Abstract
This article summarises expert discussion on the management of patients with hepatocellular carcinoma (HCC), which took place during the 24th World Gastrointestinal Cancer Congress (WGICC) in Barcelona, July 2022. A multidisciplinary approach is mandatory to ensure an optimal diagnosis and staging of HCC, planning of curative and therapeutic options, including surgical, embolisation, ablative strategies, or systemic therapy. Furthermore, in many patients with HCC, underlying liver cirrhosis represents a challenge and influences the therapeutic options.
Key words: hepatocellular carcinoma, liver transplantation, chemoembolisation, immunotherapy, radioembolisation
Highlights
-
•
The incidence of HCC will continue to rise.
-
•
HCC is curable at an early stage.
-
•
New locoregional treatments such as radioembolisation are showing impressive results.
-
•
A combination of immunotherapy is the gold standard of first-line treatment for advanced hepatocellular cancer.
-
•
There are more and more active systemic treatment options.
Introduction
With 905 700 new cases in 2020 (just under 5% of all cancers) and 830 200 deaths, liver cancer is the third leading cause of cancer deaths worldwide after lung and colorectal cancer.1 With a mortality/incidence ratio of 0.92, liver cancer is a major cancer burden with a very poor prognosis. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and it comprises 75%-85% of primary liver cancer cases.2
The most common risk factors and aetiologies for HCC are chronic infection by hepatitis B virus or hepatitis C virus, nonalcoholic fatty liver disease in the setting of obesity with/without associated diabetes mellitus type 2, and heavy alcohol consumption.3,4 Other reported aetiologies are metabolic disorders, including α1-antitrypsin deficiency, hemochromatosis, and autoimmune diseases. Contributory environmental factors include aflatoxin-contaminated food and tobacco. There is a wide variation in exposure to these different risk factors depending on geographical and sociocultural factors. Significant improvements in the knowledge and management of HCC have been seen in the past two decades. The advent of powerful antiviral treatments and lifestyle changes mostly explain the important epidemiological changes. Several clinical guidelines are available, which are endorsed by societies involved in clinical care and research in HCC. To master this rapidly evolving field, clinicians need more than streamlined guidelines. This article summarises an expert discussion on the management of HCC, which was organised during the 24th European Society for Medical Oncology (ESMO)/World Congress Gastrointestinal Cancer Congress (WCGICC) in July 2022 in Barcelona, Spain. In view of the rapid progress of knowledge in the field of HCC, it was decided in agreement with all the experts to include in this text presentations or publications that occurred after the congress.
Methods
At the 24th ESMO/WCGICC held in Barcelona in July 2022, a panel of invited experts involved in the basic science, clinical care, and clinical research for patients with HCC conducted a structured discussion on different aspects of HCC management. The included experts represented multiple disciplines involved in the care of HCC: hepatologists, medical oncologists, gastrointestinal oncologists, interventional radiologists, radiation oncologists, pathologists, and surgical oncologists. Experts were selected based on their scientific merits and their recognition as international opinion leaders. The panel was presented with a detailed questionnaire prior to the meeting. Answers were summarised and then discussed in an extended forum. Conclusions of the recommendations and expert opinions are based on published data and on clinical experience. The experts’ opinions/recommendations do not therefore represent an official guideline or true consensus statements. This publication aims to guide clinicians in the hands-on decisions encountered in the management of HCC, especially in fields where scientific evidence remains limited.
Epidemiology
In 2020, age-standardised incidence and mortality rates for liver cancer were 9.5 and 8.7 per 100 000, respectively. Age-standardised incidence and mortality rates were highest in Eastern Asia (17.8 new cases, 16.1 deaths), Northern Africa (15.2 new cases, 14.5 deaths), and South-Eastern Asia (13.7 new cases, 13.2 deaths).1 The major risks of HCC vary by geographic region, resulting in marked differences in the burden of HCC across regions.2 In the United States, the incidence of HCC has tripled since the 1980s, with >40 000 new cases in 2020.5 It should be noted that this incidence has continued to increase in recent years despite the implementation of screening for HCC in patients with liver cirrhosis. The number of new cases of HCC is predicted to increase globally by 55% between 2020 and 2040, with 1.4 million new diagnoses forecast for 2040.1 The experts considered that HCC would undoubtedly remain a global public health problem in the coming years.
In the future, it is anticipated that there will be a change in the spectrum of chronic liver disease preceding the occurrence of HCC with a decrease in viral causes related to both vaccination for hepatitis B and the implementation of curative therapy for hepatitis C, albeit a total disappearance is unlikely due to the lack of a universal vaccination and treatment strategies particularly in lower-resource settings.3,6
In summary, the experts anticipate that the observed decrease in HCC related to chronic viral liver disease will be compensated by an increase in liver disease related to nonalcoholic fatty liver disease linked to an overall increase in the incidence of obesity, as already observed in patients transplanted for HCC,7 while alcohol-related liver disease will have a stable incidence.
HCC screening
Screening of patients with liver cirrhosis to detect small and potentially curable HCCs started >35 years ago. Ultrasound examination of the liver remains the reference for screening of HCC.8 Alpha-fetoprotein (AFP) measurement was added to ultrasound examination, but its value has been debated in terms of cost-effectiveness and removed from recommendations in some countries. However, a recent meta-analysis showed that ultrasound alone detected only 45% of HCC, whereas the detection rate rose to 63% when the AFP assay was used in addition to ultrasound.9 In patients with a liver that is very difficult to examine by ultrasound, it has been proposed to use computed tomography (CT) or even magnetic resonance imaging (MRI),10 but this raises additional questions in terms of the cost of screening and the availability of equipment, as well as in terms of cost-effectiveness. Surveillance examinations are recommended every 6 months.11 On a country scale, the effect of this screening in terms of mortality reduction remains limited and debated.12 However, experts recommend the use of ultrasound and AFP testing every 6 months in patients with cirrhosis.
HCC scoring and staging systems
The Child–Pugh score13,14 remains the most widely used score in evaluating liver functional reserve in patients with liver cirrhosis. Despite its age (or perhaps because of its age, which makes it universally known), it is systematically calculated before a therapeutic decision is made. The albumin–bilirubin (ALBI) score developed more recently in the specific context of HCC is gaining ground in hepatology circles.15 This growing interest derives from the fact that it is a continuous score that is more objective in its application because it is based solely on biological data, lacks ground or ceiling effects, and makes it possible to differentiate prognosis within Child–Pugh A5 score and Child–Pugh A6 score. The ALBI score is not yet used to select or stratify patients even in the most recently reported trials with a stratification that continues to use the Child–Pugh score. However, real-life studies or post hoc analyses have confirmed the prognostic value of this score, including in patients receiving recent combination therapies such as atezolizumab bevacizumab16 or durvalumab tremelimumab,17 confirming that the ALBI score may be incorporated as a stratification factor in future studies. Post hoc studies on, for example, the evolution of the ALBI score during modern HCC treatment such as ramucirumab, have been published.18 The ALBI score was difficult to use in the past but is now accessible via a specific website.19 However, it is still criticised because the mechanical basis of its calculation are not accepted by all. As a result, a simplified version has recently been developed; however, its validation is needed in large-scale studies.20
The Model for End-Stage Liver Disease (MELD) score is less used at this stage for the assessment of hepatocellular function outside the special cases of patients for whom liver transplantation is being considered, where it is widely used to manage the waiting list.21,22
The Barcelona Clinic Liver Cancer (BCLC) prognosis and treatment strategy score is widely accepted, especially as it was updated in 2022 to reflect recent changes in the management of HCC.23 The interest of this classification system lies in its prognostic value linked to a definition of the therapeutic strategy. In addition, since its first publication in 1999,24 the BCLC score has undergone many iterations to adapt to the evolution of knowledge and treatment advances of HCC, which are led by experts beyond the original Barcelona group.23 As the versions evolved, some categories saw their heterogeneity better taken into account. Changes include category B, which was previously recommended to be treated by transarterial chemoembolisation (TACE)24 and which in the latest version is subdivided into three different groups with indications ranging from liver transplantation to systemic treatment and TACE.23 Therefore although the experts unanimously accept this score regarding prognostic use, this is not always the case in terms of therapeutic choice.
The Japanese HCC score (Japan Integrated Staging Score) has not been adopted beyond its country of origin, although it accurately defines potential treatment indications.25
In summary, the experts recommend the use of the BCLC score to assist in the management of HCC. They also recommend the use of the Child–Pugh score to assess liver function but suggest that the ALBI score should be given more prominence in future studies.
Diagnosis
Biopsy should be carried out routinely if the patient is not known to have and does not show signs of chronic liver disease. Several organisations have published guidelines for the noninvasive diagnosis of HCC in patients with defined risk, including the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver-European Organisation for Research and Treatment of Cancer (EASL-EORTC), and the Asian-Pacific Association for the Study of the Liver (APASL).26 In addition, radiologists have been working to standardise the elements of HCC diagnosis.27 These guidelines essentially use the vascular pattern of HCC: contrast uptake during the arterial phase combined with the washout of contrast media during the portal venous or the delayed phases on dynamic CT or MRI. However, biopsy is increasingly being carried out for many reasons particularly when there is no curative approach. The first is purely clinical and diagnostic: there are mixed tumours of the combined hepatocellular plus intrahepatic cholangiocarcinoma type that must be diagnosed because they require specific treatments. However, their incidence is rare [comprising 1.3% of liver tumours from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute (SEER) database in 2008].28,29 In addition, the interest of the biopsy lies in the possibility of sequencing the tumour DNA and RNA and specifying its biological subtype,4 even in the absence of trials specifically dedicated to biological subgroups in this disease, despite suggested targets.30 Biopsy is also of scientific interest as it allows a better characterisation of the disease and the identification of predictive biomarkers. The experts therefore recommend the widespread use of biopsy in the diagnosis of HCC, even though radiological diagnosis has become much more effective and should be preferred for localised forms.
Staging
Baseline imaging of localised HCC should include contrast-enhanced CT and MRI scans. Diagnostic evaluation (but also imaging in response to locoregional therapy) in HCC is currently evaluated using Liver Imaging Reporting and Data Systems version 2018, which offers a comprehensive approach for a lesion-by-lesion assessment of cirrhotic nodules.31 Liver MRI is not necessary if the cancer is extensive on CT scan and if no curative or locoregional treatment can be considered, and in these cases the CT scan is usually sufficient to assess the effectiveness of systemic treatments. The role of fluorodeoxyglucose-positron emission tomography is extremely limited in this disease, due to its lack of sensitivity32 and is not recommended. Choline PET is exceptionally used in the extension assessment of these tumours. Although it has produced some interesting results, its diffusion remains limited33 and therefore does not apply to clinical practice. Contrast-enhanced ultrasound can play a role in certain situations where CT and MRI have failed.34
A serum AFP assay should be carried out in all cases prior to treatment. In the event the AFP level is normal, carcinoembryonic antigen (CEA) and carbohydrate antigen 19 19-9 (CA19-9) assays can be carried out if the tumour has a mixed component.28 The practice of measuring other markers such as decarboxyprothrombin has not become established and is not recommended by the experts.35
Treatment of localised forms
Liver resection
Indications for liver resection may follow the EASL recommendations8 or other validated ones. Surgical resection is recommended as the treatment of choice in patients with HCC arising in a noncirrhotic liver or limited cirrhosis with preserved liver function. Measurement of liver function particularly in patients with underlying liver disease before an intended liver resection is of critical importance; further, a European guideline summarising essential tools in its assessment is being developed. Liver resection is recommended for single HCC of any size and in particular for tumours >2 cm, when hepatic function is preserved, and sufficient remnant liver volume is maintained, and when there is no portal hypertension. HCC presenting with two or three nodules within Milan criteria may be eligible for liver resection according to patient performance status, comorbidities, and the aforementioned criteria. Minimal invasive resection has become the therapy of choice in experienced hands with the main benefit of less postoperative morbidity, reduced postoperative stay, and improved long-term outcome.36
Liver transplantation
The Milan criteria (one nodule <5 cm or up to three nodules each <3 cm) remain the reference in most countries for defining the indications for liver transplantation.37 However, some prefer the somewhat broader criteria that were described later: University of San Francisco criteria38 or up-to-seven criteria.39 Recently, it has been reported that the addition of AFP to the Milan criteria improved their performance.40 This ‘AFP-score’ is used in France and in Latin America41 to prioritise transplants; in the United States, the United Network for Organ Sharing (UNOS) criteria are recognised at the national level and in many European countries the Eurotransplant criteria help in prioritisation for transplantation.
Given the difficulty of access to transplantations, most offer their patients a bridging treatment approach, in particular if the expected waiting time exceeds 6 months. Bridging treatments vary; the most commonly used are TACE, radiofrequency (or microwave) ablation (RFA/MWA), or more recently trans-arterial radioembolisation (TARE).42 For the experts, RFA or MWA techniques are on a downward trend in favour of TARE, which is gaining popularity.
The type of procedure to be selected is the one that offers the best local control-to-adverse effects ratio depending on the size and location of the tumour. This is typically a decision that should be made in a multidisciplinary setting. A recent meta-analysis evaluated these bridging techniques through 3106 records in six articles (1043 patients) that met the inclusion criteria.43 Patients with HCC listed for liver transplantation and undergoing bridging techniques had a longer waiting time to liver transplantation [mean difference = 3.77 months, 95% confidence interval (CI) 2.07-5.48] compared with the non-interventional group. However, they had higher survival rates after liver transplantation at 1 year [odds ratio (OR) = 2.00, 95% CI 1.18-3.41], 3 years (OR = 1.47, 95% CI 1.01-2.15), and 5 years (OR = 1.50, 95% CI 1.06-2.13).43
Local ablation
Ablation is used in BCLC 0 patients (i.e. with preserved hepatocellular function, good general condition, and a single tumour ≤2 cm) because there is a high level of evidence that this treatment is equivalent to surgery in terms of overall survival but the duration of the procedure and hospitalisation are shorter.44 In this situation, MWA is more frequently used because a recent meta-analysis reported that MWA resulted in a higher complete ablation rate and lower local tumour progression than RFA in treating HCC nodules.45 There was no significant difference in overall survival between the two therapeutic procedures. It is also recommended in case of three nodules <3 cm not suitable for surgical procedures. Recent evidence suggests that TARE may also play a role in this indication.46
Radiotherapy
Recent comparative studies have reported that the local control rate of stereotactic body radiotherapy (SBRT) for intrahepatic malignancies is comparable with that of RFA.47 Furthermore, a recent meta-analysis has shown that SBRT/ablative RT can yield oncologic outcomes similar to RFA, and suggested that it can be more effective for the treatment of tumours in locations where RFA is difficult to carry out or for large-sized tumours (>3 cm).48 However, it is rarely used. The experts considered that SBRT should be discussed as an option on a routine basis during a multidisciplinary board for localised tumours and especially when no other treatment can be offered, particularly when there is a vascular contact but not only in these cases.
In summary, in this situation, the experts follow the recommendations of the ESMO clinical guidelines published in 201849 and updated in 2021,50 and more closely the BCLC recommendations updated in 2022.23 A particular recommendation lies in the interest of TARE in bridging situations before liver transplantation, the results of which seem to be very interesting.
Treatment of intermediate disease (stage B BCLC)
TACE and TARE
TACE using conventional TACE with lipiodol or TACE with drug-eluting beads is the standard treatment for intermediate BCLC cases [i.e. tumour >3 cm, multinodular (≥4 nodules)] without vascular invasion, or extrahepatic disease, Eastern Cooperative Oncology Group performance status 0, Child–Pugh A, and without portal thrombosis.51 There is no substantial benefit from choosing one specific embolising procedure versus the other based on results from several randomised trials. A randomised trial has even shown that embolisation with microspheres alone was as active as the same treatment with doxorubicin-loaded microspheres.52 The use of TACE varies across centres and typically this technique applies to HCCs with two to seven nodules, the largest of which is <7 cm in size and which can be treated supra-selectively.
TARE, in particular because it has been demonstrated that real dosimetry can be carried out to improve the efficacy-to-adverse effects ratio,53 is increasingly being used in this indication. Undoubtedly, the cost of TARE treatment remains an obstacle in some countries. However, it should be noted that no cost-effectiveness study of the various treatments has been carried out.
A meta-analysis conducted on individual patient data included 17 studies comparing TACE and TARE.54 This meta-analysis was inconclusive and reported no difference in overall survival but a longer time to progression with TARE relative to TACE (mean time to progression 17.5 versus 9.8 months).54 In addition, a recent randomised phase II study comparing drug-eluting TACE (34 patients) with TARE (38 patients) found a benefit in terms of local control and overall survival in favour of TARE: 30.2 versus 15.6 months (hazard ratio = 0.46; P = 0.006).55
Intra-arterial hepatic chemotherapy
The results from one phase III trial showed that compared with TACE, hepatic arterial infusion of chemotherapy with oxaliplatin plus intravenous 5-fluorouracil (5-FU) and folinic acid significantly improved the overall survival with a significantly lower incidence of grade 3-4 adverse events for large and unresectable HCC.56 Despite these results, hepatic intra-arterial chemotherapy is not considered by experts as a treatment option that they would be likely to use.
Combination of locoregional treatments
Combination of sorafenib to TACE in the TACTICS trial did not show any overall survival advantage (median: 36.2 months with TACE plus sorafenib and 30.8 months with TACE alone, not significant).57 The combination of locoregional treatments is limited to clinical trials, some of which evaluate, for example, SBRT combined with TACE58 or immunotherapy and TACE. Some centres are already combining TACE and RFA to try to downstage tumours to make them accessible for curative treatment. This is particularly true for tumours between sizes 3 and 5 cm, as in this population a randomised trial59 and a meta-analysis60 showed that trying to reduce the tumour size to allow access to curative treatment was beneficial. Another trial, which was prematurely closed due to slow accrual after inclusion of 40 patients, suggested that the use of SBRT could result in superior local control as compared with TAE/TACE rechallenge (median duration of local control not reached versus 8 months; P = 0.0002).61
The role of systemic treatment
Systemic treatment is considered for patients who have recurrence of disease after a curative intent, or progress after locoregional therapy. Individualisation of decisions plays an important role in this type of treatment strategy. It was particularly emphasised that the transition to systemic therapy should not be too late, given the improved outcome of systemic treatment. Artificial intelligence could in the future help define the optimal moment for this switch.62 A study including 237 patients evaluated the value of adding RFA or SBRT after TACE, using an artificial intelligence system that recommended it in about half of the patients. In these patients the mean progression-free survival was 5.3 months compared to 1.8 years in untreated patients, and overall survival was also increased to 7.5 versus 5.3 years. Furthermore, the experts agree with the proposal of the new BCLC guidelines that infiltrative forms are better treated early or exclusively with new systemic combinations.23
Again, the experts follow the ESMO 2018 recommendations updated in 2021,49,50 but also especially the latest BCLC update.23 This is true in particular with regard to the subdivision of the intermediate group into three categories. The experts also emphasise the importance of (i) ensuring that systemic treatment is rapidly introduced if TACE shows initial signs of ineffectiveness, (ii) considering a global strategy involving combined systemic treatment first in the event of a borderline indication for TACE, and (iii) giving TARE an increasingly important role in this indication. By contrast, the role of intra-arterial hepatic chemotherapy remains marginal.
Treatment of advanced disease
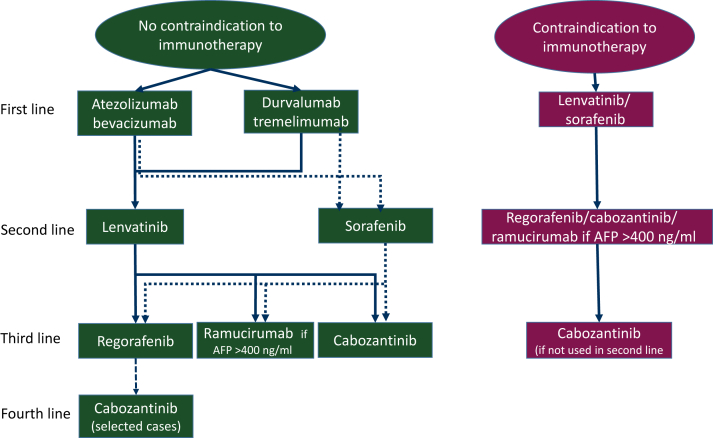
The combination of atezolizumab and bevacizumab demonstrated superior efficacy to sorafenib in terms of response rate (30% versus 11%), progression-free survival (median: 6.8 months versus 4.3 months), and overall survival (median: 19.2 versus 13.4 months).63 More recently, the combination of durvalumab and tremelimumab has also been shown to be more active than sorafenib in terms of response rate (20% versus 6%) and overall survival (median: 16.4 months versus 13.8 months) but without effect on progression-free survival (median: 3.78 versus 4.07).64 There is no direct head-to-head comparison of these two new standards of treatment, which are therefore valid first-line option. The combination of atezolizumab + bevacizumab is widely used and oncologists and hepatologists have learned to manage its toxicity. The combination of durvalumab and tremelimumab is easier to use in patients potentially at risk of gastrointestinal bleeding, and the potential toxicity of the addition of anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4) has been reduced using a single injection regimen of tremelimumab at the initiation of therapy (Figure 1).
Figure 1.
Treatment of advanced disease.
Despite the evidence of the superiority of these two combinations with immunotherapy, there will still be ∼15%-20% of patients who will only receive tyrosine kinase inhibitors (sorafenib or lenvatinib), in particular because of underlying autoimmune diseases.65 The question was also raised for treating a recurrence of HCC after transplantation in patients receiving immune suppressors. The use of immunotherapy in this context remains contraindicated. However, the situation may change in the future, as a recent trial in kidney transplant recipients found no transplant rejection and no net decrease in the efficacy of immunotherapy, which was administered (median number of cycles = 3) in 22 kidney transplant patients while immune-suppressive therapy was maintained.66
The use of immunotherapy beyond the first line is anecdotal, partly for scientific reasons, as randomised trials in patients who have not received immunotherapy in the first line gave somewhat discordant results, with the KEYNOTE 240 study being totally negative67 and the KEYNOTE 394 study including only Asian population and68 finding an improvement in median survival from 13 to 14.6 months, an improvement in median progression-free survival from 2.3 to 2.6 months.69 In addition, in many countries there is a problem of access to the drug due to a lack of funding. When used in the second line, a programmed cell death protein 1 (PD-1) inhibitor alone is most often chosen. In the United States, it is possible to administer the combination nivolumab and ipilimumab to these patients. This combination has shown significant efficacy in early-phase trials70 and pending validation from an already completed phase III clinical trial (CheckMate-9DW trial (NCT04039607).
In case of progression or intolerance of the chosen immunotherapy-based combination, the choice of second-line treatment is usually a targeted therapy previously used in the first line (i.e. sorafenib or lenvatinib). Lenvatinib, which has been shown to be noninferior to sorafenib and with a higher objective response rate,67 is gaining acceptance in this situation. In some countries, restrictions on use, such as funded access to regorafenib in the event of proven progression on sorafenib, have led to the prescription of sorafenib after failure to immunotherapy.
After the failure of first-line targeted therapy, experts in countries with access to all the molecules give either regorafenib71 or cabozantinib,68 reserving ramucirumab for patients with AFP levels >400 ng/ml.72
In the event of progression under second-line tyrosine kinase inhibitor treatment, the approach is not uniform, with some experts trying to give the most suitable patients all the available molecules (switching to cabozantinib, for example, if regorafenib fails),73 while others limiting treatment only to the second line. The lack of third-line treatment seems to be more related to the issue of financial support for expensive treatments in some countries as the pivotal study validating the use of cabozantinib included patients in third line.68 There is even some published data confirming the efficacy of cabozantinib in patients who received immunotherapy.74
BCLC staging defines patients with portal vein invasion as advanced stage (C) and recommends systemic therapy. However, SBRT has been reported to be an effective treatment for this type of situation, particularly in a series of 70 patients, resulting in repermeabilisation of the portal vein and subsequent TACE.75 In another recent study, adding hepatic intra-arterial chemotherapy with 5-FU and oxaliplatin to sorafenib improved median overall survival (13.4 months versus 7.1 months).76 Despite these results, experts consider that adequate systemic treatment should remain the rule in these patients, especially as they show major improved outcomes, for example, in the phase III IMbrave150 study that proved the efficacy of the combination of atezolizumab and bevacizumab.63 However, these patients with main portal vein thrombosis were not eligible for inclusion in the HIMALAYA trial, so there are no data on the combination of durvalumab and tremelimumab in this situation.64
In summary, again, the experts’ position is closer to the position of the recent BCLC update than to the ESMO guidelines published in 2018 and updated in 2021, as it obviously incorporates the possibility to choose between the two first-line combination treatment options.
Terminal stage (BCLC D)
The proportion of patients diagnosed at this stage remains high, in the order of 20%-30% in different countries around the world. At this stage, no anticancer treatment can be proposed. However, the most effective comprehensive management of the symptoms and patients’ needs should be provided. A specialised palliative and supportive care team should be involved whenever possible.
The future
The future of HCC treatment is exciting but also full of new challenges and opportunities. Better prevention could significantly reduce the burden of disease and better surveillance could increase the chances of curing more patients. In the treatment of localised disease, the role of each of the techniques that can be used to challenge surgery remains to be defined. The very recently reported IMbrave050 trial included 668 patients with HCC who had undergone a curative resection or ablation. They were randomly assigned to receive atezolizumab plus bevacizumab (n = 334) or to undergo active surveillance (n = 334) until disease recurrence or unacceptable toxicity. The primary endpoint was recurrence-free survival (RFS). The median RFS was not reached in either arm at a median follow-up of 17.4 months. However, the 12-month RFS rate was significantly higher in the atezolizumab–bevacizumab arm than in the active surveillance arm: 78% and 65%, respectively (hazard ratio = 0.72; 95% CI 0.56-0.93; P = 0.012). The overall survival data were immature.77 The adjuvant use of the combination of atezolizumab and bevacizumab could change the overall management of these patients. In case of early recurrence, patients should be considered resistant to this combination, which would make them potential candidates for durvalumab + tremelimumab. However, we have no data on the efficacy of this combination after failure of a combination of antiangiogenic and anti-programmed death-ligand 1 (anti-PD-L1). In patients who are going to be treated by liver transplantation, the indications of the different bridging techniques including newcomers such as TARE will have to be clarified. In intermediate forms, TARE is becoming increasingly important, but its precise indications should be better defined. The combination of new, more powerful systemic therapies with local treatment techniques (TACE or TARE) is the subject of numerous therapeutic trials. The LAUNCH trial has already tested a combination of systemic therapy and TACE, but it actually tested the value of adding TACE to lenvatinib and not vice versa. Nevertheless, this trial showed in 338 patients with advanced HCC that the addition of TACE to lenvatinib improved overall survival, 17.8 versus 11.5 months (P <0.001), and progression-free survival, 10.6 versus 6.4 months (P <0.001).78 On the contrary, the combination of sorafenib with SBRT did not improve the results of irradiation in a randomised phase III trial including 193 patients.79 Much remains to be done in this area of combining systemic and locoregional treatments, particularly with those combination therapies that have shown the best results in the treatment of advanced forms. A new anti-PD-1, tislelizumab, has recently shown its efficacy as first-line monotherapy in advanced forms (RATIONALE-301 trial),80 as has the combination of camrelizumab (another anti-PD-1) plus rivoceranib (a VEGF inhibitor tyrosine kinase).81 The respective positioning of these new treatments will have to be determined in clinical practice, even if the first line of treatment for HCC is beginning to be busy. With the abundance of systemic treatment options, the time has come in advanced forms for sequence trials and also for attempts to personalise these treatments with the search for predictive and prognostic biomarkers. The role of even more innovative therapies such as chimeric antigen receptor-T cells will also need to be clarified.
Funding
None declared.
Disclosure
MD reports participation in the advisory boards of Roche, Merck Serono, Amgen, Bayer, Servier, Pierre Fabre, BeiGene, Astra Zeneca, Daiichi Sankyo, Merck-Sharp-Dohme, Boehringer Ingelheim; served as a speaker in symposium sponsored by Roche, Merck Serono, Bayer, Merck-Sharp-Dohme, Servier, Pierre Fabre, and Daiichi Sankyo; reports institutional research funding from Roche, Merck Serono, Esteve: Roche, and PanCAN. His wife is the head of the oncology business unit at Sandoz France. GKAA reports research support to the institution from Arcus, Agios, AstraZeneca, Bayer, BioNTech, BMS, Celgene, Flatiron, Genentech Ltd./F. Hoffmann-La Roche Ltd., Genoscience, Incyte, Polaris, Puma, QED, SillaJen, and Yiviva; and receives consulting support from Agios, Alnylam, AstraZeneca, Autem, Bayer, BeiGene, Ltd., Berry Genomics, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech Ltd./F. Hoffmann-La Roche Ltd., Genoscience, Helio, Incyte, Ipsen, Legend Biotech, Merck, MINA, QED, Redhill, SillaJen, Surface Oncology, TheraBionic, twoXAR, Vector, and Yiviva. TBS reports research funding (to institution) from Agios, Arys, Arcus, Atreca, Boston Biomedical, Bayer, Eisai, Celgene, Lilly, Ipsen, Clovis, Seattle Genetics, Genentech, Novartis, Mirati, Merus, AbGenomics, Incyte, Pfizer, BMS; consulting (to institution) for Ipsen, Arcus, Pfizer, Seattle Genetics, Bayer, Genentech, Incyte, Eisai, Merus, Merck KGA, and Merck; consulting (to self) for Stemline, AbbVie, Blueprint Medicines, Boehringer Ingelheim, Janssen, Daiichi Sankyo, Natera, Treos Bio, Celularity, Caladrius Biosciences, Exact Science, Sobi, BeiGene, Kanaph, Astra Zeneca, Deciphera, Zai Labs, Exelixis, MJH Life Sciences, Aptitude Health, Illumina, Foundation Medicine, and Sanofi. Independent Data safety Monitoring Committee/Data Safety Monitoring Board: The Valley Hospital, FibroGen, Suzhou Kintor, Astra Zeneca, Exelixis, Merck/Eisai, PanCAN, and 1Globe; serves on the Scientific Advisory Board of Imugene, Immuneering, Xilis, Replimune, Artiva, and Sun Biopharma. JB reports participation in the advisory boards of Mirati, Insmed, Oxford BioTherapeutics, BioSapien, EMD Serono, Ipsen, Merck-Sharp-Dohme, Merus, BMS, Bexion; research funding (to institution) from AbbVie, Astellas, Atreca, Bayer, Dragonfly, I-Mab, Lilly, Incyte, EMD Serono, Pfizer, BMS, Transcenta Therapeutics, Tyra, Totus, Sumitomo Dainippon Pharma Oncology, 23 and me, Parthenon, and HiberCell; and serves on the data safety monitoring committee of Astra Zeneca, Novocure, and Boehringer-Ingelheim. AC reports fees paid to his institution as an invited speaker from Amgen, Foundation Medicine, Merck Serono, and Roche; fees paid to his institution for advisory board membership from Amgen, AnHeart Therapeutics, Merck Serono, Roche, and Transgene; institutional funding as principal investigator (PI) from Actuate Therapeutic, Adaptimmune, Amcure, Amgen, Astellas, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb (BMS), FibroGen, Genentech, Lilly, MedImmune, Merck Serono, Merck Sharp & Dohme (MSD), Natera, Novartis, Servier, Sierra Oncology, and Takeda; and reports a nonremunerated role as General and Scientific Director of INCLIVA Biomedical Research Institute. TdB reports receipt of grants/research supports from Galil medical; receipt of honoraria or consultation fees from Terumo, Guerbet, and GE Healthcare. PG reports receipt of honoraria from Bayer, Boston Scientific, AstraZeneca, Adaptimmune, BMS, Eisai, MSD, Sirtex, Lilly, Roche, Guerbet, and Ipsen. AL reports travel and educational support from Ipsen, Pfizer, Bayer, AAA, Sirtex, Novartis, Mylan, Delcath, Advanz Pharma, and Roche; speaker honoraria from Merck, Pfizer, Ipsen, Incyte, AAA, QED, Servier, Astra Zeneca, EISAI, Roche, and Advanz Pharma; advisory and consultancy honoraria from EISAI, Nutricia, Ipsen, QED, Roche, Servier, Boston Scientific, Albireo Pharma, AstraZeneca, Boehringer Ingelheim, GENFIT, TransThera Biosciences, and Taiho. FL reports participation in the advisory boards of Amgen, Astellas, Bayer, BeiGene, BMS, Daiichi Sankyo, Eli Lilly, MSD, Novartis, Roche; has received fees as an invited speaker from Art Tempi, AstraZeneca, BMS, Daiichi-Sankyo, Eli Lilly, Incyte, Merck Serono, MSD, Novartis, Roche, and Servier; expert testimony for BioNTech; research funding (to the institution) from BMS and Gilead. RO reports consulting fees from BMS, SERVIER, MERCK, MSD; support for attending meeting for Servier and BMS; and serves on the advisory board for Servier and BMS. EMO reports research funding to institution from Genentech/Roche, BioNTech, AstraZeneca, Arcus, Elicio, Parker Institute, NIH/NCI, and PERTZYE; consulting for Boehringer Ingelheim, BioNTech, Ipsen, Merck, Novartis, AstraZeneca, BioSapien, Astellas, Thetis, Autem, Novocure, Neogene, BMS, Tempus, FibroGen, and Merus. TG reports consulting fees from Roche, AstraZeneca, Baxter, and Servier; payment of honoraria for lectures from Incyte, Olympus, Roche, and Servier. JML reports research support from Bayer Pharmaceuticals, Eisai Inc, Bristol-Myers Squibb and Ipsen Consultancy: Bayer HealthCare Pharmaceuticals, Eisai Inc, Merck, Bristol-Myers Squibb, Eli Lilly, Roche, Genentech, Ipsen, Glycotest, AstraZeneca, Omega Therapeutics, Mina Alpha, Boston Scientific, Exelixis, Bluejay, Captor Therapeutics. TM reports consulting or advisory roles with Ability Pharma, AstraZeneca, Basilea, Baxter, BioLineRx, Celgene, Eisai, Genzyme, Incyte, Ipsen, Lilly, MSD, Novocure, QED Therapeutics, Roche, Sanofi/Aventis, Servier, and Zymeworks; and travel, accommodation, and expenses were from Celgene, H3 Biomedicine, Incyte, Merck, Sanofi, and SERVIER. DM reports institutional funding from Astellas; personal travel support/honoraria from Astellas, Janssen, Bayer, Ipsen, BMS, and MSD. KM reports honoraria from Bayer, Bristol-Myers Squibb, Chugai Pharma, Lilly Japan, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical, and Takeda; consulting or advisory role for Amgen, AstraZeneca, Chugai Pharma, and Ono Pharmaceutical; research funding from Amgen Astellas BioPharma, Astellas Pharma, Daiichi Sankyo, Gilead Sciences, Kyowa Hakko Kirin, Mediscience Planning, Merck Biopharma, MSD, Ono Pharmaceutical, Parexel International, Pfizer, Sanofi, Shionogi, Solasia Pharma, and Sumitomo Dainippon. JMO reports payment for lectures, presentations, speakers bureaus: BMS, Merck, Bayer, Astra Zeneca, MSD, Pfizer. Travel supports from Merck and Pfizer. PP reports honoraria from Celgene, Bayer, Ipsen, Merck, AstraZeneca, TriSalus Life Sciences, Blueprint Medicines, SynCoreBio, Incyte, Bristol Myers Squibb/Medarex, Guardant Health, Rafael Pharmaceuticals, and Daiichi Sankyo/Astra Zeneca; consulting or advisory role for Celgene, Ipsen, Merck, TriSalus Life Sciences, Daiichi Sankyo, SynCoreBio, and Taiho Pharmaceutical; is on the speakers’ bureau of Celgene, Bayer, Ipsen, Novartis, Incyte, and Bristol Myers Squibb/Medarex; research funding from Bayer (Inst), Incyte (Inst), Karyopharm Therapeutics (Inst), Merck (Inst), Taiho Pharmaceutical (Inst), Momenta Pharmaceuticals (Inst), Novartis (Inst), Plexxikon (Inst), Immunomedics (Inst), Regeneron (Inst), Genentech (Inst), TYME (Inst), Caris Life Sciences (Inst), ASLAN Pharmaceuticals (Inst), QED Therapeutics (Inst), Halozyme (Inst), Boston Biomedical (Inst), Advanced Accelerator Applications (Inst), Lilly (Inst), and Merus (Inst). GP reports honoraria for advisory roles or speakers fees from Servier, Bayer, Roche, Amgen, Merck, Lilly, MSD, BMS, AstraZeneca, Pierre Fabre, Incyte, and Novartis. ERG reports participation in the advisory boards of Amgen, Bayer, BMS, and Roche; is an invited speaker for Astellas, Merck, and Roche. TS reports honoraria from Amgen, Bayer, Falk Foundation, AstraZeneca, and Pierre Fabre; is on the advisory boards of Lilly, Bayer, Servier, AstraZeneca, Pierre Fabre, Cantargia, Mirati, and Scandion; has received research support (to the institution) from Boehringer Ingelheim, Sanofi, and BMS. BS reports consultancy fees from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, Eisai, Eli Lilly, Incyte, Ipsen, Novartis, MSD, Roche, Sanofi, Sirtex Medical, and Terumo; speaker fees from Astra Zeneca, Bayer, BMS, Eisai, Eli Lilly, Incyte, Ipsen, Novartis, Roche, Sirtex Medical, and Terumo; research grants (to institution) from BMS and Sirtex Medical. JT reports other ownership interests in Oniria Therapeutics; consulting or advisory role with Bayer, Boehringer Ingelheim, Lilly, MSD, Merck Serono, Novartis, Sanofi, Taiho Pharmaceutical, Peptomyc, Chugai Pharma, Pfizer, Seattle Genetics, Array BioPharma, AstraZeneca, Genentech, Menarini, Servier, HalioDx, F. Hoffmann LaRoche, Mirati Therapeutics, Pierre Fabre, Tessa Therapeutics, TheraMyc, Daiichi Sankyo, Samsung Bioepis, IQVIA, Ikena Oncology, Merus, NeoPhore, Orion Biotechnology, Hutchison MediPharma, Scandion Oncology, Ona Therapeutics, SOTIO, Inspirna, and Scorpion Therapeutics. CV reports consultancy role in or honoraria from Bayer, Roche, MSD, Ipsen, and Sirtex. EVC reports consulting or advisory role with Bayer, Lilly, Roche, Servier, Bristol Myers Squibb, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Array BioPharma, Daiichi Sankyo, Pierre Fabre, Taiho Pharmaceutical, Incyte, Astellas Pharma, GlaxoSmithKline, Nordic Group, Pfizer, Takeda, ALX Oncology, AbbVie, BeiGene, Boehringer Ingelheim, Mirati Therapeutics, Seattle Genetics, Terumo, Zymeworks, and Ipsen; research funding from Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Novartis (Inst), Roche (Inst), Ipsen (Inst), Merck (Inst), Merck KGaA (Inst), Servier (Inst), and Bristol Myers Squibb (Inst). HW reports participating in the advisory boards/is an invited speaker/participated in the meetings for Servier, Pierre Fabre, Incyte, Bayer, Pfizer, Zymeworks, Merck KGaA, Amgen, Roche/Genentech/FM, SIRTEX Medical, Erytech Pharma, BMS (Celgene), BTG, and Seagen; performs consulting for NICE/BSI expert Bayer, Pierre Fabre, ONCOSIL, Incyte, and Celgene; received research funding from MSD, Merck Serono, Pfizer, and Sirtex. All other authors have declared no conflicts of interest.
References
- 1.Rumgay H., Arnold M., Ferlay J., et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rumgay H., Ferlay J., de Martel C., et al. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 2022;161:108–118. doi: 10.1016/j.ejca.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Balogh J., Victor D., 3rd, Asham E.H., et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 6.Wong G.L., Hui V.W., Yip T.C., et al. Universal HBV vaccination dramatically reduces the prevalence of HBV infection and incidence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2022;56:869–877. doi: 10.1111/apt.17120. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z., Stepanova M., Ong J.P., et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–755 e743. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Tzartzeva K., Obi J., Rich N.E., et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154:1706–1718.e1701. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta P., Soundararajan R., Patel A., Kumar M.P., Sharma V., Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: a systematic review and meta-analysis. J Hepatol. 2021;75:108–119. doi: 10.1016/j.jhep.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Pelizzaro F., Peserico G., D'Elia M., et al. Surveillance for hepatocellular carcinoma with a 3-months interval in ‘extremely high-risk’ patients does not further improve survival. Dig Liver Dis. 2022;54:927–936. doi: 10.1016/j.dld.2021.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Kansagara D., Papak J., Pasha A.S., et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161:261–269. doi: 10.7326/M14-0558. [DOI] [PubMed] [Google Scholar]
- 13.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 14.Child C.G., Turcotte J.G. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 15.Johnson P.J., Berhane S., Kagebayashi C., et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulgenzi C.A.M., Cheon J., D’Alessio A., et al. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: results of the AB-real study. Eur J Cancer. 2022;175:204–213. doi: 10.1016/j.ejca.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Vogel A., Chan S., Furuse J., et al. O5—Outcomes by baseline liver function in patients with unresectable hepatocellular carcinoma treated with tremelimumab and durvalumab in the Phase 3 HIMALAYA study. Ann Oncol. 2022;33:S380–S381. [Google Scholar]
- 18.Kudo M., Galle P.R., Brandi G., et al. Effect of ramucirumab on ALBI grade in patients with advanced HCC: results from REACH and REACH-2. JHEP Rep. 2021;3 doi: 10.1016/j.jhepr.2020.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ALBI (Albumin-Bilirubin) Grade for Hepatocellular Carcinoma (HCC) https://www.mdcalc.com/calc/10070/albi-albumin-bilirubin-grade-hepatocellular-carcinoma-hcc Available at.
- 20.Kariyama K., Nouso K., Hiraoka A., et al. EZ-ALBI score for predicting hepatocellular carcinoma prognosis. Liver Cancer. 2020;9:734–743. doi: 10.1159/000508971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamath P.S., Wiesner R.H., Malinchoc M., et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 22.Kim W.R., Biggins S.W., Kremers W.K., et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reig M., Forner A., Rimola J., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M., Chung H., Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 26.Ayuso C., Rimola J., Vilana R., et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol. 2018;101:72–81. doi: 10.1016/j.ejrad.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Chernyak V., Tang A., Do R.K.G., et al. Liver imaging: it is time to adopt standardized terminology. Eur Radiol. 2022;32:6291–6301. doi: 10.1007/s00330-022-08769-5. [DOI] [PubMed] [Google Scholar]
- 28.Bhagat V., Javle M., Yu J., et al. Combined hepatocholangiocarcinoma: case-series and review of literature. Int J Gastrointest Cancer. 2006;37:27–34. doi: 10.1385/IJGC:37:1:27. [DOI] [PubMed] [Google Scholar]
- 29.Wachtel M.S., Zhang Y., Xu T., et al. Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin Med Pathol. 2008;1:43–47. doi: 10.4137/cpath.s500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding J.J., Nandakumar S., Armenia J., et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25:2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrero J.A., Kulik L.M., Sirlin C.B., et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 32.Kim K., Kim S.J. Diagnostic test accuracies of F-18 FDG PET/CT for prediction of microvascular invasion of hepatocellular carcinoma: a meta-analysis. Clin Imaging. 2021;79:251–258. doi: 10.1016/j.clinimag.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Chotipanich C., Kunawudhi A., Promteangtrong C., et al. Diagnosis of hepatocellular carcinoma using C11 choline PET/CT: comparison with F18 FDG, contrast enhanced MRI and MDCT. Asian Pac J Cancer Prev. 2016;17:3569–3573. [PubMed] [Google Scholar]
- 34.Vidili G., Arru M., Solinas G., et al. Contrast-enhanced ultrasound liver imaging reporting and data system: lights and shadows in hepatocellular carcinoma and cholangiocellular carcinoma diagnosis. World J Gastroenterol. 2022;28:3488–3502. doi: 10.3748/wjg.v28.i27.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefrere J.J., Gozin D., Soulier J.P., et al. Specificity of increased des-gamma-carboxyprothrombin in hepatocellular carcinoma after vitamin K1 injection. J Hepatol. 1987;5:27–29. doi: 10.1016/s0168-8278(87)80057-0. [DOI] [PubMed] [Google Scholar]
- 36.Kabir T., Tan Z.Z., Syn N.L., et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg. 2021;109:21–29. doi: 10.1093/bjs/znab376. [DOI] [PubMed] [Google Scholar]
- 37.Mazzaferro V., Regalia E., Doci R., et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 38.Yao F.Y., Ferrell L., Bass N.M., et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 39.Mazzaferro V., Llovet J.M., Miceli R., et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 40.Duvoux C., Roudot-Thoraval F., Decaens T., et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–994 e983. doi: 10.1053/j.gastro.2012.05.052. quiz e914-985. [DOI] [PubMed] [Google Scholar]
- 41.Pinero F., Tisi Bana M., de Ataide E.C., et al. Liver transplantation for hepatocellular carcinoma: evaluation of the alpha-fetoprotein model in a multicenter cohort from Latin America. Liver Int. 2016;36:1657–1667. doi: 10.1111/liv.13159. [DOI] [PubMed] [Google Scholar]
- 42.Llovet J.M., De Baere T., Kulik L., et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293–313. doi: 10.1038/s41575-020-00395-0. [DOI] [PubMed] [Google Scholar]
- 43.Di Martino M., Vitale A., Ferraro D., et al. Downstaging therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis on intention-to-treat outcomes. Cancers (Basel) 2022;14:5102. doi: 10.3390/cancers14205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takayama T., Hasegawa K., Izumi N., et al. Surgery versus radiofrequency ablation for small hepatocellular carcinoma: a randomized controlled trial (SURF Trial) Liver Cancer. 2022;11:209–218. doi: 10.1159/000521665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dou Z., Lu F., Ren L., et al. Efficacy and safety of microwave ablation and radiofrequency ablation in the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Medicine (Baltimore) 2022;101 doi: 10.1097/MD.0000000000029321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salem R., Johnson G.E., Kim E., et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology. 2021;74:2342–2352. doi: 10.1002/hep.31819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J., Shin I.S., Yoon W.S., Koom W.S., Rim C.H. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: meta-analyses and a systematic review. Radiother Oncol. 2020;145:63–70. doi: 10.1016/j.radonc.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Rim C.H., Lee J.S., Kim S.Y., Seong J. Comparison of radiofrequency ablation and ablative external radiotherapy for the treatment of intrahepatic malignancies: a hybrid meta-analysis. JHEP Rep. 2023;5 doi: 10.1016/j.jhepr.2022.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel A., Cervantes A., Chau I., et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–iv255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 50.Vogel A., Martinelli E., ESMO Guidelines Committee Electronic address: clinicalguidelines@esmo.org; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol. 2021;32:801–805. doi: 10.1016/j.annonc.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Llovet J.M., Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 52.Brown K.T., Do R.K., Gonen M., et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046–2053. doi: 10.1200/JCO.2015.64.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garin E., Tselikas L., Guiu B., et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17–29. doi: 10.1016/S2468-1253(20)30290-9. [DOI] [PubMed] [Google Scholar]
- 54.Brown A.M., Kassab I., Massani M., et al. TACE versus TARE for patients with hepatocellular carcinoma: overall and individual patient level meta analysis. Cancer Med. 2023;12:2590–2599. doi: 10.1002/cam4.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhondt E., Lambert B., Hermie L., et al. (90)Y Radioembolization versus drug-eluting bead chemoembolization for unresectable hepatocellular carcinoma: results from the TRACE phase II randomized controlled trial. Radiology. 2022;303:699–710. doi: 10.1148/radiol.211806. [DOI] [PubMed] [Google Scholar]
- 56.Li Q.J., He M.K., Chen H.W., et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol. 2022;40:150–160. doi: 10.1200/JCO.21.00608. [DOI] [PubMed] [Google Scholar]
- 57.Kudo M., Ueshima K., Ikeda M., et al. Final results of TACTICS: a randomized, prospective trial comparing transarterial chemoembolization plus sorafenib to transarterial chemoembolization alone in patients with unresectable hepatocellular carcinoma. Liver Cancer. 2022;11:354–367. doi: 10.1159/000522547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buckstein M., Kim E., Ozbek U., et al. Combination transarterial chemoembolization and stereotactic body radiation therapy for unresectable single large hepatocellular carcinoma: results from a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2022;114:221–230. doi: 10.1016/j.ijrobp.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Peng Z.W., Zhang Y.J., Chen M.S., et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 60.Wang X., Hu Y., Ren M., et al. Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: a time-to-event meta-analysis. Korean J Radiol. 2016;17:93–102. doi: 10.3348/kjr.2016.17.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Comito T., Loi M., Franzese C., et al. Stereotactic radiotherapy after incomplete transarterial (chemo-) embolization (TAE∖TACE) versus exclusive TAE or TACE for treatment of inoperable HCC: a phase III trial ( NCT02323360) Curr Oncol. 2022;29:8802–8813. doi: 10.3390/curroncol29110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mo A., Velten C., Jiang J.M., et al. Improving adjuvant liver-directed treatment recommendations for unresectable hepatocellular carcinoma: an artificial intelligence-based decision-making tool. JCO Clin Cancer Inform. 2022;6 doi: 10.1200/CCI.22.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 64.Abou-Alfa G.K., Chan S.L., Kudo M., et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40:379. [Google Scholar]
- 65.Rimassa L., Personeni N., Czauderna C., Foerster F., Galle P. Systemic treatment of HCC in special populations. J Hepatol. 2021;74:931–943. doi: 10.1016/j.jhep.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 66.Carroll R.P., Boyer M., Gebski V., et al. Immune checkpoint inhibitors in kidney transplant recipients: a multicentre, single-arm, phase 1 study. Lancet Oncol. 2022;23:1078–1086. doi: 10.1016/S1470-2045(22)00368-0. [DOI] [PubMed] [Google Scholar]
- 67.Finn R.S., Ryoo B.Y., Merle P., et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 68.Abou-Alfa G.K., Borgman-Hagey A.E., Kelley R.K. Cabozantinib in hepatocellular carcinoma. N Engl J Med. 2018;379:1384–1385. doi: 10.1056/NEJMc1810178. [DOI] [PubMed] [Google Scholar]
- 69.Qin S., Chen Z., Fang W., et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: a randomized, double-blind, phase III trial. J Clin Oncol. 2023;41:1434–1443. doi: 10.1200/JCO.22.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yau T., Kang Y.K., Kim T.Y., et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6 doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruix J., Qin S., Merle P., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 72.Zhu A.X., Kang Y.K., Yen C.J., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 73.Bruix J., Chan S.L., Galle P.R., Rimassa L., Sangro B. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75:960–974. doi: 10.1016/j.jhep.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Storandt M.H., Gile J.J., Palmer M.E., et al. Cabozantinib following immunotherapy in patients with advanced hepatocellular carcinoma. Cancers (Basel) 2022;14:5173. doi: 10.3390/cancers14215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shui Y., Yu W., Ren X., et al. Stereotactic body radiotherapy based treatment for hepatocellular carcinoma with extensive portal vein tumor thrombosis. Radiat Oncol. 2018;13:188. doi: 10.1186/s13014-018-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He M., Li Q., Zou R., et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chow P., Chen M., Cheng A.L., et al. AACR; Orlando, Florida. Philadelphia, PA: 2023. IMbrave050: phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. Proceedings of the 114th Annual Meeting of the American Association for Cancer Research. April 14-19, 2023; p. CT003. [Google Scholar]
- 78.Peng Z., Fan W., Zhu B., et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH) J Clin Oncol. 2023;41:117–127. doi: 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
- 79.Dawson L.A., Winter K.A., Knox J.J., et al. NRG/RTOG 1112: randomized phase III study of sorafenib vs. stereotactic body radiation therapy (SBRT) followed by sorafenib in hepatocellular carcinoma (HCC) J Clin Oncol. 2023;41:489. [Google Scholar]
- 80.Qin S., Kudo M., Meyer T., et al. Final analysis of RATIONALE-301: randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol. 2022;33:S808–S809. doi: 10.2217/fon-2019-0097. [DOI] [PubMed] [Google Scholar]
- 81.Qin S., Chan L.S., Gu S., et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol. 2022;33:LBA35. [Google Scholar]