Abstract
Introduction:
Type 2 diabetes mellitus (T2DM) is the risk factor for coronary artery disease (CAD).
Material and Methods:
In this study, we assessed the prevalence of CAD in asymptomatic T2DM patients and its correlation with invasive testing in treadmill testing (TMT)-positive cases. A total of 90 patients with asymptomatic T2DM were recruited and subjected to TMT TMT-positive patients were subjected to coronary angiography (CAG).
Results:
At baseline, the mean duration of T2DM (years) was 4.87 ± 4.04 with mean levels of HbA1c (%) of 7.96 ± 1.02. TMT was positive in 28 patients (31.1%) for reversible myocardial ischaemia (RMI), and among them, 16 patients consented to CAG, of which 14 required coronary angioplasty and the remaining two (7.1%) had to undergo coronary artery bypass grafting (CABG). The remaining 12 TMT positives (42.9%) were managed medically.
Conclusion:
To conclude, there is a high prevalence of silent CAD in T2DM. They need regular screening to detect the same and prevent the morbidity and mortality associated with overt CAD. Hence, it is important to screen people with type 2 diabetes, to prevent the morbidity and mortality associated with overt CAD.
Keywords: Cardiovascular disease, treadmill test, metabolic syndrome
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterised by hyperglycaemia due to either insulin resistance or insulin secretory defect resulting in microvascular or macrovascular complications. In 2021, there were 537 million adults (20–79 years) with diabetes worldwide, and by 2030, the projected estimate of adults (20–79 years) with diabetes worldwide is 643 million.[1] By the year 2030, over 85 per cent of the world’s patients with T2DM will be in developing countries. In 2021, there were 74.2 million adults (20–79 years) with diabetes in India, and by 2045, the projected estimate of adults (20–79 years) with diabetes in India is 124.9 million.[1]
According to a World Health Organization (WHO) survey, in India 24.8% of people die annually due to various cardiovascular diseases, among which coronary artery disease (CAD) is the leading cause. In 2016, a total of 28% of deaths in India were due to cardiovascular diseases.[2] T2DM being a major cardiovascular risk factor is responsible for the increased risk of CAD and is associated with poor outcome in myocardial infarction.[3] The prevalence of cardiovascular disease in T2DM is 32.2% throughout the world.[4] T2DM is considered a risk equivalent to CAD, which is often responsible for morbidity and premature mortality in T2DM patients. While the diagnosis of T2DM doubles the cardiovascular risk in men and more than triples the risk in women, hypertension quadruples the cardiovascular risk in T2DM.[5]
Previous studies have found a significant association between HbA1c levels (≥7.4%) and duration of diabetes (≥10 years) even if they were asymptomatic for CAD. The chronic enhancement of oxidative stress, IL-1b, IL-6, tumour necrosis factor (TNF)-a and C-reactive protein (CRP) have been associated with endothelial dysfunction and atherogenesis.[6] Patients with T2DM exhibit an increased risk for the development of atherosclerotic CAD due to factors such as hyperglycaemia, dyslipidaemia and insulin resistance, which lead to endothelial cell damage, vascular smooth muscle dysfunction,[7,8] impaired platelet function and abnormal coagulation.[9] Patients with T2DM have lipid-rich atherosclerotic plaques, which are more vulnerable to rupture than the plaques seen in people without T2DM.[10]
In recent years, a number of studies have shown a high risk of CAD even among asymptomatic T2DM[11-13] and Miller et al.[14] stressed the need to screen all asymptomatic patients with T2DM for the presence of CAD. In 2019, the Prospective Evaluation of Chronic Pancreatitis for Epidemiologic and Translational Studies (PROCEED) study had a 40% prevalence of CAD on screening asymptomatic T2DM patients with computed tomography (CT) coronary angiography (CAG) and assessing the coronary artery calcium score.[15] Noninvasive methods are used to improve risk stratification in patients with a clinical suspicion of CAD to guide physicians for further procedures and interventions and may enable assessment of pre-symptomatic lesions, eventually reducing the death and disability associated with CAD.[16]
The American Diabetes Association (ADA) recommends that a treadmill exercise test (TMT) being a noninvasive sophisticated screening test should be performed on all asymptomatic T2DM patients with high-risk factors for early detection of asymptomatic CAD, to prevent catastrophic cardiac events and consequent deaths. Various studies had also been conducted for risk stratification, and significant asymptomatic T2DM patients were found TMT-positive, but final outcomes of these patients were not correlated with CAG.
Our study will help to fill these lacunae, and a step further will correlate the findings of CAG with their final outcome and management of these patients. We hypothesise that the prevalence of CAD is comparably high in asymptomatic T2DM patients. Hence, our study aimed to assess the prevalence of CAD in T2DM with invasive correlation. Our objectives were as follows: 1. to study the early changes in the cardiovascular system in asymptomatic T2DM patients; 2. to correlate the hyperglycaemic changes among patients of CAD; and 3. to find the association between the level of glycaemic control and CAD.
MATERIALS AND METHODS
This prospective observational study was carried out in a tertiary care hospital in North India over one year from January 2019 to January 2020. The inclusion criteria were adult asymptomatic patients with T2DM (aged more than 30 years but less than 76 years of either sex) willing to participate. The exclusion criteria were as follows: 1) typical history of angina; 2) known noncoronary heart diseases such as congenital or acquired valvular heart disease, cognitive cardiac failure (CCF) or arrhythmia; 3) known history of abnormal electrocardiogram (ECG) finding or diagnosed CAD by invasive and noninvasive procedure; and 5) unwilling to participate in the study.
In a previous study, Khanapure et al.[17] reported the prevalence of CAD screen positivity rate to be 32.9% (~33%) in an asymptomatic T2DM population. In the present study, we also target a similar prevalence. The sample size has been calculated using the following formula:[17] n = C2xp(1-p)/e2, where P is the targeted incidence (33%), that is 0.39, C is a constant at a certain confidence level (its value at 95% confidence limit and 80% power is 1.96) and e is the allowed error (taken as 10% or 0.10). Now, putting these values in the above equation, we get 84.9378 ~ 85. Thus, the calculated sample size is 85. After adding a contingency of 5% and rounding off, we shall target a sample size of 90. The total sample size was taken as 90 with an assumed 33% prevalence of CAD in asymptomatic T2DM in a previous similar study.
Clinical features, blood biochemistry and five cardiac risk factors, such as hypertension, dyslipidaemia, family history of CAD, smoking habit and macro–micro-albuminuria, were noted. T2DM is defined based on the ADA diagnostic criteria as follows: 1) random blood glucose level >200 mg/dl and osmotic symptoms, 2) fasting blood glucose level >126 mg/dl and 3) diagnosed and receiving treatment for T2DM. The duration of T2DM was noted as the time period between diagnoses of T2DM-to-CAD evaluation in years. Diabetic retinopathy, neuropathy and nephropathy were evaluated with appropriate clinical and laboratory testing. Fasting and postprandial blood glucose with HbA1c and lipid profile were performed in every patient.
TMT was performed in all patients according to Bruce’s protocol irrespective of a number of cardiac risk factors. TMT is defined as positive if there is >1 mm horizontal/downsloping ST segment for 0.08 s after the J point. CAG was considered in those with positive TMT results to look for CAD. The data so collected were subjected to statistical analysis.
All statistical analysis was carried out using the latest version of Statistical Package for the Social Sciences (SPSS) software. The Chi-square test and independent-samples t-test were used to evaluate the data. A P value was set significantly at <0.05.Approved by institutional ethics commitee on 29 Aug 2018. The study was approved by Command Hospital (Central command) Ethical Committee, Lucknow vide letter dated 29 Aug 2018. Written informed consent was obtained for participation in the study and use of the patient data for research and educational purposes. The procedures follows the guidelines laid down in Declaration of Helsinki 2008.
RESULTS
A total of 90 patients (44 males and 46 females) of T2DM were studied. The majority of the patients (29 (32.2%)) was in the 41–50 years of age group. Twenty-five (27.8%) and 19 (21.1%) patients were in the age group of 51–60 years and below 40 years. Additionally, patients between 51 and 60 years of age were 25 (27.8%) and those beyond 60 years were 17 (18.9%). The baseline parameters of the study population are shown in Table 1.
Table 1.
Baseline parameters of the study population
| Variables | Mean±standard deviation | Median | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 50.09±10.67 | 50.0 | 24.0 | 71.0 |
| Duration of diabetes (years) | 4.87±4.04 | 4.0 | 1.0 | 26.0 |
| BMI (KG/m2) | 26.00±3.15 | 26.0 | 20.0 | 34.0 |
| Waist circumference (cm) | 86.41±8.92 | 86.0 | 69.0 | 118.0 |
| HbA1c (%) | 7.96±1.02 | 7.6 | 6.7 | 10.3 |
| Fasting blood glucose (mg/dl) | 158.88±43.72 | 142.0 | 88.0 | 284.0 |
| Postprandial blood glucose (mg/dl) | 239.03±45.38 | 221.0 | 167.0 | 389.0 |
| Serum cholesterol (mg/dl) | 223.31±66.99 | 211.0 | 98.0 | 473.0 |
| Triglyceride (mg/dl) | 136.00±54.12 | 117.0 | 60.0 | 416.0 |
| HDL (mg/dl) | 46.17±7.24 | 46.0 | 32.0 | 66.0 |
| LDL (mg/dl) | 112.90±27.97 | 110.5 | 27.0 | 184.0 |
| Non-HDL (mg/dl) | 175.07±66.55 | 162.0 | 56.0 | 426.0 |
Twenty-eight subjects were found positive for reversible myocardial ischaemia (RMI) on TMT and were advised for CAG. Among them, 10 (35.7%) patients were in the age group of 51–60 years. Cases positive for RMI were predominantly males (60.7%). Also, 18 (64.3%) cases were positive for RMI and had 6–10 years of duration of diabetes (mean duration: 8.8 years). A total of 24 cases (85.7%) positive for RMI had a body mass index (BMI) more than 25 kg/m2, while 20 (71.4%) cases had waist circumference more than the cutoff for Asian Indians (males <90 cm; females <80 cm) These various parameters of the study population are shown in Table 2.
Table 2.
Anthropometry of the study population
| Clinical examination | TMT | P | ||
|---|---|---|---|---|
|
| ||||
| RMI-positive (n=28) | RMI-negative (n=62) | |||
| Age group (years) | ≤40 years | 4 (14.3%) | 15 (24.2%) | 0.350 |
| 41–50 years | 7 (25.0%) | 22 (35.5%) | ||
| 51–60 years | 10 (35.7%) | 15 (24.2%) | ||
| >60 years | 7 (25.0%) | 10 (16.1%) | ||
| Gender | Male | 17 (60.7%) | 27 (43.5%) | 0.173 |
| Female | 11 (39.3%) | 35 (56.5%) | ||
| Duration of diabetes (years) | 0–5 | 6 (21.4%) | 60 (96.8%) | <0.001 |
| 6–10 | 18 (64.3%) | 2 (3.2%) | ||
| 11–15 | 2 (7.1%) | 0 (0.0%) | ||
| >15 | 2 (7.1%) | 0 (0.0%) | ||
| BMI (kg/m2) | 20–24.9 | 4 (14.3%) | 24 (38.7%) | <0.001 |
| 25–29.9 | 12 (42.9%) | 34 (54.8%) | ||
| ≥30 | 12 (42.9%) | 4 (6.5%) | ||
| Waist circumference | ≤85 | 8 (28.6%) | 36 (58.1%) | 0.032 |
| 86–99 | 16 (57.1%) | 22 (35.5%) | ||
| ≥100 | 4 (6.5%) | 4 (14.3%) | ||
We also found that five (17.8%) of 28 RMI-positive cases had HbA1c >10%, while eight (28.5%) had HbA1c in the range of 9 to 9.9%. A total of 92% of TMT-positive cases had fasting blood glucose levels >126 mg/dl. Of 28 RMI-positive patients, 96% had serum cholesterol >200 mg/dl and non-high-density lipoprotein (HDL) cholesterol >130 mg/dl. This association of biochemical profile with TMT positivity of the study population is shown in Table 3.
Table 3.
Laboratory parameters of the study population
| Clinical examination | Range | TMT | P | |
|---|---|---|---|---|
|
| ||||
| RMI-positive (n=28) | RMI-negative (n=62) | |||
| HbA1c (%) | ≤6.7 | 0 (0.0%) | 1 (1.6%) | <0.001 |
| 6.8–8.8 | 15 (53.6%) | 57 (91.9%) | ||
| 8.9–9.9 | 8 (28.6%) | 2 (3.2%) | ||
| ≥10 | 5 (17.9%) | 2 (3.2%) | ||
| Fasting blood glucose (mg/dl) | ≤126 | 2 (7.1%) | 13 (21.0%) | 0.046 |
| 127–199 | 17 (60.7%) | 41 (66.1%) | ||
| ≥200 | 9 (32.1%) | 8 (12.9%) | ||
| Postprandial blood glucose (mg/dl) | ≤200 | 3 (10.7%) | 8 (12.9%) | 0.201 |
| 201–299 | 19 (67.9%) | 49 (79.0%) | ||
| ≥300 | 6 (21.4%) | 5 (8.1%) | ||
| Serum cholesterol (mg/dl) | ≤200 | 1 (3.6%) | 32 (51.6%) | <0.001 |
| 201–239 | 8 (28.6%) | 17 (27.4%) | ||
| ≥240 | 19 (67.9%) | 13 (21.0%) | ||
| Triglyceride (mg/dl) | ≤150 | 17 (60.7%) | 52 (83.9%) | 0.017 |
| 151–199 | 7 (25.0%) | 9 (14.5%) | ||
| ≥200 | 4 (14.3%) | 1 (1.6%) | ||
| HDL (mg/dl) | ≤30 | 0 (0.0%) | 0 (0.0%) | 1.00 |
| 31–45 | 14 (50.0%) | 30 (48.4%) | ||
| ≤46 | 14 (50.0%) | 32 (51.6%) | ||
| LDL (mg/dl) | ≤130 | 20 (71.4%) | 47 (75.8%) | 0.585 |
| 131–159 | 5 (17.9%) | 12 (19.4%) | ||
| ≥160 | 3 (10.7%) | 3 (4.8%) | ||
| Non-HDL (mg/dl) | ≤130 | 1 (3.6%) | 21 (33.9%) | <0.001 |
| 130.1–199 | 10 (35.7%) | 35 (56.5%) | ||
| ≥200 | 17 (60.7%) | 6 (9.7%) | ||
Our study found a statistically significant (P < 0.001) association between the duration of T2DM and TMT-positive cases as shown in Table 4. Glycaemic parameters had a significant association with TMT-positive cases, while TMT-positive cases had a significant correlation with HbA1c levels as shown in Figure 1.
Table 4.
Association of glycaemic parameters with RMI-positive patients
| Glycaemic parameters | TMT | P | |
|---|---|---|---|
|
| |||
| RMI-positive (n=28) | RMI-negative (n=62) | ||
| Duration of DM (years) | 8.96±4.86 | 3.02±1.50 | <0.001 |
| Fasting blood sugar (mg/dl) | 177.25±49.66 | 150.58±38.37 | 0.007 |
| Postprandial blood sugar (mg/dl) | 254.64±53.88 | 231.98±39.46 | 0.027 |
| HBA1c (%) | 8.94±0.84 | 7.51±0.74 | <0.001 |
Figure 1.
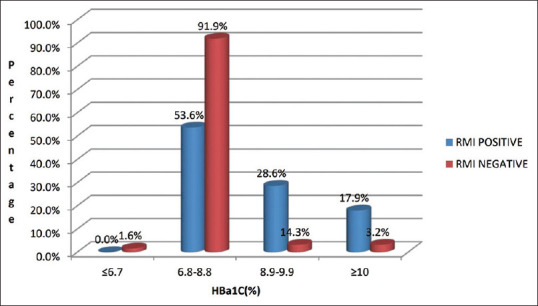
Correlation of TMT positivity with HbA1c
All TMT-positive patients were advised for CAG, but only 16 (57.1%) underwent the procedure and others were reluctant. Of 16 patients, 14 (50%) required coronary angioplasty and the remaining two (7.1%) had to undergo coronary artery bypass grafting (CABG) while the remaining 12 (42.9%) were managed medically. We optimised their medical management of CAD with antiplatelets, high-dose statins and antianginal agents with three monthly follow-ups on an outpatient basis. In addition, they were also prescribed glucagon-like receptor 1 agonists or sodium–glucose co-transporter 2 inhibitors for optimum glycaemic management with cardiovascular benefits.
DISCUSSION
T2DM is an independent and modifiable risk factor for the development of CAD. The mortality related to cardiovascular disease (CVD) is doubled in men with T2DM and quadrupled in women with T2DM over their non-diabetic counterparts. CAD silently progresses over years in them, and hence, the detection is often delayed. Silent CAD is an important cause of premature death in patients. In approximately 18% of patients with CAD, sudden death is the first and the only manifestation.[18] The prevalence of CAD in T2DM is variable and ranges from 9 to 75% in various studies depending on the used criterias for diagnosis of CAD. Our study also confirmed that diabetes was significantly associated with abnormal TMT.
In our study, the majority of the patient’s age was from 41 to 50 years (32.2%) with a mean age of 50.09 ± 10.67 years. This was in accordance with Fletcher et al.,[19] in which the mean age of asymptomatic T2DM patients with eventful TMT was 67 ± 9 years. A similar result was seen by Kim et al.,[20] wherein significant TMT events were recorded in the mean age group of 63.1 ± 9.4 years, thus implying that CAD generally occurs beyond 50 years of age. Kaul et al.[21] and Haris et al.[22] in their study showed that CAD was reported at ages more than 55 years. Age being a nonmodifiable CAD risk factor, the incidence of CAD significantly increases beyond 55 years of age.
In our study, of 90 patients 46 (51.1%) were female and 44 (48.9%) were male. Among the 28 patients positive for RMI, 11 (39.2%) were female and the remaining 17 (62.9%) were male. This was similar to the study by Premalatha et al.[23] who found that the gender-wise frequency of CAD in males is 60% and in females is 40%. Joshi et al.[24] also reported a higher prevalence of silent myocardial infarction in males (28.56%) compared with females (18.8%). The majority of aforementioned studies were conducted in patriarchal societies, and thus, the higher prevalence of CAD in males could be due to increased access to health care, as well as higher consumption of tobacco and higher smoking habits. The sample size and inferences of various studies similar to our study are shown in Table 5.
Table 5.
Various studies similar to our studies and their inferences
| Study | Sample size | Year | Inference |
|---|---|---|---|
| Gayathri[13] | 50 | 2016 | The preponderance of silent myocardial ischaemia in asymptomatic T2DM was 30%. The duration of T2DM was directly proportional to the increased risk of silent myocardial ischaemia in T2DM |
| Sharda M et al.[12] | 75 | 2016 | TMT in T2DM patients has a significant role in the detection of silent myocardial ischaemia. The prevalence of silent myocardial ischaemia in T2DM patients was 37.3%. A significant correlation exists between risk factors for CAD and evidence of ischaemia on TMT in T2DM patients |
| Khanapure SP et al.[17] | 82 | 2017 | TMT being a noninvasive sophisticated screening test should be performed on all asymptomatic T2DM patients with high-risk factors for early detection of asymptomatic CAD to prevent catastrophic cardiac events and consequent death |
| Joshi AS et al.[24] | 50 | 2017 | TMT can detect silent myocardial ischaemia in asymptomatic T2DM patients and recommended that TMT should be a part of routine management in asymptomatic patients with T2DM |
| Our study | 90 | 2020 | The prevalence of silent myocardial ischaemia in T2DM patients was 31.1%. TMT positive for RMI should undergo CAG for the detection of CAD |
In our study, 18 (90.0%) of 20 cases with T2DM of duration between 6 and 10 years (mean duration: 8.8 years) were RMI-positive, while all patients with T2DM duration of more than 10 years were RMI-positive. Similar results were observed in the study by Gayathri[13] who showed that 71.4% of RMI-positive patients had a duration of T2DM between 11 and 15 years and 80% with a duration of between 16 and 20 years. Gupta et al.[25] and Ahluwalia et al.[26] have recorded around 70% of patients with a duration of diabetes of more than 5 years associated with silent MI. Sharda et al.,[12] and Janand et al.[27] have also demonstrated a strong association between the duration of T2DM and abnormal exercise test. Sarkar et al.[28] had concluded that long-standing asymptomatic T2DM (more than 10 years) with a family history of CAD should undergo TMT for early detection of CAD. In our study, 16 patients had BMI >30 kg/m2 among whom 12 (75.0%) were positive for RMI on TMT. Only 12 of 46 patients with a BMI between 25 and 29.9 kg/m2 were positive for RMI. In a similar study by Khanapure et al.,[17] 17 TMT-positive cases of 24 had BMI between 30 and 34.9 kg/m2, while only 10 (21.27%) cases were TMT-positive of 47 with a BMI between 25 and 29.9 kg/m2.
Of a total of 28 RMI-positive cases, five (17.9%) cases had HbA1c more than 10, while eight cases (28.6%) had HbA1c between 8.9 and 9.9% and the rest 15 (53.6%) had a range of 6.8 to 8.8%. Gayathri[13] had shown statistically significant positive TMT in asymptomatic T2DM patients with HbA1c of more than 9.7%. Ravipati et al.[29] found silent MI in 27 of 54 patients, that is 50% whose HbA1c levels were ≥7.6. In a similar study, Agarwal et al.[30] reported that the mean HbA1c in their study was 7.7 ± 1.61. The former was a population-based study, and the patients were not in regular follow-up, so the prevalence of poor glycaemic control was high. This is indicative that our group of patients was a highly compliant group taking medicines regularly with regular in their follow-up visits.
Nineteen (67.9%) of 28 TMT-positive cases had serum cholesterol of more than 240 mg/dl. A triglyceride level of more than 200 mg/dl was found in 11 (39.2%) TMT-positive cases, and non-HDL levels of more than 200 mg/dl were observed in 17 (60.7%) TMT-positive cases. A similar correlation was also observed by Joshi et al.[24] who documented that 25% of asymptomatic T2DM patients with TMT-positive had serum cholesterol >240 mg/dl and low-density lipoprotein (LDL) >160 mg/dl. Gayathri[13] found statistically significant average values of TG in positive and negative cases of TMT in asymptomatic T2DM to be 176.6 mg% and 130.5 mg%, respectively, while serum cholesterol was 174.4 mg% and 180.9 mg%, respectively, in negative and positive cases of TMT. De Luca et al.[31] and Ravipati et al.[29] also found an association between elevated levels of TG and positive TMT.
Our 28 patients were found positive for RMI on TMT similar to Agarwal et al.[30] who reported silent CAD in 28.9% of patients in their study. Considering it was more similar in design to Western studies, the prevalence is comparable though a little higher than the previous studies. The wide variability in the prevalence rates reported by the various studies is expected because of the difference in population groups and the goals of the studies done.
In our study, 28 (31.1%) TMT-positive were advised for CAG of which 16 (57.1%) underwent the same, while 12 (42.9%) were reluctant and refused the procedure. However, they were started on medical management in view of the high benefit–risk ratio and were considered to be on medical management in our further follow-up. To find an invasive correlation among asymptomatic type 2 DM, we found that all 16 patients who underwent CAG had a significant CAD. Among these, two had to undergo CABG, and in the remaining 14, coronary angioplasty was needed. Afsar et al.[32] demonstrated that patients with T2DM had a higher prevalence of three-vessel disease (TVD) (32.43% versus 26.19%) and a lower prevalence of single-vessel disease (SVD) (27.02% versus 33.33%). Shakya et al.[33] reported angiographically, absolutely normal vessels were present in 6.4% and minor CAD was seen in 3.8% of patients, which was more prevalent in unstable angina (UA) followed by non-ST elevation myocardial infarction (NSTEMI). Angiographically normal coronaries were seen in 14% of cases of acute coronary syndrome (ACS) in a study by Ahmed et al.[34] In UA, many patients may have been overdiagnosed and so were false-positive. Similarly, false-positive cases in NSTEMI may be attributed to other causes of raised troponin levels apart from MI such as myocarditis, infection with sepsis and renal impairment.[35] In our study, 14 (50.0%) had underwent coronary angioplasty, 12 (42.9%) are on medical management and two (12.50%) underwent CABG.
The strength of the study is that the analysis was performed based on the consent pro forma. The limitations of the study were the small sample size and the nonusage of CTCA. The use of CTCA would have improved the compliance of the patients, and we could have compared it with the findings of CAG. Also, the study did not further analyse the actual carotid intima medial thickness, which could have further stratified the cerebrovascular risk. We recommend that further studies are needed on a large size of study population and also consider other risk factors such as family history, hypertension, smoking and other diseases. Better noninvasive techniques of detecting silent myocardial ischaemia such as stress thallium and CTCA should be used. The evaluation of newer risk factors and genetic predisposition is the point that needs to be emphasised in planning future studies. There is an urgent need to realise that there is a high prevalence of silent CAD in T2DM and they should undergo regular screening to detect the same, to prevent the morbidity and mortality associated with overt CAD.
Silent CAD in T2DM is an important cause of premature death in patients, as it silently progresses over years and eventually causes classic silent ischaemia and catastrophic cardiac events, especially in those with associated risk factors. It can be recommended that TMT should be a part of the routine investigation in asymptomatic patients with T2DM without previous clinical and electrocardiographic signs of ischaemic heart disease, especially with age >55 years, duration >10 years, long-term impaired glycaemic control and dyslipidaemia. CAG is a confirmatory diagnosis of CAD, and based on the severity of the disease, patients may be managed based on treatment options available such as medical management, coronary angioplasty and CABG.
CONCLUSION
To conclude, TMT can detect early CAD in asymptomatic T2DM. The longer the duration of diabetes mellitus, the higher the chances of having CAD. Additionally, poor glycaemic control has a significant correlation with CAD in T2DM. In patients with obesity and dyslipidaemia, there is a high risk of CAD despite asymptomatic T2DM.
We acknowledge all patients enrolled and the entire staff of cardiology and endocrinology departments of the hospital for all the support to carry out the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas:Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar AS, Sinha N. Cardiovascular disease in India:A 360 degree overview. Med J Armed Forces India. 2020;76:1–3. doi: 10.1016/j.mjafi.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt VH, Hobohm L, Münzel T, Wenzel P, Gori T, Keller K. Impact of diabetes mellitus on mortality rates and outcomes in myocardial infarction. Diabetes Metab. 2021;47:101211. doi: 10.1016/j.diabet.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes:A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 6.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update:cardiovascular disease in diabetes mellitus:Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus–mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki LA, Poot M, Gerrity RG, Bornfeldt KE. Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis:lack of direct growth-promoting effects of high glucose levels. Diabetes. 2001;50:851–60. doi: 10.2337/diabetes.50.4.851. [DOI] [PubMed] [Google Scholar]
- 8.Williams SB, Cusco JA, Roddy M-A, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 9.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24:1476–85. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 10.Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180–4. doi: 10.1161/01.cir.102.18.2180. [DOI] [PubMed] [Google Scholar]
- 11.Tavares CAF, Wajchjenberg BL, Rochitte C, Lerario AC. Screening for asymptomatic coronary artery disease in patients with type 2 diabetes mellitus. Arch Endocrinol Metab. 2016;60:143. doi: 10.1590/2359-3997000000170. [DOI] [PubMed] [Google Scholar]
- 12.Sharda M, Soni AK, Meena S, Nigam H, Singh A. A prospective study on utility of exercise treadmill test in type 2 diabetes mellitus patients. J Assoc Phys India. 2016;64:32–7. [PubMed] [Google Scholar]
- 13.Gayathri M. Study of Treadmill test in Detecting Asymptomatic Coronary Artery Disease in Type 2 Diabetes Mellitus. 2016 doi: 10.4093/dmj.2011.35.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller TD, Redberg RF, Wackers FJ. Screening asymptomatic diabetic patients for coronary artery disease:Why not? J Am Coll Cardiol. 2006;48:761–4. doi: 10.1016/j.jacc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 15.Venuraju SM, Lahiri A, Jeevarethinam A, Cohen M, Darko D, Nair D, et al. Duration of type 2 diabetes mellitus and systolic blood pressure as determinants of severity of coronary stenosis and adverse events in an asymptomatic diabetic population:PROCEED study. Cardiovasc Diabetol. 2019;18:51. doi: 10.1186/s12933-019-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim S, Choi SH, Choi E-K, Chang S-A, Ku YH, Chun EJ, et al. Comprehensive evaluation of coronary arteries by multidetector-row cardiac computed tomography according to the glucose level of asymptomatic individuals. Atherosclerosis. 2009;205:156–62. doi: 10.1016/j.atherosclerosis.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Khanapure SP, Parmar D, Bajaj G, Mural RH. Prevalence of silent coronary artery disease (CAD) in asymptomatic T2DM–A prospective study. Int J Contemp Med Res. 2017;4:2341. [Google Scholar]
- 18.Barthelemy O, Le Feuvre C, Timsit J. Silent myocardial ischemia screening in patients with diabetes mellitus. Arq Bras Endocrinol Metabol. 2007;51:285–93. doi: 10.1590/s0004-27302007000200018. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher G, Suvant R. Hursts The Heart. 9th ed. NY: McGraw-Hill; 1998. The exercise stress test; p. 522. [Google Scholar]
- 20.Kim MK, Baek KH, Song KH, Kwon HS, Lee JM, Kang MI, et al. Exercise treadmill test in detecting asymptomatic coronary artery disease in type 2 diabetes mellitus. Diabetes Metab J. 2011;35:34–40. doi: 10.4093/dmj.2011.35.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaul S, Alladi S, Mridula RK, Bandaru SV, Boddu DB, Anjanikumar D, et al. Prevalence and risk factors of carotid intima-media thickness in asymptomatic individual subjects in a tertiary care center in India. Ann Indian Acad Neurol. 2015;18:430–4. doi: 10.4103/0972-2327.165481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haris M, Hariawan H, Ismail MT, Wahab AS. Correlation between carotid intimal-media thickness and coronary artery disease severity in stable coronary artery disease patients. ACI (Acta Cardiologia Indonesiana) 2018;3:81. [Google Scholar]
- 23.Premalatha G, Anirudhan B, Mohan V, Sastry N. Treadmill (cardiac stress) test in the diagnosis of ischaemic heart disease in NIDDM patients:Usefulness and safety. Int J Diabetes Dev Ctries. 1995;15:3. [Google Scholar]
- 24.Joshi AS, Lahane CG, Kashid AA. The result of treadmill test in asymptomatic type 2 diabetes mellitus. Int J Sci Rep. 2017;3:166. [Google Scholar]
- 25.Gupta S, Pandit R. Silent myocardial ischaemia and cardiac autonomic neuropathy in diabetics. Indian Heart J. 1992;44:227–9. [PubMed] [Google Scholar]
- 26.Ahluwalia G, Jain P, Chugh S, Wasir H, Kaul U. Silent myocardial ischemia in diabetics with normal autonomic function. Int J Cardiol. 1995;48:147–53. doi: 10.1016/0167-5273(94)02233-9. [DOI] [PubMed] [Google Scholar]
- 27.Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes:Who to screen. Diabetes Care. 1999;22:1396–400. doi: 10.2337/diacare.22.9.1396. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar NC, Jain S, Sarkar P, Tilkar M, Modi N. Early detection of coronary artery disease in asymptomatic type 2 diabetes mellitus patients. Int J Adv Med. 2015;2:26–9. [Google Scholar]
- 29.Ravipati G, Aronow WS, Ahn C, Sujata K, Saulle LN, Weiss MB. Association of hemoglobin A1c level with the severity of coronary artery disease in patients with diabetes mellitus. Am J Cardiol. 2006;97:968–9. doi: 10.1016/j.amjcard.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal A, Singla S, Singla R, Lal A, Wardhan H, Yadav R. Prevalence of coronary risk factors in type 2 diabetics without manifestations of overt coronary heart disease. J Assoc Physicians India. 2009;57:135–42. [PubMed] [Google Scholar]
- 31.DeLuca AJ, Saulle LN, Aronow WS, Ravipati G, Weiss MB. Prevalence of silent myocardial ischemia in persons with diabetes mellitus or impaired glucose tolerance and association of hemoglobin A1c with prevalence of silent myocardial ischemia. Am J Cardiol. 2005;95:1472–4. doi: 10.1016/j.amjcard.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Afsar MN, Ahmed K, Rahman S. A comparative study of coronary angiographic (CAG) findings between diabetic and nondiabetic patients. Med Today. 2014;26:95–9. [Google Scholar]
- 33.Shakya A, Jha SC, Gajurel RM, Poudel CM, Sahi R, Shrestha H, et al. Clinical characteristics, risk factors and angiographic profile of acute coronary syndrome patients in a tertiary care center of Nepal. Nepal Heart J. 2019;16:27. [Google Scholar]
- 34.Ahmed M, Rubaiyat KA, Saleh MAD, Chowdhury AW, Khuda CKE, Ferdous KAF, et al. Clinical characteristics and angiographic profile of acute coronary syndrome patients in a tertiary hospital of Bangladesh. Bangladesh Heart J. 2018;33:10–5. [Google Scholar]
- 35.Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart. 2006;92:987. doi: 10.1136/hrt.2005.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]


