Abstract
Several perioperative factors are responsible for the dysregulation or suppression of the immune system with a possible impact on cancer cell growth and the development of new metastasis. These factors have the potential to directly suppress the immune system and activate hypothalamic-pituitary-adrenal axis and the sympathetic nervous system with a consequent further immunosuppressive effect.
Anesthetics and analgesics used during the perioperative period may modulate the innate and adaptive immune system, inflammatory system, and angiogenesis, with a possible impact on cancer recurrence and long-term outcome. Even if the current data are controversial and contrasting, it is crucial to increase awareness about this topic among healthcare professionals for a future better and conscious choice of anesthetic techniques.
In this article, we aimed to provide an overview regarding the relationship between anesthesia and cancer recurrence. We reviewed the effects of surgery, perioperative factors, and anesthetic agents on tumor cell survival and tumor recurrence.
Keywords: Anesthesia, Stress factors, Cancer, Cancer recurrence, Outcome
Introduction
Surgery represents one of the leading treatments for the therapeutic management of several kinds of tumors. However, at the same time, surgery can have a direct and an indirect effect on tumor cell survival leading to tumor recurrence. Surgery can lead to the release of cancer cells into the bloodstream during tumor manipulation with consequent metastatic spread to distant organs [1]. Furthermore, even with clear resected surgical margins, minimal residual disease may remain and flourish with consequent local or lymphatic spread [2]. Additionally, several perioperative factors, such as inflammatory response to surgery, hypothermia, blood transfusion, tissue hypoxia, hyperglycemia, post-operative pain, can create a state of relative immunosuppression [3, 4]. Stress factors also have the potential of activating the systemic inflammatory response and enhancing tumor growth, with consequential increasing the risk of metastatic recurrence [5]. Then, the aforementioned factors have also the potential of creating an appropriate microenvironment for tumor growth through the release of hormonal mediators (i.e., catecholamines, prostaglandins), cytokines (e.g., interleukin-6, IL-4 and IL-10, TGF-β) and the upregulated expression of the transcription factor hypoxia-inducible factor 1-alpha (i.e., HIF1A) with consequent enhancement of angiogenesis pathways, cell proliferation, and the metastatic ability of cancer cells [6–8]. Not only, surgical stress can also trigger the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system which in turn also regulates the immune response with the consequent further suppression of cell immunity [9].
Likewise, anesthesia techniques may affect metastatic progression of tumor cells [10]. In fact, anesthetic drugs can play a modulatory effect on the immune system, on systemic inflammatory response, on neuroendocrine stress response and on cancer signaling pathways [11–13]. The influence of the anesthetic technique on neuroendocrine, inflammatory, and immune responses during surgery can alter local and systemic immunity with consequent boosting the tumor growth factors production and loco-regional recurrence and metastasis [14]. Even more, anesthetic-analgesic drugs seemed also to mediate the expression of specific genes or molecular pathways involved in the control of differentiation, cell growth, and of tumor progression [11]. Interestingly, evidence suggested that propofol may have a potential antitumor effect due to the regulation of mRNA expression [15]. Several preclinical and clinical studies have already shown the potential impact of anesthetics and adjuvants on cancer recurrence and survival [10]. What seems to emerge from the existing literature is that opioids can suppress the humoral immune response and can have pro-angiogenic effects, whereas regional anesthesia techniques have been associated with lower rates of cancer recurrence [16–18]. Even more, it seemed that total intravenous anesthesia (TIVA) was associated with improved recurrence-free survival in comparison to volatile anesthesia [19]. Thus, evidence is arising about the possible relation between anesthesia technique and cancer recurrence, however, a huge limitation to the current literature is represented by the impossibility of evaluating the effect of each single drug on cancer recurrence, since anesthesia requires a combination of different classes of anesthetics (i.e., hypnotic, analgesic). Consequently, further studies are needed on this topic.
Accordingly, it is crucial for healthcare personnel to consider the possible relation and implication between anesthesia, perioperative stress factors and cancer for a future better and conscious choice of anesthetic technique with the goal of improving cancer outcome. In this article, we aimed to provide an overview regarding the relationship between anesthesia and cancer recurrence. We reviewed the effects of surgery, perioperative factor, and anesthetic agents on tumor cell survival and tumor recurrence.
Perioperative metastasis
Perioperative stress factors trigger physiological responses that in turn can create an appropriate microenvironment for the growth of pre-existing micro-metastatic, for the formation of new ones and for their spread [20]. Several perioperative variables (i.e., the inflammatory response to surgery, hypothermia, and blood transfusion) represent important risk factors responsible for creating a state of relative immunosuppression and of increasing vulnerability to cancer recurrence.
Perioperative metastasis survival and growth are mediated through various mechanisms [21]:
Increase shedding of cancer cells due to mechanical manipulations of the tumor during surgery [1];
Activation of inflammatory response [22];
Modulation of immune function [23];
Triggering the neuroendocrine and paracrine stress responses [24];
Activation of pro-angiogenic signaling pathways [25];
Expression of specific genes and/or molecular pathways [26].
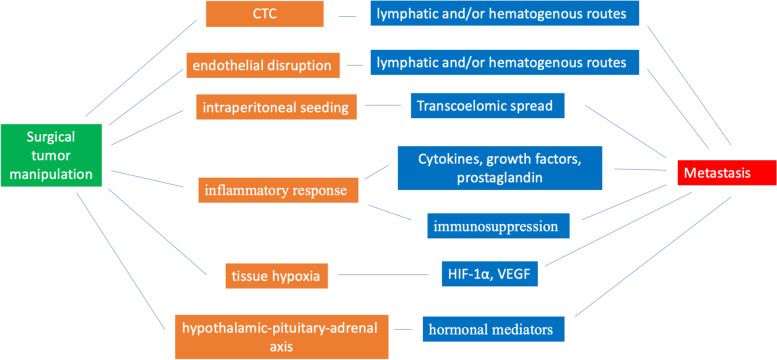
Metastasis can occur through transcoelomic, lymphatic, and/or hematogenous routes. Transcoelomic spread refers to the diffusion of cancer cells to the peritoneal cavity, due to the migration of a primary cancer of the abdomen/pelvis or due to the systemic spread of another kind of primary cancer [27]. During abdominal and pelvic operation, surgical manipulation can be responsible for intraperitoneal seeding [28]. Even more, lymphatic network is commonly increased in solid tumors, especially in tumor margin and peritumor area and lymph flow that drains tumors is often increased, with increased interstitial fluid pressure and consequent altered lymphatic drainage [29–31]. Consequently, mechanical disruption and manipulation of the cancer during surgery may facilitate the dissemination of tumor cells also through lymphatic routes [32]. In fact, surgical incision may be responsible for endothelial disruption and consequent increase in the hydrostatic and oncotic pressures, thus favoring migration of cancer cells in the lymphatic network and subsequent dissemination. Additionally, physiological response to surgical stress led to an overexpression of lymphangiogenic factors (i.e., vascular endothelial growth factor (VEGF), prostaglandins, and platelet-derived growth factor (PDGF)) with consequent further enhance of tumor dissemination [33–35]. Surgery may also increase the hematic release of circulating tumor cells (CTC); the levels of CTC were found to be increased during different kind of surgeries [36–39]. Not all the CTC are able to seed with the consequent formation of distant metastasis. To accomplish this process, CTC have to escape circulating immune defenses and to migrate and invade fertile zone to colonize. Several inflammatory mediators and hypoxic conditions are responsible of creating vulnerable areas where CTC can migrate and proliferate: the so-called pre-metastatic niche [40].
The activation of inflammatory system due to surgical stress lead to the migration of macrophages, neutrophils, fibroblasts and mesenchymal stem cells on the site of the surgery [41]. These cells secrete several factors (e.g., VEGF, PDGF, epidermal growth factor-EGF, prostaglandin, matrix metalloproteinases (MMP)), responsible for promoting cancer growth, lymphangiogenesis, angiogenesis, and consequent dissemination [42]. Prostaglandins play an important role in increasing the metastatic invasiveness of cancer cells through the activation of several receptors (e.g., B2-adrenergic, and cyclooxygenase-2 receptors) [43, 44]. Even more, MMP and VEGF are responsible for favoring tumor cell adhesion, angiogenesis, and invasiveness of cancer cells [42, 45]. Interestingly, platelet seemed to play an important role in immune escaping of cancer cells [46]. In fact, micro-clot formation can protect CTC from natural killer (NK), from cell-mediated detection, and promotes CTC adhesion to the endothelium. Even more, activated platelets can release soluble mediators (i.e., transforming-growth factor beta -TGF-β, PDGF and adenosine triphosphate) with important effects on immune system: modulation of the NK activity and of the vascular permeability [47]. Furthermore, local and systemic immune responses to surgery lead to pro-inflammatory and immunosuppressive consequences with deeply suppression of cell-mediated immunity (CMI) [6]. The consequent immunosuppression is due to the release of several mediators such as cytokines (e.g., Interleukin-6), with an inhibitory effect on NK activity. Remarkably, several trials have found an increased level of Th2 lymphocytes and decrease level of Th1 lymphocytes with altered Th1/Th2 ratio during cancer surgery [48]. These responses may represent another important aspect to consider regarding the relation between perioperative stress response and immunosuppression.
The activation of neural signaling is induced not only by surgical tissue trauma but also by other stress factors (e.g., hypothermia, tissue hypoxia, and patient anxiety). The activation of neural signaling (i.e., the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis) led to the release of stress hormones (i.e., catecholamines, opioids, and glucocorticoids) with important consequences on cancer cell invasiveness [49]. The consequent hormonal storm stimulates inflammatory and immunologic response. Afferent nerves from the site of tissue damage triggers the activation of the HPA axis and sympathetic nervous system with consequent secretion of ACTH, cortisol, catecholamines, aldosterone, vasopressin, and glucagon. Cortisol are natural steroid hormones that bind the transcription factor glucocorticoid receptor (GR). The hypersecretion of cortisol lead to the upregulation of anti-inflammatory protein and downregulation of pro-inflammatory protein expression. Even more, cortisol influences the adaptive and innate immunity systems. Because of increased cortisol production, the number of circulating monocytes, macrophage and dendritic cells are reduced. Even more, another important consequence is represented by reduction of circulating T cells, with a shift from a pro-inflammatory Th1 phenotype to an anti-inflammatory Th2 phenotype. Glucocorticoids also effects the expression of genes that regulate the inflammatory response (i.e., NF-KB and AP-1) and inhibits the activation, proliferation, and production of immunoglobulins by B cell lymphocyte [50]. Even more, the activation of the neuroendocrine response is also responsible of changing tumor microenvironment, and remodeling lymphatic and blood vasculature [51]. All these processes are implied in tumor recurrence. Stress hormones were reported to downregulate NK, cytotoxic T lymphocytes activity, and macrophage motility/phagocytosis [52, 53]. Furthermore, catecholamine bind β-adrenoceptors on cell surface with activation of calcium-cAMP signaling and consequent enhancement of pro-metastatic factors transcription (e.g., HIF, VEGF, and MMP) [54]. Beta-adrenoreceptors have been found in several cancer cells (i.e., breast, prostate, lung, liver) [54]. The activation of these signaling pathways leads to increase tumor cell growth and their invasiveness.
Finally, another important aspect is represented by the possible correlation between stress response and expression of specific genes or molecular pathways with the consequent changes in the cell signaling [26, 55, 56]. The epigenetic modification of gene expression involved during surgery is due to DNA methylation, histone modifications, chromatin, and noncoding RNAs (ncRNAs) remodeling [57]. Furthermore, the disruption of local vasculature during surgery, lead to hypoperfusion, ischemia, and hypoxia. Hypoxia stimulates the upregulated expression of the transcription factor hypoxia-inducible factor 1-alpha (i.e., HIF1A) with consequent promotion of angiogenesis, cell proliferation, and metastasis [58]. Furthermore, HIF promotes the secretion of angiogenic factors (e.g., VEGF and angiopoietin 2) with a further effect on tumor progression and metastatic spread [59]. The level of HIF1A has been correlated with tumor progression, metastatic spread and outcome [60]. Hypoxic conditions lead also to increased production of reactive oxygen species (ROS). The consequent oxidative stress can trigger several transcription factors (i.e., NF-κB, AP-1, p53, HIF-1α, PPAR-γ, β-catenin/Wnt, and Nrf2) that in turn lead to the expression of growth factors, inflammatory cytokines and chemokines [61]. The effect of surgery and of anesthetic techniques on cancer recurrence are summarized in Tables 1 and 2. A schematic representation of perioperative metastasis due to surgical manipulation is presented in Fig. 1.
Table 1.
Effects of surgery on cancer recurrence
| Effects of surgery on cancer recurrence | ||
|---|---|---|
| Action | Consequences | |
| Direct effect on tumor cell survival | Surgical tumor manipulation | Release of cancer cells into the bloodstream ➔ metastatic spread to distant organs |
| Surgical tumor manipulation | Intraperitoneal seeding➔ Transcoelomic spread | |
| Surgical tumor manipulation and incision | Endothelial disruption ➔ increase hydrostatic and oncotic pressure➔dissemination of tumor cells through lymphatic routes | |
| Minimal residual disease in surgical margins | Local or lymphatic spread | |
| Action | Consequences | |
| Indirect effect on tumor cell survival | Physiological response to perioperative stress factors | Activating the systemic inflammatory response➔ migration of macrophages, neutrophils, fibroblasts on the site of the surgery ➔ Release of cytokines, growth factors and prostaglandin➔ promoting cancer growth, lymphangiogenesis, angiogenesis, and consequent dissemination |
| Physiological response to perioperative stress factors | Activating the systemic inflammatory response➔ state of relative immunosuppression➔ immune escaping of cancer cells➔appropriate microenvironment for tumor growth | |
| Physiological response to perioperative stress factors | Trigger the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system➔ release of hormonal mediators➔ enhance tumor growth | |
| Physiological response to perioperative stress factors | Expression of specific genes and/or molecular pathways➔ promotion of angiogenesis, cell proliferation, and metastasis | |
| Physiological response to perioperative stress factors | Activation of pro-angiogenic signaling pathways➔ increasing the metastatic invasiveness | |
Table 2.
Effects of anesthetics on cancer recurrence
| Effects of anesthetics on cancer recurrence | |
|---|---|
| Type of anesthetics | Effects |
| Volatile anesthetics |
-Pro-inflammatory and immunosuppressive action -Reduces Th1/Th2 ratio -Impairs NK cell activity -Induces T cell and B cell apoptosis -Upregulation of hypoxia-inducible factors (HIF-1α, HIF-2α,) -Increase transcription of pro-metastatic factors (VEGF, angiopoietin-1, proteases MMP-2, and MMP-9) -Enhanced tumor cell proliferation -Increase angiogenesis, and cell migration |
| Intravenous anesthetics |
-Anti-inflammatory and immunosuppression properties -Suppression of prostaglandin and inflammatory cytokine production -Inhibition of cyclooxygenase (COX) activity -Stimulate the proliferation of NK cells -Increase expression of granzyme B and IFNγ -Increase cytotoxic T lymphocyte activity -Does not affect the Th1/Th2 ratio -Modulate genetic signaling pathways -Inhibits histone acetylation |
| Ketamine, Thiopental |
-Suppress the activity of NK cells -Induce apoptosis in lymphocytes -Inhibits the functional maturation of dendritic cells -Reduce the synthesis of pro-inflammatory cytokines |
| Opioids |
-Modulate wound healing -Immunosuppression effects -Inhibits natural killer cell activity -Inhibits responses of T and B cells to mitogens -Inhibits antibody production -Promotes lymphocyte apoptosis, -Reduces the differentiation of T cells -Inhibits phagocytic activity -Inhibits of the release of cytokine/ chemokine production |
| Local anesthetics |
-Activates apoptotic pathway -Inhibits tumor cell growth and migration -Increases the activity of NK -Increases the number of T-helper (Th) cells -Preserves Th1/Th2 cells ratio -Preserves IFN-gamma concentrations -Modulates gene expression -Increases IL-4 levels -Decreases IL-10, IL-8, TNF-alfa production |
| NSAIDs and COX-2 inhibitors |
-Inhibits the cyclooxygenase 1 and the cyclooxygenase 2 -Reduces prostaglandin synthesis |
| Paracetamol | -Inhibits prostaglandin endoperoxide H2 synthase and cyclooxygenase activity |
Fig. 1.
Schematic representation of perioperative metastasis due to surgical manipulation
Anesthetic agents
Volatile and intravenous anesthetics
The increasing interest in the impact of anesthetics and cancer progression has stimulated several in vivo and in vitro studies on the relation between different kinds of anesthetics used during surgery and cancer development and progression [48, 62]. Even if the evidence is conflicting, halogenated anesthetics seemed to present several pro-inflammatory and immunosuppressive effects that can have an important impact on enhancing metastasis formation [63]. Volatile anesthetic agents are implied in the upregulation of hypoxia-inducible factors [64]. Several trials are showed that the exposure of cancer cells to isoflurane and sevoflurane led to upregulation of HIF-1α, HIF-2α, growth factor and increase transcription of pro-metastatic factors (VEGF, angiopoietin-1, proteases MMP-2 and MMP-9, insulin-like growth factor IGF-1) which enhanced tumor cell proliferation, increased angiogenesis, and cell migration [65, 66]. Furthermore, halogenated anesthetics inhibit the activity of the immune system; reduces Th1/Th2 ratio, impairs NK cell activity, induces T cell and B cell apoptosis [67–69]. Consequently, the volatile anesthetic may promote immunosuppression and the creation of a pro-malignant environment that supports the growth of residual cancer cells.
On the other hand, propofol presents anti-inflammatory and immunosuppression properties [70–72]. Several studies have shown that propofol could inhibit adhesion, migration, invasiveness of cancer cells and induce apoptosis [73, 74]. Propofol presents anti-inflammatory properties through the suppression of prostaglandin and inflammatory cytokine production and the inhibition of cyclooxygenase (COX) activity [75]. Even more, propofol may prevent immunosuppression through the preservation of NK cell function. Not only propofol preserved NK activity, it seemed that propofol could also stimulate the proliferation of NK cells through the increased expression of granzyme B, IFN-γ, and activating surface receptors (e.g., CD16, NKp30, NKp44, and NKG2D) [76–78]. In fact, increased NK cell infiltration of tumors is reported after the administration of propofol. Furthermore, propofol could increase cytotoxic T lymphocyte activity and does not affect the Th1/Th2 ratio [79].
Propofol may also modulate genetic signaling pathways with important consequences on carcinogenesis:
Inhibition of HIF-1α protein synthesis induced by hypoxia [80];
Inhibition of the mRNA expression of MMP-2 and MMP-9 and p38 MAPK signaling (signaling pathway regulating proliferation, cell motility, and survival) [81];
Inhibition of the NF-κB pathway [82];
Downregulation of S100A4 in endothelial cells and suppression of VEGF expression from cancer cells with consequent anti-angiogenic effects [83, 84];
Upregulating miRNA expression (tumor suppressors and by inhibiting the expression of miRNAs that works as oncogenes) [85];
Inhibiting histone acetylation [86].
Noteworthy, signaling pathways are not usually independent and participate in a crosstalk to create a regulatory network. Consequently, propofol may affect several pathways with important regulation on genes expression. Propofol with its anti-inflammatory and pro-immunity effects has been suggested to have a positive impact on long-term survival and cancer outcome [87–90]. However, no unified conclusion has been reached and further evidence is needed to come to a clear conclusion. In 2019, a randomized controlled trial was published comparing the incidence of metastatic breast cancer recurrence in patients who received regional anesthesia and propofol versus general anesthesia with volatile anesthetic sevoflurane and opioid analgesia [91]. The studies included 2108 women who underwent breast surgery. Cancer recurrence was similar between the groups. Contrarily, a 2019 meta-analysis by Yap et al. analyzed the effects of anesthetics on cancer recurrence and survival [19]. The study included ten trials. The authors found that TIVA was associated with improved recurrence-free survival.
In 2021, Ramirez et al. performed a review describing how drugs may regulate important function on immune and cancer cells [92]. The authors presented several preclinical and clinical studies and explained the effects of anesthetics on cancer cells. The authors presented 21 retrospective and 4 RCTs studies comparing the effects of TIVA versus volatile anesthesia. They also presented 28 retrospective and 9 RCTs studies assessing the effects of regional anesthesia on long-term outcome. Preclinical evidence showed that volatile anesthesia regulates important function in cancer cells and that they can directly modify intracellular signal involved in proliferation, migration and invasion. The authors concluded that “…whether volatile anesthetics have a deleterious effect on cancer recurrence and survival remains a controversial issue…”; however, Ramirez explained how “…volatile anesthesia regulate important function in cancer cells.”. This evidence suggested that anesthetics may play a potential impact on cancer recurrence, at least from a cellular point of view. Of course, we cannot speculate that the result of preclinical studies could be translated into clinical practices.
Finally, ketamine and thiopental present immune effects. Thiopental inhibits the function of neutrophils and NK [93]. Ketamine may suppress the activity of NK cells, induce apoptosis in lymphocytes and inhibits the functional maturation of dendritic cells [94]. Ketamine may also reduce the synthesis of pro-inflammatory cytokines, (e.g., IL-6, TNF-α) [95]. However, the evidence regarding the relation between ketamine and thiopental and cancer is scarce and far to be conclusive.
Opioids
Increasing evidence suggests that, beyond their primary analgesic function, opioids present several physiological effects. Opioids modulate wound healing and cancer progression through their endothelial action and through their influence on angiogenesis [17]. Furthermore, opioids are known to act on the immune system with immunosuppression effects [16, 96]. Through the mu-opioid receptor (MOR) or non-opioid receptors (toll-like receptors) expressed by immune cells, opioids play their direct effect on the immune system, inhibiting natural killer cell activity, inhibiting responses of T and B cells to mitogens and antibody production [97–100]. Furthermore, opioids can inhibit several neutrophils and macrophages activity: inhibition of phagocytic activity and inhibition of the release of cytokine/chemokine production [101]. Moreover, opioids act indirect effects on the immune system through the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis [102, 103].
The interplay between opioids and cancer, however, is complex and far to be understood deeply. It was also observed that neutrophils, macrophages and T cells also release endogenous opioid peptides with consequent reduction of inflammation and pain through the binding of peripheral opioid receptors [96, 104]. Noteworthy, it is important to take into account that the control of pain may have a beneficial indirect effect on immunity. The balance between the immunosuppressive effect of the opioid and the reduction of immunosuppression of pain is difficult to foresee [105].
In brief, different kinds of opioids seemed to act different effects in in vitro/in vivo model:
Morphine: suppresses the activity of NK cells, promotes lymphocyte apoptosis, reduces the differentiation of T cells, and stimulates angiogenesis [99];
Fentanyl: decrease the activity of NK cells and increase the number of regulatory T cells [106];
Sufentanil: decrease the activity of NK cells, increase the number of regulatory T cells, inhibits leukocyte migration [107];
Alfentanil: decreases the activity of NK cells [108];
Remifentanil: suppress the activity of NK cells and lymphocytic proliferation [109].
Interestingly, methyl-naltrexone, an opioid antagonist, seemed to inhibit tumor cell invasion and implantation, while continuous infusion of MNTX decreases primary tumor growth and development of lung metastasis [110].
Local anesthetics
The implementation of regional anesthesia/analgesia techniques seemed to have a positive impact on reducing cancer recurrence via several mechanisms [111]:
Reduces the stress response to surgery (via pain control or sympathetic block) and reduces the levels of cortisol, β-endorphin, and epinephrine [112, 113];
Reduces the need for opioids or volatile agents (indirect effect);
Activates apoptotic pathway [114];
Inhibits tumor cell growth and migration [115];
Increases the activity of NK [116];
Increases the number of T-helper (Th) cells, preserved the ratio of Th1 to Th2 cells [117];
Preserves IFN-gamma concentrations [118];
Modulates gene expression, DNA demethylation [119];
Increases IL-4 and decreasing IL-10, IL-8, TNF-alfa [120].
Besides the possible beneficial mechanism triggered by regional anesthesia, there is no strong evidence regarding the effect of regional anesthesia on cancer recurrence. Xu et al. evaluated the effects of epidural anesthesia-analgesia on recurrence-free survival after lung cancer surgery. The authors compared two groups: general anesthesia versus general anesthesia and regional anesthesia groups [121]. The authors concluded that regional anesthesia did not improve recurrence-free survival compared with general anesthesia alone. In both groups, general anesthesia was induced with propofol, sufentanil, and rocuronium while anesthesia was maintained with propofol and/or sevoflurane (with or without nitrous oxide inhalation). Even more, dexmedetomidine was given at the discretion of anesthesiologists. Consequently, due to the high heterogeneity of drugs administered (propofol, sevoflurane, opioids, dexmedetomidine), it was not possible to come to any conclusion regarding general anesthesia. It was impossible of evaluating the effect of each single drug on cancer recurrence. Similarly, in Du et al., the authors concluded that regional anesthesia did not improve recurrence-free survival compared with general anesthesia alone [122]. Even more, general anesthesia was induced with midazolam, propofol, sufentanil, and rocuronium and maintained with either intravenous, inhalation, or combined. A 2015 meta-analysis including 10 studies showed improved overall survival when neuraxial analgesia was used in radical prostatectomy [123]. On the other hand, as aforementioned mentioned, in 2019 a randomized controlled trial did not find any difference in cancer recurrence between the groups receiving regional anesthesia and propofol versus general anesthesia with volatile anesthetic sevoflurane and opioid analgesia [91]. Several studies were conducted on this topic; however, due to the heterogeneity of the trials, it is difficult to draw any conclusion from the existing literature [118, 124–126].
NSAIDs, COX-2 inhibitors, paracetamol, alpha-2 adrenoceptor agonists
Other drugs commonly used in the perioperative period:
NSAIDs and COX-2 inhibitors: represented the most widely painkiller used for the management of perioperative analgesia. NSAIDs inhibit the cyclooxygenase 1 (COX-1) and the cyclooxygenase 2 (COX-2) enzymes with consequent anti-inflammatory, analgesic and antipyretic effects. Several trials have already shown the potential benefits of NSAIDs in the prevention of human cancer [127]. Above all, the long-term use of daily low-dose aspirin has been already related to the risk reduction of several kind of cancers: from colon, breast, lung, and prostate cancer [127, 128]. COX is frequently overexpressed in several cancers with important effects on cancer progression with an important contribution in tumorigenesis [127, 129–131]: increased production of prostaglandins, inhibition of apoptosis and promotion of angiogenesis, increased cell motility and invasion and modulation of inflammation and immune function [132, 133]. NSAIDs inhibit cyclooxygenase enzymes, leading to reduction of prostaglandin synthesis (i.e., prostaglandin E2, PGE2) and promote immune responses [134]. In particular, PGE2 plays a crucial role in promoting cancer progression; enhancement of cellular proliferation, promotion of angiogenesis, inhibition of apoptosis, stimulation of invasion/motility, and suppression of immune response [44]. Nevertheless, NSAIDs can be administered in combination with opioids or with paracetamol to increase the analgesic efficacy and to reduce the daily consumption of opioids [135]. Consequently, the possible survival benefits of receiving NSAIDs may be also due to their opioid-sparing effects of the usage of multimodality therapy in the perioperative settings [136].
Paracetamol: inhibits prostaglandin endoperoxide H2 synthase and cyclooxygenase activity with pain-relieving and antipyretic properties. However, paracetamol has no anti-inflammatory effects. Paracetamol can be administered in combination with opioids or NSAIDs to increases the analgesic efficacy and reduce daily morphine consumption [137]. Analyzing the current literature, the relationship between paracetamol usage and cancer recurrence are conflicting: increased risks for urinary tract cancers and decreased risk for ovarian cancer [138, 139]. However, the results reached so far have been inconsistent.
Alpha-2 adrenoceptor agonists: dexmedetomidine and clonidine are alpha-2 adrenoceptor agonists mainly used for sedation and as part of multimodal opioid-sparing analgesia. Alfa-adrenoceptors are found to be expressed in breast cancer, both epithelial and stromal cells [140]. Consequently, alfa-modulators may affect cancer progression and recurrence. However, evidence is scarce regarding the relation between dexmedetomidine and/or clonidine and cancer recurrence and far to be conclusive [141–143].
Discussion and conclusions
Overview articles represent a useful aid for addressing bias and concerns or to put light on the insufficiency of the current literature and to stimulate further research in a particular field. We decided to provide an overview only on the impact of anesthetic techniques and surgery on cancer recurrence because the current data are controversial and contrasting. Our aim was to summarize content from several articles and provide the reader with a general understanding of the possible relation between anesthetics and cancer.
It is also important to highlight that, up to now, the heterogeneity of the factors implied in cancer recurrence during surgery are high and the heterogeneity of the current literature on cancer and anesthesia would make impractical, or at least hard, to summarize and to come to any kind of conclusion. Not only the anesthetic technique but also several perioperative factors can influence immune surveillance and inflammatory responses and they may favor proliferation of metastasis. Furthermore, the impact of anesthetics technique depending on the type of cancer could make the discussion confusing considering the vast and divergent literature available on this topic. This would made even more difficult to come to any kind of conclusion.
Another important limitation is represented by the fact that it is impossible to evaluate the effect of each single drug on cancer recurrence, since anesthesia requires a combination of different classes of drugs (i.e., hypnotic, analgesic). The difference in baseline characteristics between groups (i.e., ASA), the different concentration of volatile anesthetics used in the clinical studies, the different duration of the surgery and the extension of surgical incision (minimally invasive vs. open surgery) represented important confounding factors. Even more, the majority of the data looking at the relationship of these techniques and cancer outcome in different kind of tumor originates from retrospective studies.
Surely, evidence is arising about the possible impact of anesthesia technique, perioperative period, cancer recurrence and long-term outcome. Even if the current data are controversial and contrasting, it is crucial to increase awareness about this topic among healthcare professionals for a future better and conscious choice of anesthetic techniques. Consequently, further trials are needed for a deeper understanding of the aforementioned mechanisms and on the actual impact of anesthetic techniques on the long-term survival. At this stage of the clinical research, we think that share awareness represents the major goal in an informative way.
Acknowledgements
None.
Authors’ contributions
Etrusca Brogi: concept and design, acquisition of data, analysis and interpretation of data, drafting/critical revision the manuscript, control, and guarantee that all aspects of the work were investigated and resolved. Francesco Forfori: study concept and design, drafting/revising the manuscript, control and guarantee that all aspects of the work were investigated and resolved, critical revision of the manuscript for important intellectual content, and study supervision. Both authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Etrusca Brogi, Email: etrusca.brogi@uslnordovest.toscana.it.
Francesco Forfori, Email: francesco.forfori@unipi.it.
References
- 1.Tohme S, Simmons RL, Tsung A (2017) Surgery for cancer: a trigger for metastases. Cancer Res 77(7):1548–1552 [DOI] [PMC free article] [PubMed]
- 2.Pierik AS, Leemans CR, Brakenhoff RH Resection margins in head and neck cancer surgery: an update of residual disease and field cancerization. Cancers (Basel):132021 [DOI] [PMC free article] [PubMed]
- 3.Vallejo R, Hord ED, Barna SA, Santiago-Palma J, Ahmed S. Perioperative immunosuppression in cancer patients. J Environ Pathol Toxicol Oncol. 2003;22(2):139–146. doi: 10.1615/JEnvPathToxOncol.v22.i2.70. [DOI] [PubMed] [Google Scholar]
- 4.Peng YP, Qiu YH. Surgical stress and immunosuppression. Sheng Li Ke Xue Jin Zhan. 2006;37(1):31–36. [PubMed] [Google Scholar]
- 5.Chen Z, Zhang P, Xu Y, Yan J, Liu Z, Lau WB, et al. Surgical stress and cancer progression: the twisted tango. Mol Cancer. 2019;18(1):132. doi: 10.1186/s12943-019-1058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264(1):73–80. doi: 10.1097/SLA.0000000000001691. [DOI] [PubMed] [Google Scholar]
- 7.Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295(1):7–16. doi: 10.1016/j.canlet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, et al. Prostate cancer cell malignancy via modulation of HIF-1α pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014;111(7):1338–1349. doi: 10.1038/bjc.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito H. Endocrine response to surgical stress. Nihon Geka Gakkai Zasshi. 1996;97(9):701–707. [PubMed] [Google Scholar]
- 10.Wall T, Sherwin A, Ma D, Buggy D. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123(2):135–150. doi: 10.1016/j.bja.2019.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponferrada AR, Orriach JLG, Manso AM, Haro ES, Molina SR, Heredia AF et al (2020) Anaesthesia and cancer: can anaesthetic drugs modify gene expression? Ecancermedicalscience 14:1080. 10.3332/ecancer.2020.1080. eCollection 2020. [DOI] [PMC free article] [PubMed]
- 12.Dang Y, Shi X, Xu W, Zuo M (2018) The effect of anesthesia on the immune system in colorectal cancer patients. Can. J Gastroenterol Hepatol 2018:7940603. 10.1155/2018/7940603. eCollection 2018. [DOI] [PMC free article] [PubMed]
- 13.Schneemilch CE, Schilling T, Bank U. Effects of general anaesthesia on inflammation. Best Pract Res Clin Anaesthesiol. 2004;18(3):493–507. doi: 10.1016/j.bpa.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22(3):263–277. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 15.Hamaya Y, Takeda T, Dohi S, Nakashima S, Nozawa Y. The effects of pentobarbital, isoflurane, and propofol on immediate-early gene expression in the vital organs of the rat. Anesth Analg. 2000;90(5):1177–1183. doi: 10.1097/00000539-200005000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Eisenstein TK. The role of opioid receptors in immune system function. Front Immunol. 2019;10:2904. doi: 10.3389/fimmu.2019.02904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ondrovics M, Hoelbl-Kovacic A, Fux DA. Opioids: modulators of angiogenesis in wound healing and cancer. Oncotarget. 2017;8:25783–25796. doi: 10.18632/oncotarget.15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le-Wendling L, Nin O, Capdevila X. Cancer recurrence and regional anesthesia: the theories, the data, and the future in outcomes. Pain Med. 2016;17(4):756–775. doi: 10.1111/pme.12893. [DOI] [PubMed] [Google Scholar]
- 19.Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. 2019;66(5):546–561. doi: 10.1007/s12630-019-01330-x. [DOI] [PubMed] [Google Scholar]
- 20.Onuma AE, Zhang H, Gil L, Huang H, Tsung A (2020) Surgical stress promotes tumor progression: a focus on the impact of the immune response. Clin Med 9(12):4096. Published online 2020 Dec 18. 10.3390/jcm9124096 [DOI] [PMC free article] [PubMed]
- 21.Neeman E, Ben-Eliyahu S. The perioperative period and promotion of cancer metastasis: New outlooks on mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(Suppl):S32–S40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margraf A, Ludwig N, Zarbock A, Rossaint J. Systemic inflammatory response syndrome after surgery: mechanisms and protection. Anesth Analg. 2020;131(6):1693–1707. doi: 10.1213/ANE.0000000000005175. [DOI] [PubMed] [Google Scholar]
- 23.Salo M. Effects of anaesthesia and surgery on the immune response. Acta Anaesthesiol Scand. 1992;36(3):201–220. doi: 10.1111/j.1399-6576.1992.tb03452.x. [DOI] [PubMed] [Google Scholar]
- 24.Okur H, Küçükaydin M, Ustdal KM. The endocrine and metabolic response to surgical stress in the neonate. J Pediatr Surg. 1995;30:626–625. doi: 10.1016/0022-3468(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 25.Kong B, Michalski CW, Friess H, Kleeff J. Surgical procedure as an inducer of tumor angiogenesis. Exp Oncol. 2010;32(3):186–189. [PubMed] [Google Scholar]
- 26.Lirk P, Fiegl H, Weber NC, Hollmann MW. Epigenetics in the perioperative period. Br J Pharmacol. 2015;172(11):2748–2755. doi: 10.1111/bph.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbolina MV (2018) Molecular mechanisms regulating organ-specific metastases in epithelial ovarian carcinoma. Cancers (Basel) 10(11):444. 10.3390/cancers10110444 [DOI] [PMC free article] [PubMed]
- 28.Bahat G, Saka B, Yenerel M, Yilmaz E, Tascioglu C, Dogan O. Peritoneal seeding and subsequent progression of mantle cell lymphoma after splenectomy for debulking. Curr Oncol. 2010;17:78–82. doi: 10.3747/co.v17i3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14(3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 30.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7(2):192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 31.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 32.Tvedskov TF, Jensen MB, Kroman N, Balslev E. Iatrogenic displacement of tumor cells to the sentinel node after surgical excision in primary breast cancer. Breast Cancer Res Treat. 2012;131(1):223–229. doi: 10.1007/s10549-011-1720-y. [DOI] [PubMed] [Google Scholar]
- 33.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201(7):1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramovitch R, Marikovsky M, Meir G, Neeman M. Stimulation of tumour growth by wound-derived growth factors. Br J Cancer. 1999;79(9-10):1392–1398. doi: 10.1038/sj.bjc.6690223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonio N, Bønnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, et al. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34(17):2219–2236. doi: 10.15252/embj.201490147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skondra M, Gkioka E, Kostakis ID, Pissimissis N, Lembessis P, Pectasides D, et al. Detection of circulating tumor cells in breast cancer patients using multiplex reverse transcription-polymerase chain reaction and specific primers for MGB, PTHRP and KRT19 correlation with clinicopathological features. Anticancer Res. 2014;34(11):6691–6699. [PubMed] [Google Scholar]
- 37.Brown DC, Purushotham AD, Birnie GD, George WD. Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription and polymerase chain reaction. Surgery. 1995;117:95–101. doi: 10.1016/S0039-6060(05)80235-1. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto M, Tanaka F, Yoneda K, Takuwa T, Matsumoto S, Okumura Y, et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg. 2014;18(6):775–783. doi: 10.1093/icvts/ivu048. [DOI] [PubMed] [Google Scholar]
- 39.Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer. 2010;102(9):1327–1334. doi: 10.1038/sj.bjc.6605651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17(5):302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 41.Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–3885. doi: 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol. 2000;50(1-2):121–137. doi: 10.1023/A:1006436624862. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, DuBois RN. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Lett. 2008;267(2):197–203. doi: 10.1016/j.canlet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55(1):115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Im JH, Fu W, Wang H, Bhatia SK, Hammer DA, Kowalska MA, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64(23):8613–8619. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 46.Amo L, Tamayo-Orbegozo E, Maruri N, Eguizabal C, Zenarruzabeitia O, Riñón M, et al. Involvement of platelet-tumor cell interaction in immune evasion. Potential role of podocalyxin-like protein 1. Front Oncol. 2014;4:245. doi: 10.3389/fonc.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlesinger M (2018) Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol 11(1):125. 10.1186/s13045-018-0669-2 [DOI] [PMC free article] [PubMed]
- 48.Ishikawa M, Nishioka M, Hanaki N, Miyauchi T, Kashiwagi Y, Ioki H, et al. Perioperative immune responses in cancer patients undergoing digestive surgeries. World J Surg Oncol. 2009;7:7. doi: 10.1186/1477-7819-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim TH, Rowat AC, Sloan EK. Neural regulation of cancer: from mechanobiology to inflammation. Clin Transl Immunol. 2016;5:e78. doi: 10.1038/cti.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenkrantz Hölmich E, Petring Hasselager R, Tvilling Madsen M, Orhan A, Gögenur I (2020) Long-term outcomes after use of perioperative glucocorticoids in patients undergoing cancer surgery: a systematic review and meta-analysis. Cancers (Basel) 12(1):76. Published online 2019 Dec 27. 10.3390/cancers12010076 [DOI] [PMC free article] [PubMed]
- 51.Dilley RJ, Schwartz SM. Vascular remodeling in the growth hormone transgenic mouse. Circ Res. 1989;65(5):1233–1240. doi: 10.1161/01.RES.65.5.1233. [DOI] [PubMed] [Google Scholar]
- 52.Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30) Clin Exp Immunol. 2005;139(2):287–296. doi: 10.1111/j.1365-2249.2004.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deitch EA, Bridges RM. Stress hormones modulate neutrophil and lymphocyte activity in vitro. J Trauma. 1987;27(10):1146–1154. doi: 10.1097/00005373-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Mravec B, Horvathova L, Hunakova L (2020) Neurobiology of cancer: the role of β-adrenergic receptor signaling in various tumor environments. Int J Mol Sci 21(21):7958. 10.3390/ijms21217958 [DOI] [PMC free article] [PubMed]
- 55.Ilango S, Paital B, Jayachandran P, Padma PR, Nirmaladevi R. Epigenetic alterations in cancer. Front Biosci (Landmark Ed) 2020;25:1058–1109. doi: 10.2741/4847. [DOI] [PubMed] [Google Scholar]
- 56.Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (Edinb) 2010;105(1):4–13. doi: 10.1038/hdy.2010.54. [DOI] [PubMed] [Google Scholar]
- 57.Sadahiro R, Knight B, James F, Hannon E, Charity J, Daniels IR, et al. Major surgery induces acute changes in measured DNA methylation associated with immune response pathways. Sci Rep. 2020;10(1):5743. doi: 10.1038/s41598-020-62262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dengler VL, Galbraith M, Espinosa JM. Transcriptional Regulation by Hypoxia Inducible Factors. Crit Rev Biochem Mol Biol. 2014;49(1):1–15. doi: 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn GO, Seita J, Hong BJ, Kim YE, Bok S, Lee CJ, et al. Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci U S A. 2014;111(7):2698–2703. doi: 10.1073/pnas.1320243111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang W, Cai J, Zabkiewicz C, Zhang H, Ruge F, Jiang WG. The effects of anesthetics on recurrence and metastasis of cancer, and clinical implications. World J Oncol. 2017;8(3):63–70. doi: 10.14740/wjon1031e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Y, Jiang W, Xie S, Xue F, Zhu X. The Role of Inhaled Anesthetics in Tumorigenesis and Tumor Immunity. Cancer Manag Res. 2020;12:1601–1609. doi: 10.2147/CMAR.S244280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi QY, Zhang SJ, Liu L, Chen QS, Yu LN, Zhang FJ, et al. Sevoflurane promotes the expansion of glioma stem cells through activation of hypoxia-inducible factors in vitro. Br J Anaesth. 2015;114:825–830. doi: 10.1093/bja/aeu402. [DOI] [PubMed] [Google Scholar]
- 65.Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119(3):593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W, Shao X. Isoflurane promotes non-small cell lung cancer malignancy by activating the Akt-mammalian target of rapamycin (mTOR) signaling pathway. Med Sci Monit. 2016;22:4644–4650. doi: 10.12659/MSM.898434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tazawa K, Koutsogiannaki S, Chamberlain M, Yuki K. The effect of different anesthetics on tumor cytotoxicity by natural killer cells. Toxicol Lett. 2017;266:23–31. doi: 10.1016/j.toxlet.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ji FH, Wang YL, Yang JP. Effects of propofol anesthesia and sevoflurane anesthesia on the differentiation of human T-helper cells during surgery. Chin Med J (Engl) 2011;124(4):525–529. [PubMed] [Google Scholar]
- 69.Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102(6):1147–1157. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- 70.Chen RM, Chen TG, Chen TL, Lin LL, Chang CC, Chang HC, et al. Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Ann N Y Acad Sci. 2005;1042:262–271. doi: 10.1196/annals.1338.030. [DOI] [PubMed] [Google Scholar]
- 71.Helmy SA, Al-Attiyah RJ. The immunomodulatory effects of prolonged intravenous infusion of propofol versus midazolam in critically ill surgical patients. Anaesthesia. 2001;56:4–8. doi: 10.1046/j.1365-2044.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- 72.Li R, Liu H, Dilger JP, Lin J. Effect of propofol on breast cancer cell, the immune system, and patient outcome. BMC Anesthesiol. 2018;18(1):77. doi: 10.1186/s12871-018-0543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Jiao H, Jiang Z, Chen R. Propofol inhibits migration and induces apoptosis of pancreatic cancer PANC-1 cells through miR-34a-mediated E-cadherin and LOC285194 signals. Bioengineered. 2020;11:510–521. doi: 10.1080/21655979.2020.1754038. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Du Q, Liu J, Zhang X, Zhu H, Wei M, Wang S (2018) Propofol inhibits proliferation, migration, and invasion but promotes apoptosis by regulation of Sox4 in endometrial cancer cells. Braz J Med Biol Res 51(4):e6803. 10.1590/1414-431x20176803. Epub 2018 Feb 26 [DOI] [PMC free article] [PubMed]
- 75.Inada T, Kubo K, Shingu K. Possible link between cyclooxygenase-inhibiting and antitumor properties of propofol. J Anesth. 2011;25(4):569–575. doi: 10.1007/s00540-011-1163-y. [DOI] [PubMed] [Google Scholar]
- 76.Zhou M, Dai J, Zhou Y, Wu J, Xu T, Zhou D, et al. Propofol improves the function of natural killer cells from the peripheral blood of patients with esophageal squamous cell carcinoma. Exp Ther Med. 2018;16:83–92. doi: 10.3892/etm.2018.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ai L, Wang H (2020) Effects of propofol and sevoflurane on tumor killing activity of peripheral blood natural killer cells in patients with gastric cancer. J Int Med Res 48(3):300060520904861. 10.1177/0300060520904861. [DOI] [PMC free article] [PubMed]
- 78.Brand JM, Schmucker P, Breidthardt T, Kirchner H. Upregulation of IFN-gamma and soluble interleukin-2 receptor release and altered serum cortisol and prolactin concentration during general anesthesia. J Interferon Cytokine Res. 2001;21(10):793–796. doi: 10.1089/107999001753238024. [DOI] [PubMed] [Google Scholar]
- 79.Liu S, Gu X, Zhu L, Wu G, Zhou H, Song Y et al (2016) Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Medicine (Baltimore) 95(49):e5479. 10.1097/MD.0000000000005479. [DOI] [PMC free article] [PubMed]
- 80.Tanaka T, Takabuchi S, Nishi K, Oda S, Wakamatsu T, Daijo H, et al. The intravenous anesthetic propofol inhibits lipopolysaccharide-induced hypoxia-inducible factor 1 activation and suppresses the glucose metabolism in macrophages. J Anesth. 2010;24(1):54–60. doi: 10.1007/s00540-009-0829-1. [DOI] [PubMed] [Google Scholar]
- 81.Wu KC, Yang ST, Hsia TC, Yang JS, Chiou SM, Lu CC, et al. Suppression of cell invasion and migration by propofol are involved in down-regulating matrix metalloproteinase-2 and p38 MAPK signaling in A549 human lung adenocarcinoma epithelial cells. Anticancer Res. 2012;32:4833–4842. [PubMed] [Google Scholar]
- 82.Zhang L, Wang N, Zhou S, Ye W, Jing G, Zhang M. Propofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2. J Exp Clin Cancer Res. 2012;31(1):66. doi: 10.1186/1756-9966-31-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo XG, Wang S, Xu YB, Zhuang J. Propofol suppresses invasion, angiogenesis and survival of EC-1 cells in vitro by regulation of S100A4 expression. Eur Rev Med Pharmacol Sci. 2015;19:4858–4865. [PubMed] [Google Scholar]
- 84.Wang Z, Cao B, Ji P, Yao F. Propofol inhibits tumor angiogenesis through targeting VEGF/VEGFR and mTOR/eIF4E signaling. Biochem Biophys Res Commun. 2021;555:13–18. doi: 10.1016/j.bbrc.2021.03.094. [DOI] [PubMed] [Google Scholar]
- 85.Ishikawa M, Iwasaki M, Sakamoto A, Ma D. Anesthetics may modulate cancer surgical outcome: a possible role of miRNAs regulation. BMC Anesthesiol. 2021;21(1):71. doi: 10.1186/s12871-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao X, Mi Y, Guo N, Luan J, Xu H, Hu Z, et al. The mechanism of propofol in cancer development: An updated review. Asia Pac J Clin Oncol. 2020;16(2):e3–e11. doi: 10.1111/ajco.13301. [DOI] [PubMed] [Google Scholar]
- 87.Xu Y, Pan S, Jiang W, Xue F, Zhu X (2020) Effects of propofol on the development of cancer in humans. Cell Prolif 53(8):e12867. Published online 2020 Jun 29. 10.1111/cpr.12867 [DOI] [PMC free article] [PubMed]
- 88.Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The choice of anaesthetic--sevoflurane or propofol--and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. 2014;119(3):251–261. doi: 10.3109/03009734.2014.922649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69(2):126–132. doi: 10.4097/kjae.2016.69.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lai HC, Lee MS, Lin C, Lin KT, Huang YH, Wong CS, et al. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma: a retrospective cohort study. Br J Anaesth. 2019;123(2):151–160. doi: 10.1016/j.bja.2019.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, Kurz A, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. 2019;394:1807–1815. doi: 10.1016/S0140-6736(19)32313-X. [DOI] [PubMed] [Google Scholar]
- 92.Ramirez MF, Cata JP (2021) Anesthesia techniques and long-term oncological outcomes. Front 11:788918. 10.3389/fonc.2021.788918. eCollection 2021. [DOI] [PMC free article] [PubMed]
- 93.Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, et al. The inhibitory effects of thiopental, midazolam, and ketamine on human neutrophil functions. Anesth Analg. 1998;86(1):159–165. doi: 10.1213/00000539-199801000-00032. [DOI] [PubMed] [Google Scholar]
- 94.Forget P, Collet V, Lavand'homme P, De Kock M. Does analgesia and condition influence immunity after surgery? Effects of fentanyl, ketamine and clonidine on natural killer activity at different ages. Eur J Anaesthesiol. 2010;27(3):233–240. doi: 10.1097/EJA.0b013e32832d540e. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Shen R, Wen G, Ding R, Du A, Zhou J et al (2017) Effects of Ketamine on Levels of Inflammatory Cytokines IL-6, IL-1β, and TNF-α in the Hippocampus of Mice Following Acute or Chronic Administration. Front Pharmacol 8:139. 10.3389/fphar.2017.00139. eCollection 2017. [DOI] [PMC free article] [PubMed]
- 96.Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol. 2018;175(14):2717–2725. doi: 10.1111/bph.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boland JW, Pockley AG. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol. 2018;175(14):2726–2736. doi: 10.1111/bph.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beilin B, Martin FC, Shavit Y, Gale RP, Liebeskind JC. Suppression of natural killer cell activity by high-dose narcotic anesthesia in rats. Brain Behav Immun. 1989;3(2):129–137. doi: 10.1016/0889-1591(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 99.Pruett SB, Han YC, Fuchs BA. Morphine suppresses primary humoral immune responses by a predominantly indirect mechanism. J Pharmacol Exp Ther. 1992;262(3):923–928. [PubMed] [Google Scholar]
- 100.Ninković J, Roy S. Role of the mu opioid receptor in opioid modulation of immune function. Amino Acids. 2013;45(1):9–24. doi: 10.1007/s00726-011-1163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6(4):442–465. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hall DM, Suo JL, Weber RJ. Opioid mediated effects on the immune system: sympathetic nervous system involvement. J Neuroimmunol. 1998;83(1-2):29–35. doi: 10.1016/S0165-5728(97)00218-X. [DOI] [PubMed] [Google Scholar]
- 103.Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanism(s) of action. J Neuroimmunol. 1998;83(1-2):19–28. doi: 10.1016/S0165-5728(97)00217-8. [DOI] [PubMed] [Google Scholar]
- 104.Kelly E, Henderson G, Bailey CP. Emerging areas of opioid pharmacology. Br J Pharmacol. 2018;175(14):2715–2716. doi: 10.1111/bph.14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Page GG. Immunologic effects of opioids in the presence or absence of pain. J Pain Symptom Manage. 2005;29(5 Suppl):S25–S31. doi: 10.1016/j.jpainsymman.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 106.Martucci C, Panerai AE, Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain. 2004;110(1-2):385–392. doi: 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 107.Peng Y, Yang J, Guo D, Zheng C, Sun H, Zhang Q, et al. Sufentanil postoperative analgesia reduce the increase of T helper 17 (Th17) cells and FoxP3(+) regulatory T (Treg) cells in rat hepatocellular carcinoma surgical model: A randomised animal study. BMC Anesthesiol. 2020;20(1):212. doi: 10.1186/s12871-020-01129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim R (2018) Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med 16(1):8. 10.1186/s12967-018-1389-7 [DOI] [PMC free article] [PubMed]
- 109.Sacerdote P, Gaspani L, Rossoni G, Panerai AE, Bianchi M. Effect of the opioid remifentanil on cellular immune response in the rat. Int Immunopharmacol. 2001;1(4):713–719. doi: 10.1016/S1567-5769(01)00005-4. [DOI] [PubMed] [Google Scholar]
- 110.Janku F, Johnson LK, Karp DD, Atkins JT, Singleton PA, Moss J. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol. 2016;27(11):2032–2038. doi: 10.1093/annonc/mdw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth. 2015;115(Suppl 2):ii34–ii45. doi: 10.1093/bja/aev375. [DOI] [PubMed] [Google Scholar]
- 112.Hahnenkamp K, Herroeder S, Hollmann MW. Regional anaesthesia, local anaesthetics and the surgical stress response. Best Pract Res Clin Anaesthesiol. 2004;18(3):509–527. doi: 10.1016/j.bpa.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 113.O'Riain SC, Buggy DJ, Kerin MJ, Watson RWG, Moriarty DC. Inhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesth Analg. 2005;100:244–249. doi: 10.1213/01.ANE.0000143336.37946.7D. [DOI] [PubMed] [Google Scholar]
- 114.Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, Cheng SP. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. 2014;9(2):e89563. doi: 10.1371/journal.pone.0089563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chamaraux-Tran TN, Mathelin C, Aprahamian M, Joshi GP, Tomasetto C, Diemunsch P, et al. Antitumor Effects of Lidocaine on Human Breast Cancer Cells: An In Vitro and In Vivo Experimental Trial. Anticancer Res. 2018;38(1):95–105. doi: 10.21873/anticanres.12196. [DOI] [PubMed] [Google Scholar]
- 116.Bar-Yosef S, Melamed R, Page GG, Shakhar G, Shakhar K, Ben-Eliyahu S. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94(6):1066–1073. doi: 10.1097/00000542-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 117.Wada H, Seki S, Takahashi T, Kawarabayashi N, Higuchi H, Habu Y, et al. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007;106:499–506. doi: 10.1097/00000542-200703000-00014. [DOI] [PubMed] [Google Scholar]
- 118.Grandhi RK, Lee S, Abd-Elsayed A. The Relationship Between Regional Anesthesia and Cancer: A Metaanalysis. Ochsner J. 2017;17(4):345–361. [PMC free article] [PubMed] [Google Scholar]
- 119.Xuan W, Hankin J, Zhao H, Yao S, Ma D. The potential benefits of the use of regional anesthesia in cancer patients. Int J Cancer. 2015;137(12):2774–2784. doi: 10.1002/ijc.29306. [DOI] [PubMed] [Google Scholar]
- 120.Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, et al. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology. 2012;117(3):548–559. doi: 10.1097/ALN.0b013e3182661977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu ZZ, Li HJ, Li MH, Huang SM, Li X, Liu QH, et al. Epidural Anesthesia-Analgesia and Recurrence-free Survival after Lung Cancer Surgery: A Randomized Trial. Anesthesiology. 2021;135:419–432. doi: 10.1097/ALN.0000000000003873. [DOI] [PubMed] [Google Scholar]
- 122.Du YT, Li YW, Zhao BJ, Guo XY, Feng Y, Zuo MZ, et al. Long-term survival after combined epidural-general anesthesia or general anesthesia alone: follow-up of a randomized trial. Anesthesiology. 2021;135:233–245. doi: 10.1097/ALN.0000000000003835. [DOI] [PubMed] [Google Scholar]
- 123.Lee BM, Singh Ghotra V, Karam JA, Hernandez M, Pratt G, Cata JP. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manag. 2015;5(5):387–395. doi: 10.2217/pmt.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109(2):180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 125.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sessler DI, Ben-Eliyahu S, Mascha EJ, Parat MO, Buggy DJ. Can regional analgesia reduce the risk of recurrence after breast cancer? Methodology of a multicenter randomized trial. Contemp Clin Trials. 2008;29(4):517–526. doi: 10.1016/j.cct.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 127.Harris RE, Beebe-Donk J, Doss H, Burr DD. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13(4):559–583. [PubMed] [Google Scholar]
- 128.Tsoi KKF, Ho JMW, Chan FCH, Sung JJY. Long-term use of low-dose aspirin for cancer prevention: A 10-year population cohort study in Hong Kong. Int J Cancer. 2019;145(1):267–273. doi: 10.1002/ijc.32083. [DOI] [PubMed] [Google Scholar]
- 129.Tomozawa S, Tsuno NH, Sunami E, Hatano K, Kitayama J, Osada T, et al. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br J Cancer. 2000;83(3):324–328. doi: 10.1054/bjoc.2000.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Okajima E, Uemura H, Ohnishi S, Tanaka M, Ohta M, Tani M, et al. Expression of cyclooxygenase-2 in primary superficial bladder cancer tissue may predict risk of its recurrence after complete transurethral resection. Aktuelle Urol. 2003;34(4):256–258. doi: 10.1055/s-2003-41610. [DOI] [PubMed] [Google Scholar]
- 131.Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005;26(5):1393–1399. [PubMed] [Google Scholar]
- 132.Schack A, Fransgaard T, Klein MF, Gögenur I. Perioperative Use of Nonsteroidal Anti-inflammatory Drugs Decreases the Risk of Recurrence of Cancer After Colorectal Resection: A Cohort Study Based on Prospective Data. Ann Surg Oncol. 2019;26(12):3826–3837. doi: 10.1245/s10434-019-07600-8. [DOI] [PubMed] [Google Scholar]
- 133.Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth. 2014;113(Suppl 1):i82–i87. doi: 10.1093/bja/aet464. [DOI] [PubMed] [Google Scholar]
- 134.Moris D, Kontos M, Spartalis E, Fentiman IS. The Role of NSAIDs in Breast Cancer Prevention and Relapse: Current Evidence and Future Perspectives. Breast Care (Basel) 2016;11(5):339–344. doi: 10.1159/000452315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen JY, Ko TL, Wen YR, Wu SC, Chou YH, Yien HW, et al. Opioid-sparing effects of ketorolac and its correlation with the recovery of postoperative bowel function in colorectal surgery patients: a prospective randomized double-blinded study. Clin J Pain. 2009;25:485–489. doi: 10.1097/AJP.0b013e31819a506b. [DOI] [PubMed] [Google Scholar]
- 136.Bailard NS, Flores RA. Could opioid sparing, rather than a direct non-steroidal anti-inflammatory drug effect, be responsible for improved survival after conservative breast surgery? Br J Anaesth. 2015;114:527. doi: 10.1093/bja/aev014. [DOI] [PubMed] [Google Scholar]
- 137.Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr Anaesth. 2013;23(6):475–495. doi: 10.1111/pan.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Friis S, Nielsen GL, Mellemkjaer L, McLaughlin JK, Thulstrup AM, Blot WJ, et al. Cancer risk in persons receiving prescriptions for paracetamol: a Danish cohort study. Int J Cancer. 2002;97(1):96–101. doi: 10.1002/ijc.1581. [DOI] [PubMed] [Google Scholar]
- 139.Weiss NS. Use of acetaminophen in relation to the occurrence of cancer: a review of epidemiologic studies. Cancer Causes Control. 2016;27(12):1411–1418. doi: 10.1007/s10552-016-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bruzzone A, Piñero CP, Rojas P, Romanato M, Gass H, Lanari C, et al. α(2)-Adrenoceptors enhance cell proliferation and mammary tumor growth acting through both the stroma and the tumor cells. Curr Cancer Drug Targets. 2011;11(6):763–774. doi: 10.2174/156800911796191051. [DOI] [PubMed] [Google Scholar]
- 141.Cata JP, Singh V, Lee BM, Villarreal J, Mehran JR, Yu J, et al. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33(3):317–323. doi: 10.4103/joacp.JOACP_299_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Forget P, Berlière M, Poncelet A, De Kock M. Effect of clonidine on oncological outcomes after breast and lung cancer surgery. Br J Anaesth. 2018;121(1):103–104. doi: 10.1016/j.bja.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 143.Lavon H, Matzner P, Benbenishty A, Sorski L, Rossene E, Haldar R, et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120(1):188–196. doi: 10.1016/j.bja.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.