Abstract
Reproductive synchrony and the consequent clustering of births are hypothesized to be regulated by seasonal changes in rainfall and food availability. Such climate-related seasonality is, however, questionable in tropical populations occupying temporally invariant habitats year round. Using the long-term data of the Cayo Santiago rhesus macaques from 1973 to 2013, this study distinguishes synchrony (a greater than chance clustering of births) from seasonality (a cluster of births during a period of the year when abiotic conditions are favorable) and shows that females are highly synchronized (>72% of births in a 3-month period) but the effects of environmental zeitgebers on reproduction are overridden by biological factors. Specifically, biotic and abiotic factors including (i) loss of immature offspring; (ii) population density; (iii) age at delivery; (iv) rainfall; and (v) changes in colony management were modeled in relation to the annual onset of births and the median birth date. Females experiencing loss of immature offspring had an interbirth interval of <365 days in average and the proportion of these females increased up to 48% due to changes in colony management overtime, although reproductive synchrony increased with increasing population density. A secular trend in both the onset of births and the median date of birth is documented and the model predicts that the median birth date will advance across all calendar-based seasons by 2050. The secular trend in reproduction appears to be triggered by changes in the age at delivery of females, the absence of physiological constraints from maternal investment due to offspring loss, shorter interbirth interval, and a higher degree of coordination due to increasing population density. This study challenges the reproductive phenology previously described for rhesus macaques highlighting the importance of long-term studies in addressing the ultimate causes of reproductive synchrony.
Keywords: interbirth interval, lactational anovulation, Macaca mulatta, reproductive phenology, seasonal reproduction, synchronization
INTRODUCTION
Reproductive phenology in mammals is regulated by changes in food availability, temperature, rainfall, and photoperiod [Bronson, 1985]. When environmental conditions vary in a dramatic and predictable way they can function as external cues timing the reproduction of organisms in a population and leading to clustering of reproduction (synchrony) [Ims, 1990a]. However, at lower tropical latitudes, annual variation in such environmental factors might not be significant enough to function as a cue for reproduction [Bronson, 2009]. For example, photo-responsiveness in long-lived mammals is thought to be enforced by photoperiod down to approximately 30° latitude, below which it weakens until disappearing completely at the equator [Bronson, 1989, 2009]. Yet, some tropical mammalian populations inhabiting spatially and temporally homogeneous environments exhibit reproductive synchronization that might be the result of biological interactions (e.g., physiological, behavioral), rather than climatic cues alone (e.g., calendar-based seasons) [Bronson, 1989; Ims, 1990a,b; Fürtbauer et al., 2011]. Thus, the ultimate cause of reproductive synchrony, whether an environmentally driven process, a biologically driven process, or a combination of both is currently a matter of debate [Clarke et al., 2012].
Sociobiological cues have also been suggested to play a major role in reproductive synchrony among mammals. Specifically, promiscuous populations with exaggerated sexual swellings, clumped spatial distribution, communal feeding, and communal living are expected to have some level of synchronized reproduction [Ims, 1990a; Nunn, 1999]. Females living in groups, and thus sharing the same space, would be exposed to the same reproduction-triggering cue simultaneously. Among mammals, primates living in multimale and multifemale social groups with strong kinship among females commonly exhibit such characteristics [Bercovitch & Goy, 1990; Okayasu, 2001; Power et al., 2013]. For instance, the nonseasonally breeding lion tailed macaque (Macaca silenus) exhibits reproductive coordination and a nonrandom distribution of births through estrous synchrony, suggesting socially driven estrous synchronization [Clarke et al., 1992]. For the rather highly seasonal Japanese macaques (Macaca fuscata) from Yakushima, social disturbances such as troop takeovers during the mating season have been described as the causal mechanism explaining its synchrony [Okayasu, 2001]. Power et al. [2013] compared the female’s “choice” of synchronization versus asynchronization in a population of seasonal nonprovisioned langur monkeys (environmentally constrained) and a nonseasonal provisioned population (not environmentally constrained). The authors concluded that females are synchronized when seasonally constrained, confusing paternity and reducing infanticide, although they desynchronize reproduction and show visible cues of receptivity enabling single males to monopolize mating when there are no seasonal food limits.
Reproduction in the provisioned colony of free-ranging rhesus macaques located on the tropical island of Cayo Santiago, Puerto Rico, which is below the hypothesized latitudinal threshold for seasonal changes, has been reported to be highly seasonal with estrous periods limited from July to January [Conaway & Koford, 1964; Koford, 1965] and most births occurring within a period of 3 months every year [Bercovitch & Goy, 1990; Bercovitch et al., 1998; Rawlins & Kessler, 1986a; Vandenbergh, 1973]. The reproductive coordination of this entire population of rhesus monkeys has been historically attributed to spring rains [Hoffman et al., 2008; Koford, 1965; Rawlins & Kessler, 1985, 1986b; Vandenbergh, 1973]. However, the variation in the distribution of births reported since establishment of this colony in 1938 suggests an ongoing shift in the onset of births advancing in time through the four different calendar-based seasons [Carpenter, 1942; Hoffman et al., 2008; Koford, 1965; Vandenbergh, 1973]. These temporal changes raise questions about the role of environmental zeitgebers versus biological cues on the timing of reproduction in this primate population.
This study challenges the previous reports on breeding seasonality in the Cayo Santiago rhesus macaques by distinguishing synchrony from seasonality. The former is defined as a greater than chance clustering of births within a birth season independent of the timing of the birth cluster, and the latter as a synchronization of births during periods of the year when ecological conditions are favorable (e.g., spring rainfall) [Clarke et al., 1992]. This study reexamines potential biotic and abiotic factors causing the observed annual advance in the median birth date for the population from 1973 to 2013 including (i) mortality of immature offspring; (ii) age at delivery; (iii) population density; and (iv) rainfall patterns. As colony management practices have been reported to influence mother–infant relationships [Berman,1989], the duration of trapping activities in days and the number of removed individuals are also examined. The impact of the removal of sexually immature individuals, which might affect female reproduction as an apparent death, is also examined. Finally, the years required for the secular trend to advance the population’s median birth date a full 12-month period from the median birth date in 1973 is modeled. The Cayo Santiago rhesus macaque colony is ideal to examine the influence of these factors on the reproductive phenology of mammals because other variables, which could potentially influence reproduction, such as food availability and predation [Bronson, 1985; Ims, 1990b], are controlled on this population.
METHODS
Study Population
Cayo Santiago, a 15.2-ha island located 1km off the southeastern coast of Puerto Rico (18°09′N, 65° 44′W), has been inhabited by a population of free-ranging rhesus monkeys (Macaca mulatta) since 1938. Its average daily temperature is 28°C and relative humidity ranges from 60% to 75% [Rawlins & Kessler, 1985]. All monkeys are descendants of the original 409 Indian founders and no additional animals have been added to the colony except through births. Each animal has a unique tattoo for identification and censuses of the entire population have been continuously conducted since 1956. Information on each individual monkey includes its identity, date of birth, sex, maternity, and date of death or date of removal from the population. Only data since 1973 were used to avoid any potential error in data collection [Sade et al., 1985].
During the study period, high protein commercial monkey diet has been provided at approximately 0.23/kg/animal/day [Kessler & Rawlins, 2015; Sade et al., 1985]. Water is collected in catchments on the island, stored in concrete or fiberglass cisterns, and filtered and chlorinated prior to distribution at automatic watering stations. The population is captured annually in order to identify (tattoo and ear notch) yearlings, bleed them for maternity and paternity confirmation, cull the population for population control and to supply monkeys to the breeding colonies of the Caribbean Primate Research Center (CPRC) Sabana Seca Field Station (SSFS). Culling strategies have changed over time. From 1973 to 1983, the colony was not culled. From 1984 through 1995, entire social groups were periodically removed for population control. Since 1996, selective culling by age has been carried out, mostly targeting sexually immature individuals (0- to 2-years old) [Hernández-Pacheco et al., 2015].
Reproductive and Mortality Data Collection
Reproductive data on females were obtained from the Cayo Santiago demographic database and included all individuals born during the 41 birth seasons from 1973 through 2013. For all individuals born in each season, the date of birth, and the mother’s identity were used to estimate the (i) total number of births; (ii) monthly distribution of births and degree of reproductive synchrony; (iii) age at delivery; and (iv) interbirth interval (IBI) of females. The IBIs were estimated for females giving birth in two consecutive seasons (females that failed to give birth in a particular season were not included). The first and last births recorded in each season were used to estimate the length of each birth season [Rawlins & Kessler, 1985]. The degree of reproductive synchrony was estimated using the percentage of births occurring within a 3-month period [Kaumanns et al., 2013; Van Schaik et al., 1999]. Note that given the temporal changes in the distribution of births the assigned year of a particular birth season is not necessarily equal to the calendar year in which it took place (e.g., “birth season 2010” took place between 2009 and 2010, see Results). The number of <1 year old individuals that died each year was recorded to estimate annual infant mortality and test for temporal variability in infant mortality. The same analysis was carried out for yearling (1 to <2 years old individuals) and juvenile (2 to <3 years old individuals) monkeys. The total number of females alive at the end of every birth season was used to estimate population density. Stillborn individuals were not included in any analysis.
Rainfall, Trapping, and Culling Data
Previous studies on the reproductive phenology of Cayo Santiago have correlated the onset of mating with the onset of the spring rainy season [Hoffman et al., 2008; Koford, 1965; Rawlins & Kessler, 1985]. To determine if rainfall modulated seasonal reproduction during the 40-year study period, daily precipitation measurements from Juncos 1 SE weather station (18.23°, 65.91°) from 1973 through 2012 were evaluated (Weather Source, LLC, http://weathersource.com ). This weather station was selected based on its proximity to Cayo Santiago (~18km) and data completeness for the study period. For each year, the total rainfall (cm) was tabulated bi-weekly from daily records and the onset of spring rains was identified by the bi-weekly period showing a significant increase in mean daily rainfall of more than two times the amount of the previous period [Rawlins & Kessler, 1985]. Once the period was identified, the date of the start of the spring rains was estimated as the first day of the period with recorded rainfall. Data from the Juncos 1 SE weather station corresponding to years 1984 and 2011 was incomplete (Weather Source, LLC, http://weathersource.com) and was not included in this analysis.
The total number of days required for trapping and the total number of monkeys captured during each annual trapping period during years of available data (1993–2013) were obtained from Cayo Santiago’s database.
Data Analysis
As the monthly births were not normally distributed in 93% of the seasons studied (Shapiro–Wilk normality test <0.05), the reproductive data (e.g., birth distribution, IBI) were described using the median as the measure of location. For all analyses, calendar dates were converted to Julian dates (e.g., January 1=day 0; December 31=day 364 or 365, if leap year). Least-squares regression analyses were performed to test for a temporal advancement (shift) in the annual onset of the birth season and the median birth date. Correlation analyses between reproductive variables and the onset of spring rains, the annual trapping duration, and the number of individuals trapped were performed. As the annual trapping occurred during or immediately after the birth season, correlation analyses were made between trapping data at a given year and reproductive data from the following year. Successful mating was estimated by subtracting 165 days from the date of each birth (average gestation length of rhesus macaques from the date of birth) [Rawlins & Kessler, 1985; Valerio et al., 1969]. To estimate the effect of immature offspring loss on IBI, differences in mean IBI of females experiencing either infant (0 to <1 years old), yearling (1 to <2 years old), or juvenile (2 to <3 years old) loss versus females giving birth to surviving offspring (no loss) were tested using nonparametric Mann–Whitney and Kruskal–Wallis tests. Immature offspring loss was defined as the loss of a <3 years old offspring due to mortality or culling between consecutive births.
To identify the set of variables that best explain the observed variation in the onset of births and median birth date, model selection was performed using multimodel inference or model averaging based on the second order Akaike’s Information Criterion () as the is recommended for sample sizes [Burnham & Anderson, 2002] (Supporting Information). Two measures associated with the ; and Akaike weights () are also included. The is a measure of each model relative to the best model, so that it represents the ratio of values for each model relative to the whole set of candidate models [Burnham & Anderson, 2002; Mazerolle, 2004]. The Akaike weight changes the scale of the to compare them on a scale of 1 and it indicates the probability that the model fits the data better among the whole set of candidate models [Burnham & Anderson, 2002; Mazerolle 2004]. All analyses were carried out using R.3.0.1, package pgirmess [Giraudoux, 2013, http://perso.orange.fr/giraudoux ; R Development Core Team, http://www.R-project.org]. All research procedures were approved by the Caribbean Primate Research Center and the Institutional Animal Care and Use Committee of the University of Puerto Rico, Medical Sciences Campus, in accordance with USDA regulations and NIH guidelines. This research also adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates.
RESULTS
Temporal Distribution of Births
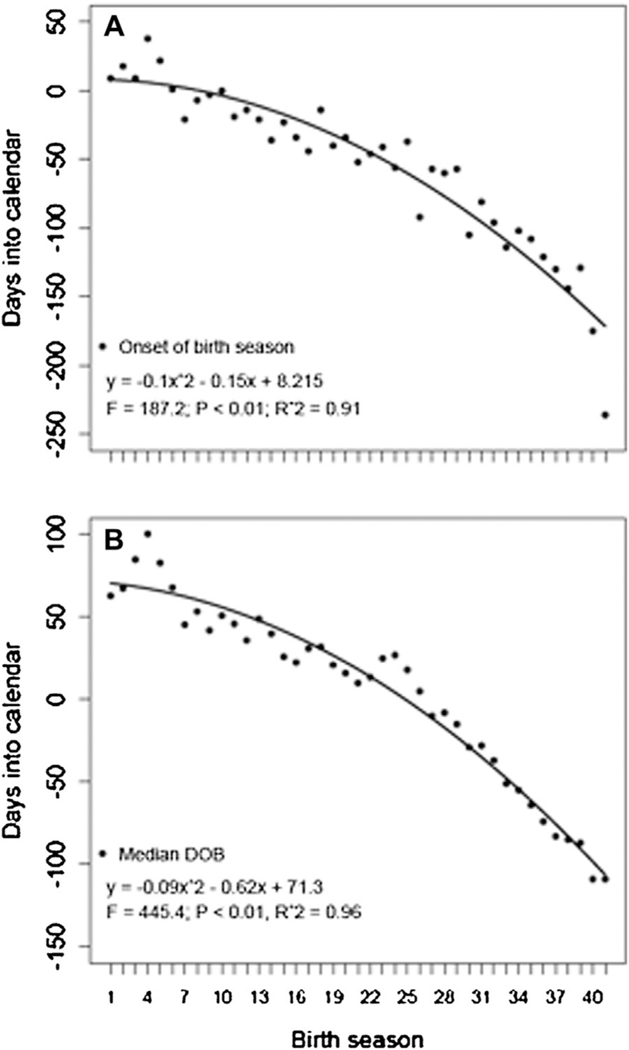
A total of 7,893 births (males = 4,011, females =3,774, unknown sex=108) were recorded from 1973 to 2013. High reproductive clustering was found in every season with more than 72% of births taking place in a 3-month period (Supporting Information). The median birth season length was 174.0 days (95% CI=129.0, 269.6days). A relatively isolated birth was recorded in September, 1980 whereas the rest of the births during the corresponding season took place three months later. Because no similar event was recorded in the 40-year study period, this isolated birth was not considered when estimating the onset of the birth season. The onset of births advanced every season, exhibiting an increase in the rate of the advance through time (, , , , ; Fig. 1A). In the same way, the median date of birth advanced every season exhibiting an increase in the rate of the advance through time (, , , , ; Fig. 1B).
Fig. 1.
Temporal advance in the onset of birth season (A) and median birth date (B) of Cayo Santiago rhesus macaques from 1973 (season 1) to 2013 (season 41).
Age at Delivery, Offspring Loss, and Population Density
Mean age of rhesus macaque females increased linearly over time (, , , ; SI III, Supporting Information). The mean age at delivery increased over time (, , , ;SIIV, Supporting Information). A negative relationship between the median age at delivery and the median date of birth was found (, , , ; SIV, Supporting Information).Mean infant mortality changed significantly through time with a higher rate of increase during the last decade (, , , , ; Supporting Information). During the entire study period, the mean annual infant mortality was (mean ± SD). Similarly, mean annual mortality of yearlings changed through time (, , , , ; Supporting Information).The mean annual mortality of yearlings was (mean ± SD). No significant variability in mean annual juvenile mortality through time was observed. The mean annual mortality of juveniles was (mean ± SD).
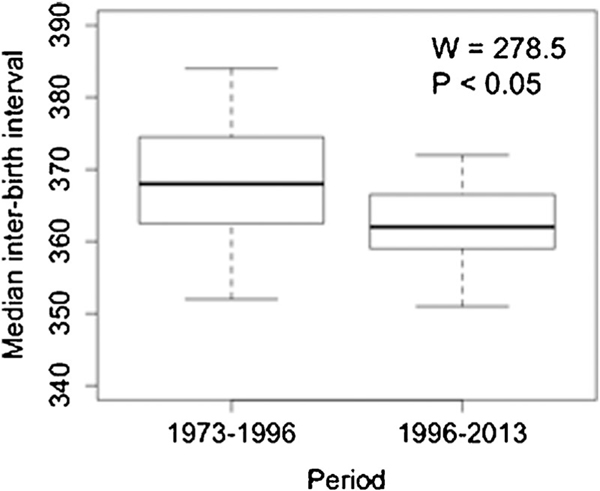
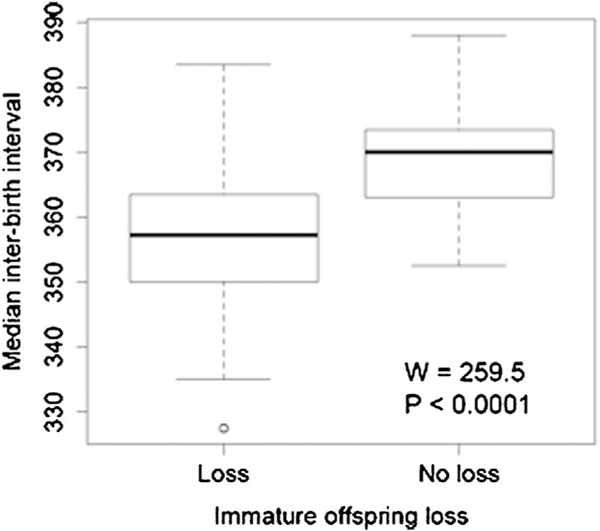
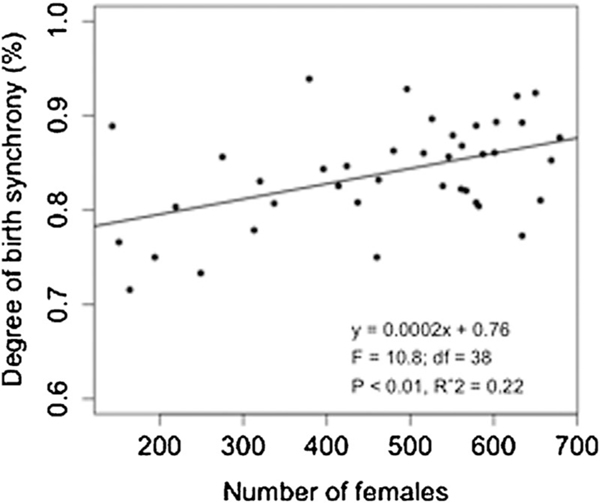
The IBI of individual females giving birth during consecutive seasons ranged from 150 to 530 days resulting in a median IBI of 366 days (95% CI=302.0, 432.0 days) for the entire study period. IBIs longer than 365 days correspond to females giving birth during the beginning and the end of consecutive seasons, respectively. The median IBI of females losing immature offspring by death or culling (353 days, 95%CI=332, 381 days) was significantly shorter than that of females with no loss (371 days, 95%CI=360, 384 days; , ; Fig. 2). A multiple comparison test after a Kruskal–Wallis indicated that females losing infants had a shorter IBI than females losing yearlings or juveniles, but females losing yearlings or juveniles had a shorter IBI than females with no loss (Kruskal–Wallis , , ). Changes in colony management starting in 1996, coupled with changes in offspring mortality resulted in an increase in the proportion of females losing immature offspring over time (Table I). The median IBI of individual females decreased significantly following 1996 (, , ; Fig. 3 for annual median values). From 1973 to 1996, only 428 of 2556 females (16.7%; annual proportion of 16.2%; 95%CI=0.11, 0.23) giving birth during consecutive seasons lost an immature offspring, whereas from 1996 to 2013 the proportion of these females increased to 48.2% (1,476 of 3,065 females; annual proportion of 50.3%; 95%CI=0.18, 0.62; Table I). Individual females losing infants had a median IBI of 350 days (95%CI=270, 437 days) whereas females losing yearlings and juveniles had a median IBI of 361 (95%CI=308, 451 days) and 361 days (95%CI=294, 452 days), respectively. The annual median IBI from 1973 to 1996 was 368 days (95%CI=353, 383 days), whereas from 1996 to 2013 it was 362 days (95%CI=353, 371 days; Table I). A decreasing temporal trend in IBI was observed only for females losing juveniles (, , , , ). The degree of synchronization of births increased linearly as female density increased (, , , , ; Fig. 4). Females experiencing the removal of more than one offspring during the same season did not present a different IBI from females experiencing the removal of only one offspring (Mann–Whitney , ).
Fig. 2.
Median interbirth interval of Cayo Santiago females giving birth in consecutive years during two periods of different culling strategies; from 1973 to 1996 entire social groups were culled, 1996–2013 culling was targeted to immature individuals mostly.
TABLE I.
Median Interbirth Interval of Female Rhesus Giving Birth During Consecutive Birth Seasons From 1973 to 2013
| Median IBI of females with consecutive births (days) | |||
|---|---|---|---|
|
| |||
| Interval | All females | No loss | Immature loss |
| 1973–1974 | 368 | 376 | 348 |
| 1974–1975 | 384 | 384 | 384 |
| 1975–1976 | 380 | 380 | 372 |
| 1976–1977 | 352 | 360 | 343 |
| 1977–1978 | 353 | 359 | 328 |
| 1978–1979 | 362 | 366 | 342 |
| 1979–1980 | 370 | 371 | 361 |
| 1980–1981 | 367 | 369 | 353 |
| 1981–1982 | 372 | 374 | 369 |
| 1982–1983 | 369 | 371 | 359 |
| 1983–1984 | 362 | 364 | 345 |
| 1984–1985 | 382 | 383 | 379 |
| 1985–1986 | 363 | 363 | 347 |
| 1986–1987 | 358 | 360 | 335 |
| 1987–1988 | 367 | 370 | 340 |
| 1988–1989 | 380 | 383 | 363 |
| 1989–1990 | 377 | 379 | 367 |
| 1990–1991 | 359 | 362 | 347 |
| 1991–1992 | 367 | 370 | 352 |
| 1992–1993 | 366 | 371 | 357 |
| 1993–1994 | 372 | 375 | 366 |
| 1994–1995 | 383 | 388 | 353 |
| 1995–1996 | 370 | 370 | 362 |
|
| |||
| Median (95%CI) | 368 (353, 383) | 371 (360, 384) | 353 (332, 381) |
| N (%) | 2,556 (100) | 2,128 (83.3) | 428 (16.7) |
|
| |||
| 1996–1997 | 365 | 373 | 361 |
| 1997–1998 | 361 | 362 | 360 |
| 1998–1999 | 363 | 367 | 356 |
| 1999–2000 | 371 | 372 | 369 |
| 2000–2001 | 367 | 370 | 356 |
| 2001–2002 | 357 | 363 | 356 |
| 2002–2003 | 366 | 368 | 363 |
| 2003–2004 | 360 | 364 | 358 |
| 2004–2005 | 357 | 358 | 355 |
| 2005–2006 | 368 | 371 | 366 |
| 2006–2007 | 362 | 367 | 358 |
| 2007–2008 | 359 | 363 | 356 |
| 2008–2009 | 359 | 362 | 352 |
| 2009–2010 | 362 | 372 | 358 |
| 2010–2011 | 372 | 376 | 364 |
| 2011–2012 | 351 | 352 | 345 |
| 2012–2013 | 369 | 371 | 366 |
|
| |||
| Median (95%CI) | 362 (353, 371) | 368 (360, 374) | 358 (348, 368) |
| N (%) | 3,065 (100) | 1,589 (51.8) | 1,476 (48.2) |
Fig. 3.
Interbirth interval of Cayo Santiago females giving birth during consecutive seasons but experiencing loss of an immature offspring (death or culling) versus females with no loss of offspring.
Fig. 4.
Degree of synchrony of reproduction among Cayo Santiago females. Degree of synchrony was estimated by calculating the percentage of births during each season occurring within three months.
Rainfall
To determine whether the rainfall data from the Juncos weather station was representative of the rainfall data from Humacao, the same correlation analysis between the onset of spring rains and the onset of mating from 1975 to 1983 used by Rawlins and Kessler [1985] was employed. During this period, a significant decreasing trend in the onset of spring rains was observed (, , , , ). The variation correlated positively with the estimated onset of mating, confirming the report by Rawlins and Kessler [1985] (, , , , ). When the same analysis was carried out using the data set from (1985 to 2013), (, , , , ) and there was no significant correlation between the variation in the onset of spring rains and onset of the mating season (, , , , ).
Annual Trapping
The temporal shift in the onset of births and median birth date did not correlate with the duration of trapping activities (, , ; , , , respectively). The duration of trapping activities from 1993 to 2012 was days (mean ± SD). The temporal shift in the onset of births and median birth date also did not correlate with the total number of monkeys trapped annually (, , , , , respectively). The total annual number of monkeys trapped from 1993 to 2012 was .
Model Selection and Predicted Advance in Reproduction
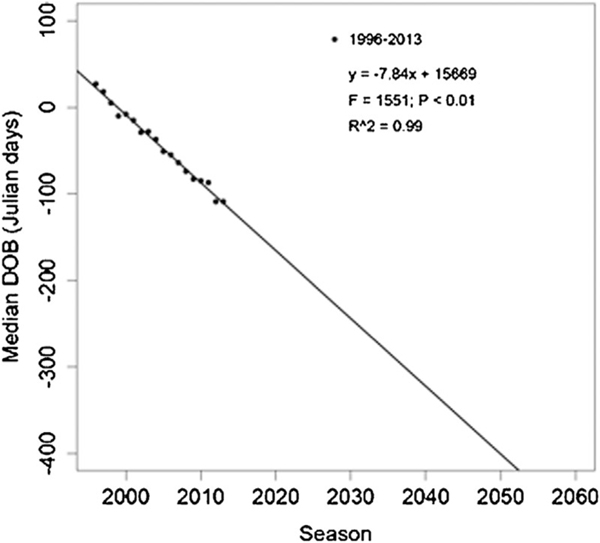
Multimodel inference selection was carried out separately for data from 1973 to 1996 and from 1996 to 2013 because not all factors were available for both periods, such as trapping and culling-related variables. Model selection indicated that variability in the age at delivery and population density provided the best explanation for the observed onset and median birth dates until 1996 (this interpretation was based on parsimony as two models resulted with the same ; Supporting Information). However, after 1996 population density, infant mortality, and variability in the IBI of females losing infants provided the best explanation for the observed onset and median birth dates (Supporting Information). The linear model of the temporal variation in median birth date predicts that, under current conditions, the distribution of births (and thus mating) would advance 12 months by 2050 (Fig. 5).
Fig. 5.
Predicted time of the population’s median birth date to advance a full 12-months period.
DISCUSSION
A secular trend in the annual onset of births and the median birth date of the Cayo Santiago rhesus macaque population was observed during the 40-year study period. Progressively earlier mating has shifted the onset of reproduction and subsequent birthing “backwards” through three different calendar-based seasons; spring to winter to autumn. Rather than climatic cues, the reproductive phenology of these macaques is better described by biological factors. These factors include the age at delivery of females, the decrease in the median IBI of the female population (<365 days) due to an increase overtime in the proportion of females losing an immature offspring (either to natural death or culling) between consecutive births, and an increase in birth synchronization due to an increase in female density.
As early as 1957, an advance in the timing of breeding synchronization was reported in the Cayo Santiago macaque population [Koford, 1965]. Variation in rainfall during the spring affecting the availability of natural foods (e.g., bark, leaves, fruits) was described as the principal cue for the timing of mating and consequent annual cluster of births [Koford, 1965, 1966; Rawlins & Kessler, 1985; Vandenbergh & Vessey, 1968]. However, these early studies were carried out for a short time frame of 2–9 years when the median birth date advanced at a slower rate and when breeding activity coincided with the calendar dates of the annual rainy season (Fig. 2). In addition, data for one of the analyses covered the decade when no culling of the population was done. Thus, a long-term secular trend across calendar-based seasons was not evident until mating advanced beyond the temporal limits of the annual rainy season. Model selection used in this study supports the conclusion that biological factors had a greater impact on reproduction of these rhesus monkeys during the period from 1973 to 1996 than climatic ones. Although, Hoffman et al. [2008] described a long-term positive relationship between the onset of spring rainy season and birth season from 1963 to 1996, this finding cannot be compared with this study, or previous ones, because different criteria were used to estimate onset of the spring rainy season (first day since January 1st in which precipitation was equal to or greater than 1in.).
The present study demonstrates that both the onset of births and the median birth date in the Cayo Santiago population have occurred beyond the temporal limits of a particular climatic season. The monkeys do forage on vegetation and they are exposed to environmental influences, but variability in the availability of provided food is absent and annual climatic conditions, vary little in contrast to wild populations [Southwick et al., 1961; Vandenbergh & Vessey, 1968]. Environmental influences at Cayo Santiago resemble those of other macaque populations that inhabit rain forests where food and rainfall do not vary much throughout the year and the annual reproductive cycle of the population is not confined to a particular calendar-based season [Kaumanns et al., 2013]. This conclusion is supported by the model’s prediction that, at the present rate of change and under the current conditions and management, the median birth dates at Cayo Santiago will advance a full year by 2050. Thus, the effects of environmental zeitgebers on reproduction at Cayo Santiago appear to be overridden by biological factors affecting females, such as maternal physical condition, previous reproductive outcome, and female density.
Since the colony’s founding in 1938, provisioning has significantly improved the physical condition and reproductive potential of females. For instance, high nutritional status has been correlated with early sexual maturation [Schwartz et al., 1988]. In fact, Cayo Santiago macaques have lower IBIs on average compared to the reported 12–24 month IBIs of other rhesus populations [Fooden, 2000].Although provisioning alone might be responsible for the secular trend, it remained constant throughout the study period and the observed change in the rate of the advance of mating and births over time suggests other factors must be influencing female reproduction [Maestripieri & Georgiev, 2015; Rogovin & Moshkin, 2007]. For instance, the rate of advance in the median birth date in 1973 was 0.80 days/year while that of 2013 was 8.00 days (Fig. 2).
Reproductive failure or infant mortality could also trigger an advance in the timing of estrus of female macaques by shortening their IBIs [Koford, 1965, 1966;Rawlins & Kessler, 1986b;Rawlins etal., 1984]. The biological mechanism behind this, lactational anovulation, in which the resumption of mating is delayed by nursing/suckling, has been described for several mammals [Lee, 1996; Williams, 1986], including Cayo Santiago macaques [Johnson et al., 1993, 1998]. However, given that only 8–12% of all parous females were nonlactating, and the fact that the year-to-year variation in the date of delivery among individual females was high, early advances in the timing of breeding synchronization in Cayo Santiago macaques were not thought to be driven principally by physiological factors affecting estrous cycles [Koford, 1965, Rawlins & Kessler, 1985]. The present study shows that not only females losing infants between consecutive births have lower IBIs, but also females losing offspring up to 2 years of age (immature offspring) have IBIs lower than the rest of the parous females (<365 days). This suggests extended maternal care, and thus energy allocation to offspring protection, also plays a major role in the mother’s future reproductive success [Clutton-Brock, 1991], a factor overlooked by focusing exclusively on infant mortality. Furthermore, given the fact that hormones affect individual behavior, including interactions between individuals, little attention has been given to the role of stress hormones in the regulation of behavior and specifically reproduction at high densities [Rogovin & Moshkin, 2007]. Thus, this study suggests that culling immature offspring might lower stress levels among females by reducing intragroup density [Aureli & De Waal, 2000] enhanc-] enhancing their chance to mate during the following season. This would create an overall population effect on interbirth intervals and, therefore, the onset of mating and the median birth date. Given the variability in immature offspring mortality and culling practices after 1996, the annual proportion of females losing offspring increased (48% on average, Table I). As a consequence, a larger number of females apparently came into estrus earlier every year shifting the annual reproductive cycle of the entire population due primarily to their high degree of synchronization.
Mean age of rhesus macaque females has increased linearly over time, showing that the population has become “older.” In the same way, the mean age at delivery has increased over time. Such linear trend relates significantly with the median date of birth. Longevity analyses show that birth rates in rhesus macaques decline with age [Bercovitch & Berard, 1993; Gagliardi et al., 2007; Johnson & Kapsalis, 1995]. Therefore, a population exhibiting a significant increase in mean age of females is expected to have a higher proportion of females failing to conceive or losing pregnancies. In such scenario, the increasing loss of offspring would promote shorter IBIs of females, a hypothesis supported by the data presented.
This high degree of synchronization in females was positively associated with population density. Although not tested in this study, population density might have affected social group size, which in turn affects interindividual proximity [Berman et al., 1997], suggesting high density stimulates female coordination. Social facilitation of mating in promiscuous primates is thought be advantageous to avoid infanticide (which has never been reported in Cayo Santiago) by confusing paternity [Nunn, 1999], with the underlying mechanism being that mating synchrony lowers the probability of a male monopolizing a single female, enabling them to mate with multiple males [Fürtbauer et al., 2011]. For instance, during the nonmating season, female rhesus in a social group exhibited copulatory behavior after other female members were experimentally brought into estrus [Vandenbergh & Drickamer, 1974]. The fact that social groups on Cayo Santiago had different birth periods [Koford, 1965] in a particular birth season also suggests that synchronization among females is socially coordinated. This implies that females mating earlier in a season would trigger a significant advance in mating activities at the population level when density is high by affecting the reproductive behavior of both females from their group and females from adjacent groups. During the last three decades population density at Cayo Santiago has increased significantly, resulting in more frequent culling, and thus, a higher proportion of females losing offspring [Hernández-Pacheco et al.,2015]. A higher proportion of females exhibiting IBIs <365 days coupled with a higher degree of synchronization over time, would cause a further advance and accelerate the change in the rate of advance in reproduction at the population level.
Heritability of reproductive traits might also influence the reproductive behavior of individual females. However, estimates of heritability for IBIs show a negligible genetic component [Blomquist, 2009; Gagliardi et al., 2010]. This is supported by the high variability in reproductive phenology presented by the different Cayo Santiago derived populations. For instance, the population at La Parguera, Puerto Rico, had a 2-month delay in mating activities compared to Cayo Santiago [Drickamer, 1974; Rawlins & Kessler, 1985; Vandenbergh & Vessey, 1968]. Similarly, monkeys translocated to Desecheo Island, Puerto Rico, in 1966 were observed to mate 1 to 2 months later than on Cayo Santiago [Morrison & Menzel, 1972; Rawlins & Kessler, 1985]. In 1979, the rhesus colony from La Parguera was translocated to Morgan Island, SC and retained the same reproductive pattern until female rhesus from other facilities were introduced to the colony, lengthening the mating season [Taub & Mehlman, 1989]. After another social group from Cayo Santiago was sent to the German Primate Center at Gottingen in 1984, the group lost its seasonality although housed indoors but recovered it 10 years later when the monkeys were moved outdoors [Kaumanns et al., 2013]. However, by the year 2000, births on Cayo Santiago peaked in December, but in Germany they peaked later in April–May. Finally, high synchronization of births in a social group of Cayo Santiago moved to the CPRC’s SSFS in 1984 and maintained in a large corral complex was observed [Bercovitch et al., 1998], but not among rhesus harem breeding groups at this facility that were disturbed. The variation in the timing of reproduction for Cayo Santiago macaques and its derived populations points towards the effects of reproductive outcome in the previous season (e.g., infant mortality) [Gordon, 1981] and social disturbances and colony disruptions [Berman, 1989]. Colony management procedures, culling, group translocation, introduction of individuals, and proximity of individuals (due to housing facility type) seem to be more significant than climatic influences or heredity factors.
Whether this secular trend in reproductive synchrony is also observed in the wild is unknown as long-term studies on wild populations are lacking. For populations living in areas with significant annual resource variability, seasonal climatic constraints probably do interact with biological factors to modulate reproduction as suggested by previous studies. The discoveries from this study of reproduction at Cayo Santiago show that the annual reproductive cycle of this population is indeed highly synchronized, but is not confined to any climatic season. It also suggests that the combined effects of (i) social mechanisms which enhance individual fitness, together with;(ii)the absence of physiological limitations of initial and extended maternal investment; and (iii) increasing population density are the principal biological factors responsible for the identified secular trend in reproduction of the Cayo Santiago macaques.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank the staff members of Cayo Santiago and the CPRC who have contributed to census data collection and provided technical and administrative support. Cayo Santiago is supported by the Office of Research Infrastructure Programs (ORIP) of the National Institute of Health, grant numbers 8P40OD012217 and 3P40OD012217-27S1 awarded to the University of Puerto Rico Medical Sciences Campus. The content of the publication is the sole responsibility of the authors and does not necessarily represent the official views of NCRR, ORIP, or UPR.
Contract grant sponsor: National Institute of Health; contract grant numbers: 8P40OD012217, 3P40OD012217-27S1.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- Aureli F, De Waal FBM. 2000. Natural conflict resolution. Berkeley and Los Angeles, California: University of California Press. p 133. [Google Scholar]
- Bercovitch FB, Berard JD. 1993. Life history costs and consequences of rapid reproductive maturation in female rhesus macaques. Behavioral Ecology and Sociobiology 32:103–109. [Google Scholar]
- Bercovitch FB, Goy RW. 1990. The socioendocrinology of reproductive development and reproductive success in macaques. In: Ziegler TE Bercovitch FB, editors. Socioendocrinology of Primate Reproduction. New York: WileyLiss. p 59–93. [Google Scholar]
- Bercovitch FB, Lebron MR, Samuel Martinez H, Kessler MJ. 1998. Primigravidity, body weight, and costs of rearing first offspring in rhesus macaques. American Journal of Primatology 46:135–144. [DOI] [PubMed] [Google Scholar]
- Berman CM. 1989. Trapping activities and mother-infant relationships on Cayo Santiago: a cautionary tale. In Kessler MJ, editor. Proceedings of the Meeting to Celebrate the 50th Anniversary of the Cayo Santiago Rhesus Monkey Colony. Puerto Rico Health Sciences Journal 8:73–78. [PubMed] [Google Scholar]
- Berman CM, Rasmussen KLR, Suomi SJ. 1997. Group size, infant development and social networks in free-ranging rhesus monkeys. Animal Behaviour 53:405–421. [Google Scholar]
- Blomquist GE. 2009. Fitness-related patterns of genetic variation in rhesus macaques. Genetica 135:209–219. [DOI] [PubMed] [Google Scholar]
- Bronson FH. 1985. Mammalian reproduction: an ecological perspective. Biology of Reproduction 32:1–26. [DOI] [PubMed] [Google Scholar]
- Bronson FH. 1989. Mammalian reproductive biology. Chicago, Illinois, USA: University of Chicago Press. p 7–27. [Google Scholar]
- Bronson FH. 2009. Climate change and seasonal reproduction in mammals. Philosophical Transactions The Royal Society 364:3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer. [Google Scholar]
- Carpenter CR. 1942. Sexual behavior of free-ranging rhesus monkeys (Macaca mulatta). Journal of Comparative Psychology 33:113–162. [Google Scholar]
- Clarke AS, Harvey NC, Lindburg DG. 1992. Reproductive coordination in a nonseasonally breeding primate species, Macaca silenus. Ethology 91:46–58. [Google Scholar]
- Clarke PMR, Henzi SP, Barrett L. 2012. Estrous synchrony in a nonseasonal breeder: adaptive strategy or population process? Behavioral Ecology 23:573–581. [Google Scholar]
- Clutton-Brock TH. 1991. The evolution of parental care. Princeton, New Jersey: Princeton University Press. p 3–29. [Google Scholar]
- Conaway CH, Koford CB. 1964. Estrous cycles and mating behavior in a free-ranging band of rhesus monkeys. Journal of Mammalogy 45:577–588. [Google Scholar]
- Drickamer LC. 1974. Ten-year summary of reproductive data for free-ranging Macaca mulatta. Folia Primatologica 21:61–80. [DOI] [PubMed] [Google Scholar]
- Fooden J.2000. Systematic review of the rhesus macaque, Macaca mulatta (Zimmermann, 1780). Fieldiana Zoologica 96:1–180. [Google Scholar]
- Fürtbauer I, Mundry R, Heistermann M, Schülke O, Ostner J. 2011. You mate, I mate: macaque females synchronize sex not cycles. PLoS ONE 6:e26144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi C, Falkenstein KP, Franke DE, Michael Kubisch H. 2010. Estimates of heritability for reproductive traits in captive rhesus macaque females. American Journal of Primatology 72:811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi C, Liukkonen JR, Phillippi-Falkenstein KM, Harrison RM, Kubisch HM. 2007. Age as a determinant of reproductive success among captive female rhesus macaques (Macaca mulatta). Reproduction 133:819–826. [DOI] [PubMed] [Google Scholar]
- Gordon TP. 1981. Reproductive behavior in the rhesus monkey: social and endocrine variables. American Zoologist 21:185–195. [Google Scholar]
- Hernández-Pacheco R, Delgado DL, Rawlins RG, Kessler MJ, Ruiz-Lambides AV, Maldonado E, Sabat AM. 2015. Managing the Cayo Santiago macaque population: the role of density. American Journal of Primatology. DOI: 10.1002/ajp.22375 [DOI] [PMC free article] [PubMed]
- Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D.2008.Sexdifferences insurvival costs of reproduction in a promiscuous primate. Behavioral Ecology and Sociobiology 62:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ims RA. 1990a. The ecology and evolution of reproductive synchrony. Trends in Ecology and Evolution 5:135–140. [DOI] [PubMed] [Google Scholar]
- Ims RA. 1990b. On the adaptive value of reproductive synchrony as a predator-swamping strategy. American Naturalist 136:485–498. [Google Scholar]
- Johnson RL, Kapsalis E. 1995. Ageing, infecundity and reproductive senescence in free-ranging female rhesus monkeys. Journal of Reproduction and Fertility 105: 271–278. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Berman CM, Malik I. 1993. An integrative model of the lactational and environmental control of mating in female rhesus monkeys. Animal Behaviour 46:63–78. [Google Scholar]
- Johnson RL, Malik I, Berman CM. 1998. On the quantification of suckling intensity in primates. American Journal of Physical Anthropology 105:33–42. [DOI] [PubMed] [Google Scholar]
- Kaumanns W, Singh M, Schwibbe M. 2013. Environmental change and housing conditions result in disappearance and return of reproductive seasonality in rhesus macaques (Macaca mulatta). Current Science 105:527–521. [Google Scholar]
- Kessler MJ, Rawlins RG. 2015. A 75-year pictorial history of the CayoSantiago rhesus monkeycolony. American Journal of Primatology. DOI: 10.1002/ajp.22381 [DOI] [PMC free article] [PubMed]
- Koford CB. 1965. Population dynamics of rhesus monkeys on Cayo Santiago. In: DeVore I, editor. Primate behavior. New York: Holt, Rinehart & Winston. p 160–174. [Google Scholar]
- Koford CB. 1966. Population changes in rhesus monkeys: Cayo Santiago, 1960–1964. Tulane Studies in Zoology 13:1–7. [Google Scholar]
- Lee PC. 1996. The meanings of weaning: growth, lactation, and life history. Evolutionary Anthropology 5:87–96. [Google Scholar]
- Maestripieri D, Georgiev AV. 2015. What cortisol can tell us about the costs of sociality and reproduction among free-ranging rhesus macaque females on Cayo Santiago. American Journal of Primatology. DOI: 10.1002/ajp.22368 [DOI] [PMC free article] [PubMed]
- Mazerolle MJ. Making sense out of Akaike’s information criterion (AIC): its use and interpretation in model selection and inference from ecological data. http://www.theses.ulaval.ca/2004/21842/apa.html
- Morrison JA, Menzel EW. 1972. Adaptation of a free-ranging rhesus monkey group to division and transplantation. Wildlife Monographs 31:5–78. [Google Scholar]
- Nunn CL. 1999. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Animal Behaviour 58:229–246. [DOI] [PubMed] [Google Scholar]
- Okayasu N.2001. Contrast of estrus in accordance with social contexts between twotroops of wild japanese macaques on Yakushima. Anthropological Sicience 109:121–139. [Google Scholar]
- Power C, Sommer V, Watts I. 2013. The seasonality thermostat: female reproductive synchrony and male behavior in monkeys, neanderthals, and modern humans. PaleoAnthropology 2013:33–60. [Google Scholar]
- Rawlins RG, Kessler MJ. 1985. Climate and seasonal reproduction in the Cayo Santiago macaques. American Journal of Primatology 9:87–99. [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. 1986a. The history of the Cayo Santiago colony. In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques: history, behavior and biology. Albany, NY: State University of New York Press. p 13–45. [Google Scholar]
- Rawlins RG, Kessler MJ. 1986b. Demography of the free-ranging Cayo Santiago macaques 1976–1983. In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques: history, behavior and biology. Albany, NY: State University of New York Press. p 46–72. [Google Scholar]
- Rawlins RG, Kessler MJ, Turnquist JE. 1984. Reproductive performance, population dynamics and anthropometrics of the free-ranging Cayo Santiago rhesus macaques. Journal of Medical Primatology 13:247–259. [PubMed] [Google Scholar]
- Rogovin KA, Moshkin MP. 2007. Autoregulation in mammalian populations and stress: an old theme revisited. Zhurnal Obshchei Biologii 68:244–267. [PubMed] [Google Scholar]
- Sade DS, Chepko-Sade DB, Schneider JM, Roberts SS, Richtsmeier JT. 1985. Basic demographic observations on free-ranging rhesus monkeys. New Haven, CT: Human-Relations Area Files. 159. [Google Scholar]
- Schwartz SM, Wilson ME, Walker ML, Collins DC. 1988. Dietary influences on growth and sexual maturation in premenarchial rhesus monkeys. Hormones and Behavior 22:31–251. [DOI] [PubMed] [Google Scholar]
- Southwick CH, Beg MA, Siddiqi MR. 1961. A population survey of rhesus monkeys in Northern India. II. Transportation routes and forest areas. Ecology 42:699. [Google Scholar]
- Taub DM, Mehlman PT. 1989. Development of the Morgan Island rhesus monkey colony. PR Health Science Journal 8:159–169. [PubMed] [Google Scholar]
- Valerio DA, Miller RL, Innes JRM, Courtney KD, Pallotta AJ, Guttmacher RM. 1969. Macaca mulatta: management of a laboratory breeding colony. New York: Academic Press. [Google Scholar]
- Vandenbergh JG, Vessey S. 1968. Seasonal breeding of free-ranging rhesus monkeys and related ecological factors. Journal of Reproduction and Fertility 15:71–79. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. 1973. Environmental influences on breeding in rhesus monkeys. Primate Reproductive Behavior 2:1–19. [Google Scholar]
- Vandenbergh JG, Drickamer LC. 1974. Reproductive coordination among free-ranging rhesus monkeys. Physiology & Behavior 13:373–376. [DOI] [PubMed] [Google Scholar]
- Van Schaik CP, van Noordwijk MA, Nunn CL. 1999. Sex and social evolution inprimates. In: Lee PC, editor. Comparative primate socioecology. Cambridge: Cambridge University Press. p 204–240. [Google Scholar]
- Williams RF. 1986. The interbirth interval in primates: effects of pregnancy and nursing. In: Benirschke K, editor. Primates: the road to self-sustaining populations. San Diego, California: Proceedings of the Zoological Society of San Diego. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.