Abstract
Hyperthermic intrathoracic chemotherapy (HITHOC) adjunct to surgery for Malignant Pleural Mesothelioma (MPM) has no definite role. The primary objective of this pilot-trial was to evaluate the feasibility for future large studies. The study design was a prospective randomized three-centric pilot trial. We recruited patients diagnosed with MPM and prospectively assigned them to two groups: Group A: Video Assisted Thoracic Surgery (VATS) talc pleurodesis or Group B: Video-assisted P/D plus HITHOC. From November-2011 to July-2017 24 males and 3 females, with a median age of 68-years were enrolled (recruitment rate 5 patients/year). Preoperative stage was I-II, and 18 had epithelioid type. 14 patients were in the Group A. Operative mortality was 0. Follow-up ranged 6–80 months. The median overall survival time started to diverge at 20 months, being 19 months (95% CI 12–25) in Group A and 28 months (95% CI 0–56) in Group B. Survival rate for the epithelioid type was 15 months (95% CI 0–34) in Group A and 45 months (95% CI 0–107) in the Group B. These findings suggest that video-assisted P/D plus HITHOC may improve survival time in MPM patients undergoing surgical treatment and support the need for a larger multicenter randomized clinical trial.
Keywords: Malignant pleural mesothelioma, Pleurectomy/decortication, Hyperthermic intrathoracic chemotherapy (HITHOC), Video assisted thoracic surgery (VATS), Debulking surgery
1. Introduction
Malignant Pleural Mesothelioma (MPM) is a rare but very aggressive cancer arising from the pleura often diagnosed in a locally advanced stage. Its worldwide incidence has been increasing. Total incidence is highest in the USA and UK although per capita, Australia and Italy also rank high [1]. Despite the main risk factor for mesothelioma is occupational exposure to asbestos, environmental exposure to these fibers also plays an important role and both contribute to the formation of clusters [2,3].
The prognosis of MPM is poor and the mean survival period of the patients is 9–12 months [1]. Analysis of mesothelioma mortality recorded in the WHO mortality database between 1994 and 2008 yielded an age-adjusted mortality rate of 4.9 per million, a mean age at death of 70 years and male to female ratio of 3.6:1 [4].
Treatments options are often limited to palliative care and only few have intention to cure. The type of treatment depends on centre or surgeon experience, cancer staging and patient's individualities such as age and performance status. There is abundant evidence to affirm that extra pleural pneumonectomy is nowadays rarely performed because the reported higher mortality [5] and because similar or even better long-term results which can be obtained with less invasive surgeries pleurectomy/decortication (P/D) or talc pleurodesis (TP) alone [[6], [7], [8]]. Pleurectomy/decortication is a lung sparing resection which permits the macroscopic removal of the tumor, and has a low surgical mortality rate with a significant risk of local recurrence. Furthermore, the mean survival for patients treated with TP alone is 14 months, and P/D, which shows a mortality of 1.8%, has a reported survival of 17 months [6,7]. Hyperthermic Intraoperative Thoracic Chemotherapy (HITHOC) is a type of adjuvant local treatment performed in the operating room immediately after surgery for MPM [9,10], and many experiences and studies have been reported to date a median survival rate ranging from 20 to 35 months [[11], [12], [13]]. Nonetheless, there is no gold standard treatment for MPM, and therefore the challenge for the future is to develop strategies that will bring good quality of life, longer disease-free interval and prolonged overall survival.
1.1. Aims
This pilot trial aimed at assess the feasibility for a larger randomized controlled trial (RCT) to compare two surgical treatments for MPM, namely VATS talc pleurodesis (Group A) and video-assisted Pleurectomy/Decortication (P/D) plus HITHOC (Group B). See details in the Methods.
The primary aim is the description of study feasibility of such large RCT through measurements of recruitment and retention rates.
The secondary aims are:
-
1.
Evaluation of survival rates of trial participants after 1, 2 and 3 years after surgery using in person or phone interview.
-
2.
Assessment of length of hospital stay with tailored case-report form.
-
3.
Assessment of perioperative complications as well as late complication after 3, 6 and 12 months from the procedures.
-
4.
Estimation of the sample size for the subsequent trial as well as of recruitment centers needed for trial implementation.
2. Materials and methods
2.1. Study design and registration
This pilot study was designed as a randomized trial using parallel-groups with allocation ratio 1:1. The study was registered with International Standard Randomized Controlled Trial Number no. ISRCTN12709516. The Ethical approval was provided by the University Research Area-Financial Research Committee on December 4, 2015 (reference no. DFB4A9).
2.2. Participants, setting
The study participants were patients diagnosed with Malignant Pleural Mesothelioma (MPM). After contacting five Sicilian Thoracic surgery centers for the implementation of the pilot trial, three centers eventually agreed to participate. In particular, the study was carried out in.
-
•
Centre A1: Thoracic surgery at Morgagni Hospital, Catania, Italy
-
•
Centre A2: University of Palermo Hospital, Palermo Italy
-
•
Centre B: University of Catania Hospital, Catania, Italy
Validation of patients’ recruitment and survival rates have been provided by the Regional Operative Centre (COR Sicily) for the Italian National Mesothelioma Registry, a national-based Institution of the Italian Government [14].
2.3. Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
2.4. Inclusion criteria
-
•
Patients who gave written consent to participate.
-
•
Patients with confirmed MPM (ICD-10-CM C38.4).
-
•
Patients with MPM with or without pleural effusion, with a performance status ≤2.
-
•
Patients who agreed to undergo VATS talc pleurodesis.
2.5. Exclusion criteria
-
•
Patients unfit for a VATS procedure (previous thoracic surgery).
-
•
Patients with proven mediastinal lymphadenopathy N2.
-
•
Clinical evidence of disease progression since diagnosis to advanced stage (stage III-IV).
From November 2011 to July 2017 all physicians and surgeons of the participant centers identified patients with MPM that met the inclusion criteria. Patients were then approached by the operating surgeon who explained the operative procedures and the study. Then the informed written consent was obtained.
2.6. Interventions
Participants received either video-assisted Pleurectomy/Decortication (P/D) plus HITHOC or VATS talc pleurodesis, according to which participating Centre they attended. This was determined by the existing expertise at each of those participating centers. All patients who were admitted to Centers A1 and A2 underwent VATS talc pleurodesis, while all patients admitted to Centre B underwent video-assisted P/D plus HITHOC. All patients received continued oncological management according to local policy, which could include chemotherapy, palliative radiotherapy, immunotherapy or further surgery.
2.6.1. The VATS talc pleurodesis arm (Group A)
Group A participants were admitted to the Morgagni Institute (Centre A) and underwent uniportal VATS talc pleurodesis [15] drainage of the pleural effusion, biopsy and frozen section. When the results of the frozen section analysis demonstrated the presence of a thoracic cancer, primary or secondary, patients received the VATS talc pleurodesis to avoid the accumulation of excess fluid, talc being the most effective sclerosant agent for treatment of malignant pleural effusion. Under direct vision, talc was inserted onto the entire lung surface. Talc pleurodesis was performed even when just a lobe was trapped [15]. A drain was kept on suction until drainage stopped in order to promote adhesions between the lung and chest wall.
2.6.2. The video-assisted P/D and HITHOC arm (Group B)
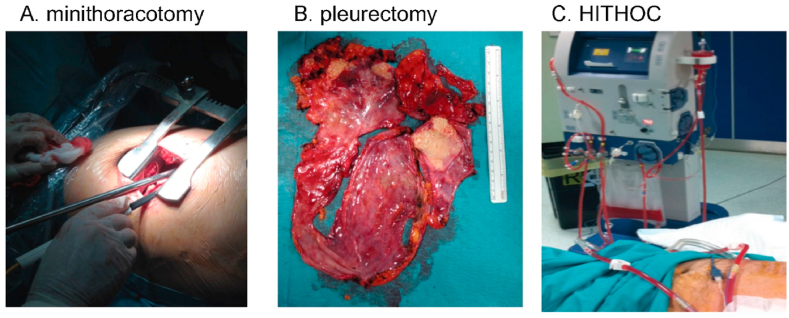
Group B participants were admitted to the University of Catania (Centre B) and underwent video-assisted P/D plus HITHOC. Pleurectomy decortication was performed via a mini-thoracotomy of 10–12 cm and a 10 mm 0° optic was used to assist the surgeon [16]. The debulking procedure is less invasive than an extra pleural pneumonectomy and includes pleurectomy (removal of parietal pleura), decortication (removal of visceral pleura) and removal of all tumor able to be seen by the human eye [11,12,17,18]. HITHOC is a concentrated dose of chemotherapy with cisplatin diluted in a normal saline solution (0.9% sodium chloride) of 2–3 L, warmed at 42.5 °C, and infused and circulated in the chest at the dose of 120 mg/m2 for 60 min. When present, the pleural effusion was aspirated at the beginning of the operation to drain excess fluid from the chest. All included patients had 100 mL of pleural effusion taken for cytological analysis at the beginning of the operation, at the end of the operation and at the end of the HITHOC. Fig. 1 demonstrates the three steps of the video-assisted P/D HITHOC method including minithoracotomy (Fig. 1A), pleurectomy (Fig. 1B) and HITHOC (Fig. 1C). All patients have been followed throughout the study using personal interview and record card including assessments and measurements to address each pilot trial aim. Patients were instructed to attend the outpatient the clinic at 3, 6, 12 months and when necessary. No changes to pilot trial assessments or measurements after the pilot trial commenced have been done.
Fig. 1.
Steps of the video-assisted P/D HITHOC method: A. minithoracotomy; B. pleurectomy; C. HITHOC.
Feasibility for a larger randomized study was the main outcome of our pilot study. Priori criteria were the following: (a) the proportion of the invited centers accepting to participate would be 50% or greater; (b) the number of the patients recruited for each centre would be minimum of three or greater per year. As this is a feasibility study a formal sample size calculation is not required, but we estimated the number of participants required by Julius’ criteria [19].
2.7. Randomization
Initially (November 2011), the study randomization was performed in-house using the envelopes method. During the first full year (2012) three patients were enrolled. Failure to recruit sufficient numbers became evident and we were not able to complete the study. We therefore contacted thoracic units in Sicily, and in January 2014 two Units joined the study. We then modified the randomization method: the nature of the study was modified and patients were randomized to one of treatment groups and alternatively referred to the study Centers according to their experience. All patients with mesothelioma referred at the Morgagni Hospital of Catania and University Hospital of Palermo (Group A) were treated with uniportal VATS talc pleurodesis, whilst those undergoing surgery at the University Hospital of Catania (Group B) were treated with video-assisted Pleurectomy/Decortication plus HITHOC. The enrolment was closed July 31, 2017 and patients were followed-up until December 31, 2020. In both centers the eligible patients were identified by the oncologists and surgeons responsible of their clinical assessment.
2.8. Statistical analysis
This is a pilot trial with the sample size based on feasibility. Given the lack of previous evidence of talc pleurodesis efficacy in terms of risk reduction, we decided to do a pilot study in small groups of 12 patients in each arm following the criteria of Julius and CONSORT extension to randomized pilot studies [19,20]. We addressed each pilot objective using quantitative method. Continuous and categorical variables were expressed as median (interquartile range-IQR) and relative frequencies (%), respectively. The probability of survival over time was estimated using the Kaplan-Meier method and expressed as median and 95% confidence interval (CI). We used as start date the date of surgery procedure. We calculated Hazard Ratios (HR) and 95% CI, with the Group A (VATS talc pleurodesis) as the reference, using a Cox proportional hazard regression model, adjusting for age, sex, histological subtype, asbestos exposure, performance status (1, 2), postoperative TNM Classification of Malignant Tumors (pTNM) [21], and Stage (1a, 1 b). For the analysis we used the intention-to-treat approach. Stratified analysis divided by smoking history have been also carried out due to potential effect modifier. We performed all statistical analyses through SPSS statistical software (Statistical Package for the Social Science-SPSS, Windows version 21.0; SPSS Inc., Chicago, IL, USA).
3. Results
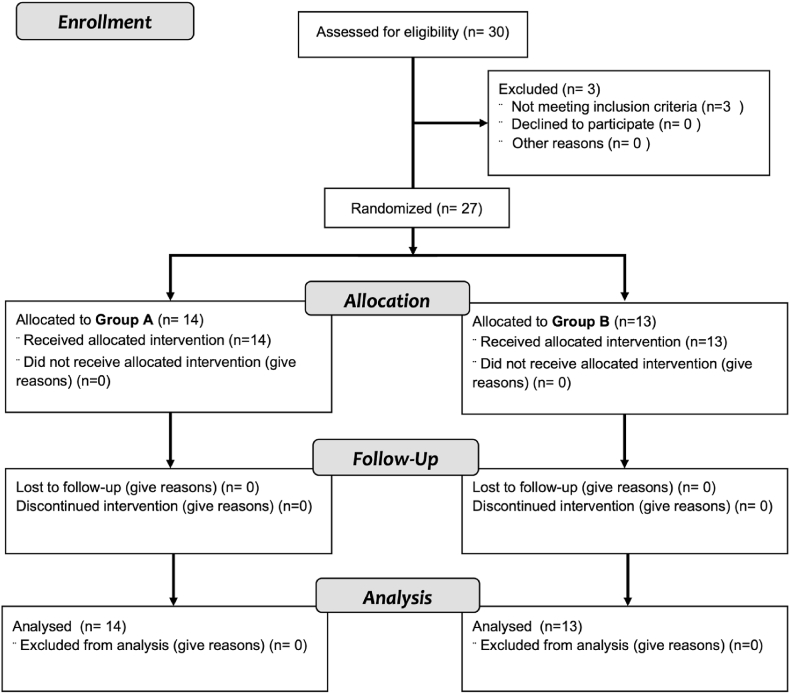
Fig. 2 shows the flow-chart of study enrollment, allocation and follow-up. We recruited patient from November 1, 2011 up to July 31, 2017, with average five patients per year enrolled. The University Hospital of Palermo contributed with 3 patients who did not meet the inclusion criteria and have been excluded, leaving 27 patients for the analysis. The retention rate was 100% as no patients were lost at follow-up.
Fig. 2.
Patients flow from enrollment to analysis.
3.1. Patients’ demographics
Overall, we included 27 patients in the trial, namely 24 men and three women. The median age was 68 years (IQR: 59–74 years) for both groups, slightly higher for Group A (69.5 years) compared to Group B (67 years) (Table 1). Study participants were similar in terms of socio-demographic characteristics. Eleven out 27 patients (40.7%) reported asbestos exposure. Thirteen patients (48.1%) were smokers. Seven patients of the Group B underwent neo-adjuvant treatment. Clinical features and staging according to pTNM divided by Groups are also reported in Table 1. All patients underwent adjuvant treatment.
Table 1.
Socio-demographic and clinical characteristics of study population divided by intervention arm (Group A: VATS talc pleurodesis arm; Group B: Video-assisted P/D and HITHOC arm). Value are number and percentages (%) when not differently indicated.
| Subject characteristics | Group A (N = 14) | Group B (N = 13) |
|---|---|---|
| Agea | 69.5 (63.5–75.3) | 67.0 (59.0–74.0) |
| Sex – male | 13 (92.9) | 11 (84.6) |
| Smoke history (yes) | 8 (57.1) | 5 (38.5) |
| Comorbidities (yes) | 13 (92.9) | 12 (92.3) |
| Asbestos exposure (yes) | 6 (42.9) | 5 (38.5) |
| Living in Biancavilla (yes) | 0 (0.0) | 4 (30.8) |
| Preoperative stage | ||
| 1a | 11 (78.6) | 2 (15.4) |
| 1 b | 3 (21.4) | 10 (76.9) |
| 3 b | 0 (0.0) | 1 (7.7) |
| Postoperative TNM | ||
| T1 N0 M0 | 8 (57.1) | 2 (15.4) |
| T1 N1 M0 | 2 (14.3) | 0 (0.0) |
| T2 N0 M0 | 2 (14.3) | 5 (38.5) |
| T3 N0 M0 | 2 (14.3) | 5 (38.5) |
| T4 N0 M0 | 0 (0.0) | 1 (7.7) |
| Histology | ||
| Epithelioid | 9 (64.3) | 9 (69.2) |
| Sarcomatoid | 2 (14.3) | 1 (7.7) |
| Biphasic | 3 (21.4) | 3 (23.1) |
| Surgery time (minutes)a | 65.2 (46.8–79.0) | 330 (248–418) |
| Hospital stay (days)a | 6 (5–10) | 8 (6–10) |
| Complications (yes) | 8 (57.1) | 7 (53.9) |
| Adjuvant chemotherapy | ||
| Carboplatin and radiotherapy | 0 (0.0) | 2 (15.4) |
| Carboplatin and pemetrexed | 4 (28.6) | 5 (38.5) |
| Cisplatin | 6 (42.9) | 5 (38.5) |
| Cisplatin and pemetrexed | 2 (14.3) | 0 (0.0) |
| Pemetrexed | 1 (7.1) | 0 (0.0) |
| Tomudex | 1 (7.1) | 0 (0.0) |
| Topotecan | 0 (0.0) | 1 (7.7) |
| Immunotherapy | ||
| Nivolumab | 0 (0.0) | 1 (7.7) |
| Survival after diagnosis | ||
| 1 year | 9 (64.3) | 9 (69.2) |
| 2 years | 4 (28.6) | 7 (53.8) |
| 3 years | 2 (14.3) | 5 (38.5) |
| Survival after treatment | ||
| 1 year | 9 (64.3) | 8 (61.5) |
| 2 years | 4 (28.6) | 6 (46.2) |
| 3 years | 2 (14.3) | 5 (38.5) |
Median (interquartile range).
3.1.1. Group A
Group A (VATS talc pleurodesis alone) consisted of 14 patients (one female, 13 males) with a median age of 70 years (IQR: 64–75). In this cohort there were six patients (42.8%) who reported asbestos exposure, and nine (57.1%) were confirmed as smokers.
The symptoms at diagnosis they reported were: cough, dyspnea, chest pain, odynophagia, asthenia, shoulder pain, dysphonia, and weight loss; one patient refers no symptoms.
None of the patients underwent neoadjuvant therapy and all patients of Group A underwent surgery for recurrent undiagnosed pleural effusion. Computed Tomography (CT) and Positron Emission Tomography (PET) scans were carried out in all patients after surgery.
Final diagnosis of MPM confirmed nine patients with epithelioid MPM, three with biphasic and two with sarcomatoid. According to pTNM, all patients of Group A were in stage IA or IB. The most common adjuvant chemotherapy regimen was cisplatin alone or in combination to pemetrexed followed by carboplatin and pemetrexed.
3.1.2. Group B
Group B (Video-assisted P/D plus HITHOC) consisted of 13 patients (two females, 11 males), with a median age of 67 years (IQR: 59–74). Five patients (38.5%) reported asbestos exposure, and four patients (30.7%) were from Biancavilla; five patients (35.5%) were confirmed as smokers. The symptoms reported were: cough, dyspnea, chest pain, asthenia, weight loss; one patient refers no symptoms.
CT and PET scans were carried out in all patients before surgery. All patients at the time of surgical treatment had a confirmed diagnosis of MPM - obtained in four patients via thoracentesis (cytological diagnosis), in two patients via CT-guided biopsy, in seven patients after video-assisted-biopsy. Epithelioid MPM was diagnosed in nine patients, biphasic in three and sarcomatoid in one patient. Seven patients underwent neoadjuvant therapy. The median time between diagnosis and surgery was 2 (IQR: 1–6) months.
According to pTNM staging, all patients in Group B were in stage IA or IB, except one patient in stage IIIB (as assessed post-operation). Several wedge resections were performed in three patients, diaphragmatic reconstruction with a patch in two, and rib resection in one. The most common adjuvant chemotherapy regimens were cisplatin alone given and the combination of carboplatin and pemetrexed, immunotherapy was performed in one patient.
3.2. Outcomes of the operations
Median surgery time for Group A was 65.2 min (IQR: 46.8–79.0). Median operative time, from the time of skin incision to the end of perfusion for Group B, was 316 min (IQR: 224–425 min). The median hospital stay was 6 (IQR: 5–10) and 8 (IQR: 6–10) days for Group A and Group B, respectively. There was no incidence of intra-operative complications in any of the 27 patients. Post-operative complication rate was 8 (51.7%) and 7 (53.9%) in Group A and B, respectively (Table 2). In five patients of the Group B the mini-thoracotomy was enlarged due to tight adhesions.
Table 2.
Detailed information of post-operative complications in the study population divided by intervention arm (Group A: VATS talc pleurodesis arm; Group B: Video-assisted P/D and HITHOC arm).
| Complications | N (%) |
|---|---|
| Group A (N = 14) | |
| Incomplete pulmonary expansion | 4 (28.6) |
| Melaena | 1 (7.1) |
| Hemothorax | 1 (7.1) |
| Pleural effusion | 2 (14.3) |
| Group B (N = 13) | |
| Anemia | 2 (15.4) |
| Tingle in the lips and nausea | 1 (7.7) |
| Postural and stay tremor | 1 (7.7) |
| Tachyarrhythmia | 1 (7.7) |
| Syncope | 1 (7.7) |
| Persistent Air-leak | 1 (7.7) |
Four patients (28.6%) of Group A presented with incomplete pulmonary expansion after uniportal VATS talc pleurodesis without affecting the implementation of the procedure in all patients. Further surgical intervention for post-operative complications was not necessary as well as we did not register in-hospital mortality. Similarly, no mortality was documented after 1 month and 2 months after the procedures. One patient subsequently underwent two further operations due to tumor recurrence. None of the patients presented renal failure after the interventions.
3.3. Follow-up
The median follow-up time was 19 months (IQR: 9–32 months) for Group A, with one patient (7.1%) out of 14 patients still alive at the end of follow-up. The median follow-up time of 28 months (IQR: 11–56 months) for Group B, with four patients (30.8%) out of 13 patients still alive at the end of follow-up.
3.4. Survival rates of participants at 1, 2, and 3 years
Table 3 shows the complete details of the follow-up after surgical procedures for both groups after 3, 6, 12, 24, 36 months. In Group A, the survival times after diagnosis and treatment were identical as diagnosis was performed at the same time of the treatment (talc pleurodesis). Conversely for the Group B, treatment was performed from 15 days up to 6 months after diagnosis, and therefore survival after treatment did not match at the survival after diagnosis, but survival times reported in Table 3 are calculated from the time of surgical procedure from all subjects.
Table 3.
Survivors during follow-up (months) from the date of the surgical procedure divided by intervention arm (Group A: VATS talc pleurodesis arm; Group B: Video-assisted P/D and HITHOC arm). Values are number and percentage (%).
| Follow-up time | Group A (N = 14) | Group B (N = 13) |
|---|---|---|
| 3 months | 14 (100) | 13 (100) |
| 6 months | 12 (85.7) | 11 (84.6) |
| 12 months | 9 (64.3) | 9 (69.2) |
| 24 months | 3 (21.4) | 7 (53.8) |
| 36 months | 1 (7.1) | 4 (30.8) |
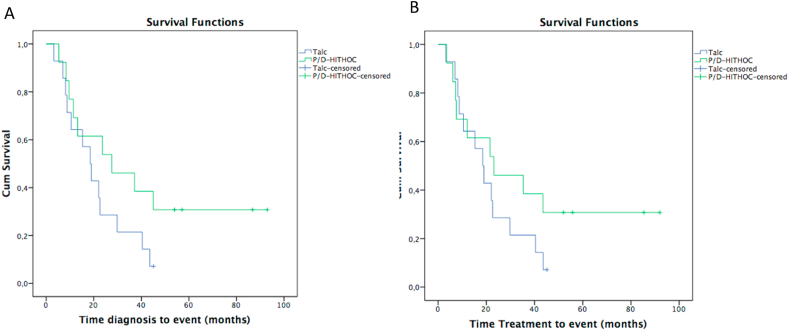
The most important difference in terms of survival from time diagnosis, even if imprecise, was detected after 20 months when the median overall survival started to diverge. The median overall survival time after diagnosis for Group A was 19 months (95% CI: 12–25) versus 28 months (95% CI: 0–56) of Group B (Fig. 3A); whereas median overall survival after treatment for Group A was 19 months (95% CI: 12–25) versus 23 months (95% CI: 0–50) of Group B (Fig. 3B). The HR for overall survival, at time diagnosis, between Groups A and B was 0.52 (95% CI: 0.22–1.23) in the crude model, and HR 0.15 (95% CI: 0.02–0.91) after adjustment for age, sex, histological subtype TNM (one patient stage T1N1M0 of Group A and one patient T4N0M0 of the Group B have not been included in the analysis because they were present only in one group), stage (IA, IB; one patient staged IIIB has not been included in the analysis because there was no corresponding patient in the other group) and asbestos exposure.
Fig. 3.
Kaplan-Meier curves comparing VATS talc pleurodesis (Group A) and Video-assisted P/D-HITHOC (Group B) at time diagnosis (A) and at time treatment (B).
Median overall survival time from diagnosis for Group A was 19 months (95% CI: 1.6–36) and 15 months (95% CI: 4–26) versus 28 months (95% CI: 9–46) and 11 months (95% CI: 7–15) for Group B for non-smokers and smokers, respectively.
For non-smoking patients in Group A, median overall survival time from treatment was 19 months (95% CI: 1.6–36) and 15 months (95% CI: 4–26) for smokers. In Group B, median overall survival time from treatment was 23 months (95% CI: 4–42) for non-smokers and 8 months (95% CI: 4–11) for smokers. In non-smoking patients, the risk was reported to be 65% lower (HR = 0.35, 95% CI: 0.09–1.33).
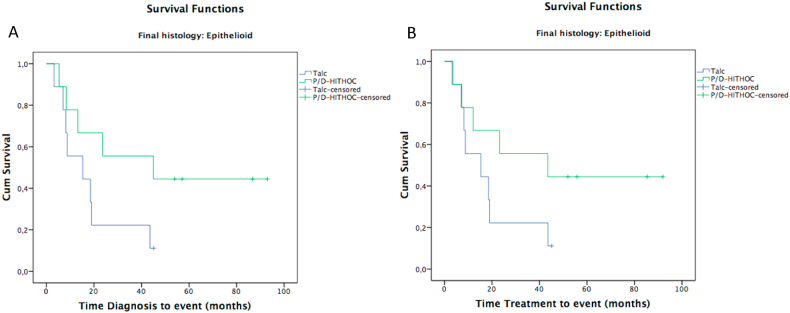
Patients with epithelioid MPM histology showed a median survival of 15 months (IQR: 0–34) in Group A and 45 months (IQR: 0–107) in Group B. The presence of epithelioid MPM post diagnosis (HR = 0.87, 95% CI: 0.32–2.4) and after treatment (HR = 0.77, 95% CI: 0.28–2.2), showed a risk of death lower than patients with biphasic histology (Fig. 4A and B, Table 4); whereas patients with sarcomatoid MPM histology both at the time of diagnosis (HR = 1.84, 95% CI: 0.44–7.70) and after treatment (HR = 1.62, 95% CI: 0.39–6.80) showed a risk of death higher than patients with a biphasic histology (Table 4).
Fig. 4.
Kaplan-Meier curves comparing video-assisted P/D-HITHOC (Group B) and VATS talc pleurodesis (Group A) at time diagnosis (A) and time treatment (B), by pleurodesis of epithelioid mesothelioma.
Table 4.
Median survival months and hazard ratio (HR) with their 95% confidence interval (CI) according to final histology divided by intervention arm (Group A: VATS talc pleurodesis arm; Group B: Video-assisted P/D and HITHOC arm).
| Histology | N | Time diagnosis |
Time treatment |
HR (95% CI) a |
|||
|---|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Time treatment | Time diagnosis | ||
| Biphasic | 6 | 30 (17–42) | 28 (1.8–53) | 30 (17–42) | 22 (0–46) | Ref. | Ref. |
| Epithelioid | 18 | 15 (0–34) | 45 (0–107) | 15 (0–34) | 43 (0–103) | 0.77 (0.28–2.2) | 0.87 (0.32–2.4) |
| Sarcomatoid | 3 | 11a | 10a | 11a | 8a | 1.62 (0.39–6.8) | 1.84 (0.44–7.7) |
Confidence interval not reported due to low frequency.
3.5. Sample size calculation for the future trial
Considering the HR for overall survival found in this pilot trial of 0.52, the needed samples size for a future trial, based on alfa significance level of 0.05 and 80% of power [22], should be of 145 patients with approximately 73 events/deaths.
4. Discussion
After years of skepticism regarding surgical treatment of patient with mesothelioma, a more positive era began in the late 1980s and alternative surgical treatment protocols started to be trialed with the aim at improving surgical outcomes for this aggressive cancer. Unfortunately, all studies to date confirmed that the role of surgery remains very uncertain due, in part, to the lack of large prospective randomized trials, which might confirm its role [[23], [24], [25], [26]]. In truth, few randomized controlled trials comparing procedures for MPM, ranging from radical (EPP) to less radical video-assisted P/D or VATS talc pleurodesis have been done, and none of them represents the gold standard for the treatment of MPM [5,6]. The questions on “why surgery does not work in Malignant Pleural Mesothelioma” (which was posed in an editorial of few years ago) remains relevant [27], and from reading the literature it is evident that extended operations for MPM such as extra pleural pneumonectomy should definitively be abandoned because long term survival is not enhanced, and in-hospital mortality is unacceptable when compared with those results obtained with less invasive approaches [28].
Thoracic surgeons are aware that in mesothelioma even the most aggressive surgery treatment cannot guarantee removal of all residual cancer cells. The main objective of the HITHOC treatment has therefore been to completely eradicate any remaining cancer cells [[10], [11], [12]]. A large study on video-assisted P/D plus HITHOC was carried out at Brigham and Women's Hospital, Harvard Medical School, Boston, in 2013 [29]. It enrolled 103 patients with a diagnosis of MPM who were treated with video-assisted P/D via a thoracotomy: 72 patients received HITHOC (Group A) and 31 patients received no HITHOC (Group B). Group A showed an overall survival time of 35.3 months compared with 22.8 months for Group B. Although these results have not been confirmed by other centers, many groups have shown an important increase in survival with a good quality of life [[30], [31], [32], [33], [34]].
Our study differs from the Harvard study and many others, as all our patients in the group B underwent video-assisted mini-thoracotomy of 10–12 cm instead of a large thoracotomy with rib removal. With this minimally invasive strategy, a zero mortality has been confirmed along with a low complication rate, low general toxicity and a total preservation of respiratory function. Moreover, another reported advantage is that this minimally invasive technique could stimulate a positive immunological patient reaction [35]. Although the duration of the VATS talc pleurodesis alone demonstrated lower surgical time in line with previous studies [36] compared to video-assisted P/D and HITHOC, it is important to note that in both Group A and Group B of our study, the difference in the amount of time spent in surgery did not affect the duration of hospital stay or postoperative complications, demonstrating the safety of the combination of video-assisted P/D and HITHOC.
We calculated survival time after diagnosis and after treatment, and the overall survival time increased in favor of video-assisted P/D plus HITHOC with a median survival of 28 months and with 30% of patients still alive at the end of follow-up on December 31, 2020. This information is important as it reinforces that overall survival could be further prolonged, and also justifies the undertaking of a larger randomized-controlled trial.
It is also noteworthy that in Group B, the median survival time in the 9 patients with epithelioid MPM undergoing video-assisted P/D plus HITHOC, was 45 months, versus 15 months in the 9 patients with epithelioid MPM undergoing VATS talc pleurodesis. It is certainly not new that patients with epithelioid MPM survive longer than those with other types of MPM, but the long-term results from our study are encouraging for the future treatment of this aggressive cancer. Nevertheless, the event that no long term survival was obtained in the no-epithelioid group suggests that HITHOC may be beneficial for epithelioid but not biphasic or sarcomatoid pleural mesothelioma. As HITHOC is part of the multimodality treatment for MPM, it is important to note that in our paper there was no inequality in the use of adjuvant treatment between both groups. Adjuvant chemotherapy was given for each patient and most of them received cisplatin, carboplatin and pemetrexed. Immunotherapy was given only in one patient later in the study.
In the future study we should pay more attention to Biancavilla, a town located in a volcanic area of eastern Sicily of special interest because of a cluster of mesothelioma cases, where our results suggest a longer survival for these patients. This could be due to the type of treatment (Video-assisted P/D plus HITHOC), to the smoking habits, but also to a different carcinogenic effect of the fibres found in Biancavilla (fluoro-edenite).
Limitations of our study include the small number of patients. To overcome this limitation, the participation in the future study will also be offered both to national and international specialist centers. In our study, approximately more than half of the eligible centers agreed to the study. This information is important for planning the future trial and because the involvement of other centers would be helpful not only to increase the sample size but because this methodology could also be applied in research settings other than the future definitive RCT. Although neoadjuvant, adjuvant chemo-radio or immunotherapy have been administered to the patients, we have no specific information about doses for every single patient. In addition, the different administration of neo-adjuvant and adjuvant therapy may have somewhat affected the results, despite the decision of the type of therapy was related to the specific modality of diagnosis, i.e. intra-operative for Group A and pre-operative for Group B, respectively. Another limitation is that we did not know how long the patients had quit smoking, so we have to add to the questionnaire more specific questions regarding smoking habits. A potential source of bias could be that the house staff (nurses and specialized nurses) of the Centre A has not been formally educated about the study while in the Centre B house staff was informed about the pilot trial. This difference could result in a better care in the Centre B. To avoid remaining uncertainty about feasibility, the described potential bias will be resolved informing the staff about the project. Nonetheless, it should be noted that physicians and surgeons were formally and adequately educated about the trial, thus decreasing sources of bias due to different care of patients.
5. Conclusions
In summary, this pilot study represents an important step forward demonstrating the feasibility for a multicenter randomized trial to compare two alterative palliative treatments (Video-assisted P/D plus HITHOC versus VATS talc pleurodesis) in the management of MPM, and to estimate the benefits on patients’ quality and quantity of life.
6. Institutional review board statement
Information about the procedure were freely available to the public domain and approved by the University Research Committee and by the Clinical Directorate (no. 2053 April 20, 2013 Chief Institutional Board of the Policlinico-Vittorio Emanuele Hospital). Written informed consent was obtained from all patients with detailed information to the possible risks and benefits of the procedures. Data were acquired in compliance with GDPR regulation (General Data Protection Regulation, European Union 2016/679) and the study was retrospectively registered in an International Database (ISRCTN12709516).
Author contribution statement
MM conceived and designed the experiments; MM, SC, CS, FB, MN; performed the experiments; MM, MFi, TF, MS, RT, RP and PV analyzed and interpreted the data; MM, MFi, MFe, TF, MS wrote the paper with contribution of all other authors. All authors approved the final manuscript.
Funding statement
This study has been funded in part by the University of Catania (Catania, Italy) 409 within the FIR Research Program 2014 no. DF84A9. TF was supported by grant “UNIMORE FAR 410 2022 Mission Oriented, Linea FOMO-Fondazione di Modena”.
Data availability statement
Data are referred to human subjects and cannot be made freely available, but data supporting the findings of this study may be available on reasonable request. A statement has been included in the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
This study is dedicated to all Pioneer Sicilian Patients affected with MPM who volunteered accepted the operation of video-assisted P/D and HITHOC at the University of Catania hospital, and to those who cannot be operated. In the memory of those who could not be helped. We thank the surgeons, doctors, nurses, radiographers, pathologists at the participating centers. We thank The Policlinico “Rodolico” Hospital for financial support covering the cost of hyperthermic intrathoracic chemotherapy. Collaborators PubMed citable Study Group on Malignant Pleural Mesothelioma (alphabetic order): Marco Aiello, Marinella Astuto, Massimo Cajozzo, Giovanna Fantaci, Ines Monte, Tommaso Nicolosi, Hector Soto Parra, Cristina Scuderi.
References
- 1.Scherpereel A., Astoul P., Baas P., Berghmans T., Clayson H., de Vuyst P., Dienemann H., Galateau-Salle F., Hennequin C., Hillerdal G., Le Péchoux C., Mutti L., Pairon J.-C., Stahel R., van Houtte P., van Meerbeeck J., Waller D., Weder W. European respiratory society/European society of thoracic surgeons task force. Guidelines of the European respiratory society and the European society of thoracic surgeons for the management of malignant pleural mesothelioma. Eur. Respir. J. 2010;35:479–495. doi: 10.1183/09031936.00063109. [DOI] [PubMed] [Google Scholar]
- 2.Nuyts V., Nawrot T., Nemery B., Nackaerts K. Hotspots of malignant pleural mesothelioma in Western Europe. Transl. Lung Cancer Res. 2018;7(5):516–519. doi: 10.21037/tlcr.2018.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paoletti L., Batisti D., Bruno C., Di Paola M., Gianfagna A., Mastrantonio M., Nesti M., Comba P. Unusually high incidence of malignant pleural mesothelioma in a town of eastern Sicily: an epidemiological and environmental study. Arch. Environ. Health. 2000;55(6):392–398. doi: 10.1080/00039890009604036. [DOI] [PubMed] [Google Scholar]
- 4.Delgermaa V., Takahashi K., Park E.K., Le G.V., Hara T., Sorahan T. Global mesothelioma deaths reported to the world health organization between 1994 and 2008. Bull. World Health Organ. 2011;89(10):716–724. doi: 10.2471/BLT.11.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treasure T., Lang-Lazdunski L., Waller D., Bliss J.M., Tan C., Entwisle J., Snee M., O'Brien M., Thomas G., Senan S., O'Byrne K., Kilburn L.S., Spicer J., Landau D., Edwards J., Coombes G., Darlison L., Peto J. MARS trialists. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12(8):763–772. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rintoul R.C., Ritchie A.J., Edwards J.G., Waller D.A., Coonar A.S., Bennett M., Lovato E., Hughes V., Fox-Rushby J.A., Sharples L.D., MesoVATS Collaborators Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet. 2014;384(9948):1118–1127. doi: 10.1016/S0140-6736(14)60418-9. [DOI] [PubMed] [Google Scholar]
- 7.Halstead J.C., Lim E., Venkateswaran R.M., Charman S.C., Goddard M., Ritchie A.J. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur. J. Surg. Oncol. 2005;31(3):314–320. doi: 10.1016/j.ejso.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhou N., Rice D.C., Tsao A.S., Lee P.P., Haymaker C.L., Corsini E.M., Antonoff M.B., Hofstetter W.L., Rajaram R., Roth J.A., Swisher S.G., Vaporciyan A.A., Walsh G.L., Mehran R.J., Sepesi B. Extrapleural pneumonectomy versus pleurectomy/decortication for malignant pleural mesothelioma. Ann. Thorac. Surg. 2022;113(1):200–208. doi: 10.1016/j.athoracsur.2021.04.078. [DOI] [PubMed] [Google Scholar]
- 9.Järvinen T., Paajanen J., Ilonen I., Räsänen J. Hyperthermic intrathoracic chemoperfusion for malignant pleural mesothelioma: systematic review and meta-analysis. Cancers. 2021;13(14):3637. doi: 10.3390/cancers13143637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migliore M., Albalkhi I., Alshammari A., Aldebakey H., Alashgar O., Gastardi J.C., Santana-Rodríguez N. A glimpse into the role of debulking surgery and hyperthermic intrathoracic chemotherapy (HITHOC) in the management of malignant pleural mesothelioma. Shanghai Chest. 2022;6:7681. doi: 10.21037/shc-22-22. [DOI] [Google Scholar]
- 11.Migliore M., Ried M., Molins L., Lucchi M., Ambrogi M., Molnar T.F., Hofmann H.S. Hyperthermic intrathoracic chemotherapy (HITHOC) should be included in the guidelines for malignant pleural mesothelioma. Ann. Transl. Med. 2020;9(11):960. doi: 10.21037/atm-20-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson A.G., Kutywayo K., Mohammed S.B., Fennel D.A., Apostolos N. Cytoreductive surgery with hyperthermic intrathoracic chemotherapy for malignant pleural mesothelioma: a systematic review. Thorax. 2023;78(4):409–417. doi: 10.1136/thoraxjnl-2021-218214. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z.Y., Zhao S.S., Ren M., Liu Z.L., Li Z., Yang L. Effect of hyperthermic intrathoracic chemotherapy on the malignant pleural mesothelioma: a systematic review and meta-analysis. Oncotarget. 2017;8(59):100640–100647. doi: 10.18632/oncotarget.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marinaccio A., Corfiati M., Binazzi A., Di Marzio D., Bonafede M., Verardo M., Migliore E., Gennaro V., Mensi C., Schallemberg G., Mazzoleni G., Fedeli U., Negro C., Romanelli A., Chellini E., Grappasonni I., Pascucci C., Madeo G., Romeo E., Trafficante L., Carrozza F., Angelillo I.F., Cavone D., Cauzillo G., Tallarigo F., Tumino R., Melis M., ReNaM Working Group The epidemiological surveillance of malignant mesothelioma in Italy (1993-2015): methods, findings, and research perspectives. Epidemiol. Prev. 2020;44(1):23–30. doi: 10.19191/EP20.1.P023.014. [DOI] [PubMed] [Google Scholar]
- 15.Migliore M. Efficacy and safety of single-trocar technique for minimally invasive surgery of the chest in the treatment of noncomplex pleural disease. J. Thorac. Cardiovasc. Surg. 2003;126(5):1618–1623. doi: 10.1016/s0022-5223(03)00592-0. [DOI] [PubMed] [Google Scholar]
- 16.Migliore M., Deodato G. Thoracoscopic surgery, video-thoracoscopic surgery, or VATS: a confusion in definition. Ann. Thorac. Surg. 2000;69(6):1990–1991. doi: 10.1016/s0003-4975(00)01302-3. [DOI] [PubMed] [Google Scholar]
- 17.Flores R.M., Pass H.I., Seshan V.E., Dycoco J., Zakowski M., Carbone M., Bains M.S., Rusch V.W. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J. Thorac. Cardiovasc. Surg. 2008;135(3):620–626. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 18.Nakas A., Martin Ucar A.E., Edwards J.G., Waller D.A. The role of video assisted thoracoscopic pleurectomy/decortication in the therapeutic management of malignant pleural mesothelioma. Europ. J. Cardiothorac. Surg. 2008;33(1):83–88. doi: 10.1016/j.ejcts.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Julious S.A. Sample size of 12 per Group rule of thumb for a pilot study. Pharmaceut. Stat. 2005;4(4):287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 20.Eldridge S.M., Chan C.L., Campbell M.J., Bond C.M., Hopewell S., Thabane L., Lancaster G.A. PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobin L.H., Gospodarowicz M.K., Wittekind Ch. seventh ed. John Wiley & Sons Ltd; Chichester, UK: 2010. TNM Classification of Malignant Tumours. [Google Scholar]
- 22.Fiorentino F., Treasure T. Sample size calculations for randomized controlled trials and for prediction models. Colorectal Dis. 2021;23:316–319. doi: 10.1111/codi.15489. [DOI] [PubMed] [Google Scholar]
- 23.Bovolato P., Casadio C., Billè A., Ardissone F., Santambrogio L., Ratto G.B., Garofalo G., Bedini A.V., Garassino M., Porcu L., Torri V., Pastorino U. Does surgery improve survival of patients with malignant pleural mesothelioma? a multicenter retrospective analysis of 1365 consecutive patients. J. Thorac. Oncol. 2014;9(3):390–396. doi: 10.1097/JTO.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 24.Pass H.I., Temeck B.K., Kranda K., Thomas G., Russo A., Smith P., Friauf W., Steinberg S.M. Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann. Surg Oncol. 1997;4(8):628–633. doi: 10.1007/BF02303746. [DOI] [PubMed] [Google Scholar]
- 25.Woodard G.A., Jablons D.M. Surgery for pleural mesothelioma, when it is indicated and why: arguments against surgery for malignant pleural mesothelioma. Transl. Lung Cancer Res. 2020;9(Suppl 1):S86–S91. doi: 10.21037/tlcr.2020.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opitz I., Lauk O., Meerang M., Jetter A., Aeschlimann B., Seifert B., Günther D., Stahel R.A., Weder W. Intracavitary cisplatin-fibrin chemotherapy after surgery for malignant pleural mesothelioma: a phase I trial. J. Thorac. Cardiovasc. Surg. 2020;159(1):330–340. doi: 10.1016/j.jtcvs.2019.07.073. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y.C. Surgical resection of mesothelioma: an evidence-free practice. Lancet. 2014;384(9948):1080–1081. doi: 10.1016/S0140-6736(14)60795-9. [DOI] [PubMed] [Google Scholar]
- 28.Batirel H.F., Metintas M., Caglar H.B., Ak G., Yumuk P.F., Yildizeli B., Yuksel M. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J. Thorac. Cardiovasc. Surg. 2016;151(2):478–484. doi: 10.1016/j.jtcvs.2015.09.121. [DOI] [PubMed] [Google Scholar]
- 29.Sugarbaker D.J., Gill R.R., Yeap B.Y., Wolf A.S., DaSilva M.C., Baldini E.H., Bueno R., Richards W.G. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J. Thorac. Cardiovasc. Surg. 2013;145(4):955–963. doi: 10.1016/j.jtcvs.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Migliore M., Calvo D., Criscione A., Palmucci S., Fuccio Sanzà G., Caltabiano R., Spatola C., Privitera G., Aiello M.M., Parra H.S., Ciancio N., Di Maria G. Pleurectomy/decortication and hyperthermic intrapleural chemotherapy for malignant pleural mesothelioma: initial experience. Future Oncol. 2015;11(24 Suppl):19–22. doi: 10.2217/fon.15.286. [DOI] [PubMed] [Google Scholar]
- 31.Ried M., Potzger T., Braune N., Diez C., Neu R., Sziklavari Z., Schalke B., Hofmann H.S. Local and systemic exposure of cisplatin during hyperthermic intrathoracic chemotherapy perfusion after pleurectomy and decortication for treatment of pleural malignancies. J. Surg. Oncol. 2013;107(7):735–740. doi: 10.1002/jso.23321. [DOI] [PubMed] [Google Scholar]
- 32.Ambrogi M.C., Bertoglio P., Aprile V., Chella A., Korasidis S., Fontanini G., Fanucchi O., Lucchi M., Mussi A. Diaphragm and lung–preserving surgery with hyperthermic chemotherapy for malignant pleural mesothelioma: a 10-year experience. J. Thorac. Cardiovasc. Surg. 2018;155(4):1857–1866. doi: 10.1016/j.jtcvs.2017.10.070. [DOI] [PubMed] [Google Scholar]
- 33.Burt B.M., Richards W.G., Lee H.S., Bartel S., Dasilva M.C., Gill R.R., Jaklitsch M.T., Johnson B.E., Swanson S.J., Bueno R., Sugarbaker D.J. A phase I trial of surgical resection and intraoperative hyperthermic cisplatin and gemcitabine for pleural mesothelioma. J. Thorac. Oncol. 2018;13(9):1400–1409. doi: 10.1016/j.jtho.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Aprile V., Lenzini A., Lococo F., Bacchin D., Korasidis S., Mastromarino M.G., Guglielmi G., Palmiero G., Ambrogi M.C., Lucchi M. Hyperthermic intrathoracic chemotherapy for malignant pleural mesothelioma: the forefront of surgery-based multimodality treatment. J. Clin. Med. 2021;10(17):3801. doi: 10.3390/jcm10173801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker W.S., Leaver H.A. Immunologic and stress responses following video-assisted thoracic surgery and open pulmonary lobectomy in early stage lung cancer. Thorac. Surg. Clin. 2007;17(2):241–249. doi: 10.1016/j.thorsurg.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Cardillo G., Facciolo F., Carbone L., Regal M., Corzani F., Ricci A., Di Martino M., Martelli M. Long-term follow-up of video-assisted talc pleurodesis in malignant recurrent pleural effusions. Eur. J. Cardio. Thorac. Surg. 2002;21(2):302–306. doi: 10.1016/s1010-7940(01)01130-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are referred to human subjects and cannot be made freely available, but data supporting the findings of this study may be available on reasonable request. A statement has been included in the manuscript.