Abstract
We sought to characterize developmental trajectories of EEG spectral power over the first 2 years after birth and examine whether family income or maternal education alter those trajectories. We analyzed EEGs (n = 161 infants, 534 EEGs) collected longitudinally between 2 and 24 months of age, and calculated frontal absolute power across 7 canonical frequency bands. For each frequency band, a piecewise growth curve model was fit, resulting in an estimated intercept and two slope parameters from 2 to 9 months and 9–24 months of age. Across 6/7 frequency bands, absolute power significantly increased over age, with steeper slopes in the 2–9 month period compared to 9–24 months. Increased family income, but not maternal education, was associated with higher intercept (2–3 month power) across delta–gamma bands (p range = 0.002–0.04), and reduced change in power between 2 and 9 months of age in lower frequency bands (delta-alpha, p range = 0.01–0.02). There was no significant effect of income on slope between 9 and 24 months. EEG intercept and slope measures did not mediate relationships between income and 24-month verbal and nonverbal development. These results add to growing literature concerning the role of socioeconomic factors in shaping brain trajectories.
Keywords: EEG, Neurodevelopment, Socioeconomic status, Infancy
1. Introduction
The brain grows rapidly during the first year after birth, more than doubling in size with rapid bursts of synaptogenesis and myelination that facilitate connectivity across the brain (Gilmore et al., 2012, Gilmore et al., 2018, Knickmeyer et al., 2008). This period of neurobiological growth and organization sets the foundation for the emergence of expected developmental milestones across childhood and adolescence. Moreover, growing evidence suggests that socioeconomic status and associated variables (SES; e.g. income level, access to resources, parental stress, parental education) may directly or indirectly impact the developing brain, especially during the first year of life (Farah, 2018, Noble et al., 2015).
There are many reasons that associations between SES-related variables and early neurodevelopment (EEG) might be observed. During early periods of rapid neurodevelopment, the brain is particularly sensitive to input from the environment(Greenough et al., 1987). Experiences that occur during those early periods fine-tune cortical development, allowing the brain to adapt to the environment it encounters (e.g., Greenough et al., 1987). As a result, early experiences—including experiences associated with family SES (e.g., family income and caregiver education)—might shape the brain in a manner that is particularly consequential and long-lasting (e.g., Nelson and Gabard-Durnam, 2020). Prior research has demonstrated associations between SES-related variables and features of children’s early environments. For example, SES-related variables, including family income and maternal education, have been associated with variation in caregiver-child interactions (e.g., responsive caregiving), the quantity, quality, and complexity of language to which children are exposed, exposure to stress, environmental factors (e.g., nutrition, pollution), and the resources to which children and families have access (e.g., medical care pre- and postnatally, safe neighborhoods, quality early childcare and education) (Evans, 2004, Hoff, 2003, Kim et al., 2013, Rowe, 2018, Shonkoff et al., 2009). SES-related variables are also associated with both structural and functional brain development in the early years; for example, in s/MRI studies, lower levels of family income are associated with reduced volume and network connectivity in multiple areas of the brain that mediate language, memory, and executive functioning (e.g., Barch et al., 2022; Farah, 2018; Noble et al., 2015), suggesting that the kinds of experiences children from varying socioeconomic backgrounds encounter may act to shape early neurodevelopment in an experience-driven manner.
Changes in brain development in infancy can also be measured non-invasively using electroencephalography (EEG), which captures network-level oscillatory activity at the scalp surface, and can thereby reflect developmental changes in neuronal activity as well as neural organization and connectivity across brain regions. EEG power, a measure of cortical activity, is often measured across canonical frequency bands (e.g., theta: 4–6 Hz, gamma: 30–55 Hz). Cortical activity within these frequency ranges is thought to reflect different cognitive and behavioral states, and variation in power is also associated with individual differences in behavior (Buzsaki and Draguhn, 2004, Mathalon and Sohal, 2015). For example, EEG power in the gamma frequency range is associated with higher-order cognitive processes such as language processing and sensory integration (Benasich et al., 2008, Engel and Singer, 2001, Wang, 2010). Developmental changes in EEG power are thought to reflect cortical growth, maturation, and organization of the developing brain (e.g.,Anderson and Perone, 2018). Changes in EEG power may arise from structural changes in the brain, including pruning and myelination that accompany changes in functional organization (Anderson and Perone, 2018, Smit et al., 2012). One illustration of these changes is a developmental shift in the ratio of low (e.g., theta) to high (e.g., beta, gamma) frequency power that has been observed across early childhood. This shift is thought to reflect cortical maturation and increasingly efficient neural organization, while a deviation from this pattern—relatively lower power in high frequency bands and higher power in low frequency bands—is proposed to reflect an experience-driven maturational lag in neurodevelopment (Anderson and Perone, 2018, Saby and Marshall, 2012). For example, reduced power in high frequency bands has been observed in children who faced severe early psychosocial adversity/neglect (Marshall et al., 2004), children whose mothers reported high levels of stress (Pierce et al., 2019), and children from low-SES backgrounds. Higher family income has been associated with higher gamma power from as early as 6–9 months after birth (Brito et al., 2016, Cantiani et al., 2019, Tomalski et al., 2013), and higher maternal education has been positively associated with beta and gamma power by 2 months (Pierce et al., 2019). Higher relative theta power, and lower relative alpha power, has also been observed for older children (7–12 years) living in a low- compared to high-SES context (Harmony et al., 1988). Importantly, reduced EEG power in these studies may have been attributable to factors more proximal to the child (e.g., prematurity) that are prevalent in low-SES contexts (Harmony et al., 1990). Recent experimental studies have also investigated the impact of altering income level through cash supplements (Troller-Renfree et al., 2022) or maternal paid leave (Kozak et al., 2021) on infant EEG power. In these studies, 3-month-old infants whose families received additional income showed increased power in higher-frequency bands (e.g., beta and gamma) compared to those who did not receive income supplements, suggesting a potential causal link between family socioeconomic status and EEG power during infancy.
EEG power during infancy has also been linked to cognitive and language outcomes. For example, alpha power at 8-months has been associated with concurrent working memory (Bell, 2001), while infant alpha power also predicts executive function at preschool and kindergarten age (Kraybill and Bell, 2013). As reported earlier, baseline gamma power in newborns predicts language and memory scores at 15 months, while baseline gamma power at 16, 24, and 36-months has been found to predict language abilities at 4 and 5 years (Benasich et al., 2008, Gou et al., 2011). Critically, baseline gamma power at 6 months has also been found to mediate associations between SES and language complexity at 24 months (Cantiani et al., 2019), suggesting a causal role of gamma oscillations in language development—perhaps due to its role in sensory integration and perceptual binding. Overall the above observations suggest that associations between SES and later developmental outcomes may in part be mediated the impact of SES-related variables on brain development.
Surprisingly, only a handful of studies have evaluated developmental trajectories of EEG power over the first 2 years of life, and most have focused on specific frequency bands at specific ages. It is therefore unclear when SES is most strongly associated with infant brain development. The few studies that have reported longitudinal EEG trajectories early in infancy have found that EEG absolute power across the broad range of frequencies (3–50 Hz) increases between 3 and 9 months and then typically plateaus (Gabard-Durnam et al., 2019, Jing et al., 2010), with the most substantial growth occurring between 3 and 6 months. In addition, longitudinal studies of infancy and early childhood have observed a shift in the frequency at which power in lower frequency ranges (4–12hz) is at its peak―specifically, the peak at 5 months is observed in the theta range (4–6 hz) and then shifts to the low-alpha range (6–9 Hz) by 2 years of age (Cellier et al., 2021, Marshall et al., 2002). Overall, such developmental changes in the power and peak frequency are thought to reflect maturation of structural and functional brain development and corresponding gains in cognitive and behavioral abilities.
The current study has three main objectives. First, we aimed to map longitudinal trajectories of spectral power over the first 2 years after birth in a socioeconomically diverse sample of infants in order to determine typical trends in neural development over this period. Second, we examined whether and how family income and maternal education are associated with trajectories of spectral power over the first two years of life. Here, we hypothesized that both maternal education and income would have significant positive associations with change in power over time (i.e., slope), especially during the first 12 months. Previous studies suggest that differences would be observed in higher-frequency power (Cantiani et al., 2019, Kozak et al., 2021, Tomalski et al., 2013, Troller-Renfree et al., 2022); however, since few studies have explored the full frequency spectrum longitudinally, we included seven canonical frequency bands (delta, theta, low alpha, high alpha, beta, gamma, and high gamma) in our analyses. Finally, for any significant predictors of EEG trajectories (income or maternal education) we aimed to test whether EEG power over time mediated the association between that SES-related variable and verbal and non-verbal developmental skills at 24 months.
2. Methods
2.1. Studies and participants
EEGs were collected as part of two longitudinal studies occurring over a 13-year period within the same lab. Sample numbers for each study are shown in Table 1. In Study 1 (IRB#P0001908), participants were recruited from the Boston Children’s Hospital Primary Care Center which predominantly serves families from low-income backgrounds. Participants were enrolled at 2 months of age and EEG data collected at 2, 6, 9, 12, and 24 months were analyzed.
Table 1.
Testing Optimal Model Fit Across Growth Models.
| EEG Construct | Chi-square | D.F. | CFI | TLI | RMSEA | SRMR | AIC | BIC | SABIC |
|---|---|---|---|---|---|---|---|---|---|
| Delta Frontal Linear Quadratic Piecewise |
64.152*** 40.179*** 14.452* |
10 6 6 |
0.187 0.487 0.873 |
0.187 0.145 0.788 |
0.183 0.188 0.094 |
0.326 0.174 0.074 |
-364.297 -380.270 -405.997 |
-333.483 -337.130 -362.858 |
-365.140 -381.450 -407.178 |
| Theta Frontal Linear Quadratic Piecewise |
79.789*** 30.049*** 8.381 |
10 6 6 |
0.000 0.647 0.965 |
0.000 0.412 0.942 |
0.208 0.158 0.050 |
0.405 0.141 0.055 |
-379.710 -421.450 -443.118 |
-348.896 -378.310 -399.978 |
-380.553 -422.630 -444.298 |
| Low Alpha Frontal Linear Quadratic Piecewise |
71.041*** 24.007*** 5.427 |
10 6 6 |
0.423 0.830 1.000 |
0.423 0.716 1.000 |
0.195 0.137 0.000 |
0.366 0.117 0.058 |
-353.723 -392.756 -411.337 |
-322.909 -349.617 -368.197 |
-354.566 -393.937 -412.517 |
| High Alpha Frontal Linear Quadratic Piecewise |
52.456*** 33.704*** 11.427 |
10 6 6 |
0.670 0.784 0.958 |
0.670 0.641 0.930 |
0.162 0.169 0.075 |
0.324 0.179 0.047 |
-464.584 -475.337 -497.613 |
-433.770 -432.197 -454.474 |
-465.427 -476.517 -498.794 |
| Beta Frontal Linear Quadratic Piecewise |
69.031*** 49.492*** 10.376 |
10 6 6 |
0.617 0.718 0.972 |
0.617 0.529 0.953 |
0.191 0.212 0.067 |
0.646 0.361 0.064 |
-446.078 -457.617 -496.733 |
-415.264 -414.478 -453.594 |
-446.921 -458.798 -497.913 |
| Gamma Frontal Linear Quadratic Piecewise |
87.563*** 34.120*** 5.827 |
10 6 6 |
0.508 0.822 1.000 |
0.508 0.703 1.000 |
0.219 0.171 0.000 |
0.819 0.292 0.068 |
-387.150 -432.592 -460.886 |
-356.336 -389.452 -417.746 |
-387.993 -433.772 -462.066 |
Note. The Sample adjusted BIC uses as sample size the following modification: [n * = (n + 2) / 24]. Quadratic and piecewise models had the same number of degrees of freedom thus the reader is advised to consult the estimates from the information criteria.
* p < .05. ** p < .01. *** p < .001
In Study 2 (IRB#X06–080374), infants were recruited from the metro Boston area starting at 3 or 6 months of age. This study recruited both infants with and without familial risk for autism spectrum disorder (ASD); however, for this analysis only children without familial risk for ASD were included. Similar to Study 1, EEG data collected 3, 6, 9, 12, 18, 24 months of age were analyzed.
For both studies, all infants had a minimal gestational age of 36 weeks, no history of prenatal or postnatal genetic, metabolic, or neurological problems, and no known genetic disorders. Infants who were later diagnosed with ASD (either by assessment during the study, or by community diagnosis disclosed by parents) were excluded from the analysis. IRB approval was obtained for combined analysis of combined data sets.
2.2. EEG data collection
Baseline (non-task-related) EEG data were collected using similar protocols and testing rooms for both studies. Recordings were completed in dimly lit, sound attenuated rooms with low electrical signal background. Continuous EEG was collected for 2–5 min using either a 64-channel Geodesic Sensor (12.5% of total EEGs) or 128-channel Hydrocel Geodesic Net (Electrical Geodesics, Inc., Eugene, OR) connected to a NetAmps 300 amplifier for Study 1, and either a NetAmps 200 or 300 amplifier for Study 2, and referenced online to a single vertex electrode (Cz). Data were sampled at 500 Hz for Study 1, and either 250 or 500 Hz for Study 2 with mean impedance kept below 100 kΩ. In Study 1, infants were 60 cm from a computer monitor and either held over their parent’s shoulder or seated forward on their parent’s lap while watching a video of infant toys while a trained research assistant engaged minimally, sometimes using bubbles or a toy to help keep the infant calm. In Study 2, no video was shown, but similar to Study 1, the infant was held by their parent while a research assistant helped keep the infant calm using bubbles or toys intermittently.
2.2.1. EEG pre-processing
Raw Netstation (Electrical Geodesics, Inc) files were exported to MATLAB (version R2017a) for preprocessing and absolute power calculations were conducted using the Batch Automated Processing Platform (BEAPP) (Levin et al., 2018) with integrated Harvard Automated Preprocessing Pipeline for EEG (HAPPE) (Gabard-Durnam et al., 2018). Using the automated BEAPP/HAPPE pipeline improves reproducibility and replicability of our analysis. The following parameters were used: For each EEG, a 1 Hz high-pass and 100 Hz low-pass filter were applied, data were sampled at 500 Hz and were resampled to 250 Hz, and then were run through the HAPPE module consisting of 60 Hz line noise removal, bad channel rejection, and artifact removal using combined wavelet-enhanced independent component analysis (ICA) and Multiple Artifact Rejection Algorithm (MARA) (Winkler et al., 2011, Winkler et al., 2015). The following channels, in addition to the 10–20 electrodes, were used for MARA: 64-channel net: 16, 9, 8, 3, 58, 57, 21, 25, 18, 30, 43, 50, 53, 32, 33, 38, 41, 45; and 128-channel net: 28, 19, 4, 117, 13, 112, 41, 47, 37, 55, 87, 103, 98, 65, 67, 77, 90, 75. These electrodes were chosen as they evenly cover all brain regions of interest (Fig. 1) and have corresponding matches across 64 and 128 channel nets. After artifact removal, channels removed during bad channel rejection were then interpolated, data were referenced to the average reference, detrended to the signal mean, and segmented into 2-second segments to allow for power calculations using multitaper spectral analysis (Babadi and Brown, 2014). Any segments with retained artifact were rejected using HAPPE’s amplitude and joint probability criteria.
Fig. 1.
Electrodes used for HAPPE preprocessing for 128- and 64-channel nets. 10–20 electrodes are in pink. Electrodes used for power analysis are circumscribed in black.
2.2.2. EEG rejection criteria
EEG recordings were rejected using the following HAPPE data quality measures and visual inspection as previously described(Gabard-Durnam et al., 2018, Gabard-Durnam et al., 2019): Fewer than 20 segments (40 s of total EEG), percent good channels < 80%, percent independent components rejected > 84%, mean artifact probability of components kept > 0.25, and percent variance retained < 20%. In addition, recordings with mean power greater or less than 2 SD from the group mean for each frequency band were visually reviewed for further rejection. Overall, this method resulted in 28 (5%) of recordings being rejected. Quality metrics of EEG data included in analyses are shown in Table 1.
2.2.3. EEG power analysis
The power spectral density at each electrode for each 2 s segment was calculated in the BEAPP PSD module using a multitaper spectral analysis (Babadi and Brown, 2014, Levin et al., 2018) using three orthogonal tapers. Previous literature has observed both frontal and whole brain associations with SES and child development, and thus both were considered for our analysis (eg. Benasich et al., 2008; Brito et al., 2016; Otero, 2003; Pierce et al., 2019; Tomalski et al., 2013; Troller-Renfree et al., 2020). Channels clustered over the frontal cortex were analyzed and channels with overlapping spatial locations for 64-channel and 128-channel nets were chosen (64-channel net: 16, 13, 8, 3, 62, 57, 9, 58; 128-channel net: 28, 24, 19, 11, 4, 124, 117, 13, 112; Fig. 1). Whole brain analysis included all blue and pink electrodes shown in Fig. 1. A statistical analysis of invariance across studies is described below (Section 2.4.1) and found that growth parameters using frontal electrodes had better parsimony allowing for single model across studies. Power across six frequency bands was calculated as mean power per Hz across the following canonical frequency ranges previously used in longitudinal infant and toddler studies (Gabard-Durnam et al., 2019, Tierney et al., 2012): delta (2–3.99 Hz), theta (4–5.99 Hz), low alpha (6–8.99 Hz), high alpha (9–12.99 Hz), beta (13–29.99 Hz), gamma (30–45 Hz), high gamma (65–90 Hz).
2.3. Developmental assessment
The Mullen Scales of Early Learning (MSEL) (Mullen, 1995), a standardized developmental assessment for children 0–68 months of age was administered by trained and reliable research assistants at 24 months. The Nonverbal and Verbal Developmental Quotients were calculated based on the age equivalents of the 4 subscales (Fine Motor, Visual Reception, Expressive Language, and Receptive Language) and the child’s chronological age.
2.4. Statistical analyses
2.4.1. Longitudinal modeling of EEG power parameters
Several growth curve models (linear, quadratic, and piecewise) were estimated to identify the optimal trajectory of EEG power. Linear and quadratic growth both offered poor model fit (comparative fit index [CFI] linear models: range 0–0.67; quadratic models: range 0.49–0.83). Given the shape of the trajectories, a piecewise model that estimated an intercept and two separate slopes (slope 1 between 3 and 9 months, and slope 2 between 9 and 24 months) was assessed. Acceptable fit was observed for the piecewise growth model across all dependent variables except for delta, as evidenced by descriptive fit indices, e.g., CFI > 0.95 (range 0.95–1), unstandardized residual values (i.e., RMSEA) less than 0.08 (range 0–0.075), and a non-significant Chi-square statistic indicative of “exact model fit”. While delta fit indices were not ideal (CFI = 0.873, RMSEA 0.094), they were better than the linear and quadratic models (Table 1). Furthermore, since comparisons between quadratic and piecewise models were not possible using inferential statistical tests (as both models had the same degrees of freedom), evidence of model superiority was provided by information criteria. Across all models, the values of the AIC, BIC and SABIC criteria were smaller in the piecewise model compared to the quadratic model, thus supporting the superiority of the piecewise model over the linear and quadratic growth models (Table 1).
Sample characteristics and age at the first EEG collection were significantly different between studies; thus, prior to estimating growth curves, we first verified that data from the two studies were invariant with respect to the intercept (first timepoint) and growth parameters within the piecewise models. A multi-group approach was implemented in which intercepts and slopes were specified to be invariant across samples from Study 1 and Study 2. Significant differences in intercepts and slopes would signal heterogeneity and prevent the use of single model that included both samples. A global test was used which contrasted two models―one in which intercept and slope parameters were allowed to freely vary across studies, and one in which they were specified to be equivalent across studies. In addition, a local test was performed in which a series of z-tests in which one parameter at a time was specified to be equivalent across studies. Results are shown in Supplemental Table 1. One global test (frontal gamma) suggested non-invariance (p = .045); however, local tests of intercept and slopes for frontal gamma were not significant (p = .06–.68). One local test (theta slope from 9 to 24 months) was significant (p = .042); however, the global test and other local tests were not significant (p = .16–.42). After False Discovery Rate (FDR) correction for multiple comparisons, no tests were significant and full invariance across study samples was confirmed. Thus, the two samples were combined into a single sample for all subsequent analyses. A similar analysis was completed for power measures averaged across the whole brain, however this did show variance between studies, and thus we proceeded with the single sample from frontal electrodes described above.
2.4.2. Mediation analysis
A mediation analysis was performed to examine the degree to which any associations between family income and verbal and non-verbal skills were mediated by EEG measures. Here, the indirect effects of income on verbal and non-verbal skills were estimated as the product of two regression slopes, one predicting EEG from family income (‘a’ path) and one predicting developmental outcomes from EEG levels (‘b’ path). The significance of each indirect effect was tested by use of a z-test and bootstrapping. All models tested for linear relationships using a saturated regression analysis model. However, because the distribution of indirect effects is likely non-normal, particularly with modest sample sizes, bias-corrected non-symmetric 95% confidence intervals were created using bootstrap standard errors from 10,000 replicated samples. Those results agreed with the results from the z-test testing the significance of the indirect effect across all instances.
2.4.3. Statistical power analysis considerations
A Monte Carlo simulation was run to estimate the statistical power for growth slopes equal to 1 and 0.5 in standardized units in order to reflect the two types of slopes, an accelerated one and one that was less steep. Furthermore, the intercept was set to − 0.5 as it was mostly negative across models. Results indicated that a sample of n = 160 participants was associated with power levels in excess of 99% for both specified slopes and the intercept term, using 1000 replicated samples. All growth curve analyses were conducted using Mplus 8.9.
Statistical power for each mediation model was estimated using a Monte Carlo simulation with paths being set to a standardized value of 0.50 (using Cohen’s d effect size convention on what constitutes a medium effect) by fixing item means and variances to 0 and 1, respectively. Based on the sample size of n = 90 and 10,000 replications, coverage of the direct and indirect paths ranged from 94.8% to 95%. Power ranged from 98.3% to 99.6%. The largest parameter bias estimate was 0.01, which was well below 1% and far below 5%− 10%, the recommended cutoff values. All mediation analyses were conducted using Mplus 8.9.
3. Results
3.1. Participant characteristics
A total of 161 infants contributed EEG data to this analysis. Demographic data are shown in Table 2 for the full sample, as well as separated for Study 1 and Study 2. 37.5% of participants identified as a race other than white, and at least 15% reported income levels below $35,000 (125% of the Federal Poverty Level for a family of 4). Study 1 had significantly lower scores on developmental assessment, as measured by the MSEL Verbal and Nonverbal Quotients.
Table 2.
Sample demographics and EEG quality metrics.
| Full Sample N = 161 |
Study 1 N = 50 |
Study 2 N = 111 |
|
|---|---|---|---|
| Sex | 87M, 74F | 26M, 24F | 61M, 50F |
| Ethnicity,n (%) | |||
| Not Hispanic or Latino | 146 (91) | 41 (82) | 105 (94.5) |
| Hispanic or Latino | 13 (8) | 8 (16) | 5 (4.5) |
| Not Answered | 2 (1) | 1 (1) | 1 (1) |
| Race,n (%) | |||
| White | 97 (60) | 6 (12) | 91 (82) |
| Black or African American | 32 (20) | 26 (52) | 6 (5) |
| Asian | 5 (3) | 2 (4) | 3 (3) |
| Mixed Race | 17 (10.5) | 7 (14) | 10 (9) |
| Other | 6 (4) | 6 (12) | 0 (0) |
| Not answered | 4 (2.5) | 3 (6) | 1(1) |
| Household income,% (n) | |||
| < $35,000 | 24 (15) | 16 (32) | 8 (7) |
| $35,000 - $75,000 | 22 (14) | 9 (18) | 13 (12) |
| > $75,000 | 84 (52) | 11 (22) | 73 (66) |
| Not answered | 31 (19) | 14 (28) | 17 (15) |
| Maternal Education,% (n) | |||
| < High School | 7 (4) | 6 (12) | 1 (1) |
| High school | 21 (13) | 20 (40) | 1 (1) |
| Associates Degree | 21 (13) | 11 (22) | 10 (9) |
| 4 year college degree | 27 (17) | 6 (12) | 21 (19) |
| > 4 year college degree | 68 (42) | 4 (8) | 64 (57.5) |
| Not answered | 17 (11) | 3 (6) | 14 (12.5) |
| Number of EEGs at each age | |||
| All ages | 534 | 196 | 338 |
| 2–3 months | 68 | 48 | 20 |
| 6 months | 126 | 43 | 83 |
| 9 months | 133 | 41 | 92 |
| 12 months | 128 | 42 | 86 |
| 24 months | 79 | 22 | 57 |
| EEG quality metrics | |||
| Number of Segments | 101.7 ± 44.6 | 129.0 ± 25.4 | 86 ± 45.8 |
| Percent Good Channels | 92.3 ± 4.7 | 92.2 ± 4.8 | 92.5 ± 4.6 |
| Percent ICs Rejected | 36.2 ± 10.2 | 36.4 ± 11.0 | 36.1 ± 9.8 |
| Percent Variance Kept of Post Waveleted Data | 64.7 ± 15.4 | 62.7 ± 16.2 | 65.8 ± 14.8 |
| Mean Artifact Probability of Kept ICs. | 0.12 ± 0.04 | 0.12 ± 0.04 | 0.12 ± 0.04 |
| 12-month Mullen Scales of Early Learningmean (SD) | |||
| N = 140–142 | N = 40–42 | N = 100 | |
| Verbal Quotient | 97.5 (13.0) | 96.4 (11.7) | 97 (13.5) |
| Nonverbal Quotient | 117 (12.6) | 109.2 (9.4) | 121 (12.1) |
| 24-month Mullen Scales of Early Learningmean (SD) | |||
| N = 105 | N = 26 | N = 79 | |
| Verbal Quotient | 107.8 (20.7) | 87.7 (20.5) | 114.4 (16.1) |
| Nonverbal Quotient | 104.6 (15.7) | 93.3 (13.7) | 108 (14.64) |
3.2. Piecewise growth models of EEG power
A total of 534 EEGs were analyzed over time. Developmental trajectories of frontal EEG power across the seven frequency bands are shown in Fig. 2, with mean trajectories for Study 1 and Study 2 shown in blue and orange, respectively. A piecewise model was found to be superior over both linear and quadratic models, and suggested the presence of two distinct slopes―a steep slope characterizing an increase in EEG power between 2 and 9 months, and a second slope with less steep but continued linear growth between 9 and 24 months (Table 1). Tests of significance for intercepts and slope were performed, and all but one (gamma slope 2) were significantly different from zero, suggesting increased power over time for both segments across all frequency bands (Table 3).
Fig. 2.
EEG absolute power from 2 to 24 months of age. Mean log of EEG power is shown across each frequency band across binned visit ages for the full data set in black, Study 1 in blue, and Study 2 in orange. Shaded area shows 95% confidence intervals around the full data set mean.
Table 3.
Intercepts and Slopes for Piecewise Growth Model Across EEG Variables.
| EEG Construct | b | S.E. | Z-test | p-value |
|---|---|---|---|---|
| Delta Frontal Intercept Slope 1 (3–9 months) Slope 2 (9–24 months) |
-0.200*** 0.080*** 0.033*** |
0.032 0.009 0.009 |
-6.331 8.857 3.603 |
< 0.001 < 0.001 < 0.001 |
| Theta Frontal Intercept Slope 1 (3–9 months) Slope 2 (9–24 months) |
-0.325*** 0.096*** 0.023** |
0.029 0.008 0.009 |
-11.097 11.360 2.657 |
< 0.001 < 0.001 0.008 |
| Low Alpha Frontal Intercept Slope 1 (3–9 months) Slope 2 (9–24 months) |
-0.477*** 0.104*** 0.071*** |
0.028 0.008 0.009 |
-17.148 12.594 8.286 |
< 0.001 < 0.001 < 0.001 |
| High Alpha Frontal Intercept Slope 1 (3–9 months) Slope 2 (9–24 months) |
-0.670*** 0.074*** 0.082*** |
0.025 0.008 0.008 |
-26.413 9.791 9.668 |
< 0.001 < 0.001 < 0.001 |
| Beta Frontal Intercept Slope 1 (3–9 months) Slope 2 (9–24 months) |
-1.002*** 0.088*** 0.036*** |
0.027 0.008 0.009 |
-36.752 11.144 4.202 |
< 0.001 < 0.001 < 0.001 |
| Gamma Frontal Intercept Slope 1 (3–9 months) Slope 2 (9–24 months) |
-1.296*** 0.095*** 0.013 |
0.027 0.008 0.009 |
-48.146 12.118 1.496 |
< 0.001 < 0.001 0.135 |
| High Gamma Frontal Intercept Slope 1 (3–9 months) Slope 2 (9–24 months) |
-1.812*** 0.055*** 0.062*** |
0.030 0.008 0.012 |
-61.165 6.488 5.342 |
< 0.001 < 0.001 < 0.001 |
3.3. Association between income and maternal education with EEG power trajectories
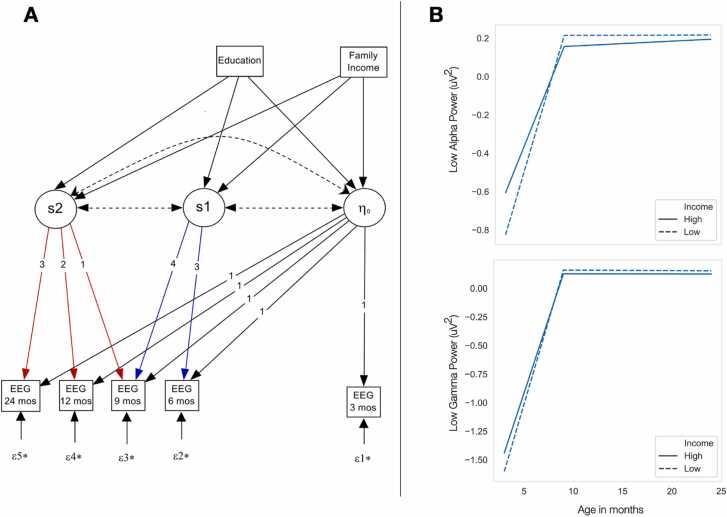
Next, we investigated the association between income and maternal education with developmental trajectories of EEG power. Specifically, we regressed the intercept and two slope parameters of the piecewise model on income and maternal education (both included as ordered categorical variables, as shown in Table 2) simultaneously to account for their potential overlap (Fig. 3A). As shown in Table 4, across almost all EEG frequencies bands (delta to gamma), increased family income was associated with significantly higher intercept (2–3 month power). Further, for lower frequency bands (delta to high alpha), increased income was also associated with a less steep positive slope between 3 and 9 months. Piecewise model estimates for low alpha and low gamma are shown in Fig. 3B. There was no significant effect of income on the second slope term. Further, maternal education was not significantly associated with any growth curve measures.
Fig. 3.
A. Piecewise model for EEG growth trajectory. B. Model estimates for Low Alpha (top) and Gamma (bottom) power for High and Low income groups.
Table 4.
Association between Family Income and Maternal Education on EEG Trajectory Measures.
| Prediction | Intercept | Slope 1: 3–9 Months | Slope 2: 9–24 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family Income | Maternal Education | Family Income | Maternal Education | Family Income | Maternal Education | |||||||
| B | P value | B | P value | B | P value | B | P value | B | P value | B | P value | |
| Delta | 0.105 | 0.013 | -0.013 | 0.647 | -0.030 | 0.021 | 0.002 | 0.833 | 0.018 | 0.282 | -0.003 | 0.759 |
| Theta | 0.098 | 0.020 | -0.006 | 0.842 | -0.29 | 0.022 | 0.001 | 0.996 | 0.021 | 0.181 | -0.002 | 0.849 |
| Low Alpha | 0.110 | 0.005 | -0.010 | 0.689 | -0.29 | 0.018 | -0.003 | 0.676 | 0.018 | 0.255 | 0.002 | 0.822 |
| High Alpha | 0.105 | 0.002 | -0.002 | 0.924 | -0.28 | 0.011 | -0.002 | 0.795 | 0.007 | 0.631 | -0.001 | 0.927 |
| Beta | 0.079 | 0.037 | 0.012 | 0.631 | -0.17 | 0.146 | -0.004 | 0.566 | 0.004 | 0.779 | 0.002 | 0.834 |
| Gamma | 0.081 | 0.043 | 0.007 | 0.791 | -0.16 | 0.182 | -0.003 | 0.713 | 0.002 | 0.918 | 0.004 | 0.693 |
| High Gamma | 0.059 | 0.197 | -0.032 | 0.282 | -0.21 | 0.120 | 0.007 | 0.411 | 0.021 | 0.284 | -0.002 | 0.875 |
3.4. Mediation analysis
Within our data set, there was a significant association between family income and developmental outcomes as measured on the MSEL (Verbal and Nonverbal Developmental Quotients). For every one unit categorical increase in family income, Developmental Quotients increased by 10–17 points (p < 0.01; see Supplemental Table 2). Given this, we next assessed whether differences in EEG intercept and/or slope (i.e. Slope 1) mediated the reassociation between income and developmental outcomes (MSEL Verbal and Nonverbal Developmental Quotients). No mediated/indirect effects of EEG measures on the association between family income and developmental outcomes were observed (Supplemental Table 2).
4. Discussion
In this study, we sought to examine the growth trajectory of EEG spectral power from 2 to 24 months after birth in a diverse sample of infants, and to determine whether family income or maternal education are associated with trajectory parameters. We then aimed to examine whether EEG trajectory parameters mediated the association between family income and/or education with developmental outcomes at the endpoint of those trajectories (i.e., 24 months). We observed that absolute EEG power across all frequency bands increased over the first two years of life and was best modeled using a piecewise fit: growth trajectories were characterized by a steep increase in power between 2 and 9 months, followed by a less steep, but continued increase in power between 9 and 24 months. We also observed that increased family income, but not maternal education, was associated with differences in trajectory parameters, in particular power at the first wave of assessment (i.e., the intercept), which corresponded to 2–3 months of age. Although family income was positively associated with both verbal and non-verbal outcomes at 24 months, neither the intercept nor slope parameters of the EEG trajectories mediated these associations.
4.1. Growth trajectories in EEG power
Absolute EEG power is comprised of both broadband neuronal firing as well as oscillatory activity occurring at the frequency band of interest (Donoghue et al., 2020, Donoghue et al., 2021). Early developmental changes in EEG activity likely represent various aspects of brain development including cortical growth and synaptogenesis, changes in excitatory and inhibitory circuitry, as well as the maturation of thalamocortical connections known to be crucial to regulating oscillatory activity in the brain (Lopes da Silva, F, 1991, Tröndle et al., 2022). Here, we observe a striking increase in absolute power between 2 and 9 months of age across all frequency bands. This global increase in power across all frequency bands prior to 9 months suggests that at least a portion of the observed increases in power are due to changes in broadband neuronal firing. We hypothesize that these changes reflect known increases in brain size, neuronal number, and synaptic connections occurring during the first year after birth. Infant MRI studies observe a doubling of brain volume and cortical gray matter in the first year of life compared to only a 15–20% increase in the second year (Gilmore et al., 2012, Knickmeyer et al., 2008). Few studies have evaluated EEG trajectories of absolute power in the first year after birth. A landmark study by Matousek and Petersen (Matousek and Petersen, 1973, Matousek and Petersén, 1971) is frequently cited as showing age-dependent decreases in low frequency (delta, theta) power and increases in high frequency (alpha, beta) bands. However, the Matousek and Peterson study starts at one year of age and extends to adolescence, missing the 2–9 month period where in our study we observe rapid increases in EEG power across the frequency spectrum. It is also difficult to directly compare our findings with previous infant longitudinal studies investigating relative power, as relative power is defined by the absolute power of a discrete frequency band divided by a larger “total” frequency band. For example, in Marshall et al. (2002), EEG data was collected longitudinally between 5 and 51 months and analyzed by focusing on relative power of 1 Hz bins between 3 and 10hz. They observed an age-dependent shift in which 1 Hz frequency bin had highest relative power; 4–6hz bins were strongest at 5 months of age, and 6–9 Hz bins were strongest from 10 to 51 months. While our study focuses on absolute power, qualitative differences in theta and alpha trajectories are observed, suggesting developmental shifts in oscillatory power occurring in the theta and alpha range. We observe that alpha power has more pronounced increases in power between 9 and 24 months compared to theta power (Fig. 2). This is also observed in the Slope 2 coefficients of our piecewise growth models (Table 3), where high-alpha coefficients are similar between Slope 1 and 2, whereas theta Slope 2 coefficients are substantially reduced compared to Slope 1. Overall, these findings are consistent with shifts in peak power from the theta to the alpha frequency range over the first year of life.
It is important to recognize that absolute spectral power represents a combination of oscillatory (i.e. periodic) activity and broadband non-oscillatory (i.e. aperiodic) activity, the latter of which follows a 1/f distribution so that absolute spectral power decreases with increasing frequencies (Donoghue et al., 2021, Voytek et al., 2015). The parameterization of aperiodic and periodic activity is relatively new (Donoghue et al., 2020); however, emerging research suggests that periodic and aperiodic components have different trajectories and functional relevance (Cellier et al., 2021, Gao et al., 2017, Voytek and Knight, 2015). Thus, while out of the scope of this study, future analyses that investigates early developmental trajectories of both the aperiodic and periodic activity will be informative to understanding physiological changes occurring during early development.
This study was also limited to frontal electrodes. Initial assessments using power averaged across the whole brain showed variance across the two studies and could not be used. It is likely that there are different patterns in EEG growth trajectories between different regions of interest, and this is an area that future longitudinal analyses should investigate.
4.2. Associations between income and early infant EEG power
We observed that family income, but not maternal education, was significantly related to EEG growth trajectories. Specifically, lower income was associated with a reduced intercept (estimated EEG power at 2–3 months) for virtually all the frequency bands examined (delta-gamma). Interestingly, lower income was also associated with a steeper slope, or greater increase in power, between 3 and 9 months of age. This was most prominent for lower frequency bands (2–13hz) such that, by 9 months of age, differences in EEG power as a function of income were no longer observed (Fig. 3B). In contrast, for beta and gamma power, income was not associated with any significant differences in Slope 1 or Slope 2.
The largest effect of family income on infant EEG power was at the earliest time point, 2–3 months after birth. This suggests that income-related differences in the prenatal and neonatal environment (e.g. malnutrition, maternal stress, toxins) may impact brain development. Consistent with this finding, Pierce et al. (2019), observed associations between maternal stress and EEG power as early as 2 months old. However, another recent study (Brito et al., 2016) observed no differences in EEG power between low and high SES groups measured in sleeping neonates (12–96 h after birth). These contrasting results suggest that either effects of SES on brain development occur within a relatively small window just after birth, or that neonatal sleep EEG measures are not commensurate with awake infant EEG. It is also possible that underlying brain differences present a birth cannot be accurately measured using scalp EEG as brain differences may be obfuscated by factors related to the fetal-neonatal transition (e.g., vaginal vs c-section delivery, medications given to mother during delivery, variable transitions in neonatal endocrine, metabolic responses) (Hillman et al., 2012), or that differences are not measurable until connections between subcortical structures are further established after birth.
Studies in both 6–9 month old infants and preschool aged children have found that reduced income is associated with lower EEG power in high frequencies (Otero, 2003, Tomalski et al., 2013). Our analyses are not directly comparable to these studies as we assessed associations between income and longitudinal growth rather than associations at discrete ages. However, our findings do support different effects of income on growth of EEG power for low versus high frequency bands―specifically, reduced income was only associated with greater growth in power (Slope 1) for low frequency but not higher frequency bands. Our observation that lower income was associated with greater increases in power between 2 and 9 months (Slope 1) may also support the “Stress Acceleration Hypothesis” posited by Callaghan and Tottenham, 2016 (Callaghan and Tottenham, 2016), where increased early adversity and stress adaptively accelerate brain development, especially in regions associated with emotional learning and reactivity. Income-related differences in Slope 1 may also represent the brain’s plasticity and natural ability to compensate for early adversities. Larger studies focused on infants from low resourced families are needed to further determine what factors outside of income influence early growth trajectories and developmental outcomes.
In contrast to income, we did not find an association between maternal education and EEG growth trajectories. This was surprising as maternal education is one of the strongest facets of SES related to developmental outcomes (Carneiro et al., 2013). One possible explanation is that income-related material conditions (e.g., nutrition, access to medical care, exposure to toxins) more strongly impact very early brain development—where most of the current effects were observed (i.e., on the intercept)—whereas maternal education more directly influences the parenting environment which translates to brain changes during later periods of childhood. It is also possible that, within our study population, income and maternal education were tightly correlated which reduced our ability to statistically identify more modest and independent effects of education on EEG power.
4.3. Mediators of developmental outcomes
Although family income was associated with both verbal and non-verbal developmental outcomes at 24 months in the current study, we did not observe any mediation effect of EEG power trajectories (intercept or slope parameters) on the relation between income and developmental outcomes. It is important to note that we tested mediation effects of each component of the piecewise model (Intercept, Slope 1, and Slope 2), and not the combined effect of these measures on EEG power. We also recognize that the largest amount of missing data occurred at the 24-month visit, when developmental measures were assessed. To assess the impact of missing data on our findings, we compared our sample-based results with the averaged estimated from 50 imputed datasets. Results for all but one finding were consistent, with the one significant finding reflecting chance (i.e., 5%) level expectations. Another recent study found that early absolute beta/gamma power (25–45hz) measured at 6 months of age mediated associations between SES and 24-month language development (Cantiani et al., 2019). Future analyses within larger longitudinal data sets can further assess whether there are critical periods during which EEG power may mediate developmental outcomes, and when changes in EEG power are most malleable.
Several lines of research suggest that higher income provided just after birth may modulate early brain development. Maternal paid leave has been associated with increased relative power across high frequency bands (9–30 Hz), but decreased relative theta (4–6 Hz) power measured at 3 months of age (Kozak et al., 2021). Similarly, a recent randomized control trial evaluated the effects of a monthly low- vs high-cash gifts to mothers on their infant’s early brain development. It was found that 3-month old infants whose mothers received high-cash gifts showed higher absolute power in high frequency bands (6–49hz) compared to infants whose mothers received a nominal monthly cash gift (Troller-Renfree et al., 2022), although effects were reduced after adjusting for multiple comparisons. Our observational study similarly supports the association between higher income and higher EEG power at least early in infancy. However the lack of mediation effect emphasizes the need for further research to determine whether income supplements have long-term positive effects on developmental outcomes.
4.4. Limitations
The current study focused on income and maternal education as measures of SES that may impact brain and child development over the first two years of life. Importantly, our findings are observational, and although longitudinal follow-up allowed us to track change over time, we are unable to infer causal effects of income on brain development. We also recognize that there are many risk factors (e.g., cumulative or specific adverse life events, parental stress or depression, infection, malnutrition, etc.) that covary with SES and may have more focal effects on brain development. Indeed, EEG studies in predominantly lower-income families have found that increased perceived maternal stress is associated with differences in infant EEG power, including increased relative theta power, reduced relative alpha power, and reduced relative or absolute gamma power (Pierce et al., 2019, Troller-Renfree et al., 2020). Several studies have also begun to investigate the complex relationships between SES factors such as maternal stress, home environment, and language exposure and how together these can influence EEG power and language outcomes (Brito et al., 2020, Pierce et al., 2021). Further research that parses SES-related factors associated with brain development and developmental outcomes is crucial to the development of effective interventions and public policies.
5. Conclusions
The current study charts the trajectory of absolute EEG power over the first two years of life in a diverse sample of infants. We demonstrate sharp increases in power over the first 9 months of life, with family income associated with higher power across multiple frequency bands at 2–3 months, but lower income associated with more growth during the first year after birth. Our findings suggest the possibility of accelerated trajectories of early brain growth in the context of socioeconomic adversity, and emphasize the potential role of socioeconomic factors in shaping brain trajectories.
Funding
This work was supported by JPB Research Network on Toxic Stress: A project of the Center on the Developing Child at Harvard University and the National Institutes of Health (R01-DC010290 to CAN; K23DC07983 to CLW; 1T32MH112510 to CLW).
CRediT authorship contribution statement
Carol Wilkinson: Conceptualization, Methodology, Software, Data curation, Writing – original and draft, Visualization, Project administration. Lara Pierce: Conceptualization, Methodology, Software, Investigation, Data Curation, Writing – resubmitted draft, Writing – review & editing. Georgios Sideridis: Methodology, Software, Formal analysis, Visualization, Writing – review & editing. Mark Wade: Conceptualization, Methodology, Writing – review & editing. Charles Nelson: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank all the children and families who generously participated in this research. We thank all the research staff involved in participant recruitment, data collection, and database administration.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101260.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
Data will be made available on request. Datasets analyzed as part of the presented research are available from the corresponding author upon reasonable request. Please note that based on the consent forms obtained for the studies included in this research, it may not be possible to accommodate all requests.
References
- Anderson A.J., Perone S. Developmental change in the resting state electroencephalogram: Insights into cognition and the brain. Brain Cognit. 2018 doi: 10.1016/j.bandc.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Babadi B., Brown E.N. A review of multitaper spectral analysis. IEEE Trans. Biomed. Eng. 2014;61(5):1555–1564. doi: 10.1109/TBME.2014.2311996. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Donohue M.R., Elsayed N.M., Gilbert K., Harms M.P., Hennefield L., Herzberg M., Kandala S., Karcher N.R., Jackson J.J., Luking K.R., Rappaport B.I., Sanders A., Taylor R., Tillman R., Vogel A.C., Whalen D., Luby J.L. Early childhood socioeconomic status and cognitive and adaptive outcomes at the transition to adulthood: the mediating role of gray matter development across five scan waves. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2022;7(1):34–44. doi: 10.1016/j.bpsc.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M.A. Brain electrical activity associated with cognitive processing during a looking version of the A-Not-B task. Infancy.: Off. J. Int. Soc. Infant Stud. 2001;2(3):311–330. doi: 10.1207/S15327078IN0203_2. [DOI] [PubMed] [Google Scholar]
- Benasich A. a, Gou Z., Choudhury N., Harris K.D. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav. Brain Res. 2008;195:215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito N.H., Fifer W.P., Myers M.M., Elliott A.J., Noble K.G. Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Dev. Cogn. Neurosci. 2016;19:144–151. doi: 10.1016/j.dcn.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito N.H., Troller-Renfree S.V., Leon-Santos A., Isler J.R., Fifer W.P., Noble K.G. Associations among the home language environment and neural activity during infancy. Dev. Cogn. Neurosci. 2020;43 doi: 10.1016/j.dcn.2020.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G., Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1930. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiani C., Piazza C., Mornati G., Molteni M., Riva V. Oscillatory gamma activity mediates the pathway from socioeconomic status to language acquisition in infancy. Infant Behav. Dev. 2019;57 doi: 10.1016/j.infbeh.2019.101384. [DOI] [PubMed] [Google Scholar]
- Carneiro P., Meghir C., Parey M. Maternal education, home environments, and the development of children and adolescents. J. Eur. Econ. Assoc. 2013:123–160. doi: 10.1111/j.1542-4774.2012.01096.x. 11(suppl_1) [DOI] [Google Scholar]
- Cellier D., Riddle J., Petersen I., Hwang K. The development of theta and alpha neural oscillations from ages 3 to 24 years. Dev. Cogn. Neurosci. 2021;50 doi: 10.1016/J.DCN.2021.100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T., Haller M., Peterson E.J., Varma P., Sebastian P., Gao R., Noto T., Lara A.H., Wallis J.D., Knight R.T., Shestyuk A., Voytek B. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020;23(12):1655–1665. doi: 10.1038/s41593-020-00744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T., Schaworonkow N., Voytek B. Methodological considerations for studying neural oscillations. Eur. J. Neurosci. 2021:1–26. doi: 10.1111/ejn.15361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A.K., Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn. Sci. 2001;5(1):16–25. doi: 10.1016/S1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Evans G.W. The environment of childhood poverty. Am. Psychol. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Farah M.J. Socioeconomic status and the brain: prospects for neuroscience-informed policy. Nat. Rev. Neurosci. 2018;19(7):428–438. doi: 10.1038/s41583-018-0023-2. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Mendez Leal A.S., Wilkinson C.L., Levin A.R. The harvard automated processing pipeline for electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front. Neurosci. 2018;12:97. doi: 10.3389/FNINS.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Wilkinson C., Kapur K., Tager-Flusberg H., Levin A.R., Nelson C.A. Longitudinal EEG power in the first postnatal year differentiates autism outcomes. Nat. Commun. 2019;10(1):4188. doi: 10.1038/s41467-019-12202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, R., Peterson, E.J., Voytek, B., 2017. Inferring synaptic excitation/inhibition balance from field potentials. 10.1016/j.neuroimage.2017.06.078. [DOI] [PubMed]
- Gilmore J.H., Shi F., Woolson S.L., Knickmeyer R.C., Short S.J., Lin W., Zhu H., Hamer R.M., Styner M., Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb. Cortex. 2012;22(11):2478–2485. doi: 10.1093/cercor/bhr327. (N. Y., N. Y.: 1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Knickmeyer R.C., Gao W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018;19(3):123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z., Choudhury N., Benasich A.A. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behav. Brain Res. 2011;220(2):263–270. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W.T., Black J.E., Wallace C.S. Experience and brain development. Child Dev. 1987;58(3):539–559. [PubMed] [Google Scholar]
- Harmony T., Hinojosa G., Marosi E., Becker J., Rodriguez M., Reyes A., Rocha C. Correlation between EEG spectral parameters and an educational evaluation. Int. J. Neurosci. 1990;54(1–2):147–155. doi: 10.3109/00207459008986630. [DOI] [PubMed] [Google Scholar]
- Hillman N., Kallapur S.G., Jobe A. Physiology of transition from intrauterine to extrauterine life. Clin. Perinatol. 2012;39(4):769–783. doi: 10.1016/j.clp.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff E. The specificity of environmental influence: socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 2003;74(5):1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Jing H., Gilchrist J.M., Badger T.M., Pivik R.T. A longitudinal study of differences in electroencephalographic activity among breastfed, milk formula-fed, and soy formula-fed infants during the first year of life. Early Hum. Dev. 2010;86(2):119–125. doi: 10.1016/j.earlhumdev.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Kim P., Evans G.W., Angstadt M., Ho S.S., Sripada C.S., Swain J.E., Liberzon I., Phan K.L. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. USA. 2013;110(46):18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak K., Greaves A., Waldfogel J., Angal J., Elliott A.J., Fifier W.P., Brito N.H. Paid maternal leave is associated with better language and socioemotional outcomes during toddlerhood. Infancy. 2021;26(4):536–550. doi: 10.1111/infa.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraybill J.H., Bell M.A. Infancy predictors of preschool and post-kindergarten executive function. Dev. Psychobiol. 2013;55(5):530–538. doi: 10.1002/dev.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A.R., Méndez Leal A.S., Gabard-Durnam L.J., O’Leary H.M. BEAPP: the batch electroencephalography automated processing platform. Front. Neurosci. 2018;12:513. doi: 10.3389/fnins.2018.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva, F Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalogr. Clin. Neurophysiol. 1991;79(2):81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- Marshall P.J., Fox N.A., Bucharest Early Intervention Project Core Group A comparison of the electroencephalogram between institutionalized and community children in Romania. J. Cogn. Neurosci. 2004;16(8):1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- Marshall P.J., Bar-Haim Y., Fox N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113(8):1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Mathalon D.H., Sohal V.S. Neural oscillations and synchrony in brain dysfunction and neuropsychiatric disorders: it’s about time. JAMA Psychiatry. 2015;72(8):840–844. doi: 10.1001/jamapsychiatry.2015.0483. [DOI] [PubMed] [Google Scholar]
- Matousek, M., Petersen, I., 1973. Frequency analysis of the electro encephalogram in normal children and adolescents. Kellaway, Peter And Ingemar Petersen (Ed) Automation Of Clinical Electroencephalography Proceedings Of A Conference Viii+318p Illus Raven Press, Publishers, 75–102.
- Matousek M., Petersén I. [Frequency analysis of EEG registrations in normal children 1-16 years old] Nord. Med. 1971;85(20):637–638. [PubMed] [Google Scholar]
- Mullen E.M. American Guidance Service; 1995. Mullen Scales of Early Learning. [Google Scholar]
- Nelson C.A., Gabard-Durnam L.J. Early adversity and critical periods: neurodevelopmental consequences of violating the expectable environment. Trends Neurosci. 2020;43(3):133–143. doi: 10.1016/j.tins.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Schork N.J., Murray S.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kennedy D.N., Van Zijl P., Sowell E.R. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero G. EEG development in children with sociocultural disadvantages: a follow-up study. Clin. Neurophysiol. 2003;114(10):1918–1925. doi: 10.1016/S1388-2457(03)00173-1. [DOI] [PubMed] [Google Scholar]
- Pierce L.J., Thompson B.L., Gharib A., Schlueter L., Reilly E., Valdes V., Roberts S., Conroy K., Levitt P., Nelson C.A. Association of perceived maternal stress during the perinatal period with electroencephalography patterns in 2-month-old infants. JAMA Pediatr. 2019;173(6):561. doi: 10.1001/jamapediatrics.2019.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce L.J., Reilly E., Nelson C.A. Associations between maternal stress, early language behaviors, and infant electroencephalography during the first year of life. J. Child Lang. 2021;48(4):737–764. doi: 10.1017/S0305000920000501. [DOI] [PubMed] [Google Scholar]
- Rowe M.L. Understanding socioeconomic differences in parents’ speech to children. Child Dev. Perspect. 2018;12(2):122–127. [Google Scholar]
- Saby J., Marshall P.J. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012;29(6):997–1003. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J.P., Boyce W.T., McEwen B.S. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Smit D.J.A., Boomsma D.I., Schnack H.G., Hulshoff Pol H.E., de Geus E.J.C. Individual differences in EEG spectral power reflect genetic variance in gray and white matter volumes. Twin Res. Hum. Genet.: Off. J. Int. Soc. Twin Stud. 2012;15(3):384–392. doi: 10.1017/thg.2012.6. [DOI] [PubMed] [Google Scholar]
- Tierney A.L., Gabard-Durnam L., Vogel-Farley V., Tager-Flusberg H., Nelson C.A. Developmental trajectories of resting eeg power: an endophenotype of autism spectrum disorder. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P., Moore D.G., Ribeiro H., Axelsson E.L., Murphy E., Karmiloff-Smith A., Johnson M.H., Kushnerenko E. Socioeconomic status and functional brain development—Associations in early infancy. Dev. Sci. 2013;16(5):676–687. doi: 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Troller-Renfree S.V., Brito N.H., Desai P.M., Leon-Santos A.G., Wiltshire C.A., Motton S.N., Meyer J.S., Isler J., Fifer W.P., Noble K.G. Infants of mothers with higher physiological stress show alterations in brain function. Dev. Sci. 2020;23(6) doi: 10.1111/DESC.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S.V., Costanzo M.A., Duncan G.J., Magnuson K., Gennetian L.A., Yoshikawa H., Halpern-Meekin S., Fox N.A., Noble K.G. The impact of a poverty reduction intervention on infant brain activity. Proc. Natl. Acad. Sci. 2022;119(5) doi: 10.1073/pnas.2115649119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröndle M., Popov T., Dziemian S., Langer N. Decomposing the role of alpha oscillations during brain maturation. ELife. 2022;11 doi: 10.7554/eLife.77571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Knight R.T. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry. 2015;77(12):1089–1097. doi: 10.1016/j.biopsych.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Kramer M.A., Case J., Lepage K.Q., Tempesta Z.R., Knight R.T., Gazzaley A. Age-related changes in 1/f neural electrophysiological noise. J. Neurosci. 2015;35(38):13257–13265. doi: 10.1523/JNEUROSCI.2332-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 2010;90(3):1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I., Haufe S., Tangermann M. Automatic classification of artifactual ICA-Components for artifact removal in EEG signals. Behav. Brain Funct. 2011;7(1):30. doi: 10.1186/1744-9081-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler, I., Debener, S., Muller, K.R., & Tangermann, M. (2015). On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, 2015-Novem, 4101–4105. https://doi.org/10.1109/EMBC.2015.7319296. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request. Datasets analyzed as part of the presented research are available from the corresponding author upon reasonable request. Please note that based on the consent forms obtained for the studies included in this research, it may not be possible to accommodate all requests.