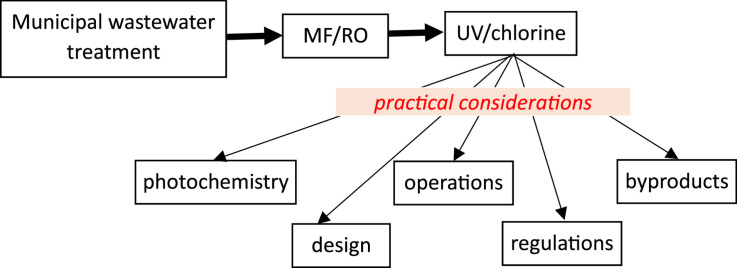
Highlights
-
•
This is a summary of theory and practical experience with UV-Cl.
-
•
UV-Cl theory not well understood making predictive modeling difficult.
-
•
Operational experience is limited but important lessons are emerging.
-
•
Ammonia/chloramines in RO permeate are complicating factors.
-
•
There is a need for a better, science-based regulatory environment.
Keywords: UV, Chlorine, AOP, Reuse, Ammonia, Chloramines
Abstract
This paper reports conclusions from a recent study completed for the Water Research Foundation and the State of California to offer guidance on UV-chlorine advanced oxidation for potable water reuse. The fundamentals of UV-chlorine advanced oxidation are discussed, and lessons learned from some of the early adopters of this technology are presented. Important highlights include the significant impact of ammonia and chloramines on UV-chlorine treatment, challenges associated with predicting UV-chlorine performance due to complex photochemistry, and an ongoing need to monitor potential byproducts and transformation products when employing any form of advanced oxidation for potable reuse.
Graphical abstract
Introduction
The ultraviolet (UV)-chlorine (Cl) advanced oxidation process (AOP) can be cost-effective for potable water reuse treatment and is growing in popularity, particularly following reverse osmosis (RO) as part of the so-called “full advanced treatment (FAT)” train. Of the active-design and under-construction potable reuse UV-AOP projects in the United States recently reported, 76% were pursuing UV-chlorine versus UV-hydrogen peroxide (H2O2) (Festger et al., 2021). While UV-chlorine has many similarities to UV-H2O2 treatment, there are important differences. The municipal water industry is familiar with UV-H2O2 treatment due to several decades of experience, but UV-chlorine is new and there is a need to disseminate the recent lessons learned, as well as to highlight current information gaps to allow the process to be improved.
The authors recently completed a study funded by the Water Research Foundation and the State of California on “UV-Chlorine AOP in Potable Reuse: A Guidance Manual to Assessment and Implementation” (WRF, 2022). This mini-review summarizes some of the key conclusions from this study. It is assumed that the reader has a basic understanding of UV-based AOP, so the focus is on specific technical details that differentiate UV-chlorine from other AOPs. For additional general information, the reader is directed to the original full report (WRF, 2022), or to “Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications” (Stefan, 2018).
Important fundamentals of UV-chlorine photochemistry
Several important fundamental principles of UV-chlorine photochemistry are highlighted in this section as reminders to help in understanding subsequent concepts presented in this review.
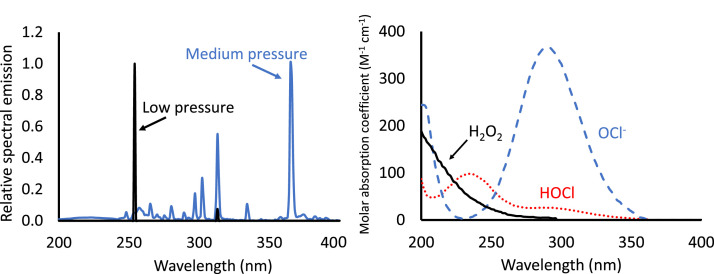
UV-based AOPs work by adding an oxidant (chlorine or H2O2) that absorbs UV photons and decays to form a reactive product that can destroy contaminants. In the case of H2O2 decomposition, the main useful product is the hydroxyl radical (•OH). Chlorine photolysis also produces •OH, but it also produces several reactive chlorine species (RCS), including Cl• and ClO•. The efficiency by which UV photolysis forms these useful products depends on how well each oxidant absorbs UV photons, and in turn, the efficiency by which the resulting photolysis produces the •OH and the RCS—i.e., the quantum yield. Fig. 1 illustrates that when using low pressure (LP) UV lamps that emit primarily at 254 nm, both hypochlorous acid (HOCl) and hypochlorite (OCl−) absorb photons about three times more efficiently than H2O2 on a per-mole basis. Medium pressure (MP) lamps emit photons not only near 254 nm but also in the 290–350 nm range, which are absorbed by HOCl and OCl− but not effectively by H2O2.
Fig. 1.
Typical UV lamp emission spectra (left) and H2O2 and chlorine adsorption spectra (right) Low pressure and medium pressure lamp emissions are not to scale: medium pressure emission is typically much higher than low pressure (from WRF, 2022).
When one mole of H2O2 absorbs one mole of photons at 254 nm, it produces 1.11 mol of •OH radicals (i.e., the quantum yield is 1.11) (Goldstein et al., 2007). The quantum yield of •OH formation from chlorine photolysis is less certain, reportedly ranging between 0.46–1.4 when HOCl undergoes photolysis (Stefan, 2018; Chuang et al., 2017; Bulman et al., 2019), and from 0.12 to 0.61 when OCl− is photolyzed (Stefan, 2018). Furthermore, the quantum yield of production of RCS via chlorine photolysis at 254 nm is uncertain, with Stefan et al. (2018) and Chuang et al. (2017) reporting a quantum yield of Cl• formation from OCl− and HOCl in the order of 0.3–0.6 at 254 nm. The quantum yields associated with MP UV (i.e., with wavelengths other than 254 nm) are also unreported. These uncertainties suggest that models to predict the amount of •OH and RCS available to treat the water under different UV-chlorine treatment conditions may be inaccurate. This is a current weakness of UV-chlorine treatment design: performance must be predicted largely on empirical and uncertain evidence. More research in this area is needed.
An additional factor that governs both UV-chlorine and UV-H2O2 performance is the scavenging of the radicals and reactive species by the chlorine and H2O2 themselves. •OH reacts at least 4.5 times more quickly with OCl− than with HOCl (Buxton and Subhani, 1972; Anastasio and Matthew, 2006), which is a contributing factor that may lead UV-chlorine to be more effective at pH values below the pKa of chlorine (about 7.5 at 25 °C) where HOCl predominates. This has helped UV-chlorine to become an attractive option for water reuse treatment that employs reverse osmosis (RO). RO permeate typically has a pH of 5–6 due to anti-fouling acid injection, and may make UV-chlorine more cost effective relative to UV-H2O2. Ongoing research is also demonstrating that RCS species may be more effective at higher pH, potentially widening the pH envelope of UV-chlorine relative to UV-H2O2 depending on the susceptibility of a specific contaminant to RCS reaction (Guo et al., 2017, 2022). UV-chlorine may also be more efficient at higher pH if using MP UV lamps. OCl− is a very effective absorber of photons emitted at the higher wavelengths by MP lamps (Fig. 1) and, in particular, the photolysis of OCl− leads to not only •OH but also to RCS (Zhou et al., 2019). There is little reported about the performance of MP lamps at higher pH to treat contaminants, particularly those susceptible to RCS reaction.
Research needs. At present, UV-chlorine treatment design is largely empirical. Many of the details of chlorine photochemistry remain unknown. More data must be developed to allow accurate treatment models to be advanced. Such models could then be used to optimize process design.
Ammonia, chloramines, nitrate, and nitrite
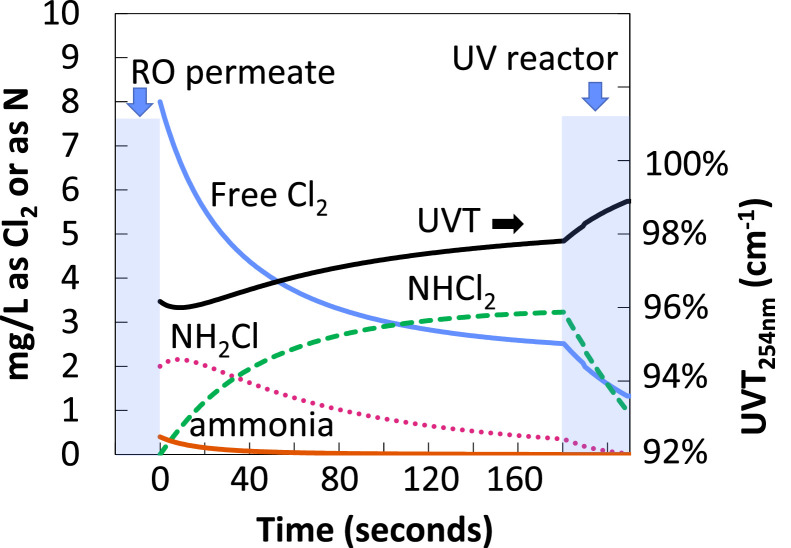
The degree of nitrification/denitrification that precedes RO and advanced oxidation can be erratic unless the plant is well designed and operated for this purpose. As a result, the concentrations of ammonia, nitrate, and nitrite arriving at the RO can be widely variable, and RO is an incomplete barrier against these species. Furthermore, chloramines are often applied as an antifoulant for the RO. As such there can be a mixture of all these compounds in the RO permeate. The implications are discussed in the following text and in Fig. 2, which shows model simulations of the reactions of a fictive scenario whereby free chlorine is applied in a RO permeate containing NH3 and/or NH2Cl 180 s upstream of the UV reactor (WRF, 2022).
Fig. 2.
Simulation of the effect of nitrogen species on water entering a fictive UV-chlorine reactor
(from WRF, 2022).
Ammonia: If ammonia concentrations are high (e.g., > 1 to 2 mg-N/L), UV-chlorine treatment may be cost-prohibitive due to the free chlorine demand of the ammonia. Lower ammonia concentrations may be acceptable, but they will still exert a chlorine demand. The reaction between ammonia and free chlorine is essentially instantaneous at neutral and higher pH, but at pH 5.5 the reaction may take tens of seconds to complete (Fig. 2). Therefore, one must account for the chlorine demand exerted by the ammonia as the water travels from the chlorine injection point to both the location of the chlorine analyzer, and the entry to the UV reactor, in order to accurately establish the desired free chlorine concentration entering the reactor.
Chloramines: Just like ammonia, chloramines exert a free chlorine demand that is not instantaneous. In particular, monochloramine reacts with free chlorine over several minutes at pH 5.5 to form dichloramine. Thus, the concentration of monochloramine decreases and the concentration of dichloramine increases (Fig. 2). This has three important implications:
-
(1)
It is another complication in measuring the amount of free chlorine entering the UV reactor. The free chlorine measurements must account for the travel time from the chlorine injection site to both the chlorine analyzer and the UV reactor, otherwise the measured chlorine will not correspond to what is actually entering the reactor.
-
(2)
Monochloramine is a very strong •OH scavenger (k = 1.0 × 109 M − 1s−1), while dichloramine is weaker (k = 0.62 × 109 M − 1s−1) (Anastasio and Matthew, 2006). The contribution of both species must be considered in terms of the overall •OH scavenging in the water in the reactor, but if the travel time between free chlorine addition and the reactor increases, the chloramine speciation becomes arguably more favorable, as monochloramine is converted to dichloramine. An example of a fictive RO permeate containing a mixture of chloramines, nitrate, and nitrite, is shown in Table 1, demonstrating that the chloramines are contributing about 30% to the overall •OH scavenging. UV-chlorine performance will therefore be strongly influenced by the amount of chloramines, and the ratio of mono- versus dichloramine.
-
(3)
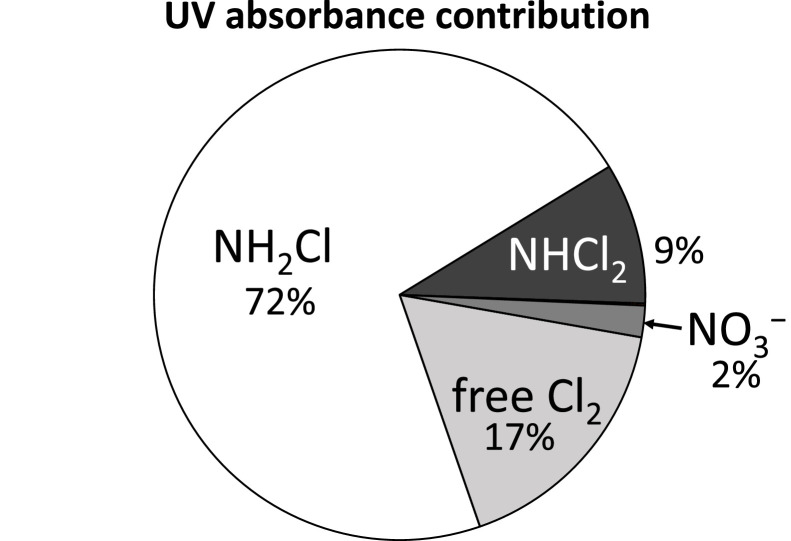
Monochloramine has a molar absorption coefficient of about twice that of dichloramine at 254 nm (WRF, 2022). This is shown in Fig. 3 for a fictive RO permeate, illustrating that monochloramine may be the dominant UV absorber, preventing more efficient chlorine photolysis to drive the treatment process. This suggests that, as with point (2), allowing more time for free chlorine to convert monochloramines to dichloramine prior to the water entering the UV reactor is beneficial. However, more reaction time also allows more free chlorine to be consumed, which is detrimental to treatment performance. At present, there are no readily available models that a designer can use to easily simulate and determine the optimum reaction time between the point of chlorine injection and the UV reactor for different combinations of free chlorine dose and ammonia/chloramine concentration.
Table 1.
•OH scavenging of nitrogen species post-RO (pH 5.5).
| Species | Concentration | k•OH (M − 1s − 1) | Contribution to •OH scavenging |
Refs. |
|---|---|---|---|---|
| Free chlorine | 3 mg/L as Cl2 | 2.0 × 109 HOCl, 8.8 × 109 OCl− |
71% | 2006 Anastasio and Matthew, 2006 |
| Monochloramine | 2 mg/L as Cl2 | 1.0 × 109 | 23% | Anastasio and Matthew, 2006 |
| Dichloramine | 1.5 mg/L as Cl2 | 6.2 × 108 | 6% | Anastasio and Matthew, 2006 |
| Nitrate | 0.8 mg/L as N | 4.0 × 105b | < 1% | Yin et al. 2020b |
| Nitrite | Variablea | 1.1 × 1010 | Variablea | Yin et al., 2020 |
M − 1s−1 = per molar per second.
Nitrite is likely to be present only in some systems with UV-H2O2, since free chlorine quickly converts nitrite to nitrate.
This reaction rate coefficient is reported in a secondary source from an ambiguous primary source, but evidence suggests that the rate is negligible.
Fig. 3.
Example of percent of photons absorbed at 254 nm in a fictive RO permeate containing 5 mg/L free chlorine, 3 mg/L monochloramine, 4 mg/L dichloramine, and 1.5 mg-N/L nitrate (from WRF, 2022).
Nitrite: While nitrite is unlikely to exist in a UV-chlorine system since it is quickly oxidized by free chlorine to nitrate, it can be a problem for UV-H2O2 systems. Nitrite is an extremely strong •OH scavenger (Table 1). As it is a very strong radical scavenger, it has a strong impact on system sizing. Nitrite removal prior to UV-H2O2 treatment is essential. There is also evidence that for very high UVT water (e.g., 98% cm−1, consistent with RO permeate), there is potential for nitrite formation inside the UV reactor from nitrate and chloramines, especially for MP systems, but also from LP systems (Stefan, 2021).
Research needs. The mathematical models used here to illustrate the influence of chloramines on UV-chlorine performance were developed from experiments conducted at much longer reaction times (e.g., tens of minutes) than is relevant for UV-chlorine, where the kinetics over seconds to several minutes is critical. There is a need to validate or recalibrate these models under short reaction times and other conditions (e.g., pH, temperature) relevant to water reuse, so that the impact of ammonia and chloramines on UV-chlorine treatment can be accurately predicted and mitigated.
Regulatory issues and design goals
Treatment processes must be designed to meet clear treatment goals. If a UV-AOP is to treat water containing a specific contaminant, such as a groundwater polluted with trichloroethylene (TCE), the goal is comparatively straightforward: the AOP system can be designed based on the known or tested reactivity of that contaminant with UV and the reactive species being produced, and then eventual performance can be confirmed by measuring the concentration of that contaminant in the treated water. For water reuse, the situation is far more complex. The UV-AOP is to serve as a barrier against potential contaminants that may be in the water, possibly with irregular frequency. There is therefore complexity in establishing the initial treatment level, and then also in how to monitor ongoing treatment performance once the system is installed.
There is no consensus on treatment goals for UV-AOP systems in water reuse. In California, UV-AOP for indirect potable reuse treatment following RO is required to achieve 6-log “virus” inactivation and 0.5-log 1,4-dioxane as a surrogate for organic microcontaminant removal. If NDMA is present, a UV dose capable of reducing it to below the 10 ng/L notification level may be a secondary treatment target. As jurisdictions continue to develop and promulgate regulations other indicator compounds may be suggested.
The selection of treatment goals should be carefully considered because there may be unintended consequences on the resulting preference for UV-chlorine treatment versus UV-H2O2 or other methods, and in turn, on actual protection against the spectrum of pathogens and chemical contaminants. For example, If the treatment goal for chemical destruction were to be set at 0.5-log 1,4-dioxane reduction, then both UV-chlorine and UV-H2O2 systems could be designed to achieve that goal. While 1,4-dioxane is susceptible to destruction by •OH (k = 2.8 × 109 M − 1s−1, Patton et al., 2017), it is relatively inert to RCS (Chuang et al., 2017). If UV-chlorine and UV-H2O2 were both designed to achieve the same level of •OH production to achieve the same level of 1,4-dioxane destruction, the RCS that are also being formed by the UV-chlorine system would be reacting with and destroying some contaminants that may be in the water and which are reactive with RCS. Thus, in reality, the UV-chlorine system would provide a broader treatment barrier than UV-H2O2, despite both achieving equal regulatory credit based on 1,4-dioxane destruction. If UV-H2O2 were installed instead of UV-chlorine based on cost and due only to their similar ability to destroy 1,4-dioxane, the added benefit of the role of RCS would be overlooked.
UV-chlorineUV-chlorineResearch needs. Very few jurisdictions have established treatment requirements for potable water reuse. The lack of clear treatment goals makes it difficult to design UV-chlorine systems (or any treatment system). Such goals need to be developed, and they should avoid unintended bias against forms of treatment that may be at present poorly understood, but which could ultimately prove to be advantageous (e.g., the role of RCS, wavelengths other than 254 nm, etc.).
Disinfection credit
A benefit of UV-AOP treatment for water reuse is the combination of chemical destruction and disinfection from UV. There is no industry consensus about the degree to which a UV-AOP reactor for water reuse treatment might be required to provide disinfection, but in California, there is a proposal to use UV-AOP to gain a 6-log inactivation credit for Giardia, Cryptosporidium, and viruses (Waterboards, 2021). Demonstrating 6-log inactivation of an organism in a UV reactor through reactor validation testing is at the boundary of what is possible given the need to spike a challenge organism at a concentration high enough to measure a million-fold reduction. An alternative method to solve this problem is the “combined variable approach” for UV reactor validation (Wright et al., 2020). Here, one or (ideally) more challenge organisms are used to benchmark UV reactor disinfection performance and certain operating conditions required to achieve 6-log inactivation of target pathogens are extrapolated. There is some debate among experts about the conditions under which such extrapolation should be allowed, but at present it is arguably the best method available to demonstrate 6-log inactivation of target organisms given the practical challenges in doing so directly.
Another consideration for disinfection using UV-AOP is the action of the oxidant itself. H2O2 is a relatively weak disinfectant on its own (e.g., Labatiuk et al., 1993) and is generally not recognized as a viable primary disinfectant, but chlorine has well-established CT inactivation kinetics for predicting its contribution to disinfection. At present, there has been no known discussion among regulators about allowing the oxidant to be given regulatory disinfection credit. It is also unclear whether the radicals and reactive species would contribute to disinfection, with some studies suggesting that their concentration would be too low to contribute meaningfully (Mamane et al., 2007; Rattanakul and Oguma, 2017). Even if their contribution were significant, there would need to be a method to monitor radical/reactive species concentrations in real time to allow disinfection credit to be obtained, which is not possible, at present.
Research needs. Methods to validate and monitor UV-AOP reactor performance for disinfection, and the role of the oxidant in disinfection, need to be considered.
Treatment performance monitoring
A treatment goal for water reuse might be defined based on the theoretical destruction of a contaminant(s) (e.g., 0.5-log 1,4-dioxane, 6-log virus), but in practice the actual destruction would not be routinely tracked since the contaminants may not be present, or the analysis would be too challenging. Instead, treatment performance is tracked using some combination of UV and oxidant dose monitoring, coupled with data such as flow rate and UV transmittance (UVT). There are generally three approaches that can be used to monitor performance:
-
(1)
Control by minimum electrical energy dose (EED). EED (kWh/volume) measures the power used by the UV lamps, normalized to flow rate. The required EED is based on performance testing at different flow/UVT/oxidant dose/lamp power combinations that are used to define the operating window. This approach assumes some minimum allowable chlorine concentration and UVT, and perhaps an allowable pH range. As such, unless these parameters are all at their limits, the system is always overdosing to some extent. This is the simplest and most conservative approach.
-
(2)
Control by minimum dose. The UV system's programmable logic controller (PLC) calculates the required UV dose applied to the water as a function of UV lamp power, flow rate, and UVT using an algorithm validated via field testing similar to the EED approach. It uses a fixed minimum oxidant dose and potentially limits to other parameters, such as pH.
-
(3)
Control by calculated log reduction. This is the most complex algorithm, but theoretically achieves the most efficient treatment performance. The PLC calculations account for UV lamp power, flow rate, UVT, oxidant concentration, and potentially other water quality parameters, to output the predicted log reduction of the target contaminant for those instantaneous conditions using an algorithm validated via field testing like the previous two methods. As of the time of this article, there is one site in California with an operating permit that uses this approach.
As the control method increases in complexity, so does the required validation testing (i.e., number of test conditions) to verify its ability to maintain required treatment. From the discussion above, it's evident that a UV-chlorine system must use multiple sensors to ensure sufficient treatment. Some important considerations are the following:
UVT: UVT can be monitored prior to the chlorine addition point, or at the entry to the UV reactor. Chlorine has a strong absorbance at 254 nm and at other wavelengths (relevant if a MP lamp is used) (Fig. 1), so if UVT is monitored only prior to chlorine addition, the PLC must estimate the change in UVT due to the chlorine addition. It is important to recognize that if ammonia/chloramines are present in the water, the UVT may change considerably as they react with chlorine in the travel time between the sampling line and the UVT monitor, making the UVT reading an inaccurate estimate of what is entering the UV reactor. As such, travel times to the UVT monitor should either be kept very short, or similar to the travel time between the sampling point and the UV reactor inlet. UVT can also be monitored at the reactor exit since some UV dose algorithms can use both influent/effluent values for calculation. Some RO permeate may have UVT values greater than 97% cm−1, requiring special UVT monitors with longer path lengths (e.g., 2 - 5 cm) to achieve accuracy. It is not uncommon for algorithms not to take credit for UVT values above 98% due to the uncertainty in the measurement in the very high UVT range.
Free chlorine: The issues associated with measuring free chlorine when ammonia and chloramines are present have already been discussed. In short, care must be taken to provide a conservative estimate of free chlorine concentration entering the UV reactor, as there are ongoing reactions occurring between the species as the water is travelling to both the monitor and the reactor from the sampling point. As with UVT monitoring, travel times to the chlorine analyzer should either be kept very short, or similar to the travel time between the sampling point and the UV reactor inlet. It should be repeated, however, that this complication only exists if ammonia/chloramines are present in the RO permeate, otherwise free chlorine application and monitoring is greatly simplified.
Research needs. Practical experience with monitoring UV-chlorine processes is limited. As more systems are brought online, stakeholders should disseminate the lessons learned. There is limited information about real-world UV-chlorine installations in peer-reviewed literature or trade journals.
O&M costs
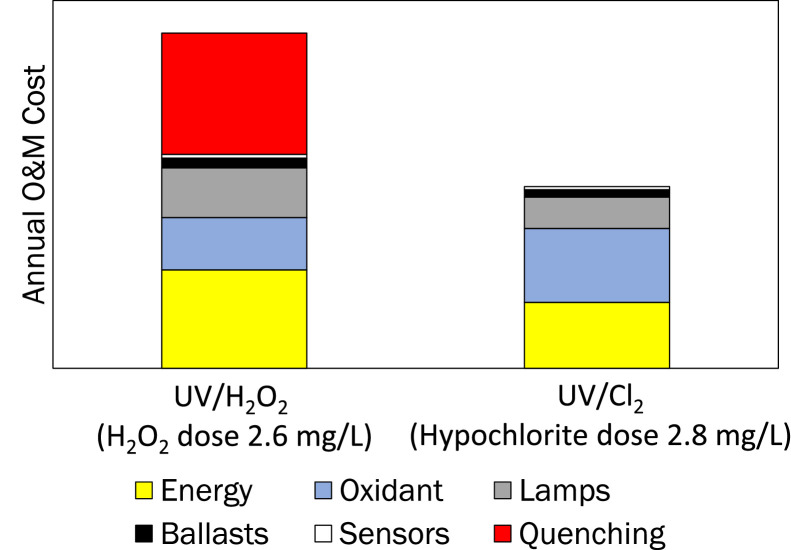
UV-chlorine system costing is very reactor-, location-, and configuration-specific. WRF (2022) reported an example comparison of estimated annual operations and maintenance costs (in California) for UV-chlorine relative to UV-H2O2 treatment of potable reuse water following RO, when treating to achieve 0.5-log 1,4-dioxane destruction and 1.2-log NDMA reduction (Fig. 4). It predicts that energy costs are lower for UV-chlorine than UV-H2O2, but that oxidant costs for treatment are slightly higher. The major difference in the costs shown in Fig. 4 is for quenching residual H2O2. It is assumed that such quenching is achieved by application of chlorine. If there is no need to quench residual H2O2, the cost of UV- H2O2 will drop significantly, making the two options more comparable.
Fig. 4.
Comparison of annual O&M Costs for UV-chlorine and UV-H2O2 for a 12-mgd reuse installation with low ammonia and nitrite; LPHO lamp-based UV-AOP systems (from WRF, 2022).
WRF (2022) reported that in general, low pressure high output UV systems will have a higher purchase price than MP due to the greater number of lamps and ballasts required, but that annual O&M costs for MP systems may be in the order of 50% higher. This is based on very limited data. The relative cost of LP vs. MP is also likely to be a function of treatment targets. If NDMA photolysis is required, MP is more inefficient than LP because the photons emitted by MP at wavelengths greater than 254 nm are poorly absorbed by NDMA, however they may be efficiently absorbed by chlorine to drive chemical destruction. As such, MP might be more cost-competitive if treatment requires only chemical oxidation and not NDMA destruction. More research is needed to test this theory.
Source water quality considerations
The municipal water that supplies potable reuse projects can vary widely over time depending on weather events, season and sources of contribution to the collection system. Much of this variability may be reduced through the upstream treatment train, but nonetheless careful consideration should be given to properly characterizing the range of water quality to be treated by the UV-chlorine system. Ammonia and chloramines are particularly important to characterize if they are present in the RO permeate for reasons already explained. The range of expected UVT is critical. AOP performance is heavily influenced by the •OH (and RCS) scavenging capacity in the water. In RO permeate, the contribution of such scavenging by effluent organic matter (EFOM) is likely to be small compared to the scavenging due to the chlorine and chloramines, whose concentrations can be measured or accurately predicted at the design stage. As such, the degree of •OH scavenging in the water can be accurately predicted and its impact on treatment performance determined. In contrast, the scavenging of RCS by these compounds is largely unknown, making overall UV-chlorine treatment models uncertain.
Research needs. The role of RCS for treatment performance, and their scavenging by constituents in the water, is very poorly understood. Until more is learned, it will be difficult to quantify the added benefit of these potentially useful species when designing UV-chlorine treatment beyond demonstrating log removal of the target compound (typically 1,4-dioxane).
Byproducts and toxicity
There is concern that the relatively high chlorine concentrations needed for UV-chlorine treatment (e.g., 2–5 mg/L), coupled with photolysis and the generation of RCS, might lead to the formation of toxic byproducts. Partially mitigating this fear is that the chlorine reaction time available to form byproducts is small. This is because chlorine is typically added immediately upstream of the UV reactor, and a well-designed reactor may lead to the almost complete destruction of the chlorine within tens of seconds. Nevertheless, thisan area of current research.
There are only a few reported studies of the formation of conventional drinking water disinfection byproducts (DBPs) from UV-chlorine. Wang et al. (2015, 2019) and evaluated conventional DBP formation in bench-, pilot-, and full-scale UV-chlorine drinking water systems treating surface waters (TOC ranging from 1.5 - 3.5 mg-C/L, chlorine ranging up to 10 mg/L), and generally reported minimal THM and HAA formation (< 13 μg/L total formation) across the UV reactors. Pisarenko et al. (2013) reported similar minimal THM/HAA formation when treating Colorado River water at bench-scale using UV-chlorine. There is some consensus (albeit with very preliminary data) that haloacetonitrile formation may be enhanced across UV-chlorine, but still in the order of < 10 μg/L. Nevertheless, despite not being regulated in jurisdictions known to the authors, these N-containing byproducts are reported to be more toxic than common regulated DBPs (Muellner et al., 2007; Plewa et al., 2008).
Interestingly, Wang et al. (2019) found that adsorbable organohalide formation (AOX—a measure of all organohalide byproducts) increased by 70 μg-Cl/L across UV (MP)-Cl treatment compared to a chlorine-only control, but only at pH 6.5 and not pH 8.0. This implies that the increase in AOX precursors was due to the role of •OH rather than RCS, since RCS is formed more significantly at higher pH.
Research has also examined the potential formation of toxicity as measured by bioassays across UV-chlorine. Wang et al. (2018) suggested that •OH has a theoretical > 80% probability of generating transformation products from a survey of a large number of different micropollutants. In experiments, however, Huang et al. (2017) and Li et al. (2016) both reported a reduction in cytotoxicity and estrogenicity of specific micropollutants due to UV-chlorine, and Sun et al. (2019) reported that UV-chlorine led to 22–27% less overall cytotoxicity and genotoxicity than UV-H2O2 after post-chlorination. In contrast, Chaves et al. (2020) reported that transformation products of 17β-estradiol and 17α-ethinylestradiol were more cytotoxic following UV-chlorine than UV-H2O2. Plewa et al. (2012) found that LP and MP UV-chlorine treatment of reclaimed Ohio River water were equally or less genotoxic and cytotoxic than water treated in chlorine-alone controls. Hua et al. (2021) reported that UV-chlorine treatment of secondary wastewater effluent led to a 19–76% reduction in acute toxicity and genotoxicity, respectively.
Of unique concern to UV-chlorine treatment is the potential presence of chlorate and bromate. Chlorate can be found in sodium hypochlorite, especially if it is stored for long periods, with jurisdictions setting limits in the order of 0.7–1.0 mg/L (WHO, 2017; Health Canada, 2008). The application of relatively high chlorine doses for UV-chlorine treatment can therefore directly lead to chlorate in the finished water since it is not destroyed by the AOP. Furthermore, UV- chlorine photolysis inevitably forms chlorate, with the molar yields reportedly ranging from 5 to 20% of the chlorine that undergoes photolysis (Buxton and Subhani, 1972; Wang et al., 2015). If bromide is present in the source water, the UV-chlorine process can convert it to bromate, which can have a low drinking water limit (10 μg/L) in some jurisdictions (USEPA, 2023). The chemistry of this formation pathway is complex, with some water conditions being much more susceptible to bromate formation than others. The reader is referred to WRF (2022) for details of this phenomenon, and mitigation measures.
Research needs. Our understanding of byproducts, transformation products, and toxicity, when applying UV-chlorine is limited. However, the research to date generally shows no indications of any such outcomes that would prevent the use of UV-chlorine for potable reuse. However, much more research on this topic is warranted, but similar research is also warranted for other oxidation strategies that could be applied in the context of potable water reuse—or indeed, for other reuse applications such as crop irrigation.
Conclusions
UV-chlorine is a new treatment technology that is well suited for potable reuse treatment following RO. While this mini-review points to uncertainties and challenges associated with UV-chlorine, none of these prevent its more widespread adoption. The community of end users are encouraged to continue a dialog to share the lessons learned as its use grows. The research community is encouraged to try to resolve some of the key unanswered questions associated with chlorine photochemistry to advance best practices and process optimization.
Author contributions
Authors Mackey and Hofmann assembled the entirety of this manuscript, using information developed and contributed by all the remaining authors listed, in addition to original information developed by Mackey and Hofmann themselves.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Ron Hofmann reports financial support was provided by Water Research Foundation and the State of California.
Acknowledgments
This work was supported by Water Research Foundation and the California State Water Resources Control Board under Proposition 1Author: Please provide missing Proposition 1. The authors thank these organizations for their financial, technical, and administrative assistance. This material does not necessarily reflect the views and policies of the funders. The authors also appreciate the assistance provided by Trojan Technologies, De Nora, Xylem, Hampton Roads Sanitation District, Region of Peel, Los Angeles Department of Water & Power, San Diego Pure Water Division, Cornwall Ontario, Gwinnett County Department of Water Resources, Orange County Water District, Region of Waterloo Ontario, Southern Water, and the Water Replenishment District of Southern California.
Data availability
No data was used for the research described in the article.
References
- Anastasio C., Matthew B.M. A chemical probe technique for the determination of reactive halogen species in aqueous solution: part 2–Chloride solutions and mixed bromide/chloride solutions. Atmosph. Chem. Phys. 2006;6(9):2439–2451. [Google Scholar]
- Bulman D.M., Mezyk S.P., Remucal C.K. The impact of pH and irradiation wavelength on the production of reactive oxidants during chlorine photolysis. Environ. Sci. Technol. 2019;53(8):4450–4459. doi: 10.1021/acs.est.8b07225. [DOI] [PubMed] [Google Scholar]
- Buxton G.V., Subhani M.S. Radiation chemistry and photochemistry of oxychlorine ions. Part 2.—photodecomposition of aqueous solutions of hypochlorite ions. J. Chem. Soc. Faraday Trans. 1: Phys. Chem. Condens. Phases. 1972;68:958–969. [Google Scholar]
- Chaves F.P., Gomes G., Della-Flora A., Dallegrave A., Sirtori C., Saggioro E.M., Bila D.M. Comparative endocrine disrupting compound removal from real wastewater by UV/Cl and UV/H2O2: effect of pH, estrogenic activity, transformation products and toxicity. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141041. [DOI] [PubMed] [Google Scholar]
- Chuang Y.H., Chen S., Chinn C.J., Mitch W.A. Comparing the UV/monochloramine and UV/free chlorine advanced oxidation processes (AOPs) to the UV/hydrogen peroxide AOP under scenarios relevant to potable reuse. Environ. Sci. Technol. 2017;51(23):13859–13868. doi: 10.1021/acs.est.7b03570. [DOI] [PubMed] [Google Scholar]
- Festger A., Stefan M., Royce A., Kwon M. Proceedings of the WateReuse Annual Conference (Virtual) 2021. Selecting your UV/AOP: UV/peroxide, UV/chloramine, or UV/chlorine?”. March 16-25. [Google Scholar]
- Goldstein S., Aschengrau D., Diamantm Y., Rabani J. Photolysis of aqueous H2O2: quantum yield and applications for polychromatic UV actinometry in photoreactors. Environ. Sci. Technol. 2007;41(21):7486–7490. doi: 10.1021/es071379t. [DOI] [PubMed] [Google Scholar]
- Guo K., Wu Z., Shang C., Yao B., Hou S., Yang X., Song W., Fang J. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water. Environ. Sci. Technol. 2017;51(18):10431–10439. doi: 10.1021/acs.est.7b02059. [DOI] [PubMed] [Google Scholar]
- Guo K., Wu Z., Chen C., Fang J. UV/chlorine process: an efficient advanced oxidation process with multiple radicals and functions in water treatment. Acc. Chem. Res. 2022;55(3):286–297. doi: 10.1021/acs.accounts.1c00269. [DOI] [PubMed] [Google Scholar]
- Health Canada . Healthy Environments and Consumer Safety Branch, Health Canada; Ottawa, Ontario: 2008. Guidelines For Canadian Drinking Water Quality: Guideline Technical Document — Chlorite and Chlorate. Water Quality and Health Bureau.https://publications.gc.ca/collections/collection_2009/sc-hc/H128-1-08-549E.pdf [Google Scholar]
- Hua Z., Li D., Wu Z., Wang D., Cui Y., Huang X., Fang J., An T. DBP formation and toxicity alteration during UV/chlorine treatment of wastewater and the effects of ammonia and bromide. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116549. [DOI] [PubMed] [Google Scholar]
- Huang N., Wang T., Wang W.L., Wu Q.Y., Li A., Hu H.Y. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: synergistic effect, transformation products and toxicity evaluation. Water Res. 2017;114:246–253. doi: 10.1016/j.watres.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Labatiuk C.W., Belosevic M., Finch G.R. Inactivation of Giardia muris using ozone and ozone-hydrogen peroxide. Ozone: Sci. Eng. 1993;16(1):67–78. [Google Scholar]
- Li M., Xu B., Liungai Z., Hu H.Y., Chen C., Qiao J., Lu Y. The removal of estrogenic activity with UV/chlorine technology and identification of novel estrogenic disinfection by-products. J. Hazard. Mater. 2016;307:119–126. doi: 10.1016/j.jhazmat.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Mamane H., Shemer H., Linden K.G. Inactivation of E. coli, B. subtilis spores, and MS2, T4, and T7 phage using UV/H2O2 advanced oxidation. J. Hazard. Mater. 2007;146(3):479–486. doi: 10.1016/j.jhazmat.2007.04.050. [DOI] [PubMed] [Google Scholar]
- Muellner M.G., Wagner E.D., McCalla K., Richardson S.D., Woo Y.T., Plewa M.J. Haloacetonitriles vs. regulated haloacetic acids: are nitrogen-containing DBPs more toxic? Environ. Sci. Technol. 2007;41(2):645–651. doi: 10.1021/es0617441. [DOI] [PubMed] [Google Scholar]
- Patton S., Li W., Couch K.D., Mezyk S.P., Ishida K.P., Liu H. Impact of the ultraviolet photolysis of monochloramine on 1, 4-dioxane removal: new insights into potable water reuse. Environ. Sci. Technol. Lett. 2017;4(1):26–30. [Google Scholar]
- Pisarenko A.N., Stanford B.D., Snyder S.A., Rivera S.B., Boal A.K. Investigation of the use of chlorine based advanced oxidation in surface water: oxidation of natural organic matter and formation of disinfection byproducts. J. Adv. Oxid. Technol. 2013;16(1):137–150. [Google Scholar]
- Plewa M.J., Muellner M.G., Richardson S.D., Fasano F., Buettner K.M., Woo Y.T., McKague A.B., Wagner E.D. Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: an emerging class of nitrogenous drinking water disinfection byproducts. Environ. Sci. Technol. 2008;42(3):955–961. doi: 10.1021/es071754h. [DOI] [PubMed] [Google Scholar]
- Plewa M.J., Wagner E.D., Metz D.H., Kashinkunti R., Jamriska K.J., Meyer M. Differential toxicity of drinking water disinfected with combinations of ultraviolet radiation and chlorine. Environ. Sci. Technol. 2012;46(14):7811–7817. doi: 10.1021/es300859t. [DOI] [PubMed] [Google Scholar]
- Rattanakul S., Oguma K. Analysis of hydroxyl radicals and inactivation mechanisms of bacteriophage MS2 in response to a simultaneous application of UV and chlorine. Environ. Sci. Technol. 2017;51(1):455–462. doi: 10.1021/acs.est.6b03394. [DOI] [PubMed] [Google Scholar]
- Stefan M.I. Ed. IWA Publishing; London, UK: 2018. Advanced Oxidation Processes For Water Treatment: Fundamentals and Applications. [Google Scholar]
- Stefan M.I. Presented at the 2021 IUVA Asia Workshop; 2021. UV/AOPs For Water Reuse: From Science to Implementation. February 19, 2021. [Google Scholar]
- Sun P., Meng T., Wang Z., Zhang R., Yao H., Yang Y., Zhao L. Degradation of organic micropollutants in UV/NH2Cl advanced oxidation process. Environ. Sci. Technol. 2019;53(15):9024–9033. doi: 10.1021/acs.est.9b00749. [DOI] [PubMed] [Google Scholar]
- USEPA (2023) National primary drinking water regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Byproducts. Accessed February 24, 2023.
- Wang D., Bolton J.R., Andrews S.A., Hofmann R. UV/chlorine control of drinking water taste and odor at pilot and full-scale. Chemosphere. 2015;136:239–244. doi: 10.1016/j.chemosphere.2015.05.049. [DOI] [PubMed] [Google Scholar]
- Wang W.L., Wu Q.Y., Huang N., Xu Z.B., Lee M.Y., Hu H.Y. Potential risks from UV/H2O2 oxidation and UV photocatalysis: a review of toxic, assimilable, and sensory-unpleasant transformation products. Water Res. 2018;141:109–125. doi: 10.1016/j.watres.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Wang C., Moore N., Bircher K., Andrews S., Hofmann R. Full-scale comparison of UV/H2O2 and UV/Cl2 advanced oxidation: the degradation of micropollutant surrogates and the formation of disinfection byproducts. Water Res. 2019;161:448–458. doi: 10.1016/j.watres.2019.06.033. [DOI] [PubMed] [Google Scholar]
- Waterboards (2021). Derivation of log removal values for the addendum to a framework for regulating direct potable reuse, presenting an early draft of the anticipated criteria for DPR.
- WRF . The Water Research Foundation; Denver, CO: 2022. UV-Chlorine AOP in Potable Reuse: A Guidance Manual to Assessment and Implementation.https://www.waterrf.org/research/projects/uvchlorine-aop-potable-reuse-assessment-applicability-operational-issues-and Accessed February 24, 2023. [Google Scholar]
- Wright H., Heath M., Brooks T., Adams J. U.S. Environmental Protection Agency; Washington, DC: 2020. Innovative Approaches for Validation of Ultraviolet Disinfection Reactors for Drinking Water Systems; p. 2020.EPA/600/R-20/094 [Google Scholar]
- Yin R., Blatchley III E.R., Shang C. UV photolysis of mono-and dichloramine using UV-LEDs as radiation sources: photodecay rates and radical concentrations. Environ. Sci. Technol. 2020;54(13):8420–8429. doi: 10.1021/acs.est.0c01639. [DOI] [PubMed] [Google Scholar]
- Zhou S., Zhang W., Sun J., Zhu S., Li K., Meng X., Luo J., Shi Z., Zhou D., Crittenden J.C. Oxidation mechanisms of the UV/free chlorine process: kinetic modeling and quantitative structure activity relationships. Environ. Sci. Technol. 2019;53(8):4335–4345. doi: 10.1021/acs.est.8b06896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.