Abstract
Background
Issues remain on the optimal management of subarachnoid hemorrhage (SAH) patients once they are admitted to the referring center, before and after the aneurysm treatment. To address these issues, we created a consensus of experts endorsed by the Italian Society of Anesthesia and Intensive Care (SIAARTI). In this manuscript, we aim to provide a list of experts’ recommendations regarding the early management of SAH patients from hospital admission, in a center with neurosurgical/neuro-endovascular facilities, until securing of the bleeding aneurysm.
Methods
A multidisciplinary consensus panel composed of 24 physicians selected for their established clinical and scientific expertise in the acute management of SAH patients with different background (anesthesia/intensive care, neurosurgery, and interventional neuroradiology) was created. A modified Delphi approach was adopted.
Results
Among 19 statements discussed. The consensus was reached on 18 strong recommendations. In one case, consensus could not be agreed upon and no recommendation was provided.
Conclusions
This consensus provides practical recommendations for the management of SAH patients in hospitals with neurosurgical/neuroendovascular facilities until aneurysm securing. It is intended to support clinician’s decision-making and not to mandate a standard of practice.
Keywords: Subarachnoid hemorrhage, Hemodynamic management, Surgical management, Blood pressure, Aneurysm treatment
Background
Aneurysmal subarachnoid hemorrhage (SAH) represents an important cause of mortality and morbidity, with half of the survivors experiencing persistent neurological deficits [1, 2]. The prognosis of SAH patients depends on multiple non-modifiable factors (as patients’ pre-injury characteristics, the extent of primary cerebral damage, etc.) and on therapeutic interventions [1–3]. The primary goal of the treatment of aneurysmal SAH is the exclusion of the ruptured aneurysm, as rebleeding importantly increases the risk of mortality and poor clinical outcome [1, 2, 4, 5]. Recently, we developed a consensus with clinical recommendations of the Italian Society of Anesthesia and Intensive Care (SIAARTI) on the early management of patients with aneurysmal subarachnoid hemorrhage in a hospital without neurosurgical/neuro-endovascular facilities, and we identified a total of 13 recommendations to provide clinicians with a pragmatic approach in such clinical situations [6]. However, questions remain on the optimal management of SAH patients once they are admitted to the referring center, before and after the aneurysm treatment. Large, multicenter, randomized trial data confirming the effectiveness of treatments are lacking for many of the interventions applied even in this phase, and currently, only a few aspects of SAH management are supported by high-quality evidence [7, 8].
Therefore, we created a consensus of experts endorsed by the SIAARTI regarding the management of SAH patients after admission to the hospital with neurosurgical/neuroradiological facilities, and for clarity, we decided to split the recommendations and discussion considering the clinical approach before and after aneurysm treatment. Specifically, in this manuscript (part 1), we aim to provide a list of experts’ recommendations regarding the early management of SAH patients from hospital admission, in a center with neurosurgical/neuro-endovascular facilities, until the aneurysm treatment.
Methods
The steering committee (EP, CR, FR) selected a multidisciplinary panel of experts according to their established clinical and scientific expertise in the management of SAH, including a methodologist, neurointensivists, neuroanesthesiologists, neurosurgeons, and neuroradiologists. Two experts were identified as an advisory committee for their clinical and scientific expertise in the field (NS, GC). The Executive Committee of the SIAARTI commissioned the project and supervised the methodology and structure of the consensus. The Steering Committee conceived the project, defined the aims, the timeline and the methodology (engaged with the research group of the SIAARTI for the development of the consensus), set the agenda for meetings and Delphi rounds, and ensured communications with the panel. The steering committee met weekly via teleconferences, from August 2021 to February 2022, using the Zoom platform (Zoom Video Communications), and had 2 meetings with the Research Committee of SIAARTI for the definition of the methodology and an assessment of the on-going work.
Delphi process
The group conducted a non-systematic review regarding the clinical management of SAH patients admitted to a hospital with neurosurgical/neuro-endovascular facilities, until the aneurysm treatment and afterwards the panel and the steering committee identified the domains and generated a list of questions to be addressed by the panel. An initial list of statements was distributed to the experts for discussion and refinement. According to the panel’s feedback, a set of questions was generated. We used an online tool to conduct the modified iterative Delphi process [9, 10]. Using three online surveys—distributed from September 2021 to January 2022—the Consensus panel members were asked to express their degree of agreement and voted independently, with the possibility to add specific comments during the first two voting rounds. Comments were used to further refine the questions. Finally we proposed to vote on practical statements (recommendations) based on the answer to the questions. Three thresholds were pre-defined: when more than 85% of the panelist agreed on a specific statement, we issued a strong recommendation, while a weak recommendation could be possible in case of agreement by 75–85% of the panelists. If less than 75% of the participant endorsed a statement, no recommendation could be issued.
After each round, we analyzed answers to spot heterogeneity and inconsistent patterns of individual members. For each Delphi round, panelists were provided with results and the frequency distribution of responses recorded in the previous round, and were then invited to evaluate their previous answers and revise them if needed.
The analysis of voting results was performed by a non-voting methodologist (CR).
Results
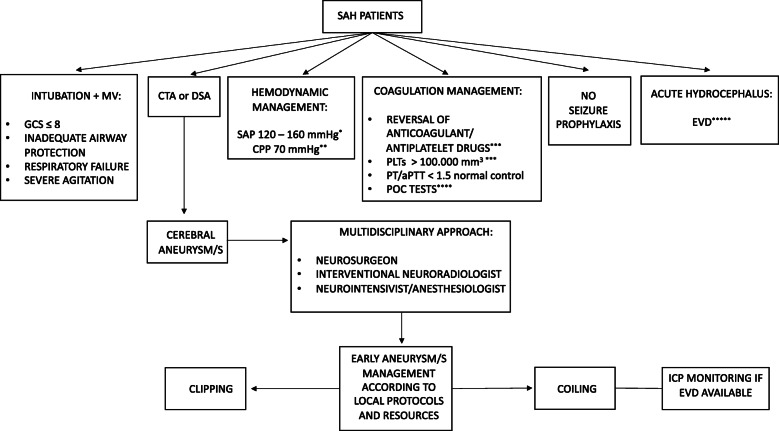
This consensus provided 19 statements (Table 1): 18 were strong recommendations, endorsed by more than 85% of participants, while we were unable to reach a consensus in one case. Figure 1 summarizes the recommendations in an operational flow chart. The consensus recommendations are listed below with the percentage of agreement.
Table 1.
List of consensus recommendations
| N. | Recommendation | Level |
|---|---|---|
| 1 | We recommend that all salvageable patients (i.e. patients who may recover, at least to some extent, with appropriate treatment) with a spontaneous SAH, according to local expertise and availability, must undergo a CTA or DSA. | Strong recommendation |
| 2 | We recommend that SAH patients in coma (GCS score ≤ 8) and/or with inadequate airway protection or respiratory failure need to be sedated, intubated and mechanically ventilated. | Strong recommendation |
| 3 | We recommend that SAH patients with severe agitation, despite mild sedation and pain control, need to be sedated, intubated and mechanically ventilated. | Strong recommendation |
| 4 | We recommend the maintenance of a PLTs count > 100.000/mm3 in all salvageable SAH patients candidates for neurosurgical intervention. | Strong recommendation |
| 5 | We recommend the reversal of antiplatelet drugs in all salvageable SAH patients, candidates for neurosurgical intervention. | Strong recommendation |
| 6 | We recommend the maintenance of a PT/aPTT value of < 1.5 normal control in all salvageable SAH patients. | Strong recommendation |
| 7 | We recommend the reversal of anticoagulant drugs in all salvageable SAH patients candidates for neurosurgical intervention. | Strong recommendation |
| 8 | We recommend, if available, the utilization of POC tests (e.g. TEG and ROTEM) to assess and optimize the coagulation function in salvageable SAH patients taking the NOACs and/or antiplatelets drugs. | Strong recommendation |
| 9 | We are unable to provide any recommendation regarding the routine administration of tranexamic acid for a short-term therapy (< 24 h from SAH) before aneurysm treatment to prevent rebleeding. | No recommendation |
| 10 | We recommend in all salvageable comatose SAH patients with acute hydrocephalus to rapidly undergo EVD placement before aneurysm management. | Strong recommendation |
| 11 | We recommend, before aneurysm treatment, for the management of intracranial hypertension related to acute hydrocephalus, the drainage (EVD available) of small volumes of CSF to reduce the risk of rebleeding. | Strong recommendation |
| 12 | We recommend, in case of EVD placement before aneurysm/s management, the ICP monitoring, during endovascular coiling. | Strong recommendation |
| 13 | We recommend the maintenance of a SAP between 120 and 160 mmHg to avoid aneurysmal rebleeding and to ensure an adequate CPP. Individualized arterial pressure targets considering patient’s clinical history (i.e., arterial hypertension) and/or radiological signs of intracranial hypertension seem reasonable. | Strong recommendation |
| 14 | We recommend the maintenance of SAP values close to the lower limit (120 mmHg) in patients without a history of arterial hypertension and/or radiological signs of elevated ICP. | Strong recommendation |
| 15 | We recommend the maintenance of SAP values close to the upper limit (160 mmHg), avoiding fluctuations, for patients with a history of arterial hypertension and/or radiological signs of elevated ICP. | Strong recommendation |
| 16 |
We recommend, in case of ICP monitoring, the maintenance of a CPP of 70 mmHg*. * for an accurate CPP estimation the arterial transducers need to be zeroed at the level of the tragus. |
Strong recommendation |
| 17 | We recommend against seizure prophylaxis in salvageable SAH patients without seizures (observed clinically and/or with EEG). | Strong recommendation |
| 18 | We recommend that ruptured cerebral aneurysm/s be secured early according to local protocols and resources. | Strong recommendation |
| 19 | We recommend a strict collaboration between the interventional neuroradiologist, the neurosurgeon, the neurointensivist/anesthesiologist to find the best strategy (clips or coils) to secure the ruptured cerebral aneurysm/s. | Strong recommendation |
SAH subarachnoid hemorrhage, CTA computed tomography angiography, DSA digital subtraction angiography, POC pint-of-care, TEG thromboelastography, ROTEM rotational thromboelastometry, NOACs novel oral anticoagulants, EVD external ventricular drain, CSF cerebrospinal fluid, EEG electroencephalogram, ICP intracranial pressure, GCS Glasgow coma scale, CPP cerebral perfusion pressure, SAP systolic arterial pressure, PLTs platelets, PT prothrombin time, aPTT activated partial thromboplastin time
Fig. 1.
Consensus flow-chart. * according to patient’s clinical history (i.e., arterial hypertension, etc.) and/or radiological signs of intracranial hypertension. ** in case of ICP monitoring; for an accurate CPP estimation the arterial transducers need to be zeroed at the level of the tragus. *** SAH patients candidates for neurosurgical intervention. **** if available, to assess and optimize the coagulation function in salvageable SAH patients taking the NOACs and/or antiplatelet drugs. ***** before aneurysm treatment, in case of intracranial hypertension, drain small volumes of CSF to reduce the risk of rebleeding. SAH subarachnoid hemorrhage, CTA computed tomography angiography, DSA digital subtraction angiography, POC pint-of-care, EVD external ventricular drain, ICP intracranial pressure, GCS Glasgow coma scale, CPP cerebral perfusion pressure, SAP systolic arterial pressure, PLTs platelets, PT prothrombin time, aPTT activated partial thromboplastin time, MV mechanical ventilation
Recommendation 1
We recommend that all salvageable patients (i.e., patients who may recover, at least to some extent, with appropriate treatment) with a spontaneous SAH, according to local expertise and availability, must undergo a computed tomography angiography (CTA) or digital subtraction angiography (DSA) (agreement 100%, strong recommendation).
Recommendation 2
We recommend that SAH patients in coma [Glasgow Coma Scale (GCS) score ≤ 8] and/or with inadequate airway protection or respiratory failure need to be sedated, intubated and mechanically ventilated (agreement 95.5%, strong recommendation).
Recommendation 3
We recommend that SAH patients with severe agitation, despite mild sedation and pain control, need to be sedated, intubated, and mechanically ventilated (agreement 86.5%, strong recommendation).
Recommendation 4
We recommend the maintenance of a platelets (PLTs) count > 100.000/mm3 in all salvageable SAH patients candidates for neurosurgical intervention (agreement 100%, strong recommendation).
Recommendation 5
We recommend the reversal of antiplatelet drugs in all salvageable SAH patients, candidates for neurosurgical intervention (agreement 86.5%, strong recommendation).
Recommendation 6
We recommend the maintenance of a prothrombin time (PT)/ activated partial thromboplastin time (aPTT) value of < 1.5 normal control in all salvageable SAH patients (agreement 86.5%, strong recommendation).
Recommendation 7
We recommend the reversal of anticoagulant drugs in all salvageable SAH patients candidates for neurosurgical intervention (agreement 100%, strong recommendation).
Recommendation 8
We recommend, if available, the utilization of point-of-care (POC) tests [e.g., thromboelastography (TEG) and rotational thromboelastometry ROTEM] to assess and optimize the coagulation function in salvageable SAH patients taking the novel oral anticoagulants (NOACs) and/or antiplatelet drugs (agreement 91%, strong recommendation).
Recommendation 9
We are unable to provide any recommendation regarding the routine administration of tranexamic acid for a short-term therapy (< 24 h from SAH) before aneurysm treatment to prevent rebleeding (agreement 58%, no recommendation).
Recommendation 10
We recommend in all salvageable comatose SAH patients with acute hydrocephalus to rapidly undergo external ventricular drain (EVD) placement before aneurysm management (agreement 91%, strong recommendation).
Recommendation 11
We recommend, before aneurysm treatment, for the management of intracranial hypertension related to acute hydrocephalus, the drainage (EVD available) of small volumes of cerebrospinal fluid (CSF) to reduce the risk of rebleeding (agreement 86%, strong recommendation).
Recommendation 12
We recommend, in case of EVD placement before aneurysm/s exclusion, the intracranial pressure (ICP) monitoring, during endovascular coiling (agreement 91%, strong recommendation).
Recommendation 13
We recommend the maintenance of a systolic arterial pressure (SAP) between 120 and 160 mmHg to avoid aneurysmal rebleeding and to ensure an adequate cerebral perfusion pressure (CPP). Individualized arterial pressure targets considering the patient’s clinical history (i.e., arterial hypertension) and/or radiological signs of intracranial hypertension seem reasonable (agreement 95.5%, strong recommendation).
Recommendation 14
We recommend the maintenance of SAP values close to the lower limit (120 mmHg) in patients without a history of arterial hypertension and/or radiological signs of elevated ICP (agreement 95.5%, strong recommendation).
Recommendation 15
We recommend the maintenance of SAP values close to the upper limit (160 mmHg), avoiding fluctuations, for patients with a history of arterial hypertension and/or radiological signs of elevated ICP (agreement 91%, strong recommendation).
Recommendation 16
We recommend, in case of ICP monitoring, the maintenance of a CPP of 70 mmHg*
(agreement 86.5%, strong recommendation).
* For an accurate CPP estimation, the arterial transducers need to be zeroed at the level of the tragus.
Recommendation 17
We recommend against seizure prophylaxis in salvageable SAH patients without seizures [observed clinically and/or with electroencephalogram (EEG)] (agreement 86.5%, strong recommendation).
Recommendation 18
We recommend that ruptured cerebral aneurysm/s be secured early according to local protocols and resources (agreement 91%, strong recommendation).
Recommendation 19
We recommend a strict collaboration between the interventional neuroradiologist, the neurosurgeon, the neurointensivist/anesthesiologist to find the best strategy (clips or coils) to secure the ruptured cerebral aneurysm/s (agreement 100%, strong recommendation).
Discussion
Once admitted to a referral center, SAH patients may present a number of problems, either directly caused by the aneurysmal bleeding or depending on comorbidities, medications, etc. To assist in some simple, but crucial, decisions (who and when requires tracheal intubation, for instance), panelists have formulated a list of practical recommendations which may orient the treating team in various treatment steps, as illustrated in Fig. 1. These recommendations do not cover the full spectrum of airways, hemodynamic and coagulation problems; for aspects not peculiar of SAH, we refer to previous consensus and clinical recommendations of the SIAARTI [6]. Furthermore, a few items deserve to be discussed:
Imaging before aneurysm management
Vessel imaging to identify the bleeding source is a key step in patients with SAH [7, 8, 11]. In this regard, DSA is considered the gold standard [7, 8, 11]. However, DSA is invasive, time-consuming, costly, and associated with neurologic complications [11, 12]. CTA, demonstrating a good sensitivity and specificity when compared to DSA [13], is increasingly utilized to detect cerebral aneurysm; in this regard, in many cases neurosurgeons can make the decision to proceed to clip based only on CTA [12], and recommend DSA only if CTA is inconclusive [7, 8]. Based on this, the panel agreed that the choice of performing CTA or DSA to identify the bleeding source and to choose the best treatment strategy should be made according to local availability and expertise.
EVD and CSF drainage before aneurysm management
Acute hydrocephalus, occurring in up to 30% of SAH patients, can be associated with intracranial hypertension and reduced CPP [11]. In this situation, the placement of an EVD can be lifesaving [7, 8, 11]. However, excessive or rapid CSF drainage can be associated with an increase in the intra-aneurysmal transmural pressure precipitating rebleeding and need to be avoided [14, 15]. Considering the above, we recommend the placement of an EVD in all salvageable comatose SAH patients with acute hydrocephalus and the careful, progressive drainage of CSF to minimize the risk of rebleeding.
ICP monitoring during endovascular coiling and CPP management
Episodes of elevated ICP and reduced CPP have been recently observed during endovascular coiling and could be associated with poor neurological outcomes [16]. Although more data are needed on this topic, we recommend ICP monitoring during endovascular coiling when an EVD has been inserted before aneurysm/s management.
CPP values < 70 mmHg have been associated with an increased risk of cerebral ischemia in poor-grade SAH patients [15, 17]. We therefore recommend the maintenance of a CPP of 70 mmHg. For an accurate CPP measurement, we suggest that the arterial transducer be zeroed at the level of the tragus according to recommendations for traumatic brain injury (TBI) patients [18].
Seizure prophylaxis
Seizures can occur after SAH [11, 12, 15]. Recognized risk factors include thick subarachnoid clot, intracerebral hematoma, delayed infarction, and middle cerebral artery’s aneurysms [11, 12, 15]. Despite the association of seizures with poor neurological outcomes and rebleeding, there is a lack of consensus regarding the administration of prophylactic anticonvulsant therapy after SAH [7, 8]. In particular, phenytoin utilization has been associated not only with worse long-term cognitive outcomes [19] but also with reduced nimodipine plasma concentrations by induction of CYP450 [20]. Data regarding newer anticonvulsant drugs are needed. Based on the limited available evidence, we recommend against seizure prophylaxis in salvageable SAH patients without seizures (clinically observed and/or documented with the EEG).
Aneurysm management
Aneurysm repair with surgical clipping or endovascular coiling is the only effective treatment to prevent rebleeding and should be performed as early as feasible [7, 8]. A recently published retrospective observational study on 575 aneurysmal SAH patients showed that more favorable outcomes (discharge at home and survival at 12 months) were achieved when surgical treatment occurred at approximately 12.5 h from SAH [21]. After the publication of the International Subarachnoid Aneurysm Trial (ISAT), comparing coils vs. clips utilization after SAH, the aneurysm treatment has mostly shifted from surgical clipping to endovascular coiling [11, 12, 15, 22, 23]. Patients in the coils group had a better neurological outcome 1 year after SAH and a lower risk of seizures compared to the clips group. Even after 10 years, patients in the endovascular coiling group had a better outcome in terms of probability of death and disability, despite a lower incidence of incomplete aneurysm occlusion and a lower risk of rebleeding in the surgical group [24]. The treatment of choice, however, has to be individualized and depends on multiple factors [11, 12, 15]. Clearly, this topic is outside the aims of this consensus. What has to be emphasized, however, is the importance of a multidisciplinary approach, where neurosurgeons, interventional neuroradiologists, and neurointensivists work together [7, 8, 11] and co-operate for an early aneurysm closure.
Limitations
This consensus is not based on a systematic literature review, and this limitation has to be clearly acknowledged. We opted for a consensus based on clinical expertise, well aware that this approach has weaknesses [25].
Conclusions
Our aim has been to provide practical suggestions on topics where the published evidence did not support strong statements. While deeper analyses of the literature are always welcome, we felt useful to indicate choices based on the panel’s expertise.
Acknowledgements
We wish to thank SIAARTI, Emiliano Tizi, Martina Castaldo, and Cristina Cacciagrano for the assistance and the support during the consensus development.
Abbreviations
- SAH
Subarachnoid hemorrhage
- SIAARTI
Italian Society of Anesthesia and Intensive Care
- CTA
Computed tomography angiography
- DSA
Digital subtraction angiography
- GCS
Glasgow coma scale
- PLTs
Platelets
- PT
Prothrombin time
- aPTT
Activated partial thromboplastin time
- POC
Point-of-care
- TEG
Thromboelastometry
- ROTEM
Rotational thromboelastography
- NOACs
Novel oral anticoagulants
- EVD
External ventricular drain
- CSF
Cerebrospinal fluid
- ICP
Intracranial pressure
- CPP
Cerebral perfusion pressure
- SAP
Systolic arterial pressure
- EEG
Electroencephalogram
Authors’ contributions
The steering committee (EP, CR, FR) selected a multidisciplinary panel of experts according to their established clinical and scientific expertise in the management of SAH, including a methodologist, neurointensivists, neuroanesthesiologists, neurosurgeons, and neuroradiologists. Two experts were identified as an advisory committee for their clinical and scientific expertise in the field (NS, GC). All authors read and approved the final manuscript.
Funding
None.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389(10069):655–666. doi: 10.1016/S0140-6736(16)30668-7. [DOI] [PubMed] [Google Scholar]
- 2.Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med. 2017;377(3):257–266. doi: 10.1056/NEJMcp1605827. [DOI] [PubMed] [Google Scholar]
- 3.Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76(5):588–597. doi: 10.1001/jamaneurol.2019.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stienen MN, Germans M, Burkhardt JK, Neidert MC, Fung C, Bervini D, Zumofen D, Röthlisberger M, Marbacher S, Maduri R, Robert T, Seule MA, Bijlenga P, Schaller K, Fandino J, Smoll NR, Maldaner N, Finkenstädt S, Esposito G, Schatlo B, Keller E, Bozinov O, Regli L, Swiss SOS Study Group Predictors of in-hospital death after aneurysmal subarachnoid hemorrhage: analysis of a nationwide database (Swiss SOS [Swiss study on aneurysmal subarachnoid hemorrhage]) Stroke. 2018;49(2):333–340. doi: 10.1161/STROKEAHA.117.019328. [DOI] [PubMed] [Google Scholar]
- 5.Germans MR, Coert BA, Vandertop WP, Verbaan D. Time intervals from subarachnoid hemorrhage to rebleed. J Neurol. 2014;261(7):1425–1431. doi: 10.1007/s00415-014-7365-0. [DOI] [PubMed] [Google Scholar]
- 6.Picetti E, Berardino M, Bertuccio A, Bertuetti R, Boccardi EP, Caricato A, Castioni CA, Cenzato M, Chieregato A, Citerio G, Gritti P, Longhi L, Martino C, Munari M, Rossi S, Stocchetti N, Zoerle T, Rasulo F, Robba C. Early management of patients with aneurysmal subarachnoid hemorrhage in a hospital without neurosurgical/neuroendovascular facilities: a consensus and clinical recommendations of the Italian Society of Anesthesia and Intensive Care (SIAARTI) J Anesth Analg Crit Care. 2021;1:10. doi: 10.1186/s44158-021-00012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, American Heart Association Stroke Council. Council on Cardiovascular Radiology and Intervention. Council on Cardiovascular Nursing. Council on Cardiovascular Surgery and Anesthesia. Council on Clinical Cardiology Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 8.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, European Stroke Organization European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35(2):93–112. doi: 10.1159/000346087. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins PM, Cooke PJ, Clarke RC, Guttormsen AB, Platt PR, Dewachter P, Ebo DG, Garcez T, Garvey LH, Hepner DL, Khan DA, Kolawole H, Kopac P, Krøigaard M, Laguna JJ, Marshall SD, Mertes PM, Rose MA, Sabato V, Savic LC, Savic S, Takazawa T, Volcheck GW, Voltolini S, Sadleir PHM. Consensus clinical scoring for suspected perioperative immediate hypersensitivity reactions. Br J Anaesth. 2019;123(1):e29–e37. doi: 10.1016/j.bja.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, van heet Loo M, McDonnell J, Vader JP, Kahan JP. The RAND/UCLA appropriateness method user’s manual. Santa Monica: RAND; 2001. [Google Scholar]
- 11.Chou SH. Subarachnoid hemorrhage. Continuum (Minneap Minn) 2021;27(5):1201–1245. doi: 10.1212/CON.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 12.Etminan N, Macdonald RL. Neurovascular disease, diagnosis, and therapy: subarachnoid hemorrhage and cerebral vasospasm. Handb Clin Neurol. 2021;176:135–169. doi: 10.1016/B978-0-444-64034-5.00009-2. [DOI] [PubMed] [Google Scholar]
- 13.Westerlaan HE, van Dijk JM, Jansen-van der Weide MC, de Groot JC, Groen RJ, Mooij JJ, Oudkerk M. Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis—systematic review and meta-analysis. Radiology. 2011;258(1):134–145. doi: 10.1148/radiol.10092373. [DOI] [PubMed] [Google Scholar]
- 14.Cagnazzo F, Gambacciani C, Morganti R, Perrini P. Aneurysm rebleeding after placement of external ven- tricular drainage: a systematic review and meta-analysis. Acta Neurochir (Wien) 2017;159:695–704. doi: 10.1007/s00701-017-3124-1. [DOI] [PubMed] [Google Scholar]
- 15.Sharma D. Perioperative management of aneurysmal subarachnoid hemorrhage. Anesthesiology. 2020;133(6):1283–1305. doi: 10.1097/ALN.0000000000003558. [DOI] [PubMed] [Google Scholar]
- 16.Imberti R, Picetti E, Rossi S, Capaccio E, Accetta G, Klersy C, Lafe E, Pietrobono L, Cimino F, Frattini L, Grappa E, Casagli S, Crobeddu E, Iotti GA. Intracranial pressure monitoring in poor-grade patients with aneurysmal subarachnoid hemorrhage treated by coiling. World Neurosurg. 2021;156:e206–e214. doi: 10.1016/j.wneu.2021.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt JM, Ko SB, Helbok R, Kurtz P, Stuart RM, Presciutti M, Fernandez L, Lee K, Badjatia N, Connolly ES, Claassen J, Mayer SA. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke. 2011;42:1351–1356. doi: 10.1161/STROKEAHA.110.596874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas E, NACCS. Czosnyka M, Hutchinson P, SBNS Calculation of cerebral perfusion pressure in the management of traumatic brain injury: joint position statement by the councils of the Neuroanaesthesia and Critical Care Society of Great Britain and Ireland (NACCS) and the Society of British Neurological Surgeons (SBNS) Br J Anaesth. 2015;115(4):487–488. doi: 10.1093/bja/aev233. [DOI] [PubMed] [Google Scholar]
- 19.Naidech AM, Kreiter KT, Janjua N, Ostapkovich N, Parra A, Commichau C, Connolly ES, Mayer SA, Fitzsimmons BF. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 2005;36(3):583–587. doi: 10.1161/01.STR.0000141936.36596.1e. [DOI] [PubMed] [Google Scholar]
- 20.Rosengart AJ, Huo JD, Tolentino J, Novakovic RL, Frank JI, Goldenberg FD, Macdonald RL. Outcome in patients with subarachnoid hemorrhage treated with antiepileptic drugs. J Neurosurg. 2007;107(2):253–260. doi: 10.3171/JNS-07/08/0253. [DOI] [PubMed] [Google Scholar]
- 21.Buscot MJ, Chandra RV, Mainguard J, Nichols L, Blizzard L, Stirling C, Smith K, Lai L, Asadi H, Froelich J, Reeves MJ, Thani N, Thrift A, Gall S. Association of onset-to-treatment time with discharge destination, mortality, and complications among patients with aneurysmal subarachnoid hemorrhage. JAMA Netw Open. 2022;5(1):e2144039. doi: 10.1001/jamanetworkopen.2021.44039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–1274. doi: 10.1016/S0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 23.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 24.Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT) Lancet. 2015;385(9969):691–697. doi: 10.1016/S0140-6736(14)60975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD, GRADE working group GRADE evidence to decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]