Abstract
Selective functional group interconversions in complex molecular settings underpin many of the challenges facing modern organic synthesis. Currently, a privileged subset of functional groups dominates this landscape, while others, despite their abundance, are sorely underdeveloped. Amines epitomize this dichotomy; they are abundant but otherwise intransigent toward direct interconversion. Here, we report an approach that enables the direct conversion of amines to bromides, chlorides, iodides, phosphates, thioethers, and alcohols, the heart of which is a deaminative carbon-centered radical formation process using an anomeric amide reagent. Experimental and computational mechanistic studies demonstrate that successful deaminative functionalization relies not only on outcompeting the H-atom transfer to the incipient radical but also on the generation of polarity-matched, productive chain-carrying radicals that continue to react efficiently. The overall implications of this technology for interconverting amine libraries were evaluated via high-throughput parallel synthesis and applied in the development of one-pot diversification protocols.
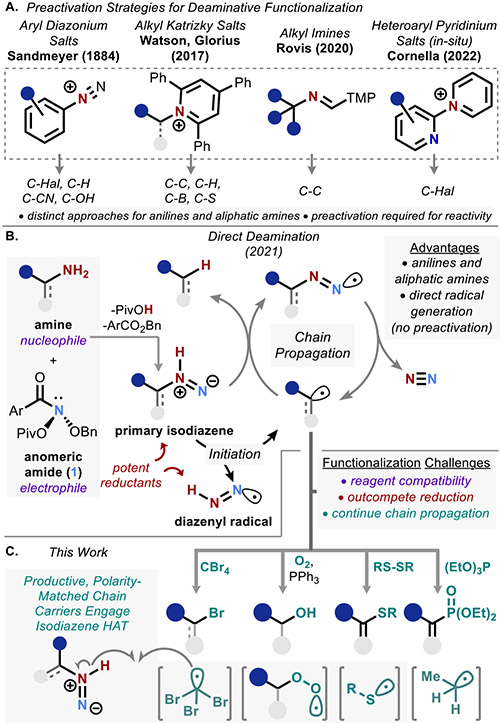
The functional group concept is central to organic chemistry, allowing classification of compounds on the basis of reactive substructures.1-6 Some functional groups are clearly privileged in their ability to serve as points of diversification, with halides, carboxylic acids, organoboron compounds, and alcohols serving critical (and growing) roles in this capacity.7-11 As such, it comes as no surprise that these groups comprise the bulk of fragment libraries used in drug discovery.12-14 Accordingly, a great deal of effort has been undertaken to coax other nontraditional functional groups to behave more like their more diversifiable counterparts.15-18 Despite their significant abundance in nature and in medicinal chemistry libraries,19 amines have remained one of the most challenging groups for functional group interconversion, likely as a consequence of their low heterolytic nucleofugality, high homolytic C─N bond dissociation energy, and characteristic basicity.20-24
With few exceptions, transformations of amines into other functional groups rely on preactivation strategies, thus requiring a minimum of two distinct transformations to accomplish one desired interconversion (Figure 1A). The classical example of this is conversion of the amino group to a diazonium ion,25,26 with more recent protocols employing Katrizky-type pyridinium salts27-35 or electron-rich imines.36,37 A rare instance of a direct deaminative functionalization was recently reported by Cornella,38,39 in which in situ formation of a pyridinium ion allows deaminative functionalization via an SNAr mechanism for electron-poor (hetero)aromatics. The rarity of one-step amine diversification processes is a stark reminder of the inadequacies in this area of synthetic chemistry.
Figure 1.
Introduction. (A) State-of-the-art deaminative functionalization methods requiring preactivation. TMP = trimethoxyphenyl. (B) Previously reported direct deamination. (C) Challenges for radical trapping and successful direct deaminative functionalization relying on productive chain carrier generation.
Beyond the necessity for prefunctionalization, each of these prior examples is limited to either aliphatic or aromatic amines due to the instability of key intermediates and the underlying differences in reactivity between aromatic and aliphatic amines. For example, aliphatic diazonium ions are prone to elimination rather than substitution. Likewise, radical generation from Katrizky salts tends to be limited to sp3-centered reactivity.20
To address this shortfall, we hypothesized that the anomeric amide-promoted deamination method previously reported by some members of our team,40,41 which productively engages both aliphatic and aromatic amines (Figure 1B), could provide a unified solution to the question of deaminative functionalization. In particular, we suspected that the free radical intermediates involved in this process could be intercepted for productive bond formations beyond hydrogen atom transfer (HAT). Several challenges to this direct deaminative functionalization needed to be overcome for this hypothesis to be realized (Figure 1C). First, the nucleophilicity and basicity of amines as well as the electrophilicity and oxidizing properties of 1 introduce compatibility constraints that rule out common radical trapping agents (e.g., Selectfluor and B2Pin2). Second, suitable reagents would need to outcompete the competitive HAT process—a nontrivial hurdle given the dramatic reducing capacity of the isodiazene intermediates involved in the original chain mechanism.41 A third, more subtle requirement was ultimately uncovered through our mechanistic study (vide infra): the byproducts of the radical trapping process must be productive chain carriers in order to minimize the number of high-barrier initiation events required for reactivity. We report here the successful realization of this strategy, providing a method for deaminative bromination of both aromatic and aliphatic amines, along with more focused strategies allowing aromatic phosphonylation and thiolation as well as aliphatic hydroxylation.
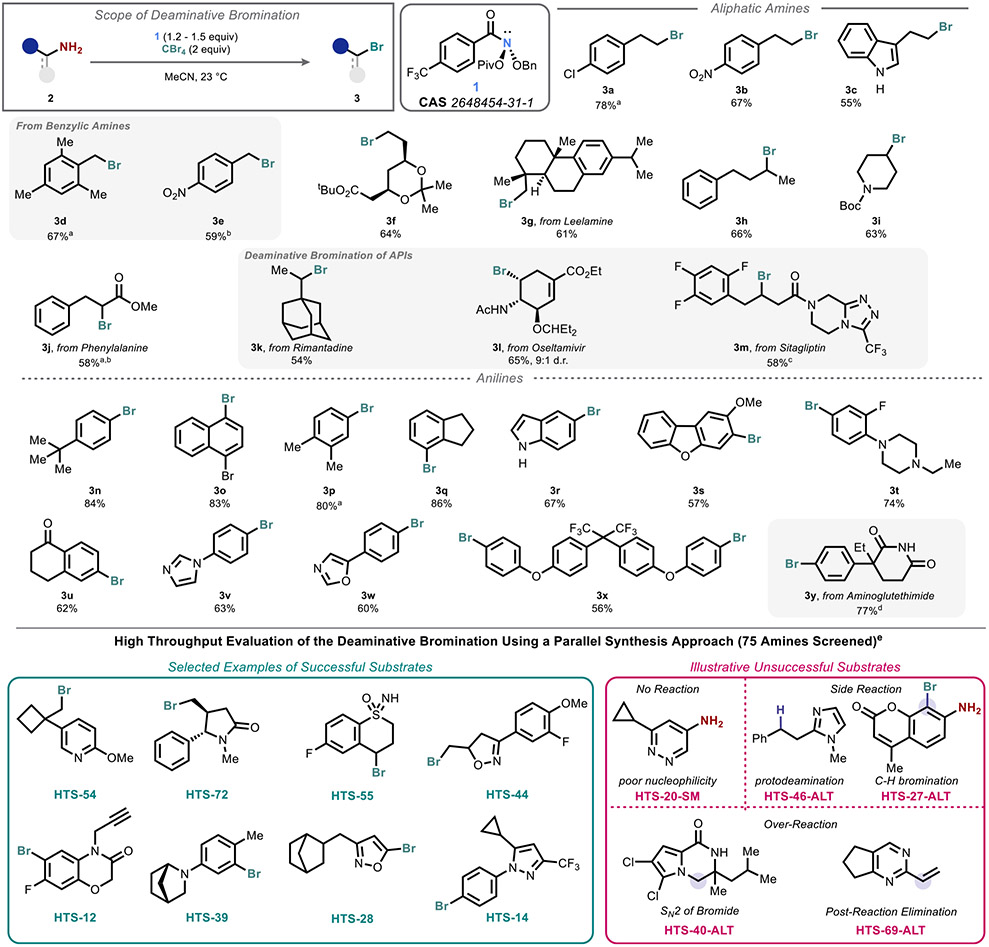
Initial investigations uncovered CBr4 as a trapping reagent that affords high yields of the corresponding bromodeamination products.42,43 Given the utility of converting a nucleophilic handle into an electrophilic handle and the difficulty of accomplishing aliphatic bromodeamination by traditional means,44,45 we sought to probe the generality of this method with respect to amine structure (Figure 2).
Figure 2.
Scope of the deaminative bromination and high-throughput screening data. Conditions: 1 (aliphatic amines −1.2 equiv, aromatic amines −1.5 equiv), CBr4 (2 equiv), CH3CN (0.1 M), 23 °C, 3 h (aliphatic) or 10 h (aromatic). Isolated yields unless otherwise indicated. aNMR yield. bDeamination also observed. cIsolated as TFA salt. d24 h. eSee the Supporting Information for details.
Both α-primary and α-secondary primary aliphatic amines as well as anilines (including several active pharmaceutical ingredients 2k, 2l, 2m, and 2y) were smoothly converted, even enabling multiple bromodeamination events on a single substrate (3x). Remarkably, despite the simplicity of the conditions, this deaminative bromination process exhibits a broader scope under milder conditions than our previously reported hydrodeamination protocol. For example, benzylic substrates (e.g., 2d and 2e) were generally not productively deaminated in the absence of CBr4 but afford benzylic bromides under the conditions employed here. Ambient temperatures could be used for both amine classes, which stands in contrast to our prior work wherein deletion of aromatic amines typically required heating.
We then leveraged parallel synthesis and high-throughput purification platforms to further survey the reaction scope with a set of 75 commercially available amines. A diverse set of amines were chosen by selecting across multiple clusters in a self-organizing map,46 while enriching for pharmaceutically relevant motifs, including saturated and unsaturated heterocycles, hydrogen bond donors, and hydrogen bond acceptors. A subset of the results are summarized in the bottom panel of Figure 2 with full details in the Supporting Information.47 Together, our traditional and high-throughput scope evaluations offer insight into the merits and limitations of this method. In particular, we have categorized common failure modes that include poor nucleophilicity, competitive deamination, C–H bromination (a reaction that is not suppressed by the addition of halide scavengers and whose origin is unknown), spontaneous cyclization, and elimination. Further analysis of the high-throughput data can be found in the Supporting Information.
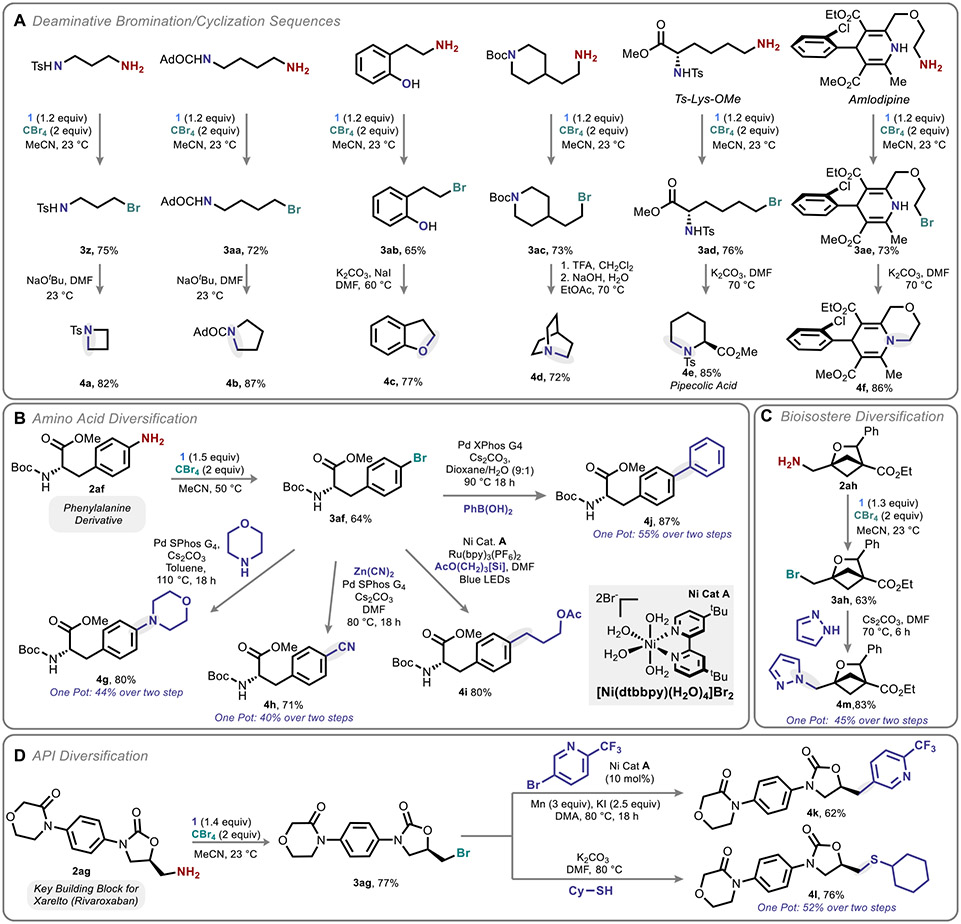
Inspired by the outcome of HTS-40-ALT, which undergoes spontaneous cyclization, we applied our deaminative bromination protocol to substrates bearing internal nucleophiles, with subsequent base-promoted cyclization affording the corresponding heterocycle for 4- (4a), 5- (4b, 4c), and 6-membered (4d) ring formation (Figure 3A). Notably, this protocol could be applied to the calcium channel blocker Amlodipine, affording cyclized analogue 4f in 62% overall yield over two steps, and to a lysine derivative, affording the far rarer pipecolic acid skeleton (4e).48
Figure 3.
Implications of deaminative bromination for downstream diversification. See the Supporting Information for detailed reaction conditions. [Si] = diisopropylammonium bis(catechol)silicate.
Intermolecular reactivity was next explored, specifically in the context of medicinally relevant motifs (Figure 3B-D). A commercially available aniline-based phenylalanine (Phe) derivative (2af) could be converted to a small library of unnatural Phes (4g–4j, Figure 3A). This process could be telescoped by the introduction of a simple liquid–liquid extraction to purge residual CBr4 and related species.49,50 Aliphatic amine building blocks could similarly be elaborated, including a recently identified, commercially available arene bioisosteric building block51 (Figure 3C) and a precursor of the anticoagulant Xarelto (Rivaroxaban, 2ag, Figure 3D).
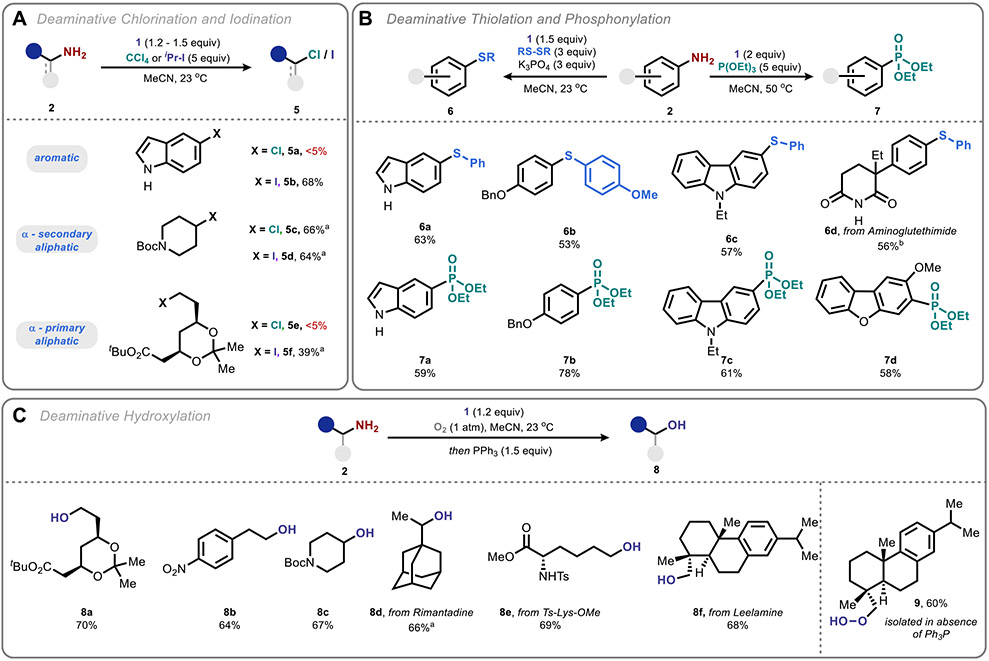
We next surveyed additional trapping agents that would allow direct conversion of amines to other functional groups (Figure 4). Though none of the examined trapping reagents retained the generality of CBr4, a number of valuable direct transformations were identified. CCl4, for example, was found to chlorinate α-secondary aliphatic amines effectively but was unable to outcompete deamination for α-primary or aromatic amine substrates (Figure 4A). Isopropyl iodide was effective for both α-secondary and aromatic amines but afforded somewhat diminished yields for α-primary amines.52,53
Figure 4.
Beyond bromination: aromatic phosphonylation, aromatic thiolation, chlorination, iodination, and aliphatic hydroxylation. See the Supporting Information for detailed reaction conditions. aNMR yield. b50 °C.
Moving beyond halogenation, anilines were found to undergo productive phosphonylation with triethyl phosphite as well as thiolation with disulfides (Figure 4B), with the latter conditions requiring the addition of anhydrous inorganic base to suppress competitive hydrodeamination (and reduction of the anomeric amide) by the liberated thiol.54-57 Aliphatic amines afforded lower yields with greater amounts of competitive hydrodeamination under these conditions.
Conversely, we found that saturation of the reaction solution with O2 prior to addition of the amine substrate resulted in the formation of peroxide products, which could be reduced to the corresponding alcohols prior to isolation through treatment with triphenylphosphine (Figure 4C).58 This net amine to alcohol conversion is of particular significance for medicinal chemistry as it allows one to retain a hydrogen bond donor (and acceptor) while drastically altering basicity. The process is applicable to a range of aliphatic substrates but was unfortunately poorly applicable to anilines. We attribute the divergence in these three methods to radical polarity effects, wherein nucleophilic aliphatic radicals more effectively engage triplet oxygen, while the electrophilic aromatic radicals are productively trapped by phosphites and disulfides.59,60
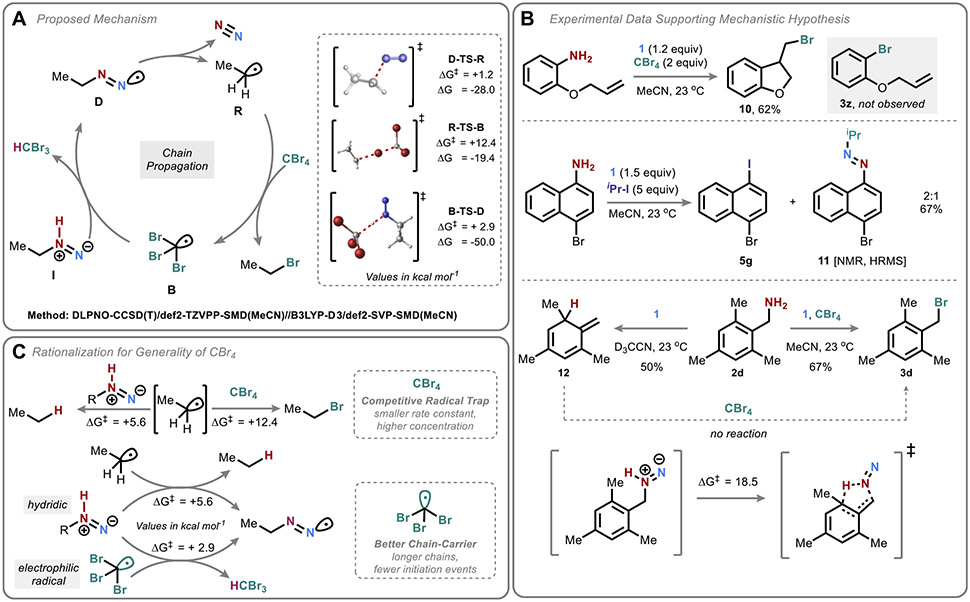
The peculiarities among these deaminative functionalization reactions with respect to amine structure, especially compared to the surprising broadness of the deaminative bromination, warranted a deeper mechanistic investigation. The proposed chain mechanism (omitting initiation and termination steps) is shown in Figure 5A; it is based on the analogous chain process previously determined to be operative in the parent deamination process41 and is supported by computations at the DLPNO-CCSD(T)/def2-TZVPP-SMD(MeCN)//B3LYP-D3/def2-SVP-SMD(MeCN) level of theory.61,62
Figure 5.
Mechanistic and computational study. (A) Proposed mechanism with computed energetics. (B) Experimental mechanistic evidence. (C) Rationalization of the generality of CBr4.
Experimental data also support this proposed mechanistic pathway (Figure 5B). Observation of 10 from a radical clock substrate as the sole product of deaminative bromination supports the intermediacy of an aryl radical. (Similar results were obtained for the hydroxylation and thiolation; see the Supporting Information for details.) Additionally, in one attempted deaminative iodination with isopropyl iodide, a mixed aryl-isopropyl azo compound 11 was observed (and isolated as a mixture with the expected iodide). Together, these experiments are consistent with the generation of both carbon-centered (R) and diazenyl (D) radical intermediates.
In the absence of CBr4, most benzylic amines underwent nonspecific decomposition, but in certain cases such as the mesityl substrate 2d, the corresponding isotoluene product 12 can be detected by 1H NMR as the major product (Figure 5B, bottom). Results from our computations suggest that this product could be formed via a concerted [2,3]-sigmatropic rearrangement of the intermediate isodiazene. Interestingly, the addition of carbon tetrabromide subverts this pathway and instead affords the benzylic bromide product 3d. Addition of carbon tetrabromide to the isotoluene after its formation does not result in the formation of 3d, suggesting that the CBr4-mediated pathway intercepts the isodiazene intermediate prior to its rearrangement. This result, in turn, suggests that •CBr3 is in fact a more effective chain carrier than the parent benzylic radical, undergoing more rapid HAT with the isodiazene.
Indeed, the computed rate constant for bromine atom transfer to ethyl radical is ~105 times slower than HAT (Figure 5C), suggesting that it is the far higher concentration of CBr4 in comparison to the isodiazene that affords competitive radical trapping. Instead, the efficiency of this process seems to be driven by the capacity for •CBr3 to carry the radical chain (via B-TS-D, ΔΔG‡ = 2.7 kcal/mol compared to HAT by ethyl radical).63 This model further accords with the observation that aromatic amines can be deaminated at lower temperatures in the presence of CBr4 than in its absence—longer chains require fewer initiation events. Importantly, this facet of the CBr4 radical chemistry serves to illustrate a general lesson: a compound that successfully outcompetes reduction but does not generate a productive chain carrier is unlikely to be effective.
This chain-carrying effectiveness can be attributed to radical polarity effects. The isodiazene is hydridic, such that electrophilic radicals (•CBr3, •OOR, and •SR) undergo more rapid HAT64 (see the Supporting Information for a detailed computational analysis). Phosphites, on the other hand, afford chain carriers of near-equal efficiency to the parent hydrodeamination process (•Et), consistent with the necessity to heat these reactions.
In summary, a suite of direct functional group interconversions for amines have been developed that complements the abundant nature of this functionality. By identifying productive chain carriers, various anomeric amide-mediated deaminative functionalization reactions were realized, with the tribromomethyl radical being uniquely effective for enabling bromodeamination of both aliphatic and aromatic amine substrates. The innate value of this process was benchmarked in a high-throughput library assay and used for the diversification of API building blocks. Deaminative phosphonylation, thiolation, and hydroxylation were shown to be possible applying the same mechanistic manifold (albeit to more specific amine classes). Mechanistic studies rationalized the disparity between deaminative bromination and other functionalization modes, supporting the superior chain-carrying nature of the polarity-matched carbon tribromide radical. As demonstrated, the deaminative functionalization processes here provide practitioners with a new tool for amine diversification and will ultimately accelerate drug discovery efforts.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Dong and Rawal groups for lending chemicals and Adam M. Weiss (UChicago) for assistance with preparative HPLC. We also thank Bo Hao (Janssen HTE) for technical assistance and Scott Wolkenberg (Janssen PMC) and Jaume Balsells (Janssen DPR) for their constructive feedback. We thank Bas-Jan Van Der Leede, Woute Van Bellegem, Riley Svec, and Callie Bryan of Janssen Research & Development LLC for conducting Ames II testing on compound 1 and for assistance in the interpretation of the results.
Funding
M.D.L. thanks the Packard, Sloan, and Dreyfus Foundations for support. K.J.B. thanks the George Van Dyke Tiers Foundation for fellowship support. O.G. thanks the NIGMS NIH (R35GM137797), Camille and Henry Dreyfus Foundation, and the Welch Foundation (A-2102–20220331) for funding and Texas A&M University HPRC resources (https://hprc.tamu.edu), UMD Deepthought2, MARCC/BlueCrab HPC clusters, and XSEDE (CHE160082 and CHE160053) for computational resources.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c11453.
Experimental procedures, characterization data, and computational details (PDF)
Calculated properties of high throughput substrates (XLSX)
Contributor Information
Balu D. Dherange, Department of Chemistry, University of Chicago, Chicago, Illinois 60637, United States
Mingbin Yuan, Department of Chemistry and Biochemistry, University of Maryland, College Park, Maryland 20742, United States.
Christopher B. Kelly, Discovery Process Research, Janssen Research & Development LLC, Spring House, Pennsylvania 19477, United States
Christopher A. Reiher, Parallel Medicinal Chemistry, Janssen Research & Development LLC, Spring House, Pennsylvania 19477, United States
Cristina Grosanu, High Throughput Purification, Janssen Research & Development LLC, Spring House, Pennsylvania 19477, United States.
Kathleen J. Berger, Department of Chemistry, University of Chicago, Chicago, Illinois 60637, United States
Osvaldo Gutierrez, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States.
Mark D. Levin, Department of Chemistry, University of Chicago, Chicago, Illinois 60637, United States
REFERENCES
- (1).Afagh NA; Yudin AK Chemoselectivity and the Curious Reactivity Preferences of Functional Groups. Angew. Chem., Int. Ed 2010, 49 (2), 262–310. [DOI] [PubMed] [Google Scholar]
- (2).Shenvi RA; O’Malley DP; Baran PS Chemoselectivity: The Mother of Invention in Total Synthesis. Acc. Chem. Res 2009, 42 (4), 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Schwan J; Christmann M Enabling Strategies for Step Efficient Syntheses. Chem. Soc. Rev 2018, 47 (21), 7985–7995. [DOI] [PubMed] [Google Scholar]
- (4).Crossley SWM; Shenvi RA A Longitudinal Study of Alkaloid Synthesis Reveals Functional Group Interconversions as Bad Actors. Chem. Rev 2015, 115 (17), 9465–9531. [DOI] [PubMed] [Google Scholar]
- (5).Corey EJ; Cheng X-M The Logic of Chemical Synthesis, 1st ed.; Wiley: 1995. [Google Scholar]
- (6).Mahjour B; Shen Y; Liu W; Cernak T A Map of the Amine–Carboxylic Acid Coupling System. Nature 2020, 580 (7801), 71–75. [DOI] [PubMed] [Google Scholar]
- (7).Sandford C; Aggarwal VK Stereospecific Functionalizations and Transformations of Secondary and Tertiary Boronic Esters. Chem. Commun 2017, 53 (40), 5481–5494. [DOI] [PubMed] [Google Scholar]
- (8).El-Faham A; Albericio F Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev 2011, 111 (11), 6557–6602. [DOI] [PubMed] [Google Scholar]
- (9).Goldfogel MJ; Huang L; Weix DJ Cross-Electrophile Coupling. In Nickel Catalysis in Organic Synthesis; John Wiley & Sons, Ltd: 2020; pp 183–222. [Google Scholar]
- (10).Juliá F; Constantin T; Leonori D Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev 2022, 122 (2), 2292–2352. [DOI] [PubMed] [Google Scholar]
- (11).Fletcher S. The Mitsunobu Reaction in the 21 St Century. Org. Chem. Front 2015, 2 (6), 739–752. [Google Scholar]
- (12).Brenk R; Schipani A; James D; Krasowski A; Gilbert IH; Frearson J; Wyatt PG Lessons Learnt from Assembling Screening Libraries for Drug Discovery for Neglected Diseases. ChemMedChem. 2008, 3 (3), 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schneider G. Automating Drug Discovery. Nat. Rev. Drug Discovery 2018, 17 (2), 97–113. [DOI] [PubMed] [Google Scholar]
- (14).Campos KR; Coleman PJ; Alvarez JC; Dreher SD; Garbaccio RM; Terrett NK; Tillyer RD; Truppo MD; Parmee ER The Importance of Synthetic Chemistry in the Pharmaceutical Industry. Science 2019, 363 (6424), No. eaat0805. [DOI] [PubMed] [Google Scholar]
- (15).Dong Z; MacMillan DWC Metallaphotoredox-Enabled Deoxygenative Arylation of Alcohols. Nature 2021, 598 (7881), 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Sandfort F; O’Neill MJ; Cornella J; Wimmer L; Baran PS Alkyl–(Hetero)Aryl Bond Formation via Decarboxylative Cross-Coupling: A Systematic Analysis. Angew. Chem., Int. Ed 2017, 56 (12), 3319–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Guo L; Rueping M Decarbonylative Cross-Couplings: Nickel Catalyzed Functional Group Interconversion Strategies for the Construction of Complex Organic Molecules. Acc. Chem. Res 2018, 51 (5), 1185–1195. [DOI] [PubMed] [Google Scholar]
- (18).Xu Y; Qi X; Zheng P; Berti CC; Liu P; Dong G Deacylative Transformations of Ketones via Aromatization-Promoted C─C Bond Activation. Nature 2019, 567 (7748), 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wang Y; Haight I; Gupta R; Vasudevan A What Is in Our Kit? An Analysis of Building Blocks Used in Medicinal Chemistry Parallel Libraries. J. Med. Chem 2021, 64 (23), 17115–17122. [DOI] [PubMed] [Google Scholar]
- (20).Berger KJ; Levin MD Reframing Primary Alkyl Amines as Aliphatic Building Blocks. Org. Biomol. Chem 2021, 19, 11–36. [DOI] [PubMed] [Google Scholar]
- (21).Kong D; Moon PJ; Lundgren RJ Radical Coupling from Alkyl Amines. Nat. Catal 2019, 2 (6), 473–476. [Google Scholar]
- (22).Li Y-N; Xiao F; Guo Y; Zeng Y-F Recent Developments in Deaminative Functionalization of Alkyl Amines. Eur. J. Org. Chem 2021, 2021 (8), 1215–1228. [Google Scholar]
- (23).Laurence C; Brameld KA; Graton J; Le Questel J-Y; Renault E The PKBHX Database: Toward a Better Understanding of Hydrogen-Bond Basicity for Medicinal Chemists. J. Med. Chem 2009, 52 (14), 4073–4086. [DOI] [PubMed] [Google Scholar]
- (24).Tshepelevitsh S; Kütt A; Lõkov M; Kaljurand I; Saame J; Heering A; Plieger PG; Vianello R; Leito I On the Basicity of Organic Bases in Different Media. Eur. J. Org. Chem 2019, 2019 (40), 6735–6748. [Google Scholar]
- (25).Mo F; Dong G; Zhang Y; Wang J Recent Applications of Arene Diazonium Salts in Organic Synthesis. Org. Biomol. Chem 2013, 11 (10), 1582–1593. [DOI] [PubMed] [Google Scholar]
- (26).Mukhopadhyay S; Batra S Applications of Sodium Nitrite in Organic Synthesis. Eur. J. Org. Chem 2019, 2019 (38), 6424–6451. [Google Scholar]
- (27).Klauck FJR; James MJ; Glorius F Deaminative Strategy for the Visible-Light-Mediated Generation of Alkyl Radicals. Angew. Chem., Int. Ed 2017, 56 (40), 12336–12339. [DOI] [PubMed] [Google Scholar]
- (28).Basch CH; Liao J; Xu J; Piane JJ; Watson MP Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C─N Bond Activation. J. Am. Chem. Soc 2017, 139 (15), 5313–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Pang Y; Moser D; Cornella J Pyrylium Salts: Selective Reagents for the Activation of Primary Amino Groups in Organic Synthesis. Synthesis 2020, 52 (4), 489–503. [Google Scholar]
- (30).Martin-Montero R; Yatham VR; Yin H; Davies J; Martin R Ni-Catalyzed Reductive Deaminative Arylation at Sp3 Carbon Centers. Org. Lett 2019, 21 (8), 2947–2951. [DOI] [PubMed] [Google Scholar]
- (31).Yi J; Badir SO; Kammer LM; Ribagorda M; Molander GA Deaminative Reductive Arylation Enabled by Nickel/Photoredox Dual Catalysis. Org. Lett 2019, 21 (9), 3346–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sun S-Z; Romano C; Martin R Site-Selective Catalytic Deaminative Alkylation of Unactivated Olefins. J. Am. Chem. Soc 2019, 141 (41), 16197–16201. [DOI] [PubMed] [Google Scholar]
- (33).Ni S; Li C-X; Mao Y; Han J; Wang Y; Yan H; Pan Y Ni-Catalyzed Deaminative Cross-Electrophile Coupling of Katritzky Salts with Halides via C─N Bond Activation. Sci. Adv 2019, 5 (6), No. eaaw9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pulikottil FT; Pilli R; Suku RV; Rasappan R Nickel-Catalyzed Cross-Coupling of Alkyl Carboxylic Acid Derivatives with Pyridinium Salts via C─N Bond Cleavage. Org. Lett 2020, 22 (8), 2902–2907. [DOI] [PubMed] [Google Scholar]
- (35).Zhang X; Qi D; Jiao C; Liu X; Zhang G Nickel-Catalyzed Deaminative Sonogashira Coupling of Alkylpyridinium Salts Enabled by NN2 Pincer Ligand. Nat. Commun 2021, 12 (1), 4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ashley MA; Rovis T Photoredox-Catalyzed Deaminative Alkylation via C─N Bond Activation of Primary Amines. J. Am. Chem. Soc 2020, 142 (43), 18310–18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Dorsheimer JR; Ashley MA; Rovis T Dual Nickel/Photoredox-Catalyzed Deaminative Cross-Coupling of Sterically Hindered Primary Amines. J. Am. Chem. Soc 2021, 143 (46), 19294–19299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Ghiazza C; Faber T; Gómez-Palomino A; Cornella J Deaminative Chlorination of Aminoheterocycles. Nat. Chem 2022, 14 (1), 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ghiazza C; Wagner L; Fernández S; Leutzsch M; Cornella J Bio-Inspired Deaminative Hydroxylation of Aminoheterocycles and Electron-Deficient Anilines. Angew. Chem., Int. Ed 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kennedy SH; Dherange BD; Berger KJ; Levin MD Skeletal Editing through Direct Nitrogen Deletion of Secondary Amines. Nature 2021, 593 (7858), 223–227. [DOI] [PubMed] [Google Scholar]
- (41).Berger KJ; Driscoll JL; Yuan M; Dherange BD; Gutierrez O; Levin MD Direct Deamination of Primary Amines via Isodiazene Intermediates. J. Am. Chem. Soc 2021, 143 (42), 17366–17373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kharasch MS; Jensen EV; Urry WH Reactions of Atoms and Free Radicals in Solution. X. The Addition of Polyhalomethanes to Olefins. J. Am. Chem. Soc 1947, 69 (5), 1100–1105. [Google Scholar]
- (43).Strekowski L; Kiselyov AS; Fokin AA; Schreiner PR Carbon Tetrabromide. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd: 2007. [Google Scholar]
- (44).Palmieri G Oxidation of N-Alkyl-N’-Tosylhydrazines with Bromine. Tetrahedron 1983, 39 (24), 4097–4101. [Google Scholar]
- (45).White EH; Ryan TJ; Hahn BS; Erickson RH N-Nitroso- and N-Nitrotrialkylureas and Their Decomposition. J. Org. Chem 1984, 49 (25), 4860–4866. [Google Scholar]
- (46).Digles D; Ecker GF Self-Organizing Maps for In Silico Screening and Data Visualization. Mol. Inform 2011, 30 (10), 838–846. [DOI] [PubMed] [Google Scholar]
- (47).Definition of success: 0.5 mg at >85% purity was used as the cutoff for success because (i) semi-automated, mass directed purification prioritizes speed and purity over yield and (ii) the work-up procedure was unoptimized and may have contributed to diminished yield.
- (48).Gatto GJ; Boyne MT; Kelleher NL; Walsh CT Biosynthesis of Pipecolic Acid by RapL, a Lysine Cyclodeaminase Encoded in the Rapamycin Gene Cluster. J. Am. Chem. Soc 2006, 128 (11), 3838–3847. [DOI] [PubMed] [Google Scholar]
- (49).Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59 (10), 4443–4458. [DOI] [PubMed] [Google Scholar]
- (50).Jouffroy M; Primer DN; Molander GA Base-Free Photoredox/Nickel Dual-Catalytic Cross-Coupling of Ammonium Alkylsilicates. J. Am. Chem. Soc 2016, 138 (2), 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Levterov VV; Panasyuk Y; Pivnytska VO; Mykhailiuk PK Water-Soluble Non-Classical Benzene Mimetics. Angew. Chem., Int. Ed 2020, 59 (18), 7161–7167. [DOI] [PubMed] [Google Scholar]
- (52).Kharasch MS; Jensen EV; Urry WH Addition of Carbon Tetrachloride and Chloroform to Olefins. Science 1945, 102 (2640), 128–128. [DOI] [PubMed] [Google Scholar]
- (53).Alvarez EM; Karl T; Berger F; Torkowski L; Ritter T Late-Stage Heteroarylation of Hetero(Aryl)Sulfonium Salts Activated by α-Amino Alkyl Radicals. Angew. Chem., Int. Ed 2021, 60 (24), 13609–13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Liu J; Xiao H-Z; Fu Q; Yu D-G Advances in Radical Phosphorylation from 2016 to 2021. Chem. Synth 2021, 1 (1), 9. [Google Scholar]
- (55).Chmiel AF; Williams OP; Chernowsky CP; Yeung CS; Wickens ZK Non-Innocent Radical Ion Intermediates in Photoredox Catalysis: Parallel Reduction Modes Enable Coupling of Diverse Aryl Chlorides. J. Am. Chem. Soc 2021, 143 (29), 10882–10889. [DOI] [PubMed] [Google Scholar]
- (56).Dénès F; Pichowicz M; Povie G; Renaud P Thiyl Radicals in Organic Synthesis. Chem. Rev 2014, 114 (5), 2587–2693. [DOI] [PubMed] [Google Scholar]
- (57).Teders M; Henkel C; Anhäuser L; Strieth-Kalthoff F; Gómez-Suárez A; Kleinmans R; Kahnt A; Rentmeister A; Guldi D; Glorius F The Energy-Transfer-Enabled Biocompatible Disulfide–Ene Reaction. Nat. Chem 2018, 10 (9), 981–988. [DOI] [PubMed] [Google Scholar]
- (58).Maillard B; Ingold KU; Scaiano JC Rate Constants for the Reactions of Free Radicals with Oxygen in Solution. J. Am. Chem. Soc 1983, 105 (15), 5095–5099. [Google Scholar]
- (59).Pryor WA; Pickering TL Reactions of Radicals. Rates of Chain Transfer of Disulfides and Peroxides with the Polystyryl Radical. J. Am. Chem. Soc 1962, 84 (14), 2705–2711. [Google Scholar]
- (60).Parsaee F; Senarathna MC; Kannangara PB; Alexander SN; Arche PDE; Welin ER Radical Philicity and Its Role in Selective Organic Transformations. Nat. Rev. Chem 2021, 5 (7), 486–499. [DOI] [PubMed] [Google Scholar]
- (61).Riplinger C; Neese F An Efficient and near Linear Scaling Pair Natural Orbital Based Local Coupled Cluster Method. J. Chem. Phys 2013, 138 (3), 034106. [DOI] [PubMed] [Google Scholar]
- (62).Riplinger C; Pinski P; Becker U; Valeev EF; Neese F Sparse Maps—A Systematic Infrastructure for Reduced-Scaling Electronic Structure Methods. II. Linear Scaling Domain Based Pair Natural Orbital Coupled Cluster Theory. J. Chem. Phys 2016, 144 (2), 024109. [DOI] [PubMed] [Google Scholar]
- (63).The carbon tribromide radical builds to high enough concentrations that pentabromoethane is often observed as a byproduct in crude reaction mixtures (presumably via the hexabromoethane dimer, which can similarly undergo bromine transfer).
- (64).Liu F; Ma S; Lu Z; Nangia A; Duan M; Yu Y; Xu G; Mei Y; Bietti M; Houk KN Hydrogen Abstraction by Alkoxyl Radicals: Computational Studies of Thermodynamic and Polarity Effects on Reactivities and Selectivities. J. Am. Chem. Soc 2022, 144 (15), 6802–6812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.