Abstract
Background:
Behavioral and emotional dyscontrol commonly occur following traumatic brain injury (TBI). Neuroimaging and electrophysiological correlates of dyscontrol have not been systematically summarized in the literature to date.
Objective:
To complete a systematic review of the literature examining neuroimaging and electrophysiological findings related to behavioral and emotional dyscontrol due to TBI.
Methods:
A PRISMA compliant literature search was conducted in PubMed (MEDLINE), PsycINFO, EMBASE, and Scopus databases prior to May 2019. The database query yielded 4392 unique articles. These articles were narrowed based on specific inclusion criteria (e.g. clear TBI definition, statistical analysis of the relationship between neuroimaging and dyscontrol).
Results:
A final cohort of 24 articles resulted, comprising findings from 1,552 patients with TBI. Studies included civilian (n=12), military (n=10), and sport (n=2) samples with significant variation in the severity of TBI incorporated. Global and region-based structural imaging was more frequently used to study dyscontrol than functional imaging or diffusion tensor imaging. The prefrontal cortex was the most common neuroanatomical region associated with behavioral and emotional dyscontrol, followed by other frontal and temporal lobe findings.
Conclusion:
Frontal and temporal lesions are most strongly implicated in the development of post-injury dyscontrol symptoms, although they are also the most frequently investigated regions of the brain for these symptom categories. Future studies can make valuable contributions to the field by 1) emphasizing consistent definitions of behavioral and emotional dyscontrol, 2) assessing pre-morbid dyscontrol symptoms in subjects, 3) utilizing functional or structural connectivity-based imaging techniques, or 4) restricting analyses to more focused brain regions.
Keywords: Traumatic Brain Injury, Neuroimaging, Impulsivity, Aggression, Dyscontrol
INTRODUCTION
Traumatic brain injury (TBI) is an alteration in brain function caused by an external force to the head 1. TBI is common – in the U.S. alone, 1.4 million people each year sustain a TBI, and approximately 2% of the population is living with a TBI 2. New research suggests that TBI is a disease process rather than an isolated event, as it consists of both acute and chronic downstream consequences 3. Due to its severity and chronicity, TBI is a major source of disability both in the United States and worldwide 2.
Neuropsychiatric disturbances following TBI account for a significant portion of the disability and impairment associated with the injury. These symptoms can occur acutely post-injury in the context of a post-traumatic encephalopathy or develop more gradually and insidiously after other acute symptoms appear to resolve. Although some acute neuropsychiatric manifestations of TBI, such as coma, delirium, or subsyndromal delirium, are gravely disabling, they are often transient.4. After the acute post-traumatic encephalopathy resolves, protracted neuropsychiatric sequelae develop in as many as half the TBI patients 5. This review specifically addresses these more chronic neuropsychiatric sequelae of TBI. These symptoms can be either new-onset psychiatric complications or exacerbation of pre-existing, previously well-controlled conditions. Many types of neuropsychiatric symptoms can develop after TBI, including mood changes, personality changes, psychosis, sleep changes, or changes in one’s ability to regulate behavior and emotion. The inability to regulate behavior and emotion constitutes an impairing class of symptoms described by Arciniegas and Wortzel 6 and frequently encountered in patients post-TBI. This construct, commonly referred to as behavioral and emotional dyscontrol, includes symptoms such as aggression, impulsivity, disinhibition, irritability, affective lability, agitation, and pathological laughing and crying. It is one of the most challenging consequences of TBI faced by patients and families, and one of the most difficult to manage for providers 7.
Neuroimaging modalities are commonly used in the study of TBI and psychiatric disorders, although they are rarely studied in conjunction. For example, TBI has been linked to brain volume loss due to degradation of parenchyma in the acute, subacute, and chronic time periods 8. This volume loss has been reported both globally 9 and in discrete brain regions such as the caudate 10. Mild TBI has also been associated with changes in task-mediated activation on functional MRI (fMRI) in the dorsolateral prefrontal cortex (PFC), ventrolateral PFC, and basal ganglia 8.
Separately, a growing literature exists attempting to link neuropsychiatric symptoms with their neuroimaging correlates in various disease processes 11, again using both structural and functional imaging techniques. Electroencephalography (EEG) has also been used to correlate electrophysiological findings with neuropsychiatric symptoms. Though EEG is better classified as an electrophysiological modality, rather than a traditional neuroimaging modality, it can be similarly useful for characterizing and localizing brain-behavior relationships. For conciseness, when neuroimaging is referenced generically in this manuscript, it is referring to both traditional neuroimaging and EEG. Such research on neuroimaging findings associated with neuropsychiatric symptoms of TBI has yet to be systematically compiled. Understanding these relationships has the potential to impact clinical decision-making surrounding post-TBI prognosis and management. For example, one promising study found that cognitive benefits of methylphenidate post-TBI were only seen in those patients who had low caudate dopamine transporter levels as measured with 123I-ioflupane single-photon emission computed tomography (SPECT) 12.
Over the past several years, members of a TBI Special Interest Group comprised of clinicians, researchers, and trainees in neuropsychiatry undertook a large research effort designed to systematically review the existing literature on the topic of neuropsychiatric symptoms (NPS) due to TBI, specifically as they relate to neuroimaging findings. This paper is a product of that larger research effort and focuses on symptoms of behavioral and emotional dyscontrol in the setting of TBI. To our knowledge, this review is the first to summarize the neuroimaging literature investigating behavioral and emotional dyscontrol in those who have experienced TBI. This study will further characterize the relationship between imaging and TBI-related behavioral and emotional dyscontrol through the following aims: 1) Identifying literature trends based on imaging modality, 2) Highlighting patterns based on pertinent TBI variables (i.e., severity, occurrence, population), 3) Describing relevant findings related to neuroimaging in TBI-associated behavioral and emotional dyscontrol, and 4) Outlining the current trends in research practice including an assessment of potential bias in common study designs.
MATERIALS AND METHODS
Search Strategy
A structured literature search strategy was designed to identify articles with neuroimaging and NPS components in human TBI samples. Articles were extracted from PubMed (MEDLINE), PsycINFO, EMBASE, and Scopus databases. Boolean searches were kept broad in the interest of reflecting all neuroimaging modalities and in order to capture broad domains of neuropsychiatric symptomatology. A more general approach was also necessitated by the current state of the TBI literature, which comprises many disparate approaches to definition, severity, population, and timing of assessment. We employed 41 imaging-related keywords, 46 NPS-related keywords, and 15 TBI-related keywords. Exact search phrases and MeSH search field qualifiers are outlined in Appendix 1. For this particular review on the topic of dyscontrol, we narrowed our search to 19 NPS-related keywords: aggression, agitation, behavioral dyscontrol, disinhibition, emotional dyscontrol, emotional dysregulation, emotional incontinence, forced crying, impulsivity, inappropriate laughter, involuntary crying, involuntary emotional expression disorder, irritability, lability, pathological emotionalism, pathological emotionality, pathological laughing and crying, pathological laughter, and pseudobulbar affect.
Review Protocol
This review adhered to PRISMA 13 guidelines for implementation and reporting of systematic reviews. A summary of the review protocol, including the number of articles included and excluded in each step, can be found in Figure 1. In the first level of the screening process, titles and abstracts were reviewed in parallel for determination of inclusion or exclusion. Individuals in dyads were blind to each other’s determinations and an identical data extraction sheet was utilized by all reviewers. Discrepancies and cases when a reviewer was unsure were routed to a third-party reviewer for a final decision. All included articles were then subjected to a full-text review by dyads, again followed by a reappraisal if necessary. The resulting article cohort was then split up into six NPS domains: depression, anxiety, post-traumatic stress disorder, sleep disturbance, behavioral and emotional dyscontrol, and psychosis. The present review focuses on the NPS domain of behavioral and emotional dyscontrol. A series of subsequent reviews focused on the other NPS domains will be published from these same efforts.
Figure 1.
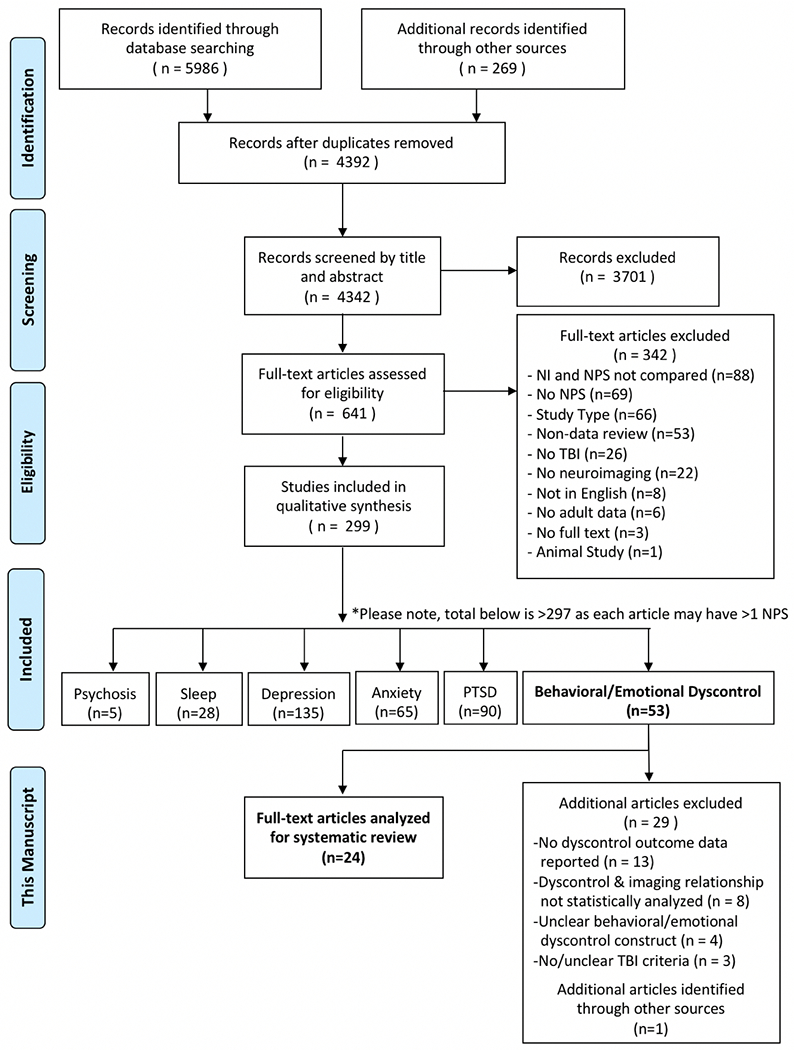
Article Selection Process
Inclusion and Exclusion Criteria
For both title/abstract and full-text reviews, a standardized set of inclusion and exclusion criteria were applied. Articles were excluded if they 1) Lacked any one of the three key elements (neuroimaging, NPS, or TBI); 2) Were of an ineligible study type (i.e., case reports/case series with n < 5, editorials, commentary letters, replies to editor, book reviews, non-peer-reviewed articles, conference proceedings, poster abstracts, dissertations); 3) Were not written in English; and/or 4) The study population had no human subjects or adult data (<18 years). Articles were not judged on the basis of TBI severity, singularity or reoccurrence of TBI, acuity or chronicity of NPS, neuroimaging modality, or if neuroimaging was conducted in the acute, sub-acute, or chronic time period post-TBI. This information was, however, collected on all articles.
Final articles selected for the present review focused on behavioral and emotional dyscontrol symptoms subsequent to TBI. These articles met all of the following additional criteria: 1) Statistically analyzed the relationship between neuroimaging findings and behavioral or emotional dyscontrol in individuals with TBI; 2) Had a clear TBI definition for participants included in the study (formalized or study-specific criteria with any combination of Glasgow Coma Scale score, loss/alteration in consciousness, and/or post-traumatic amnesia); and 3) Reported a clear behavioral or emotional dyscontrol outcome. Articles that satisfied these criteria were then reviewed for individual analyses between behavioral and emotional dyscontrol and neuroimaging findings. These analyses were then organized based on imaging modality, presence or absence of lesion, and neuroanatomical localization. Articles drawing from unique subsamples of the same patient cohort were summed without removal of overlapping data for demographic transparency.
Article Quality Review
Included articles were rated for bias and quality by two independent reviewers using the Newcastle-Ottawa Scale 14, with specific focus on study design as applicable for dyscontrol outcomes of interest. Studies with significant limitation in quality or bias were included in the review with notation of their limitations.
RESULTS
Following application of inclusion and exclusion criteria, the final cohort of articles consisted of 24 studies published between 1986-2018. There were 1,552 total patients with TBI and 335 non-TBI comparisons represented in the cohort of articles, for an aggregated sample size of 1,887. Twenty-two of the 24 articles reported statistically significant findings related to behavioral and emotional dyscontrol. Population of Interest: Civilian populations (12 articles) were the most commonly studied, followed by military (10 articles), and sport (2 articles). TBI characteristics: Seven studies included all levels of TBI severity, whereas other articles restricted inclusion to mild TBI (8 articles), severe TBI (4 articles), moderate and severe TBI (4 articles), or mild and moderate TBI (1 article). Thirteen articles studied patients with a singular TBI, 10 articles included participants with either singular or recurrent TBI, and one article studied only patients with recurrent TBI. Chronicity: The majority of articles studied participants using neuroimaging acquired greater than six months post-TBI (13 articles), followed by any time less than six months (3 articles), between two weeks and six months (2 articles), and less than two weeks (2 articles). Four articles did not specify the timing of neuroimaging acquisition. For the purposes of evaluating risk of bias, eight of the articles were formulated as case-control studies, and 16 were evaluated as cohort studies. A summary of article characteristics can be found in Table 1.
Table 1.
Summary of Article Characteristics (n = 24)
| Variable | % |
|---|---|
| Study Type | |
| Case-control | 33 |
| Cohort | 67 |
|
| |
| Population | |
| Civilian | 50 |
| Military | 42 |
| Sport | 8 |
|
| |
| TBI Severity | |
| Mild | 33 |
| Mild & Moderate | 4 |
| Moderate & Severe | 17 |
| Severe | 17 |
| Any severity | 29 |
|
| |
| TBI Occurrence | |
| Single | 54 |
| Recurrent | 4 |
| Single & Recurrent | 42 |
|
| |
| Imaging Timing Post-TBI † | |
| Acute/subacute | 8 |
| Acute/subacute & Intermediate | 13 |
| Intermediate & Chronic | 8 |
| Chronic | 54 |
| Unclear | 17 |
Note: Acute/subacute = 0 hours–2 weeks, intermediate = 2 weeks –6 months, and chronic = >6 months.
Findings by Imaging Modality
Structural Imaging
Key findings are summarized in Table 2 and imaging modality breakdown is displayed in Figure 2. Fifteen of the studies used structural imaging analyses; six utilized computed tomography (CT), six magnetic resonance imaging (MRI), and three both CT and MRI. These studies included 1,285 TBI participants and 229 non-TBI comparisons in total. Thirteen of the 15 structural imaging studies reported significant findings relating neuroimaging findings to behavioral and emotional dyscontrol. In studies that investigated nonspecific lesion versus non-lesion, lesions were positively associated with greater impulsivity15, disinhibition 16, and agitation 17, but not hostility 15. Studies that looked at the brain globally found that traumatic axonal injury (defined as a trauma-induced white matter lesion identified with a combination of imaging modalities) was positively associated with emotional dyscontrol 18, volume loss was positively associated with disinhibition 19, and left greater than right hemispheric lesions were positively associated with aggression 20. Localization of lesions are presented in Figure 3.
Table 2.
Articles Comparing Neuroimaging and Neuropsychiatric Symptoms of Behavioral and Emotional Dyscontrol in Traumatic Brain Injury (n = 24)
| Article | Study Type** | Sample Size | Population | TBI Diagnostic Criteria | TBI Severity; Occurrence | Timing of Neuroimaging Since TBI | Neuroimaging Modality | Neuropsychiatric Outcome Measure(s) | Key Findings * |
|---|---|---|---|---|---|---|---|---|---|
| Borek et al., 2001 | Cohort | 98 | Civilian | Non-penetrating brain injury, defined by duration of LOC, GCS, and PTA; severe = LOC > 24 hours or GCS < 9, or PTA > 1 week | All (majority severe); Single | Unclear | EEG, CT, MRI | Presence/absence of aggression and irritability recorded from case notes | Left hemisphere injury more associated with aggression than right hemisphere lesion (58% vs. 41%, p = 0.02). No association for irritability. |
| Cristofori et al., 2016 | Case-control | 145 (112 TBI, 33 non-TBI) | Military | Penetrating head injuries from the Caveness Vietnam Head Injury Study phase 4 | Severe; Single | About 40 years | CT | Implicit Association Test (IAT) focused on implicit attitudes towards violence/aggression, Aggression Questionnaire, Attitudes Towards Guns and Violence Questionnaire (AGVQ), State-Trait Anger Expression Inventory (STAXI) | More positive implicit attitude towards violence associated with lesions of the left posterior inferior temporal cortex, bilateral DLPFC, and bilateral OFC. Less positive implicit attitude toward violence associated with lesions of the middle and superior OFC. |
| Dailey et al., 2018 (a) | Cohort | 26 (10 TBI; 16 non-TBI) | Sport | Based on VA/DoD and ACRM criteria (LOC < 30 min, PTA < 24 hours, AOC, focal neurological damage that may or may not be transient) | Mild; Both | 6 months or 12 months | MRI (DTI) | Buss-Perry Aggression Questionnaire (BPAQ), Personality Assessment Inventory (PAI) | Reduced white matter integrity (increased radial diffusivity) in the corpus callosum associated with greater aggression on BPAQ total score. Physical aggression associated with higher radial diffusivity and lower fractional anisotropy in splenium of CC. Aggressive attitude associated with higher radial diffusivity in body of CC. |
| Dailey et al., 2018 (b) | Cohort | 34 (17 TBI, 17 non-TBI) | Civilian | Based on ACRM and VaDoD Criteria (GCS = 13-15, LOC < 30 min., PTA < 24 hours, transient AOC) | Mild; Single | ≥ 6 months (M = 290.40 days, SD = 91.87) | rsfMRI | Buss-Perry Aggression Questionnaire (BPAQ) | Elevated aggression associated with increased right hippocampus to midcingulate cortex connectivity in TBI compared to controls. |
| Epstein et al., 2016 | Case-control | 82 (55 TBI, 27 non-TBI) | Military | OSU TBI-ID (injury to the head followed by AOC or LOC); ACRM guidelines (dizziness, confusion, or LOC < 30 min. and PTA < 24 hours) | Mild; Both | ≥ 12 months (M = 107.3 months, SD = 93.3 months) | MRI (MPRAGE) | Buss-Perry Aggression Questionnaire (BPAQ) | Aggression was not significantly related to OFC morphometry after Bonferroni correction. |
| Finnanger et al., 2015 | Cohort | 139 (67 TBI, 72 non-TBI) | Civilian | Head Injury Severity Scale (HISS) criteria | Moderate, severe; Single | Median = 10 days, range = 1-120 days | MRI | Behavioral Regulation Index including inhibition and emotional control subscales (BRIEF-A), Achenbach System of Empirically Based Assessment (ASEBA) Adult Self-Report form | Presence of traumatic axonal injury on MRI correlated with total scores on BRIEF-A and ASEBA Adult Self-Report form. |
| Formisano et al., 1991 | Cohort | 48 | Civilian | LOC/AOC 3 weeks to 2 months, Innsbruck Coma Scale (Gerstenbrand 1982) score 15-20, GCS 3-9 | Severe; Single | 1-2 years | CT | Fragebogen zur Erfassung von Aggressivitatsfaktoren (FAF) | No significant differences in FAF scores between temporal and non-temporal groups; no pathological scores in any group. |
| Goswami et al., 2016 | Cohort | 36 (19 TBI, 17 non-TBI) | Sport | Patient self-report operationalized by the International Consensus Statements | Mild; Recurrent | Unclear | MRI (DTI), rsfMRI | Personality Assessment Inventory (PAI) aggression scale, Sustained Attention to Response Task (SART) | Negative correlation between right OFC thickness and aggression, impulsivity (via task errors on go/no-go); negative correlation between uncinate fasciculus axial diffusivity and aggression; impulsivity (via task errors on go/no-go). rsfMRI showed no significant associations between left/right OFC and left/right ATL. |
| Grafman et al., 1986 | Cohort | 103 (52 TBI; 51 non-TBI) | Military | Vietnam Head Injury Study criteria; presence of lesion on neuroimaging | Moderate, severe; Both | About 15-20 years | CT | Profile of mood states (“ready to fight,” “angry,” “grouchy,” “bad-tempered,” “rebellious”) | Patients with left dorsofrontal lesions more likely to endorse “angry”, “ready to fight”, “grouchy”, “bad-tempered” compared to left OFC, non-frontal, control groups; right OFC more “angry”, “ready to fight”, “grouchy” than left OFC, bilateral OFC, right dorsofrontal or non-frontal lesions or controls. |
| Knutson et al., 2015 | Cohort | 177 | Military | Caveness Vietnam Head Injury Study Phase 3 | All; Both | 33-39 years | CT | Neuropsychiatric Inventory (NPI) behavioral disinhibition scale | Disinhibition scores showed associations with lesions to the right OFC, bilateral insula, right temporal lobe, left frontal, precentral and postcentral regions, and bilateral gyrus rectus. Patients with higher disinhibition scores also had a greater percentage of volume loss throughout the brain in general. |
| Koponen et al., 2006 | Cohort | 58 | Civilian | Head trauma causing neurologic symptoms (headache and nausea) ≥ 1 week and at least one of the following: LOC ≥ 1 min., PTA ≥ 30 min., neurological symptoms (other than headache and nausea) in first 3 days post-injury, neuroradiological findings | All (plus very severe); Both | M = 31.5 years | MRI | “Organic personality syndrome” assessed using DSM-III-R criteria (labile, aggressive, disinhibited subtypes) | Presence of contusions on MRI associated with disinhibited organic personality syndrome. Frontal lesions associated with organic personality syndrome and disinhibited subtype. |
| Lee et al., 1997 | Cohort | 72 (41 with CNS lesions, 31 without) | Civilian | Admitted to emergency department for head injury following traffic accident, patient/family member report of trauma history | All; Single | ≥ 5 months | MRI | Barratt Impulsiveness Scale (BIS); Symptom Checklist 90-R (SCL-90-R) | Non-planning impulsivity more associated with brain lesions vs. no-lesion group. No significant difference between lesion and non-lesion groups for SCL-90-R hostility measure. |
| Lopez-Larson et al., 2013 | Case-control | 74 (40 TBI only, 19 TBI + suicidal behavior, 15 non-TBI) | Military | Report of an injury event to the head followed by AOC or LOC ≤ 30 min.; OSU TBI-ID | Mild; Both | Unclear | MRI (DTI) | Barratt Impulsiveness Scale (BIS) | Positive correlation between BIS total score and right anterior thalamic radiation fractional anisotropy. Positive regressions found for fractional anisotropy of the bilateral anterior thalamic radiation and BIS total, BIS planning, and BIS attention. |
| McGlade et al., 2015 | Cohort | 41 | Military | OSU TBI-ID; Patient report of head injury followed by AOC or LOC; Belanger et al., 2009 criteria (mild = AOC ≤ 24 hours or LOC ≤ 30 min.; moderate = AOC 24 hours to 7 days or LOC 30 min. to 24 hours; severe = AOC > 7 days or LOC > 24 hours) | All; Both | Unclear | MRI | Buss-Perry Aggression Questionnaire (BPAQ) physical aggression subscale, Displaced Aggression Questionnaire (DAQ) revenge planning scale, Profile of Mood States (POMS) | All in males only: BPAQ associated with decreased left OFC to left angular gyrus connectivity; DAQ associated with increased right OFC to right cerebellum and right angular gyrus connectivity, as well as associated with decreased right OFC to right mid-occipital cortex connectivity. |
| Moore et al., 2016 | Cohort | 81 (52 TBI, 29 non-TBI) | Military | American Academy of Neurology criteria | Mild; Both | 11-50 months (M = 22.5 months) | EEG | Profile of Mood States (anger/hostility) | Frontal beta asymmetry on EEG associated with self-reported anger/aggression in athletes post-concussion. |
| Nathan et al., 2015 | Cohort | 27 (15 TBI, 12 non-TBI) | Military | Based on VA/DoD Criteria (PTA < 24 hours, LOC < 15 min.) | Mild; Single | 2-10 months (M = 147 days | rsfMRI | Personality Assessment Inventory (PAI) aggression and borderline feature scales | Aggression scores correlated with right cerebellar lobule VII spatial information within default mode network; borderline features correlated with left cerebellar lobule I-IV. |
| Oder et al., 1992 | Case-control | 36 | Civilian | Closed head injury based on GCS | Severe; Single | Within 2 months | SPECT | Giessen test (disinhibition and aggression components) | Disinhibited behavior was associated with low regional cerebral blood flow in the frontal lobes. Aggressive behavior was associated with low blood flow in right brain regions. |
| Pardini et al., 2014 | Cohort | 170 (141 TBI, 29 non-TBI) | Military | Penetrating TBI; Caveness Vietnam Head Injury Study phase 3 | Moderate, severe; Single | 36-39 years | CT | Neuropsychiatric Inventory (NPI) agitation and aggression subscale | ANCOVA on NPI revealed an interaction effect for dopamine receptor D1 genotype x lesion location; no significant main effects for lesion location group. Dopamine receptor D1 carriers had higher NPI scores than G/G carriers in the medial PFC group and lower NPI scores in the lateral PFC group. ANCOVA on NPI scores for dopamine receptor D2 x lesion location and catechol-O-methyltransferase x lesion location showed no significant main effects, interaction effects, or covariate effects with other genotypes. |
| Spikman et al., 2016 | Cohort | 186 | Civilian | GCS 9-12 (moderate TBI) or GCS 3-8 (severe TBI) | Moderate, severe; Single | Upon presentation to emergency department | CT | Presence/absence of anger as reported on study-specific structured survey | Patient and proxy ratings of anger were higher for the frontal TBI group as opposed to the nonfrontal group. |
| Stern et al., 2004 | Case-control | 37 | Civilian | LOC < 24 hours, GCS 13-15, normal CT scan, normal skull x-ray, and EEG with no focal signs | Mild; Single | M = 21 months | EEG | “Extraverted-aggressive” classification by semi-structured interview | Enhanced alpha/theta ratio in the frontal and parietal leads for extraverted aggressive group vs. introverted-withdrawn and low-complaint groups. |
| Tateno et al., 2004 | Case-control | 92 | Civilian | GCS, PTA | All | Upon admission for TBI | CT, MRI | Pathological Laughing and Crying Scale | Patients with PLC had a greater frequency of frontal lobe injury than patients without PLC (p=0.04). Diffuse lesions were more common in the no-PLC group. No significant between group differences in frequency of lesions in other brain areas. Lateral aspect of the left frontal lobe was associated with the presence of PLC with logistic regression analysis (p=0.03). |
| Van Der Naalt, et al., 2000 | Cohort | 67 | Civilian | GCS 9-14, PTA > 1 hour | Mild, moderate; Single | Median = 45 days, range = < 1 hour to 3 months | CT, MRI | Presence/absence of agitation, inappropriate behavior as reported by nurse and/or treating physician clinical observation | Agitation was related to lesions on CT and number of lesions. A similar relationship was seen for relating symptoms to presence and number of lesions on MRI for agitation and inappropriate behavior). More than 2x as many lesions were seen on MRI and CT for patients with restlessness and/or agitation compared to patients without behavioral disturbance. Restlessness, agitation, and inappropriate behavior were associated with more frontotemporal lesions on CT and MRI (approximately half of patients with behavioral disturbance). |
| Yamaki et al., 2018 | Case-control | 26 | Civilian | Field GCS ≤ 8 and severe verbal disturbance | Severe; Both | M = 623 days | 18F-FDG-PET/CT | Brief Psychiatric Rating Scale (BPRS) uncooperativeness subscale | Thalamic glucose metabolism imbalanced (R > L) and lateralized in 6 patients who exhibited uncooperativeness. |
| Yurgelun-Todd et al., 2011 | Case-control | 32 (15 TBI, 17 non-TBI) | Military | Patient report of injury to the head followed by AOC or LOC; OSU TBI; mild = LOC ≤ 30 min., moderate/severe = LOC > 30 min. | All; Both | 104.5-192.2 months | MRI (DTI) | Barratt Impulsiveness Scale (BIS) | Total cingulum fractional anisotropy, right genu fractional anisotropy, and right cingulum fractional anisotropy positively correlated with BIS total score; total cingulum and right genu correlated with BIS attention subscale measures of impulsivity; right genu fractional anisotropy positively correlated with BIS planning subscale. |
Note:
Key findings are statistically significant unless otherwise noted.
Study type as it applies to outcome of interest, may be different than overall study design.
18F-FDG-PET = 18F fluorodeoxyglucose positron emission tomography, ACRM = American Congress of Rehabilitation Medicine, ANCOVA = analysis of covariance, AOC = alteration of consciousness, ATL = anterior temporal lobe, cc = corpus callosum, CT = computed tomography, DLPFC = dorsolateral prefrontal cortex, DTI = diffusion tensor imaging, DWI = diffusion weighted imaging, EEG = electroencephalogram, GCS = Glasgow Coma Scale, LOC = loss of consciousness, MRI = magnetic resonance imaging, MPRAGE = magnetization-prepared rapid gradient-echo, non-TBI = controls without traumatic brain injury, OFC = orbitofrontal cortex, OSU TBI-ID = Ohio State University TBI Identification Method, PFC = prefrontal cortex, PLC = pathological laughing and crying), PTA = post-traumatic amnesia, rsfMRI = resting state functional magnetic resonance imaging, SPECT = single-photon emission computerized tomography, TBI = traumatic brain injury, VA/DoD = Veterans Affairs/Department of Defense.
Figure 2.
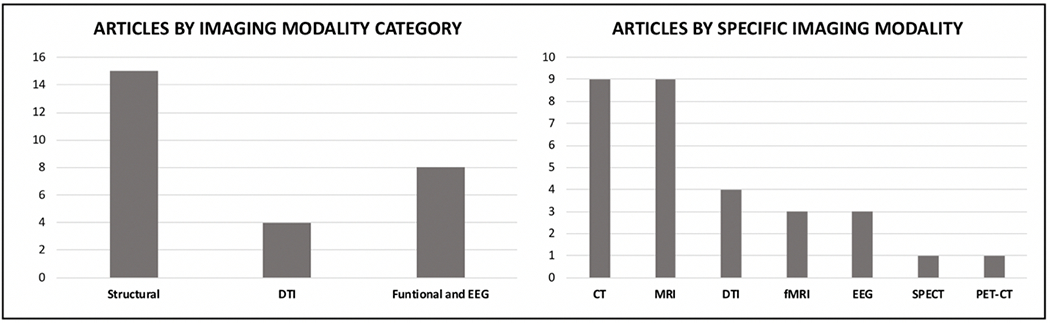
Findings Stratified by Imaging Modality
Figure 3.
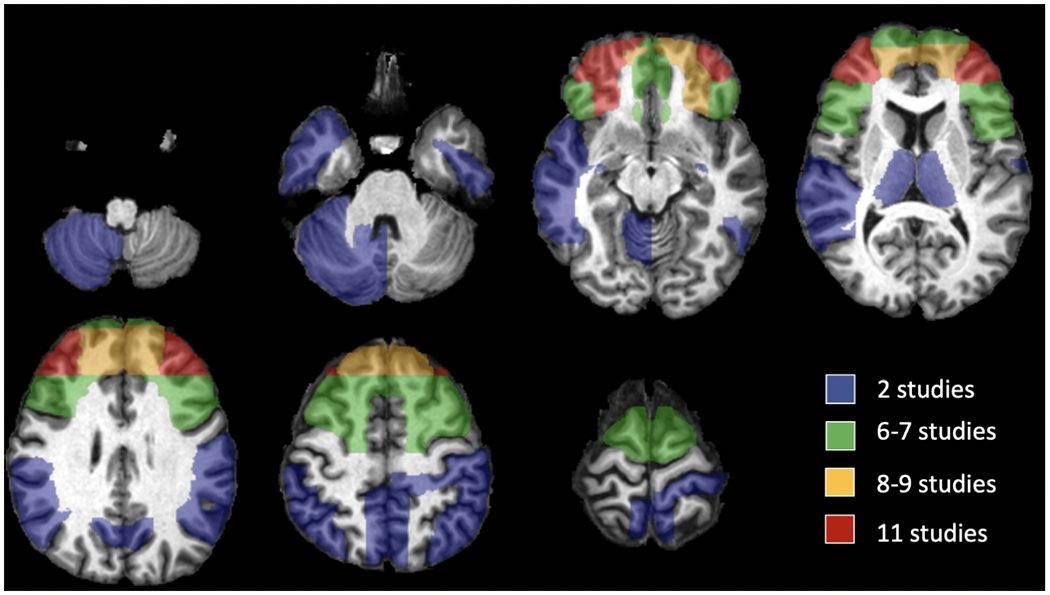
Brain Map Representing the Approximate Locations of Replicated Neuroimaging Findings in TBI-related Behavioral and Emotional Dyscontrol
The PFC was the most common site of neuroimaging findings associated with dyscontrol. Specifically, four studies found positive associations between dyscontrol and lesions to the right orbitofrontal cortex (OFC) 19,21–23, one with the left OFC 23, and one with the OFC bilaterally 24. Another study 25, however, found that aggression was no longer significantly related to OFC morphometry after Bonferroni correction. One study found that aggression was positively associated with medial PFC lesions26. Another study found that the bilateral dorsolateral PFC lesions24 were positively associated with violent attitudes, though a contrasting study found a negative association between lateral PFC and aggression 26.
Regarding other brain regions, six studies found frontal lesions were positively associated with dyscontrol16,17,19,21,27,28. Three studies found positive associations between temporal lesions and dyscontrol 17,19,24. One contrasting study 29 found no significant differences in aggression scores between temporal and non-temporal lesion groups. Finally, isolated significant relationships were found between dyscontrol and the bilateral gyrus rectus, bilateral insula, and precentral and postcentral regions 19, as well as the left and right angular gyri, right cerebellum, and right mid-occipital cortex 23.
Diffusion Tensor Imaging
Four of the studies used diffusion tensor imaging (DTI) analyses. These studies included 103 TBI participants and 65 non-TBI comparisons in total. All four DTI studies reported significant findings relating neuroimaging findings to behavioral and emotional dyscontrol. One study 30 found that greater aggression (Buss-Perry Aggression Questionnaire) was positively correlated with increased radial diffusivity in the corpus callosum; physical aggression in particular was associated with increased diffusivity in the splenium, while aggressive attitude was associated with increased diffusivity in the body of the corpus callosum. A second study showed an association of decreased uncinate fasciculus axial diffusivity with increased aggression and impulsivity scores 22. The remaining two studies found that fractional anisotropy was positively correlated with impulsivity (Barratt Impulsiveness Scale). One 31 found the correlation for both the right and bilateral anterior thalamic radiations, while the other 32 noted the same relationship for the total cingulum, right cingulum, and right genu.
Functional Imaging and EEG
Five of the studies used functional imaging analyses; three utilized fMRI, one SPECT, and one positron emission tomography (PET). Three more studies utilized EEG. Collectively, these eight studies included 300 TBI participants and 75 non-TBI comparisons. Seven of the eight studies reported significant findings related to behavioral and emotional dyscontrol. Two of the three resting state fMRI studies showed significant results – one found aggression was positively associated with increased right hippocampus to mid-cingulate cortex connectivity 33 and another found aggression was positively associated with connectivity between the right cerebellar lobule VII region and the default mode network 34. A third study examined left and right OFC to anterior temporal lobe (ATL) connectivity and found no association with aggression 22. Both studies that utilized EEG exclusively 35,36 found frontal lobe abnormalities to be positively associated with aggression. The SPECT study 37 similarly found that low regional cerebral blood flow in the frontal lobes was positively associated with disinhibited behavior. The PET-CT study 38 found that right lateralized thalamic glucose metabolism was positively associated with uncooperativeness.
Quality Metrics
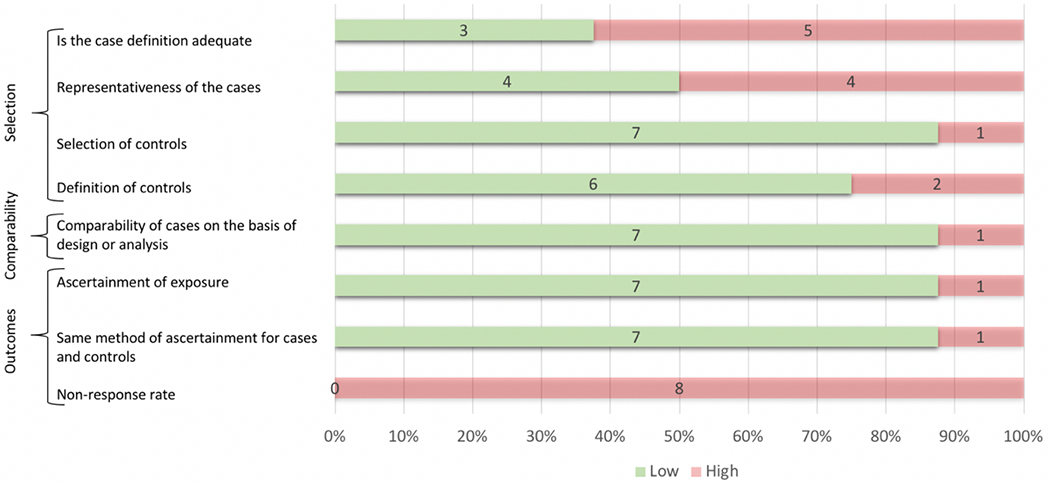
The Newcastle-Ottawa Scale results are shown in Figure 4. Risk of bias for each criterion was considered to be high for explicit failure to meet the criterion or if the reporting was unclear. Notable areas of high risk for potential bias introduction across cohort studies included: 14 of 16 studies failed to demonstrate that the outcome of interest was not present at the start of the study; 10 of 16 studies did not clearly delineate adequate follow-up; and 10 of 16 studies had an exposed cohort that was not clearly representative of the community of interest. For case-control studies, the most common areas of potential bias introduction included: 8 of 8 studies failed to adequately report non-response rates; 5 of 8 did not provide adequate case definition; and 4 of 8 studies did not have cases that were clearly representative of the community of interest.
Figure 4a.
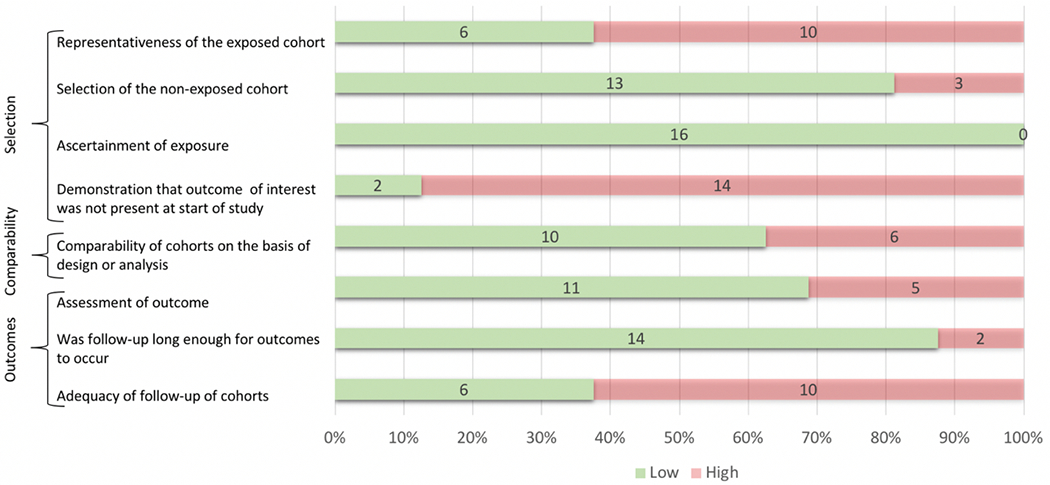
Sources of Bias in Cohort Studies (n = 16)
DISCUSSION
This systematic review summarizes the literature on neuroimaging correlates of TBI-associated behavioral and emotional dyscontrol. The conceptualization of this symptom cluster was based largely on a construct championed in the literature by Arciniegas and Wortzel6. This review is part of a larger project that outlines the neuroimaging correlates of several neuropsychiatric symptoms that commonly present after TBI.
The findings of this review support several conclusions. First, they strengthen the notion that neuropsychiatric symptoms such as behavioral and emotional dyscontrol have identifiable imaging correlates. The studies discussed here suggest that patients who have an identifiable structural lesion on neuroimaging following TBI are more likely to experience behavioral and emotional dyscontrol than patients who have no identifiable lesion. This is of significant clinical relevance as impulsivity following TBI, especially in the setting of emotional dysregulation or depression, can contribute to greater suicide risk as discussed by Lopez-Larson et al. (2013).
Second, this review recapitulates previous work suggesting that dyscontrol symptoms are a common subtype of personality disturbance after frontal lobe injury 39. The PFC was the neuroanatomical location most commonly associated with neuroimaging changes in subjects demonstrating behavioral and emotional dyscontrol symptoms post-TBI. The OFC in particular had six positive associations with dyscontrol 19,21–24. The orbitofrontal circuit has been described as the “neocortical representation of the limbic system” and helps to determine the appropriate time, place, and strategy for environmentally-elicited behavioral responses 40. It is no surprise then that abnormalities or damage in this neuroanatomical region would lead to dysregulated behavioral and emotional responses to stimuli 41. Moreover, the term disinhibition syndrome has been used to describe a similar cluster of symptoms including emotional lability and impulsivity, reminiscent of features seen in mania, attention deficit hyperactivity disorder, antisocial and borderline personality disorders, and substance abuse 42. Disinhibition syndrome results from disruption of the OFC, which is again consistent with the findings of the present review.
Additionally, frontal lobe changes were the most common location of abnormalities associated with behavioral and emotional dyscontrol observed in functional neuroimaging. A previous systematic review by Brower and Price found that the literature supports an association between frontal lobe dysfunction and increased aggressive and antisocial behavior 43. Despite this strong association between the prefrontal cortex and dyscontrol, we must keep in mind that various other subtypes of personality change can occur following prefrontal injury, including executive dysfunction, depression, hypo-emotionality, and apathy 39. Such findings highlight the importance of continued neuroimaging research focusing on more spatially and functionally specific brain regions in order to parse out the unique and complex personality changes that can occur following a TBI or other brain lesion affecting the frontal lobe.
A third conclusion from this review is that temporal lobe structures are also frequently implicated in dyscontrol syndromes, often in combination with the frontal lobe. These results are consistent with findings in frontotemporal degenerative illnesses, such as Pick’s disease, non-specific frontotemporal degeneration, and motor neuron disease, or postsurgical effects in those regions 44. For example, Alsemari and Malloy found that dyscontrol symptoms were more prevalent in frontotemporal dementia when compared to both Alzheimer’s disease and non-TBI controls45.
Some studies in this review also implicate brain regions in post-TBI dyscontrol disorders which are less expected, such as the cerebellum 23. This is consistent with the previously mentioned work of Alsemari and Malloy, which showed that cerebellar changes identified with both structural and functional imaging are associated with dyscontrol 45. This is consistent with literature on cerebellar cognitive affective syndrome 46, a neuropsychiatric presentation of cerebellar dysfunction or stroke 47 involving emotional dyscontrol and aggression in addition to deficits in executive function, visuospatial cognition, and language 48.
Another noteworthy observation from this review is that the studies included are highly heterogeneous in terms of the tools used to measure dyscontrol, the tools used for neuroimaging, and the details of the TBI itself and the population being studied. For example, 20 different measures of dyscontrol were employed across the 23 studies, making generalization of findings across studies challenging. These different measures have different methods for categorizing and classifying dyscontrol and its associated findings. Moreover, there were variations in the definitions of agitation and aggression in different studies included in this review, as well as differences between subtypes of behavioral and emotional dyscontrol. A unique challenge amongst these studies is that behavioral and emotional dyscontrol outcome measures are often self-report scales (or report from a close relative or proxy). Heterogeneity among statistical methods and sample sizes further complicated the task of objectively comparing statistical associations or the lack thereof. In order to mitigate the impact of this limitation, the articles underwent quality review using the Newcastle-Ottawa Scale and each of their sample sizes is listed in Table 2. An effort was made to limit the scope of this paper to reduce some of this variability. Consequently, this review did not look at cognitive or task-based measures of disinhibition or executive dysfunction, which was formulated as a separate symptom category. Finally, this paper excluded studies that focused on depression or anxiety as outcomes – although these symptom clusters could broadly be considered emotional dyscontrol entities, we reserve examination of them individually and in greater detail elsewhere.
In terms of variability in imaging across studies, a notable observation is the variation in chronicity of TBI at time of imaging. Some studies used imaging taken immediately after the TBI, others greater than six months after the TBI, and still others used neuroimaging from a more variable range of time points. A paper from Han et al. concluded that Apolipoprotein E showed compensatory changes in the first three years following TBI49. This is important for our review, as variations in timing of neuroimaging may elucidate different aspects of TBI, whether they be the immediate effects of TBI or subsequent compensatory changes. The use of different imaging modalities such as MRI, CT, EEG, and functional imaging modalities to study TBI-associated changes also contributes to the difficulty of comparing results across studies.
The variable aspects of TBI chronicity and severity are also outlined in detail in Table 2. These studies looked at subjects with TBIs of various severities and frequencies, with various definitions for what was classified as a TBI, and at symptoms observed post-TBI over varying lengths of time. All these factors make generalizable conclusions difficult if not impossible to draw. This does, however, help to guide future research in this topic area by providing researchers with a list of important factors to consider and control for in designing new studies.
The Newcastle-Ottawa Scale 14 was applied in this study to offer a general idea of where the challenges and opportunities lie for future work in neuroimaging correlates of TBI-associated dyscontrol symptoms. Notably, the scale was applied based on the article’s study design as it applied to our outcome of interest. As such, some studies were formulated as case-control or cohort design based on which it most closely resembled in regards to the dyscontrol and imaging associations. This scale served to further highlight some of the research challenges mentioned previously and also identified additional areas for improvement. First, very few of the studies in our review accounted for pre-morbid functioning or personality traits in the study population. This makes it impossible to know with certainty to what degree the dyscontrol symptoms result from TBI or neuroimaging changes. Future work should consider prospective studies in high-risk TBI populations, looking at pre-morbid neuroimaging and neuropsychiatric functioning, to minimize this complicating factor and also provide a highly similar non-TBI comparison group.
Second, amongst these studies, many were studies of convenience samples or retrospective studies that took advantage of available data on TBI patients or took a subsample of data from a larger cohort study. This raises concern for potential sampling bias and generalizability. For example, many articles had study populations that included only males or veterans and thus were poorly representative of the TBI population as a whole. Prospective studies again could mitigate some of these problems, although an inherent problem in this research will always be the inability to ethically “randomize” patients to a TBI exposure. Finally, this review revealed potential reporting bias for studies with positive results, as only two of 23 articles reported negative results.
This paper offers a review of neuroimaging findings and modalities studied in behavioral and emotional dyscontrol disorders following TBI. Neuroimaging is becoming one of the most common tools for studying the pathophysiological underpinnings of neuropsychiatric illness. As the field grows ever more sophisticated, imaging techniques discussed in this paper are now being utilized in combination, with multimodal neuroimaging improving our understanding of clinical symptoms 50. However, the present study did find a surprising lack of white matter tract connectivity or functional imaging results. Both areas represent promising avenues for future research.
Conclusions
In summary, we performed a systematic review of neuroimaging literature examining TBI and co-occurring behavioral and emotional dyscontrol. We found that the majority of studies focused on global / regional structural neuroimaging and that the PFC is the neuroanatomical region most frequently associated with dyscontrol symptoms. Future studies that place additional emphasis on consistent definitions of behavioral and emotional dyscontrol symptoms, as well as those that account for pre-morbid functioning, will be valuable contributions to the literature.
Figure 4b.
Sources of Bias in Case-control Studies (n = 8)
Acknowledgements
The study team would like to thank Akshay Krieg, B.S., William Tobolowsky, M.D., Allison Bailey, M.D., Gardner McCullough, LMSW, Tejus Pradeep, M.D., Alexandra Pletnikova, B.S., and Emily Berich-Anastasio, MPH, for their meaningful contributions to the work presented in this manuscript.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. NTT is supported in general by departmental funding from the Department of Psychiatry at the University of Iowa, Iowa City, IA and the INSPIRE Training Grant 5T32MH019113.
APPENDICES
Appendix A. Search Terms
(“Magnetic Resonance Imaging”[mh:noexp] OR “Diffusion Magnetic Resonance Imaging”[mh] OR “Magnetic Resonance Angiography”[mh] OR “magnetic resonance imaging” OR “magnetic resonance imaging” OR “MRI” OR “fMRI” OR “MR imaging” OR “diffusion imaging” OR “diffusion tensor imaging” OR “DTI” OR “tractography” OR “magnetic resonance angiography” OR “perfusion weighted imaging” OR “perfusion imaging” OR “Tomography, X-Ray Computed”[mh:noexp] OR “Four-Dimensional Computed Tomography”[mh] OR “Tomography, Spiral Computed”[mh] OR “Multidetector Computed Tomography”[mh] OR “computer assisted tomography” OR “computed tomographic angiography” OR “computed tomography” OR “electron beam tomography” OR “computer tomography” OR “optical tomography” OR “susceptibility weighted imaging” OR “SWI” OR “Positron-Emission Tomography”[mh] OR “Positron emission tomography” OR “PET” OR “EEG” OR “electroencephalogram” OR “electroencephalography”[mh] OR “electroencephalography” OR “MEG” OR “magnetoencephalography” OR “magnetoencephalography”[mh] OR “Spectroscopy, Near-Infrared”[mh] OR “infrared imaging” OR “near-infrared spectroscopy” OR “neuroimaging”[mh] OR “neuroimaging” OR “voxel-based morphometry” OR “VBM” OR “SPECT”)
AND
(“neuropsychiatric” OR “psychiatric” OR “delusion” OR “hallucination” OR “psychotic Disorders”[mh] OR “affective Disorders, Psychotic”[mh] “psychotic” OR “aggression” OR “agitation” OR “emotional dyscontrol” OR “behavioral dyscontrol” OR “dysphoria” OR ”depression”[mh] OR “Depressive disorder”[mh] OR “depressive” OR “Anxiety”[mh] OR “mania” OR “elation” OR “euphoria” OR “apathy”[mh] OR “disinhibition” OR “lability” OR “irritability” OR “stress disorders, Post-Traumatic”[mh] OR “post-traumatic stress disorder” OR “PTSD” OR “Sleep Wake Disorders”[mh] OR “Sleep Disorders, Circadian Rhythm”[mh] OR “sleep disorder” OR “sleep disorders” OR “Circadian Rhythm” OR “sleep apnea” OR “executive dysfunction” OR “impulsivity” OR “personality” OR “pathological laughing and crying” OR “pseudobulbar affect” OR “pathological laughter” OR “inappropriate laughter” OR “involuntary crying” OR “emotional incontinence” OR “pathological emotionalism” OR “pathological emotionality” OR “emotional dysregulation” OR “forced crying” OR “involuntary emotional expression disorder”)
AND
(“Brain Injuries”[mesh] OR “Brain Hemorrhage, Traumatic”[mh] OR “Diffuse Axonal Injury”[mh] OR “traumatic brain” OR “cerebral trauma” OR “brain trauma” OR “diffuse axonal injury” OR “brain injury” OR “brain injuries” OR “Brain Concussion”[mh] OR “concussion” OR “concussed” OR “Brain Injury, Chronic”[mh] OR “traumatic brain injury” OR “TBI” OR “head injury”)
NOT
(“animals”[mesh] NOT (“animals”[mesh] AND “humans”[mesh]))
Footnotes
CRediT Author Statement
Barry R. Bryant*, M.D.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization, Project administration
Lisa N. Richey, B.A.: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization, Project administration
Sahar Jahed, D.O.: Conceptualization, Methodology, Writing - Original Draft, Writing - Review and Editing
Amanda Heinzerling, B.S.: Methodology, Validation, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization
Daniel A. Stevens, M.D., Ph.D.: Conceptualization, Methodology, Writing - Original Draft, Writing - Review and Editing
Benjamin D. Pace, M.S.: Formal analysis, Investigation, Writing - Original Draft, Writing - Review and Editing
Jerry Tsai, B.A.: Conceptualization, Methodology, Writing - Review & Editing, Visualization
Michael J.C. Bray, M.S.: Conceptualization, Methodology, Writing - Review & Editing
Aaron I. Esagoff, B.S.: Methodology, Writing - Review and Editing, Visualization
Jaxon Adkins: Methodology, Formal analysis, Investigation, Writing - Review and Editing
Ilana Cohen, M.D.: Conceptualization, Methodology, Writing - Review and Editing
Bharat R. Narapareddy, M.D.: Conceptualization, Methodology, Writing - Review and Editing
Carla P. Rodriguez, B.S.: Conceptualization, Methodology, Writing - Review and Editing
Melissa B. Jones, M.D.: Conceptualization, Methodology, Writing - Review and Editing
Carrie Roper, Psy.D.: Conceptualization, Methodology, Writing - Review and Editing
Eric L. Goldwaser, D.O., Ph.D.: Conceptualization, Methodology, Writing - Review & Editing
Katie Lobner, MLIS: Conceptualization, Methodology, Writing - Review and Editing
Shan Siddiqi, M.D.: Conceptualization, Methodology, Writing - Review & Editing
Haris I. Sair, M.D.: Writing - Review and Editing, Visualization
Margo Lauterbach, M.D.: Conceptualization, Methodology, Writing - Review and Editing
Licia P. Luna, M.D.: Conceptualization, Methodology, Formal analysis, Writing - Review & Editing, Visualization
Matthew E. Peters, M.D.: Conceptualization, Methodology, Formal analysis, Writing - Review & Editing, Visualization, Project administration
Nicholas T. Trapp, M.D., M.S.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization, Project administration
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
REFERENCES
- 1.Menon DK, Schwab K, Wright DW, Maas AI: Position statement: Definition of traumatic brain injury. Archives of Physical Medicine and Rehabilitation 2010: 1637–40. Doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Prevention C for DC and: Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. CDC and NIH Report to Congress; 2015. Doi: 10.3171/2009.10.JNS091500. [DOI] [PubMed] [Google Scholar]
- 3.Masel BE, DeWitt DS: Traumatic Brain Injury: A Disease Process, Not an Event. Journal of Neurotrauma 2010; 27(8): 1529–40. Doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 4.Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Medicine 2020; 46(5): 1020–2. Doi: 10.1007/S00134-019-05907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koponen S, Taiminen T, Portin R, et al. Axis I and II Psychiatric Disorders After Traumatic Brain Injury: A 30-Year Follow-Up Study. American Journal of Psychiatry 2002; 159(8): 1315–21. Doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- 6.Arciniegas DB, Wortzel HS: Emotional and Behavioral Dyscontrol After Traumatic Brain Injury. Psychiatric Clinics of North America 2014; 37(1): 31–53. Doi: 10.1016/j.psc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Rochat L, Beni C, Billieux J, Annoni JM, Van Der Linden M: How impulsivity relates to compulsive buying and the burden perceived by caregivers after moderate-to-severe traumatic brain injury. Psychopathology 2011; 44(3): 158–64. Doi: 10.1159/000322454. [DOI] [PubMed] [Google Scholar]
- 8.Bigler ED: Traumatic brain injury, neuroimaging, and neurodegeneration. Frontiers in Human Neuroscience 2013: 395. Doi: 10.3389/fnhum.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Kierans A, Kenul D, et al. Mild traumatic brain injury: Longitudinal regional brain volume changes. Radiology 2013; 267(3): 880–90. Doi: 10.1148/radiol.13122542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernick C, Banks S: What boxing tells us about repetitive head trauma and the brain. Alzheimer’s Research & Therapy 2013; 5(3): 23. Doi: 10.1186/alzrt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boublay N, Schott AM, Krolak-Salmon P: Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: a review of 20 years of research. European Journal of Neurology 2016: 1500–9. Doi: 10.1111/ene.13076. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins PO, De Simoni S, Bourke NJ, et al. Stratifying drug treatment of cognitive impairments after traumatic brain injury using neuroimaging. Brain 2019; 142(8): 2367–79. Doi: 10.1093/brain/awz149. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339(jul21 1): b2700–b2700. Doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. vol. 7. NIHR Journals Library; 2003. [DOI] [PubMed] [Google Scholar]
- 15.Lee Jae Kwang; Hong YP: Psychiatric Symptoms of the Head Trauma Patients Lesions By CNS Lesions Detected by MRI. Journal of Traffic Medicine 1997; 25(3–4): 97–102. [Google Scholar]
- 16.Koponen S, Taiminen T, Kurki T, et al. MRI findings and Axis I and II psychiatric disorders after traumatic brain injury: A 30-year retrospective follow-up study. Psychiatry Research: Neuroimaging 2006; 146(3): 263–70. Doi: 10.1016/j.pscychresns.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Naalt J, Van Zomeren AH, Sluiter WJJ, et al. Acute behavioural disturbances related to imaging studies and outcome in mild-to-moderate head injury. Brain Injury 2000; 14(9): 781–8. Doi: 10.1080/026990500421895. [DOI] [PubMed] [Google Scholar]
- 18.Finnanger TG, Olsen A, Skandsen T, et al. Life after Adolescent and Adult Moderate and Severe Traumatic Brain Injury: Self-Reported Executive, Emotional, and Behavioural Function 2–5 Years after Injury. Behavioural Neurology 2015; 2015: 2–5. Doi: 10.1155/2015/329241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutson KM, Dal Monte O, Schintu S, et al. Areas of Brain Damage Underlying Increased Reports of Behavioral Disinhibition. The Journal of Neuropsychiatry and Clinical Neurosciences 2015; 27(3): 193–8. Doi: 10.1176/appi.neuropsych.14060126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borek LL, Butler R, Fleminger S: Are neuropsychiatric symptoms associated with evidence of right brain injury in referrals to a neuropsychiatric brain injury unit? Brain Injury 2001; 15(1): 65–9. Doi: 10.1080/02699050118431. [DOI] [PubMed] [Google Scholar]
- 21.Grafman J, Vance SC, Weingartner H, Salazar AM, Amin D: The effects of lateralized frontal lesions on mood regulation. Brain 1986; 109(6): 1127–48. Doi: 10.1093/brain/109.6.1127. [DOI] [PubMed] [Google Scholar]
- 22.Goswami R, Dufort P, Tartaglia MC, et al. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Structure and Function 2016; 221(4): 1911–25. Doi: 10.1007/s00429-015-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGlade E, Rogowska J, Yurgelun-Todd D: Sex differences in orbitofrontal connectivity in male and female veterans with TBI. Brain Imaging and Behavior 2015; 9(3): 535–49. Doi: 10.1007/s11682-015-9379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristofori I, Zhong W, Mandoske V, et al. Brain Regions Influencing Implicit Violent Attitudes: A Lesion-Mapping Study. The Journal of Neuroscience 2016; 36(9): 2757–68. Doi: 10.1523/JNEUROSCI.2975-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein DJ, Legarreta M, Bueler E, King J, McGlade E, Yurgelun-Todd D: Orbitofrontal cortical thinning and aggression in mild traumatic brain injury patients. Brain and Behavior 2016; 6(12): e00581. Doi: 10.1002/brb3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pardini M, Krueger F, Hodgkinson CA, et al. Aggression, DRD1 polymorphism, and lesion location in penetrating traumatic brain injury. CNS Spectrums 2014; 19(5): 382–90. Doi: 10.1017/S1092852914000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spikman JM, Timmerman ME, Coers A, van der Naalt J: Early Computed Tomography Frontal Abnormalities Predict Long-Term Neurobehavioral Problems But Not Affective Problems after Moderate to Severe Traumatic Brain Injury. Journal of Neurotrauma 2016; 33(1): 22–8. Doi: 10.1089/neu.2014.3788. [DOI] [PubMed] [Google Scholar]
- 28.Tateno A, Jorge RE, Robinson RG: Pathological laughing and crying following traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences 2004; 16(4): 426–34. Doi: 10.1176/JNP.16.4.426. [DOI] [PubMed] [Google Scholar]
- 29.Formisano R, Schmidhuber-Eiler B, Saltuari L, Cigany E, Birbamer G, Gerstenbrand F: Neuropsychological outcome after traumatic temporal lobe damage. Acta Neurochirurgica 1991; 109(1–2): 1–4. Doi: 10.1007/BF01405688. [DOI] [PubMed] [Google Scholar]
- 30.Dailey NS, Smith R, Bajaj S, et al. Elevated Aggression and Reduced White Matter Integrity in Mild Traumatic Brain Injury: A DTI Study. Frontiers in Behavioral Neuroscience 2018; 12(June): 1–11. Doi: 10.3389/fnbeh.2018.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Larson M, King JB, McGlade E, et al. Enlarged Thalamic Volumes and Increased Fractional Anisotropy in the Thalamic Radiations in Veterans with Suicide Behaviors. Frontiers in Psychiatry 2013; 4(AUG): 1–13. Doi: 10.3389/fpsyt.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yurgelun-Todd DA, Bueler CE, McGlade EC, Churchwell JC, Brenner LA, Lopez-Larson MP: Neuroimaging correlates of traumatic brain injury and suicidal behavior. Journal of Head Trauma Rehabilitation 2011; 26(4): 276–89. Doi: 10.1097/HTR.0b013e31822251dc. [DOI] [PubMed] [Google Scholar]
- 33.Dailey NS, Smith R, Vanuk JR, Raikes AC, Killgore WDS: Resting-state functional connectivity as a biomarker of aggression in mild traumatic brain injury. NeuroReport 2018; 29(16): 1413–7. Doi: 10.1097/WNR.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 34.Nathan DE, Oakes TR, Yeh PH, et al. Exploring variations in functional connectivity of the resting state default mode network in mild traumatic brain injury. Brain Connectivity 2015; 5(2): 102–14. Doi: 10.1089/brain.2014.0273. [DOI] [PubMed] [Google Scholar]
- 35.Moore RD, Sauve W, Ellemberg D: Neurophysiological correlates of persistent psycho-affective alterations in athletes with a history of concussion. Brain Imaging and Behavior 2016; 10(4): 1108–16. Doi: 10.1007/s11682-015-9473-6. [DOI] [PubMed] [Google Scholar]
- 36.Stern B, Glicksohn J, Stern M, Myslobodsky MS: Profiles of Patients with a History of Mild Head Injury. International Journal of Neuroscience 2004; 114(9): 1223–37. Doi: 10.1080/00207450490475742. [DOI] [PubMed] [Google Scholar]
- 37.Oder W, Goldenberg G, Spatt J, Podreka I, Binder H, Deecke L: Behavioural and psychosocial sequelae of severe closed head injury and regional cerebral blood flow: a SPECT study. Journal of Neurology, Neurosurgery & Psychiatry 1992; 55(6): 475–80. Doi: 10.1136/jnnp.55.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaki T, Suzuki K, Sudo Y, et al. Association between uncooperativeness and the glucose metabolism of patients with chronic behavioral disorders after severe traumatic brain injury: a cross-sectional retrospective study. BioPsychoSocial Medicine 2018; 12(1): 6. Doi: 10.1186/s13030-018-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrash J, Stuss DT, Aksan N, et al. “Frontal lobe syndrome”? Subtypes of acquired personality disturbances in patients with focal brain damage. Cortex 2018; 106: 65–80. Doi: 10.1016/j.cortex.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters ME, Moussawi K, Rao V: Teaching Clinical Reasoning with an Example Mnemonic for the Neuropsychiatric Syndromes of Traumatic Brain Injury. Academic Psychiatry 2018; 42(5): 686–9. Doi: 10.1007/s40596-017-0831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAllister TW: Neurobiological consequences of traumatic brain injury. Dialogues in Clinical Neuroscience 2011; 13(3): 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinn D, Katzman J: “The Wizard of Oz:” A Depiction of TBI-Related Neurobehavioral Syndromes. Academic Psychiatry 2012; 36(4): 340. Doi: 10.1176/appi.ap.11010014. [DOI] [PubMed] [Google Scholar]
- 43.Brower MC, Price BH: Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: A critical review. Journal of Neurology Neurosurgery and Psychiatry 2001: 720–6. Doi: 10.1136/jnnp.71.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boronat S, Newberry P, Mehan W, Thiele EA, Duhaime AC: Klüver-Bucy syndrome after unilateral frontotemporal resection in a child with tuberous sclerosis. Child’s Nervous System 2013; 29(8): 1391–4. Doi: 10.1007/s00381-013-2127-3. [DOI] [PubMed] [Google Scholar]
- 45.Alsemari A, Malloy PF: The behavioral dyscontrol scale in the differential diagnosis of behavioral variant of frontotemporal dementia and Alzheimer disease. Clinical Neuropsychologist 2019: 1–10. Doi: 10.1080/13854046.2019.1701709. [DOI] [PubMed] [Google Scholar]
- 46.Schmahmann JD, Sherman JC: The cerebellar cognitive affective syndrome. vol. 121. 1998. [DOI] [PubMed] [Google Scholar]
- 47.Greve K: Cognitive and Emotional Sequelae of Cerebellar Infarct: A Case Report. Archives of Clinical Neuropsychology 1999; 14(5): 455–69. Doi: 10.1016/S0887-6177(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 48.Argyropoulos GPD, van Dun K, Adamaszek M, et al. The Cerebellar Cognitive Affective/Schmahmann Syndrome: a Task Force Paper. Cerebellum 2020: 102–25. Doi: 10.1007/s12311-019-01068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han SD, Drake AI, Cessante LM, et al. Apolipoprotein e and traumatic brain injury in a military population: Evidence of a neuropsychological compensatory mechanism? Journal of Neurology, Neurosurgery and Psychiatry 2007; 78(10): 1103–8. Doi: 10.1136/jnnp.2006.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tulay EE, Metin B, Tarhan N, Arıkan MK: Multimodal Neuroimaging: Basic Concepts and Classification of Neuropsychiatric Diseases. Clinical EEG and Neuroscience 2019; 50(1): 20–33. Doi: 10.1177/1550059418782093. [DOI] [PubMed] [Google Scholar]


