Abstract
The roles of plant growth-promoting rhizobacteria in promoting plant growth and soil health, including alteration in plant metabolism and production of phytohormones such as indole-3-acetic acid (IAA) and the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase, are indisputable. This study aimed to isolate and characterize beneficial bacteria isolated from the rhizosphere of pineapple from distinct stress-inducing habitats, including water excess-, herbicide-over-treated-, and pathogen-infected areas at PT Great Giant Foods located in Lampung, Indonesia. The isolated bacteria were screened based on IAA production and ACC deaminase activities. Six selected isolates produced IAA with concentrations of up to 36.93 mgL−1. The highest value belongs to Bacillus sp. NCTB5I, followed by Brevundimonas sp. CHTB 2C (13.13 mgL−1) and Pseudomonas sp. CHTB 5B (6.65 mgL−1). All isolates were detected with ACC deaminase activities with Brevundimonas sp. CHTJ 5H consuming 88% of ACC over 24 h, the highest among all. Brevundimonas sp. CHTB 2C was detected with the highest ACC deaminase activity with the value of 13,370 nm α-ketobutyrate mg−1h−1. In another experiment, it was revealed that all selected isolates promote soybean growth. These bacteria are potential to be developed in the future as bioagents to promote plant growth, especially under stressful environmental conditions.
Keywords: Bacillus, Brevundimonas, Burkholderia, Exiguobacterium, Plant growth-promoting rhizobacteria, Pseudomonas
1. Introduction
Plant growth-promoting rhizobacteria (PGPR) accommodate the acquisition of key nutrients for their host plants, such as nitrogen (N) and phosphorus (P), and modulate regulating substances (phytohormones) to improve growth and productivity [[1], [2], [3]]. Plant growth promotion effects by PGPR are achieved both directly and indirectly [4]. Direct mechanisms include nitrogen fixation, phosphate solubilization, iron absorption, and production of phytohormones namely auxin (incl. IAA), cytokinin, and gibberellins whereas indirect mechanisms refer to bacterial ability to inhibit phytopathogen growth via production of ACC deaminase, antimicrobial compounds, cell wall inhibitors, and siderophore [[5], [6], [7], [8]]. Moreover, PGPR can prevent root diseases such as root withering and rotting [9].
Auxin orchestrates many processes within plant tissues, specifically the ones that are important for plant growth and development [10]. As early as 1996, Patten and Glick has reported that approximately 80% of microorganisms in the rhizosphere were able to synthesize and release auxin as a secondary metabolite product, although the study lacked appropriate approaches to target unculturable microorganisms [11]. Among IAA beneficial effects are lateral root formation and root hair growth promotion [12].
In addition to the auxin-related plant growth promotion, the activity of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase of PGPR might stimulate plant growth by reducing ethylene level [13,14]. Ethylene affects plants in many ways and is responsible for several processes related to plant growth and development [15]. Depending on the concentration, it might promote root initiation, inhibit root elongation, activate phytohormone synthesis, influence plant-microbe symbiosis in different stages, and activate response mechanisms against biotic and abiotic stresses [16,17]. Adverse environmental conditions, for instance extreme temperatures, drought, flooding, high-salinity, radiation, and pathogen presence might be accompanied with increase in ethylene synthesis [18,19]. ACC deaminase produced by PGPR will degrade the ethylene precursor ACC and therefore inhibit plant senescence and promote plant growth [20].
Although IAA-producing bacteria are essential for plant growth, IAA might elicit ACC synthesis that induce plant senescence symptoms [21,22]. Thus, combination of IAA- and ACC deaminase-producing bacteria might be a better solution since the latter potentially degrade ACC [23]. In-depth knowledge on the plant response after IAA- and ACC deaminase-producing bacteria admission would be rewarding, especially to define the best strategy for PGPR application in the field. Moreover, this approach encourages sustainable agriculture practices as the utilization of chemical fertilizer is subjugated and thus safer from the ecological point of view.
This study focuses on the isolation of beneficial bacteria from pineapple of both healthy and unhealthy plants under different abiotic and biotic stress sources and subsequent characterization, particularly on the activity of IAA and ACC deaminase. The isolated bacteria with both activities are potential bioagents that could be used in the future to promote plant growth specifically under stressful conditions. They might also maintain soil health and further advocate sustainable agriculture. Correspondingly, admission of these bacteria in the future might benefit farmers and mitigate environmental deterioration caused by human activities.
2. Materials and methods
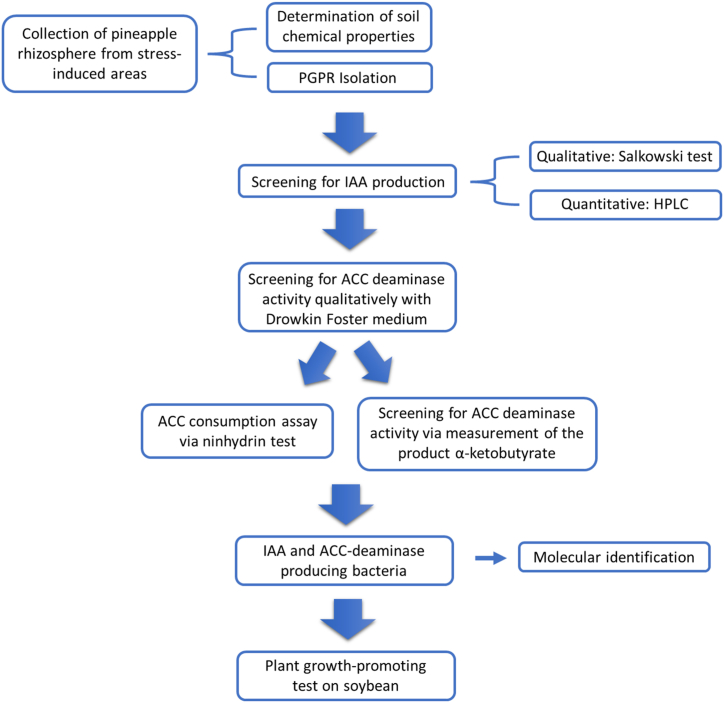
As presented in Fig. 1, the current study was carried out in several steps. It started from collection of pineapple rhizospheres from the stress-induced areas of PT. Great Giant Foods, Central Lampung, Indonesia. Bacteria were isolated from the soil samples and the soil chemical property analysis was conducted in parallel. Isolated bacteria were then screened based on the ability to produce IAA and ACC deaminase. The potential isolates were further tested for their ability to promote soybean growth.
Fig. 1.
Experimental steps of the current study, from soil collection to plant growth-promoting test.
2.1. Collection of soil samples from stress-induced areas
Soil samples were collected from the pineapple rhizospheres of stress-induced areas. These include collection of rhizosphere soils of healthy and unhealthy plant aged between 6 and 12 months from flooded-areas (CFTB and CFTJ; TB for healthy and TJ for unhealthy plants, respectively), herbicide-affected (CHTB and CHTJ), and Phytophthora-infected areas (CPTB and CPTJ). In addition, rhizosphere soil from healthy plants in the non-stress areas was also collected for comparison (NCTB). For each area, the soil samples were taken using a simple randomized sampling approach with the depth of 20 cm. Each of the soil samples was placed in a polyethylene bag and transferred to the laboratory in a cool condition.
2.2. Analysis of soil chemical properties
Soil chemical properties, including C-organic, total nitrogen, phosphorus and potassium content, along with C:N ratio, were analyzed accordingly. Total C-organic content was determined using Walkley Black method [24]. Total nitrogen content was measured using Kjeldahl method [25]. The phosphorus and potassium content were determined using HCl 25% extraction method [26].
2.3. Soil respiration, urease, and phosphatase activities
Soil respiration was determined by measuring oxygen uptake in the soil, following the method described by Schinner et al. [27]. Urease activity was measured using the Nessler method [28] whereas phosphomonoesterase activity was determined following the method of Tabatabai and Bremner [29].
2.4. Bacterial isolation
Bacteria were isolated from the rhizosphere of pineapple plants. Surrounding soil was separated via mild shaking of the roots. Rhizosphere soil was then collected by submerging the roots in sterilized water combined with gentle-shaking. The soil suspension was inoculated onto the Tryptic Soy Agar (TSA) media, employing serial dilution technique and pour plate method, and incubated at 28–31 °C for 2–3 days. The inoculation was performed in three replicates for each sample.
2.5. Characterization of bacterial isolates
Bacterial isolates were characterized based on their metabolic capacities to produce IAA, degrade ACC, and release ACC deaminase.
2.6. Qualitative tests of IAA production and ACC deaminase activity
Bacterial colonies were transferred into fresh TSA and purified using the quadrant-streak plate method. Pure colonies were subjected to qualitative IAA and ACC deaminase tests. For all the subsequent analysis, bacterial isolate TPK5B2 collected from rice with IAA-producing and ACC deaminase activities was used as the positive control/comparison.
IAA measurement was performed with a colorimetric method using Salkowski reagent [30]. IAA-positive bacteria were subsequently grown in Dworkin Foster (DF) salt minimal medium consisting of either 2.5 mmol L−1 ACC or 2.5 mmol L−1 ammonium sulfate as the only nitrogen source [31]. Positive growth indicates the presence of ACC deaminase. Simultaneously, bacteria were grown in a basic DF salts minimal medium without the addition of any nitrogen source to test if the bacteria were diazotrophic.
2.7. IAA production assay
The IAA produced by the isolated bacteria was measured quantitatively using High-Performance Liquid Chromatography (HPLC) [32]. A total of 4 mL of sample was centrifuged at 10,000 rpm for 15 min. Supernatant was collected and the pH was adjusted to the value of 2.8 prior to 3-time-reflux extraction using ethyl acetate with the ratio of 1:1 (v/v). The extract was concentrated using a rotary evaporator and later re-suspended in 1 mL of ethanol absolute. The suspension was filtered using 0.2 μm PTFE membrane filter and injected to HPLC (Shimadzu) using C18 column (Supelco) and methanol:acetic acid:double distilled water (30:1:70 v/v/v) as the mobile phase. The flow rate and the wavelength were set to 1 mL/min and 280 nm, respectively. The data were processed accordingly.
2.8. Screening for ACC-degrading bacteria via ninhydrin assay
The ninhydrin assay follows the method described by Li et al. [33]. Bacterial isolates were grown in the LB-rich medium and incubated for 24 h. Cells were harvested and washed in DF medium. Cell suspension was inoculated into liquid DF medium supplemented with ACC and incubated at room temperature for 24 h in a shaker with the speed of 100 rpm. Cells were later harvested via centrifugation at 8000 g for 5 min. A total of 1 mL supernatant was transferred into a test tube, added with 2 mL of ninhydrin reagent, homogenized, incubated in a water bath for 15 min, re-incubated back in room temperature for 2 min, and homogenized for 30 s. The suspension was left for 10 min at room temperature before OD measurement at 570 nm. The standard curves were prepared accordingly. Blank solution used was DF medium without ACC.
2.9. Measurement of ACC deaminase activity
ACC deaminase activity was measured following the method described by Honma and Shimomura (1978) with modification [34]. The enzyme activity was measured indirectly via quantification of the product α-ketobutyrate. Cell pellet was prepared in a 1.5 mL tube, re-suspended in 1 mL 0.1 M Tris-HCl (pH 7.6), and centrifuged at 16,000 g for 5 min. After the supernatant was discarded, 600 μL 0.1 N Tris HCL (pH 8) and 30 μL toluene were added to the tube. The suspension was then vortexed at a high speed for 30 s. A total of 100 μL suspension was separated and stored at 4 °C for subsequent protein assay following the method described by Bradford (1976) [35]. The remaining suspension was used for ACC deaminase assay. A total of 200 mL suspension was placed into a 1.5 mL tube, added with 20 μL 0.5 M ACC, vortexed, and incubated at 30 °C for 15 min. Addition of 1 mL of 0.56 M HCl was done subsequently and the tube was vortexed and centrifuged at 16,000 g for 5 min. A total of 1 mL supernatant was transferred, added with 800 μL of 0.56 M HCl, and vortexed prior to mixing with 2,4-dinitrophenylhydrazine reagent (0.2% 2,4- dinitrophenylhydrazine in 2 M HCl). The tube was vortexed, incubated at 30 °C for 30 min, added with 2 mL 2 N NaOH and re-vortexed. The absorbance was then measured at the wavelength of 540 nm.
2.10. Qualitative protease, phosphate solubilizing, and nitrogen-fixing assay
Protease activity was tested by checking the growth of the isolates on skim milk agar. After 3 days, the formation of a clear zone was inspected. Phosphate-solubilizing bacteria was isolated using the selective National Botanical Research Institute's Phosphate (NBRIP) medium [36]. In addition, nitrogen-fixing activity was tested through inoculation of the isolates on semi solid nitrogen-free medium [37]. Positive result was defined with medium color changes to blue and formation of a circular ring after approximately 2-week-incubation incubation time.
2.11. Molecular identification of selected bacterial isolates
IAA- and ACC-deaminase producing isolates were identified based on their partial 16 S ribosomal RNA gene sequences. The closest relatives were determined via alignment to Eztaxon (www.ezbiocloud.net) [38]. Multiple sequence alignment (MSA) was performed using Clustal W and the result was inspected manually. The maximum-likelihood tree was constructed using MEGA 7.0 [39]with 1000 bootstrap replication. TN93 + G + I model for nucleotide substitution was applied for the tree.
2.12. The effect of bacterial isolates on the growth of soybean
The experiment employed a factorial completely randomized design (CRD) with two factors and three replicates for each treatment. The first factor was water content, which included a non-stress level (field capacity level) and a stress level (50% field capacity level). The second factor was the bacterial isolate, which consisted of seven IAA- and ACC deaminase-producing isolates (including one isolate collected previously from rice rhizosphere) and one inactive isolate as control. A total of 48 experimental units of three soybean plants grown in a bottle jar were used in this experiment.
Soybean seeds were first washed in flowing water and surface-sterilized using ethanol for 3 min and dipped into sterile water for 30 s. The last step was done three times. The selected bacterial isolates were grown on Nutrient Broth (NB) medium for 48 h at room temperature on a shaker with the speed of 100 rpm. The liquid culture was prepared in the concentrations of 108–109 CFU mL−1. Cells were harvested and re-suspended using Hoagland solution. Soybean seeds were dipped into the solution for 10 min and subsequently transferred into a sterile jar bottle containing 150 g of sterile (autoclaved) beach sand. After 14 days, measurement of plant height and dry weight, along with the root length and weight, was done accordingly.
2.13. Data analysis
Data analysis was performed using R (version 4.2.1.; R core team 2022) [40]. Unless otherwise mentioned, all plots were constructed using the R “ggplot2” package [41]. The data on the soil respiration, soil urease and phosphomonoesterase activity, ACC consumption, and alpha-ketobutyrate production were subjected to a one-way analysis of variance (ANOVA) followed with Tukey's all pairwise multiple comparison test [42]. For the soybean experiment, the data in plant height, root length, and dry weight of above and below ground biomass were analyzed using a two-way ANOVA test followed by the Duncan's new multiple range test [43].
3. Results
3.1. Soil physicochemical properties
Total nitrogen content across soil samples from flooded-areas (CFTB and CFTJ; TB for healthy and TJ for unhealthy plants, respectively), herbicide-affected (CHTB and CHTJ), Phytophthora-infected (CPTB and CPTJ) and non-stress areas (NCTB) was similar to the smallest value of 0.44% detected in the soil of healthy plants in the herbicide-affected areas (Table 1). The highest value of 0.46% was observed for the soil of the healthy plants in the flood-affected area and the unhealthy plants in the Phytophthora-infected area. Total phosphorus content was higher in soil exposed to either abiotic or biotic stresses compared to control (137.61 ppm), with the highest value detected from the rhizosphere of unhealthy pineapple plants in the herbicide-affected areas (189.94 ppm). The lowest and highest values for total potassium (K) were 80.87 ppm and 242.60 ppm, respectively, both of which belonged to the herbicide-affected areas. The first is for the healthy pineapple plant rhizosphere while the latter corresponds to the unhealthy pineapple plants. Furthermore, C-organic content was similar across samples with the values of up to 4.94% from the soil of the healthy plants in the non-stress area. The lowest value of C-organic came from the herbicide-affected area with 4.76% and 4.55% for the unhealthy and healthy plants, respectively. Similarly, the C:N ratio was the highest in the non-stress areas with the value of 11.08 while the ratios of the flood-affected areas were slightly lower (10.82 for the unhealthy plants and 10.70 for the healthy plants). The rhizosphere of the healthy plants in the herbicide-affected area gave the lowest value of 10.35.
Table 1.
Soil chemical properties across samples.
| No. | Parameter | Unit | Soil samples |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CFTB | CFTJ | CHTB | CHTJ | CPTB | CPTJ | NCTB | |||
| 1 | Total N | % | 0.46 | 0.45 | 0.44 | 0.45 | 0.45 | 0.46 | 0.45 |
| 2 | Total P | ppm | 161.91 | 154.43 | 153.50 | 189.94 | 161.91 | 146.02 | 137.61 |
| 3 | Total K | ppm | 181.95 | 161.73 | 80.87 | 242.60 | 202.17 | 161.73 | 202.17 |
| 4 | C-organic | % | 4.88 | 4.89 | 4.55 | 4.76 | 4.82 | 4.82 | 4.94 |
| 5 | C:N ratio | 10.70 | 10.82 | 10.35 | 10.64 | 10.61 | 10.41 | 11.08 | |
CFTB = flood-affected, healthy plants; CFTJ = flood-affected, unhealthy plants; CHTB = herbicide-affected, healthy plants; CHTJ = herbicide-affected unhealthy plants; CPTB = Phytophthora-infected, healthy plants; CPTJ = Phytophthora-infected, unhealthy/diseased plants; NCTB = non-stress areas, healthy plants.
3.2. Soil respiration, urease, and phosphomonoesterase activities
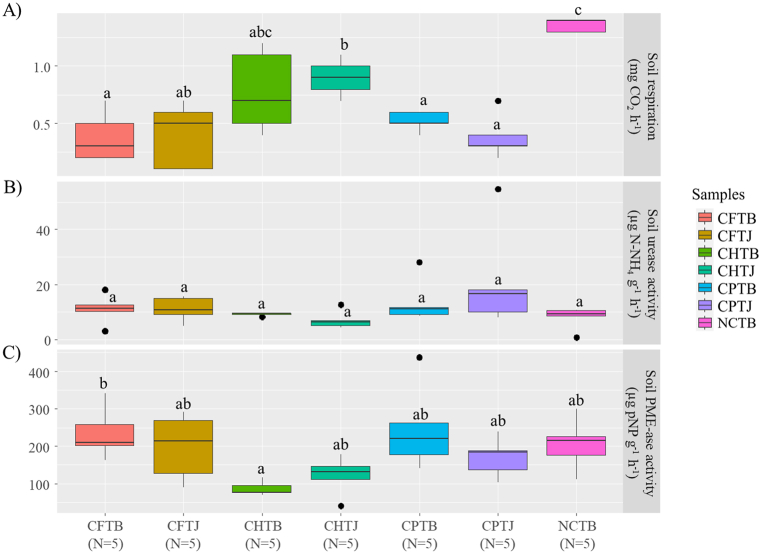
One-way ANOVA test revealed a statistically significant difference between at least two groups on soil respiration and PME-ase activity and thus the data were subjected to the Tukey's multiple comparison test. Of the rhizosphere soil samples from flooded-areas, herbicide-affected, Phytophthora-infected and non-stress areas, the last one was found with the highest soil respiration with the value of 1.362 ± 0.056 mg CO2 h−1 (Fig. 2A). Flooding was found to decrease soil respiration the most, with the values of 0.385 ± 0.225 mg CO2 h−1 and 0.400 ± 0.277 mg CO2 h−1 for the healthy and unhealthy plants, respectively. In general, among samples in the stress-induced areas, the herbicide-exposed areas gave significantly higher respiration rate. In term of soil urease activities, no significant differences could be observed between distinct areas (Fig. 2B) with the smallest value of 7.298 ± 3.224 μg N–NH4 g−1 h−1 (herbicide areas around unhealthy plants) and the highest value of 21.574 ± 19.032 μg N–NH4 g−1 h−1 (Phytophthora-infected areas around unhealthy/diseased plants). Soil phosphatase activities varied among samples, although herbicide-exposed areas were found with the lowest values of 87.092 ± 19.015 μg pNP g−1 h−1 in the rhizosphere of healthy plants and 122.219 ± 51.264 μg pNP g−1 h−1 in the rhizosphere of unhealthy plants (Fig. 2C). The two highest values were detected in the rhizosphere of diseased plant from flood-affected and the rhizosphere of healthy plant from Phytophthora-infected areas with 206.327 ± 69.475 μg pNP g−1 h−1, 235.649 ± 68.736 μg pNP g−1 h−1, and 248.206 ± 115.178 μg pNP g−1 h−1, respectively.
Fig. 2.
Soil respiration (A), urease (B), and phosphatase/PME-ase (C) activities in the rhizosphere of pineapple plants collected from flood-affected (CFTB and CFTJ for healthy and unhealthy plants, respectively), herbicide-exposed (CHTB and CHTJ for healthy and unhealthy plants, respectively), Phytophthora-infected (CPTB and CPTJ for healthy and unhealthy/diseased plants, respectively), and non-stress induced areas (NCTB for healthy plants). Each data point represents the mean of five replicates. Different letters denote significant differences (p < 0.01; multcomp test).
3.3. Isolation, screening, and IAA-producing capacity of bacterial isolates
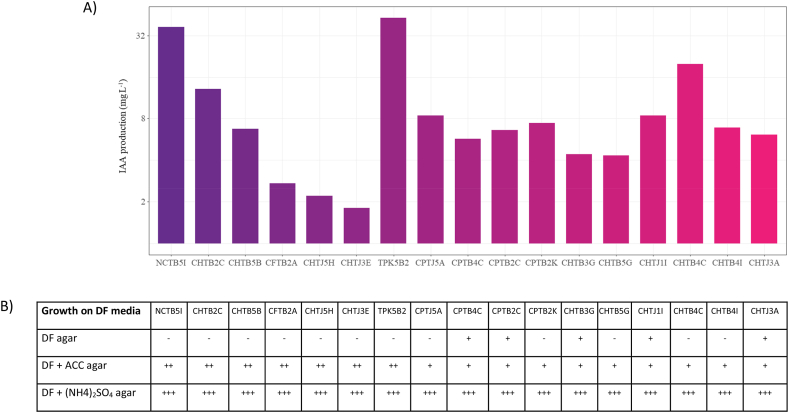
A total of 171 isolates were successfully isolated from the rhizosphere of pineapples. Of these, 73 isolates could form indole based on the pink colorization in the growth medium after administration of Salkowski reagent. The isolates were further screened based on their morphology and color intensity, leaving 17 isolates ready for subsequent analysis. The ability to produce IAA for 48 h varied among the 18 isolates. The lowest IAA production was detected on CHTJ 3E with the value of 1.8 mg L−1 whereas the highest was achieved by control TPK5B2, isolated from rice, with the value of 43.0 mg L−1 (Fig. 3A). Among bacterial isolates from the pineapple rhizosphere, NCTB5I produced the highest IAA content with the value of 36.9 mg L−1. However, the production was higher for the control isolate TPK5B2 with the value of 42.98 nmoL mg−1 h−1.
Fig. 3.
IAA production of 16 bacterial isolates from the rhizosphere of pineapple plants and one control isolate from the rhizosphere of rice (TPK5B2) (A) and ACC deaminase assay using Dworkin-Foster (DF) medium with the addition of ACC and (NH4)2SO4 as the substrate (B). Different colors represent different bacterial isolates.
3.4. ACC-deaminase activities of bacterial isolates
All isolates grew on DF medium, either with supplementation of ACC or ammonium sulfate, indicating the positive activity of ACC deaminase. Interestingly, 5 isolates grew on a basic DF medium without any N source, indicating that they were able to perform nitrogen fixation. The isolates were CPTB4C, CPTB2C, CHTB 3G, CHTJ 1I, and CHTJ 3A. A total of 6 isolates with better growth on the DF-ACC medium, along with the control isolate TPK5B2, were chosen for qualitative analysis of the ACC deaminase. These included NCTB5I, CHTB 2C, CHTB 5B, CFTB2A, CHTJ 5H, and CHTJ 3E (Fig. 3B).
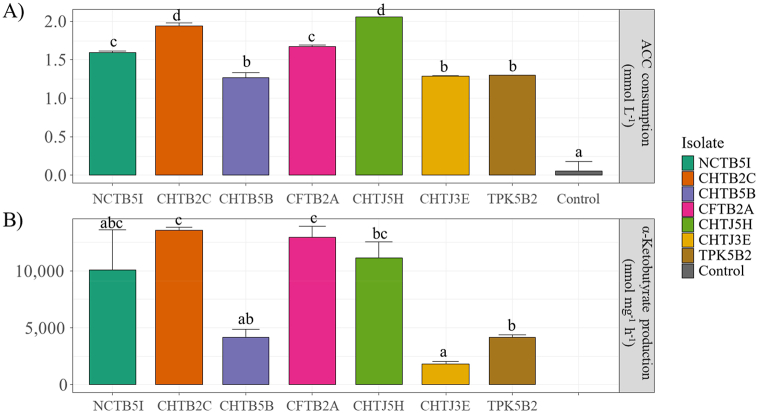
Significant differences were also detected among treatments of the ACC consumption and α-ketobutyrate production experiments. The isolates CHTJ 5H, CHTB 2C, dan CFTB2A were among the fastest to consume ACC during the first 24 h with the values of 2.2 mmol L−1 (88%), 2.1 mmol L−1 (84%) and 1.8 mmol L−1 (72%), respectively (Fig. 4A). Additionally, the bacterium CHTB 5B consumed the lowest ACC with the value of 1.3 mmol L−1 (52%). ACC deaminase activity was determined by measuring the product α-ketobutyrate. Among the pineapple bacteria, isolates CHTB 2C, CFTB2A and CHTJ 5H produced highest concentration of α-ketobutyrate with the values of 13,370 nmoL mg−1 h−1, 12,255 nmoL mg−1 h−1, and 10,233 nmoL mg−1 h−1, respectively (Fig. 4B).
Fig. 4.
ACC consumption of the selected PGPR isolates in the DF-ACC medium administered with 2.5 mmol L−1 ACC after incubation for 24 h and subsequent measurement using ninhydrin assay (A). ACC deaminase activity of each bacterial isolate measured using the 2,4-dinitrophenylhydrazine assay (measurement of α-Ketobutyrate) after induction in the DF-ACC medium for 24 h (b). Each data point represents the mean of two replicates. ACC, 1- aminocyclopropane-1-carboxylate. Different letters denote significant differences (p < 0.01; multcomp test).
3.5. Protease, phosphate solubilizing, and nitrogen-fixing activity
The isolate CHTB 2C and the control TPK5B2 had a higher proteolytic activity compared to the others based on the diameter of the clear zone, followed by the isolates CHTJ 3E and CHTJ 5H (Table 2). For the phosphate solubilizing trait, the isolates CHTB 5B and CFTB2A were confirmed to have the enzymatic activity. None of the two isolates, however, was observed with nitrogen-fixing capability.
Table 2.
Plant growth-promoting traits of the bacterial isolates.
| No. | Bacterial isolates | Plant growth-promoting activities |
||
|---|---|---|---|---|
| Proteolytic | Phosphate-solubilizing | Nitrogen-fixing | ||
| 1 | NCTB5I | - | - | - |
| 2 | CHTB 5B | - | + + | - |
| 3 | CHTJ 5H | + | - | - |
| 4 | CHTJ 3E | + + | - | - |
| 5 | CFTB2A | - | + | - |
| 6 | CHTB 2C | + + + | - | - |
| 7 | TPK5B2 (Control) | + + + | - | - |
3.6. Molecular identification of bacterial isolates
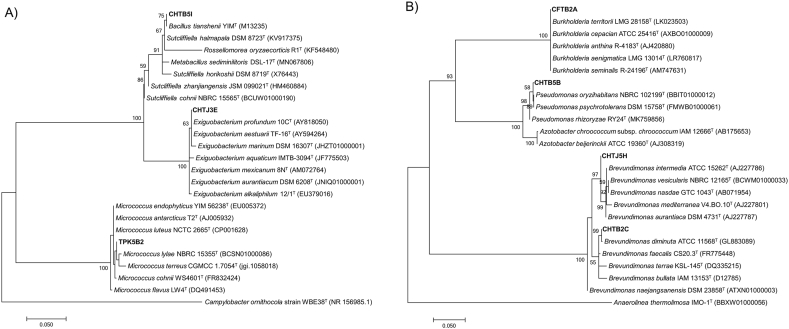
The partial 16 S rRNA gene sequences of the bacterial isolates were aligned against EzBiocloud database and the closest type strain sequences were retrieved for the construction of the phylogenetic tree. Of the gram-positive isolates, CHTB 5I was closest to Bacillus tianshenii YIM M13235T (99.9% similarity), CHTJ 3E to Exiguobacterium profundum 10C T (100% similarity), and the control isolate from rice, TPK5B2 to Micrococcus luteus NCTC 2665T (99.53% similarity) (Fig. 5A). Of the gram-negative isolates, CFTB2A was closest to Burkholderia territorii LMG 28158T (100% similarity), CHTB 5B to Pseudomonas oryzihabitans NBRC 102199T and P. psychrotolerans DSM 15758T (both with 99.47% similarity), CHTB 2C to Brevundimonas diminuta ATCC 11568T (99.89% similarity), and CHTJ 5H to Br. Intermedia ATCC 15262T (99.19% similarity) (Fig. 5B). The phylogenetic affiliation of the isolates to their corresponding genera was supported with high bootstrap values and therefore confirmed their affiliation to those specific genera.
Fig. 5.
Phylogenetic tree of 6 bacterial isolates from the rhizosphere of pineapple plants and one control isolate from rice (TPK5B2) based on partial 16 S rRNA gene sequences. The phylogenetic tree was separated between the gram positive (A) and the gram negative (B) bacteria. The tree was built using the maximum-likelihood (ML) method and bootstrap analysis was performed using 1000 replications. Only bootstrap values above 50% were depicted on the tree nodes. Bar, 0.050 substitutions per position.
3.7. The beneficial effects of bacterial isolates on the growth of soybean
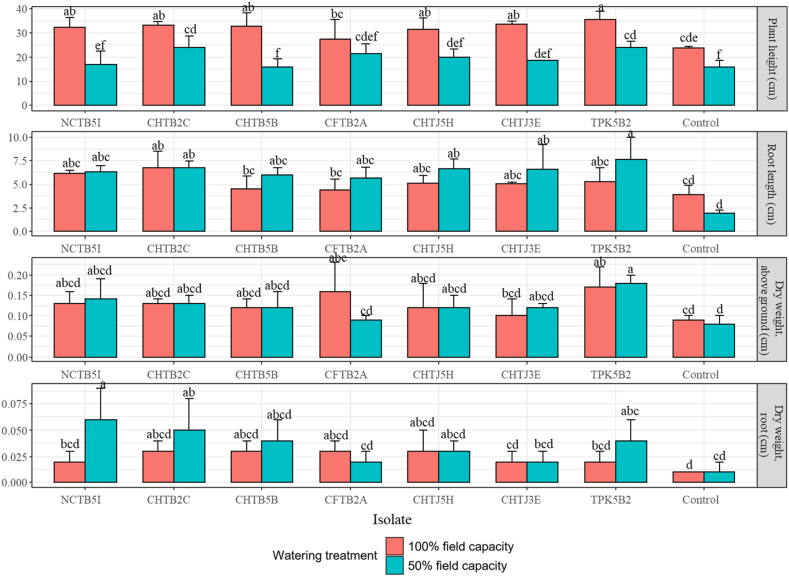
The effects of the six bacterial isolates along with the rice isolate TPK5B2 and one inactive isolate (control) on soybean growth were tested subsequently. The decrease in the watering level, from 100% to 50% field capacity, was shown to lower the plant height (Fig. 6). Under watering with 100% field capacity, the inoculation of all bacteria was shown to increase plant height compared to control and the increase was significant except for CFTB2A. Under the watering with 50% capacity, the treatment with CHTB 2C and TPK5B2 significantly increased plant height. For root length, only the treatment with CHTB 2C significantly increased the value compared to the control for 100% watering capacity (Fig. 6). However, when changing to 50% field capacity, inoculation of all bacteria was shown to increase root length significantly. Furthermore, only the treatment with the control TPK5B2 significantly increased the above ground biomass compared to control for both 100% and 50% watering capacity. Of dry root weight, a significant increase was shown for the inoculation treatment with NCTB5I and CHTB 2C, in comparison to control, for 50% watering capacity. No significant differences could be observed for the watering treatment with 100% capacity. Interestingly, more than half of inoculation treatments show tendency of higher root biomass under 50% watering capacity.
Fig. 6.
The effect of the bacterial inoculation on the growth of soybean, including plant height, root length, dry above ground biomass, and dry root weight. Different letters denote significant differences (p < 0.05, Duncan's new multiple range test).
4. Discussion
Abiotic and biotic stresses such as drought, flooding, extreme temperatures, saline condition, acidity, and the presence of pathogens threaten to decrease plant productivity by more than 50% [[44], [45], [46], [47], [48]]. Abiotic stresses might be triggered by changes in global climate along the decades [49,50]. These stresses potentially change plant morphology, physiology, and biochemistry, that consequently alter plant growth and productivity [[51], [52], [53], [54], [55]]. In addition, plant germination is highly affected by salinity and high-temperature where the conditions could lead to a delayed or failed germination of the seeds [50,51]. The effect of bacterial inoculation under abiotic stress might be beneficial for the host plants, as depicted by tomatoes subjected to flooding and inoculated with Pseudomonas putida UW4 [56]. The strain is known to harbor ACC deaminase activity. Tomato-treated plants are shown to adapt better during flooding, indicating an increase in tolerance against the abiotic stress.
The pineapple plantation of PT. Great Giant Foods located in Lampung has several abiotic and biotic stress-induced areas due to floods, herbicide-exposure, and the presence of plant pathogen Phytophthora. Water-excess or flooding areas potentially increase ethylene biosynthesis within plant root and stem. Moreover, ACC would be synthesized in the root and transported to shoot, where it will be converted into ethylene by ACC oxidase [57] that could lead to appearance of senescence symptoms including leaf necrosis and abscission.
In this study, isolation of bacteria with beneficial properties, namely IAA production and ACC deaminase activities, was conducted from the rhizosphere of healthy and unhealthy pineapple plants within stress-induced areas of PT. Great Giant Foods. Soil chemical analysis reveals that stressed conditions significantly decrease soil respiration, especially with flooding and the presence of Phytophthora. High water content in soil has reportedly decreased soil respiration rate, as it restricts the microbial aerobic respiration [58,59]. The presence of foliar fungal pathogens and insect herbivores has also been reported to decrease soil respiration [60]. Furthermore, this study observed no effect of abiotic or biotic stresses on the soil urease activities. Another study reveals an increase of urease activities during flooding [61] whereas herbicide treatment leads to weaker enzymatic activity [62]. Phosphorus content was the highest in the rhizosphere of unhealthy pineapple plants in the herbicide-affected areas, which corresponds to lower value of phosphatase enzyme activity. In this case, high phosphorus content may probably inhibit the activity of the enzyme. However, lower enzymatic activity was also observed for the healthy plants in the same areas, even with lower phosphorus concentration. The result may indicate the influence of some other factors on the soil enzymatic activities. Another study reveals the influence of soil moisture, organic matter, C:N ratio, temperature, climate, and plant species to the soil enzymatic activities [63,64]. We observed that abiotic or biotic stresses alter physicochemical properties of soil, including the decrease of soil C-organic and C:N ratio and an increase of total phosphorus content compared to control. The interplay of these stresses, soil physicochemical properties, and most likely soil microbial communities, will possibly determine the direction and magnitude of the soil enzymatic activities.
Burkholderia, Pseudomonas, Bacillus, Exiguobacterium, Brevundimonas, and Micrococcus are among the identified genera of the IAA- and ACC deaminase-producing bacteria investigated in this study. Members of these genera have been reported to produce IAA for their host plant [65,66]. In a similar work (Marfungah et al., unpublished), reported that P. aeruginosa RE81 produces the highest concentration of IAA among PGPR isolated from the Eucalyptus pellita rhizosphere. The isolate has a strong growth inhibitory effect against Xanthomonas sp., the causal agent of the bacterial leaf blight disease on eucalyptus. Micrococcus has been reported with plant growth promoting traits such as phosphate solubilization, IAA production, ACC deaminase activities, and siderophore production, as depicted in Micrococcus sp. NII-0909 that promotes the growth of cowpea and increases root colonization of the seedlings [67]. Similarly, Bacillus is known to foster many PGPR strains including the endophytic B. mojavensis PRN2 and B. subtilis LK14 isolated from pea and tomato plants, [68,69]. Both strains possess the ability to produce IAA and ACC deaminase. However not all members of the genus are gifted with both abilities. For example, Bacillus sp. HYT-12-1 that produces ACC deaminase but lacks in IAA production. Likewise, Brevundimonas sp. CTEM2.9 is detected with ACC deaminase activity but not IAA production [70]. The ability of PGPR to produce IAA varies across different species and strains and may depend on culture conditions, growth phases, and substrate availability as well, as discussed by Mirza et al. (2004) [71]. Interestingly, two of the three isolates with pronounced ACC deaminase activities, namely Brevundimonas sp. and Burkholderia sp. were taken from the healthy plants in the herbicide-affected areas, which may indicate a profound ACC deaminase activity to endure the abiotic stress.
The plant stress response is closely associated with phytohormones namely abscisic acid (ABA), salicylic acid (SA), jasmonic acid (JA), and ethylene, in which all present in a very small quantity but have a significant role for plant defense mechanisms [53,72]. One of the plant responses to environmental stress stimuli is to increase the level of ethylene that potentially kills plant pathogens. However, high ethylene levels are also dangerous for the plant as it increases the risk of cell and tissue death [23]. On the other hand, phytohormones such as indole acetic acid (IAA), gibberellic acid (GA), and cytokinins (CA) play crucial roles to promote plant growth [45].
Plant growth-promoting rhizobacteria (PGPR) promote plant growth, productivity, and development [2,7,8] via nutrition provision, production of phytohormones, siderophores and growth factors, or via induced systemic resistance (ISR) to increase plant resistance against phytopathogens [1,50,[73], [74], [75], [76]]. The phytohormone indole-3-acetic acid (IAA) affects root proliferation, elongation, and nodulation [77,78]. On the other hand, ACC-deaminase will convert ACC to α-ketobutyrate and ammonia and prevent ethylene production that cause early leaf senescence and abscission. Moreover, ACC-producing bacteria could provide the host plant with nitrogen and energy, increase root length, and improve water uptake during stress conditions [50]. Although each IAA and ACC deaminase are beneficial for the host plant, they might not necessarily work in sync. IAA excess might potentially lead to activation of ACC synthase that converts ACC into ethylene [79]. An increase in ethylene level may have negative feedback on IAA production and therefore reduced plant growth-promoting impact of the phytohormone. To maintain IAA production and conserve the beneficial impact on the host plant, the utilization of bacteria with both IAA- and ACC deaminase-producing capacities could be performed. In this case, the ethylene precursor ACC will be degraded by ACC deaminase [26] and thus will not negatively impact IAA production. Moreover, some reports highlight the antagonistic activity of ACC deaminase-producing bacteria against pathogenic microorganisms, including inhibition of Scelerotium rolfsii on tomato plants [75]. In general, the cross-talk between IAA production and ACC deaminase activities is most likely to define the host plant response against environmental stresses or pathogen attack. The utilization of bacteria with both IAA and ACC deaminase activities, including Burkholderia, Pseudomonas, Bacillus, Exiguobacterium, Brevundimonas, and Micrococcus in this study, should then be employed further to investigate their impact on the host plants growth and productivity, especially under stressful environmental conditions.
The isolates of Brevundimonas, Bacillus, Pseudomonas, and Micrococcus are found to be effective to increase plant height, root length, and both above- and below-ground (root) biomasses of soybean, even under lower water availability (specifically in the roots) compared to the control. Interestingly, the top three of the selected isolates (Brevundimonas sp. CHTB 2C, and Bacillus sp. NCTB5I, along with the control isolate Micrococcus sp. TPK5B2) are among the best IAA producers with a positive ACC deaminase activities. Apart from the rice origin Micrococcus sp. TPK5B2, the other two were isolated from healthy plants of either conducive or stressful environments. The excreted IAA and ACC deaminase activities are thought to play key roles in mitigating the negative effects of the stressful conditions on plant growth and heath. Although the effect is not seen for all parameters, the amendment of other bacterial isolates gives a significantly good impact on one or two parameters. Brevundimonas is known to possess plant growth-promoting and disease suppression traits [80]. Plant growth promoting traits of Brevundimonas include siderophore production, phosphate solubilization, IAA production, ACC deaminase activity, as depicted in B. diminuta NBRI012 that promote growth of rice and decrease arsenic accumulation in the edible part of the plant [81]. Furthermore, B. diminuta MYS6 promotes root elongation, shoot growth, improves leaf chlorophyll, and increases dry and fresh weight of sunflower plant, under Cu stress [82]. In addition, Micrococcus yunnanensis RS222 is reported to increase root elongation and plant dry weight of canola seedlings under salinity stress conditions [83], whereas M. luteus AKAD 3-5 promotes soybean growth and has biocontrol activity against Fusarium oxysporum [84].
The indole-3-acetic acid (IAA) produced by PGPR is categorized as a type of auxin and could be synthesized using tryptophan that is released as a component of root exudate. This phytohormone is an important molecule for signaling between bacteria and the host plant. IAA-producing bacteria will boost the auxin to optimum level and thus improve plant health and productivity [85,86]. The phyto-stimulatory effect of IAA is directed on root development where the increase of surface area will be promoted, including lateral root formation, increase of the root hair length, and development of adventitious roots, that will lead to refinement in the water and nutrient uptake [87]. On the other hand, the increase of ethylene triggered by different kinds of abiotic stresses will negatively impact seed germination, root development, and the plant overall growth. The role of ACC-deaminase-producing bacteria will be crucial in cleaving ACC, the ethylene precursor and hence the maintenance of plant growth. The mitigation of heat stress by ACC deaminase-producing bacteria has been proven for Bacillus cereus. In addition to having positive impact on the plant physiology aspect such as shoot and root length, biomass, and leaf surface area, the bacteria also increase plant chlorophyll content and antioxidant activity, and improve water and nutrient uptake [58]. Interestingly, member of Bacillus has been known as a strong biocontrol agent. Three strains of Bacillus subtilis have been shown to exhibit antagonistic activity against Fusarium in a study concerning cotton plantation [88]. The inoculation of those bacteria, either individually or in a consortium, has increased antioxidants, phenol, and flavonoid levels, increase stem and root length, promote lateral root formation which might be due to increase in IAA level, and also increase gibberellic acid concentration during the exposure to biotic stress. These results emphasize multiple courses of action of bacteria to alleviate biotic and abiotic stress. The resistance mechanism is most likely involving different aspects and thus careful and thorough investigation must be conducted, for example in the study of the dynamic between IAA and ACC deaminase. The information could be utilized in the future to implement the best strategy to increase crop resistance against stress stimuli.
This study emphasizes the potential future application of six beneficial bacteria isolates, including Brevundimonas sp. CHTB 2C, Brevundimonas sp. CHTJ 5H, Bacillus sp. NCTB5I, Burkholderia sp. CFTB2A, Pseudomonas sp. CHTB 5B, and Exiguobacterium sp. CHTJ 3E, along with the control isolate of rice-origin Micrococcus sp. TPK5B2. All are found with IAA production and ACC deaminase activity and promote soybean growth. Although the control isolate showed a higher IAA production, Bacillus sp. NCTB5I follows closely behind. Brevundimonas sp. CHTJ 5H and Brevundimonas sp. CHTB 2C were observed with the highest ACC consumption and alpha-ketobutyrate production, respectively. Moreover, Brevundimonas sp. CHTB 2C and Exiguobacterium sp. CHTJ 3E were also detected with a notable protease activity, and Pseudomonas sp. CHTB 5B with phosphate solubilizing activity. Their potential might change under different environmental conditions, depending on the metabolic capabilities of each bacterium. Although the control isolate had better results in specific aspects for soybean growth, the result would not be necessarily true for the application to the pineapple plantation, from where the isolates originated. Therefore, these isolates should be developed further in the near future as potential bioagents to promote plant growth especially under abiotic or biotic stress conditions, more specifically for application in the rhizosphere of pineapple plants.
5. Conclusion
Our study highlights the activity of six bacterial isolates from the rhizosphere of pineapple plants, and one control isolate from rice in producing phytohormone IAA and ACC deaminase, that potentially increase tolerance against abiotic or biotic stresses. The isolates include Brevundimonas sp. CHTB 2C, Brevundimonas sp. CHTJ 5H, Bacillus sp. NCTB5I, Burkholderia sp. CFTB2A, Pseudomonas sp. CHTB 5B, and Exiguobacterium sp. CHTJ 3E, along with the control isolate of rice-origin Micrococcus sp. TPK5B2, all with the capacity to produce IAA, positive ACC deaminase activities, and promotion of soybean growth. These are potential bacteria to be utilized in the near future as bioagents to promote plant growth especially under stressful environmental conditions. The following experiments should include the investigation of the positive impacts these bacteria have for the growth and development of pineapple plantation.
Author contribution statement
Hanim R. Ratnaningsih, Supriyono Loekito, Suryo Wiyono, Sarjiya Antonius: Conceived and designed the experiments.
Hanim R. Ratnaningsih, Tirta K. Dewi: Performed the experiments.
Hanim R. Ratnaningsih, Zahra Noviana, Tirta K. Dewi, Abdul Gafur, Sarjiya Antonius: Analyzed and interpreted the data.
Zahra Noviana, Sarjiya Antonius: Contributed reagents, materials, analysis tools or data.
Hanim R. Ratnaningsih, Zahra Noviana, Tirta K. Dewi, Supriyono Loekito, Suryo Wiyono, Abdul Gafur, Sarjiya Antonius: Wote the paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by Indonesian Institute of Sciences (presently National Research and Innovation Agency - BRIN) and the RISPRO-LPDP Funding Program. We also gratefully acknowledge BRIN for providing facility and logistics to support the research.
We are immensely grateful to PT Great Giant Foods, especially Mr. Hardono, for the unwavering support and sampling permission on the site.
Contributor Information
Abdul Gafur, Email: gafur@uwalumni.com.
Sarjiya Antonius, Email: sarj.antonius@gmail.com.
References
- 1.Pii Y., Mimmo T., Tomasi N., Terzano R., Cesco S., Crecchio C. Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review, Biol Fertil Soils. 2015;51:403–415. doi: 10.1007/s00374-015-0996-1. [DOI] [Google Scholar]
- 2.Das P.P., Singh K.R., Nagpure G., Mansoori A., Singh R.P., Ghazi I.A., Kumar A., Singh J. Plant-soil-microbes: a tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022;214 doi: 10.1016/j.envres.2022.113821. [DOI] [PubMed] [Google Scholar]
- 3.Batool T., Ali S., Seleiman M.F., Naveed N.H., Ali A., Ahmed K., Abid M., Rizwan M., Shahid M.R., Alotaibi M., Al-Ashkar I., Mubushar M. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-73489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glick B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995;41:109–117. doi: 10.1139/m95-015. [DOI] [Google Scholar]
- 5.Frampton R.A., Pitman A.R., Fineran P.C. Advances in bacteriophage-mediated control of plant pathogens. Internet J. Microbiol. 2012 doi: 10.1155/2012/326452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleem M., Arshad M., Hussain S., Bhatti A.S. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotechnol. 2007;34:635–648. doi: 10.1007/s10295-007-0240-6. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya P.N., Jha D.K. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 8.Beneduzi A., Ambrosini A., Passaglia L.M.P. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. 2012. www.sbg.org.br [DOI] [PMC free article] [PubMed]
- 9.Chakraborty B.N., Allay S., Chakraborty A.P., Chakraborty U. PGPR in managing root rot disease and enhancing growth in Mandarin (Citrus reticulata Blanco.) seedlings. J. Hortic. Sci. 2017;11:104–115. [Google Scholar]
- 10.Zhu Z., Zhang H., Leng J., Niu H., Chen X., Liu D., Chen Y., Gao N., Ying H. Isolation and characterization of plant growth-promoting rhizobacteria and their effects on the growth of Medicago sativa L. under salinity conditions, Antonie van Leeuwenhoek. Int. J. Gener. Molec. Microbiol. 2020;113:1263–1278. doi: 10.1007/s10482-020-01434-1. [DOI] [PubMed] [Google Scholar]
- 11.Patten C.L., Glick B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996;42:207–220. doi: 10.1139/m96-032. [DOI] [PubMed] [Google Scholar]
- 12.Batista B.D., Dourado M.N., Figueredo E.F., Hortencio R.O., Marques J.P.R., Piotto F.A., Bonatelli M.L., Settles M.L., Azevedo J.L., Quecine M.C. The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom) Arch. Microbiol. 2021;203:3869–3882. doi: 10.1007/s00203-021-02361-z. [DOI] [PubMed] [Google Scholar]
- 13.Penrose D.M., Glick B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plantarum. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 14.Ali S., Kim W.C. Plant growth promotion under water: decrease of waterlogging-induced ACC and ethylene levels by ACC deaminase-producing bacteria. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois M., Van den Broeck L., Inzé D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018;23:311–323. doi: 10.1016/j.tplants.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camehl I., Oelmüller R. Do ethylene response factors-9 and -14 repress PR gene expression in the interaction between piriformospora indica and arabidopsis? Plant Signal. Behav. 2010;5:932–936. doi: 10.4161/psb.5.8.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galland M., Gamet L., Varoquaux F., Touraine B., Touraine B., Desbrosses G. The ethylene pathway contributes to root hair elongation induced by the beneficial bacteria Phyllobacterium brassicacearum STM196. Plant Sci. 2012;190:74–81. doi: 10.1016/j.plantsci.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Li N., Han X., Feng D., Yuan D., Huang L.J. Signaling crosstalk between salicylic acid and ethylene/Jasmonate in plant defense: do we understand what they are whispering? Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S.U., Lee C.J., Park S.C., Nam K.J., Lee K.L., Kwak S.S., Kim H.S., Kim Y.H. Flooding tolerance in sweet potato (Ipomoea batatas (L.) lam) is mediated by reactive oxygen species and nitric oxide. Antioxidants. 2022;11 doi: 10.3390/antiox11050878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orozco-Mosqueda M. del C., Glick B.R., Santoyo G. ACC deaminase in plant growth-promoting bacteria (PGPB): an efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020;235 doi: 10.1016/j.micres.2020.126439. [DOI] [PubMed] [Google Scholar]
- 21.Glick B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S., Pandey S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French Bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glick B.R., Todorovic B., Czarny J., Cheng Z., Duan J., McConkey B. Promotion of plant growth by bacterial ACC deaminase. CRC Crit. Rev. Plant Sci. 2007;26:227–242. doi: 10.1080/07352680701572966. [DOI] [Google Scholar]
- 24.Horowitz W., Latimer G. Md. AOAC International; Gaithersburg: 2006. Official Methods of Analysis of AOAC International. [Google Scholar]
- 25.Kjeldahl J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. für Anal. Chem. 1883;22:366–382. doi: 10.1007/BF01338151. [DOI] [Google Scholar]
- 26.Tobing W.L.T., Magdalena Kolo M., Bria D., Putra Purba M., Maria Paulo Soares E. Soil characterization in ex-manganese mining land in north-central timor district, east nusa tenggara. Int. J. Serv. Technol. Manag. 2022;3:1753–1762. doi: 10.46729/ijstm.v3i6.631. [DOI] [Google Scholar]
- 27.Schinner Franz, Öhlinger Richard, Kandeler Ellen, Margesin Rosa. Springer Berlin Heidelberg; 1996. Methods in Soil Biology. [Google Scholar]
- 28.Koch F.C., McMeekin T.L. A new direct nesslerization micro-kjeldahl method and a modification of the nessler-folin reagent for ammonia. J. Am. Chem. Soc. 1924;46:2066–2069. doi: 10.1021/ja01674a013. [DOI] [Google Scholar]
- 29.Tabatabai M.A., Bremner J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969;1:301–307. doi: 10.1016/0038-0717(69)90012-1. [DOI] [Google Scholar]
- 30.Gordon S.A., Weber R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26 doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dworkin M., Foster J.W. Experiments with some microorganisms WHICH utilize ethane and hydrogen. J. Bacteriol. 1958;75:592–603. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehnaz S., Lazarovits G. Inoculation effects of Pseudomonas putida, Gluconacetobacter azotocaptans, and Azospirillum lipoferum on corn plant growth under greenhouse conditions. Microb. Ecol. 2006;51:326–335. doi: 10.1007/s00248-006-9039-7. [DOI] [PubMed] [Google Scholar]
- 33.Li Z., Chang S., Lin L., Li Y., An Q. A colorimetric assay of 1-aminocyclopropane-1-carboxylate (ACC) based on ninhydrin reaction for rapid screening of bacteria containing ACC deaminase. Lett. Appl. Microbiol. 2011;53:178–185. doi: 10.1111/j.1472-765X.2011.03088.x. [DOI] [PubMed] [Google Scholar]
- 34.Honma M., Shimomura T. Metabolism of 1-Aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978;42:1825–1831. [Google Scholar]
- 35.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 36.Nautiyal C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 37.Baldani J.I., Reis V.M., Videira S.S., Boddey L.H., Baldani V.L.D. The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil. 2014;384:413–431. doi: 10.1007/s11104-014-2186-6. [DOI] [Google Scholar]
- 38.Yoon S.-H., Ha S.-M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team. Language R.A. 2021. And Environment for Statistical Computing. [Google Scholar]
- 41.Wickham H. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 42.Hothorn T., Bretz F., Westfall P. Simultaneous inference in general parametric models. Biom. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 43.Popat Raj, Banakara Kanthesh, doebioresearch Analysis of design of experiments for biological research. 2020. https://cran.r-project.org/web/packages/doebioresearch/ (accessed March 25, 2023)
- 44.Wood A.J. Plant Abiotic Stress. John Wiley & Sons, Ltd; 2005. Eco-physiological adaptations to limited water enviornments; pp. 1–13. [DOI] [Google Scholar]
- 45.Seleiman M.F., Talha Aslam M., Ahmed Alhammad B., Umair Hassan M., Maqbool R., Umer Chattha M., Khan I., Ireri Gitari H., Uslu O.S., Roy R., Leonardo Battaglia M. Salinity stress in wheat: effects, mechanisms and management strategies. Phyton. 2022;91:667–694. doi: 10.32604/phyton.2022.017365. [DOI] [Google Scholar]
- 46.Badawy S.A., Zayed B.A., Bassiouni S.M.A., Mahdi A.H.A., Majrashi A., Ali E.F., Seleiman M.F. Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa l.) under salinity conditions. Plants. 2021;10 doi: 10.3390/plants10081657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seleiman M.F., Kheir A.M.S. Saline soil properties, quality and productivity of wheat grown with bagasse ash and thiourea in different climatic zones. Chemosphere. 2018;193:538–546. doi: 10.1016/j.chemosphere.2017.11.053. [DOI] [PubMed] [Google Scholar]
- 48.Taha R.S., Seleiman M.F., Alotaibi M., Alhammad B.A., Rady M.M., Mahdi A.H.A. Exogenous potassium treatments elevate salt tolerance and performances of Glycine max L. By boosting antioxidant defense system under actual saline field conditions. Agronomy. 2020;10 doi: 10.3390/agronomy10111741. [DOI] [Google Scholar]
- 49.Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability. Springer; New York: 2012. [Google Scholar]
- 50.Mukhtar T., ur Rehman S., Smith D., Sultan T., Seleiman M.F., Alsadon A.A., Amna, Ali S., Chaudhary H.J., Solieman T.H.I., Ibrahim A.A., Saad M.A.O. Mitigation of heat stress in solanum lycopersicum l. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: effects on biochemical profiling. Sustainability. 2020:12. doi: 10.3390/su12062159. [DOI] [Google Scholar]
- 51.Alkharabsheh H.M., Seleiman M.F., Hewedy O.A., Battaglia M.L., Jalal R.S., Alhammad B.A., Schillaci C., Ali N., Al-Doss A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: a review. Agronomy. 2021;11 doi: 10.3390/agronomy11112299. [DOI] [Google Scholar]
- 52.Seleiman M.F., Al-Suhaibani N., Ali N., Akmal M., Alotaibi M., Refay Y., Dindaroglu T., Haleem Abdul-Wajid H., Leonardo Battaglia M. Drought stress impacts on plants and different approaches to alleviate its adverse effects. 2021. [DOI] [PMC free article] [PubMed]
- 53.Yu Z., Duan X., Luo L., Dai S., Ding Z., Xia G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020;25:1117–1130. doi: 10.1016/j.tplants.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Al-shareef N.O., Tester M. ELS. John Wiley & Sons, Ltd; 2019. Plant salinity tolerance; pp. 1–6. [DOI] [Google Scholar]
- 55.Roy R., Núñez-Delgado A., Sultana S., Wang J., munir A., Battaglia M.L., Sarker T., Seleiman M.F., Barmon M., Zhang R. Additions of optimum water, spent mushroom compost and wood biochar to improve the growth performance of Althaea rosea in drought-prone coal-mined spoils. J. Environ. Manag. 2021;295 doi: 10.1016/j.jenvman.2021.113076. [DOI] [PubMed] [Google Scholar]
- 56.Grichko V.P., Glick B.R. Flooding tolerance of transgenic tomato plants expressing the bacterial enzyme ACC deaminase controlledby the 35S, rolD or PRB-1b promoter. Plant Physiol. Biochem. 2001;39:19–25. doi: 10.1016/S0981-9428(00)01217-1. [DOI] [Google Scholar]
- 57.Bray E.A. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. doi: 10.1016/S1360-1385(97)82562-9. [DOI] [Google Scholar]
- 58.Sánchez-Andrés R., Sánchez-Carrillo S., Ortiz-Llorente M.J., Álvarez-Cobelas M., Cirujano S. Do changes in flood pulse duration disturb soil carbon dioxide emissions in semi-arid floodplains? Biogeochemistry. 2010;101:257–267. doi: 10.1007/s10533-010-9472-z. [DOI] [Google Scholar]
- 59.McNicol G., Silver W.L. Separate effects of flooding and anaerobiosis on soil greenhouse gas emissions and redox sensitive biogeochemistry. J Geophys Res Biogeosci. 2014;119:557–566. doi: 10.1002/2013JG002433. [DOI] [Google Scholar]
- 60.Mitchell C.E. Trophic control of grassland production and biomass by pathogens. Ecol. Lett. 2003;6:147–155. doi: 10.1046/j.1461-0248.2003.00408.x. [DOI] [Google Scholar]
- 61.Gu C., Zhang S., Han P., Hu X., Xie L., Li Y., Brooks M., Liao X., Qin L. Soil enzyme activity in soils subjected to flooding and the effect on nitrogen and phosphorus uptake by oilseed rape. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saha S., Dutta D., Karmakar R., Ray D.P. Structure–toxicity relationship of chloroacetanilide herbicides: relative impact on soil microorganisms. Environ. Toxicol. Pharmacol. 2012;34:307–314. doi: 10.1016/j.etap.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Brockett B.F.T., Prescott C.E., Grayston S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012;44:9–20. doi: 10.1016/j.soilbio.2011.09.003. [DOI] [Google Scholar]
- 64.Singh A., Ghoshal N. Impact of herbicide and various soil amendments on soil enzymes activities in a tropical rainfed agroecosystem. Eur. J. Soil Biol. 2013;54:56–62. doi: 10.1016/j.ejsobi.2012.10.003. [DOI] [Google Scholar]
- 65.Donate-Correa J., León-Barrios M., Pérez-Galdona R. 2004. Screening for Plant Growth-Promoting Rhizobacteria in Chamaecytisus Proliferus (Tagasaste), a Forage Tree-Shrub Legume Endemic to the Canary Islands. [Google Scholar]
- 66.Dinesh R., Anandaraj M., Kumar A., Bini Y.K., Subila K.P., Aravind R. Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol. Res. 2015;173:34–43. doi: 10.1016/j.micres.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 67.Dastager S.G., Deepa C.K., Pandey A. Isolation and characterization of novel plant growth promoting Micrococcus sp NII-0909 and its interaction with cowpea. Plant Physiol. Biochem. 2010;48:987–992. doi: 10.1016/j.plaphy.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Khan A.L., Halo B.A., Elyassi A., Ali S., Al-Hosni K., Hussain J., Al-Harrasi A., Lee I.-J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 2016;21:58–64. doi: 10.1016/j.ejbt.2016.02.001. [DOI] [Google Scholar]
- 69.Maheshwari R., Bhutani N., Suneja P. Isolation and characterization of ACC deaminase producing endophytic bacillus mojavensis PRN2 from pisum sativum. Iran. J. Biotechnol. 2020;18:11–20. doi: 10.30498/IJB.2020.137279.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bessadok K., Navarro-Torre S., Pajuelo E., Mateos-Naranjo E., Redondo-Gómez S., Caviedes M.Á., Fterich A., Mars M., Rodríguez-Llorente I.D. The acc-deaminase producing bacterium variovorax sp. ct7.15 as a tool for improving calicotome villosa nodulation and growth in arid regions of Tunisia. Microorganisms. 2020;8 doi: 10.3390/microorganisms8040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sajjad Mirza M., Ahmad W., Latif F., Haurat J., Bally R., Normand P., Malik K.A. 2001. Isolation, Partial Characterization, and the Effect of Plant Growth-Promoting Bacteria (PGPB) on Micro-propagated Sugarcane in Vitro. [Google Scholar]
- 72.Kurotani K.I., Hayashi K., Hatanaka S., Toda Y., Ogawa D., Ichikawa H., Ishimaru Y., Tashita R., Suzuki T., Ueda M., Hattori T., Takeda S. Elevated levels of CYP94 family gene expression alleviate the Jasmonate response and enhance salt tolerance in rice. Plant Cell Physiol. 2015;56:779–789. doi: 10.1093/pcp/pcv006. [DOI] [PubMed] [Google Scholar]
- 73.Dobbelaere S., Vanderleyden J., Okon Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. CRC Crit. Rev. Plant Sci. 2003;22:107–149. doi: 10.1080/713610853. [DOI] [Google Scholar]
- 74.Domenech J., Reddy M.S., Kloepper J.W., Ramos B., Gutierrez-Mañero J. Combined application of the biological product LS213 with Bacillus, Pseudomonas or chryseobacterium for growth promotion and biological control of soil-borne diseases in pepper and tomato. BioControl. 2006;51:245–258. doi: 10.1007/s10526-005-2940-z. [DOI] [Google Scholar]
- 75.Dixit R., Agrawal L., Gupta S., Kumar M., Yadav S., Chauhan P.S., Nautiyal C.S. Southern blight disease of tomato control by 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing Paenibacillus lentimorbus B-30488. Plant Signal. Behav. 2016;11 doi: 10.1080/15592324.2015.1113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srivastava P., Sahgal M., Sharma K., El Enshasy H.A., Gafur A., Alfarraj S., Ansari M.J., Sayyed R.Z. Optimization and identification of siderophores produced by Pseudomonas monteilii strain MN759447 and its antagonism toward fungi associated with mortality in Dalbergia sissoo plantation forests. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.984522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glick B.R., Penrose D.M., Li J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 78.Spaepen S., Vanderleyden J., Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 79.Glick B.R. Plant growth-promoting bacteria: mechanisms and applications. Sci. Tech. Rep. 2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun Z., Yang L., Zhang L., Han M. An investigation of Panax ginseng Meyer growth promotion and the biocontrol potential of antagonistic bacteria against ginseng black spot. J Ginseng Res. 2018;42:304–311. doi: 10.1016/j.jgr.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh N., Marwa N., Mishra S.k., Mishra J., Verma P.C., Rathaur S., Singh N. Brevundimonas diminuta mediated alleviation of arsenic toxicity and plant growth promotion in Oryza sativa L. Ecotoxicol. Environ. Saf. 2016;125:25–34. doi: 10.1016/j.ecoenv.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 82.Rathi M., Yogalakshmi K.N. Brevundimonas diminuta MYS6 associated Helianthus annuus L. for enhanced copper phytoremediation. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.128195. [DOI] [PubMed] [Google Scholar]
- 83.Siddikee M.A., Chauhan P.S., Anandham R., Han G.H., Sa T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J. Microbiol. Biotechnol. 2010;20:1577–1584. doi: 10.4014/jmb.1007.07011. [DOI] [PubMed] [Google Scholar]
- 84.Dubey A., Kumar A., Khan M.L., Payasi D.K. Plant growth-promoting and bio-control activity of Micrococcus luteus strain AKAD 3-5 isolated from the soybean (Glycine max (L.) merr.) rhizosphere. Open Microbiol. J. 2022;15:188–197. doi: 10.2174/1874285802115010188. [DOI] [Google Scholar]
- 85.Olanrewaju O.S., Glick B.R., Babalola O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017;33 doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wagi S., Ahmed A. Bacillus spp.: potent microfactories of bacterial IAA. PeerJ. 2019;2019 doi: 10.7717/peerj.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu S.F., Wei J.Y., Chen H.W., Liu Y.Y., Lu H.Y., Chou J.Y. Indole-3-acetic acid: a widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015;10 doi: 10.1080/15592324.2015.1048052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdelmoteleb A., Moreno-Ramírez L., Valdez-Salas B., Seleiman M.F., El-Hendawy S., Aldhuwaib K.J., Alotaibi M., González-Mendoza D. New Bacillus subtilis strains isolated from prosopis glandulosa rhizosphere for suppressing Fusarium spp. and enhancing growth of gossypium hirsutum L. Biology. 2022;12:73. doi: 10.3390/biology12010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.