Abstract
Background
C-reactive protein (CRP) is a marker of inflammation and infection. The main proinflammatory cytokine that leads to CRP gene expression is IL-6. The study aimed to compare CRP level between patients who were treated with Tocilizumab (TCZ), an il-6 receptor blocker, and other advanced anti-inflammatory treatments (AAIT), as well as with other admitted and non-admitted populations.
Methods
A cross-sectional study of all patients (≥18 years) hospitalized at tertiary medical center between December 2009 and February 2020 and treated before hospitalization with (AAIT). Only the first hospitalization of each patient was included. Women admitted to obstetrics department were excluded. Demographic data, first blood tests results, and comorbidities were collected.
Results
The study included 563 patients treated with AAIT (2.5% received TCZ). Patients treated with TCZ were older (median 75 vs. 50 years, p < 0.001), had higher Charlson score (median 5 vs. 1, p < 0.001) and more infectious diseases at admission (50% vs. 23.4%, p = 0.05). Patients treated with TCZ had lower CRP levels (median 0.5 vs. 25 mg/l, p < 0.001) and more common normal values (64.3% vs. 20.8%, p < 0.001) compared to patients treated with other AAIT.
CRP level in patients treated TCZ (median 0.5 mg/l) was lower than that of 58,548 patients admitted to the hospital between 2010 and 2020 (median 12.55 mg/l, p < 0.001) and not statistically different from 140 non-admitted randomly selected individuals without acute disease (1.33 mg/l, p = 0.294).
Conclusion
Tocilizumab is associated with lower levels of CRP in patients admitted to acute care hospital. This finding must be considered by treating physician to avoid misinterpretation of CRP results.
Keywords: Tocilizumab, Infection, Rheumatoid arthritis, C-Reactive protein, Inflammation, Admission
Abbreviations
- IHD
Ischemic heart disease
- CVD
Cardiovascular diseases
- CHF
Congestive heart failure
- DM
Diabetes mellitus
- COPD
Chronic obstructive pulmonary disease
- PVD
Peripheral Vascular Disease
- AIDS
Acquired immunodeficiency syndrome
- TNF
Tumor necrosis factor
- JAK
Janus kinase inhibitors
- CRP
C-reactive protein
- WBC
White blood cells
- PLT
Platelets
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- TCZ
Tocilizumab
- IL
Interleukin
- tsDMARDS
Targeted synthetic disease-modifying antirheumatic drugs
- RA
Rheumatoid arthritis
- GCA
Giant Cell Arteritis
- IFX
Infliximab
- ADA
Adalimumab
- ETN
Etanercept
- wrCRP
Wide range CRP
- AAIT
advanced anti-inflammatory treatments
1. Introduction
C-reactive protein (CRP) is an acute phase protein produced mostly in the liver in response to inflammation and infection. Within 24–72 h of significant tissue injury, CRP levels in plasma may rise from around 1 mg/l to over 500 mg/l [1]. Increased levels of inflammatory cytokines, particularly interleukin-6 (IL-6), cause transcriptional activation of the CRP gene, which occurs mostly in hepatocytes in the liver. IL-6, as well as IL-1 and Tumor Necrosis Factor-alpha, are the primary proinflammatory cytokines that cause CRP gene expression [2].
CRP plays an important role in inflammatory response mainly via complement system activation and in host defense mechanisms against infections [3]. The recent introduction of biologic and targeted synthetic disease-modifying antirheumatic drugs (tsDMARDS) for the treatment of autoimmune diseases, acting via different molecular mechanisms (including mechanisms that interfere with interleukins that are involved in CRP production) has led to significantly better control of inflammatory disease activity and subsequently to lowering of CRP levels in treated patients as a laboratory indicator of chronic inflammation. These agents are widely used for the treatment of different inflammatory diseases like rheumatoid arthritis, psoriasis and psoriatic arthritis, ankylosing spondylitis, inflammatory bowel disease and hematological diseases. CRP is an important indicator of infection [4], even in immunocompromised patients [5,6] and was found to be an effective screening measure for sepsis in a meta-analysis of critically immunosuppressed patients [7].
We previously reported 9 cases of patients on Tocilizumab treatment that were admitted to the hospital due to infectious diseases and their CRP levels were normal or only slightly elevated. We also performed a literature search and found 28 case reports on this specific issue, in 15 out of 28 reported cases, CRP was normal or only slightly elevated [8]. Tocilizumab is primarily used in rheumatology and is approved for the treatment of rheumatoid arthritis (RA) and Giant Cell Arteritis (GCA), and it was recommended by the World Health Organization for the treatment of severe COVID-19 disease [9,10].
Different DMARDS may influence CRP levels due to their specific mechanisms of action and alter its usage as an important indicator of acute infection/inflammation. In the study by Listing, mean CRP levels in RA patients treated with TNF-inhibitors (Etanercept or Infliximab) were slightly elevated compared to the control group of RA patients on DMARD (mean CRP, ETA: 24.8 ± 29.9 mg/l, IFX: 28.8 ± 49.8 mg/l for IFX, control: 23.7 ± 30.6 mg/l) [11].
Evaluation of CRP level is an essential part of the assessment of patients in the emergency department. Therefore, the primary objective of this study was to compare the CRP levels measured at admission to an acute care hospital between patients treated with TCZ and patients treated with other biological or targeted DMARDs. The secondary objectives were to compare the CRP levels of the admitted patients receiving TCZ to all other patients admitted with acute disease and to individuals without acute illnesses.
2. Methods
2.1. Study design, participants and setting
A cross-sectional study of all patients aged 18 years and above who were hospitalized at the Tel Aviv Sourasky Medical Center (TASMC) between December 2009 and February 2020 and treated before hospitalization with biological or targeted DMARDs. TASMC is a tertiary referral university affiliated 1500 beds medical center located in the center of Israel. Patients who were admitted to the obstetrics ward were excluded. Only the first hospitalization of each patient during the study period was included. The study was approved by the ethical committee of Tel Aviv Sourasky Medical Center (0491-17-TLV).
2.2. Variables and data source
Data was obtained from the TASMC data warehouse using MDClone software (version 5.5.0.5). MDClone is a query tool that provides comprehensive patient-level data of wide-ranging variables in a defined time frame around an index event. Data on age, gender, comorbidities, Charlson score, first blood tests results and first vital signs were extracted.
First white blood cells count, neutrophils count, platelets count, hemoglobin, c-reactive protein, creatinine and albumin during hospitalization were collected. Comorbidities were defined according to Charlson score criteria [12]. Heart rate, systolic and diastolic blood pressure and body temperature were also extracted. Chemistry blood tests were measured by the ADVIA, Siemens Healthcare Diagnostic Inc. The ADVIA chemistry Wide-Range C-Reactive Protein (wrCRP) method measures CRP in serum and plasma by latex-enhanced immunoturbidimetric assay. The wrCRP laboratory methods were described previously [13]. Blood cells count was performed by using the Beckman Coulter UniCel. wrCRP values were also categorized as normal and above normal using 5 mg/L as a threshold value.
Data on background DMARDS treatment was collected and then categorized to one of the two following groups: tocilizumab treatment and other treatments. In further investigation, the other treatments groups were divided into 3 subgroups: anti-TNF subgroup which included Infliximab (IFX), Adalimumab (ADA), etanercept (ETN), Golimumab, and Certolizumab, JAK-inhibitor subgroup which included Tofacitinib and Baricitinib, and another subgroup which included Rituximab, Abatacept and Anakinra.
2.3. Sample size
The study sample size was determined by the number of people who met the inclusion and exclusion criteria. Fourteen patients were treated with TCZ while 549 were treated with other advanced anti-inflammatory agents. We used effect size d to calculate the power of the study. A difference of at least 0.8 standard deviations (equal to d = 0.8) between groups was applied to calculate the power of the study. Using a significance level of 5% revealed a power of 84%.
2.4. Additional comparison groups for the secondary objectives
To compare the CRP levels of admitted TCZ patients to those of other admitted patients with acute illness, we obtained data on first CRP levels of all admitted patients to our hospital during years 2010–2020. The data was obtained using MDClone tool.
In order to compare the CRP level in the admitted patients receiving TCZ to that of individuals without acute disease, a second comparison group was selected from the Tel Aviv Medical Center Inflammation Survey (TAMCIS) database. This database includes individuals who have attended the Tel Aviv Medical Center for a routine annual health check-up and agreed to participate in the inflammation survey. wrCRP was evaluated on a regular basis for all participants [[13], [14], [15]]. Since the mean age of the participants in the routine annual examination was lower, we divided the participants into age groups (<50, 50–59, 60–69, 70–79 and 80+ years) and randomly selected 10 participants in the same age group for each patient who received TCZ.
2.5. Statistical methods
Categorical variables were summarized as frequency and percentage. Continuous variables were evaluated for normal distribution using histogram and Q-Q plot. Normality distributed variables were described as mean and standard deviation (SD) while other variables were reported as median and interquartile range (IQR). Chi-square test and Fisher's exact test were applied to compare categorical variables between groups while independent samples T-test, Mann Whitney test, Analysis of Variance (ANOVA) and Kruskal Wallis test were used to compare continuous variables. All statistical tests were two sided and p-value<0.05 was considered as statistically significant. SPSS software (IBM SPSS Statistics for Windows, Version 27, Armonk, NY, USA, 2020) was used for all statistical analyses.
3. Results
3.1. Study population
The study included 563 patients (median age 51.1 years, 46% male), of whom 343 were hospitalized in internal medicine wards and 113 in surgical wards. All other patients (107) were hospitalized in other departments. Study population characteristics are summarized in Table 1(A). Two hundred and twelve patients (37.7%) had CTDs (Rheumatoid Arthritis: 122, 21.7%; Axial Spondyloarthritis: 38, 6.7%; Psoriatic Arthritis: 36, 6.7%; other CTDs: 26, 12.2%).
Table 1.
Patients’ characteristics: (A) demographic and clinical, (B) advanced anti-inflammatory treatments, (C) blood tests and vital signs.
|
A - Demographic, comorbidities and admission parameters |
N = 563 |
| Age (years), median (IQR) | 51.1 (33.4–66.26) |
| Male | 46% |
| Department of hospitalization | |
| Internal medicine wing | 60.9% |
| Dermatology | 11.0% |
| Surgery wing | 20.1% |
| Orthopedics | 3.0% |
| Gynecology | 3.9% |
| Intensive care | 1.1% |
| Reason for hospitalization | |
| Infectious | 23.6% |
| Non infectious | 76.4% |
| Comorbidities* | |
| IHD | 2.3% |
| CVD | 4.2% |
| CHF | 3.6% |
| DM | 12.3% |
| COPD | 11.0% |
| PVD | 1.9% |
| Renal disease | 6.1% |
| Dementia | 0.0% |
| Liver disease | 3.9% |
| Tumor | 5.8% |
| Lymphoma | 1.3% |
| Peptic ulcer | 1.6% |
| AIDS | 0.3% |
| *Noted in 303 patients in whom Charslon score was available in the data search results | |
|
B - Chronic advanced anti-inflammatory treatment | |
| Tocilizumab | 2.5% |
| Anti-TNF | |
| Infliximab | 23.1% |
| Adalimumab | 43.2% |
| Etanercept | 14.9% |
| Certolizumab | 1.6% |
| Golimumab | 2.8% |
| JAK-inhibitor | |
| Tofacitinib | 3.7% |
| Baricitinib | 0.2% |
| Others | |
| Rituximab | 4.3% |
| Abatacept | 2.3% |
| Anakinra |
1.4% |
|
C - Blood tests and vital signs | |
| Blood tests | |
| CRP (mg/dL), median (IQR) | 24.7 (6.5–93.6) |
| WBC (K/μL), median (IQR), | 8.6 (6.3–11.1) |
| Neutrophils (K/μL), median (IQR) | 5.6 (3.8–8.3) |
| PLT (K/μL), median (IQR) | 236 (180–306) |
| Albumin (g/L), mean (SD) | 36.7 (5.8) |
| Creatinine (mg/dL), median (IQR) | 0.80 (0.65–0.99) |
| Vital signs | |
| Heart rate (bpm), mean (SD) | 81 (16.03) |
| SBP (mmHg), mean (SD) | 127 (21.56) |
| DBP (mmHg), mean (SD) | 73 (19.92) |
| Temperature (C०), mean (SD) | 36.8 (0.47) |
IHD - Ischemic heart disease; CVD - Cardiovascular diseases; CHF - Congestive heart failure; DM - Diabetes mellitus; COPD - Chronic obstructive pulmonary disease; PVD - Peripheral Vascular Disease; AIDS - Acquired immunodeficiency syndrome; TNF - Tumor necrosis factor; JAK - Janus kinase inhibitors; CRP - C-reactive protein; WBC - White blood cells; PLT - Platelets; SBP - Systolic blood pressure; DBP - Diastolic blood pressure.
Two and half percent of the study population received TCZ (appendix 1). Adalimumab was the most widely used medicine in the study population (43.2%), followed by IFX (23.1%) and ETA (14.9%). All other treatments were relatively rarely used (<5%) as described in Table 1(B).
Median CRP in the whole study population was above the normal range (24.7 mg/l) and overall elevated in 78% of patients. Median WBC, neutrophil, lymphocytes, PLT count and creatinine as well as mean albumin level were in the normal range. Blood tests and vital signs at time of hospitalization are reported in Table 1(C). The characteristics of the patients who had RA and CTDs are summarized in appendixes 2 and 5, respectively.
3.2. Tocilizumab versus all other treatments
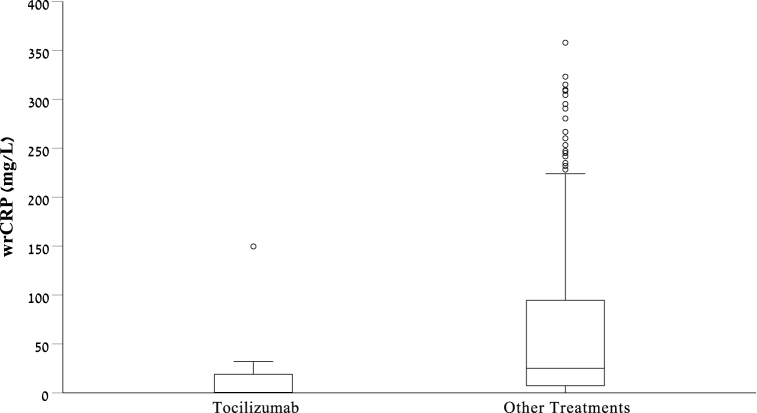
Patients treated with TCZ were older (median 75 vs. 50 years, p < 0.001), and had higher Charlson scores (median 5 vs. 1, p < 0.001). Comparison of demographic characteristics and comorbidities are summarized in Table 2(A). Patients treated with TCZ had lower CRP levels than those treated with other medications (median 0.5 vs 25 mg/l, p < 0.001, Fig. 1). In both treatment groups, CRP level was taken in a median time of approximately 16 h after admission (p = 0.558). WBC parameters (total, neutrophils and lymphocytes) as well as creatinine and albumin levels, were not statistically different between groups. Platelet level was lower in the TCZ group (149 vs. 238, p < 0.001). Vital signs were not statistically significantly different between groups. Comparison of blood tests and vital signs between those who were treated with TCZ and other treatments are summarized in Table 2(B).
Table 2.
Comparison between the patients treated with TCZ and those who treated with other advanced anti-inflammatory drugs.
| Anti-inflammatory treatment |
p-value | ||
|---|---|---|---|
| Tocilizumab | Other | ||
| Age (years), median (IQR) | 75.3 (65.3–85.2) | 50.2 (33.3–65.6) | <0.001 |
| Male | 35.7% | 46.3% | 0.434 |
| Diagnosis of infectious disease on admission | 50.0% | 23.4% | 0.050 |
| Concurrent steroids use | 35.7% | 34.6% | >0.999 |
| Comorbidities | |||
| IHD | |||
| CVD | 0.0% | 4.4% | >0.999 |
| CHF | 14.3% | 3.1% | 0.083 |
| DM | 7.1% | 12.5% | >0.999 |
| COPD | 42.9% | 9.5% | 0.002 |
| PVD | |||
| Renal disease | 14.3% | 5.8% | 0.210 |
| Liver disease | 0.0% | 4.1% | >0.999 |
| Tumor | 7.1% | 5.8% | 0.576 |
| Lymphoma | 7.1% | 1.0% | 0.170 |
| Peptic ulcer | 0.0% | 1.7% | >0.999 |
| AIDS | 0.0% | 0.3% | >0.999 |
| Charlson Score, median (IQR) | 5 (4.8–6.3) | 1 (0–3) | <0.001 |
| Blood tests | |||
| CRP (mg/dL), median (IQR) | 0.5 (0.05–21.21) | 25.06 (7.25–94.86) | <0.001 |
| WBC (K/μL), median (IQR), | 9.3 (6.4–11.5) | 8.5 (6.3–11.1) | 0.720 |
| Neutrophils (K/μL), median (IQR) | 6.6 (4.0–8.9) | 5.6 (3.8–8.3) | 0.711 |
| Lymphocytes (K/μL), median (IQR) | 1.7 (0.9–2.1) | 1.7 (1.2–2.3) | 0.631 |
| PLT (K/μL), median (IQR) | 149 (124–210) | 238 (184–308) | <0.001 |
| Albumin (g/L), mean (SD) | 38 (3.9) | 36.6 (5.8) | 0.384 |
| Creatinine (mg/dL), median (IQR) | 0.78 (0.6–1.17) | 0.80 (0.65–0.99) | 0.988 |
| Vital signs | |||
| Heart rate, mean (SD), bpm | 80.7 (13.4) | 81.2 (16.1) | 0.896 |
| SBP, mean (SD), mmHg | 136.9 (18.7) | 126.9 (21.6) | 0.085 |
| DBP, mean (SD), mmHg | 75.1 (10.5) | 73.7 (14.0) | 0.748 |
| Body temperature, mean (SD),C० | 36.6 (0.4) | 36.8 (0.5) | 0.176 |
IHD - Ischemic heart disease; CVD - Cardiovascular diseases; CHF - Congestive heart failure; DM - Diabetes mellitus; COPD - Chronic obstructive pulmonary disease; PVD - Peripheral Vascular Disease; CRP - C-reactive protein; WBC - White blood cells; PLT - Platelets; SBP - Systolic blood pressure; DBP - Diastolic blood pressure.
Fig. 1.
Box and whisker plot demonstrating the distribution of CRP level in patients treated with Tocilizumab and patients treated with other anti-inflammatory medications.
When we categorized CRP into normal and abnormal values, 64.3% of patients who were treated with TCZ had normal CRP values while only 20.8% of the patients treated with other medications had normal values (p < 0.001). To notice, the patients who were treated with TCZ, had two times more infectious diagnoses on admission compared to patients treated with other medicines (50% vs 23.4%, p = 0.05). In patients treated with TCZ and hospitalized with infectious disease (7 patients), only one patient had significant CRP elevation (149 mg/l), 4 patients had intermediately increased CRP levels (6.1–31.9 mg/l) and 3 patients had CRP levels within the normal range.
In further analyses, only patients who had RA and CTDs were included. The comparison of demographic characteristics, comorbidities, blood tests and vital signs between those who were treated with TCZ and other treatments are summarized in appendixes 3 and 6.
3.3. Tocilizumab versus anti-TNF, JAK-i and other treatment groups
Patients treated with TCZ were also compared to patients treated with anti-TNF (n = 482), JAK-I (n = 22) and other treatments (n = 45). Age, gender, infectious disease diagnosis, WBC count and CRP levels in each treatment group are summarized in Table 3.
Table 3.
Influence of TCZ in comparison with other treatment groups.
| Anti-inflammatory treatment |
p-value | ||||
|---|---|---|---|---|---|
| TCZ | Anti-TNF | JAK-i | Other | ||
| Age (years), median (IQR) | 75.28 (65.3–85.24) | 45.79 (31.93–64.06) | 64.01 (51.66–81.16) | 62.1 (56.17–79.63) | <0.001 |
| Male | 35.7% | 49.0% | 22.7% | 28.9% | 0.006 |
| Diagnosis of infectious disease on admission | 50.0% | 22.4% | 38.1% | 26.7% | 0.040 |
| CRP (mg/dL), median (IQR) | 0.5 (0.05–21.21) | 24.09 (6.54–91.81) | 19.64 (6.65–76.71) | 93.32 (16.95–172.16) | <0.001 |
| WBC (K/μL), median (IQR), | 9.25 (6.4–11.53) | 8.6 (6.4–11.1) | 7.45 (5.08–9.88) | 7.8 (5.95–12.95) | 0.315 |
TCZ - Tocilizumab; TNF - Tumor necrosis factor; JAK - Janus kinase inhibitors; CRP - C-reactive protein; WBC - White blood cells.
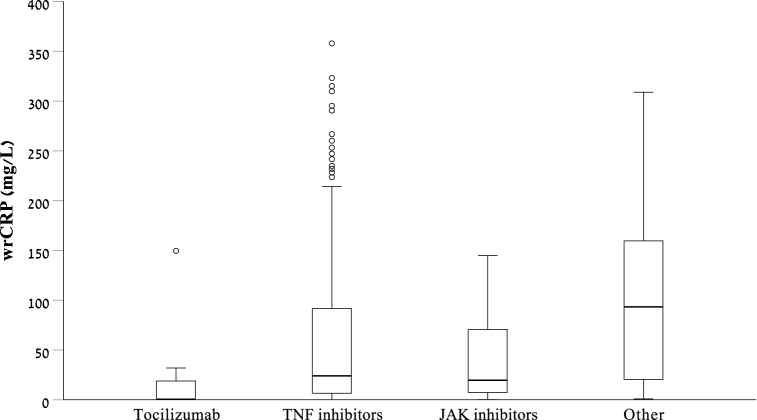
CRP level in the TCZ group (median 0.5 mg/l) was lower than that measured in anti-TNF group (24.09 mg/l, p < 0.001), JAK-i group (19.64 mg/l, p = 0.008), and other treatment group (93.32 mg/l, p < 0.001, Fig. 2).
Fig. 2.
Box and whisker plot demonstrating the distribution of CRP level in patients treated with Tocilizumab and patients treated with TNF-i, JAK-i and other anti-inflammatory medications.
As previously, further analyses that included only patients who had RA and CTDs were performed. These analyses are summarized in appendixes 4 and 7 respectively.
3.4. CRP level in patients treated with Tocilizumab in comparison to all other admissions
CRP level in patients treated with Tocilizumab was also compared to the CRP level of 58,548 patients that were admitted to the hospital between 2010 and 2020 and CRP level at admission was available. Median first CRP in the 58548 admissions was significantly higher (median 12.55 mg/l, IQR 2.65–54.84) the those treated with TCZ (median 0.5 mg/l, IQR 0.05–21.21, p < 0.001).
3.5. CRP level in admitted patients treated with Tocilizumab in comparison to non-admitted individuals without acute disease
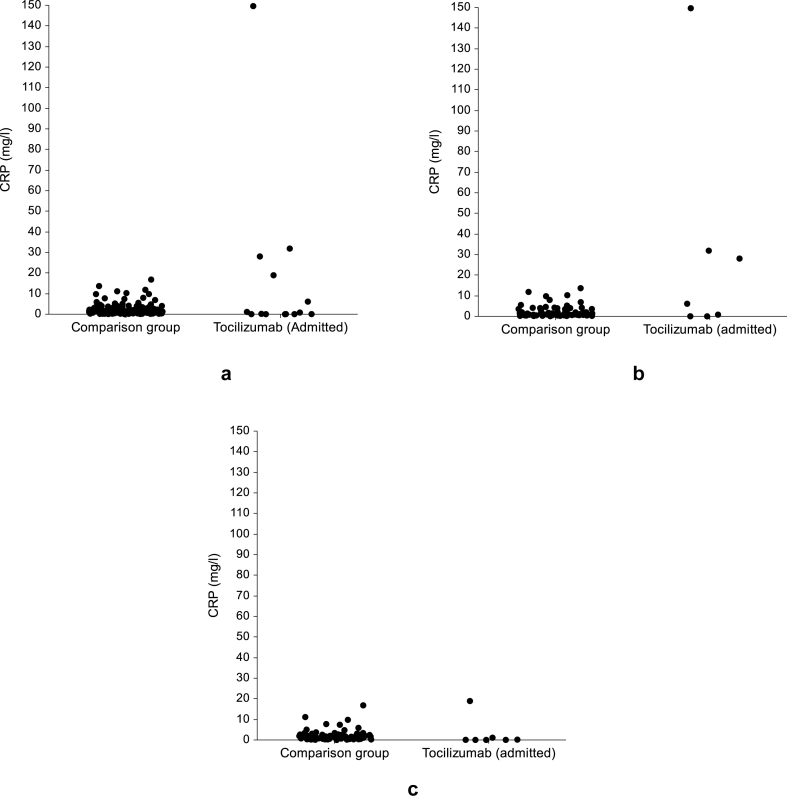
The median CRP level at the 140 selected TAMCIS participants (1.33 mg/l, IQR 0.61–2.28) was not statistically different (p = 0.294, Fig. 3a) of the admitted patients receiving TCZ (0.50 mg/l, IQR 0.05–21.21). When we compared separately TCZ patients (n = 7) who admitted and diagnosed with an acute infection (median 6.12, IQR 0.03–31.89) to 70 selected TAMCIS participants (1.07 mg/l, IQR 0.62–3.50), no statistically different was observed (p = 0.348, Fig. 3b). However, when we compared separately TCZ patients (n = 7) who were admitted and diagnosed with other acute illnesses (median 0.08, IQR 0.05–1.11) to 70 selected TAMCIS participants (1.43 mg/l, IQR 0.58–2.62), a statistically significant difference was observed (p = 0.013, Fig. 3c).
Fig. 3.
Dot plot demonstrating the distribution of CRP level in: (a) admitted patients treated with Tocilizumab and non-admitted individuals with no acute disease, (b) in patients admitted due to infectious disease, and (c) in patients admitted due to non-infectious disease.
4. Discussion
TCZ, a medication that is primarily used in rheumatology practice, may decrease CRP levels due to its specific mechanism of action related to IL-6 inhibition [16].
To our knowledge, this is the first study in patients admitted to acute care hospital that compares CRP levels between patients treated with TCZ and patients treated with other types of biologic and targeted synthetic DMARDS, as well as, comparison with other admitted patients and non-admitted non-acute illness individuals.
Evaluation of CRP levels is essential and especially in the early stages of an urgent medical referral. The level of CRP taken from patients in the emergency department was found in a previous study at our center to be associated with recurrent referrals within 7 days [17]. Understanding whether certain medications affect CRP levels is crucial in order to know how to interpret the test result.
There is scarcity of data regarding the influence of the advanced biological treatments on the c-reactive protein levels at hospital admission. Typically patients present to their treating physician during the acute phase of their illness (for example active RA or inflammatory bowel disease exacerbation) with high CRP levels and are treated with advanced anti-inflammatory drugs which are expected to reduce the inflammation and thus lower CRP levels. The existing data mainly refers to decrease of CRP levels due to improvement of the inflammatory disease activity, but there is a lack of data about CRP levels at acute illness which requires hospital admission. TNF-i decreases CRP levels, generally slightly more than csDMARDs in equivalent patient populations [18]. CRP levels may be reduced by roughly 10 mg/l using JAK inhibitors that target downstream signaling pathways of IL-6 and other cytokines, with the reduction being dose dependent [19,20].
In this study we showed that levels of CRP at admission in patients treated with TCZ are significantly lower (0.5 mg/l, IQR 0.05–21.21) in comparison to patients treated with other biologic and targeted DMARDS (25.06 mg/l, IQR 7.25–94.86, p < 0.001). Even this study had limited power due to the small sample size, we were able to demonstrate a significant difference between these two groups of patients.
We also demonstrated that CRP level was lower in TCZ treated patients compared to patients treated with TNFα-i (24.09 mg/l, IQR 6.54–91.81, p < 0.001), JAK-i (19.64 mg/l, IQR 6.65–76.71, p = 0.008) and other group which included RTX, ABA and Anakinra (93.32 mg/l, IQR 16.95–172.16, p < 0.001). This study also provides evidence that CRP level in patients treated with TCZ is significantly lower in comparison with the general population admitted to acute care hospital. The median CRP level of the admitted patients who were treated with TCZ was lower that of non-admitted individuals without acute disease, however it was not statistically different. When we compared separately, TCZ patients with acute infection to the individuals without acute disease the median CRP was slightly higher (not statistically significant) but when TCZ patients with other acute disease were compared they had significantly lower CRP level that the individuals with acute disease. These findings highlight the importance of the accurate interpretation of CRP level in patients admitted to the hospital, as CRP is an important laboratory marker of inflammation and infection.
It has been suggested that medications of JAK-i class may also decrease CRP levels dramatically due to influence of JAK inhibition on production of IL-6 [21]. In our study, CRP levels in the JAK-i treated group were not significantly different from that of the Anti-TNF treated group, 19.64 mg/l (IQR 6.65–76.71) vs 24.09 mg/l (IQR 6.54–91.81, p = 0.42). CRP levels were significantly lower in TCZ treated group versus JAK-i treated group which emphasizes the action of TCZ.
Another important finding is that PLT level in TCZ treated patients was significantly lower compared with the other agents. This may be explained by the fact Interleukin 6 (IL-6) stimulates megakaryocytopoiesis in the bone marrow, increasing platelet numbers in the circulation and thus, blocking the il-6 pathway may decrease PLT levels [22].
The study had several limitations. First, the study is based on electronic medical records, which were scanned by using ICD-9 codes and codes of the medications, documented during the patients hospitalization, and the files were not evaluated by the researchers. TCZ patients as well as all other patients were scanned using the same methods, which reduces the probability of information bias.
Second, we did not have data of the timing of administration of the last dose of advanced anti-inflammatory drug and therefore we could not evaluate the association between time from administration and CRP level. Third, the study had limited power however a significant difference in CRP level was found between patients treated with TCZ and other advanced anti-inflammatory drugs. This finding demonstrates the significant difference between groups of treatments.
In the last 2 years use of TCZ in clinical practice has strikingly increased due to COVID-19 pandemic. The findings of this study can be generalized to the community of patients who had TCZ treatment for COVID-19, with the caveat that CRP levels may not accurately reflect these patients' infectious status.
As CRP may not be a reliable indicator to identify inflammation in patients receiving TCZ treatment, other methods have been proposed [8]. In patients with suppressed CRP levels an increased IL-6 levels during TCZ therapy could serve as a potential marker of inflammation [23]. Another study demonstrated that the neutrophil-to-lymphocyte ratio (NLR) could be useful for predicting bacterial infections in RA patients treated with TCZ [24]. Procalcitonin, an independent marker of bacterial infections not influenced by IL-6, has been proposed as a preferred surrogate marker during TCZ treatment to detect bacterial infections [25]. In addition, monitoring neutrophil CD64 levels may also assist in early identification of infection among patients undergoing interleukin-6 receptor antagonist therapy [26].
In conclusion, Tocilizumab, an IL-6 receptor blocker, was associated with lower levels of CRP versus other advanced immunosuppressive treatments in patients admitted to acute care hospitals even though these patients were older and had more infectious diseases. This fact may potentially lead to inaccurate assessment of patients presenting with infectious disease and underestimate the intensity of their infectious process.
In conclusion, Tocilizumab, an IL-6 receptor blocker, was associated with lower levels of CRP in patients admitted to an acute care hospital than that of admitted patients that were treated with other advanced immunosuppressive treatments as well as all other admissions. Admitted patients treated with TCZ were not found to be different in their CRP level from a non-hospitalized and non-acute disease population. This fact may potentially lead to misinterpretation of CRP level in patients presenting with acute illnesses to emergency department and the treating physician must consider it.
Author contribution statement
Mark Berman, M.D.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Shlomo Berliner, Prof.: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Nancy Bashouti, Ph.D.; Ori Elkayam, Prof: Conceived and designed the experiments.
Tomer Ziv-Baran: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Funding
This work was supported in part by the Dalia and Arye Pershkovsky grant for Biomedical research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16665.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang D., Sun M., Samols D., Kushner I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J. Biol. Chem. 1996;271(16):9503–9509. doi: 10.1074/jbc.271.16.9503. [DOI] [PubMed] [Google Scholar]
- 3.Du Clos T.W. Function of C-reactive protein. Ann. Med. 2000;32(4):274–278. doi: 10.3109/07853890009011772. PMID: 10852144. [DOI] [PubMed] [Google Scholar]
- 4.Healy B., Freedman A. Infections. BMJ. 2006;332(7545):838–841. doi: 10.1136/bmj.332.7545.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroesen S., Widmer A.F., Tyndall A., Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatology. 2003;42(5):617–621. doi: 10.1093/rheumatology/keg263. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg S.B. Infections in the immunocompromised rheumatologic patient. Crit. Care Clin. 2002;18(4):931–956. doi: 10.1016/s0749-0704(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira V.M., Moraes R.B., Stein A.T., Wendland E.M. Accuracy of C - Reactive protein as a bacterial infection marker in critically immunosuppressed patients: a systematic review and meta-analysis. J. Crit. Care. 2017;42:129–137. doi: 10.1016/j.jcrc.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Berman M., Ben-Ami R., Berliner S., et al. The effect of tocilizumab on inflammatory markers in patients hospitalized with serious infections. Case series and review of literature. Life. 2021;11(3):258. doi: 10.3390/life11030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.REMAP-CAP Investigators. Gordon A.C., Mouncey P.R., et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N. Engl. J. Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recovery Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Listing J., Strangfeld A., Kary S., et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52(11):3403–3412. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chron. Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Ziv-Baran T., Wasserman A., Goldiner I., et al. Characteristics of apparently healthy individuals with a very low C-reactive protein. Clin. Chim. Acta. 2019;495:221–226. doi: 10.1016/j.cca.2019.04.073. [DOI] [PubMed] [Google Scholar]
- 14.Ziv-Baran T., Shenhar-Tsarfaty S., Etz-Hadar I., et al. The ability of the wide range CRP assay to classify individuals with low grade inflammation into cardiovascular risk groups. Clin. Chim. Acta. 2017;471:185–190. doi: 10.1016/j.cca.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Shenhar-Tsarfaty S., Shapira I., Toker S., et al. Weakened cholinergic blockade of inflammation associates with diabetes-related depression. Mol. Med. 2016;22:156–161. doi: 10.2119/molmed.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimoto N., Kishimoto T. Interleukin 6: from bench to bedside. Nat. Clin. Pract. Rheumatol. 2006;2(11):619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 17.Ziv-Baran T., Wasserman A., Shteinvil R., et al. C-reactive protein and emergency department seven days revisit. Clin. Chim. Acta. 2018;481:207–211. doi: 10.1016/j.cca.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Choy E.H., Bernasconi C., Aassi M., Molina J.F., Epis O.M. Treatment of rheumatoid arthritis with anti-tumor necrosis factor or tocilizumab therapy as first biologic agent in a global comparative observational study. Arthritis Care Res. 2017;69(10):1484–1494. doi: 10.1002/acr.23303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Vollenhoven R.F., Fleischmann R., Cohen S., et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 2012;367(6):508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 20.Genovese M.C., Kremer J., Zamani O., et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 2016;374(13):1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 21.McInnes I.B., Byers N.L., Higgs R.E., et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res. Ther. 2019;21(1):183. doi: 10.1186/s13075-019-1964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams N., Bertoncello I., Jackson H., Arnold J., Kavnoudias H. The role of interleukin 6 in megakaryocyte formation, megakaryocyte development and platelet production. Ciba Found. Symp. 1992;167:160–173. doi: 10.1002/9780470514269.ch10. [DOI] [PubMed] [Google Scholar]
- 23.Berger C.T., Recher M., Daikeler T. Interleukin-6 flags infection in tocilizumab-treated giant cell arteritis. Rheumatology. 2017;57:196–197. doi: 10.1093/rheumatology/kex336. [DOI] [PubMed] [Google Scholar]
- 24.Nagai Y., Yokogawa N., Shimada K., Sugii S. Utility of the neutrophil-to-lymphocyte ratio for predicting bacterial infection in patients with rheumatoid arthritis receiving Tocilizumab. Rheumatol. Int. 2020;40:2039–2046. doi: 10.1007/s00296-020-04705-2. [DOI] [PubMed] [Google Scholar]
- 25.Gaensbauer J.T., Press C.A., Hollister J.R. Astur. E.J. Procalcitonin. Pediatr. Infect. Dis. J. 2013;32:1040. doi: 10.1097/INF.0b013e318295a3d0. [DOI] [PubMed] [Google Scholar]
- 26.Matsui T., Komiya A., Shimada K., Nakayama H., Tohma S. Neutrophil CD64 as a marker of infection in patients treated with tocilizumab. Mod. Rheumatol. 2009;19:696–697. doi: 10.3109/s10165-009-0223-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.