Abstract
We examined the patterns of antibiotic prescribing by medical and non‐medical prescribers (dentists, nurse practitioners, and midwives) in Australia. We explored trends in the dispensed use of antibiotics (scripts and defined daily dose [DDD] per 1000 population/day) by Australian prescribers over the 12‐year period, 2005–2016. We obtained data on dispensed prescriptions of antibiotics from registered health professionals subsidized on the Pharmaceutical Benefits Scheme (PBS). There were 216.2 million medical and 7.1 million non‐medical dispensed prescriptions for antibiotics over 12 years. The top four antibiotics for medical prescribers were doxycycline; amoxicillin, amoxicillin plus clavulanic acid, and cefalexin, constituting 80% of top 10 use in 2005 and 2016; the top three for non‐medical were amoxicillin, amoxicillin plus clavulanic acid and metronidazole (84% of top 10 use in 2016). The proportional increase in antibiotic use was higher for non‐medical than medical prescribers. While medical prescribers preferentially prescribed broad‐spectrum and non‐medical prescribers moderate‐spectrum antibiotics, there was a large increase in the use of broad‐spectrum antibiotics over time by all prescribers. One in four medical prescriptions were repeats. Overprescribing of broad‐spectrum antibiotics conflicts with national antimicrobial stewardship initiatives and guidelines. The proportional higher increase in antibiotic use by non‐medical prescribers is a concern. To reduce inappropriate use of antibiotics and antimicrobial resistance, educational strategies targeted at all medical and non‐medical prescribers are needed to align prescribing with current best practice within the scope of practice of respective prescribers.
Keywords: anti‐infective agents, dentistry, microbial, midwifery, nursing, prescribing
Abbreviations
- AMH
Australian Medicines Handbook
- AMR
antimicrobial resistance
- AMS
antimicrobial stewardship
- ATC
anatomical therapeutic chemical (classification)
- DDD
defined daily dose
- eTG
electronic Therapeutic Guidelines
- GP
general practitioner
- MW
midwives
- NP
nurse practitioners
- PBS
Pharmaceutical Benefits Scheme
1. INTRODUCTION
Antibiotics are a finite resource. By 2050, it is estimated that more deaths will be attributable to antimicrobial resistance than cancer. 1 Antimicrobial resistance (AMR) is an urgent public health concern; and inappropriate antibiotic prescribing is linked to increased AMR. 2 As Australia's use of antibiotics is one of the highest in the developed world, 3 the Australian Government released a National Antimicrobial Resistance Strategy in 2015 to guide the response to this threat from antibiotic misuse and resistance. 4 The main aim of the strategy is to provide antimicrobial stewardship (AMS) to all sectors of health care.
AMS is the coordinated set of actions designed to promote and increase the appropriate use of antibiotics and is a key strategy to conserve antibiotic effectiveness. 4 Judicious AMS strategies lead to improved infection outcomes that range from reducing the quantum and improving the appropriateness of antibiotic prescribing, to reducing infection rates through immunization. 5 Successful AMS models improve the management of infections, and reduce institutional resistance rates, morbidity, mortality, and health care costs. 6 Most AMS strategies, have, however, been undertaken in hospitals rather than in the community. 5
There is an exponential rise in non‐medical healthcare professionals prescribing worldwide and in Australia. Non‐medical prescribers in Australia include nurse practitioners (NP), midwives (MW), dentists, and optometrists, with dentists comprising the largest group within non‐medical prescribers. 7
Understanding the patterns of antibiotic prescribing, by health discipline, is the first step in developing effective strategies to improve the quality use of antibiotics in primary care. We aimed to examine the patterns of antibiotic prescribing by non‐medical and medical prescribers in Australia by class of antibiotics, spectrum, and prescription type, over time.
2. METHODS
We purchased data from the Department of Human Services Medicare 8 for each formulation of each systemic antibacterial dispensed on the Pharmaceutical Benefits Scheme (PBS) by prescriber type and prescription type (original, repeat) between January 2005 and September 2016. First introduced in 1948, the PBS is a national formulary that subsidizes a comprehensive range of registered medicines to Australian citizens. PBS medicines are mostly prescribed in the community and are intended to be used by patients at home. Hospital prescriptions eligible for subsidy are limited to non‐admitted patients and at hospital discharge. 9 As such, certain intravenous antibiotics prescribed for serious infections are unlikely to be included in the dataset.
There are two levels of PBS co‐payments—one for general beneficiaries (AU$38.30 in 2016) 10 and a lower one for concessional beneficiaries (those on social security) (AU$6.20 in 2016). Some medicines are priced below the co‐payment for general beneficiaries (under co‐payment, i.e., not PBS‐subsidized) and dispensing data for those are collected in a different way to PBS‐subsidized data. The data do not include non‐subsidized use of medicines dispensed with a private prescription (patient pays the full cost) but this is likely to be negligible for these products. Before July 2012, PBS data did not include dispensings for medicines that fell below the consumer co‐payment level. No medicines cost less than the concessional co‐payment, so all medicines dispensed to concessional beneficiaries were captured. However, some dispensings to general beneficiaries fell below the co‐payment level and were excluded from data capture. From July 2012 onwards, information on all PBS dispensings (including those that cost less than the consumer co‐payment level) is captured in the PBS database. 11
We calculated dispensed medicine use for medicines in Anatomical Therapeutic Chemical (ATC) codes J01A (antibacterials for systemic use). 12 These are broadly classified as: tetracyclines; amphenicols; beta‐lactam antibacterials; sulfonamides and trimethoprim; macrolides, lincosamides and streptogramins; aminoglycosides; quinolones and other antibacterials. We excluded non‐antibiotic antimicrobials in J01XX such as hexamine hippurate, as these are not generally used to treat new active infections. We used the WHO standardized methodology of defined daily dose (DDD, maintenance dose for the main indication in adults) per 1000 population per day between 2005 and 2016 (12 years) to calculate dispensed use. 12
We collated the classes of health professionals with prescribing rights in Australia into medical and non‐medical. Medical practitioners were defined as general practitioner (family doctor or primary care physician), physician (medical specialists in internal medicine) and surgeon (medical specialists in surgery), with non‐medical prescribers classified as dentist, nurse practitioner, midwife, and optometrist. In Australia, dentists, nurse practitioners, midwives, and optometrists are not considered medical prescribers, and there are relatively few physician assistants.
Many medications on the PBS are subsidized for a specific patient group or indication. There are three restriction categories: (1) Unrestricted benefits (no restrictions apply to their therapeutic use); (2) Restricted benefits (can only be prescribed for specific therapeutic uses); and (3) Authority required benefits (prescriber must gain approval from Services Australia for the prescription to be valid). We analyzed dispensed use of these systematic antibacterial agents within this context.
We allocated all antibiotics to a spectrum and class, assessed use by prescription type, and compared use of the ‘top 6’ antibiotics between medical and non‐medical prescribers. There is a lack of consensus to define antibiotic spectrum class (narrow, moderate, broad), so we compared the spectrum category of individual antibiotics prescribed in the community using three main sources: (1) the Australian eTherapeutic Guidelines (eTG) 13 , 14 ; (2) the Australian Medicines Handbook (AMH) 15 ; and (3) the Sanford Guide to Antimicrobial Therapy 16 supplemented by other sources. 17 , 18 , 19
The eTG contains comprehensive national guidelines for antibiotic prescribing in hospitals and general practice, and both eTG and AMH are accepted Australian national community resources that primary care health professionals would have ready access to. It was only where there was discordance between the resources for a specific antibiotic that we sought expert opinion to establish spectrum categories from a panel, consisting of a GP widely published in the area of judicious antibiotic prescribing, a member of the Cochrane Collaboration Respiratory Tract Infection subgroup, an Antimicrobial Stewardship Pharmacist and an Infectious Diseases medical microbiologist (Table 1). For example, amoxicillin and cefalexin were classified as moderate spectrum, with doxycycline and amoxicillin plus clavulanic acid as broad spectrum.
TABLE 1.
Categorization of antibiotics and class by spectrum.
| Spectrum | Antibiotics | Antibacterial class |
|---|---|---|
| Narrow |
Benzathine penicillin, Benzylpenicillin Phenoxymethypenicillin, Procaine penicillin |
Penicillin–narrow |
| Dicloxacillin, Flucloxacillin | Anti‐Staphylococcal penicillin | |
| Clindamycin, Lincomycin | Lincosamide | |
| Sodium fusidate | Other—Narrow | |
| Gentamicin, Tobramycin | Aminoglycoside | |
| Vancomycin | Glycopeptide | |
| Hexamine Hippurate | Other—Narrow | |
| Moderate | Amoxicillin, Ampicillin | Aminopenicillin |
| Cefalexin, Cefaclor, Cefalothin, Cefazolin, Cefuroxime | 1st generation Cefalosporin | |
| Broad | Amoxicillin + Clavulanic acid | Aminopenicillin—extended |
| Cefepime | 4th generation Cefalosporin | |
| Cefoxitin | 2nd generation Cefalosporin | |
| Cefotaxime, Ceftriaxone | 3rd generation Cefalosporin | |
| Chloramphenicol | Other | |
| Ciprofloxacin, Gatifloxacin, Moxifloxacin, Norfloxacin | Quinolone | |
| Doxycycline, Minocycline, Tetracycline | Tetracycline | |
| Azithromycin, Clarithromycin Erythromycin, Roxithromycin | Macrolide | |
| Metronidazole, Tinidazole | Nitroimidazole | |
| Imipenem, Meropenem | Carbapenem | |
| Nitrofurantoin | Nitrofuran | |
| Piperacillin + Tazobactam, Ticarcillin + Clavulanate | Ureidopenicillin | |
|
Trimethoprim, Trimethoprim + Sulfamethoxazole |
Other Other + Sulfonamide |
In order to have more complete capture of all antibiotics, we analyzed data from concessional beneficiaries (all medicines priced above the concessional co‐payment). 20 We obtained the mid‐year Australian resident population values from the Department of Social Services annual reports. 21
We analyzed the data using descriptive statistics (Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). This was a retrospective analysis of routinely‐collected aggregated data where no individual could be identified, hence no ethical approval was necessary.
3. RESULTS
3.1. Top six antibiotics
There were 216 226 713 antibiotic prescriptions dispensed that were written by medical prescribers (96.8%) and 7 085 882 prescriptions by non‐medical prescribers (3.2%) over the study period (2005–2016). Of these prescriptions, 89.5% originated from general practitioners, 4.8% from physicians, 2.5% from surgeons, 3.1% from dentists, and less than 0.1% from the remaining non‐medical prescribers (nurse practitioners, midwives (after 2011), and optometrists).
The antibiotics prescribed by medical prescribers differed from non‐medical prescribers. The top four antibiotics for medical prescribers were doxycycline; amoxicillin, amoxicillin plus clavulanic acid, and cefalexin, constituting 80% of top 10 use in both 2005 and 2016 (Figure 1).
FIGURE 1.
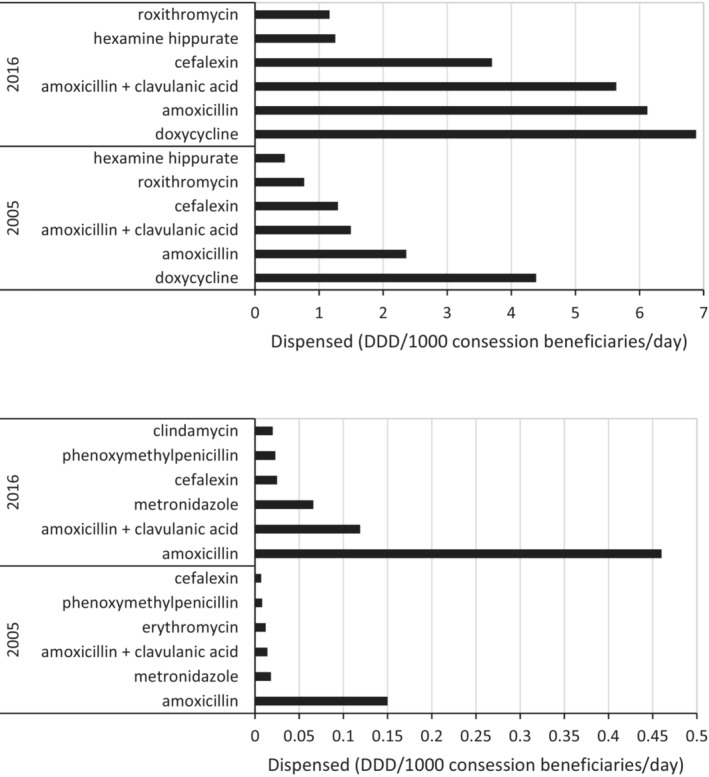
Top six antibiotics by dispensed use (DDD/1000 concession beneficiaries/day) for medical prescribers (upper panel) and non‐medical prescribers (lower panel) for 2005 and 2016.
The top six antibiotics accounted for 89% of top 10 use in 2016. The use of the top four antibiotics increased substantially between 2005 and 2016 (Figure 2). Amoxicillin plus clavulanic acid increased 277% (to 5.636 DDD/1000/day) and cefalexin increased 186% (to 3.698 DDD/1000/day) in 2016 (Figure 1). These two antibiotics accounted for 78% of top 10 use in 2016.
FIGURE 2.
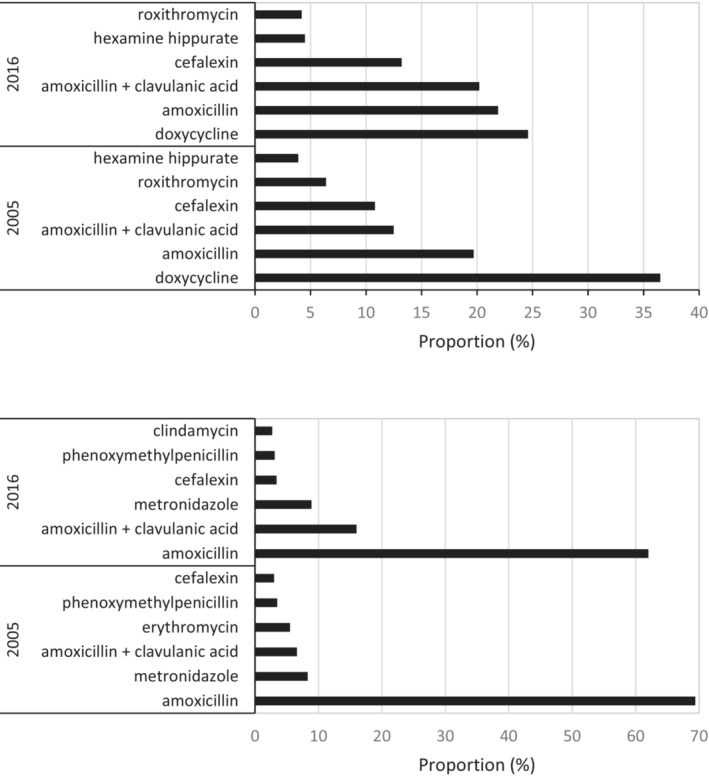
Top six antibiotics—proportion of top 10 (as measured by dispensed use DDD/1000 concession beneficiaries/day) for medical prescribers (upper panel) and non‐medical prescribers (lower panel) for 2005 and 2016.
Non‐medical prescribers preferentially prescribed amoxicillin, amoxicillin plus clavulanic acid and metronidazole, constituting 84% of top 10 use in 2005 and 87% in 2016 (Figure 2). The use of the top three antibiotics increased by 207%, 561% and 371%, respectively, between 2005 and 2016.
3.2. Antibiotics by spectrum
The profile of antibiotic use by spectrum differed between medical and non‐medical prescribers. For medical prescribers, broad‐spectrum antibiotics increased from 22% in 2005 to 63% in 2016. Overall, broad‐ and moderate‐spectrum agents accounted for 92% of all antibiotic use in 2016 (Figure 3). Use of broad‐spectrum antibiotics increased by 111% between the two time periods (with 157% for moderate spectrum).
FIGURE 3.
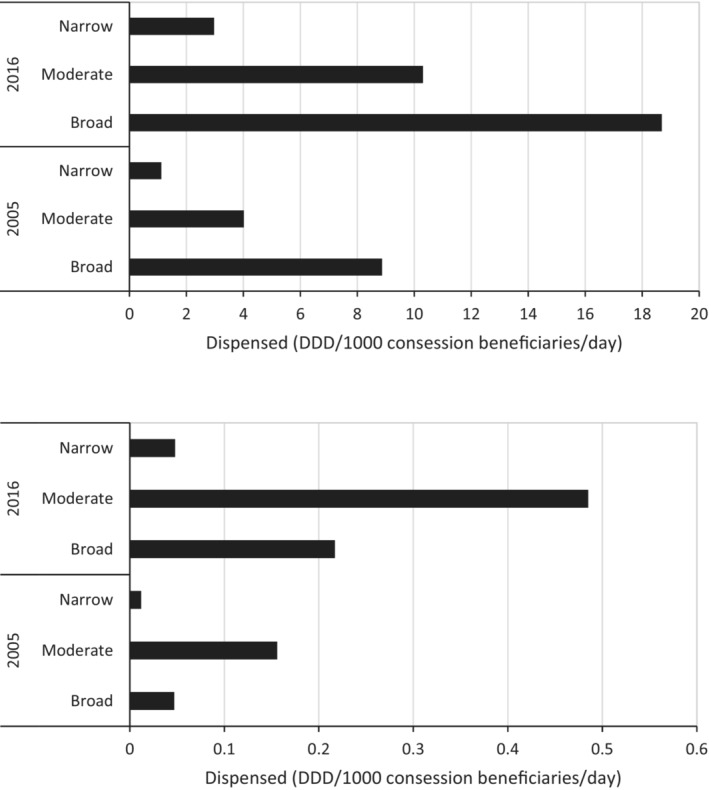
Antibiotic dispensed use (DDD/1000 concession beneficiaries/day) by spectrum (narrow, moderate and broad) for medical prescribers (upper panel) and non‐medical prescribers (lower panel) for 2005 and 2016.
For non‐medical prescribers, moderate‐spectrum antibiotics were the most commonly prescribed (72% in 2005, 65% in 2016, Figure 4). Together, broad‐ and moderate‐spectrum antibiotics constituted 94% in both years. The rate of non‐medical prescribing increased across all spectrum classes between 2005 and 2016: broad by 362%; moderate by 211%; and narrow by 300% (Figure 4).
FIGURE 4.
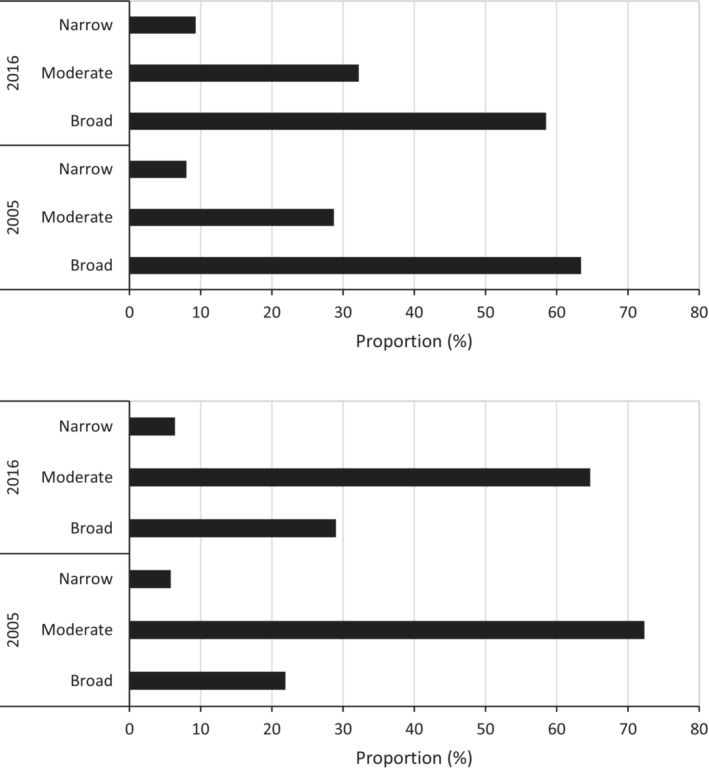
Antibiotic dispensed use (proportion as measured by dispensed use DDD/1000 concession beneficiaries/day) by spectrum (narrow, moderate and broad) for medical prescribers (upper panel) and non‐medical prescribers (lower panel) for 2005 and 2016.
3.3. Use by prescription type
During the study period, medical prescribers were permitted to issue repeat prescriptions for antibiotics. Between 2005 and 2011, 24% of all dispensed prescriptions were repeats (Figure 5). Although the absolute number of prescriptions rose markedly after 2011 to a peak of 27.484 million prescriptions per year in 2015, the proportion of repeat prescriptions fell to 20% of the total in 2016 (Figure 3). NP and midwives were permitted to write repeat prescriptions, dependent on the antibiotic and dose. 7 While dentists were not (routinely) approved to prescribe repeat prescriptions during the study period, we identified 2700 repeat prescriptions dispensed over this period.
FIGURE 5.
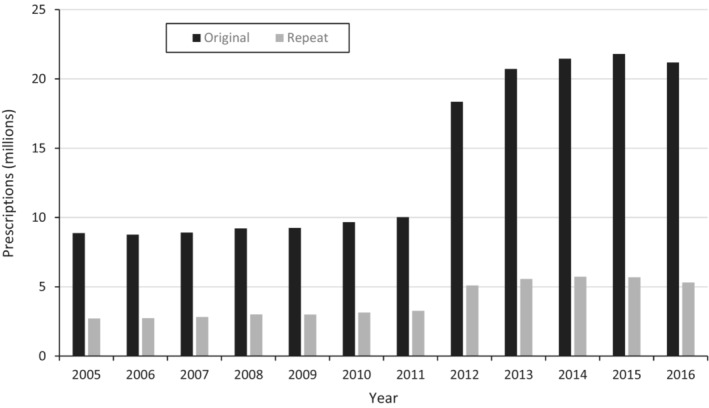
Antibiotic dispensed use (prescriptions counts of concession beneficiaries) by prescription type (original or repeat) for medical prescribers between 2005 and 2016.
4. DISCUSSION
Antibiotic use increased markedly over time, with a large increase in the use of broad‐spectrum antibiotics over time by all prescribers. Some 24% of medical prescriptions were repeats. For medical prescribers, the most dispensed antibiotics were doxycycline, amoxicillin, and amoxicillin plus clavulanic acid, while for non‐medical prescribers, amoxicillin and amoxicillin + clavulanic acid were most common. In contrast to countries such as the United Kingdom 22 , 23 and the USA, 24 broad spectrum macrolide antibiotics such as roxithromycin and azithromycin did not feature in the Australian list of top ranked antibiotics dispensed.
Australian national guidelines for antibiotic prescribing in general practice and hospitals 14 consistently place macrolides as a lower rank choice for many indications and there are national restrictions placed on azithromycin prescribing through the PBS. These stewardship strategies have contributed to reducing the use of this antibiotic class. As early as 2006, Jarvinen and colleagues commented in a letter to the editor on the “PBS ‐ limitations on macrolides” in the Medical Journal of Australia. 25 They stated that widespread use of newer macrolides in the community is not advisable because of the propensity of macrolides to induce antibiotic resistance, and their greater cost. They also referred to the restrictions placed on specifically azithromycin prescribing through the PBS having important implications for the effective and safe management of pertussis in Australia.
While non‐medical prescribers accounted for only 3.2% of total antibiotic prescriptions, this group showed the greatest proportional increase over time. Medical prescribers preferentially prescribed broad‐spectrum antibiotics, whereas non‐medical prescribers preferred moderate‐spectrum antibiotics.
This is the first study to examine medical and non‐medical prescribing of antibiotics in Australia. We acknowledge some limitations. We could only analyze use in concession beneficiaries prior to July 2012, 11 but this constitutes 92% of dispensed use (by volume) on the PBS. 26 It is unlikely that patterns of prescribing would differ markedly between general and concessional beneficiaries. We categorized antibiotics by spectrum using published sources and expert opinion, but we acknowledge that using other sources may result in slightly different classifications.
Our study reaffirms that general medical practitioners (GPs) remain the dominant medical antibiotic prescribers in Australia. 27 They were responsible for nine in 10 dispensed prescriptions over the 12‐year study period, which is well above the comparable figure of 65% for GPs in Denmark in 2015 and 2016. 28 Del Mar and colleagues highlighted that the antibiotic crisis is not directly obvious to GPs working in the community. They advocated for improved surveillance for monitoring community antibiotic resistance rates by indication, together with regulatory interventions such as changing the default in electronic prescribing to ‘no repeats’, changing packaging to facilitate tailored amounts for the specified indications, and restricting access to particular antibiotics. 29
Prescriptions from dentists constituted only 3.1% of all antibiotics dispensed; almost 7 million prescriptions over 12 years. Based on official data from the Australian Health Practitioner Regulation Agency, there were 21 838 general dentists and 1804 dental specialists registered in Australia in September 2020. This gives an average of 24 prescriptions per year per dental registrant. By comparison, the medical workforce is five times larger (43 710 general medical practitioners and 71 167 medical specialists), and accounted for over 216 million prescriptions over 12 years, at an average of 157 prescriptions per year per registrant. Of concern, amoxicillin was the most commonly prescribed antibiotic by dentists, 20 , 30 which does not align with the Therapeutic Guidelines for dental indications for treating infections, or with best practice for antimicrobial stewardship (AMS).
Outside of dentists, other non‐medical prescribing in Australia (i.e., nurse practitioners and midwives) is in its infancy. A comparable descriptive study of national data on antibiotic prescriptions from Scottish nurse prescribers over 2007–2013 showed considerable variability in prescribing patterns and the volume of antibiotics prescribed. Non‐medical prescribing could become an increasing contributor to antibiotic prescribing in primary care settings. 31
To reduce AMR, non‐medical prescribing should be aligned to the national AMR strategy, both for treatment of infections and for antibiotic prophylaxis (including post‐surgical prophylaxis). 4 , 32 The present data show similar issues to those identified from the 2013 National Antimicrobial Prescribing Survey, 33 with unnecessary use of broad spectrum antibiotics. The current regulations for permitted repeat prescriptions focus more on the discipline than recognizing chronicity or severity of infection. We need to better address the issue of repeat prescriptions and requisite focus of future monitoring of antibiotic use. This reinforces the need for further initiatives in best practice prescribing principles to conserve antibiotics as a valuable resource, targeted to the rapidly expanding non‐medical prescriber cohort and in settings where that are identified challenges to implement AMS.
Around 28% of the Australian population (7 million people) live in rural and remote areas. This poses unique challenges due to their geographic location, and they often have poorer health outcomes than people living in metropolitan areas. People living in rural and remote areas have higher rates of hospitalizations, deaths, and injury, and also poorer access to, and use of, primary health care services, than people living in major cities. 34 The National Centre for Antimicrobial Stewardship in Australia reported on regional and rural hospitals in Australia having context‐specific needs and challenges relating to antimicrobial stewardship (AMS). Moreover, there are disparities in AMS implementation, reflecting broader differences in healthcare delivery between metropolitan and regional and rural settings. 35 , 36 , 37 The Hospital National Antimicrobial Prescribing Survey (NAPS) suggests that, compared with major‐city hospitals, regional and rural hospitals have higher levels of inappropriate antimicrobial prescribing for particular antimicrobials (e.g., ceftriaxone) and common infections (e.g., cellulitis and sepsis). 35 Two qualitative studies by Bishop et al. 38 , 39 found that barriers to the implementation of AMS programs in rural settings include competing demands for resources; difficulty in recruiting staff; lack of training and education; limited resources for information technology; limited pharmacy resources; distance (resulting in isolation from the larger centres); and lack of support from some medical professionals. These findings build on other Australian work in rural settings. 36 , 40 , 41 , 42
Healthcare professionals in rural and regional areas would benefit greatly from AMS training, resources, service support, and education. Such areas often have limited resources, and less or no access to expert AMS advice. It is easier to influence new behaviors in novice prescribers than change established prescribing practices. 43 , 44
Education and lifelong learning in relation to antibiotic prescribing is a critical strategy in the global fight against AMR. 45 There is a need to improve prescribers' awareness of AMS principles, to address gaps in education, including inconsistent teaching on the management of infectious diseases in clinical curricula, and improve skills in prescribing antibiotics. 46 It is vital to have consistency among health professions in applying AMS principles. What, how, and where healthcare professionals are taught shapes the readiness and resilience of a health system. 46 We need to develop effective interdisciplinary programs on best practice in prescribing. 47 All prescribers (medical and non‐medical) need core competencies in prescribing medicines. 48 This is particularly so for some professions (e.g., midwives and nurse practitioners) who can have quite wide scopes of practice. 49 Nurse practitioners have been able to prescribe from the PBS since 2010; they are often asked to prescribe antibiotics in primary care and hospital emergency departments. 50 Midwives were given prescribing rights in 2010; and two in five are endorsed. 49 Midwives can prescribe antibiotics in the prenatal, intrapartum and post‐natal stages of pregnancy. 51
Our finding of a strong preference for broad spectrum antibiotics by dental prescribers did not align with the guidelines for antibiotic treatments. 52 , 53 Such issues could be addressed in three main ways: (a) education within the curriculum for current students; (b) continuing professional development (CPD) for current practitioners; and (c) policy changes such as accreditation of dental practices placing greater stress on medication safety and prescribing patterns.
Overall, non‐medical prescribing of antibiotics is small compared to medical prescribing, but nevertheless it is important for AMS. Antibiotic choices should align with the most recent Therapeutic Guidelines. 14 There is a growing group of prescribers, including NPs and midwives, with expanding scope. AMS efforts need to take into account the growing number of non‐medical prescribers, and work with the respective professions to promote AMS and eliminate the excessive use of antibiotics. 45 The impact of changes to curricula and from CPD programs on AMS needs to be assessed, so that preparedness for safe and optimal prescribing practices is enhanced. 46
AUTHOR CONTRIBUTIONS
Samantha Hollingworth, Treasure McGuire, and Mieke van Driel conceptualized the project, interpreted the analyses, wrote, edited, and reviewed the main text, and approved the final edition of the manuscript. Nelufa Begum analyzed the data, reviewed, and approved the final edition of the manuscript. Pauline Ford, Glenda Hawley, and Laurence Walsh contributed to the interpretation of the analyses, reviewed, and approved the final edition of the manuscript.
FUNDING INFORMATION
We thank the University of Queensland Faculty of Health and Behavioral Sciences for funding this research with a Research Collaboration Seeding Grant. The funder had no role in the study, and the researchers were independent from the funder.
DISCLOSURE
The authors have no conflicts of interest to declare.
ETHICS APPROVAL STATEMENT
No ethics approval was required for using these de‐identified data. No individual could have been identified from the data provided by the data custodian (Australian Government).
ACKNOWLEDGMENTs
Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Hollingworth SA, McGuire T, Van Driel M, et al. Antimicrobial stewardship: Prescribing across the primary care health professions. Pharmacol Res Perspect. 2023;11:e01106. doi: 10.1002/prp2.1106
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available for purchase from the Australian Government Services Australian (formerly the Department of Human Services). Restrictions apply to the availability of these data, which were used with permission for this study.
REFERENCES
- 1. Review on Antimicrobial Resistance . Tackling drug‐resistant infections globally: final report and recommendations. 2016. Accessed 23 Nov 2022. https://amr‐review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
- 2. Department of Health and Aged Care . Antimicrobial resistance (AMR). 2022. Accessed 23 Nov 2022. https://www.amr.gov.au/
- 3. McKenzie D, Rawlins M, Del Mar C. Antimicrobial stewardship: what's it all about? Aust Prescr. 2013;36:116‐201. [Google Scholar]
- 4. Australian Government Department of Health, Department of Agriculture . Responding to the threat of antimicrobial resistance: Australia's First Antimicrobial Resistance Strategy 2015–2019. 2015. Accessed 23 Nov 2022. https://www.amr.gov.au/resources/responding‐threat‐antimicrobial‐resistance‐australias‐first‐national‐antimicrobial‐resistance‐strategy‐2015‐2019
- 5. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. doi: 10.1002/14651858.CD003543.pub3 [DOI] [PubMed] [Google Scholar]
- 6. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159‐177. doi: 10.1086/510393 [DOI] [PubMed] [Google Scholar]
- 7. Department of Health . Pharmaceutical Benefits Scheme. 2022. Accessed 23 Nov 2022. http://www.pbs.gov.au/pbs/home
- 8. Department of Human Services . Statistical information and data. 2016. Accessed 23 Nov 2022. https://www.humanservices.gov.au/corporate/statistical‐information‐and‐data
- 9. Department of Health . Australian statistics on medicines 2015. Commonwealth Government. 2015. Accessed 23 Nov 2022. https://www.pbs.gov.au/info/statistics/asm/asm‐2015
- 10. Department of Human Services . Pharmaceutical Benefit Schedule item statistics. 2016. Accessed 23 Nov 2022. http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp
- 11. Paige E, Kemp‐Casey A, Korda R, Banks E. Using Australian pharmaceutical benefits scheme data for pharmacoepidemiological research: challenges and approaches. Public Health Res Pract. 2015;25(4):e2541546. [DOI] [PubMed] [Google Scholar]
- 12. WHO Collaborating Centre for Drug Statistics Methodology . ATC/DDD Index. 2022. Accessed 23 Nov 2022. http://www.whocc.no/atc_ddd_index/
- 13. Therapeutic Guidelines . Antibiotic prescribing in primary care: Therapeutic Guidelines summary table. 2022. Accessed 23 Nov 2022. https://www.tg.org.au/news/antibiotic‐summary‐table/
- 14. Therapeutic Guidelines Limited . Therapeutic Guidelines Antibiotic, Version 16. Therapeutic Guidelines; 2020. [Google Scholar]
- 15. Australian Medicines Handbook Pty Ltd . Australian Medicines Handbook. 2022. Accessed 23 Nov 2022. https://amhonline.amh.net.au/auth
- 16. Antimicrobial Therapy Inc . The Sanford Guide to Antimicrobial Therapy. Antimicrobial Therapy, Inc. 50th ed. 2020. [Google Scholar]
- 17. Bronzwaer SL, Cars O, Buchholz U, et al. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis. 2002;8(3):278‐282. doi: 10.3201/eid0803.010192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riedel S, Beekmann SE, Heilmann KP, et al. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae. Eur J Clin Microbiol Infect Dis. 2007;26(7):485‐490. doi: 10.1007/s10096-007-0321-5 [DOI] [PubMed] [Google Scholar]
- 19. Steinman MA, Landefeld CS, Gonzales R. Predictors of broad‐spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289(6):719‐725. doi: 10.1001/jama.289.6.719 [DOI] [PubMed] [Google Scholar]
- 20. Ford PJ, Saladine C, Zhang K, Hollingworth SA. Prescribing patterns of dental practitioners in Australia from 2001 to 2012. Antimicrobials Aust Dent J. 2017;62(1):52‐57. doi: 10.1111/adj.12427 [DOI] [PubMed] [Google Scholar]
- 21. Department of Social Services . Annual reports. 2016. Accessed 23 Nov 2022. http://www.dss.gov.au/about‐the‐department/publications‐articles/corporate‐publications/annual‐reports
- 22. Courtenay M, Gillespie D, Lim R. Patterns of dispensed non‐medical prescriber prescriptions for antibiotics in primary care across England: a retrospective analysis. J Antimicrob Chemother. 2017;72(10):2915‐2920. doi: 10.1093/jac/dkx230 [DOI] [PubMed] [Google Scholar]
- 23. Dolk FCK, Pouwels KB, Smith DRM, Robotham JV, Smieszek T. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother. 2018;73(suppl_2):ii2‐ii10. doi: 10.1093/jac/dkx504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308‐1316. doi: 10.1093/cid/civ076 [DOI] [PubMed] [Google Scholar]
- 25. Jarvinen KA, McCall BJ, Nourse CB, McCormack JG, Tilse MH. Pharmaceutical benefits scheme limitations on macrolides: implications for pertussis management. Med J Aust. 2006;184(6):309. doi: 10.5694/j.1326-5377.2006.tb00251.x [DOI] [PubMed] [Google Scholar]
- 26. Department of Health . Expenditure and Prescriptions twelve months to 30 June 2016. 2016. Accessed 23 Nov 2022. https://www.pbs.gov.au/info/statistics/pbs‐expenditure‐prescriptions‐30‐june‐2016
- 27. Australian Commission on Safety and Quality in Health Care . AURA 2016: First Australian Report on Antimicrobial Use and Resistance in Human Health ‐ Full Report. 2016. https://www.safetyandquality.gov.au/publications/aura‐2016‐first‐australian‐report‐on‐antimicroibal‐use‐and‐resistance‐in‐human‐health
- 28. Høg BB, Sönksen UW. DANMAP 2015 ‐ Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2016. file:///C:/Users/uqsholl1/AppData/Local/Temp/Report‐DANMAP‐2015.pdf
- 29. Del Mar CB, Scott AM, Glasziou PP, et al. Reducing antibiotic prescribing in Australian general practice: time for a national strategy. Med J Aust. 2017;207(9):401‐406. doi: 10.5694/mja17.00574 [DOI] [PubMed] [Google Scholar]
- 30. Teoh L, Stewart K, Marino RJ, McCullough MJ. Part 1. Current prescribing trends of antibiotics by dentists in Australia from 2013 to 2016. Aust Dent J. 2018;63:329‐337. doi: 10.1111/adj.12622 [DOI] [PubMed] [Google Scholar]
- 31. Ness V, Malcolm W, McGivern G, Reilly J. Growth in nurse prescribing of antibiotics: the Scottish experience 2007‐13. J Antimicrob Chemother. 2015;70(12):3384‐3389. doi: 10.1093/jac/dkv255 [DOI] [PubMed] [Google Scholar]
- 32. Australian Government . Australia's National Antimicrobial Resistance Strategy ‐ 2020 and Beyond . 2020.
- 33. Australian Commission on Safety and Quality in Health Care . Antimicrobial prescribing practice in Australia: results of the 2013 National Antimicrobial Prescribing Survey. 2014.
- 34. Australian Institute of Health and Welfare . Rural Remote Health. 2022. Accessed 23 Nov 2022. https://www.aihw.gov.au/reports/rural‐remote‐australians/rural‐and‐remote‐health
- 35. Australian Commission on Safety and Quality in Health Care . Third Australian Atlas of Healthcare Variation. 2018. Accessed 23 Nov 2022. https://www.safetyandquality.gov.au/publications‐and‐resources/australian‐atlas‐healthcare‐variation‐series
- 36. James R, Luu S, Avent M, Marshall C, Thursky K, Buising K. A mixed methods study of the barriers and enablers in implementing antimicrobial stewardship programmes in Australian regional and rural hospitals. J Antimicrob Chemother. 2015;70(9):2665‐2670. doi: 10.1093/jac/dkv159 [DOI] [PubMed] [Google Scholar]
- 37. Thursky KA, Hardefeldt LY, Rajkhowa A, et al. Antimicrobial stewardship in Australia: the role of qualitative research in programme development. JAC Antimicrob Resist. 2021;3(4):dlab166. doi: 10.1093/jacamr/dlab166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bishop JL, Schulz TR, Kong DCM, Buising KL. Qualitative study of the factors impacting antimicrobial stewardship programme delivery in regional and remote hospitals. J Hosp Infect. 2019;101(4):440‐446. doi: 10.1016/j.jhin.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 39. Bishop JL, Schulz TR, Kong DCM, James R, Buising KL. Similarities and differences in antimicrobial prescribing between major city hospitals and regional and remote hospitals in Australia. Int J Antimicrob Agents. 2019;53(2):171‐176. doi: 10.1016/j.ijantimicag.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 40. Avent ML, Hall L, Davis L, et al. Antimicrobial stewardship activities: a survey of Queensland hospitals. Aust Health Rev. 2014;38(5):557‐563. doi: 10.1071/AH13137 [DOI] [PubMed] [Google Scholar]
- 41. Broom A, Broom J, Kirby E, Gibson A, Davis M. Antibiotic optimisation in 'the bush': local know‐how and core‐periphery relations. Health Place. 2017;48:56‐62. doi: 10.1016/j.healthplace.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 42. Ervin KE, Tse KC, Reid C, Smith E. Exploring barriers to and enablers of antimicrobial stewardship in rural health services. Infect Dis Health. 2021;26(1):11‐21. doi: 10.1016/j.idh.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 43. Avent ML, Walker D, Yarwood T, et al. Implementation of a novel antimicrobial stewardship strategy for rural facilities utilising telehealth. Int J Antimicrob Agents. 2021;57(6):106346. doi: 10.1016/j.ijantimicag.2021.106346 [DOI] [PubMed] [Google Scholar]
- 44. Sharman LS, Avent ML, Lyall V, et al. Improving paediatric antimicrobial stewardship in remote and regional Queensland hospitals: development and qualitative evaluation of a tailored intervention for intravenous‐to‐oral antibiotic switching. BMJ Open. 2022;12(12):e064888. doi: 10.1136/bmjopen-2022-064888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pulcini C, Gyssens IC. How to educate prescribers in antimicrobial stewardship practices. Virulence. 2013;4(2):192‐202. doi: 10.4161/viru.23706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rocha‐Pereira N, Lafferty N, Nathwani D. Educating healthcare professionals in antimicrobial stewardship: can online‐learning solutions help? J Antimicrob Chemother. 2015;70(12):3175‐3177. doi: 10.1093/jac/dkv336 [DOI] [PubMed] [Google Scholar]
- 47. Hawes L, Buising K, Mazza D. Antimicrobial stewardship in general practice: a scoping review of the component parts. Antibiotics (Basel). 2020;9(8):498. doi: 10.3390/antibiotics9080498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morris S, Coombes I. The right to prescribe: towards core prescribing competencies for all prescribers. Australian Prescriber. 2011;34(5):136‐137. [Google Scholar]
- 49. Small K, Sidebotham M, Gamble J, Fenwick J. Exploring midwifery prescribing in Australia. Women Birth. 2016;29(5):436‐442. doi: 10.1016/j.wombi.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 50. McIntosh T, Stewart D, Forbes‐McKay K, McCaig D, Cunningham S. Influences on prescribing decision‐making among non‐medical prescribers in the United Kingdom: systematic review. Fam Pract. 2016;33:572‐579. doi: 10.1093/fampra/cmw085 [DOI] [PubMed] [Google Scholar]
- 51. Nursing and Midwifery Board of Australia . Endorsement for scheduled medicines for midwives. 2022. Accessed 23 Nov 2022. https://www.nursingmidwiferyboard.gov.au/registration‐standards/endorsement‐for‐scheduled‐medicines‐for‐midwives.aspx
- 52. Oral and Dental Expert Group . Therapeutic Guidelines: Oral and Dental. Version 2. Therapeutic Guidelines Limited; 2016. [Google Scholar]
- 53. Walsh LJ, Ford PJ, McGuire T, van Driel M, Hollingworth SA. Trends in Australian dental prescribing of antibiotics: 2005‐2016. Aust Dent J. 2021;66(Suppl 1):S37‐S41. doi: 10.1111/adj.12846 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available for purchase from the Australian Government Services Australian (formerly the Department of Human Services). Restrictions apply to the availability of these data, which were used with permission for this study.


