Abstract
The eukaryotic cytosolic Fe-S protein assembly (CIA) machinery inserts iron-sulfur (Fe-S) clusters into cytosolic and nuclear proteins. In the final maturation step, the Fe-S cluster is transferred to the apo-proteins by the CIA-targeting complex (CTC). However, the molecular recognition determinants of client proteins are unknown. We show that a conserved [LIM]-[DES]-[WF]-COO− tripeptide present at the C-terminus of clients is necessary and sufficient for binding to the CTC in vitro and directing Fe-S cluster delivery in vivo. Remarkably, fusion of this TCR (target complex recognition) signal enables engineering of cluster maturation on a non-native protein via recruitment of the CIA machinery. Our study significantly advances our understanding of Fe-S protein maturation and paves the way for bioengineering applications.
One-Sentence Summary:
A C-terminal tripeptide guides eukaryotic iron-sulfur cluster insertion into cytosolic and nuclear proteins.
Iron-sulfur (Fe-S) proteins catalyze transformations vital for life-sustaining processes including photosynthesis, respiration, and nitrogen fixation. Although Fe-S cofactors are readily inserted into apo-proteins in vitro, their biosynthesis requires dedicated pathways that assemble the metalloclusters and shepherd these cofactors to apo-protein clients (1). Cluster biogenesis is essential for iron homeostasis, energy metabolism, genome stability, and other critical cellular processes impacting human health (2–5). The diversity of transformations catalyzed by Fe-S enzymes also makes them attractive candidates for bioengineering of novel pathways, however, inefficient cluster delivery to non-native proteins often frustrates pharmaceutical and biofuel applications (table S1) (6, 7). Although progress toward understanding the apo-client recognition has been made in some systems (7–9), there are no plug-and-play solutions to overcome bottlenecks associated with inefficient metallocofactor maturation.
Eukaryotic Fe-S biogenesis is complex because each subcellular compartment houses its own dedicated machinery (1). Cytosolic and nuclear Fe-S protein assembly (CIA) requires several mitochondrial iron-sulfur cluster (ISC) maturation components, including the Nfs1 cysteine desulfurase, and at least 9 additional cytosolic proteins. The ISC machinery synthesizes a molecule containing iron and/or sulfur (XS; Fe-Sint) (10, 11). Upon export, several protein factors use this molecule for [4Fe-4S] cluster assembly on the Cfd1-Nbp35 CIA scaffold (Fig. 1A) (12, 13). Nar1, a 2x[4Fe-4S] protein acts as the Fe-S cluster carrier, associates with the CIA targeting complex (CTC, Fig. 1A) and provides the cluster to be inserted into apo-Fe/S targets (14). These targets are recognized by the CTC, a complex comprising Met18/MMS19 (yeast/human nomenclature), Cia1/CIAO1, and Cia2/CIAO2 (15–18).
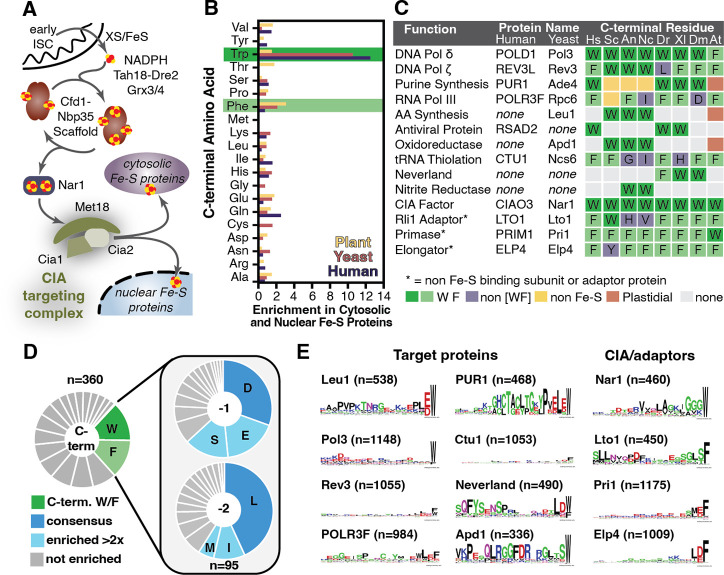
Fig. 1. A conserved [LIM]-[DES]-[WF] tripeptide is found at the C-terminus of CIA clients.
(A) Overview of the CIA pathway (yeast nomenclature). In the final step, the CIA targeting complex (CTC) identifies the CIA clients through direct, or adaptor mediated, protein-protein interactions and delivers the Fe-S cluster. (B) The enrichment of C-terminal amino acids (frequency in dataset/frequency in C-terminal proteome) in cytosolic and nuclear Fe-S proteomes of plants (Arabidopsis thaliana, yellow), yeast (Saccharomyces cerevisiae, pink), and in humans (Homo sapiens, purple). (C) The C-terminal residue of Fe-S proteins, CIA factors, and adaptors terminating in W/F in H. sapiens (Hs), S. cerevisiae (Sc), Aspergillus nidulans (An), Neurospora crassa (Nc), Danio rerio (Dr), Xenopus laevis (Xl), Drosophilia melonogaster (Dm), and A. thaliana (At). (D) Pie charts showing frequency of amino acids found at the C-terminus (n=360 sequences), and the −1 (penultimate) and −2 positions (n=95 sequences, those from the C-terminal dataset terminating in W/F). Amino acids indicated are enriched >2-fold (light green or blue) or >3-fold (dark green or blue) relative to their frequency in the human proteome. (E) WebLogos depicting conservation of C-termini for [4Fe-4S] proteins (Leu1, Pol3, Rev3, POLR3F, PUR1, Ctu1), [2Fe-2S] proteins (Neverland and Apd1), and the CIA factors/adaptors (Nar1, Lto1, Pri1, Elp4).
One major question addressed herein is how the CTC identifies its >30 clients, which include Fe-S proteins essential for DNA replication, transcription, translation, and other cellular processes (19). Proteomics and gene depletion studies have suggested that some clients only require a single CTC component for their maturation (e.g.Viperin (RSAD1) requires only Cia1), whereas other targets depend on two or more CTC subunits (20, 21). Additionally, the CIA client Rli1 does not directly bind the CTC but depends on the Yae1-Lto1 adaptor for CIA machinery recruitment (22). The diversity of the CIA proteome, combined with the incomplete catalog of CIA clients requiring adaptors, have made it challenging to unravel the cryptic codes driving substrate recognition by the CTC.
Despite these challenges, some recent studies indicated that some targets might exploit signals at their C-termini to recruit the CIA machinery. The clients, Viperin and Apd1, and the Lto1 adaptor terminate in a tryptophan residue that is critical for in vivo cofactor maturation (22–24). These studies suggest the tantalizing possibility that CIA clients use conserved recognition motifs to recruit the CTC, reminiscent of the recent proposal that an “LYR” tripeptide motif guides Fe-S cluster delivery from ISC (8). However, experimental support for this proposal is lacking, particularly regarding the number of eukaryotic Fe-S proteins bearing such signals, whether additional other residues in the primary or tertiary structure contribute to recognition, and whether these determinants are sufficient for CIA machinery recruitment. Here we show that >30% of CIA clients or their adaptors have a [LIM]-[DES]-[WF]-COO− tripeptide motif that recruits the CIA machinery and demonstrate using integrative in vitro and in vivo approaches that this Targeting Complex Recognition (TCR) signal is both necessary and sufficient for binding the CTC and delivering Fe-S clusters from the CIA machinery.
A C-terminal aromatic residue is enriched and conserved in CIA clients
By cataloging the C-terminal residue of every Fe-S protein in Fungi (Saccharomyces cerevisiae) and Metazoa (Homo sapiens) (25), we found that a C-terminal tryptophan residue was uniquely enriched in cytosolic and nuclear Fe-S proteins. Six (24%) of the yeast and five (13%) of the human CIA clients, factors, and adaptors terminated in a tryptophan (Fig. 1B, Data S1). For comparison, only 1.8% and 1.2% of all yeast and human proteins, respectively, end with a tryptophan. For plant (Arabidopsis thaliana) CIA clients (26), the most enriched C-terminal residue was phenylalanine, occurring in seven (17%) of 42 non-glutaredoxin cytosolic and nuclear Fe-S proteins (Fig. 1B, Data S1). Remarkably, none of the 164 yeast, human, or plant mitochondrial or plastidial Fe-S proteins terminated with a tryptophan or phenylalanine (fig. S1, Data S1). Similarly, just two (0.8%) of the 248 Fe-S proteins from Bacteria (E. coli) and Archaea (Methanocaldococcus jannaschii) terminate with tryptophan and ten (4%) with phenylalanine (fig. S1D, Data S1) (27–29). Therefore, a C-terminal tryptophan or phenylalanine is a hallmark of CIA clients – the first clear example of a putative CIA-targeting sequence.
Next, 360 CIA clients from 10 model organisms were analyzed challenge the universality of this hallmark and uncover any additionally conserved features (Fig. 1C, Data S2). Of the 95 sequences terminating in W or F (26% of 360 sequences), all had conserved amino acids in the penultimate (−1) and antepenultimate (−2) (Fig. 1D, Data S2). A negatively charged residue (48%) or a serine (15%) were preferred in the penultimate position, whereas a hydrophobic residue occurs at the antepenultimate in 59% of the sequences. Sequence alignments and analysis revealed conservation is limited to the −1 and −2 positions (Fig. 1E, Data S2). We named this tripeptide ([LIM]-[DES]-[WF]) motif the Targeting Complex Recognition (TCR) signal, due to its role in recruitment of the CTC (vide infra).
When CIA clients terminating in the TCR signal were compared to their homologs that are matured by other Fe-S biogenesis machineries, we only found the tripeptide motif to be exclusively conserved in CIA clients. For example, fungal isopropylmalate isomerases (Leu1) are cytosolic [4Fe-4S] proteins with a TCR signal (Fig. 1E), but bacterial, archaeal, mitochondrial and plastidial orthologs are missing the tripeptide (fig. S2, A and B). Analogous correspondences occur for other classes of Fe-S proteins, including: the CIA factor Nar1; the [4Fe-4S] protein Viperin, Ctu1, and nitrite reductase; and the [2Fe-2S] proteins Apd1, Neverland, and choline monooxygenase (fig. S2). The length of the TCR-tail varies between ~3 and 45 amino acid residues (fig. S2, boxed), suggesting the tail length tunes positioning of different clients. Available proteomic mass spectrometry data and structures of Fe-S proteins isolated from the native host demonstrates that the TCR motif remains attached after maturation (table S2), in contrast to mitochondrial import.
Additionally, some Fe-S clusters play a structural or regulatory role and therefore their cluster binding residues can be lost with evolutionary drift. Remarkably, the TCR signal and Fe-S ligands show strong correlation. For example, many glutamine phosphoribosylpyrophosphate amidotransferases, such as PUR1 in humans, are [4Fe-4S] proteins and they terminate with a TCR signal (Fig. 1E). In contrast, TCR signals are absent in the plant enzymes, which are plastidial, and thus are not matured by CIA, and in their fungal homologs, which lack Fe-S clusters (fig. S3A). RNA polymerases displayed similar co-conservation of TCR and Fe-S ligands (fig. S3B). These correlations strongly suggest that the TCR signal is functionally related to CIA.
Next, we truncated the TCR motif at the C-terminus of Pol3, the catalytic subunit of DNA polymerase δ (30). Pol3 is essential for yeast viability upon DNA damage with methylmethane sulfonate (MMS) (Fig. 2A). Chromosomal POL3 was placed under control of a galactose-regulatable promoter (Gal-POL3). This strain’s phenotypic growth defect in the presence of MMS and glucose was alleviated by episomal Pol3 expression at endogenous levels, but not by modified genes encoding truncated Pol3 variants (Fig. 2A). In similar experiments, C-terminally truncated variants of Apd1, Nar1, and Leu1 were unable to restore normal growth when expressed at low levels in Gal-regulatable (GAL-NAR1) or deletion (Δapd1, Δleu1) strains (Fig. 2, B to D; fig. S4 and S5). In all cases, removal or substitution of just the C-terminal tryptophan was sufficient to elicit the growth defect (Fig. 2). Thus, when a the TCR motif is present, it is universally required for the Fe-S protein function.
Fig. 2. Deletion of the C-terminal tryptophan of CIA clients results in defective Fe-S cluster delivery.
(A-C) The indicated yeast strains, wild-type W303, apd1 deletion, or Gal-regulable POL3 or NAR1, were transformed with a centromeric plasmid for expression of Pol3 (A), Apd1 (B), or Nar1 (C) from their natural promoters. The Δ indicates the number of C-terminal amino acids that were removed, ø indicates the empty vector control, and WT indicates the full length wild-type insert. Yeast cells were grown overnight in glucose (Glc) containing medium, then spotted after serial dilution onto Glc medium with the indicated concentrations of methylmethane sulfonate (MMS) or gallobenzophenone (Gallo). (D) Plasmid-borne Leu1 was expressed from the weak (RET2) promoter in a Δleu1 strain with a functional LEU2 allele. Yeast were grown as in (A-C), but in the absence of leucine. (E and F) Determination of Leu1 enzymatic activity in yeast crude extracts and 55Fe incorporation to quantify Fe-S cluster insertion into Leu1. W303 or Δleu1 strains harboring the indicated Leu1 variant (expressed from its natural promoter) were grown as in (A-C). For de novo 55Fe incorporation cells were grown in Fe-free medium overnight, followed by incubation with 55Fe and immunoprecipitation of Leu1. (G) Fe-S cluster insertion by the E. coli ISC machinery (cell extract, dark gray) or by chemical reconstitution of Leu1’s Fe-S cluster following purification (light gray) are unaffected by C-terminal truncation up to 17 amino acids.
Focusing on Leu1, the benchmark enzyme for quantitative measurements of CIA function (31), centromeric plasmids encoding Leu1 truncations were expressed in a Δleu1 strain. Deletion of W779 from the TCR (Leu1-Δ1), and a further truncation up to 17 amino acids, led to 60 % loss of Leu1 activity without decreasing the Leu1 expression level. (Fig. 2E; fig. S6). Analysis of de novo 55Fe incorporation into Leu1 was similarly diminished by TCR truncation (Fig. 2F). We speculate that the remaining activity of Leu1-Δ1 results from a second CTC binding determinant (32). At low expression levels, both the TCR and this second determinant are required for efficient maturation, but increased expression can drive CTC-Leu1- Δ1 or -Nar1- Δ1 complex formation (Fig. 2, C and D; fig. S4). Additional controls demonstrated the decreased Leu1- Δ1 activity was independent of the transcriptional promoter and terminator sequences (fig. S7). To rule out the possibility that the Leu1 activity loss results from defective protein folding, the Leu1 truncations were expressed in E. coli, which does not encode the CIA machinery. Leu1 activity in E. coli crude extracts or following its purification and Fe-S cluster reconstitution was undiminished upon removal of ≤17 amino acids (Fig. 2G). These data establish that the Leu1 activity loss upon TCR signal truncation results from a specific defect in cluster delivery from the CIA system.
The TCR motif mediates interaction with the CIA Targeting Complex
The CIA targeting complex (CTC) identifies apo-clients and thus it is the likely TCR signal receptor. To test this hypothesis, we used an affinity copurification assay to analyze the TCR-CTC interaction, employing the [2Fe-2S] Apd1 and [4Fe-4S] Leu1 client proteins. Both proteins copurified with the CTC, but variants lacking the indole moiety had a defect in CTC binding (Fig. 3, A to C; fig. S8). Thus, the TCR tail is critical for CTC interaction in vitro, explaining why TCR truncation leads to a defective Fe-S protein maturation in vivo.
Fig. 3. CTC binding in vitro and Fe-S cluster maturation in vivo depend on the C-terminal tryptophan and its carboxy terminus.
(A) Apd1 (wild-type; WT; W316A variant, or none; -), was mixed with yeast CTC subunits (Met18, Strep-tagged Cia1, and truncated Cia2D102). Apd1 associated with the CTC was evaluated via SDS-PAGE analysis after Streptactin affinity purification. (B) Variants (red) of wild type (WT) Leu1 tested in panels (C-E). Non-bold numbers refer to the positions in the amino acid sequence. (C) The Leu1 variants (bolded numbers) were mixed with the yeast CTC (Met18, Cia1, and Strep-tagged Cia2). Leu1 associated with the CTC was evaluated by SDS-PAGE after Streptactin affinity purification. (D) Leucine-independent growth of yeast (as in Fig. 2D) depends on the C-terminal W of Leu1. (E) Determination of Leu1 variant enzymatic activity in yeast cell extract as in Fig. 2E. (F) Affinity copurification of C-terminal Leu1 truncations as in (B). (G) The last 18 amino acids of S. cerevisiae Leu1 (gray, 10) were replaced with Leu1 tails from S. pombe (green, 11) or A. nidulans (purple, 12). The specific activities of the resulting tail transplant variants were analyzed as in Fig. 2E.
To confirm the functional relevance of the TCR to client protein maturation in vivo, the Leu1 W779 variants were expressed at low levels in a Δleu1 strain to evaluate growth in a leucine deficient medium and at higher levels to quantify Leu1 activity in crude extracts (Fig. 3, D and E; fig. S9). The W779A complemented strain grew at a 2-fold slower rate than strains expressing the WT protein and exhibited a 2-fold reduction in Leu1 specific activity (Fig. 3, D and E; fig. S9). Western blotting confirmed the decreased activity was not due to differences in protein levels (fig. S10). Leu1 with W779F/Y substitutions did not pull down with the CTC in the stringent in vitro assay (Fig. 3C), but the in vivo functionality of these TCR variants was less severely affected (Fig. 3, D and E; fig. S9). Altogether, these results demonstrate that a C-terminal tryptophan is the preferred residue for yeast TCR signals, but TCRs that include a phenylalanine residue can also be recognized.
Next, we extended the C-terminus of Leu1 by three amino acids to determine whether an internal TCR signal retained functionality. This construct was clearly unable to bind the CTC or recruit the CIA machinery in vivo (Fig. 3). Since the D778A, Q777A, and H776A substitutions neither impacted yeast growth nor CTC binding (Fig. 3), our data identify the aromatic side chain and its C-terminal position as the most critical determinants for CTC recruitment.
To examine whether TCR tail length impacts its functionality, we replaced the last 10 or 20 amino acids of Leu1 with a single tryptophan residue. As neither variant copurified with the CTC (Fig. 3F), we concluded that either additional amino acids of the tail are critical for recognition by the CTC or the TCR signal has a certain spatial requirement to allow for correct positioning in the CTC complex. To distinguish between these possibilities, we replaced the last 18 amino acids of the S. cerevisiae Leu1 with those from Schizosaccharomyces pombe or Aspergillus nidulans proteins. Importantly, these three sequences do not share any conserved residues besides the terminal [DE]-W motif (Fig. 3G). Because these tail transplant variants were efficiently matured by the CTC (Fig. 3G), we concluded that in addition to the primary sequence determinants, the TCR tail length impacts client positioning for optimal interaction and Fe-S delivery from the CTC.
The TCR signal is sufficient for recruitment of CTC to non-native targets.
To eliminate any additional sequence determinants within CIA clients that might contribute to CTC recruitment, we fused various TCR tails to SUMO, a small well folded protein with an accessible C-terminus. These SUMO peptide carriers (SPC) for Leu1759–779, Nar1482–491, Pol31088–1097 and Rev31495–1504 each bound to the CTC in a tryptophan dependent manner (Fig. 4A). Moreover, the Leu1 tail could be further truncated from 20 to just 3 amino acids (QDW) without disrupting CTC interaction (Fig. 4A).
Fig. 4. An engineered TCR signal is sufficient to recruit non-native proteins to the CTC and for Fe-S cluster delivery.
(A) Cartoon of SUMO peptide carrier (SPC) fusions used in copurification, as in Fig. 3C. Streptactin elution fractions were analyzed by SDS-PAGE and immunoblotting against the SPC N-terminal His-tag. (B) A non-native Fe-S protein, E. coli LeuCD, was engineered for cluster delivery from the CIA machinery. The E. coli subunits were fused with a linker (yellow) and the TCR-tail (salmon) of yeast Leu1 was attached. Protein structures are AlphaFold models. (C) In vivo cytosolic [4Fe-4S] cluster insertion into engineered E. coli LeuCD depends on the TCR signal with an appropriate tail length. Specific activities in cell extracts were determined as in Fig. 2E. Each LeuCD variant was expressed in a Δleu1 yeast strain (light gray), or a Δleu1 strain with galactose-regulatable genes (dark gray). Growth on Glc medium for 40 h led to depletion (↓) of the indicated Fe-S cluster biosynthesis protein. (D) Modelling of the PRIM1-PRIM2 (AlphaFold, orange and sand) and CTC (6TC0, blue hues) into the CryoEM density of the Primase-CTC complex (32). This model shows that the TCR signal (KDF, salmon) interacts with the proposed client binding site on blade 3 of CIAO1.
If the TCR motif is sufficient for association with the CTC, then it should be possible to direct Fe-S cluster delivery to a non-native, heterologous Fe-S protein in yeast. To test this prediction, we focused on the E. coli LeuC-LeuD heterodimer, the homolog of yeast Leu1 (Fig. 4B). Because the [4Fe-4S] cluster of LeuC-LeuD is biosynthesized by the bacterial ISC system, it does not possess the TCR-extension (fig. S2, A and B). The E. coli leuC and leuD genes were fused with linker derived from yeast Leu1 (yellow, Fig. 4B). When the last 30 amino acids of yeast Leu1 were fused to the C-terminus of this LeuCD construct, its enzymatic activity increased >3-fold (Fig. 4C). To demonstrate this increased activity was due to CIA machinery recruitment, several control experiments were performed. First, we determined that the increased activity of LeuCD-TCR fusion depends on the C-terminal tryptophan (Fig. 4C). Second, fractionation of yeast demonstrated LeuCD activity is cytosolic, not mitochondrial where ISC is functional (fig. S11). Third, GAL-regulatable expression of Fe-S assembly factors in Δleu1 strains confirmed the increased activity depends on the CIA machinery and the early ISC machinery providing XS/[Fe-S]int (Fig. 4C).
To identify the minimal length required to position LeuCD for cluster delivery, we shortened the TCR tail to 3 or 10 amino acids, but these variants were unable to guide Fe-S maturation (Fig. 4C). However, when Leu1 tail residues 753–776 were replaced with (SSG)8 spacer, 47±9% activity was recovered (Fig. 4C), corroborating our observation that the TCR tail length is critical for proper positioning (Fig. 3, F and G). Thus, the TCR signal can be exploited to guide non-native Fe-S proteins to the CIA machinery for metallocofactor delivery.
Discussion
N- or C-terminal primary sequence determinants are important signals for subcellular trafficking and post-translational modifications (33). Herein, we establish that cytosolic and nuclear Fe-S protein maturation is another key process controlled through short linear peptide motifs presented on the solvent-exposed C-terminus. The [LIM]-[DES]-[WF] tripeptide is ubiquitous in the eukaryotic domain of life, and consistent with our proposal it serves as a universal signal directing cytosolic and nuclear Fe-S protein maturation. Since CIA is proposed to biosynthesize [4Fe-4S] proteins (1, 34), we thus were initially surprised to identify TCR motifs in four bis-histidinyl coordinated [2Fe-2S] proteins, including Apd1 which requires its C-terminal tryptophan for CTC interaction and in vivo function (Fig. 2B, Fig. 3A). Consequently, our data imply that [2Fe-2S] proteins are also matured by the CTC.
Surprisingly, we uncovered “cryptic” TCR motifs in ELP4 and PRIM1 (Fig. 1E, fig. S12), which are non-metal binding subunits of the elongator and primase complexes. We speculate ELP4 and PRIM1 serve as adaptors for ELP3 and PRIM2, the [4Fe-4S] subunits, similar to Lto1-Yae1 for Rli1 maturation (22). First, PRIM1’s TCR signal is well conserved in Eukaryotes, but absent in archaeal homologs (fig. S12), following the pattern observed for other TCRs (fig. S2). Second, PRIM1 was found to be required for formation of the primase-CTC complex (32), but the molecular basis for this requirement was puzzling. By docking of PRIM1 into the available cryoEM density of the CTC-PRIM1-PRIM2 complex (32), we discovered that PRIM1’s TCR signal is properly positioned to interact with the CIAO1 and CIAO2 subunits of the CTC (Fig. 4D). This previously unnoted finding not only corroborates our proposal that PRIM1 can function as an adaptor for PRIM2, but it indicates that the TCR motif of clients and adaptors anchors in this region of the CTC (32, 35). Altogether, our data explain how ~30% of clients are identified by the CIA machinery: 19% (6 of the 32 human CIA clients) directly interact with the CTC via their TCR, and an additional 9% (ELP3, PRIM2 and Rli1) depend on an adaptor.
One important question remains unresolved: how do the remaining cytosolic and nuclear Fe-S proteins, those without TCR signals or adaptors, recruit the CTC? Our findings combined with previous data indicates that at least two TCR determinants are present in every CIA client and the successful approach described herein provides a framework for discovery of these additional motifs. Finally, it is notable that just one determinant is sufficient to recruit the CIA machinery, as the [LIM]-[DES]-[WF] motif can be exploited to engineer cluster delivery to a non-native Fe-S enzyme (Fig. 4). As deficiencies in cluster maturation frustrate efforts to engineer novel biosynthetic pathways requiring Fe-S enzymes, our study provides a roadmap for overcoming these bottlenecks and demonstrates how an improved understanding of the fundamental biochemistry can impact metalloenzyme bioengineering.
Supplementary Material
Acknowledgments:
We thank Sheena Vasquez for assistance in generating the model shown in Figure 4D that was based on a cryo-EM map deposited by Kassube and Thomä. We are grateful to Roland Lill’s lab, particularly Martin Stümpfig, for 55Fe incorporation analysis. We also thank Gary Sawers, Sean Elliott, Sheena Vasquez, Catherine Drennan, and Dan Kahne for helpful discussions.
Funding:
National Institutes of Health grant 1R01GM121673 (DLP)
National Science Foundation Graduate Research Fellowship (MDM, DGE-1247312) and CAREER (DLP, CHE-1555295)
German Research Foundation grant PI610/2-1 and 2 (AJP) from the Priority Program 1927
Footnotes
Competing interests: Authors declare that they have no competing interests.
Data and materials availability:
All data are available in the main text or the supplementary materials and materials used in the analysis are available upon request any researcher for purposes of reproducing or extending the analysis.
References
- 1.Braymer J. J., Freibert S. A., Rakwalska-Bange M., Lill R., Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim Biophys Acta Mol Cell Res 1868, 118863 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Weon J. L., Yang S. W., Potts P. R., Cytosolic Iron-Sulfur Assembly Is Evolutionarily Tuned by a Cancer-Amplified Ubiquitin Ligase. Mol Cell 69, 113–125.e116 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Wachnowsky C., Fidai I., Cowan J. A., Iron-sulfur cluster biosynthesis and trafficking - impact on human disease conditions. Metallomics 10, 9–29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maio N. et al. , Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets. Science 373, 236–241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honarmand Ebrahimi K. et al. , Iron-sulfur clusters as inhibitors and catalysts of viral replication. Nat Chem 14, 253–266 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Biz A., Mahadevan R., Overcoming Challenges in Expressing Iron-Sulfur Enzymes in Yeast. Trends Biotechnol 39, 665–677 (2021). [DOI] [PubMed] [Google Scholar]
- 7.D’Angelo F. et al. , Cellular assays identify barriers impeding iron-sulfur enzyme activity in a non-native prokaryotic host. Elife 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maio N. et al. , Cochaperone binding to LYR motifs confers specificity of iron sulfur cluster delivery. Cell Metab 19, 445–457 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy E. L., Booker S. J., Destruction and reformation of an iron-sulfur cluster during catalysis by lipoyl synthase. Science 358, 373–377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey A. K., Pain J., Dancis A., Pain D., Mitochondria export iron-sulfur and sulfur intermediates to the cytoplasm for iron-sulfur cluster assembly and tRNA thiolation in yeast. J Biol Chem 294, 9489–9502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kispal G., Csere P., Prohl C., Lill R., The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J 18, 3981–3989 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netz D. J., Pierik A. J., Stümpfig M., Muhlenhoff U., Lill R., The Cfd1-Nbp35 complex acts as a scaffold for iron-sulfur protein assembly in the yeast cytosol. Nat. Chem. Biol. 3, 278–286 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan V., Pierik A. J., Lill R., Crystal structures of nucleotide-free and glutathione-bound mitochondrial ABC transporter Atm1. Science 343, 1137–1140 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Balk J., Pierik A. J., Netz D. J., Mühlenhoff U., Lill R., The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron-sulphur proteins. EMBO J. 23, 2105–2115 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gari K. et al. , MMS19 links cytoplasmic iron-sulfur cluster assembly to DNA metabolism. Science 337, 243–245 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Stehling O. et al. , MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 337, 195–199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balk J., Netz D. J. A., Tepper K., Pierik A. J., Lill R., The essential WD40 protein Cia1 is involved in a late step of cytosolic and nuclear iron-sulfur protein assembly. Mol. Cell. Biol. 25, 10833–10841 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weerapana E. et al. , Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Netz D. J. A., Mascarenhas J., Stehling O., Pierik A. J., Lill R., Maturation of cytosolic and nuclear iron-sulfur proteins. Trends Cell. Biol. 24, 303–312 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Stehling O. et al. , Human CIA2A-FAM96A and CIA2B-FAM96B integrate iron homeostasis and maturation of different subsets of cytosolic-nuclear iron-sulfur proteins. Cell Metab. 18, 187–198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vo A. et al. , Identifying the Protein Interactions of the Cytosolic Iron-Sulfur Cluster Targeting Complex Essential for Its Assembly and Recognition of Apo-Targets. Biochemistry 57, 2349–2358 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Paul V. D. et al. , The deca-GX3 proteins Yae1-Lto1 function as adaptors recruiting the ABC protein Rli1 for iron-sulfur cluster insertion. Elife 4, e08231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhyay A. S. et al. , Viperin is an iron-sulfur protein that inhibits genome synthesis of tick-borne encephalitis virus via radical SAM domain activity. Cell Microbiol. 16, 834–848 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Stegmaier K. et al. , Apd1 and Aim32 are prototypes of bis-histidinyl-coordinated non-Rieske [2Fe-2S] proteins. J. Am. Chem. Soc. 114, 5753–5765 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Andreini C., Banci L., Rosato A., Exploiting Bacterial Operons To Illuminate Human Iron-Sulfur Proteins. J Proteome Res 15, 1308–1322 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Przybyla-Toscano J., Boussardon C., Law S. R., Rouhier N., Keech O., Gene atlas of iron-containing proteins in Arabidopsis thaliana. Plant J 106, 258–274 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Bak D. W., Weerapana E., Monitoring Fe-S cluster occupancy across the E. coli proteome using chemoproteomics. Nat Chem Biol 19, 356–366 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lénon M., Arias-Cartín R., Barras F., The Fe-S proteome of Escherichia coli: prediction, function, and fate. Metallomics 14, (2022). [DOI] [PubMed] [Google Scholar]
- 29.Andreini C., Rosato A., Banci L., The Relationship between Environmental Dioxygen and Iron-Sulfur Proteins Explored at the Genome Level. PLoS One 12, e0171279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netz D. J. A. et al. , Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 8, 125–132 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierik A. J., Netz D. J., Lill R., Analysis of iron-sulfur protein maturation in eukaryotes. Nat. Protoc. 4, 753–766 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Kassube S. A., Thomä N. H., Structural insights into Fe-S protein biogenesis by the CIA targeting complex. Nat Struct Mol Biol 27, 735–742 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Kumar M. et al. , The Eukaryotic Linear Motif resource: 2022 release. Nucleic Acids Res 50, D497–d508 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Netz D. J. A. et al. , A bridging [4Fe-4S] cluster and nucleotide binding are essential for function of the Cfd1-Nbp35 complex as a scaffold in iron-sulfur protein maturation. J. Biol. Chem. 287, 12365–12378 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan V. et al. , Structure of the yeast WD40 domain protein Cia1, a component acting late in iron-sulfur protein biogenesis. Structure 15, 1246–1257 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials and materials used in the analysis are available upon request any researcher for purposes of reproducing or extending the analysis.