Abstract
BACKGROUND
In June 2019, the Bolivian Ministry of Health reported a cluster of cases of hemorrhagic fever that started in the municipality of Caranavi and expanded to La Paz. The cause of these cases was unknown.
METHODS
We obtained samples for next-generation sequencing and virus isolation. Human and rodent specimens were tested by means of virus-specific real-time quantitative reverse-transcriptase–polymerase-chain-reaction assays, next-generation sequencing, and virus isolation.
RESULTS
Nine cases of hemorrhagic fever were identified; four of the patients with this illness died. The etiologic agent was identified as Mammarenavirus Chapare mammarenavirus, or Chapare virus (CHAPV), which causes Chapare hemorrhagic fever (CHHF). Probable nosocomial transmission among health care workers was identified. Some patients with CHHF had neurologic manifestations, and those who survived had a prolonged recovery period. CHAPV RNA was detected in a variety of human body fluids (including blood; urine; nasopharyngeal, oropharyngeal, and bronchoalveolar-lavage fluid; conjunctiva; and semen) and in specimens obtained from captured small-eared pygmy rice rats (Oligoryzomys microtis). In survivors of CHHF, viral RNA was detected up to 170 days after symptom onset; CHAPV was isolated from a semen sample obtained 86 days after symptom onset.
CONCLUSIONS
M. Chapare mammarenavirus was identified as the etiologic agent of CHHF. Both spillover from a zoonotic reservoir and possible person-to-person transmission were identified. This virus was detected in a rodent species, O. microtis. (Funded by the Bolivian Ministry of Health and others.)
CHAPARE VIRUS (CHAPV; GENUS MAMmarenavirus, family Arenaviridae) causes Chapare hemorrhagic fever (CHHF). Mammarenaviruses cause viral hemorrhagic fevers throughout South America. Surveillance is challenging because of a lack of diagnostic testing and because of the clinical similarity between CHHF and other endemic diseases.1-3 In 1963, Machupo virus (MACV) was identified as the cause of Bolivian hemorrhagic fever in the department of Beni, Bolivia.4,5 In 2003, cases of hemorrhagic fever were reported in the department of Cochabamba, approximately 350 km from Beni. Specimens obtained from one patient who had died contained a novel arenavirus, CHAPV.6 Data on cases of CHHF have been lacking since then.
Natural transmission of CHAPV to humans probably occurs through exposure to infected rodents or their excreta, although the reservoir is unknown.2,6 Human-to-human transmission of other mammarenaviruses, but not CHAPV, has been documented.1,2,5,6 Lassa virus, an Old World mammarenavirus, has been detected in urine, breast milk, semen, and cerebrospinal fluid, and it may persist after clinical recovery, but such persistence of New World mammarenaviruses is undocumented.7,8
In June 2019, the Bolivian Ministry of Health reported a cluster of cases of hemorrhagic fever of unknown cause that started in the municipality of Caranavi and expanded to La Paz; some clinical features had been described previously.9 Here, we describe evidence of nosocomial transmission of CHAPV, additional clinical data on CHHF, the development of new CHAPV diagnostic assays, evidence of a likely rodent reservoir, complete genome sequencing of the 2019 strains, and CHAPV persistence in patients who had recovered from CHHF.
METHODS
Laboratory Testing
Specimens were collected in accordance with the outbreak response protocols of the Bolivian Ministry of Health. At the Bolivian Center for Tropical Diseases (Centro Nacional de Enfermedades Tropicales), the specimens tested negative on molecular assays, serologic assays, or both for hantavirus, MACV, dengue virus, yellow fever virus, chikungunya virus, Zika viruses, and leptospira. They were then sent to the Centers for Disease Control and Prevention for further testing in a biosafety level 4 laboratory. Extracted RNA was analyzed by means of next-generation sequencing. Specific real-time quantitative reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assays targeting the L and S segments of CHAPV were designed and used to test specimens obtained from humans and rodents. Virus isolation was attempted with the use of Vero E6 cells. Serologic testing was performed with the use of Junin virus, MACV, and CHAPV antigens.
ECOLOGIC INVESTIGATION
Samples from small mammals (rodents and a marmoset) were obtained in the municipalities of Caranavi and Guanay, where the index case patient (Patient S1-1) lived and farmed (Fig. 1). Rodent trapping and specimen collection were conducted from July 7 through July 9, 2019, in accordance with standard published methods.10 Protocols for the testing of animals were approved by the Bolivian Ministry of Health.
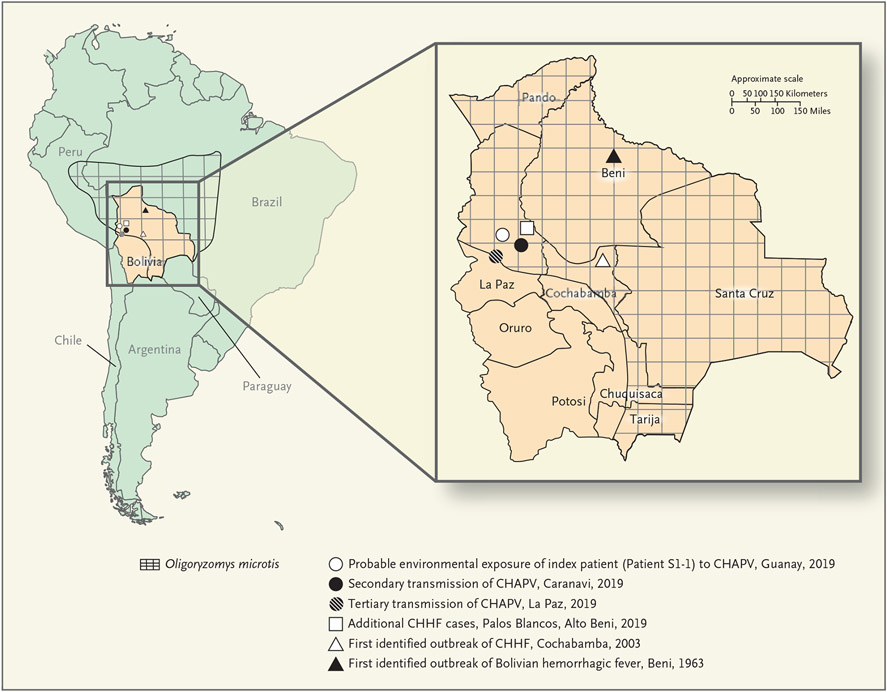
Figure 1. Chapare Virus in Bolivia.
This map of South America shows the location of Bolivia and the distribution of the possible rodent reservoir of Chapare virus (CHAPV), small-eared pygmy rice rats (Oligoryzomys microtis). The inset shows the history of transmission of Bolivian hemorrhagic fever and Chapare hemorrhagic fever (CHHF) and the locations of CHAPV transmission in 2019.
Research involving human subjects was approved by the Bolivian Ministry of Health and adhered to outbreak response protocols. Detailed methods are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Results
CLINICAL FEATURES OF CHHF AND NOSOCOMIAL TRANSMISSION
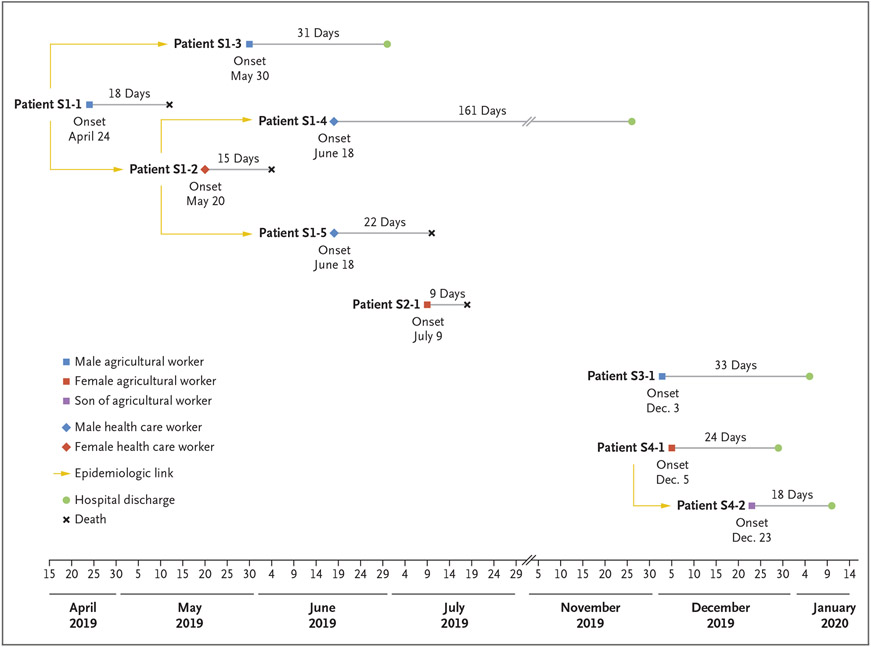
Between April 24 and June 18, 2019, viral hemorrhagic fever–like syndromes developed in five persons, and they were hospitalized. Clinical and epidemiologic data are summarized in Table 1 and Figure 2; detailed case summaries are provided in the Supplemental Results section in the Supplementary Appendix. The suspected sequence associated with possible nosocomial transmission is shown in Figure S1 in the Supplementary Appendix.
Table 1.
Epidemiologic and Clinical Characteristics of Patients with Chapare Hemorrhagic Fever (CHHF) in 2019.*
| Patient No. |
Age | Sex | Occupation or Description |
Type and Location of Exposure |
Onset Date; Incubation Period |
Presenting Symptoms |
Laboratory Findings |
Unique Features | Outcome | Method of CHHF Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| yr | ||||||||||
| S1-1 † | 65 | M | Agricultural worker | Unknown environmental exposure in Guanay | April 24; unknown | Fever, headache, myalgia, arthralgia, abdominal pain, nausea, retro-orbital pain, lumbar pain, diarrhea | ND | Gingival hemorrhage | Died on May 12 (18 days after onset) | None (probable case) |
| S1-2 † | 25 | F | Medical intern | Attended to Patient S1-1 during hospitalization in Caranavi | May 20; 9 days | Fever, headache, malaise, myalgia, arthralgia, lumbar pain, abdominal pain, nausea, vomiting, gingival hemorrhage | Anemia, leukopenia, thrombocytopenia, elevated aminotransferase levels, coagulopathy | Gingival and vaginal hemorrhage, generalized seizure, hemorrhagic shock | Died on June 4 (15 days after onset) | NGS, RT-PCR |
| S1-3 † | 25 | M | Agricultural worker | Unknown environmental exposure in Guanay or contact with Patient S1-1 while Patient S1-1 was symptomatic in Caranavi | May 30; ≥19 days | Fever, headache, malaise, myalgia, arthralgia, retro-orbital pain, vomiting, abdominal pain, gingival hemorrhage, irritability, psychomotor excitation, disorientation | Anemia, leukopenia, thrombocytopenia, elevated aminotransferase levels, coagulopathy | Generalized seizure, confusion, agitation, hemiparesis on left side, hemorrhagic signs (gingival, GI, intracerebral, and injection-site bleeding; epistaxis; hematuria; petechiae and ecchymoses), hemorrhagic shock, multiorgan failure, hypoxemic respiratory failure, prolonged neurologic deficits for several months after discharge | Discharged on June 30 (31 days after onset) | RT-PCR, virus isolation, NGS on virus isolation, anti-CHAPV IgM and IgG |
| S1-4 † | 48 | M | Physician | Attended to Patient S1-2 during ambulance transfer from Caranavi to La Paz | June 18; 16 days | Malaise, fatigue, odynophagia, arthralgia, myalgia, muscle weakness in legs, inability to walk | Leukopenia, thrombocytopenia, elevated aminotransferase levels, acute kidney injury | Paraparesis, hypoxemic respiratory failure, gingival hemorrhage, confusion, agitation, prolonged muscular and neurologic deficits for several months after discharge | Discharged on Nov. 26 (161 days after onset)‡ | NGS, RT-PCR, virus isolation, anti-CHAPV IgM and IgG |
| S1-5 † | 42 | M | Physician | Performed upper GI endoscopy for Patient S1-2 in La Paz | June 18; 14 days | Fever, headache, myalgia, arthralgia, diarrhea | Anemia, leukopenia, thrombocytopenia, elevated aminotransferase levels, acute kidney injury, coagulopathy | Generalized seizure, confusion, agitation, hyporeflexia of legs, hemorrhagic signs (gingival, GI, and injection-site bleeding; petechiae and ecchymoses), multiorgan failure, hemorrhagic shock | Died on July 10 (22 days after onset) | NGS, RT-PCR, virus isolation |
| S2-1 † § | 29 | F | Agricultural worker | Unknown environmental exposure in Palos Blancos | July 9; unknown | Fever, headache, myalgia, retro-orbital pain, abdominal pain, vomiting, lethargy, gingival hemorrhage | Anemia, thrombocytopenia, elevated aminotransferase levels | Icterus, cervical lymphadenopathy, respiratory distress, gingival hemorrhage | Died on July 18 (9 days after onset) | NGS, RT-PCR, NGS on virus isolation |
| S3-1 | 47 | M | Agricultural worker | Unknown environmental exposure in Alto Beni | Dec. 3; unknown | Headache, retro-orbital pain, arthralgia, myalgia, neurologic deficits, gingival hemorrhage | ND | Neurologic deterioration, gingival hemorrhage, ND on recovery | Discharged on Jan. 5, 2020 (33 days after onset) | NGS, RT-PCR, anti-CHAPV IgM and IgG |
| S4-1 ¶ | 27 | F | Agricultural worker | Unknown environmental exposure in Caranavi | Dec. 5; unknown | Headache, epistaxis, malaise | ND | Gingival hemorrhage, uncomplicated childbirth, ND on recovery from CHHF | Discharged on Dec. 29 (24 days after onset) | NGS, RT-PCR |
| S4-2 | 7 | M | Child of Patient S4-1 | Unknown environmental exposure or contact with Patient S4-1 in Caranavi | Dec. 23; unknown | Fever, headache, vomiting, lethargy, gingival hemorrhage | ND | Gingival hemorrhage, ND on recovery | Discharged on Jan. 10, 2020 (18 days after onset) | RT-PCR |
CHAPV denotes Chapare virus, GI gastrointestinal, ND no data available, NGS next-generation sequencing, and RT-PCR real-time quantitative reverse-transcriptase–polymerase chain reaction.
Additional clinical data for this patient are provided in the Supplemental Results section in the Supplementary Appendix.
Hospitalization of Patient S1-4 was prolonged out of precaution because of continued detection of CHAPV RNA in body fluids.
This patient was pregnant (16 weeks’ gestation) at the time of symptom onset.
This patient was pregnant (5 weeks’ gestation) at the time of symptom onset.
Figure 2. Transmission Chains Indicating Epidemiologic Linkages of Probable and Confirmed CHHF Cases in 2019.
On April 24, 2019, symptoms developed in the index case patient, Patient S1-1, a 65-year-old male agricultural worker in Guanay Municipality (Fig. 1). He presented twice to hospitals in Caranavi Province with persistent fever, myalgia, retro-orbital pain, headache, nausea, and abdominal and lumbar pain. He received a clinical diagnosis of dengue and was not admitted to the hospital during either visit. On May 7, he presented again and was admitted to the hospital with worsening symptoms and gingival hemorrhage, and he remained hospitalized until he died on May 12. No specimens were obtained for diagnostic testing; therefore, this case was categorized as probable CHHF.
Patient S1-2, a 25-year-old female medical intern in a hospital in Caranavi Province, was in direct contact with Patient S1-1 on May 11. Nine days later (on May 20), she had nausea, vomiting, fever, headaches, myalgia, arthralgia, abdominal pain, lumbar pain, and generalized malaise that worsened over the next week. Subsequent gingival and vaginal hemorrhage, generalized seizure, and hemorrhagic shock, developed, and she was admitted to a local hospital on May 27 and transferred to a tertiary care hospital in La Paz on June 2. She died on June 4 with a clinical diagnosis of dengue.
Patient S1-3, a 25-year-old male agricultural worker in Guanay, shared a home with Patient S1-1 while the patient was ill and was in direct contact with him during his hospitalization on May 11. On May 30, at 19 days after his last exposure to Patient S1-1, Patient S1-3 became ill and was admitted to a hospital in Caranavi. He was then transferred to a tertiary care hospital in La Paz, where he recovered.
Patient S1-4, a 48-year-old male physician, assisted with the ambulance transfer of Patient S1-2 from Caranavi to La Paz on June 2 and had direct exposure to her body fluids. Sixteen days after this exposure (on June 18), symptoms developed, and he was subsequently hospitalized and recovered.
Patient S1-5, a 42-year-old male gastroenterologist in La Paz, performed an endoscopy for Patient S1-2 on June 4. Symptoms developed in Patient S1-5 a total of 14 days later (on June 18). He was hospitalized, and he died on July 10.
Nosocomial transmission was suspected in at least three of these five patients (Patients S1-2, S1-4, and S1-5). These three patients were health care workers with no known risk factors for CHAPV exposure other than contact with an ill patient in a health care setting. Two patients (Patients S1-1 and S1-3) were agricultural workers; environmental exposure was suspected in Patient S1-1. Patient S1-3 had contact with Patient S1-1 while the latter patient was symptomatic; however, an environmental exposure cannot be ruled out (Table 1). All five patients were previously healthy, with no known underlying conditions; three had initial suspected diagnoses of dengue, one of postinfectious encephalomyelitis, and one of viral myopathy or Guillain–Barré syndrome. All initially reported persistent myalgia and arthralgia. Four of the five patients were persistently febrile throughout their illness; Patient S1-4 had no documented fever. Gingival hemorrhage for which transfusion support was warranted developed in all five patients. Of the four patients with available laboratory data, three had anemia, and all four had leukopenia, thrombocytopenia, and elevated aminotransferase levels during hospitalization (Section S2.1 in the Supplementary Appendix).
Neurologic manifestations developed in four of the five patients, and they received mechanical ventilation for airway protection. Patient S1-2 had no neurologic symptoms on initial presentation but had one generalized seizure 10 days after symptom onset. Patient S1-3 had psychomotor excitation and altered mental status on initial presentation, complicated by one generalized seizure and hemiparesis on the left side of his body. His altered mentation persisted for several months after discharge. Patient S1-4 had bilateral paraparesis on presentation (with deep-tendon reflexes that remained intact); this presentation prompted suspicion of viral myopathy or Guillain–Barré syndrome. The paraparesis persisted for several months after discharge. Confusion, agitation, and hyporeflexia of the legs developed in Patient S1-5, and he had one generalized seizure 8 days after symptom onset.
Three of the five patients died during hospitalization. Patient S1-3 was discharged 31 days after symptom onset, and Patient S1-4 was discharged 161 days after symptom onset. The incubation period ranged from 9 to 16 days in the three health care workers (Patients S1-2, S1-4, and S1-5) with known dates of exposure.
IDENTIFICATION OF CHAPV AND DEVELOPMENT OF NEW DIAGNOSTIC TESTS
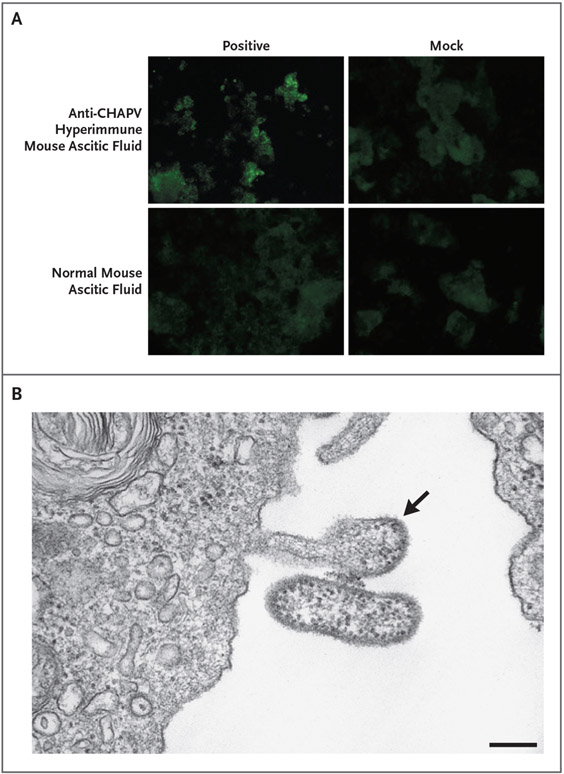
With the use of next-generation sequencing and pathogen-discovery pipelines, CHAPV sequences were consistently identified in complementary DNA libraries prepared from specimens and viral isolates, but not in mock RNA extraction or mock infection controls. No other pathogen sequences were consistently identified by next-generation sequencing in the patient specimens or viral isolates that were analyzed. Specific quantitative RT-PCR assays were developed. Multiple independent viral isolates were obtained and identified as arenaviruses with the use of an indirect immunofluorescence assay and electron microscopy and confirmed as CHAPV by means of quantitative RT-PCR (Fig. 3 and Sections S1.3 and S1.7).
Figure 3. Detection of CHAPV by an Indirect Immunofluorescence Assay and Electron Microscopy.
In Panel A, an indirect immunofluorescence assay shows CHAPV Bolivia 2019 isolate obtained from Patient S1-2 (passage 1 in Vero E6 cells, 7 days after inoculation). In Panel B, electron microscopy of CHAPV Bolivia 2019 isolate shows typical arenavirus morphologic features with pleomorphic virions containing ribosomes. The virus isolate was obtained from Patient S1-4 (passage 2 in Vero E6 cells, 7 days after inoculation). The arrow indicates a budding viral particle. The scale bar represents 200 nm.
DETECTION OF ADDITIONAL CASES OF CHHF
With improved laboratory and surveillance capacity, four additional cases of CHHF were prospectively detected in 2019 and 2020 (Table 1 and Fig. 2). Clinical details were largely unavailable; additional data for Patient S2-1 are provided in the Supplementary Appendix. Patient S2-1 was a 29-year-old pregnant (16 weeks’ gestation) agricultural worker from Palos Blancos Municipality (Fig. 1). Fever and diarrhea developed in this patient on July 9. On July 15, she presented to a local hospital with fever, headache, myalgia, lethargy, retro-orbital pain, abdominal pain, vomiting, diarrhea, and gingival hemorrhage. She died on July 18.
Patient S3-1, a 47-year-old male agricultural worker from Alto Beni Municipality, was admitted to a local hospital on December 3 with headache, retro-orbital pain, arthralgia, myalgia, neurologic deterioration, and gingival hemorrhage. He recovered and was discharged on January 5, 2020.
Patient S4-1, a 27-year-old pregnant (5 weeks’ gestation) agricultural worker from Caranavi, presented on December 5 with headache, epistaxis, and malaise. On December 23, fever, headache, vomiting, and lethargy, followed by gingival hemorrhage, developed in Patient S4-2, a 7-year-old son of Patient S4-1. He was admitted to the hospital on December 31. Patients S4-1 and S4-2 recovered and were discharged on December 29, 2019, and January 10, 2020, respectively. Patient S4-1 later had an uncomplicated childbirth.
Of these additional patients, only Patient S2-1 died; no nosocomial infections were reported. Aside from Patients S4-1 and S4-2, no epidemiologic links were identified among these patients or between these patients and any patients in the initial 2019 cluster.
Identification of a Potential Rodent Reservoir
CHAPV RNA was detected in samples of tissues obtained from 9 of 31 rodents (29%). CHAPV-positive rodent species were identified as small-eared pygmy rice rats (Oligoryzomys microtis) with the use of cytochrome b sequences for O. microtis (GenBank accession number, FJ374766).11 Additional data are provided in Section S2.2.
Phylogenetic Analysis
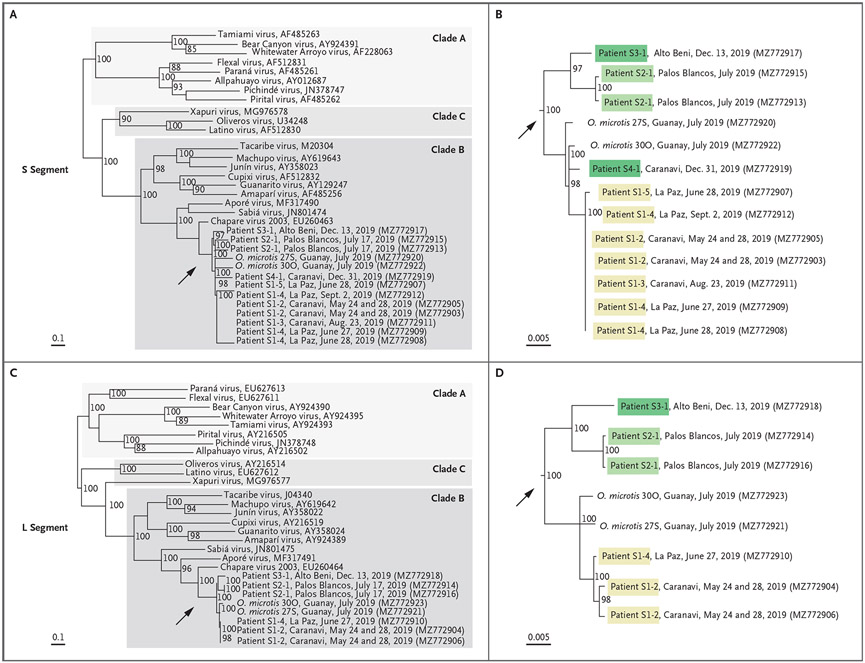
Whole-genome sequencing was attempted with the use of RNA extracted from patients, viral isolates, and CHAPV-positive rodents. Complete S segments were generated from seven of eight patients, and complete L segments were generated from four of eight patients; both segments were generated from two of nine rodents. Phylogenetic trees (Fig. 4) show that these sequences are members of the mammarenavirus genus, New World lineage, clade B, which is most closely related to the Chapare virus 2003 strain, which was originally described in Cochabamba. These new viruses belong to the Chapare mammarenavirus species, and they share more than 89% nucleotide sequence identity in the S segment, more than 88% in the L segment, and more than 88% nucleo-protein amino acid sequence identity with the Chapare virus 2003 strain.
Figure 4. Phylogenetic Analysis of New World Mammarenaviruses, Including Chapare mammarenavirus Species.
The phylogenetic tree shows relatedness between representative New World mammarenavirus S segments (Panels A and B) and L segments (Panels C and D) from clades A, B, and C, and 2019 Chapare virus sequences obtained from seven patients and two rodents (Oligoryzomys microtis) in the 2019 CHHF outbreak in Bolivia. The GenBank accession numbers of the sequences used in the phylogenetic analysis are listed in parentheses after the patient number and the location and date of specimen collection. The GenBank numbers of other viruses follow the virus names. The scale bars denote the genetic distance in nucleotide substitutions per site. The arrows in Panels A and C indicate the expanded sections shown in Panels B and D.
Phylogenetic clustering (with strong bootstrap support) showed that a strain of CHAPV circulated within the municipality of Caranavi and that cases in humans from May through December 2019 were closely related to rodent-derived viral sequences. Taken together with epidemiologic evidence, environmental transmission to Patient S1-1 with subsequent human-to-human transmission to Patients S1-2, S1-3, S1-4, and S1-5 in May through June 2019 was supported with a closely related CHAPV sequence; however, a common source of environmental exposure in Patients S1-1 and S1-3, both of whom were agricultural workers in Guanay, cannot be ruled out. An additional unique CHAPV strain that appeared to have been circulating in the Alto Beni and Palos Blancos municipalities caused disease in humans in July and December 2019.
CHAPV DETECTION IN BODY FLUIDS, VIRAL PERSISTENCE, AND SEROCONVERSION
CHAPV RNA was detected by means of RT-PCR in whole blood, serum, and urine samples, as well as in nasopharyngeal and oropharyngeal swabs, bronchoalveolar-lavage fluid, and conjunctiva and semen specimens. CHAPV RNA was detectable 113 days (in Patient S1-3) and 170 days (in Patient S1-4) after symptom onset. CHAPV was isolated from a semen sample obtained from Patient S1-3 at 86 days after symptom onset. Anti-CHAPV IgM and IgG seroconversion was detected in Patients S1-3, S1-4, and S3-1, all of whom recovered (Table S1). Additional details are provided in Section S3.1.
DISCUSSION
A total of 16 years after the initial identification of CHAPV in 2003, we found CHAPV, evidence of human-to-human transmission (including nosocomial transmission among health care workers), and a potential rodent reservoir of this virus. Cases of CHHF were identified with the use of a combination of RT-PCR (in eight patients), serologic analysis (in three), and virus isolation (in five); one case of CHHF (in Patient S1-1) remains probable.
Dengue was the initial suspected diagnosis in the early cases of CHHF in 2019. An alternative diagnosis of viral hemorrhagic fever was suspected only after the death of Patient S1-2, the subsequent illnesses in physicians in Caranavi and La Paz, and negative diagnostic tests for common causes of hemorrhagic conditions. Consequently, early supportive care and infection-prevention and infection-control protocols were delayed. CHHF and Bolivian hemorrhagic fever should be considered in patients in this geographic area, particularly in persons with risk factors for environmental or nosocomial exposure. Dengue, CHHF, and Bolivian hemorrhagic fever share many similarities (notably, fever and nonspecific symptoms such as myalgia, arthralgia, petechiae, and ecchymoses), as well as laboratory findings of leukopenia, thrombocytopenia, and elevated aminotransferase levels.12,13 A common unique feature of dengue is defervescence in the critical phase before the onset of shock, followed by organ dysfunction and severe bleeding.13 Persistence of fever, myalgia, arthralgia, weakness, or neurologic symptoms more than 7 days after symptom onset, with continued hemorrhagic manifestations, may be suggestive of CHHF. Additional data are needed to further describe the typical course of CHHF.
The treatment of arenaviruses largely relies on early diagnosis and involves supportive care. Data are lacking regarding the use of ribavirin or other antiviral agents in patients with CHHF; however, treatment with intravenous ribavirin early in the course of illness improves outcomes in patients infected with Junin virus, MACV, and Lassa virus.14-16 The limited availability of ribavirin in Bolivia poses a challenge for the treatment of acute CHHF and Bolivian hemorrhagic fever. Argentine hemorrhagic fever has been successfully treated with convalescent plasma.17 Although CHAPV serologic assays are promising new tools, further research is needed before convalescent plasma will be a possible treatment option for CHHF.
The detection of CHAPV RNA in multiple body fluids obtained from patients with CHHF highlights the importance of early implementation of infection-control measures. Our results suggest that CHAPV may persist in patients for several months after recovery. Detection of infectious CHAPV in semen obtained from a patient 86 days after the onset of symptoms suggests the possibility of sexual transmission after recovery. Although data are lacking regarding transmission of mammarenaviruses after patients have recovered from infection, transmission of other hemorrhagic fever viruses such as Ebola virus is well documented.18-21 Follow-up programs for patients who have recovered from CHHF are important to ensure access to health care and support, including psychosocial services, because survivors may have social stigmatization and longterm health consequences.22,23 Data on longterm sequelae of CHHF are lacking, but persistent neurologic, auditory, and ocular deficits have been reported in persons who have recovered from Lassa fever.24-26
In Guanay, 29% of the rodents captured for this analysis tested positive for CHAPV. Our data suggest that O. microtis may be a rodent reservoir of CHAPV; however, additional investigations are needed. O. microtis, a habitat generalist that is well adapted to peridomestic settings, is widely distributed in this region and the North La Paz Valley (Fig. 1) and is a reservoir for Rio Mamoré virus.27-34 Agricultural activity is common in the region, so further spillover of CHAPV to humans may occur.
Since the development of CHAPV-specific RT-PCR assays and improved laboratory and surveillance capacity in Bolivia, additional confirmed cases of CHHF have been identified by the Bolivian Ministry of Health. Our findings highlight the important role of international partnerships in supporting public health responses and the need for enhanced surveillance for viral hemorrhagic fever and laboratory capacity to look beyond dengue in South America.
Supplementary Material
Acknowledgments
We thank René A. Ríos Andrade, Guillermo Aliaga Gutiérrez, Joel Uzqueda Blajos, and Alinda N. Espinoza Quevedo from the Bolivian Ministry of Health and Juan D. Paredes Velasco and Edgar Quispe Rodríguez from Centro Nacional de Enfermedades Tropicales (CENETROP) for their work and contributions to rodent capture; Gabriela Añez Aguilera and Eliana Gil Colque from CENETROP for laboratory support; Tania Vacaflores Hochkofler and Nestor F. Subieta, authorities in rodentborne infections and epidemiology programs at the Bolivian Ministry of Health, for their guidance and support; Bolivian survivors and their physicians for kindly sharing information; Meredith New-love, Deirdre Launt, and Cesar Rivera from the Graphic Services Branch of the Centers for Disease Control and Prevention (CDC) for their contributions to the illustrations and data visualization design; and Stephen Welch from the Viral Special Pathogens Branch of the CDC for laboratory support.
Supported by the Bolivian Ministry of Health, Centro Nacional de Enfermedades Tropicales, the Emerging Infections Program of the Centers for Disease Control and Prevention, and in-kind funding from the Pan American Health Organization.
Footnotes
The opinions expressed by the authors do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
This article is dedicated to the memory of coauthor Carla Romero, who was instrumental in this work.
Contributor Information
R. Loayza Mafayle, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
M.E. Morales-Betoulle, Centers for Disease Control and Prevention, Atlanta
C. Romero, Unidad de Epidemiología, Bolivia.
C.M. Cossaboom, Centers for Disease Control and Prevention, Atlanta
S. Whitmer, Centers for Disease Control and Prevention, Atlanta
C.E. Alvarez Aguilera, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
C. Avila Ardaya, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
M. Cruz Zambrana, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
A. Dávalos Anajia, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
N. Mendoza Loayza, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
A.-M. Montaño, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
F.L. Morales Alvis, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
J. Revollo Guzmán, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
S. Sasías Martinez, Centro Nacional de Enfermedades Tropicales, Santa Cruz de la Sierra, Bolivia
G. Alarcón De La Vega, Unidad de Epidemiología, Bolivia
A. Medina Ramírez, Unidad de Epidemiología, Bolivia
J.T. Molina Gutiérrez, Unidad de Epidemiología, Bolivia
A.J. Cornejo Pinto, Unidad de Gestión de Riesgos en Salud Ambiental, Emergencias y Desastres, Bolivia
R. Salas Bacci, Instituto Nacional de Laboratorios de Salud “Dr. Néstor Morales Villazón”, Bolivia
J. Brignone, Instituto Nacional de Enfermedades Virales Humanas “Dr. Julio I. Maiztegui,” Pergamino, Argentina
J. Garcia, Instituto Nacional de Enfermedades Virales Humanas “Dr. Julio I. Maiztegui,” Pergamino, Argentina
A. Añez, Ministerio de Salud, the Pan American Health Organization, Bolivia
J. Mendez-Rico, Pan American Health Organization, Washington, D.C.
K. Luz, Universidade Federal do Rio Grande do Norte, Natal, Brazil
A. Segales, Hospital Obrero No. 1, Caja Nacional de Salud, Bolivia
K.M. Torrez Cruz, Hospital Militar Central, Hospital Municipal Boliviano Holandés, Bolivia
A. Valdivia-Cayoja, Hospital de Clínicas, Bolivia
B.R. Amman, Centers for Disease Control and Prevention, Atlanta
M.J. Choi, Centers for Disease Control and Prevention, Atlanta
B.-R. Erickson, Centers for Disease Control and Prevention, Atlanta
C. Goldsmith, Centers for Disease Control and Prevention, Atlanta
J.C. Graziano, Centers for Disease Control and Prevention, Atlanta
A. Joyce, Centers for Disease Control and Prevention, Atlanta
J.D. Klena, Centers for Disease Control and Prevention, Atlanta
A. Leach, Centers for Disease Control and Prevention, Atlanta
J.H. Malenfant, Centers for Disease Control and Prevention, Atlanta
S. T. Nichol, Centers for Disease Control and Prevention, Atlanta
K. Patel, Centers for Disease Control and Prevention, Atlanta
T. Sealy, Centers for Disease Control and Prevention, Atlanta
T. Shoemaker, Centers for Disease Control and Prevention, Atlanta
C.F. Spiropoulou, Centers for Disease Control and Prevention, Atlanta
A. Todres, Centers for Disease Control and Prevention, Atlanta
J.S. Towner, Centers for Disease Control and Prevention, Atlanta
J.M. Montgomery, Centers for Disease Control and Prevention, Atlanta
REFERENCES
- 1.Radoshitzky SR, Kuhn JH, Jahrling PB, Bavari S. Hemorrhagic fever-causing mammarenaviruses. In: Bozue J, ed. Medical aspects of biological warfare. Fort Sam Houston, TX: Borden Institute, 2018:517–45. [Google Scholar]
- 2.Buchmeier MJ, de la Torre JC, Peters CJ. Arenaviridae. In: Knipe DM, Howley PM, eds. Fields virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:1283–303. [Google Scholar]
- 3.Maes P, Alkhovsky SV, Bào Y, et al. Taxonomy of the family Arenaviridae and the order Bunyavirales: update 2018. Arch Virol 2018;163:2295–310. [DOI] [PubMed] [Google Scholar]
- 4.Peters CJ, Kuehne RW, Mercado RR, Le Bow RH, Spertzel RO, Webb PA. Hemorrhagic fever in Cochabamba, Bolivia, 1971. Am J Epidemiol 1974;99:425–33. [DOI] [PubMed] [Google Scholar]
- 5.Bolivian hemorrhagic fever — El Beni Department, Bolivia, 1994. MMWR Morb Mortal Wkly Rep 1994;43:943–6. [PubMed] [Google Scholar]
- 6.Delgado S, Erickson BR, Agudo R, et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog 2008;4(4):e1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raabe VN, Kann G, Ribner BS, et al. Favipiravir and ribavirin treatment of epidemiologically linked cases of Lassa fever. Clin Infect Dis 2017;65:855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lunkenheimer K, Hufert FT, Schmitz H. Detection of Lassa virus RNA in specimens from patients with Lassa fever by using the polymerase chain reaction. J Clin Microbiol 1990;28:2689–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escalera-Antezana JP, Rodriguez-Villena OJ, Arancibia-Alba AW, Alvarado-Arnez LE, Bonilla-Aldana DK, Rodríguez-Morales AJ. Clinical features of fatal cases of Chapare virus hemorrhagic fever originating from rural La Paz, Bolivia, 2019: a cluster analysis. Travel Med Infect Dis 2020;36:101589. [DOI] [PubMed] [Google Scholar]
- 10.Mills JN, Childs JE, Ksiazek TG, Peters CJ, Velleca WM. Methods for trapping and sampling small mammals for virologic testing. Atlanta: Department of Health and Human Services, 1995. [Google Scholar]
- 11.Wilson DE, Cole FR. Common names of mammals of the world. Washington, DC: Smithsonian Institution Press, 2000. [Google Scholar]
- 12.Patterson M, Grant A, Paessler S. Epidemiology and pathogenesis of Bolivian hemorrhagic fever. Curr Opin Virol 2014;5:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dengue: guidelines for patient care in the region of the Americas. 2nd ed. Washington, DC: Pan American Health Organization, 2016:157. [Google Scholar]
- 14.Kilgore PE, Ksiazek TG, Rollin PE, et al. Treatment of Bolivian hemorrhagic fever with intravenous ribavirin. Clin Infect Dis 1997;24:718–22. [DOI] [PubMed] [Google Scholar]
- 15.Veliziotis I, Roman A, Martiny D, et al. Clinical management of Argentine hemorrhagic fever using ribavirin and favipiravir, Belgium, 2020. Emerg Infect Dis 2020;26:1562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrillo-Bustamante P, Nguyen THT, Oestereich L, Günther S, Guedj J, Graw F. Determining ribavirin’s mechanism of action against Lassa virus infection. Sci Rep 2017;7:11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enria DA, Briggiler AM, Sánchez Z. Treatment of Argentine hemorrhagic fever. Antiviral Res 2008;78:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diallo B, Sissoko D, Loman NJ, et al. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis 2016;63:1353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deen GF, Broutet N, Xu W, et al. Ebola RNA persistence in semen of Ebola virus disease survivors — final report. N Engl J Med 2017;377:1428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christie A, Davies-Wayne GJ, Cordier-Lassalle T, et al. Possible sexual transmission of Ebola virus — Liberia, 2015. MMWR Morb Mortal Wkly Rep 2015;64:479–81. [PMC free article] [PubMed] [Google Scholar]
- 21.Mbala-Kingebeni P, Pratt C, Mutafali-Ruffin M, et al. Ebola virus transmission initiated by relapse of systemic Ebola virus disease. N Engl J Med 2021;384:1240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James PB, Wardle J, Steel A, Adams J. An assessment of Ebola-related stigma and its association with informal health-care utilisation among Ebola survivors in Sierra Leone: a cross-sectional study. BMC Public Health 2020;20:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overholt L, Wohl DA, Fischer WA II, et al. Stigma and Ebola survivorship in Liberia: results from a longitudinal cohort study. PLoS One 2018;13(11):e0206595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezeomah C, Adoga A, Ihekweazu C, et al. Sequelae of Lassa fever: postviral cerebellar ataxia. Open Forum Infect Dis 2019;6(12):ofz512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ficenec SC, Percak J, Arguello S, et al. Lassa fever induced hearing loss: the neglected disability of hemorrhagic fever. Int J Infect Dis 2020;100:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li AL, Grant D, Gbakie M, et al. Ophthalmic manifestations and vision impairment in Lassa fever survivors. PLoS One 2020;15(12):e0243766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patton JL, Pardiñas UFJ, D’Elía G, eds. Mammals of South America. Vol. 2. Rodents. Chicago: University of Chicago Press, 2015. [Google Scholar]
- 28.Carroll DS, Mills JN, Montgomery JM, et al. Hantavirus pulmonary syndrome in central Bolivia: relationships between reservoir hosts, habitats, and viral genotypes. Am J Trop Med Hyg 2005;72:42–6. [PubMed] [Google Scholar]
- 29.Mills JN. Biodiversity loss and emerging infectious disease: an example from the rodent-borne hemorrhagic fevers. Biodiversity 2006;7:9–17. [Google Scholar]
- 30.Emmons LH, Feer F. Neotropical rainforest mammals: a field guide. Chicago: University of Chicago Press, 1997. [Google Scholar]
- 31.Nowak RM, Walker EP. Walker’s mammals of the world. Baltimore: Johns Hopkins University Press, 1999. [Google Scholar]
- 32.Bharadwaj M, Botten J, Torrez-Martinez N, Hjelle B. Rio Mamore virus: genetic characterization of a newly recognized hantavirus of the pygmy rice rat, Oligoryzomys microtis, from Bolivia. Am J Trop Med Hyg 1997;57:368–74. [DOI] [PubMed] [Google Scholar]
- 33.Powers AM, Mercer DR, Watts DM, et al. Isolation and genetic characterization of a hantavirus (Bunyaviridae: Hantavirus) from a rodent, Oligoryzomys microtis (Muridae), collected in northeastern Peru. Am J Trop Med Hyg 1999;61:92–8. [DOI] [PubMed] [Google Scholar]
- 34.Richter MH, Hanson JD, Cajimat MN, Milazzo ML, Fulhorst CF. Geographical range of Rio Mamoré virus (family Bunyaviridae, genus Hantavirus) in association with the small-eared pygmy rice rat (Oligoryzomys microtis). Vector Borne Zoonotic Dis 2010;10:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.