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang S., Lyu C., Zhao P., Li W., Kong W., Huang C., Genin G.M., Du Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat. Commun. 2019;10:3491. doi: 10.1038/s41467-019-11397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yin Y., He X.T., Wang J., Wu R.X., Xu X.Y., Hong Y.L., Tian B.M., Chen F.M. S Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl. Mater. Today. 2020;18 [Google Scholar]
- 114.Johnson C.D., Fischer D., Smith I.M., Aranda-Espinoza H., Fisher J.P. Hyperglycemic conditions enhance the mechanosensitivity of proinflammatory RAW264.7 macrophages. Tissue Eng. 2023;29:172–184. doi: 10.1089/ten.tea.2022.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Previtera M.L., Sengupta A. Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dutta B., Goswami R., Rahaman S.O. TRPV4 plays a role in matrix stiffness-induced macrophage polarization. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sridharan R., Cavanagh B., Cameron A.R., Kelly D.J., O'Brien F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47–59. doi: 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 118.Zhuang Z.M., Zhang Y., Sun S.N., Li Q., Chen K.W., An C.F., Wang L.B., van den Beucken J., Wang H.N. Control of matrix stiffness using methacrylate-gelatin hydrogels for a macrophage-mediated inflammatory response. ACS Biomater. Sci. Eng. 2020;6:3091–3102. doi: 10.1021/acsbiomaterials.0c00295. [DOI] [PubMed] [Google Scholar]
- 119.Yuan P., Luo Y., Ma L. NIR-triggered hydrogel with dynamic stiffness via ion chelation to modulate macrophage phenotypes. J. Polym. Sci. 2022;60:3176–3185. [Google Scholar]
- 120.Somers N., Lasgorceix M. In: Encyclopedia of Materials: Technical Ceramics and Glasses. Pomeroy M., editor. Elsevier; Oxford: 2021. Surface treatment of bioceramics; pp. 701–715. [Google Scholar]
- 121.Yang Y., Lin Y.J., Zhang Z.C.A., Xu R.G., Yu X.R., Deng F.L. Micro/nano-net guides M2-pattern macrophage cytoskeleton distribution via Src-ROCK signalling for enhanced angiogenesis. Biomater. Sci. 2021;9:3334–3347. doi: 10.1039/d1bm00116g. [DOI] [PubMed] [Google Scholar]
- 122.Zheng X., Xin L., Luo Y., Yang H., Ye X., Mao Z., Zhang S., Ma L., Gao C. Near-infrared-triggered dynamic surface topography for sequential modulation of macrophage phenotypes. ACS Appl. Mater. Interfaces. 2019;11:43689–43697. doi: 10.1021/acsami.9b14808. [DOI] [PubMed] [Google Scholar]
- 123.McWhorter Frances Y., Wang T., Nguyen P., Chung T., Liu Wendy F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schoenenberger A.D., Tempfer H., Lehner C., Egloff J., Mauracher M., Bird A., Widmer J., Maniura-Weber K., Fucentese S.F., Traweger A., Silvan U., Snedeker J.G. Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon in vitro and in vivo. Biomaterials. 2020;249 doi: 10.1016/j.biomaterials.2020.120034. [DOI] [PubMed] [Google Scholar]
- 125.Barth K.A., Waterfield J.D., Brunette D.M. The effect of surface roughness on RAW 264.7 macrophage phenotype. J. Biomed. Mater. Res., Part A. 2013;101:2679–2688. doi: 10.1002/jbm.a.34562. [DOI] [PubMed] [Google Scholar]
- 126.Lee S., Park J., Kim S., Ok J., Yoo J.I., Kim Y.S., Ahn Y., Kim T.-i., Ko H.C., Lee J.Y. High-performance implantable bioelectrodes with immunocompatible topography for modulation of macrophage responses. ACS Nano. 2022;16:7471–7485. doi: 10.1021/acsnano.1c10506. [DOI] [PubMed] [Google Scholar]
- 127.Milan P.B., Lotfibakhshaiesh N., Joghataie M.T., Ai J., Pazouki A., Kaplan D.L., Kargozar S., Amini N., Hamblin M.R., Mozafari M., Samadikuchaksaraei A. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016;45:234–246. doi: 10.1016/j.actbio.2016.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou W., Duan Z., Zhao J., Fu R., Zhu C., Fan D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022;17:1–17. doi: 10.1016/j.bioactmat.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jia G., Li Z., Le H., Jiang Z., Sun Y., Liu H., Chang F. Green tea derivative-based hydrogel with ROS-scavenging property for accelerating diabetic wound healing. Mater. Des. 2023;225 [Google Scholar]
- 130.Farhan A., Hassan G., Ali S., Yousaf Z., Shafique K., Faisal A., Younis B., Mirza S. Spontaneous NETosis in diabetes: a role of hyperglycemia mediated ROS and autophagy. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1076690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hicks J.M., Halkerston R., Silman N., Jackson S.K., Aylott J.W., Rawson F.J. Real-time bacterial detection with an intracellular ROS sensing platform. Biosens. Bioelectron. 2019;141 doi: 10.1016/j.bios.2019.111430. [DOI] [PubMed] [Google Scholar]
- 132.Yang L., Zhang L., Hu J., Wang W., Liu X. Promote anti-inflammatory and angiogenesis using a hyaluronic acid-based hydrogel with miRNA-laden nanoparticles for chronic diabetic wound treatment. Int. J. Biol. Macromol. 2021;166:166–178. doi: 10.1016/j.ijbiomac.2020.10.129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.