Abstract
Diabetes is one of the most prevalent epidemic metabolic disorders, responsible for a significant amount of physical, psychological and economic loss in human society. Diabetic foot ulcer (DFU) is one of the extreme pathophysiological consequences of diabetes. Bacterial infection is the most important cause of chronic DFU. Bacterial species or their biofilms show multidrug resistance, which complicates DFU and consequently leads to amputation of the infected part. Since the Indian population comprises diverse ethnic and cultural groups, this could influence the aetiology of diabetic foot infections and bacterial diversity. We reviewed 56 articles published from 2005 to 2022 on the microbiology of DFU and extracted the data on study location, number of patients analysed in the study, pathophysiological complications, age of the patients, sex of the patient, type of bacteria, type of infection (mono or polymicrobial), predominant bacteria (Gram-positive or Gram-negative), predominant isolates and multiple drug resistance (tested or not). We analysed data and described aetiological trends in diabetic foot infections and bacterial diversity. The study revealed that Gram-negative bacteria are predominant as compared to Gram-positive bacteria in individuals with diabetes with DFU in India. Escherichia coli, Pseudomonas aeruginosa, Klebsiella sp. and Proteus sp. were the most predominant Gram-negative bacteria, while Staphylococcus aureus and Enterococcus sp. were the major Gram-positive bacteria in DFU. We discuss bacterial infections in DFU in the context of bacterial diversity, sampling methods, demography and aetiology.
Keywords: Bacterial diversity, diabetic foot ulcer, epidemiology, aetiology, Gram-negative bacteria
DIABETIC FOOT ULCER
Diabetes is one of the worst global health crises of the century and the ninth major cause of death worldwide, claiming 1.6 million fatalities in 2019.[1-4] Diabetes has several adverse metabolic consequences, which further develop pathophysiological complications including foot ulcers, neuropathy, and atherosclerosis.[5] Nearly 12–25% of individuals with diabetes are prone to developing diabetic foot ulcers (DFUs).[6,7] DFU is a complex cellulitis or osteomyelitis situation caused by interaction between the host immune system and colonizing bacteria,[8] which has devastating consequences on health, economy and psychology. Once DFUs are infected with external agents, mainly bacteria, the situation gets worse, and finally, patients are advised to get hospitalized. It is estimated that approximately 44–68% of patients admitted to hospitals develop osteomyelitis, which eventually leads to amputation of the infected part.[9,10] To avoid further complications, inclusive therapies comprising the use of antibiotics, neuropathic drugs, growth factors and inflammatory modulators have been suggested.[11,12] However, one of the major obstacles to treating DFU is bacterial colonization and antibiotic resistance.
Microbiology of DFU
Recent studies reported that bacterial infection plays a central role in the chronicity of DFU.[13] DFU generally gets infected by skin surface bacteria and further establishes colonies with complex bacterial polycultures. Although the skin surface is a common source for bacterial introduction in DFU, the environment created by early invaders eventually accommodates obligatory non-native bacteria.[14,15] Bacteria inhibiting DFU sometimes secrete toxins. The toxins secreted by bacteria increase the severity of wounds and hamper the healing process.[16,17]
In addition, the isolation of pathogenic bacteria or bacterial strains is another hindrance to DFU treatments. It is difficult to determine the role of individual bacteria or a combination of different bacterial species in DFU infections. Bacteria that may not be harmful can provide a platform for other pathogenic bacteria.[18,19] A combination of collaborating bacteria synchronizes and forms functional pathogenic groups, which are essentially responsible for the maintenance of chronic DFU.[20] The symbiotic association of various co-aggregated bacteria acts synergistically to form the biofilm.[13] Such bacterial infections are resistant to anti-microbial treatments, interfere with the host’s immune system, increase the chronicity of DFU and delay healing. In more than 70% of DFU cases, bacterial infections are found to be multidrug resistant.[21,22] Therefore, it is necessary to identify bacterial diversity, biofilm existence and multidrug resistance while treating chronic DFU.[23] The microbiology of DFU has been studied and fairly discussed in the literature; however, the Indian scenario has never been discussed.[7,14,23]
Indian scenario
India is one of the leading countries, with more than 77 million individuals with diabetes, and that number is estimated to rise to 35.7 million by 2045. Diabetes is prevalent in 8.9% of the Indian population, with an estimated 1 million diabetes-related deaths each year.[1,24] Singh[7] and Shankhdhar et al.[25] estimated that nearly 25% of individuals with diabetes patients in India will develop DFU. This situation may degrade further owing to lack of general awareness, medical infrastructure and economic limitations.[26,27] The Indian population comprises diverse ethnic and genetic groups, which may have a considerable influence on the aetiology of diabetes, physiological consequences and responses to diabetic treatments.[24,28,29] India harbours a diverse cultural population in different geographical regions with variations in cultural beliefs and sanitary practices.[30] Therefore, there may be aetiological and epidemiological differences in diabetes-related complications (including DFU), which need to be investigated. Currently, DFU-related problems are rising in India and are associated with the prevalence of diabetes.[31,32] Several factors, including socioeconomics and lifestyle, contribute to the occurrence of DFU in India.[33] Moreover, the aetiological trends and the demography of DFU have never been discussed from the Indian perspective.[15,30,31]
OBJECTIVE
In this article, we reviewed studies on the microbiology of diabetic foot infections in India. We summarized demographic trends in aetiology and bacterial diversity in DFU.
METHODS
We searched for articles related to the current topic in four databases, Web of Science, PubMed, Google Scholar and Science Direct, using key words ‘diabetic foot ulcer’, ‘diabetic foot infection’, ‘microbiology’, ‘bacteria’, and ‘India’ with ‘and’ and ‘or’ Boolean operators. We selected articles indexed in PubMed, Web of Science and the journals indexed in Scopus. We searched for articles published from January 1980 to July 2022. In addition, separately for each year, we searched for articles related to the present topic in Google Scholar using the combinations of phrases and words mentioned above. In Google Scholar searches, we screened the first hundred articles published in each year. Among the total number of articles searched, we screened for research articles on the microbiology of DFU in India. We included a total of 56 studies for further analysis. From the selected articles, we extracted information on the study location, number of patients analysed in the study, pathophysiological complications, number of DFU positive for bacterial infection, age of the patients, sex of the patients, type of bacterial species (mono or polymicrobial), type of predominant bacteria (Gram-positive or Gram-negative), predominant isolates and multiple drug resistance (tested or not). We presented the extracted data in table and graphical formats.
Demography
The first microbiological study of DFU was reported in 2005 by Sivanmaliappan and Sevanan.[34] Since then, an increasing trend in the number of studies has been observed, with the highest number of studies carried out in 2018 [Figure 1]. Among the 56 studies, the highest number of studies was carried out in South India, followed by North India [Figure 2]. Surprisingly, there are no reports of microbial infection in DFU from central India. DFU microbiological studies are reported from 12 states, among which the highest number of studies are reported from Karnataka, followed by Uttar Pradesh and Tamil Nadu [Figure 3]. All 56 studies were reported from urban cities.
Figure 1.
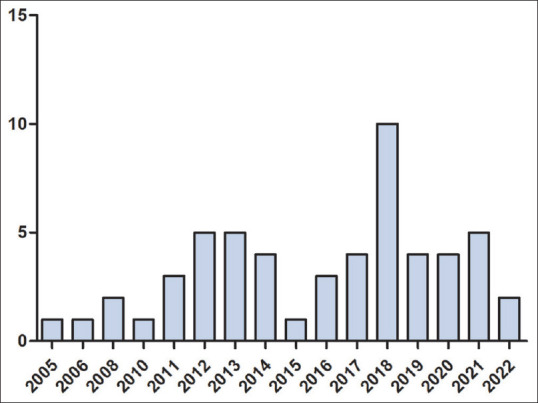
The chronological (year-wise) trend in the number of studies (total 56 studies)
Figure 2.

Relative region-wise studies on the microbiology of diabetic foot ulcers in India. SI: South India, NI: North India, WI: West India, EI: East India and N-EI: North-East India
Figure 3.
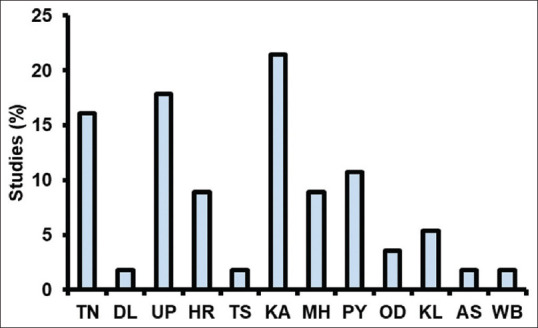
State-wise studies (%) on microbiology of diabetic foot ulcers in India. TN: Tamil Nadu, DL: Delhi, UP: Uttar Pradesh, HR: Haryana, TS: Telangana, KA: Karnataka, MH: Maharashtra, PY: Pondicherry, OD: Odisha, KL: Kerala and AS: Assam
Aetiology
Our analysis revealed that neuropathy and peripheral vascular disease (PVD) were the most common pathological complications in the patients reported with DFU, followed by nephropathy, retinopathy, hypertension and osteomyelitis [Table 1]. Among 56 studies, 6 studies reported the position of the DFU in patients [Table 2]. The heel and toe were the most common sites of DFU in Indian patients [Table 2]. Fifty-three studies reported sampling methods employed to isolate the bacteria [Figure 4]. Most of the studies used tissue samples (58.49% of them) for bacterial isolation, followed by pus samples (47.16%). A percentage of 58.49 of the studies collected samples using swabs [Figure 4].
Table 1.
Pathophysiological complications in the patients with diabetic foot infection in India
| Study | Nephropathy | Neuropathy | PVD | Osteomyelitis | Retinopathy | Hypertension | Ischaemia | Gangrene |
|---|---|---|---|---|---|---|---|---|
| Gadepalli et al. 2006[35] | 75 | 86.2 | 85 | 62.5 | 72.5 | |||
| Shankar et al. 2005[36] | 27.2 | 56.8 | 10.3 | 25.9 | 20.7 | |||
| Zubair et al. 2010[37] | 39 | 66.6 | 55.8 | |||||
| Bansal et al. 2008[38] | 76.6 | 30 | 57 | |||||
| Kumar et al. 2020[39] | 14.1 | 68.2 | 24.7 | 16.4 | 1.1 | 57.5 | 22.3 | |
| ShankarRao et al. 2022[40] | 100 | 45.5 | 84.4 | |||||
| Zubair and Ahmad 2019[41] | 62.85 | |||||||
| Noor et al. 2018[42] | 70 | 58 | 56 | 46 | 90 | 56 | ||
| Kateel et al. 2018[43] | 25 | 35 | 39 | 28.3 | 58.3 | |||
| Shettigar et al. 2018[44] | ||||||||
| Sasikumar et al. 2018[45] | 34.5 | 70.4 | ||||||
| Rastogi et al. 2017[22] | 69.2 | 92 | 23.2 | 64.4 | 92.3 | |||
| Suryaletha et al. 2018[46] | 69 | 24 | ||||||
| Noor et al. 2016[47] | 90.35 | 31.65 | 65.9 | 57 | 74.25 | 42.1 | 38.3 | |
| Malik et al. 2013[48] | 54.4 | 50.6 | 12.3 | 50.6 | 56 | |||
| Banoo et al. 2012[49] | 65 | 23 | 6 | |||||
| Zubair et al. 2011[50] | 62.7 | 46 | 26.4 | 52.9 | 67.6 | |||
| Ramakant et al. 2011[51] | 65 | 58 | 72 | 77 | ||||
| Mohanasoundaram 2012[52] | 25 | 63.2 | 16.1 | |||||
| Patil et al. 2018[53] | 83.5 | 16.5 | 75.4 | |||||
| Raghu et al. 2016[54] | 72.66 | 34 | 16 | 74.7 | 14.7 |
PVD: Peripheral vascular disease
Table 2.
Position of diabetic foot ulcer in Indian patients
| Study | Toe | Sole/Plantar | Heel | Lateral | Interdigit | Ankle | Shin | Dorsum | Metatarsal | Phalynx | Forefoot | Midfoot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shankar et al. 2005[36] | 71 | 27 | ||||||||||
| Zubair et al. 2010[37] | 21.6 | 16.6 | 33.3 | |||||||||
| Kateel et al. 2018[43] | 24 | 20 | 13 | 8 | 17 | 18 | ||||||
| Sasikumar et al. 2018[45] | 18.3 | 23 | 7.7 | 1.9 | 34.6 | |||||||
| Shekhar et al. 2014[55] | 16.7 | 16.7 | 22.2 | |||||||||
| Parvez et al. 2012[56] | 36.7 | 20 | 20 | 26.6 | 16.7 | |||||||
| Elamurugan et al. 2018[57] | 33.33 | |||||||||||
| Patil et al. 2018[53] | 20 | 9 | 15 | 38 |
Figure 4.
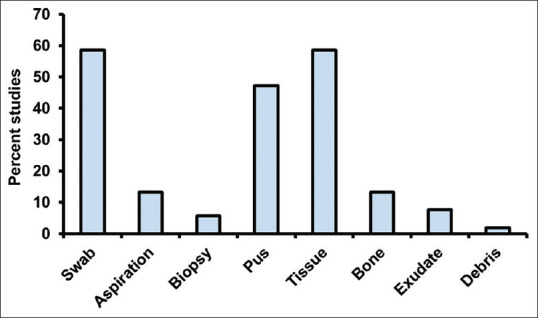
Methods employed for bacterial sampling from diabetic foot ulcers in a total of 53 studies
Overall, male patients were predominantly reported with a bacterial infection in DFU (71.59% males and 28.39% females; SE = 1.41; n = 43). The highest proportion of male patients was reported to be 92.6%,[58] and the lowest proportion of male patients was reported at 54%.[59] The average age of the patients with DFU in India is 56.39 (n = 23 studies). The median age of DFU patients with bacterial infections was 55.4 (n = 20 studies).
Bacterial diversity
Among the patients reported with DFU, 85.01% (SE = 2.34; n = 23) of patients were positive for bacterial infection. The highest proportion of patients positive for microbial infection (100%) was reported by Appapalam et al 2021.,[60] Haldar et al., 2017.[61] and Raghu et al., 2016,[54] while the lowest proportion of patients positive for microbial infection (55.38%) was reported by Seth et al., 2019[62] [Figure 5]. Generally, the number of bacteria isolated in a particular study exceeded the number of patients in the respective study, with an average of 1.66 isolates per patient (SE = 0.16; n = 31). Exceptionally, three studies by Ishwarya et al 2019,[63] Noor et al.[47] and Insan et al. 2013[64] reported fewer isolates than the number of patients studied for bacterial infection in DFU. Further, DFUs were found to be infected by single or multiple bacteria [Figure 5]. Overall, 49.74% (SE = 3.7; n = 33) DFUs were infected by a single bacterium, while 42.99% (SE = 3.65; n = 38) DFUs were infected by multiple bacteria [Figure 5].
Figure 5.
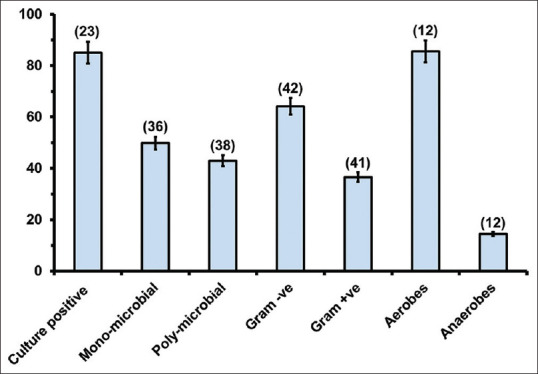
The proportion (mean ± SE) of the number of cases with bacterial infection, mono-microbial infections, polymicrobial infections, Gram-positive and Gram-negative isolates, aerobic and anaerobic isolates. Numbers in parenthesis indicate the number of studies considered for the respective analysis
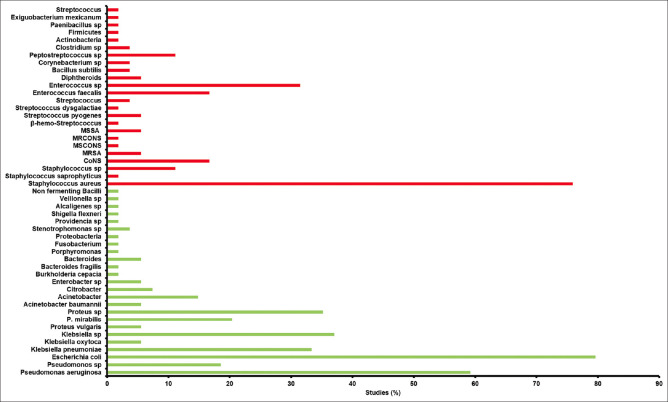
Among the bacterial isolates, Gram-negative bacteria (64.06%; SE = 1.29; n = 42) were predominant as compared to Gram-positive bacteria [36.51%; SE = 1.2; n = 41; Figure 5]. The lowest proportion of Gram-negative isolates (45.5%) was reported by Chitra et al. 2016[65] and the highest proportion of Gram-negative isolates (86.95%) was reported by Shahi et al 2013.[66] Among the studies included in this article, 12 studies isolated both aerobic and anaerobic bacteria. Aerobic isolates (85.6%; SE = 2.8) were predominant as compared to anaerobic (15.11%; SE = 2.8) isolates [Figure 5]. The highest proportion (95.9%) of aerobic isolates was reported by Rastogi et al. 2017,[22] while the highest (31.4%) proportion of anaerobic isolates was reported by Zubair et al. 2011.[50] Among 56 studies, 6 studies used molecular methods to identify isolates from DFU. A total of three studies used 16S rRNA meta-genomic methods for the identification of the total inhabitants in DFU.[36,42,46] Among the Gram-negative bacteria, E. coli was predominantly isolated in 79.62% of studies, followed by P. aeruginosa (59.25%), Klebsiella sp. (37.03%), Proteus sp. (35.18%) and so on [Figure 6]. S. aureus (75.92%) and Enterococcus sp. (31.48%) were the predominant Gram-positive bacteria isolated in different studies [Figure 6]. There is no chronological trend in the reporting of different isolates in different studies [Table 3].
Figure 6.
Predominant isolates reported in a total 54 studies. Predominant isolates are considered those with more than 5% of the total isolates in individual studies. The graph represents the number of studies (%) in which a particular bacterium was predominantly isolated. MRSA: methicillin-resistant Staphylococcus aureus, CoNS: coagulase-negative Staphylococcus, MSCONS: methicillin-sensitive coagulase-negative staphylococci, MRCONS: methicillin-resistant coagulase-negative staphylococci, MSSA: methicillin-susceptible Staphylococcus aureus
Table 3.
Chronological details of the predominant isolates reported in different studies (54 studies)
| Isolates | 2005–2012 | 2013–2017 | 2018–2022 |
|---|---|---|---|
| Pseudomonas aeruginosa | 61.53 | 50 | 65.21 |
| Pseudomonas ssp. | 23.07 | 22.22 | 13.04 |
| Escherichia coli | 92.3 | 77.77 | 73.91 |
| Klebsiella pneumoniae | 53.84 | 16.66 | 34.78 |
| Klebsiella oxytoca | 7.69 | 11.11 | |
| Klebsiella sp. | 38.46 | 33.33 | 39.13 |
| Proteus vulgaris | 15.38 | 4.34 | |
| P. mirabilis | 38.46 | 5.55 | 21.73 |
| Proteus sp. | 53.84 | 38.88 | 21.73 |
| Acinetobacter baumannii | 7.69 | 8.69 | |
| Acinetobacter | 7.69 | 11.11 | 21.73 |
| Citrobacter | 11.11 | 8.69 | |
| Enterobacter sp. | 5.55 | 8.69 | |
| Burkholderia cepacia | 4.34 | ||
| Bacteroides fragilis | 4.34 | ||
| Bacteroides | 7.69 | 11.11 | |
| Porphyromonas | 4.34 | ||
| Fusobacterium | 4.34 | ||
| Proteobacteria | 5.55 | ||
| Stenotrophomonas sp. | 11.11 | ||
| Providencia sp. | 5.55 | ||
| Shigellaflexneri | 5.55 | ||
| Alcaligenes sp. | 5.55 | ||
| Veillonella sp. | 5.55 | ||
| Non fermenting bacilli | 7.69 | ||
| Staphylococcus aureus | 92.3 | 55.55 | 82.6 |
| Staphylococcus saprophyticus | 4.34 | ||
| Staphylococcus sp. | 22.22 | 8.69 | |
| CoNS | 23.07 | 11.11 | 17.39 |
| MRSA | 5.55 | 8.69 | |
| MSCONS | 4.34 | ||
| MRCONS | 4.34 | ||
| MSSA | 7.69 | 5.55 | 4.34 |
| β-Hemo-Streptococcus | 7.69 | ||
| Streptococcus pyogenes | 7.69 | 5.55 | 4.34 |
| Streptococcus dysgalactiae | 4.34 | ||
| Streptococcus | 8.69 | ||
| Enterococcus faecalis | 15.38 | 16.66 | 17.39 |
| Enterococcus sp. | 30.76 | 33.33 | 30.43 |
| Diphtheroids | 5.55 | 8.69 | |
| Bacillus subtilis | 8.69 | ||
| Corynebacterium sp. | 5.55 | 4.34 | |
| Peptostreptococcus sp. | 23.07 | 5.55 | 8.69 |
| Clostridium sp. | 7.69 | 4.34 | |
| Actinobacteria | 5.55 | ||
| Firmicutes | 5.55 | ||
| Paenibacillus sp. | 5.55 | ||
| Exiguobacterium mexicanum | 5.55 | ||
| Streptococcus | 7.69 |
CoNS: Coagulase-negative staphylococci, MRSA: Methicillin-resistant Staphylococcus aureus, MSCONS: Methicillin-sensitive coagulase-negative staphylococci, MRCONS: Methicillin-resistant coagulase-negative staphylococci, MSSA: Methicillin-sensitive S. aureus, The values represent the proportion (%) of the studies reporting predominant bacterial isolates during different years
DISCUSSION
In this article, we summarized the aetiology and microbiology of DFIs and presented their trends. In the Indian population, DFU was reported in 4.5% of patients with newly diagnosed diabetes.[67] The proportion of DFU patients among diabetic patients is much lower in India than that in the Western world.[30,31,68,69] The possible occurrence of low DFU patients could be due to under-reporting, the lack of awareness, younger age or as shorter duration of diabetes.[58,70] Microbiological studies of DFU were reported from urban cities, irrespective of the prevalence of individuals with diabetes patients in those regions.[1] In India, diabetes is more prevalent in urban areas of the states of Tripura, Chandigarh, Tamil Nadu, Jharkhand and Andhra Pradesh.[1] Contrastingly, DFU microbiology studies were predominantly reported from Karnataka, Uttar Pradesh and Tamil Nadu. Previously, Vishwanathan et al., 2005,[32] Rastogi and Bhansali 2016[30] and Jayaprakash et al., 2009.[68] reported that neuropathy and PVD were the most common pathophysiological complications in the patients reported with DFU. Following these previous studies, neuropathy and PVD seem to be the most common pathophysiological complications in patients with DFU.
Conventional culture-based methods combined with molecular methods for bacterial identification are important for the proper identification of isolates, their metabolic characterization and the study of their drug resistance.[71-73] Moreover, advanced genomic methods provide detailed information on the diversity of culturable and non-culturable bacteria,[15] which has implications for understanding the complexity of infection, bacterial co-aggregation and biofilm formation. We identified only 6 studies (of the total of 56 studies included in the present review) in which molecular methods were used to identify bacterial isolates. Among the total number of studies included in this study, a few studies investigated biofilm formation by bacteria inhabiting DFU.[48,50,74-76] Biofilm formation in DFU and its nature are independent of the type and diversity of bacteria, and possibly the result of metabolic cooperation, horizontal gene transfer and so on.[13] Biofilm formation is an important aspect that needs to be further explored extensively to counter the problem of antibacterial drug resistance in the bacteria residing in the DFU. Bacteria inhabiting DFU have shown to be resistant to antibacterial treatment.[12,22,77,78] Among the studies considered for the present review, 45 studies tested multidrug resistance in isolates. Antibiotic resistance in bacteria is a potential cause of chronic DFU.[21,79] Few studies reported the patterns of bacterial diversity in the samples obtained from different tissues[22,56,66,57] and wound properties.[42,60,66,80] Further studies need to consider these important aspects of DFU infection as they provide valuable etiological information necessary for understanding the complexity of infection.
The present review highlights that Gram-negative bacteria were more prevalent in DFU in Indian patients than Gram-positive.[23] Macdonald et al., 2021[23] reported that the prevalence of Gram-positive and Gram-negative bacteria is associated with income status of people. Patients from middle-income and lower middle-income countries were reported to predominantly Gram-negative bacteria. The difference in the prevalence of Gram-positive and Gram-negative bacteria can be further associated with the sanitation and hygiene of the people in their respective countries.[23,51,69]
The bacterial species reported in DFU in various studies differ considerably. E. coli, P. aeruginosa and S. aureus were reported to be the most predominant bacteria in different studies. The aetiological causes of the diversity reported in DFU are diverse and may have links with hygiene practices, cultural diversity, geographical variations, awareness, antibacterial treatment and so on.[69,81,82] Sampling methods are also reported to influence bacterial diversity.[8,83,84] Since the abundance of aerobic/anaerobic and Gram-positive/Gram-negative bacteria reside at different sites of DFU, the sampling methods also contribute to bacterial diversity patterns.[71,57,85] Most of the studies included in the present analysis employed swabs and tissues for bacterial sampling [Figure 4].
This article provides a comprehensive review of an important and neglected diabetes-related complication, diabetic foot infections. We believe that this article has the potential to serve as collective baseline data and trends on the microbiology of DFU, which could help in designing further strategic studies focusing on DFU and anti-microbial therapies. Antibiotic drug resistance and biofilm formation seem to be the most thriving future research areas in DFU in India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69:2932–8. doi: 10.4103/ijo.IJO_1627_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed J, Bain S, Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab Syndr Obes. 2021;14:3567–602. doi: 10.2147/DMSO.S319895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas:Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes. Endocrinol Metab Clin North Am. 2021;50:337–55. doi: 10.1016/j.ecl.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Grunfeld C. Diabetic foot ulcers:Etiology, treatment, and prevention. Adv Intern Med. 1992;37:103–32. [PubMed] [Google Scholar]
- 6.Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased Mortality associated with diabetic foot ulcer. Diabet Med. 1996;13:967–72. doi: 10.1002/(SICI)1096-9136(199611)13:11<967::AID-DIA266>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Singh N. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. Clin Infect Dis. 2004;39(Supplement_2):S83–6. doi: 10.1086/383267. [DOI] [PubMed] [Google Scholar]
- 9.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation:Basis for prevention. Diabetes Care. 1990;13:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 10.van Asten SAV, La Fontaine J, Peters EJG, Bhavan K, Kim PJ, Lavery LA. The microbiome of diabetic foot osteomyelitis. Eur J Clin Microbiol Infect Dis. 2016;35:293–8. doi: 10.1007/s10096-015-2544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 12.Karri VVSR, Kuppusamy G, Talluri SV, Yamjala K, Mannemala SS, Malayandi R. Current and emerging therapies in the management of diabetic foot ulcers. Cur Med Res Opin. 2016;32:519–42. doi: 10.1185/03007995.2015.1128888. [DOI] [PubMed] [Google Scholar]
- 13.Versey Z, da Cruz Nizer WS, Russell E, Zigic S, DeZeeuw KG, Marek JE, et al. Biofilm-innate immune interface:Contribution to chronic wound formation. Front Immunol. 2021;12:648554. doi: 10.3389/fimmu.2021.648554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jneid J, Lavigne JP, La Scola B, Cassir N. The diabetic foot microbiota:A review. HumMicrobiome J. 2017;5-6:1–6. [Google Scholar]
- 15.Noor S, Zubair M, Ahmad J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9:192–9. doi: 10.1016/j.dsx.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Dow G, Browne A, Sibbald RG. Infection in chronic wounds:Controversies in diagnosis and treatment. Ostomy Wound Manage. 1999;45:23–7. 29-40;quiz 41-42. [PubMed] [Google Scholar]
- 17.Dunyach-Remy C, NgbaEssebe C, Sotto A, Lavigne JP. Staphylococcus aureus toxins and diabetic foot ulcers:Role in pathogenesis and interest in diagnosis. Toxins. 2016;8:209. doi: 10.3390/toxins8070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagoba B, Gavkare A, Rayate A, Mumbre S, Rao A, Warad B, et al. Role of an acidic environment in the treatment of diabetic foot infections:A review. World J Diabetes. 2021;12:1539–49. doi: 10.4239/wjd.v12.i9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. Staphylococcus aureus shifts toward commensalism in response to Corynebacteriumspecies. Front Microbiol. 2016;7:1230. doi: 10.3389/fmicb.2016.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobialnature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX ampliconpyrosequencing (bTEFAP) PLoS One. 2008;3:e3326. doi: 10.1371/journal.pone.0003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matta-Gutiérrez G, García-Morales E, García-Álvarez Y, Álvaro-Afonso FJ, Molines-Barroso RJ, Lázaro-Martínez JL. The influence of multidrug-resistant bacteria on clinical outcomes of diabetic foot ulcers:A systematic review. J Clin Med. 2021;10:1948. doi: 10.3390/jcm10091948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastogi A, Sukumar S, Hajela A, Mukherjee S, Dutta P, Bhadada SK, et al. The microbiology of diabetic foot infections in patients recently treated with antibiotic therapy:A prospective study from India. J Diabetes Complications. 2017;31:407–12. doi: 10.1016/j.jdiacomp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections:A meta-analysis. BMC Infect Dis. 2021;21:770. doi: 10.1186/s12879-021-06516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hills AP, Arena R, Khunti K, Yajnik CS, Jayawardena R, Henry CJ, et al. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6:966–78. doi: 10.1016/S2213-8587(18)30204-3. [DOI] [PubMed] [Google Scholar]
- 25.Shankhdhar K, Shankhdhar LK, Shankhdhar U, Shankhdhar S. Diabetic foot problems in India:An overview and potential simple approaches in a developing country. Curr Diab Rep. 2008;8:452–7. doi: 10.1007/s11892-008-0078-y. [DOI] [PubMed] [Google Scholar]
- 26.Beran D. The impact of health systems on diabetes care in low and lower middle income countries. Curr Diab Rep. 2015;15:20. doi: 10.1007/s11892-015-0591-8. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan V, Rao VN. Managing diabetic foot infection in India. Int J Low Extrem Wounds. 2013;12:158–66. doi: 10.1177/1534734613486153. [DOI] [PubMed] [Google Scholar]
- 28.Asharani PV, Lau JH, Roystonn K, Devi F, Peizhi W, Shafie S, et al. Health literacy and diabetes knowledge:A nationwide survey in a multi-ethnic population. Int J Environ Res Public Health. 2021;18:9316. doi: 10.3390/ijerph18179316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh PN, Arthur KN, Orlich MJ, James W, Purty A, Job JS, et al. Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am J ClinNutr. 2014;100(Suppl_1):359S–64S. doi: 10.3945/ajcn.113.071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastogi A, Bhansali A. Diabetic foot infection:An Indian scenario. J Foot Ankle Surg. 2016;3:71–9. [Google Scholar]
- 31.Viswanathan V. Epidemiology of diabetic foot and management of foot problems in India. Int J Low Extrem Wounds. 2010;9:122–6. doi: 10.1177/1534734610380026. [DOI] [PubMed] [Google Scholar]
- 32.Viswanathan V, Thomas N, Tandon N, Asirvatham A, Rajasekar S, Ramachandran A, et al. Profile of diabetic foot complications and its associated complications--a multicentric study from India. J Assoc Physicians India. 2005;53:933–6. [PubMed] [Google Scholar]
- 33.Verma M, Sharma N, Rashi, Arora V, Bashar MA, Nath B, et al. Diabetic foot care knowledge and practices in rural north India:Insights for preventive podiatry. J Assoc Physicians India. 2021;69:30–4. [PubMed] [Google Scholar]
- 34.Sivanmaliappan TS, Sevanan M. Antimicrobial susceptibility patterns of Pseudomonas aeruginosa from diabetes patients with foot ulcers. Int J Microbiol. 2011;2011:1–4. doi: 10.1155/2011/605195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gadepalli R, Dhawan B, Sreenivas V, Kapil A, Ammini AC, Chaudhry R. A clinico-microbiological study of diabetic foot ulcers in an indian tertiary care hospital. Diabetes Care. 2006;29:1727–32. doi: 10.2337/dc06-0116. [DOI] [PubMed] [Google Scholar]
- 36.Shankar EM, Mohan V, Premalatha G, Srinivasan RS, Usha AR. Bacterial etiology of diabetic foot infections in South India. Eur J Intern Med. 2005;16:567–70. doi: 10.1016/j.ejim.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Zubair M, Malik A, Ahmad J. Clinico-bacteriology and risk factors for the diabetic foot infection with multidrug resistant microorganisms in north India. Biol Med. 2010;2:22–34. [Google Scholar]
- 38.Bansal E, Garg A, Bhatia S, Attri A, Chander J. Spectrum of microbial flora in diabetic foot ulcers. Indian J Pathol Microbiol. 2008;51:204–8. doi: 10.4103/0377-4929.41685. [DOI] [PubMed] [Google Scholar]
- 39.Kumar V, Surender G, Sowjanya G, Archana A. Study of bacterial spectrum in diabetic foot ulcers. Indian J Public Health Res Dev. 2020;11:174–81. [Google Scholar]
- 40.ShankarRao AG, Behera PK, Tripathy KP, Nair AA. Clinico-microbiological profile and culture sensitivity pattern of micro-organisms isolated from diabetic foot ulcers:Study from a tertiary care centre. J Assoc Physicians India. 2022;70:11–12. [PubMed] [Google Scholar]
- 41.Zubair M, Ahmad J. Potential risk factors and outcomes of infection with multidrug resistance among diabetic patients having ulcers:7 years study. Diabetes Metab Syndr. 2019;13:414–8. doi: 10.1016/j.dsx.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Noor S, Raghav A, Parwez I, Ozair M, Ahmad J. Molecular and culture based assessment of bacterial pathogens in subjects with diabetic foot ulcer. Diabetes Metab Syndr. 2018;12:417–21. doi: 10.1016/j.dsx.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Kateel R, Augustine AJ, Prabhu S, Ullal S, Pai M, Adhikari P. Clinical and microbiological profile of diabetic foot ulcer patients in a tertiary care hospital. Diabetes Metab Syndr. 2018;12:27–30. doi: 10.1016/j.dsx.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Shettigar S, Shenoy S, Sevitha S, Rao P. Microbiological profile of deep tissue and bone tissue in diabetic foot osteomyelitis. J Clin Diagn Res. 2018;12:DC20–2. [Google Scholar]
- 45.Sasikumar K, Vijayakumar C, Jagdish S, Kadambari D, Raj Kumar N, Biswas R, et al. Clinico-microbiological profile of septic diabetic foot with special reference to anaerobic infection. Cureus. 2018;10:e2252. doi: 10.7759/cureus.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suryaletha K, John J, Radhakrishnan MP, George S, Thomas S. Metataxonomic approach to decipher the polymicrobial burden in diabetic foot ulcer and its biofilm mode of infection. Int Wound J. 2018;15:473–81. doi: 10.1111/iwj.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noor S, Ahmad J, Parwez I, Ozair M. Culture-based screening of aerobic microbiome in diabetic foot subjects and developing non-healing ulcers. Front Microbiol. 2016;7:1792. doi: 10.3389/fmicb.2016.01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malik A, Mohammad Z, Ahmad J. The diabetic foot infections:Biofilms and antimicrobial resistance. Diabetes Metab Syndr. 2013;7:101–7. doi: 10.1016/j.dsx.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Banoo S, Shashidhar V, Shubha D, Venkatesha D. Bacterial and clinical profile of diabetic foot patients. Ann Trop Med Public Health. 2012;5:69. [Google Scholar]
- 50.Zubair M, Malik A, Ahmad J. Clinico-microbiological study and antimicrobial drug resistance profile of diabetic foot infections in North India. Foot. 2011;21:6–14. doi: 10.1016/j.foot.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Ramakant P, Verma AK, Misra R, Prasad KN, Chand G, Mishra A, et al. Changing microbiological profile of pathogenic bacteria in diabetic foot infections:Time for a rethink on which empirical therapy to choose? Diabetologia. 2011;54:58–64. doi: 10.1007/s00125-010-1893-7. [DOI] [PubMed] [Google Scholar]
- 52.Mohanasoundaram K. The microbiological profile of diabetic foot infections. J Clin Diagn Res. 2012;6:409–11. [Google Scholar]
- 53.Patil A, More D, Patil A, Jadhav KA, Vijil Mejia ME, et al. Clinical, etiological, anatomical, and bacteriological study of “diabetic foot”patients:Results of a single center study. Cureus. 2018;10:e2498. doi: 10.7759/cureus.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raghu R, Padma U, Sasankan V, Puthur S, Jose J. A microbiological study of diabetic foot ulcer in a south Indian tertiary care hospital. Int J Pharm Sci Rev Res. 2016;37:167–70. [Google Scholar]
- 55.Sekhar S, Vyas N, Unnikrishnan M, Rodrigues G, Mukhopadhyay C. Antimicrobial susceptibility pattern in diabetic foot ulcer:A pilot study. Ann Med Health Sci Res. 2014;4:742. doi: 10.4103/2141-9248.141541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parvez N, Dutta P, Ray P, Shah VN, Prakash M, Khandelwal N, et al. Microbial profile and utility of soft tissue, pus, and bone cultures in diagnosing diabetic foot infections. Diabetes Technol Ther. 2012;14:669–74. doi: 10.1089/dia.2012.0039. [DOI] [PubMed] [Google Scholar]
- 57.Elamurugan TP, Jagdish S, Kate V, Chandra Parija S. Role of bone biopsy specimen culture in the management of diabetic foot osteomyelitis. Int J Surg. 2011;9:214–6. doi: 10.1016/j.ijsu.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Wasnik RN, Marupuru S, Mohammed ZA, Rodrigues GS, Miraj SS. Evaluation of antimicrobial therapy and patient adherence in diabetic foot infections. Clin Epidemiol Glob Health. 2019;7:283–7. [Google Scholar]
- 59.Sugandhi P, ArvindPrasanth D. Microbiological profile of bacterial pathogens from diabetic foot infections in tertiary care hospitals, Salem. Diabetes Metab Syndr. 2014;8:129–32. doi: 10.1016/j.dsx.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Appapalam TS, Muniyan A, Vasanthi Mohan K, Panchamoorthy R. A study on isolation, characterization, and exploration of multiantibiotic-resistant bacteria in the wound site of diabetic foot ulcer patients. Int J Low Extrem Wounds. 2021;20:6–14. doi: 10.1177/1534734619884430. [DOI] [PubMed] [Google Scholar]
- 61.Haldar J, Mukherjee P, Mukhopadhyay S, Maiti P. Isolation of bacteria from diabetic foot ulcers with special reference to anaerobe isolation by simple two-step combustion technique in candle jar. Indian J Med Res. 2017;145:97. doi: 10.4103/ijmr.IJMR_1436_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seth A, Attri A, Kataria H, Kochhar S, Seth S, Gautam N. Clinical profile and outcome in patients of diabetic foot infection. Int J App Basic Med Res. 2019;9:14. doi: 10.4103/ijabmr.IJABMR_278_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishwarya, Kalyani M, Neelusri P. Bacteriological profile and their antimicrobial susceptibility from diabetic foot infections in a tertiary care centre from Kancheepuram, India. Saudi J Pathol Microbiol. 2019;4:134–41. [Google Scholar]
- 64.Insan N, Payal N, Singh M, Yadav A, Chaudhary B, Srivastava A. Post operative wound infection:Bacteriology and antibiotic sensitivity pattern. Int J Cur Res Rev. 2013;5:74–9. [Google Scholar]
- 65.Chitra N, Madhu C, Sudhir S, Srinivasarangan M. Clinico-microbiological profile of diabetic foot infections. Indian J Public Health Res Dev. 2016;7:133–8. [Google Scholar]
- 66.Shahi SK, Kumar A, Gupta SK, Singh SK. Occurrence of multiple antibiotic resistance phenotype and class 1 integron in bacteria isolated from diabetic foot ulcers. Afr J Microbiol Res. 2013;7:5424–32. [Google Scholar]
- 67.Sinharay K, Paul UK, Bhattacharyya AK, Pal SK. Prevalence of diabetic foot ulcers in newly diagnosed diabetes mellitus patients. J Indian Med Assoc. 2012;110:608–11. [PubMed] [Google Scholar]
- 68.Jayaprakash P, Bhansali S, Bhansali A, Dutta P, Anantharaman R. Magnitude of foot problems in diabetes in the developing world:A study of 1044 patients. Diabet Med. 2009;26:939–42. doi: 10.1111/j.1464-5491.2009.02781.x. [DOI] [PubMed] [Google Scholar]
- 69.Mishra SC, Chhatbar KC, Kashikar A, Mehndiratta A. Diabetic foot. BMJ. 2017;359:j5064. doi: 10.1136/bmj.j5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morbach S, Lutale JK, Viswanathan V, Möllenberg J, Ochs HR, Rajashekar S, et al. Regional differences in risk factors and clinical presentation of diabetic foot lesions. Diabet Med. 2004;21:91–5. doi: 10.1046/j.1464-5491.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 71.Abdulbasith K, Bhaskar M, Munisamy M, Nagarajan R. Study of fine-needle aspiration microbiology versus wound swab for bacterial isolation in diabetic foot infections. Indian J Med Res. 2020;152:312. doi: 10.4103/ijmr.IJMR_1151_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brownlee M. The pathobiology of diabetic complications. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 73.Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4:271–85. doi: 10.1155/EDR.2003.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Banu A, Hassan MMN, Rajkumar J, Srinivasa S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation:A prospective study. Australas Med J. 2015;8:280–5. doi: 10.4066/AMJ.2015.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jain S, Barman R. Bacteriological profile of diabetic foot ulcer with special reference to drug-resistant strains in a tertiary care center in North-East India. Indian J Endocr Metab. 2017;21:688. doi: 10.4103/ijem.IJEM_546_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagpal S, Singh V, Kumar H, Pandey A, Mehta S, Bala R. Microbiological profile of diabetic wound infection. Indian J Public Health Res Dev. 2020;11:968–74. [Google Scholar]
- 77.Gupta S, Mujawdiya P, Maheshwari G, Sagar S. Dynamic role of oxygen in wound healing:A microbial, immunological, and biochemical perspective. Arch Razi Inst. 2022;77:512–23. doi: 10.22092/ARI.2022.357230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramirez-Acuña JM, Cardenas-Cadena SA, Marquez-Salas PA, Garza-Veloz I, Perez-Favila A, Cid-Baez MA, et al. Diabetic foot ulcers:Current advances in antimicrobial therapies and emerging treatments. Antibiotics. 2019;8:193. doi: 10.3390/antibiotics8040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Husain M, Agrawal YO. Antimicrobial remedies and emerging strategies for the treatment of diabetic foot ulcers. Curr Diabetes Rev. 2023;19:5–17. doi: 10.2174/1573399818666220228161608. [DOI] [PubMed] [Google Scholar]
- 80.Durgad S, Koticha A, Nataraj G, Deshpande A, Mehta P. Diabetic foot ulcers—where do we stand microbiologically? Int J Diabetes Dev Ctries. 2014;34:169–73. [Google Scholar]
- 81.Jnana A, Muthuraman V, Varghese VK, Chakrabarty S, Murali TS, Ramachandra L, et al. Microbial community distribution and core microbiome in successive wound grades of individuals with diabetic foot ulcers. Appl Environ Microbiol. 2020;86:e02608–19. doi: 10.1128/AEM.02608-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kunimitsu M, Kataoka Y, Nakagami G, Weller CD, Sanada H. Factors related to the composition and diversity of wound microbiota investigated using culture-independent molecular methods:A scoping review. Drug Discov Ther. 2021;15:78–86. doi: 10.5582/ddt.2021.01036. [DOI] [PubMed] [Google Scholar]
- 83.Lipsky BA. A current approach to diabetic foot infections. Curr Infect Dis Rep. 1999;1:253–60. doi: 10.1007/s11908-999-0027-1. [DOI] [PubMed] [Google Scholar]
- 84.Travers HC, Dawson J, Muthusami A, Wall ML. Review of microbiological sampling in diabetic foot disease. Br J Diabetes. 2021;21:233–6. [Google Scholar]
- 85.Huang Y, Cao Y, Zou M, Luo X, Jiang Y, Xue Y, et al. A comparison of tissue versus swab culturing of infected diabetic foot wounds. Int J Endocrinol. 2016;2016:8198714. doi: 10.1155/2016/8198714. [DOI] [PMC free article] [PubMed] [Google Scholar]