Abstract
Purpose
Continuous kidney replacement therapy (CKRT) is an increasingly common intervention for critically ill patients with kidney failure. Because CKRT affects body temperature, detecting infections in patients on CKRT is challenging. Understanding the relation between CKRT and body temperature may facilitate earlier detection of infection.
Methods
We retrospectively reviewed adult patients (≥ 18 years) admitted to the intensive care unit at Mayo Clinic in Rochester, Minnesota, from December 1, 2006, through November 31, 2015, who required CKRT. We summarized central body temperatures for these patients according to the presence or absence of infection.
Results
We identified 587 patients who underwent CKRT during the study period, of whom 365 had infections, and 222 did not have infections. We observed no statistically significant differences in minimum (P = .70), maximum (P = .22), or mean (P = .55) central body temperature for patients on CKRT with infection vs. those without infection. While not on CKRT (before CKRT initiation and after cessation), all three body temperature measurements were significantly higher in patients with infection than in those without infection (all P < .02).
Conclusion
Body temperature is insufficient to indicate an infection in critically ill patients on CKRT. Clinicians should remain watchful for other signs, symptoms, and indications of infection in patients on CKRT because of expected high infection rates.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-023-03225-y.
Keywords: Dialysis, Fever, Hypothermia, Sepsis, Thermoregulation
Introduction
Nearly 25% of patients admitted to intensive care units (ICUs) have acute kidney injury, associated with increased mortality rates [1]. Depending on the severity of the injury, 10% to 50% of critically ill patients with acute kidney injury require kidney replacement therapy (KRT), and approximately 2% to 4% of patients admitted to the ICU require continuous KRT (CKRT). CKRT has become the treatment of choice for such patients [2, 3], but CKRT can increase infection risk while also making detecting sepsis more challenging [4].
Body temperature is closely monitored in the ICU because alterations in body temperature are common and are associated with poor clinical outcomes, which include infection and death [5]. Com serum mon ICU interventions, such as antipyretic and intravenous fluid administration, may affect body temperature [6]. CKRT may also alter body temperature with cooling at initiation and warming at cessation [7]. However, how often fever develops in patients who undergo CKRT and have a concurrent infection is unknown.
Patients on CKRT commonly develop infections in the ICU [8, 9]. The presence of a dialysis catheter, for example, is a risk factor for infection [10]. Catheter-related bloodstream infections occur in 5% of patients on CKRT; overall infection rates are as high as 50% for those on CKRT [11, 12]. CKRT alters physiologic processes in the body, which can interfere with the clinical and biological parameters monitored for infection diagnoses. Body temperature is a particularly affected parameter [13–15]. This effect is most likely mediated through multiple mechanisms. Filtration of proinflammatory cytokines and heat loss in the extracorporeal circuit are some of the most important factors affecting body temperature [16–21]. Hypothermia occurs more commonly in patients on CKRT than in patients undergoing other modes of KRT, such as intermittent hemodialysis [22, 23].
Early detection and treatment of infection in the ICU are associated with improved patient outcomes and decreased mortality rates [24]. Patients on CKRT, however, are a specific population that may have atypical characteristics of infection. Therefore, it is essential to study commonly used markers of infection, such as body temperature, in this population. This study aims to describe the body temperature dynamics of patients with and without infection who underwent CKRT in the ICU at our institution.
Methods
The Mayo Clinic Institutional Review Board exempted this study with a waiver for informed consent. In this historical cohort study, we retrospectively searched the electronic health records of adult patients (≥ 18 years) who required CKRT in the ICU at Mayo Clinic, Rochester, Minnesota, between December 1, 2006, and November 31, 2015. All patients in the study underwent continuous venovenous hemofiltration CKRT. Patients who declined access to their health records for research purposes were excluded from the study. Only data collected during the first ICU admission were analyzed for patients with more than 1 ICU admission with CKRT during the study period. Central body temperatures were measured via intravenous, bladder, rectal, or esophageal sensors. Patients without documented central body temperatures were also excluded from the study.
Physician reviewers determined clinical infections (D.W.C., X.G., and S.T.). The physician reviewers used a standardized protocol to assess patient health records for the presence of infection, type of infection, and estimated date of infection onset during the ICU stay (Supplemental Figure). Patients were considered to be in the infection group if the onset of infection occurred either in the five days leading to CKRT initiation or during CKRT. For patients with multiple sites of infection, we recorded all potential sources. We reviewed a random selection of approximately 10% of the abstracted health record data a second time to assess interrater reliability, measured by the Cohen κ coefficient. Central body temperature data were summarized as the median (IQR) of the minimum, maximum, mean, and SD values measured during the entire ICU stay. Body temperatures measured both before initiation and after cessation of CKRT were considered to be recorded when the patient was off CKRT. Conversely, body temperatures measured during CKRT were considered to be recorded while the patient was on CKRT. Each patient was also assessed for the presence of a single central body temperature higher than 38.0 °C and 38.3 °C, which are 2 common threshold measurements for fever. Other continuous variables were summarized as median (IQR). Categorical variables were summarized as frequency (%). All continuous data were compared with Kruskal–Wallis rank sum tests, and categorical data were compared with Fisher exact tests. Mean (95% CI) body temperature measurements recorded 24 h before initiation of CKRT (i.e., off CKRT) and 48 h after initiation (i.e., on CKRT) were plotted only for patients who had body temperature measurements documented during this period. Statistical analyses were performed with R v4.1.2 (The R Foundation) and Python 3 (Python Software Foundation) [25, 26]. P values less than 0.05 were considered statistically significant.
Results
We identified 587 patients who underwent CKRT in the ICU during the study period, of whom 348 (59.3%) were men. The median (IQR) age of all patients was 62 (52–71) years, and 295 (50.3%) patients received a consultation with the infectious diseases department during their hospitalization. The median (IQR) ICU length of stay and time to CKRT after ICU admission was 10 (6–16) days and 1.4 (0.7–2.7) days, respectively. The median (IQR) duration of CKRT was 5.1 (2.9–9.3) days. The physician review determined that 365 (62.2%) patients had an infection in the ICU, with 278 (76.2%) of these infections occurring before CKRT initiation. The physician reviewers agreed 95% of the time, with a Cohen κ coefficient of 0.905 (P < 0.01).
A total of 381 infections occurred in 365 patients. Infections in most patients involved the pulmonary (n = 134, 36.7%) or gastrointestinal (n = 86, 23.6%) systems. Other sites of infection in these patients included skin and soft tissue (n = 35, 9.6%), the cardiovascular system (n = 64, 17.5%), the central nervous system (n = 3, 0.8%), the urinary system (n = 25, 6.8%), and other sites (n = 17, 4.7%). No infection source was identified for 17 patients, but the physician reviewers concluded that an infection was most likely present. Therefore, these patients were included in the with infection group.
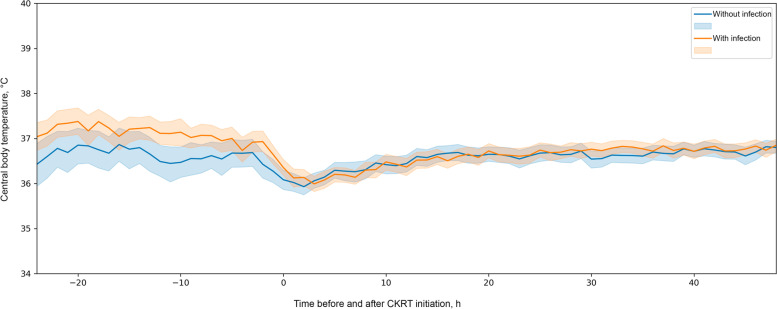
The body temperatures of patients with and without infections while on and off CKRT are reported in the Table. During CKRT, the minimum (P = 0.70), maximum (P = 0.22), and mean (P = 0.55) central body temperatures did not significantly differ between patients with or without infections. However, the body temperature SD was larger for patients with infection (0.64 °C) than for those without infection (0.55 °C), which indicates that body temperature variability was significantly greater for patients with infection (P = 0.01). When the patients were off CKRT, the median (IQR) minimum (36.0 [34.7–36.7] °C vs. 35.8 [34.3–36.4] °C), maximum (37.9 [37.2–38.7] °C gvs 37.5 [37.0–38.2] °C), and mean (36.9 [36.3–37.5] °C vs. 36.7 [36.0–37.1] °C) body temperatures were all significantly higher in patients with infection than in those without infection (all P < 0.02). Temporal changes in mean body temperatures 24 h before and 48 h after initiating CKRT for patients with and without infection are depicted in Fig. 1.
Fig. 1.
Central Body Temperature Measurement in Patients on Continuous Kidney Replacement Theraphy (CKRT) in the Intensive Care Unit. Mean body temperature measurements recorded 24 h before initiation of CRKT (off CKRT) and 48 h after initiation (on CKRT) were plotted only for patients who had measurements documented during this time period. Solid lines indicate mean temperature, and shaded regions indicate 95% CI
The number of patients with a body temperature measurement higher than 38.0 and 38.3 °C is reported in Table 1. Of the patients with infection, 152 (46.1%) had a body temperature higher than 38.0 °C while off CKRT. While on CKRT, this number decreased to 124 (34.0%). The number of patients with a recorded body temperature higher than 38.3 °C was significantly higher in patients with infection than in those without infection, both on CKRT (P = 0.03) and off CKRT (P < 0.001).
Table 1.
Summary of Patient body temperatures in the intensive care unit according to the presence of infection and use of CKRTa
| CKRT status | Body temperature measurement, °C | Total | Without infection | With infection | P |
|---|---|---|---|---|---|
| On CKRT | (N = 587) | (n = 222) | (n = 365) | ||
| Minimum | 35.1 (33.9–35.9) | 35.1 (33.6–35.8) | 35.1 (34.1–35.9) | .70b | |
| Maximum | 37.7 (37.1–38.3) | 37.7 (37.2–38.1) | 37.7 (37.1–38.3) | .22b | |
| Mean | 36.5 (36.2–36.9) | 36.5 (36.2–36.9) | 36.5 (36.1–37.0) | .55b | |
| SD | 0.60 (0.42–0.83) | 0.55 (0.40–0.76) | 0.64 (0.45–0.84) | .01b | |
| > 38.0 | 182 (31.0) | 58 (26.1) | 124 (34.0) | .05c | |
| > 38.3 | 129 (22.0) | 38 (17.1) | 91 (24.9) | .03c | |
| Off CKRT | (n = 536)d | (n = 206)d | (n = 330)d | ||
| Minimum | 35.9 (34.6–36.6) | 35.8 (34.3–36.4) | 36.0 (34.7–36.7) | .02b | |
| Maximum | 37.8 (37.1–38.6) | 37.5 (37.0–38.2) | 37.9 (37.2–38.7) | < .001b | |
| Mean | 36.9 (36.2–37.4) | 36.7 (36.0–37.1) | 36.9 (36.3–37.5) | < .001b | |
| SD | 0.52 (0.32–0.82) | 0.53 (0.31–0.82) | 0.52 (0.32–0.82) | .95b | |
| > 38.0 | 211 (39.4) | 59 (28.6) | 152 (46.1) | < .001c | |
| > 38.3 | 169 (31.5) | 47 (22.8) | 122 (37.0) | < .001c |
Abbreviation: CKRT continuous kidney replacement therapy
aContinuous data (minimum, maximum, mean, and SD) are summarized as median (IQR), and categorical data (> 38.0 °C and > 38.3 °C) are summarized as No. (%) of patients
bP value determined with Kruskal–Wallis rank sum test
cP value determined with Fisher exact test for count data
dSome patients did not have central body temperatures measured while off CKRT
Discussion
Body temperature differences between patients with and without infection appeared to be minimized in patients on CKRT. SD was the only body temperature measurement that differed between patients with and without infection during CKRT. However, the body temperature SD was only approximately 0.1 °C higher in those with infection than in those without infection while on CKRT. Therefore, the clinical relevance of SD to the detection of infection-related fever is limited. Nevertheless, the apparent differences in minimum, maximum, and mean body temperature measurements between patients with and without infection while off CKRT suggest that measuring body temperature before initiating CKRT may be most useful for detecting infection. When infection is suspected in patients on CKRT, a brief disruption may unmask changes in body temperature.
Defining a threshold of 38.3 °C allowed us to distinguish between patients with and without infection, even while on CKRT. However, only 34.8% of patients with infection met this criterion while on CKRT. This reinforces the value of thoughtful clinical evaluation and use of signs and symptoms other than body temperature when assessing for the presence of infection during CKRT.
To our knowledge, this study is the first to evaluate body temperature dynamics in patients with infection while on CKRT. Additionally, the abstraction of infection status by expert physician reviewers allowed for curating high-quality outcome data. Therefore, this study provides a reliable estimate of the potential effects of CKRT on central body temperature. Despite these strengths, this study also has several limitations that must be addressed in follow-up investigations. Because this was a historical cohort study, it was subject to biases. Determining the presence of an infection post hoc is challenging. The determination of infection relied heavily upon the evaluation of care team documentation. If a patient had an infection at any time in the five days before CKRT or while on CKRT, they were included in the infection group, which does not account for patients who may have suffered from a transient infection that resolved quickly. Although measures were taken to provide a strong case definition of infection with high interrater reliability, the generalizability of this definition in other contexts is unclear. In addition, all patients in the study underwent continuous venovenous hemofiltration. Because other types of CKRT were not analyzed, the applicability of our findings to other centers may be limited. Lastly, there are a multitude of other factors besides fever that may impact a patient's body temperature that were not examined in this study. Future studies should focus on the prospective evaluation of body temperature dynamics and include other factors that may affect body temperature (e.g., pyrolytics, cooling devices, presence of pulmonary emboli).
Conclusions
As CKRT continues to be used with increasing frequency in ICUs, knowledge about how its use may affect markers of infection is helpful for its interpretation by treating clinicians. Our findings indicate that body temperature is insufficient as a sole indicator of the presence of infection in critically ill patients on CKRT. Therefore, clinicians should remain vigilant for infection in patients on CKRT because of its association with high infection rates. All available clinical data should be used, and cultures of relevant sites should be obtained if an infection is suspected.
Supplementary Information
Acknowledgements
The Scientific Publications staff at Mayo Clinic provided editorial consultation and proofreading, administrative, and clerical support.
Abbreviations
- CKRT
Continuous kidney replacement therapy
- ICU
Intensive care unit
- KRT
Kidney replacement therapy
Authors’ contributions
Douglas Challener, John O'Horo, and Kianoush Kashani contributed to the study conception and design. Material preparation, data collection, and data analysis were performed by Douglas Challener, Xiaolan Gao, and Shahrzad Tehranian. The first draft of the manuscript was written by Douglas Challener, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Availability of data and materials
All relevant data supporting the findings of this study are reported in the article.
Declarations
Ethics approval and consent to participate
This is an observational study. The Mayo Clinic Institutional Review Board confirmed that no ethical approval is required. The Mayo Clinic Institutional Review Board exempted this study with a waiver for informed consent. All methods were performed in accordance with the relevant guidelines and regulations as published by the Committee on Publication Ethics (COPE) and the International Committee of Medical Journal Editors (ICMJE).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 2.Tandukar S, Palevsky PM. Continuous renal replacement therapy: who, when, why, and how. Chest. 2019;155(3):626–638. doi: 10.1016/j.chest.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S, Lee S, Jo HA, Han K, Kim Y, An JN, et al. Epidemiology of continuous renal replacement therapy in Korea: results from the National Health Insurance Service claims database from 2005 to 2016. Kidney Res Clin Pract. 2018;37(2):119–129. doi: 10.23876/j.krcp.2018.37.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin BR, Wu C, O'Horo JC, Faubel S, Jalal D, Kashani K. The association of platelet decrease following continuous renal replacement therapy initiation and increased rates of secondary infections. Crit Care Med. 2021;49(2):e130–e139. doi: 10.1097/CCM.0000000000004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laupland KB, Shahpori R, Kirkpatrick AW, Ross T, Gregson DB, Stelfox HT. Occurrence and outcome of fever in critically ill adults. Crit Care Med. 2008;36(5):1531–1535. doi: 10.1097/CCM.0b013e318170efd3. [DOI] [PubMed] [Google Scholar]
- 6.Young P, Saxena M, Bellomo R, Freebairn R, Hammond N, van Haren F, et al. Acetaminophen for fever in critically Ill patients with suspected infection. N Engl J Med. 2015;373(23):2215–2224. doi: 10.1056/NEJMoa1508375. [DOI] [PubMed] [Google Scholar]
- 7.Challener D, Frank R, Kashani K, O'Horo J. Body temperature trends of critically Ill patients on continuous renal replacement therapy: a single-center retrospective study. Am J Med Sci. 2022;364(4):404–408. doi: 10.1016/j.amjms.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Donowitz LG, Wenzel RP, Hoyt JW. High risk of hospital-acquired infection in the ICU patient. Crit Care Med. 1982;10(6):355–357. doi: 10.1097/00003246-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Kovvuru K, Velez JCQ. Complications associated with continuous renal replacement therapy. Semin Dial. 2021;34(6):489–494. doi: 10.1111/sdi.12970. [DOI] [PubMed] [Google Scholar]
- 10.Parienti JJ, Dugue AE, Daurel C, Mira JP, Megarbane B, Mermel LA, et al. Continuous renal replacement therapy may increase the risk of catheter infection. Clin J Am Soc Nephrol. 2010;5(8):1489–1496. doi: 10.2215/CJN.02130310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhoundi A, Singh B, Vela M, Chaudhary S, Monaghan M, Wilson GA, et al. Incidence of adverse events during continuous renal replacement therapy. Blood Purif. 2015;39(4):333–339. doi: 10.1159/000380903. [DOI] [PubMed] [Google Scholar]
- 12.Santiago MJ, Lopez-Herce J, Vierge E, Castillo A, Bustinza A, Bellon JM, et al. Infection in critically ill pediatric patients on continuous renal replacement therapy. Int J Artif Organs. 2017;40(5):224–229. doi: 10.5301/ijao.5000587. [DOI] [PubMed] [Google Scholar]
- 13.Bell M, Ronco C, Hansson F, Broman M. Hypothermia during CRRT, a comparative analysis. Acta Anaesthesiol Scand. 2020;64(8):1162–1166. doi: 10.1111/aas.13616. [DOI] [PubMed] [Google Scholar]
- 14.Finkel KW, Podoll AS. Complications of continuous renal replacement therapy. Semin Dial. 2009;22(2):155–159. doi: 10.1111/j.1525-139X.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- 15.Honore PM, Jacobs R, Hendrickx I, De Waele E, Van Gorp V, Spapen HD. 'Biomarking' infection during continuous renal replacement therapy: still relevant? Crit Care. 2015;19:232. doi: 10.1186/s13054-015-0948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu TS, Palmer EG, Batts SG, Lentz-Kapua SL, Uyehara-Lock JH, Uyehara CF. Continuous renal replacement therapy to reduce inflammation in a piglet hemorrhage-reperfusion extracorporeal membrane oxygenation model. Pediatr Res. 2012;72(3):249–255. doi: 10.1038/pr.2012.69. [DOI] [PubMed] [Google Scholar]
- 17.Peng Y, Yuan Z, Li H. Removal of inflammatory cytokines and endotoxin by veno-venous continuous renal replacement therapy for burned patients with sepsis. Burns. 2005;31(5):623–628. doi: 10.1016/j.burns.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Clark WR, Leblanc M, Levin NW. The acute dialysis quality initiative–part IV: membranes for CRRT. Adv Ren Replace Ther. 2002;9(4):265–267. doi: 10.1053/jarr.2002.35568. [DOI] [PubMed] [Google Scholar]
- 19.Yagi N, Leblanc M, Sakai K, Wright EJ, Paganini EP. Cooling effect of continuous renal replacement therapy in critically ill patients. Am J Kidney Dis. 1998;32(6):1023–1030. doi: 10.1016/S0272-6386(98)70078-2. [DOI] [PubMed] [Google Scholar]
- 20.Manns M, Maurer E, Steinbach B, Evering HG. Thermal energy balance during in vitro continuous veno-venous hemofiltration. ASAIO J. 1998;44(5):M601–M605. doi: 10.1097/00002480-199809000-00060. [DOI] [PubMed] [Google Scholar]
- 21.Constantinescu C, Pasca S, Tat T, Teodorescu P, Vlad C, Iluta S, et al. Continuous renal replacement therapy in cytokine release syndrome following immunotherapy or cellular therapies? J Immunother Cancer. 2020;8(1):e000742. doi: 10.1136/jitc-2020-000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinsonneau C, Camus C, Combes A , Costa de Beauregard MA, Klouche K, Boulain T. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368(9533):379–85. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 23.Nishimi S, Sugawara H, Onodera C, Toya Y, Furukawa H, Konishi Y, et al. Complications during continuous renal replacement therapy in critically Ill neonates. Blood Purif. 2019;47(Suppl 2):74–80. doi: 10.1159/000496654. [DOI] [PubMed] [Google Scholar]
- 24.Pruinelli L, Westra BL, Yadav P, Hoff A, Steinbach M, Kumar V, et al. Delay within the 3-hour surviving sepsis campaign guideline on mortality for patients with severe sepsis and septic shock. Crit Care Med. 2018;46(4):500–505. doi: 10.1097/CCM.0000000000002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 26.Wickham H, Averick M, Bryan J, Chang W, D'Agostino McGowan L, Francois R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data supporting the findings of this study are reported in the article.