Highlights
-
•
No dual-task training studies were eligible for inclusion.
-
•
First study examining effects of differing exercise modalities on MDS-UPDRS;Part I scores.
-
•
Most effective interventions involved longer durations and greater exercise times.
-
•
Adoption and adherence of exercise in early stages of PD, may be most effective.
-
•
Findings hold potential clinical implications immediately following a diagnosis.
Keywords: Movement disorder, Neurodegenerative, Physical activity, Non-motor symptoms
Abstract
Introduction
Whilst non-motor experiences of daily living (NMeDL) reduce quality of life (QoL) in people with Parkinson’s Disease (PD), research dedicated to NMeDL is lacking compared to motor symptoms. The aim of this Network Meta-Analysis (NMA) was to compare and determine the effects of exercise and dual-task training interventions on NMeDL for people with early-to-mid stage PD.
Methods
Eight electronic databases were systematically searched, identifying randomised control trials (RCTs) that assessed the effect of interventions on the Movement Disease Society - Unified Parkinson’s Disease Rating Scale (MDS-UPDRS); Part I scores. A fixed-effect pairwise and NMA were completed and confidence in estimates were assessed using the Confidence in Network Meta-Analysis (CINeMA) framework.
Results
Five RCTs involving exercise were identified, involving 218 participants. No dual-tasking studies were suitable. Pairwise comparisons favoured tango and mixed-treadmill training (TT) when compared to control, however 95% Confidence Intervals (CI) crossed the line of no effect (MD = 0). Indirect comparisons revealed tango had clinically meaningful reductions in Part I scores compared to speed-TT and body-weight resistance training, (MD −4.47; 95% CI −8.50 to −0.44 and MD −4.38; 95% CI −7.86 to –0.90), indicating improved NMeDL. Compared to control, low confidence evidence suggests tango and mixed-TT improves NMeDL.
Conclusions
Tango and mixed-TT are the most effective exercise interventions for improving NMeDL. Adoption of an exercise program in the early stages of PD, irrespective of modality, may be effective and holds potential clinical importance immediately following a diagnosis of PD.
Other: Prospero Registration Number; CRD42022322470.
1. Introduction
Parkinson’s Disease (PD) is the fastest-growing neurological disorder in the world [1], [2], with estimates that it will affect over 12 million individuals by 2040 [3], [4]. In the past 20 years there has been an 81% increase in loss of disability-adjusted life years, which equates to a loss of 5.8 million years, in addition to a 100% increase in deaths attributed to PD, resulting in 329,000 deaths [2]. With an ageing population and no current known cure, the ‘Parkinson’s Pandemic’ is a major contributor to the increasing prevalence of neurodegenerative disease [3], [5], [6], [7], and is becoming a global public health concern [8]. Whilst investigations examining motor symptoms have been a focal point of PD research for many years, non-motor symptoms (NMs) are gaining increased attention [9], [10] as they develop years prior to motor symptoms, progress and accumulate through the early-to-mid stages of PD and consequently reduce quality of life (QoL) [11], increase disability [9] and contribute to a loss of independence [10], [12]. Although motor symptoms and NMs both affect QoL, NMs are suggested to greater impact QoL[13]. NMs affect 100% of people with PD [14], [15], [16] and can include (but not limited to) psychosis, hallucinations, delusions, apathy, excessive drooling, constipation, nausea and vomiting, urinary incontinence, erectile dysfunction, orthostatic hypotension and autonomic dysfunction, sleep disorders including insomnia, excessive daytime sleepiness, rapid eye movement (REM) sleep behaviour disorder, restless leg syndrome, obstructive sleep apnoea, cognitive impairments and excessive sweating [9], [11], [17], [18], [19], [20]. Additionally, people with PD more likely to experience depression, anxiety, pain and fatigue [11], [20], [21], [22].
The Movement Disease Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) is a comprehensive scale for the evaluation of various symptoms and complications experienced in PD, while also assessing the extent and burden of PD [23]. The MDS-UPDRS was revised in 2008 to better evaluate the broad range and extent of NMs experienced in PD [23], [24] and is comprised of four sections, including Part I, NMeDL; Part II, motor experiences of daily living; Part III, motor examination; and Part IV, motor complications [23]. The MDS-UPDRS is the most widely used clinical rating scale for people with PD, and has demonstrated reliability, validity and sensitivity to change [23], [24]. With specific focus on NMeDL (Part I), previous work has demonstrated that MDS-UPDRS scores attain a high correlation with scores of other validated scales that assess NMs individually [25]. In addition to providing an accurate assessment of the wide range of the NMs burdens experienced in people with PD, Part I of the MDS-UPDRS has a favourable application time of approximately 10 min [25].
Current management strategies for PD include the prescription of pharmaceuticals such as dopamine precursors, dopamine agonists and monoamine oxidase, however such medications offer little symptomatic relief of NMs [26]. Additionally, disease progression and waning pharmaceutical effectiveness over time often leads to dosage increases and additional side effects [26]. These limitations have resulted in the advocation of non-pharmacological and complimentary strategies to be incorporated into management of PD [27], such as participation in regular exercise and dual tasking. Exercise is one of the most well studied non-pharmacological strategies in the management of PD [28]. Rodent studies utilising neurotoxins to induce parkinsonism have provided evidence for the neuroprotective benefits of exercise and its role in mitigating PD progression [26], [29], [30]. For people with PD, exercise has been shown to improve QoL and decrease symptom severity, including improvements in sleep quality and quantity[9], autonomic function [31], [32], cognition [33], depression, anxiety and QoL [34]. In contrast to exercise, the effectiveness of dual tasking on NMs is less clear, with indications that it may affect gait and NMs, while other studies have reported no effect on NMs [35] and adverse outcomes [36]. Notwithstanding, Tibar, El Bayad examined the effect of NMs on QoL in 117 patients with a mean Hoehn and Yahr stage of 2 and identified the most common symptoms were autonomic and sleep related [37]. Given these findings, and the authors; suggesting that further understanding the pathophysiology of NMs should be at the forefront of interventional research aiming to improve symptoms and QoL in people with PD, this network meta-analysis (NMA) will further explore the role of exercise and dual-tasking as interventions that may be supportive of people with early- to mid-stage PD.
The effects of exercise and dual tasking are well documented; however, limitations have restricted the ability to undertake correlational research, resulting in limited high-quality literature. Study limitations include poor methodological design, reduced participant numbers, exclusion of healthy control groups and/or human subjects, lack of demographic reporting and examination of isolated NMs [9]. Although pairwise comparisons involving exercise and/or dual-task interventions are abundant, little is known regarding the most effective intervention for the improvement of NMeDL for those living with PD. Therefore, the objective of this NMA was to compare and determine the effects of exercise and dual-task training interventions on NMeDL for people with early to mid-stage stage PD and will simultaneously compare multiple treatments in a single analysis via combination of both direct and indirect evidence.
2. Methods
The NMA was guided by the Cochrane Collaboration Handbook [38] and reported in conjunction with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension incorporating NMA (PRISMA-NMA) [39], [53]. The protocol for this NMA was registered with the International Prospective Register of Systematic Reviews (PROSPERO); CRD42022322470.
2.1. Eligibility criteria and study search
Eight electronic databases were systematically searched (CENTRAL, CINAHL, Emcare, Medline (OVID), ProQuest, PubMed, Scopus and Web of Science) from inception until March 2022, with search terms used provided in Table S1. Inclusion criteria included: (i) participants: human subjects with early to mid-stage PD, classified as per the Hoehn and Yahr (H&Y) Scale (Stage I-III); (ii) intervention: exercise or dual-task training; (iii) outcome: MDS-UPDRS Part I scores; NMeDL[23]; and (iv) study design: full text RCTs, provided in English. Studies were excluded when they (i) included participants with other neurological conditions/diseases, included people with advanced-stage PD (H&Y > Stage 4), (ii) outcome measures other than MDS-UPDRS Part I were used, such as the original UPDRS or Part 2, 3 or 4 exclusively, (iii) did not include a control group or involved a single acute bout of training, (iv) were feasibility, efficacy, pilot or study protocols and/or designs, abstracts or conference presentation posters.
2.2. Screening and data extraction
Two authors (PT, MS) independently screened titles, abstracts and the full text of potentially eligible RCTs, with disagreements resolved by consensus. Data extracted was completed by the primary author and included data pertaining to: 1) characteristics of participants, including sample size (number of males), age (years ± SD), duration of disease (DoD) (years ± SD), H&Y Stage (mean ± SD), MDS-UPDRS; Part I and Total MDS-UPDRS scores (mean ± SD), Levodopa medication dosage (mg) (mean ± SD) and medication stage (ON or OFF) during testing, and 2) characteristics of training interventions including, type and details of interventional and control groups, characteristics of each group and dosage of each intervention, including durations of each intervention (weeks), sessions per week and time of each session. Contact was made (PT) with authors from studies that reported sections of the MDS-UPDRS other than Part I, in aim to identify additional eligible studies.
2.3. Risk of bias assessment
Risk of bias (RoB) was completed independently by two authors (PT, TM) and was assessed using the revised Cochrane Collaboration tool for RCTs (RoB v2.0) [38], according to 6 domains including bias 1) arising from the randomisation process, 2) due to deviation from intended interventions, 3) due to missing outcome data, 4) in measurement of the outcome, 5) in the selection of the reported result and 6) overall bias [40]. Discrepancies were resolved via consensus. Overall bias was considered “low risk of bias”, “some concerns” or “high risk of bias”. Low risk of bias studies presented a “low risk” judgement in all six domains, studies with some concerns presented “some concerns” in at least one domain but no “high risk” domains and studies with “high risk of bias” attained at least one “high risk of bias” judgment across the six domains.
2.4. Assessment of homogeneity and transitivity
Clinical and methodological heterogeneity was assessed through the examination of study and trial characteristics across all eligible studies. NMA validity assumes that any participant included in the network could be randomised to any of the available interventions (transitivity) [41] and assessed via consideration and distribution of the major effect modifiers across the comparisons, including baseline disease severity, methods utilised for classifying disease severity and duration of interventions [42].
2.5. Data synthesis
Direct comparisons were obtained via the visual presentation of a network plot [43], where the node size represents sample size, colour represents RoB judgements, thickness of the line connecting the nodes is proportional to the number of studies and line colour (yellow) indicates mean indirectness. A pairwise meta-analysis was performed for each direct comparison. Data from trials were pooled to estimate the overall effect on NMeDL of any intervention compared to control. A fixed-effect NMA was performed as there was only one exercise intervention per comparison identified. Multi-arm studies were treated as independent comparisons. For each comparison, continuous outcomes were calculated as mean difference (MD) and 95% confidence intervals (CIs).
2.6. Confidence in the evidence
Confidence in the results was evaluated using the CINeMA (Confidence in Network Meta-Analysis) framework and web application. CINeMA is an adaptation of the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach for a NMA [44]. The credibility of treatment effects was evaluated via consideration of study limitations, indirectness, inconsistency (heterogeneity and incoherence), imprecision and publication bias. Each domain was graded by combining the direct evidence with respective statistical contributions to the network results and were then summarised to obtain a confidence judgement for each pairwise effect estimate. Publication bias was assessed via consideration of search completeness, however, due to small-study effects (<10), evaluation of funnel plot symmetry was not completed, and therefore could bias I2 estimates and add to uncertainty [45].
3. Results
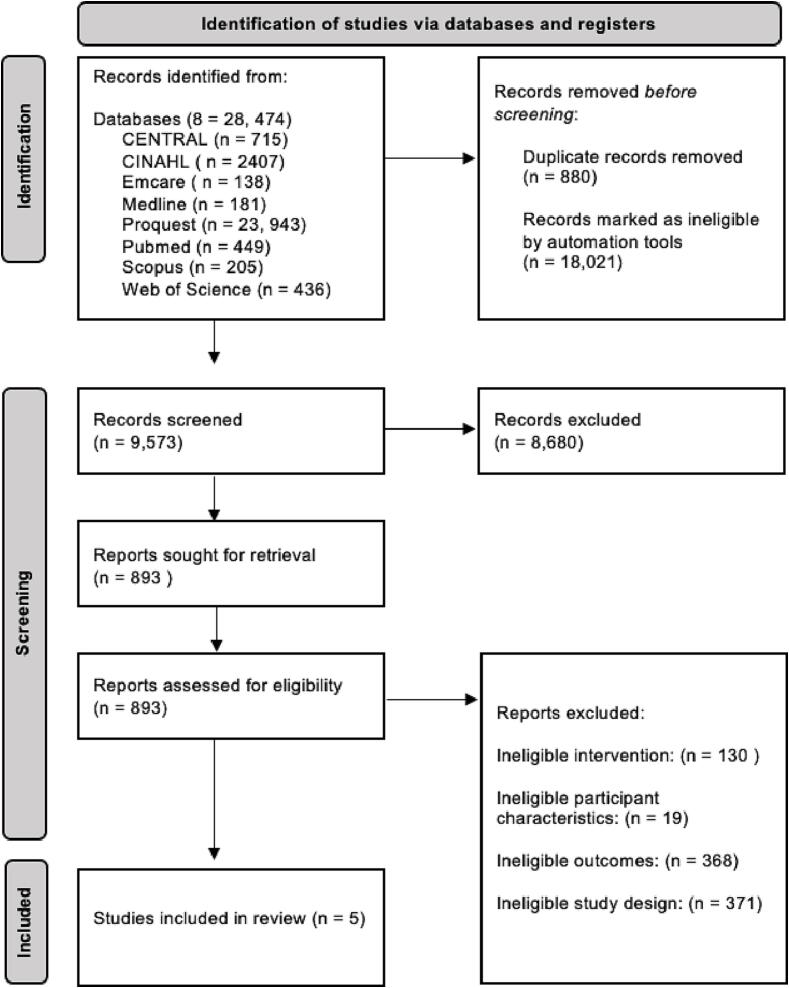
The search retrieved 9,573 studies, of which 5 were identified as being eligible for inclusion in the NMA (Fig. 1). A list of eligible and included studies are provided in Table S2. As shown in Figure S1, one closed loop was identified within the network, containing a single multi-arm study. Included studies consisted of six interventional groups with 119 participants, five control groups with 99 participants, 61% of which were male. Of the available information, participants’ mean ± standard deviation (SD) age was 67 ± 6 years, disease duration was 5.8 ± 2.5 years and H&Y stage was 2.20 ± 0.5. Mean ± SD MDS-UPDRS; Part I scores were 8 ± 4 and mean ± SD MDS-UPDRS total scores were 51 ± 16. Mean ± SD levodopa medication dosage was 571.50 mg ± 349.81 mg. Intervention durations ranged from 10 to 52 weeks and involved 1 to 3 sessions per week for 1 h in duration (Table 1). Due to the low number of studies and their differing interventions, each intervention remained independent in the analysis, including a multi-arm study which was treated as two separate interventions, (6) including, Balance and Gait (BG), 1; Dance and Movement Therapy (DMT), 1; Mixed Treadmill Training (MTT), 1; Speed Treadmill Training (STT), 1; Resistance Training and Body Weight Functional Training (RTBW), 1; Tango (T), 1 as shown in in the network plot (Figure S1).
Fig. 1.
The PRISMA Screening Flow Chart used for to identify eligible studies for inclusion, using the updated guideline for reporting systematic reviews [52]. The systematic search identified 28, 747 studies, of which 9,573 were screened at the title and abstract level. 893 studies were then screened in full text, to identify 5 eligible studies for inclusion.
Table 1.
Study, Sample Size, Participant and Intervention Characteristics of Included Studies.
| Study (year) |
Sample Size (n = m) |
Participant Characteristics |
Intervention Characteristics |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MeanAge (SD) |
DoD (SD) |
H&Y (SD) |
MDS-UPDRS;I (SD) |
MDS-UPDRS Total (SD) | LDOPA (mg) (SD) | On/ Off State |
Intervention | Characteristics | Dose | ||
| Albrecht et al., 2021 [48] | IG: 34 (20) |
70.2 (5.8) | 5.7 (4.5) |
2.1 (0.3) |
8.9 (5.4) |
52.3 (20.7) |
610.5 (355.8) |
On | IG1: HiBalance | IG: Small group sessions of gait and balance training | IG: 10w-2 × 1hr/w |
| CG: 31 (20) | 70.4 (6.1) | 4.5 (3.4) | 2.2 (0.4) |
8.9 (5.5) |
46.7 (16.0) |
458.3 (293.2) | On | CG: Active Control | CG: Speech training + Active control | CG: 10w-1 × 1hr/w | |
| Amara et al., 2020 [47] | IG: 27 (16) | 65.3 (8.1) | 8.8 (1.0) |
56.4 (18.1) |
IG1: Combined RT + BWFM | IG: Exercises (x5) to improve strength and muscle mass (3 s @ 8-12reps) + Trunk to improve postural stability + 3-4BW exercises to improve power and balance (50 reps each) | IG: 16w-3sx/w | ||||
| CG: 28 (19) | 65.8 (5.1) | 9.7 (1.2) |
50.0 (20.9) |
CG: Sleep Hygiene | CG: Discussion with sleep medicine physician | CG: 30-60mins every 4w | |||||
| Duncan et al., 2012 [49] | IG: 26 (15) | 69.3 (1.9) |
5.8 (1.1) | 2.6 (1.0) |
10.6 (4.9) |
Off | IG1: Community-Based Tango Program | IG: Participants altered between leader and follower, learnt new steps and changed partners frequently throughout 12mnths. | IG: 52w- 2 × 1hr/w | ||
| CG: 26 (15) | 69.0 (1.5) | 7.0 (1.0) | 2.5 (1.0) |
12.4 (6.7) |
Off | CG: Usual Care Control | CG: usual care, no prescribed exercise | NA | |||
| Michels et al., 2018 [50] | IG: 9 | 66.4 | 2.1 (0.3) |
11.8 (5.4) |
55.4 (15.8) |
IG1: Dance Therapy | IG: Combined dance and talk therapy | IG: 10w- 1hr /w | |||
| CG: 4 | 75.5 | 2.5 (1.0) |
10 (3.6) |
65.7 (13.0) |
CG: Control (Support) Group | CG: Group discussions of PD interventions and interventional management strategies | CG: 10w- 1hr/w | ||||
| Nadeau et al., 2014 [46] | IG1: 12 (8) | 64.0 (6.6) | 1.9 (0.2) |
3.5 (2.3) |
53.5 (16.0) |
IG1: Speed Treadmill Training | IG1: 5 min warm up, 45 min session (walking only), 5 min cool down. Commenced at 80% of participants preferred walking speed. When participants reached 100% + RPE was ≤ 4 on modified BORG scale + <75% HRm midway through previous session, speed was increased 0.2 km・h−1 in next session | IG1: 24w- 3 × 1hr/e | |||
| IG2: 11 (10) | 60.0 (6.8) | 1.9 (0.1) |
1.4 (1.0) |
40.5 (8.3) |
IG2: Mixed Treadmill Training | IG2: Same progression criteria as IG1. When criteria were met, treadmill incline (+1%) and treadmill speed (0.2 km・h-1) were alternately increased |
IG2: 24w- 3 × 1hr/e | ||||
| CG: 11 (9) | 64.3 (5.6) | 1.8 (0.2) |
2.6 (2.5) |
34.3 (12.9) |
CG: Active Control | CG: “Viactive” program - low-intensity at home exercise | CG: 24w- 2 × 1hr (home) | ||||
| Abbreviations: Control Group, CG; Hour; hr; Heart Rate Max, HRm; Interventional Group 1, IG1; Interventional Group 2, IG2; Standard Deviation, SD; Week, w. Grey shaded areas indicate data was not available and/or provided. | |||||||||||
3.1. Risk of bias assessment
Of the five (5) studies assessed, as outlined in Table S3, two studies were judged to be high RoB due to missing outcome data and RoB in the measurement of the outcome [46], [47]. The remaining three studies were judged to be of ‘some concerns’, due to bias arising from the randomisation process, deviations from intended audience, missing outcome data and bias in the selection of the reported result [48], [49], [50] (Table S3).
3.2. Assessment of clinical heterogeneity and transitivity
As there were only one study per comparison, an in-depth assessment and evaluation of heterogeneity and transitivity were not possible. Of the information provided within each of the included studies, variability in clinical characteristics was not identified; including the age of participants, disease duration and disease severity including the MDS-UPDRS; Part I score and total MDS-UPDRS scores. All studies indicated disease severity by incorporating the MDS-UPDRS total score and/or classification as per the H&Y Scale. Variability in intervention duration was identified between included studies, with duration ranging from 10 to 52 weeks. No other variation in effect modifiers was identified.
3.3. Pairwise and network-meta analysis
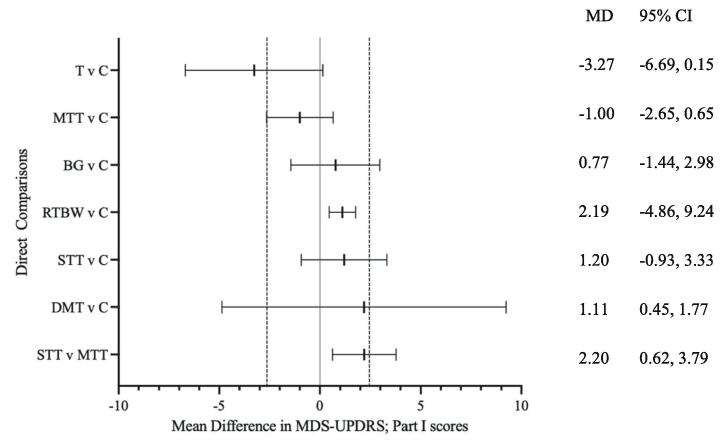
Compared to baseline, a decrease of ≥ 2.64 (−2.64) in mean MDS-UPDPRS; Part I scores represents a minimal, yet clinically meaningful improvement in symptoms, whilst an increase of ≥ 2.45 (+2.45) indicates a clinically meaningful worsening of symptoms [51]. Results from the pairwise meta-analysis are presented in Fig. 2. Tango and mixed treadmill training interventions were more effective than control in reducing MDS-UPDRS; Part 1 scores (MD − 3.27; 95% CI − 6.69 to 0.15, MD −1.00; 95% CI − 2.65 to 0.65), respectively (Fig. 2). The remaining interventions, including balance and gait training, combined resistance training and body weight functional training, speed treadmill training and dance and movement therapy all had a mean difference beyond the no effect line (MD > 0) (MD 0.77; 95% CI −1.44 to 2.98, MD 2.19; 95% CI −4.86 to 9.24, MD 1.20; −0.93 to 3.33), respectively (Fig. 2). Additionally, speed treadmill training was not favoured when compared to mixed treadmill training (MD 2.20, 95% CI 0.62 to 3.79) (Fig. 2).
Fig. 2.
Forest Plot of direct comparisons in Network Meta-Analysis. Forest plot of pairwise analysis results of mean change in MDS-UPDRS scores in Direct Comparisons. Black lines represent confidence intervals (CIs) for mean difference (MD) for each comparison. The centre line is the line of no effect (MD = 0). MD < 0 favour the first intervention, and MD > 0 favours the second. Space between dashed lines is the range of equivalence, extending from MD = −2.64 to MD = 2.45. MD values that extend beyond this range represent minimal, yet clinically-meaningful improvement (<−2.64) or worsening (>2.45) on non-motor experiences of daily living (MDS-UPDRS; Part I) [51]. Predictive intervals were not available since a fixed-effect model was used. MD: Mean difference; CI: Confidence Interval; BG; Balance and Gait training; DMT: Dance and Movement Therapy; MTT: Mixed Treadmill Training; RTBW: Resistance Training and Body Weight Functional Training; STT: Speed Treadmill Training; T: Tango.
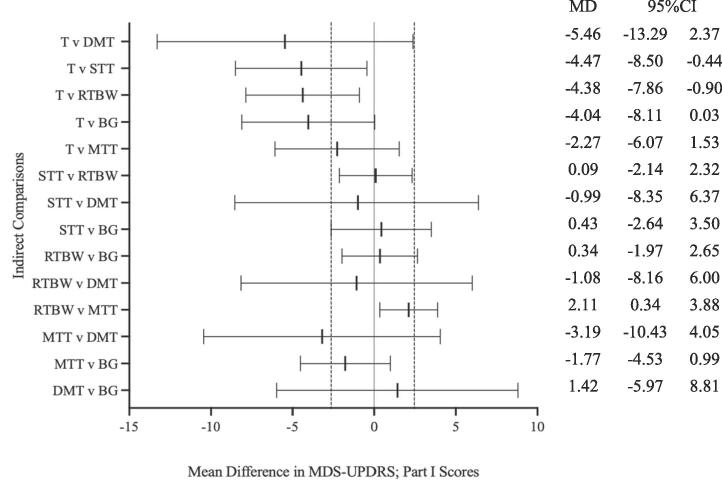
Results from the network meta-analysis are presented in Fig. 3. Of all (14) indirect comparisons, two comparisons did not cross, and remained on the left-hand side of, the ‘line of no effect’ (MD = 0) (Fig. 3), including tango versus speed treadmill training and tango versus combined resistance training and body weight functional training comparisons (MD − 4.47; 95% CI − 8.50 to − 0.44 and MD −4.38; 95% CI − 7.86 to – 0.90), respectively. All other (12) indirect comparisons crossed the line of no effect (MD > 0) (Fig. 3).
Fig. 3.
Forest Plot of indirect comparisons in Network Meta-Analysis. Forest plot of network meta-analysis results of mean change in MDS-UPDRS scores in Indirect Comparisons. Black lines represent confidence intervals (CI) for mean difference (MD) for each comparison. The centre line is the line of no effect (MD = 0). MD < 0 favour the first intervention, and MD > 0 favours the second. Space between dashed lines is the range of equivalence, extending from MD = −2.64 to MD = 2.45. MD values that extend beyond this range represent minimal, yet clinically-meaningful improvement (<−2.64) or worsening (>2.45) on non-motor experiences of daily living (MDS-UPDRS; Part I) [51]. Predictive intervals were not available since a fixed-effect model was used. MD: Mean difference; CI: Confidence Interval; BG; Balance and Gait training; DMT: Dance and Movement Therapy; MTT: Mixed Treadmill Training; RTBW: Resistance Training and Body Weight Functional Training; STT: Speed Treadmill Training; T: Tango.
3.4. Assessment of statistical heterogeneity and inconsistency
Heterogeneity for pairwise comparisons of any intervention versus control was considered moderate (I2 = 55.13); however, this estimation must be interpreted with caution as there were very few studies available to complete a more robust heterogeneity estimation. The number of studies in this NMA was small, thus, the estimations of heterogeneity may be inaccurate. Since the only closed loop was derived from a single multi-arm study [46], a statistical evaluation of the consistency assumption could not be completed.
3.5. Confidence in the evidence
Confidence in estimates for change in mean MDS-UPDRS; Part I scores are considered low to very low due to study limitations, imprecision, heterogeneity, indirectness and publication bias (Table S4). Studies were downgraded for study limitations due to RoB judgements. The small number of studies resulted in wide CIs, equating to 71.4% of all (14) indirect comparisons identifying as some (6; 42.8%) or major (4; 28.6%) concerns with imprecision. Predictive interval estimate and assessment of incoherence could not be completed due to small sample size and missing mixed evidence, and therefore inconsistency was downgraded. Indirectness was downgraded to account for variation in one effect modifier identified (length of intervention), potentially undermining the assumption of transitivity. Publication bias was downgraded due to bias in selection of the reported result. The assessment of small-study-effects could not be completed.
4. Discussion
The aim of this Network Meta-Analysis (NMA) was to compare and determine the effects of exercise and dual-task training interventions on NMeDL for people with early- to mid-stage PD. No dual-task training studies were identified as eligible for inclusion. Screening revealed that only five studies examined the effects of exercise on NMeDL, when compared to control. Low confidence evidence suggests that these exercise interventions had no clinically meaningful effect on the NMeDL in people with early- to mid-stage PD. Of the examined interventions, tango and mixed (incline and speed progression) treadmill training were the most effective in attenuating NMeDL.
The current NMA is the first to examine the effects of exercise interventions on the NMeDL in people with early- to mid-stage PD and includes a protocol registration within the PROSPERO database, reporting in alignment with the PRISMA-NMA extension and the comprehensive and systematic search of eight electronic databases, including CENTRAL. Further, confidence in estimates were assessed using the newly developed CINeMA framework and application. Limitations that arose in this study are attributable to the restricted literature available for inclusion, including the total number of eligible studies, lack of head-to-head trials and studies incorporating a control group, resulting in a scarce network plot. Additionally, the limited number of studies prevented a thorough statistical evaluation of required assumptions, adding to uncertainty in our conclusions. Emphasis was placed on the clinical assessment of transitivity, which comprised a strict inclusion criterion pertaining to patient and study characteristics. Some of the included studies did not report all desired participant characteristics such as H&Y stage, and most studies did not report if participants were in the ON or OFF medication phase when completing physical assessments and exercise training. Exploration of heterogeneity via meta-regression techniques was not possible due to lack of adequate trials [51]. Lastly, only studies that reported NMeDL, using the MDS-UPDRS; Part I, were included and, therefore, some bias could not be avoided.
Results of this study highlights the need for further robust RCTs examining the effect of exercise and dual-task training on NMs in PD and several areas and opportunities for interventional research in the examination of non-motor symptoms and NMeDL for people with PD. Total MDS-UPDRS scores are the sum of all four sections in examining severity and progression of PD, with higher scores indicating increased severity and progression of the disease. Although people included in the analysis were all classified as early- to mid-stage (H&Y: 1–3) PD, total MDS-UPDRS scores differed. Interestingly, the most effective interventions for attenuating NMeDL, were conducted with participants that had decreased baseline MDS-UPDRS scores. Unfortunately, total scores for tango were not attainable, however baseline participant scores for the second most effective intervention (mixed treadmill training) were 41 in comparison to the least effective intervention (combined resistance and body weight functional training) where baseline participant scores were 56. These differences may suggest that adoption of an exercise program in the early stages of the PD progression may be most effective and holds potential clinical implications immediately following a diagnosis. Furthermore, it may suggest that favouring or choosing one exercise modality for those with PD may not be of particular importance, but adherence to an exercise intervention, irrespective of modality, may be more important than the mode of exercise itself. Whilst further research is required, the confirmation of this theory would have marked implications for clinical management guidelines for PD.
Given the findings of this NMA, future interventional research should directly compare the effectiveness of a given intervention in very early stage or recently diagnosed PD to those with moderate-stage PD. Another avenue for further investigation and research is the total duration, including weeks and total time (hours), of exercise required to produce clinically meaningful change. The most effective interventions were those that were longer in duration and involved a greater amount of exercise time. Tango, shown to be most effective, was conducted over 52 weeks and involved 104 h of total exercise time. Following tango, mixed treadmill training included 24 weeks in duration and involved 72 h of total exercise time. In contrast to tango and mixed treadmill training, combined resistance and body-weight functional training was the least effective, which was 16 weeks in duration and only involved 48 h of total exercise time. Of note, participants in the ‘least effective’ intervention were classified as having the most advanced PD of all included studies, and therefore could have influenced the results. Further research should investigate resistance and body-weight functional training with earlier stage PD, including those with MDS-UPDRS scores < 40. The screening process revealed that a large proportion of publications since 2008 are using the original UPDRS (1987) assessment. In addition to using the revised MDS-UPDRS, including Part I (NMeDL), future research should focus on examining head-to-head trials and increasing the variety of interventions, via the inclusion of exercise and dual-task training interventions. Given PD is extremely heterogenous, all information pertaining to participant demographics, stage and duration of disease, medication states during assessment and medication quantity are pertinent to increasing transitivity in future research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding information
Publishing of this work was financially supported by the Charles Sturt University Tri-Faculty Open Access Publishing Grant.
Data availability statement
Data sharing is not applicable to this paper as no new data were created or analysed in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2023.100203.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Dorsey R., Sherer T., Okun M.S., Bloem B.R. The Rise of Parkinson's Disease. Am Sci. 2020;108(3):176–183. [Google Scholar]
- 2.World Health Organization. Parkinson disease: a public health approach: technical brief. 2022. ISBN 978-92-4-005098-3 (electronic version).
- 3.Dorsey S., Okun B. The emerging evidence of the Parkinson pandemic. J. Parkinson's Dis. 2018;8(s1):S3–S8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotty G.F., Schwarzschild M.A. Chasing Protection in Parkinson’s Disease: Does Exercise Reduce Risk and Progression? Front Aging Neurosci. 2020;12:186. doi: 10.3389/fnagi.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorsey E., Nichols A.-A., Abdelalim A., et al. Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. doi: 10.1016/S1474-4422(18)30295-3/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorsey, Bloem The Parkinson pandemic—a call to action. JAMA Neurol. 2018;75(1):9–10, doi:. doi: 10.1001/jamaneurol.2017.3299. 10.001/jamaneurol.2017.3299. [DOI] [PubMed] [Google Scholar]
- 7.Alamri Y., Pitcher T., Anderson T.J. Variations in the patterns of prevalence and therapy in Australasian Parkinson’s disease patients of different ethnicities. BMJ. 2020;2(1) doi: 10.1136/bmjno-2019-000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivy C.C., Lockmiller M.-C., McKay M., Landess K., Manning J., Denney L. The impact of exercise on sleep in people with Parkinson’s disease a scoping review. J. Clin Neurosci. 2021;86:223–229. doi: 10.1016/j.jocn.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 9.Amara A.W., Memon A.A. Effects of exercise on non-motor symptoms in Parkinson’s disease. Clin ther. 2018;40(1):8–15. doi: 10.1016/j.clinthera.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.T.D. Ellis, C. Colón-Semenza, T.R. DeAngelis, C.A. Thomas, M.-H. Saint Hilaire, G.M. Earhart et al. (Eds.), Evidence for Early and Regular Physical Therapy and Exercise in Parkinson's Disease. Semi Neurol. Thieme Medical Publishers, Inc., 2021. doi: 10.1055/s-0041-1725133. [DOI] [PMC free article] [PubMed]
- 11.Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323(6):548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y.-S., Yang S.-D., Tan Z.-X., Wang M.-M., Xing Y., Dong F., et al. The benefits and mechanisms of exercise training for Parkinson's disease. Life Sci. 2020;245 doi: 10.1016/j.lfs/2020.117345. [DOI] [PubMed] [Google Scholar]
- 13.Berganzo K., Tijero B., González-Eizaguirre A., Somme J., Lezcano E., Gabilondo I., et al. Motor and non-motor symptoms of Parkinson's disease and their impact on quality of life and on different clinical subgroups. Neurología (English Edition). 2016;31(9):585–591. doi: 10.1016/j.nrl.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Carroll V., Rossiter R., Blanchard D. Non-motor symptoms of Parkinson’s disease. Aust J Gen Pract. 2021;50(11):812–817. doi: 10.31128/AJGP-07-21-6093. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhuri J.R., Mridula K.R., Bandaru S. Prevalence of Non-motor Symptoms in Parkinson's Disease: A Study from South India. Turk Noroloji Derg. 2021:27(1). doi: 10.4277/tnd.2021.52993. [DOI] [Google Scholar]
- 16.Gulunay A., Cakmakli G.Y., Yon M.I., Ulusoy E.K., Karakoc M. Frequency of non-motor symptoms and their impact on the quality of life in patients with Parkinson's disease: a prospective descriptive case series. Psychogeriatrics. 2020;20(2):206–211. doi: 10.1111/psyg.12489. [DOI] [PubMed] [Google Scholar]
- 17.McGregor M.M., Nelson A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron. 2019;101(6):1042–1056. doi: 10.1016/j.neuron.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 18.J. Mitrofanis, The Run and the Light: the dynamic duo, Morgan & Claypool Publishers, 2019 Aug 5. doi: 10.1088/2053-2571/ab2f70ch4.
- 19.Church F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules. 2021;11(4):612. doi: 10.3390/biom11040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalia L.V., Lang A.E., Hazrati L.-N., Fujioka S., Wszolek Z.K., Dickson D.W., et al. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA neurol. 2015;72(1):100–105. doi: 10.1001/jamaneurol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadastik-Eerme L., Rosenthal M., Paju T., Muldmaa M., Taba P. Health-related quality of life in Parkinson's disease: a cross-sectional study focusing on non-motor symptoms. Health Qual Life Outcomes. 2015;13:83. doi: 10.1186/s12955-015-0281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prisnie J.C., Sajobi T.T., Wang M., Patten S.B., Fiest K.M., Bulloch A.G.M., et al. Effects of depression and anxiety on quality of life in five common neurological disorders. Gen Hosp Psychiatry. 2018;52:58–63. doi: 10.1016/j.genhosppsych.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 24.Goetz C.G., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stebbins G.T., et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher D.A., Goetz C.G., Stebbins G., Lees A.J., Schrag A. Validation of the MDS-UPDRS Part I for nonmotor symptoms in Parkinson’s disease. Mov Disord. 2012;27(1):79–83. doi: 10.1002/mds.23939. [DOI] [PubMed] [Google Scholar]
- 26.Zahoor I., Shafi A., Haq E. In: Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet] Stoker T.B., Greenland J.C., editors. Codon Publications; Brisbane (AU): 2018. Pharmacological Treatment of Parkinson’s Disease. [DOI] [Google Scholar]
- 27.Monteiro-Junior R.S., Cevada T., Oliveira B.R., Lattari E., Portugal E.M., Carvalho A., et al. We need to move more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses. 2015;85(5):537–541. doi: 10.1016/j.mehy.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Bloem B.R., de Vries N.M., Ebersbach G. Nonpharmacological treatments for patients with Parkinson's disease. Mov Disord. 2015 Sep 15;30(11):1504–1520. doi: 10.1002/mds.26363. [DOI] [PubMed] [Google Scholar]
- 29.Schwarting R., Huston J. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog. Neurobiol. 1996;50(2–3):275–331. doi: 10.1016/S0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 30.Jakowec M.W., Petzinger G.M. 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned model of parkinson's disease, with emphasis on mice and nonhuman primates. Comp med. 2004;54(5):497–513. [PubMed] [Google Scholar]
- 31.Kanegusuku H., Silva-Batista C., Peçanha T., Nieuwboer A., Silva N.D., Jr., Costa L.A., et al. Effects of progressive resistance training on cardiovascular autonomic regulation in patients with Parkinson disease: a randomized controlled trial. Arch. Phys. M. 2017;98(11):2134–2141. doi: 10.1016/j.apmr.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Costa C., Chaves L., Sousa T., Prado R., Asano R., Novais T., et al. Effects of a Dynamic Combined Training Method on Autonomic Cardiac Control in Patients with Parkinson's Disease. J. Exerc. Physiol. 2019;22:23. ISSN 1097-9751. [Google Scholar]
- 33.Nascimento C.M., Pereira J.R., de Andrade L.P., Garuffi M., Talib L.L., Forlenza O.V., et al. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res. 2014;11(8):799–805. doi: 10.2174/156720501108140910122849. [DOI] [PubMed] [Google Scholar]
- 34.Dashtipour K., Johnson E., Kani C., Kani K., Hadi E., Ghamsary M., et al. Effect of exercise on motor and nonmotor symptoms of Parkinson’s disease. Parkinson’s Dis. 2015;2015 doi: 10.1155/2015/586378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brauer S.G., Morris M.E. Can people with Parkinson's disease improve dual tasking when walking? Gait Posture. 2010 Feb 1;31(2):229–233. doi: 10.1016/j.gaitpost.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M., Gan Y., Wang X., Wang Z., Feng T., Zhang Y. Gait performance and non-motor symptoms burden during dual-task condition in Parkinson’s disease. Neurol Sci. 2022 Sep;20:1. doi: 10.1007/s10072-022-06411-2. [DOI] [PubMed] [Google Scholar]
- 37.Tibar H., El Bayad K., Bouhouche A., Ait Ben Haddou E.H., Benomar A., Yahyaoui M., et al. Non-Motor Symptoms of Parkinson's Disease and Their Impact on Quality of Life in a Cohort of Moroccan Patients. Front. Neurol. 2018;9:170. doi: 10.3389/fneur.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins J.P., Savović J., Page M.J., Elbers R.G., Sterne J.A. Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions. 2019:205. doi: 10.1002/9781119536604.ch8. [DOI] [Google Scholar]
- 39.Hutton B., Catala-Lopez F., Moher D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med Clin (Barc). 2016;147(6):262–266. doi: 10.1016/j.medcle.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Sterne J.A., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:366. doi: 10.1136/bmj.I4898. [DOI] [PubMed] [Google Scholar]
- 41.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods. 2012;3(2):80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 42.Rhodes K.M., Turner R.M., Higgins J.P. Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J. Clin. Epidemiol. 2015;68(1):52–60. doi: 10.1016/j.jclinepi.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salanti G., Ades A., Ioannidis J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Paschos P., Katsoula A., Salanti G., Giouleme O., Athanasiadou E., Tsapas A. Systematic review with network meta-analysis: the impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment Pharmacol Ther. 2018;48(11–12):1174–1185. doi: 10.1111/apt.15005. [DOI] [PubMed] [Google Scholar]
- 45.Mikolajewicz N., Komarova S.V. Meta-analytic methodology for basic research: a practical guide. Front. Physiol. 2019;10:203. doi: 10.3389/fphys.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nadeau A., Pourcher E., Corbeil P. Effects of 24 weeks of treadmill training on gait performance in Parkinson disease. Med Sci Sports Exerc. 2014;46(4):645–655. doi: 10.1249/MSS.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 47.Amara A.W., Wood K.H., Joop A., Memon R.A., Pilkington J., Tuggle S.C., et al. Randomized, controlled trial of exercise on objective and subjective sleep in Parkinson's disease. Mov Disord. 2020;35(6):947–958. doi: 10.1002/mds.28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albrecht F., Pereira J.B., Mijalkov M., Freidle M., Johansson H., Ekman U., et al. Effects of a Highly Challenging Balance Training Program on Motor Function and Brain Structure in Parkinson’s Disease. J Parkinsons Dis. 2021(Preprint):1–15. doi: 10.3233/jpd-212801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan R.P., Earhart G.M. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132–143. doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- 50.Michels K., Dubaz O., Hornthal E., Bega D. “Dance Therapy” as a psychotherapeutic movement intervention in Parkinson’s disease. Complement Ther Med. 2018;40:248–252. doi: 10.1016/j.ctim.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Horváth K., Aschermann Z., Kovács M., Makkos A., Harmat M., Janszky J., et al. Minimal clinically important differences for the experiences of daily living parts of movement disorder society–sponsored unified Parkinson's disease rating scale. Mov Disord. 2017;32(5):789–793. doi: 10.1002/mds/26960. [DOI] [PubMed] [Google Scholar]
- 52.Efthimiou O., Debray T.P., van Valkenhoef G., Trelle S., Panayidou K., Moons K.G., et al. GetReal in network meta-analysis: a review of the methodology. Res. Synth. Methods. 2016;7(3):236–263. doi: 10.1002/jrsm.1195. [DOI] [PubMed] [Google Scholar]
- 53.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this paper as no new data were created or analysed in this paper.