Abstract
Dietary restriction (DR) is the most powerful intervention to enhance health and lifespan across species. However, recent findings indicate that DR started in late life has limited capacity to induce health benefits. Age-dependent changes that impair DR at old age remain to be delineated. This requires a better mechanistic understanding of the different aspects that constitute DR, how they act independently and in concert. Current research efforts aim to tackle these questions: Are fasting periods needed for the induction of DR's health benefits? Does the improvement of cellular and organismal functions depend on the reduction of specific dietary components like proteins or even micronutrients and/or vitamins? How is the aging process intervening with DR-mediated responses? Understanding the evolutionary benefits of nutrient stress responses in driving molecular and cellular adaptation in response to nutrient deprivation is likely providing answers to some of these questions. Cellular memory of early life may lead to post-reproductive distortions of gene regulatory networks and metabolic pathways that inhibit DR-induced stress responses and health benefits when the intervention is started at old age. Inhere we discuss new insights into mechanisms of DR-mediated health benefits and how evolutionary selection for fitness in early life may limit DR-mediated improvements at old age.
Keywords: Aging, Dietary restriction, Nutrition, Metabolism, Nutrient stres response, Epigenetic memory, Reprogramming, Evolution
1. Introduction
Nutrient restriction has been shown to elongate lifespan across species ranging from yeast, over simple laboratory models, to rodents and non-human primates [1]. In mice, lifelong, moderate dietary restriction (DR) attenuates aging-related physiological decline and increases lifespan by 20–30% compared to ad libitum fed (AL) control mice [[2], [3], [4]]. DR improves overall metabolic homeostasis characterized by insulin sensitivity, glucose tolerance, lower levels of hepatic lipid accumulation; moreover, it attenuates or delays aging-related disease phenotypes like cancer, inflammation, and frailty [[1], [2], [3], [4], [5]].
Under a classic DR feeding regimen, mice receive a single food portion corresponding to ∼60–80% of the normal ad libitum intake by weight once a day [[2], [3], [4]]. This restricts all components of the diet – not only calories – even though the terms “dietary restriction” and “caloric restriction” are commonly used interchangeably [[2], [3], [4]]. DR-treated mice receive less proteins, carbohydrates and lipids, but also lowered amounts of micronutrients such as vitamins. Restriction of the dietary intake of a specific nutrient or altered ratios of macronutrients have been shown to be sufficient to activate nutrient stress response pathways that contribute to DR-induced health benefits. This has spurred research investigating the mechanisms by which dietary composition and/or specific nutrients may contribute to DR-mediated effects. When mice have access to surplus food (i.e. AL feeding) they consume up to 25% of their entire food intake during their rest (light) phase, which abolishes any prolonged fasting [6]. In contrast DR-fed mice rapidly devour their single food portion, thus self-imposing a prolonged (∼20 h) fast [6]. Therefore, it is necessary to distinguish not only between the effects of reduced caloric intake and lower levels of macro-/micronutrients but also and the effect of fasting when studying DR.
Another conceptually important aspect of DR-mediated health benefits is that the capacity of DR to induce such effects seems to be abolished when the intervention is started in late life. It has been shown that aging abrogates lifespan prolonging effects of DR in mice [4], of the DR-mimetic, metformin, in mice [7] and C. elegans [8] and of lowering growth factor signaling in mice [9] and C. elegans [10]. It is currently not well understood why the capacity of DR declines at older age. This review will highlight that nutrient stress response pathways have been selected during evolution to protect the maintenance and thus the reproductive success of the organism primarily during early life. Post-reproductive distortion of these nutrient sensing pathways by itself likely impact on the loss of DR-mediated improvements in health and lifespan during later life. In addition, we will discuss that unrestricted food intake and elevated growth signaling during early life could install a cellular memory that perturbs the induction of nutrient stress signaling response at later life (see below).
Together, a mechanistic understanding of factors that contribute to DR-mediated health benefits (including feeding regiments and restriction of specific nutrient components) as well as the understanding of factors that limit DR-mediated health benefits in late life will help to understand the nature of the aging process itself as well as the mechanisms required to slow down aging by DR interventions. In the following we will discuss important findings and conceptual ideas that center around these key areas of aging and DR research.
2. About time
All photo-sensitive organisms have evolved diurnal changes in behaviour to anticipate daily environmental fluctuation and optimize resource utilization by the proper timing of physiological processes: sleep-/wake-cycles, locomotion, body temperature, food intake, hormone secretion, etc. [6,[11], [12], [13]]. On a cellular level the core molecular clock consists of two transcription factors (CLOCK, BMAL1) that – among a plethora of “clock-controlled” target genes – induce transcription of their repressors (PER, CRY), thereby establishing a transcription-translation feedback loop that oscillates with a period of approximately 24 h [11]. The core clock is involved in the regulation of all metabolic pathways and receives important feedback from them [11]. For example, high fat diet (HFD) disrupts circadian behaviour patterns and HFD and DR have both been shown to alter genes which follow a rhythmic expression pattern in the liver [11,13]. Interestingly, DR enhanced the amplitude and number of rhythmic genes [13]. The ability to maintain rhythmic gene expression and lysine acetylation in the liver of aged mice deteriorates, and diurnal behavioural patterns are disrupted, which may contribute to age-related “disruption of metabolic homeostasis“ [11,13,14]. However, it remains unclear whether abnormal metabolism disturbs metabolic feedback on the molecular clock and thus diminishes its output, or whether an aging-induced loss of rhythmicity drives age-related pathologies [11]. This raised the question how DR's effect on circadian rhythms mediated through fasting periods might contribute to protect against age-related pathologies.
Classic DR sets cycles of high nutrient availability and scarcity, which alter metabolic functions on a behavioural and molecular level in a rhythmic fashion [2,3,12]. Food is a strong Zeitgeber because it regulates nutrient/energy availability and thereby the coordination of catabolic and anabolic processes, and abundance of their by-products like reactive oxygen species (ROS), carbon dioxide (CO2), acyl carnitines, and the ratio of oxidized (NAD+) and reduced (NADH) nicotinamide adenine dinucleotide to name but a few [[11], [13]]. In mice, once-a-day DR feeding enhances rhythmic transcription in the liver both in terms of the amplitude of the expression pattern and the number of oscillatory genes, which is linked to altered circadian patterns of histone acetylation and a “sharpened control” of the daily rhythms of NAD+, NADH and other NAD metabolites [13].
The cycling genes in the livers of young and old DR-treated mice strongly overlap with the SIRT1-dependent hepatic transcriptome [13], which does not occur under AL feeding. SIRT1 belongs to the sirtuin family of NAD + -dependent protein deacetylases that have been established to transmit a range of DR's metabolic benefits in various tissues: increased mitochondrial biogenesis (via PGC1α), inhibition of NFκB signaling and therefore lower levels of inflammation, increased resilience to cellular stress (via FOXO and p53 signaling), enhanced oxidative metabolism and protection against ROS [5,[15], [16], [17]]. Recently Levine et al., demonstrated that treating mice with 30% DR – given over a 12 h window – alters the ratio of NAD+ and NADH dependent on the time of the measurement, which in turn dictates diurnal variations in SIRT1 activity: high levels of NADH inhibit SIRT1 whereas high levels of its substrate NAD + induce the enzyme [11]. The authors could also show that the regulation of SIRT1 activity through the NAD+:NADH ratio governs the DR-induced reprogramming of the rhythmic transcriptome in murine liver [11]. DR is believed to increase the NAD + available for sirtuins, through the induction of NAD + synthesis via nicotinamide phosphoribosyltransferase (NAMPT), which expression follows a robust circadian oscillation also in the liver [5,11,13]. At the same time the liver is the site of biosynthetic reactions like lipid synthesis that consume vast amounts of NADH in proportion to nutrient availability [5,18]. In both, classic DR and time restricted feeding regimens fuel utilization follows a distinct pattern in which upon feeding, the mice immediately burn some of the carbohydrates, then utilize them for lipid synthesis, and ultimately metabolize these lipids during fasting [2]. Therefore, it seems plausible that fasting alters metabolic activity of the liver, which produces sharp peaks and troughs of the NAD+:NADH ratio that respectively induce or inhibit SIRT1-activity in a rhythmic manner (Fig. 1b) [11]. SIRT1 governs Bmal1 levels and chromatin occupancy, which dictates circadian gene expression in the liver, and thus diurnal changes in the NAD+:NADH ratio imposed by fasting could feedback to the core circadian oscillator [14].
Fig. 1.
Dietary restriction is not equivalent to caloric restriction. a) Classic once-a-day DR feeding regimens reduce the mice's caloric intake. The mice rapidly devour their food portion, thus self-impose prolonged fasting (∼20 h) in addition to a reduction in calories, which may impact circadian rhythms in the liver through the mechanism explained in panel b. Additionally it has been shown that feeding the mice in the beginning of their active period (night) increases their lifespan more substantially than if they are fed in the beginning of their rest phase (day). The distinct or overlapping mechanisms by which meal timing, caloric restriction and fasting contribute to life-/healthspan elongation remain to be elucidated. b) Proposed molecular mechanism by which fasting enhances hepatic oscillation. Feeding fasting cycles generate rhythmic cell respiration – characterized by alterations in the rate of metabolic reactions including glycolysis/gluconeogenesis, TCA cycle, electron transport chain, β-oxidation and lipid synthesis, which drive diurnal changes in the NAD+:NADH ratio (and other metabolic byproducts such as ROS). Increased NADH levels inhibit Sirt1 activity, while relatively higher levels of NAD + induce Sirt1 activity. This leads to cyclic patterns of protein de-/acetylation (AMPK, histones, Per2) that affect gene expression and the period of the core molecular oscillator. Thereby, fasting enhances cyclic gene expression in the liver and presumably rhythmic changes in liver function. Aging is associated with a diminished NAD + pool and decreased sirtuin activity, which may hamper the circadian effects of DR in old mice.
The connection between metabolism and circadian rhythmicity suggests that fasting could have effects independent of lower energy intake. To study the effect of reduced energy intake without fasting DR mice can either be provided smaller food portions throughout the day [3] or fed with chow that is ‘diluted’ with indigestible cellulose [2]. This approach has recently been used by Pak et al.: they show that 30% DR achieved by diluting the chow with cellulose (no fasting) could not elongate lifespan nor protect against age-related frailty and cancer despite lower adiposity/weight and improved glucose tolerance [2]. On the other hand mice trained to consume ∼100% of the AL calories within a 3 h time window exhibited many of the beneficial adaptations observed in classic DR mice: improved insulin sensitivity, fatty acid utilization and transcriptional reprogramming of the liver including key pathways such as AMPK and PPAR signaling [2]. Lifespan of these mice was not tested [2], however, another group found that male C57BL/6J mice fed with ∼100% of AL calories over the course of 12–15 h (i.e. 12-9 h fast between meals) had a significantly increased median lifespan (13% increase in lifespan compared to AL controls with unrestricted access to food) that was smaller than that of a concurrently generated DR-treated cohort (30% increase in lifespan compared to AL controls) [19]. In these studies circadian rhythmicity was largely overlooked – in Ref. [2] the restricted mice received food during the light period (contrary to their natural diurnal behaviour), which has been shown to cause misalignment between feeding and activity rhythms in other studies [3], and no different time points were considered for the molecular analyses, which prohibits the study of rhythmic expression patterns. A series of recent experiments separately investigated the effect of reduced caloric intake, duration of fasting period, and timing of the fasting/feeding with respect to the mice's active phase: Acosta-Rodriguez et al., utilized an automated feeding systems that could regulate the duration of the feeding/fasting periods by altering the duration between the release of food pellets [[3], [6]]. Here, the authors investigated the lifespan of mice that received AL, classic DR or a variation of DR, in which food portions were provided over the course of 12 h (short fast DR), or the entire day (DR without fasting) [3]. Importantly, they had two groups of DR and two groups of short fast DR mice (5 experimental groups in total), which were either fed in the beginning of the light or the dark period [3]. Contrary to the observations by Pak and colleagues, who used cellulose-diluted chow to achieve DR without fasting [2], in the present study the mice receiving less calories without fasting exhibited a significant 10% increase in lifespan [3]. The authors attribute the conflicting results to the difference in diet composition [3]. The mice that underwent fasting for either 12 h or 20 h lived substantially longer than the group receiving DR without fasting [3]. Strikingly, the duration of the fast did not influence lifespan but the time at which the mice received their meal/s did: the mice fed during the light period lived about 20% longer than the AL group (similar to the DR group in Ref. [2]), whereas the DR and short fast DR groups fed in the dark lived more than 30% longer [3]. In fact, mice receiving classic DR at the beginning of the dark period lived significantly longer than their counterparts fed in the beginning of the light period [3]. Therefore, fasting and meal timing seem to contribute to the DR-mediated effects on lifespan.
Interestingly, time restricted feeding during the mice's active phase was shown to ameliorate HFD-induced fatty liver disease in mouse models without changing overall food intake [20]. Increasing NAD + levels by dietary supplementation also attenuates HFD-induced metabolic dysregulation through SIRT1 signaling [17]. A recent study on the effect of NAD + supplementation on aging-related loss of circadian rhythms, demonstrated that NAD + supplementation was sufficient to largely restore rhythmic patterns of gene expression and mitochondrial respiration in the liver and to rescue diurnal activity rhythms [14]. Thus, there could be a connection between the decline of NAD + levels observed in aging [21,22] and the diminished effect of DR on circadian gene expression in the livers of old mice (Fig. 1b) [13]. It remains to be tested whether the aging-induced deterioration of cellular NAD + stores hampers DR's ability to reprogram the circadian transcriptome of the liver and if so whether that could help to explain why DR fails to elongate lifespan of already aged mice [4]. The findings discussed above underscore that reduced energy intake and fasting independently contribute to the physiological adaptation to DR, and that an elongated fast (at least 12 h) potentiates DR's lifespan effect. Furthermore, DR more effectively improves lifespan when the meals are provided in synchrony with organismal activity rhythms, which is in line with the observation that restriction of HFD to the mice's active phase ameliorates diet-induced metabolic syndrome. This knowledge will ultimately inform decisions about the treatment of human patients with dietary interventions.
3. It's not all about calories
Besides the reduction of food intake and the timing of feeding the composition of nutrients in the experimental chow is an important aspect that influences the outcome of DR interventions.
Most of the studies on rodents use chows that are optimized for high breeding performance and provide excess amounts of macro nutrients such as carbohydrates, lipids and proteins as wells as excessive amounts of micronutrients such as vitamin A and folate [23]. While these chows might provide optimal conditions during development and at young age, they elevate the risk of obesity and metabolic disorders in late life [24]. A suggested mechanism of DR mediated lifespan extension in mice is therefore the protection against over-eating induced metabolic diseases in aging cohorts [25]. This assumption however is challenged by experiments in progeroid DNA repair deficient mice, which upon DR show dramatic lifespan extension despite pre-existing cachexia and under-nutrition phenotypes [26]. In wildtype mice, there seems to be no correlation between body weight and lifespan and different variations of DR regimes could improve lifespan independently of the body weight [3]. The lifespan extending effect of DR has been associated with the regulation of multiple nutrient sensing pathways such as IGF-1 and mTOR, increased autophagy, and improved metabolic functions, such as insulin sensitivity. Therefore, weight reduction does not appear to be the primary cause of DR-mediated improvements. Indeed, the maintenance of fat mass under DR correlates positively with lifespan extension [4]. Together, these findings raise the questions whether a targeted reduction of single food components would be sufficient to elicit the same benefits as classic DR, what mechanisms and pathways are induced by the restriction of specific nutrient components, and how these mechanisms and pathways act in combination in classic DR (Fig. 2)?
Fig. 2.
Models of dietary restriction (DR) with improvement of the health- and lifespan. Diet is composed of macronutrients such as protein, carbohydrates and fat as wells as micronutrients such as amino acids or vitamins. Classically, DR is applied by a reduction of a complete diet intake (middle). All components of the diet are reduced to the same extend and initial ratios between the nutrients remain. DR leads to a metabolic response including but not limited to a reduced mTOR activity and elevated levels of FGF21 or β-hydroxybutyrate. The metabolic response promotes healthy aging and longevity. Key aspects of the metabolic response of the classic DR model can also be mimicked by changing the ratio of nutrients (left) or the specific reduction of singe nutrients (right). It remains of great interest to further elucidate different combinations of the DR models and to determine more nutrients whose restriction could be beneficial.
Studies in fruit flies and mice have shown that AL diets with a reduced protein:carbohydrate ratio could significantly improve key parameters of aging and lifespan [27]. Furthermore, it was observed that a ketogenic diet, which is completely depleted from carbohydrates and only contains fat and proteins as calorie source, significantly elongated the lifespan of mice [28]. Both the low protein/high carbohydrate and the ketogenic diet do not lead to a loss of body weight, suggesting once more that weight reduction might not be a mechanistic necessity for beneficial effects of DR. Further, selective restriction of branched chain amino acids (BCAA) in AL fed mice was shown to elongate the lifespan of progeria mouse models and of male wild type mice without lowering the energy intake of the animals [29]. These findings suggest that it is not necessarily the reduced caloric intake nor prolonged fasting periods that achieve lifespan extending effects (see above). Similarly, restriction of the dietary intake of the essential amino acid methionine elegantly demonstrates that even the reduced availability of a single amino acid can be sufficient to activate DR-like responses and extend lifespan without reducing the overall energy intake [30]. However, the restriction of dietary amino acids has been associated with reduced body fat content and lower body weight due to increased energy expenditure compared to animals in the control group [30].
In principle, it is possible that the general benefit of DR relies on a state of temporal metabolic stress, which forces the cells to rewire its genetic and metabolic networks. It would be of great interest to further investigate single molecules that can be reduced or left out from the diet in order to stimulate beneficial responses for the health and lifespan. Vitamins that are usually consumed at large or even excessive quantities could be promising candidates. Vitamin A for example is highly abundant in the standard animal chow but also in the human western diet [23,31]. Moreover, reports from rodent and human studies indicate a gradual accumulation of Vitamin A in the liver during aging [32,33]; and metabolic disorders such as non-alcoholic fatty liver disease (NAFLD) were shown to be associated with an aberrant Vitamin A accumulation in the liver [34].
Using restriction of specific nutrients to treat diseases in humans may have advantages compared to general DR. Clinical trials revealed that there are big compliance issues to adhere to a DR intervention over prolonged periods of time. For example, in patients with NAFLD, DR is the treatment of choice, and it can achieve high cure rates, however, only 10% of the patients are compliant and achieve to conduct long-term DR to achieve the therapy goal in losing 10% of their body weight [35]. Moreover, DR can have unwanted side effects. Particularly in elderly people nutrient restriction requires tight monitoring of health parameters in order to avoid malnutrition or the induction of a catabolic state, which could aggravate age-related organ atrophy, such as skeletal muscle atrophy. It is important to decipher the molecular mechanisms that underlie the health-promoting effects of DR. This knowledge may allow clinical researchers to develop dietary interventions that reduce specific nutrient components rather than the overall intake of all food components (Fig. 2). Such focused approaches may lower the risk of undernutrition or diet-induced diminishment in the quality of life as compared to conventional DR.
Experiments on conventional DR have identified key-mechanisms and factors that seem to contribute to lifespan elongation in response to DR. Examples include the reduction of mTOR activity as well as the systemic elevation of FGF21 or the ketone body β-hydroxybutyrate in blood serum. Animal experiments have proven that inhibition of mTOR [36] as well as the increase of FGF21 [37] or β-hydroxybutyrate [38] ameliorate aging associated impairments of health and lead to lifespan extension. The development of dietary interventions that target specific food components or specific effector pathways downstream of dietary interventions hold the promise to develop new interventions to improve health during aging. Such targeted dietary interventions may activate specific pathways that are included in the general set of responses that are targeted by classical DR (Fig. 2). As such these studies could improve our understanding which components of the diet contribute to induction of health-promoting signals/mechanisms in response to DR. Moreover, it is possible that restriction of specific dietary components could have better compliance rates than a generalized food restriction as conducted under classical DR. As such the restriction of specific food components could have important roles to achieve DR-mediated health benefits without a classic/strict DR regimen in the aging human population. However, cross-species differences have to be investigated. Evidence is emerging that ketogenic diets in humans may have adverse effects on cardiovascular health [39], which may counteract effects on lifespan extension as seen in laboratory mice (see above).
Finally, it is worth to note that classic DR regimes at least in mice have shown to fail to extend lifespan of mice when applied late in life [4]. Thus, also for humans it might be required to adhere to a life-long DR regimen in order to benefit from its life- and health span extending effects. Nutrient specific DR models such as intermittent methionine restriction [30] or the specific targeting of molecular pathways by drugs such as rapamycin, acarbose [40] or 17-A-estradiol [41] have shown to cause beneficial effects even if applied periodically or only at late life. For feasibility reasons these specific dietary interventions might be favorable over classic DR.
4. Evolution of nutrient stress responses and the fading of DR induced health benefits at old age
To understand mechanisms of DR-mediated health benefits and the failure of DR to induce such responses when started at old age it would be important to determine how evolution has selected gene regulatory networks and metabolic pathways that respond to DR or to dietary restriction of specific nutrient components. This could improve our mechanistic understanding of dietary restriction responses and may also give us new answers on changes in the efficacy of dietary restriction approaches to mediate health benefits when applied at different stages of the life cycle.
The disposable soma theory of aging propose that “aging” itself is not strongly selected during evolution. Instead, evolution selects for traits that are advantageous during maturation, reproduction, and rearing the next generation [42]. This biological principal imposes a strong selection pressure to optimize the “reproductive lifespan” of a species, in order to compete with others. All genetic, epigenetic, molecular and metabolic pathways are optimized to fulfill this main biological function of a species in a given time-window. According to this theoretical framework, “aging” was not selected during evolution. Instead, “aging” is the result of a drift of regulatory networks beyond the point of reproduction/rearing of the next generation (Fig. 3). Any other wiring of gene regulatory networks (for example to avoid molecular damage beyond reproductive lifespan) would be a waste of resources, and thus reduce the species’ competitiveness. There have been reports that extended lifespan has advantages for a species by enabling the help of the grand-parent generation in the upbringing of the next-next generation [43]. However, the pressure for selection of such evolutionary traits might be lower than the selection of pathways ensuring reproduction and fitness of the parental generation.
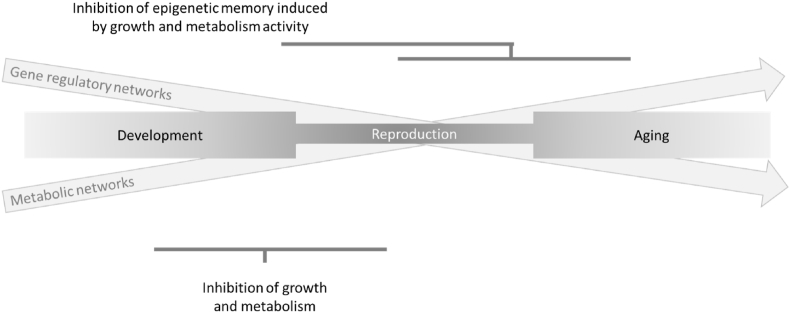
Fig. 3.
Targeting evolutionary selected regulation of gene and metabolism networks and its effects on epigenetic memory to combat aging. According to the disposable soma theory on aging gene and metabolism regulatory networks were primarily selected to coordinate development and reproduction of a species at an optimized speed. Drifts in these networks at the post-reproductive phase of life were an unwanted albeit non-selected byproduct of evolutionary selection of early life fitness. These network drifts contribute to organism aging and may also limit the induction of health-promoting stress responses by dietary restriction (DR). According to this hypothesis it may be possible to reactivate the capacity of DR to prolong organism health and lifespan in two ways: (i) DR at early life could slow down growth signaling and metabolism networks, thereby postponing the time point when networks start to drift apart, (ii) Metabolism and growth activity at young age could lead molecular damages (such as DNA damage) and thereby to deteriorations in the epigenetic landscape that impair the induction of health promoting stress response by DR. Erasure of deteriorations in the epigenetic landscapes could postponing drifts in gene and metabolism regulatory networks at post-reproductive age thereby reinstalling DR-mediated health-promoting stress responses.
In line with the hypothesis that post-reproductive drifts in regulatory networks are drivers of organismal aging, it was shown that the stoichiometry (the balance in the expression) of functionally connected gene sets is increasingly perturbed during aging, which appears to contribute to limited longevity [44]. Similarly, alteration in the stoichiometry also occur in protein complexes in aging tissue [45] and alterations in the stoichiometry of the complex of DNA-replication proteins were linked to functional declines of aging stem cells [46]. Based on the experimental support for the hypothesis outlined above, it would be important to decipher mechanisms that are responsible for the induction of age-related network deteriorations at post-reproductive age. This knowledge could guide the development of interventions that postpone the initiation or progression of age-related regulatory network drifts, which could allow for healthful aging.
Studies in mice revealed that hallmarks of hematopoietic stem cell (HSC) aging are linked to the activity of pathways that control growth and regeneration at young age. Specifically, the Lin28/let7/Igf2bp2 pathway mediates induction of mitochondrial metabolism, protein synthesis, and growth signaling in HSCs during early adult life [47]. While this activity is needed for full functionality of HSCs at young age, it also contributes to myeloid skewing of HSC differentiation - a hallmark of HSC aging [47]. The study implies that growth signalling and metabolic activity in HSCs during early life induces a memory effect that negatively impinges on HSC function at later life stages. The nature of a cellular memory that is induced in response to metabolism and growth activities during early life and how it influences tissue aging in late life remains yet to be identified.
In mice, transient induction of DNA damage leads to premature aging, which is mediated by the deterioration of the epigenetic landscape [48]. It is possible that metabolic and cell growth activity lead to DNA damage, for example by generation of ROS, which in turn aggravate the deterioration of the epigenetic landscape thereby leading to age-associated drifts in regulatory networks in late life (Fig. 3). It is well possible that such deterioration in the epigenetic landscape could also impair the regulation of gene regulatory networks that are known to contribute to DR-mediated improvements in health, such as the regulatory mechanisms that control the circadian rhythmicity in gene expression (see above). In this way, epigenetic deterioration could limit the responsiveness of aged cells and tissues to induce health promoting stress signals in response to DR ([4] and see above). Since damage induced deterioration in the epigenetic landscape likely proceeds in a random fashion, it might be necessary to employ single cell analyses in order to delineate such changes. Importantly, such techniques are now becoming more accessible for researchers [49].
An important question of future research is whether age-related drifts in gene regulatory networks (Fig. 3) could be reverted to a more youthful state? In vivo studies in mice revealed that partial reprogramming by transient induction of pluripotency factors reverts aging-associated epigenetic alterations thereby re-establishing youthful gene expression patterns, which included the regulation of stress response pathways [50]. Partial reprogramming was also shown to counteract the erosion of epigenetic landscape changes in mouse tissues that were transiently exposed to DNA damage [48]. It remains yet to be investigated whether partial reprogramming of the epigenome could also reactivate health-promoting stress responses to DR. It also remains enigmatic to what extend epigenetic reprogramming could rescue other aspects of aging that are not directly related to epigenetic alterations, as for example metabolic insults, such as mitochondrial dysfunction or declines in NAD and/or NADH levels. It is possible that some of these age-related, metabolic defects would persist even when alteration in the epigenetic control of transcriptional regulation would be reverted. If that would indeed be the case, it might be necessary to counter several aging-associated mechanisms to achieve improvements in cellular stress responses to DR at old age.
According to the disposable soma theory of aging, health-promoting stress responses (as for example to DR) were primarily selected to prolong cellular and organism function during the reproductive phase of life. The responses were not meant to prevent “aging”. We now know that health promoting signals in response to DR vanish at older age possibly due to disturbed regulation of genetic and metabolic networks at post-reproductive life cycle stages (Fig. 3). At these life cycle stages, the gene regulatory networks (also those that regulate stress responses) drift apart simply because evolution selected for the best usage of resources during early life – to speed up development, reproduction, and upbringing of the next generation thereby increasing the chance of a species to prevail against others. This evolutionary perspective appears to be of fundamental importance to understand mechanisms that inhibit DR-mediated induction of stress response at old age. Understanding evolutionary selected mechanisms of genetic and metabolic network maintenance at young age will give us new answers on how these networks deteriorate at post reproductive age. In turn, this knowledge could help to develop therapeutic interventions that aim to reinstall genetic and metabolic network stability at older age, which could rescue DR's capacity to induce health benefits even when started late in life.
5. Conclusion
DR and the induction of DR-induced nutrient stress responses is the most robust intervention to ameliorate the development of aging-associated morbidity and mortality across species. In order to enable its translation to the human population, it is important to understand, which dietary protocols are most efficient to induce health-benefits. Current research on the optimization of DR intervention focusses on the timing of such intervention (including length of fasting periods) (Fig. 1) as well as the investigation of specific food components and specific pathways that are triggered by the reduction of specific nutrients (Fig. 2). It will also be important to understand how these different pathways are connected to find the least invasive and most beneficial treatment regimens. Moreover, the failure of DR to induce health-benefits, when started in late life, is an essential concern. In human populations, lifelong DR would not be practicable due to a lack of compliance. However, efficient interventions that improve health at later stages of life would likely be accepted by a larger percentage of the population. Thus, a new perspective lies in targeting underlying causes of the late-life failure of DR in inducing health benefits. If it becomes feasible to ameliorate drifts in the regulation of nutrient stress responses at post-reproductive age (Fig. 3), it might be possible to reactivate DR-mediated improvements in late life.
Author contributions
Friedrich Becker wrote the manuscript.
Marthe Behrends wrote the manuscript.
K. Lenhard Rudolph wrote the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Friedrich Becker, Email: Friedrich.Becker@leibniz-fli.de.
Marthe M. Behrends, Email: marthebehrends@gmail.com.
K. Lenhard Rudolph, Email: Lenhard.Rudolph@leibniz-fli.de.
Data availability
No data was used for the research described in the article.
References
- 1.Green Cara L., et al. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. Jan. 2022;23(1):56–73. doi: 10.1038/s41580-021-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pak Heidi H., et al. Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat. Metab. Oct. 2021;3(10):1327–1341. doi: 10.1038/s42255-021-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acosta-Rodríguez Victoria, et al. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science (New York, N.Y.) June 2022;376(6598):1192–1202. doi: 10.1126/science.abk0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn Oliver, et al. A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat. Metab. Nov. 2019;1(11):1059–1073. doi: 10.1038/s42255-019-0121-0. 2019/10/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watroba Mateusz, Szukiewicz Dariusz. Sirtuins at the service of healthy longevity. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.724506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acosta-Rodríguez Victoria A., et al. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metabol. July 2017;26(1):267–277.e2. doi: 10.1016/j.cmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anisimov Vladimir N., et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3(2):148. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espada Lilia, et al. Loss of metabolic plasticity underlies metformin toxicity in aged Caenorhabditis elegans. Nat. Metab. 2020;2(11):1316–1331. doi: 10.1038/s42255-020-00307-1. [DOI] [PubMed] [Google Scholar]
- 9.Bartke Andrzej, et al. Early life interventions can shape aging. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.797581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen Ehud, et al. Temporal requirements of insulin/IGF‐1 signaling for proteotoxicity protection. Aging Cell. 2010;9(2):126–134. doi: 10.1111/j.1474-9726.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine Daniel C., Kuo Hsin-Yu, et al. NADH inhibition of SIRT1 links energy state to transcription during time-restricted feeding. Nat. Metab. Dec. 2021:1621–1632. doi: 10.1038/s42255-021-00498-1. 2021/12/13, vol. 3, no. 12, Nature Publishing Group UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine Daniel C., Ramsey Kathryn M., et al. Circadian NAD(P)(H) cycles in cell metabolism. Semin. Cell Dev. Biol. 2022;126:15–26. doi: 10.1016/j.semcdb.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato Shogo, et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. Aug. 2017;170(4):664–677.e11. doi: 10.1016/j.cell.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine Daniel C., Hong Heekyung, et al. NAD+ controls circadian reprogramming through PER2 nuclear translocation to counter aging. Mol. Cell. 2020;78(5):835–849.e7. doi: 10.1016/j.molcel.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Xiaolei, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metabol. Dec. 2010;12(6):662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Mouchiroud Laurent, et al. The NAD+/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantó Carles, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabol. June 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Danica, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. July 2008;22(13):1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell Sarah J., et al. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metabol. Jan. 2019;29(1):221–228. doi: 10.1016/j.cmet.2018.08.011. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatori Megumi, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabol. June 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camacho-Pereira Juliana, et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metabol. June 2016;23(6):1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McReynolds Melanie R., et al. NAD(+) flux is maintained in aged mice despite lower tissue concentrations. Cell Syst. Dec. 2021;12(12):1160–1172. doi: 10.1016/j.cels.2021.09.001. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves Philip G., Nielsen Forrest H., Fahey George C., Jr. 1993. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet; pp. 1939–1951. [DOI] [PubMed] [Google Scholar]
- 24.Martin Bronwen, et al. Control” laboratory rodents are metabolically morbid: why it matters. Proc. Natl. Acad. Sci. USA. 2010;107(14):6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf Alexander M. Rodent diet aids and the fallacy of caloric restriction. Mech. Ageing Dev. 2021;200 doi: 10.1016/j.mad.2021.111584. [DOI] [PubMed] [Google Scholar]
- 26.Vermeij W.P., et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537(7620):427–431. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solon-Biet, Samantha M., et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metabol. 2014;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts Megan N., et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metabol. 2017;26(3):539–546. doi: 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson Nicole E., et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nature Aging. 2021;1(1):73–86. doi: 10.1038/s43587-020-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plummer Jason D., Johnson Jay E. Intermittent methionine restriction reduces IGF‐1 levels and produces similar healthspan benefits to continuous methionine restriction. Aging Cell. 2022;21(6) doi: 10.1111/acel.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carazo Alejandro, et al. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. 2021;13(5):1703. doi: 10.3390/nu13051703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Njr Raica, Scott J., Lowry L., Sauberlich H.E. Vitamin A concentration in human tissues collected from five areas in the United States. Am. J. Clin. Nutr. 1972;25:291–296. doi: 10.1093/ajcn/25.3.291. [DOI] [PubMed] [Google Scholar]
- 33.van der Loo Bernd, et al. Age-related changes of vitamin A status. J. Cardiovasc. Pharmacol. 2004;43(1):26–30. doi: 10.1097/00005344-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Saeed Ali, et al. Impaired hepatic vitamin A metabolism in NAFLD mice leading to vitamin A accumulation in hepatocytes. Cell. Mol. Gastroenterol. Hepatol. 2021;11(1):309–325. doi: 10.1016/j.jcmgh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero-Gómez Manuel, Zelber-Sagi Shira, Trenell Michael. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017;67(4):829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Shindyapina Anastasia V., et al. Rapamycin treatment during development extends life span and health span of male mice and Daphnia magna. Sci. Adv. 2022;8(37) doi: 10.1126/sciadv.abo5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Yuan, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1 doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Young-Min, Ramprasath Tharmarajan, Zou Ming-Hui. β-hydroxybutyrate and its metabolic effects on age-associated pathology. Exp. Mol. Med. 2020;52(4):548–555. doi: 10.1038/s12276-020-0415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J.E., et al. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr. Metabol. Cardiovasc. Dis. 2019;29(5):513–517. doi: 10.1016/j.numecd.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Herrera Jonathan J., et al. Early or late-life treatment with acarbose or rapamycin improves physical performance and affects cardiac structure in aging mice. J. Gerontol.: Series A. 2023;78(3):397–406. doi: 10.1093/gerona/glac221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison David E., et al. 17‐a‐estradiol late in life extends lifespan in aging UM‐HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell. 2021;20(5) doi: 10.1111/acel.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkwood Thomas BL. Evolution of ageing. Nature. 1977;270(.5635):301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 43.Pavard Samuel, Coste Christophe FD. Evolutionary demographic models reveal the strength of purifying selection on susceptibility alleles to late-onset diseases. Nat. Ecol. Evol. 2021;5(3):392–400. doi: 10.1038/s41559-020-01355-2. [DOI] [PubMed] [Google Scholar]
- 44.Rangaraju Sunitha, et al. Suppression of transcriptional drift extends C. elegans lifespan by postponing the onset of mortality. Elife. 2015;4 doi: 10.7554/eLife.08833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacramento Kelmer, Erika, et al. Reduced proteasome activity in the aging brain results in ribosome stoichiometry loss and aggregation. Mol. Syst. Biol. 2020;16(6) doi: 10.15252/msb.20209596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flach Johanna, et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512(7513):198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suo Miaomiao, et al. Age-dependent effects of Igf2bp2 on gene regulation, function, and aging of hematopoietic stem cells in mice. Blood, J. Am. Soc. Hematol. 2022;139(17):2653–2665. doi: 10.1182/blood.2021012197. [DOI] [PubMed] [Google Scholar]
- 48.Yang Jae-Hyun, et al. Loss of epigenetic information as a cause of mammalian aging. Cell. 2023;186(2):305–326. doi: 10.1016/j.cell.2022.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trapp A., Kerepesi C., Gladyshev V.N. Profiling epigenetic age in single cells. Nat Aging. 2021;1:1189–1201. doi: 10.1038/s43587-021-00134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Browder Kristen C., et al. In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice. Nature Aging. 2022;2(3):243–253. doi: 10.1038/s43587-022-00183-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.