Abstract
Context:
Although patients with acute myeloid leukemia (AML) experience significant toxicities and poor outcomes, few studies have quantified patients’ experience.
Methods:
A community-centered approach was used to develop an AML-specific best–worst scaling (BWS) instrument involving 13 items in four domains (psychological, physical, decision-making, and treatment delivery) to quantify patient worry. A survey of patients and caregivers was conducted using the instrument. Data were analyzed using conditional logistic regression.
Results:
The survey was completed by 832 patients and 237 caregivers. Patients were predominantly white (88%), married/partnered (72%), and in remission (95%). The median age was 55 years (range: 19–87). Median time since diagnosis was 8 years (range: 1–40). Patients worried most about “the possibility of dying from AML” (BWS score = 15.5, confidence interval [CI] [14.2–16.7]) and “long-term side effects of treatments” (14.0, CI [12.9–15.2]). Patients found these items more than twice as worrisome as all items within the domains of care delivery and decision-making. Patients were least worried about “communicating openly with doctors” (2.50, CI [1.97–3.04]) and “having access to the best medical care” (3.90, CI [3.28–4.61]). Caregiver reports were highly correlated to patients’ (Spearman’s ρ = 0.89) though noted significantly more worry about the possibility of dying and spending time in the hospital.
Conclusion:
This large convenience sample demonstrates that AML patients have two principal worries: dying from their disease and suffering long-term side effects from treatment. To better foster patient-centered care, therapeutic decision-making and drug development should reflect the importance of both potential outcomes. Further work should explore interventions to address these worries.
Keywords: Anxiety, Caregivers/psychology, Decision Making, Drug Development/*methods, Leukemia, Myeloid, Acute, Logistic Models, Patient Preference, Patient-Centered Care, Psychooncology, Risk Assessment
1 |. INTRODUCTION
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by proliferation of clonal abnormally differentiated premature myeloid cells leading to progressive bone marrow failure.1 Over 21,000 patients are diagnosed each year in the United States and most will eventually die of their disease.2 Long-term survival is often only achieved through intense chemotherapy often combined with hematopoietic cell transplant (HCT).1 Patients experience substantial short- and long-term side effects, and quality of life substantially suffers following therapy.3,4
Over the last several decades, there has been an increasing interest in incorporating the patient experience into all aspects of healthcare delivery.5,6 This has been motivated, in large part, by an appreciation for the lack of quality in the healthcare system, especially in patient-centered care, where the patient’s specific healthcare needs, values, and preferred outcomes inform and guide clinical decisions.7,8
Incorporating the patient experience into the delivery of care in AML has many challenges. Until recently, there have been few effective therapeutic options, which limited the ability for providers to tailor therapy to patients’ preferences.9 In addition, most patients experience emotional shock at diagnosis and have difficulty understanding and contributing to chemotherapy decisions, compromising shared decision-making.10 Furthermore, there is substantial discordance in the perception of the risks and benefits of treatments between oncologists and patients, and oncologists struggle to predict what is most important to patients.11,12 Emerging methodology to quantify the experiences of patients and caregivers may be useful to improve patient-centered care.
Worry can be conceptualized as the experience of negative feelings or a chain of negative thoughts about the potential realization of unpleasant events.13,14 Risk perception of adverse events has been shown to be a predictor of cancer patients’ worry implying that interventions to address misperceptions of risk may improve patients’ experience and reduce worry.14–16 Describing the worries of cancer patients, therefore, is important to direct appropriate risk communication and supportive care interventions.15–17 This study sought to capture and quantify the worries of AML patients to inform patient-centered care and policy.
2 |. METHODS
2.1 |. Study purpose
This study was designed with the Leukemia & Lymphoma Society (LLS) to quantify the patient experience in AML to inform patient-focused drug development (PFDD).4,18–21 Study governance included an executive committee of researchers and LLS staff, and two different stakeholder groups: a committee of AML experts and a community committee comprised of AML patients and caregivers. Study design was organized across five predetermined stages: engagement, development, pretesting, pilot testing, and presentation resulting in a national survey of AML patients using an AML-specific best–worst scaling (BWS) instrument. This manuscript contains data from the national survey. The data were presented to the Food and Drug Administration (FDA) on 30 April 2018 to inform PFDD. The data that support the findings of this study are available from the corresponding author upon reasonable request.
2.2 |. Instrument development
BWS is an analytically efficient stated-preference method that allows for the quantification of the relative importance of a group of items (attributes) based on a series of choice tasks.22,23
We designed a BWS instrument to elicit patient worries from patients and their caregivers based on the lived experience of patients and the current clinical environment. A detailed description of instrument development has been published.20 Development and all revisions, including determination of worry domains and items, were informed by engagement with the stakeholders. The final instrument included 13 items in four experience domains—decision-making, treatment delivery, physical impacts, and psychosocial effects. A balanced incomplete block BWS case 1 experimental design was developed.
Participants complete 14 choice tasks. In each task, they evaluate, among four items, the one they are “most worried about” and the one they are “least worried about” over the course of their disease experience. Stakeholders determined that capturing worry over the entire disease course rather than a specific point in time was most important. Three-level Likert ratings are also collected for each item. Caregivers received instructions to respond about their perception of the patient’s worry. Figure 1 displays an example choice task.
FIGURE 1.
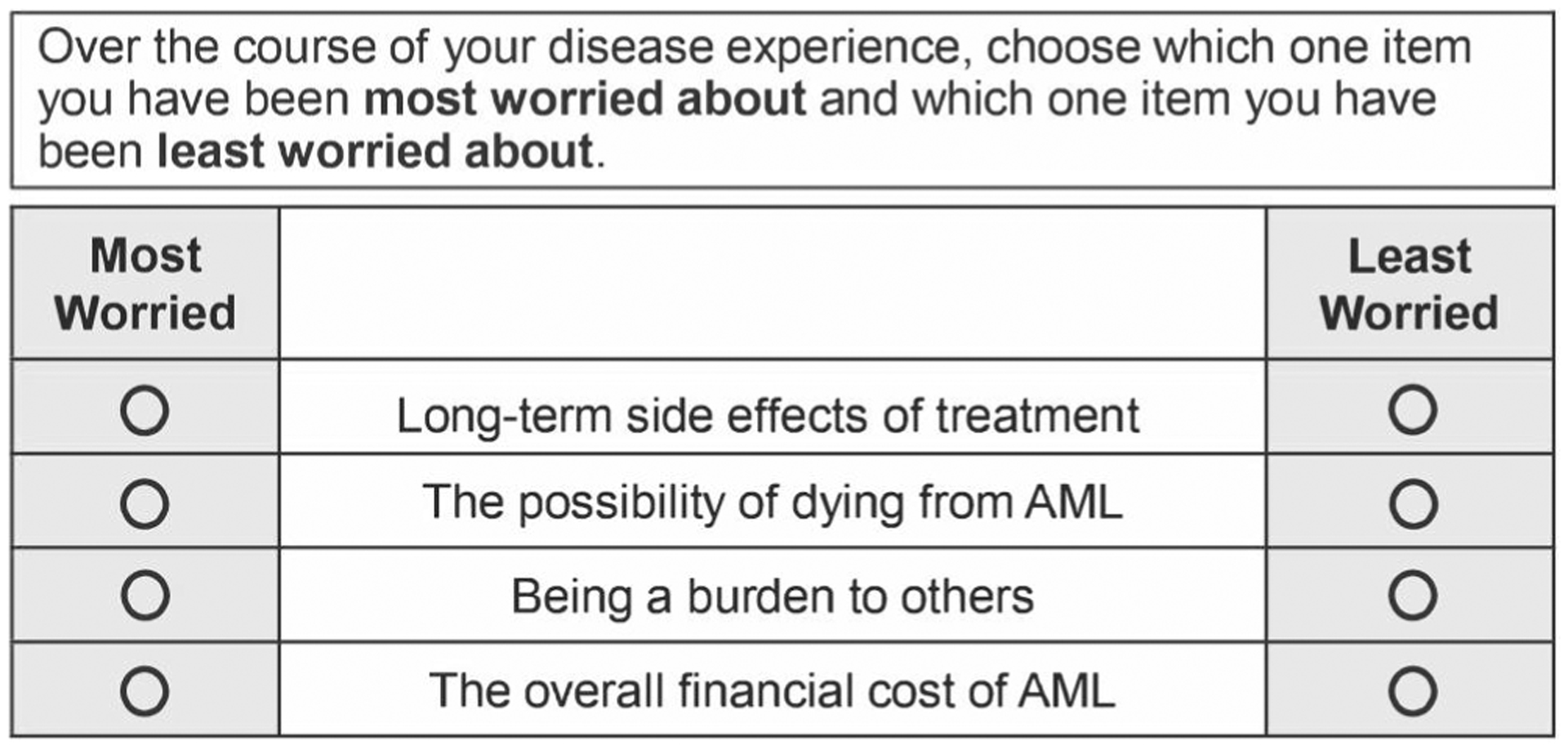
Example best–worst scaling choice task. Participants completed 14 choice tasks that included individual worry items shown in Table S1
2.3 |. Participants
Patients with AML or caregivers of patients with AML were eligible. Eligible patients were required to be at least 18 years of age at the time of the survey and have a self-reported diagnosis of AML. Eligible caregivers were required to either currently be providing or have previously provided care for an adult AML patient and be at least 18 years old. A convenience sample of individuals from an LLS database were invited by email to participate. Surveys were administered electronically using Qualtrics. Two reminder emails were sent in 3 weeks intervals in the case of nonresponse. This study was deemed as exempt human subjects research by the Johns Hopkins Bloomberg School of Public Health IRB (#7200) in accordance with 45 CFR part 46 and was conducted in accordance with the criteria set by the Declaration of Helsinki. The survey introduction included the purpose of the study, the discomfort participants could experience from completing the survey, and that participation was voluntary. The completion of the survey items was regarded as consent to participate.
2.4 |. Statistical analyses
Descriptive statistics were reported for the clinical and sociodemo-graphic information. Categorical variables were summarized by frequency statistics, continuous variables using means, standard deviations, and ranges.
The dependent variable was the participant’s judgment about the extreme attributes in each of the 14 choice tasks. An aggregate conditional logit analysis using a sequential best–worst assumption and effects coding was used resulting in positive and negative coefficients.19,22,24 We rescaled the coefficients on a standardized ratio score where all scores sum to 100 (BWS score).25 Relative, not absolute, differences on this scale are meaningful. For example, compared to a BWS score of 10, 20 is twice as influential.
Analyses were conducted using StataIC 14 (StataCorp) and Sawtooth (Sawtooth Software Inc.).
3 |. RESULTS
The survey was sent to 5353 eligible participants. Of these, 832 patients and 237 caregivers completed all items (response rate 18%). Table 1 summarizes demographic and clinical characteristics.
TABLE 1.
Demographic and clinical characteristics of patients with acute myeloid leukemia, by informant Abbreviations: HCT, hematopoietic cell transplant; GED, Graduate Equivalency Degree.
| Caregiver (n = 235) | |||||
|---|---|---|---|---|---|
| Informant | Patient (n = 892)a | Of living patient | Of deceased patient | Total | p Valueb |
| N | 892 | 140 (60%) | 95 (40%) | 235 | |
| Median age in years (range) | 55 (19–87) | 48 (17‐82) | 59 (17–91) | 52 (17–91) | 0.87 |
| Female gender | 502 (60%) | 57 (41%) | 29 (31%) | 0 | <0.001 |
| Race/ethnicity | 0.38 | ||||
| White/Caucasian | 735 (88%) | 119 (85%) | 81 (85%) | 200 (85%) | |
| Black/African American | 31 (4%) | 6 (4%) | 2 (2%) | 8 (3%) | |
| Hispanic | 34 (4%) | 7 (5%) | 8 (8%) | 15 (6%) | |
| Asian | 20 (2%) | 3 (2%) | 1 (1%) | 4 (2%) | |
| Native American | 1 (0%) | 2 (1%) | 0 | 2 (1%) | |
| Pacific Islander | 2 (0%) | 0 | 1 (1%) | 1 (0%) | |
| Other | 8 (1%) | 3 (2%) | 2 (2%) | 5 (2%) | |
| Marital status | <0.001 | ||||
| Single, never married | 99 (12%) | 41 (29%) | 12 (13%) | 53 (23%) | |
| Married or domestic partnership | 595 (72%) | 88 (63%) | 76 (80%) | 164 (70%) | |
| Widowed | 21 (3%) | 3 (2%) | 4 (4%) | 7 (3%) | |
| Divorced | 116 (14%) | 8 (6%) | 3 (3%) | 11 (5%) | |
| Education | 0.90 | ||||
| Less than high school | 5 (1%) | 18 (13%) | 10 (11%) | 28 (12%) | |
| High school/GED | 66 (8%) | 29 (21%) | 19 (20%) | 48 (20%) | |
| Some college | 210 (25%) | 37 (26%) | 24 (25%) | 61 (26%) | |
| College or graduate/professional school | 549 (66%) | 56 (40%) | 42 (44%) | 98 (42%) | |
| Employment | <0.001 | ||||
| Employed for wages | 384 (46%) | 23 (16%) | 14 (15%) | 36 (15%) | |
| Retired | 181 (22%) | 16 (11%) | 30 (32%) | 46 (20%) | |
| On disability | 211 (25%) | 65 (46%) | 38 (40%) | 103 (44%) | |
| Unemployed | 38 (5%) | 7 (5%) | 4 (4%) | 11 (5%) | |
| Student | 15 (2%) | 29 (21%) | 9 (9%) | 38 (16%) | |
| Insurance | 0.024 | ||||
| Public insurance | 165 (20%) | 31 (22%) | 34 (36%) | 66 (29%) | |
| Private insurance | 654 (79%) | 101 (72%) | 59 (62%) | 164 (71%) | |
| Median years since diagnosis (range) | 8 (1–40) | 6 (1–31) | 6 (1–19) | 6 (1–31) | <0.001 |
| Disease status | <0.001 | ||||
| In remission | 849 (95%) | 138 (88%) | 12 (10%) | 150 (60%) | |
| Not in remission | 31 (3%) | 13 (8%) | 89 (74%) | 102 (40%) | |
| Treatment | |||||
| Allogeneic HCT | 568 (64%) | 89 (64%) | 62 (65%) | 151 (64%) | 0.43 |
| Autologous HCT | 45 (5%) | 6 (4%) | 1 (1%) | 7 (3%) | 0.074 |
| Palliative care | 38 (4%) | 9 (6%) | 43 (45%) | 52 (22%) | <0.001 |
892 patients started the survey, however, only 832 completed all choice tasks.
Comparing total patient sample to total caregiver sample.
The median age of patients was 55 years (range: 19–87) at the time of the survey. Most were white (88.4%), married (71.6%), female (60.4%), college-educated (66.1%), and privately insured (79.0%). Most (95.0%) reported being in remission with about two-thirds (63.7%) previously receiving an allogeneic HCT. Patients self-reported being diagnosed with AML a median of 8.0 years ago (range: 1–40). A third (32.8%) participated in a clinical trial.
Caregivers tended to be white (86%), women (79%), the spouse (56%) or the child (24%) of the patient, and college-educated or higher (65%). The median age of patients they cared for was 52 years (range: 17–91). Most of these patients were white (85%), married or partnered (70%), male (63%), and had private insurance (70%). Demographics and clinical characteristics of patients they cared for varied from the patient-reported sample (Table 1). Caregivers cared for patients who were still living (56.5%) and who had passed away (43.5%). Most patients they cared for who were still alive were in remission (88%) while 74% of deceased patients were not in remission at the time of death. As compared to the patient-reported sample, these patients were more likely to be male, to be diagnosed more recently (mean 6 vs. 8 years), to not be in remission, and to have received palliative care (all p < 0.001).
Standardized BWS scores from the patient-reported sample are presented in Figure 2. Patients worried most about “the possibility of dying from AML” (BWS score = 15.5, confidence interval [CI] [14.2–16.7]), followed by “long-term side effects of treatments” (14.0, CI [12.9–15.2]), “being a burden to others” (9.00, CI [8.02–9.96]), “returning to daily activities” (9.00, CI [8.00–9.94]), “spending too much time in the hospital” (8.40, CI [7.45–9.33]), and “coping with the emotional demands of AML” (8.40, CI [7.42–9.31]). Patients were less worried about “short-term side effects of treatments” (6.70, CI [5.87–7.57]), “the overall financial cost of AML” (6.20, CI [5.34–6.97]), “knowing about all the treatment options” (6.10, CI [5.28–6.91]), and “choosing a treatment in a short amount of time” (6.00, CI [5.18–6.79]). Patients were least worried about “communicating openly with doctors” (2.50, CI [1.97–3.04]), “having access to the best medical care” (3.90, CI [3.29–4.61]), and “having enough information about AML” (4.40, CI [3.66–5.05]). BWS scores were highly correlated with Likert ratings (Spearman’s ρ = 0.93).
FIGURE 2.
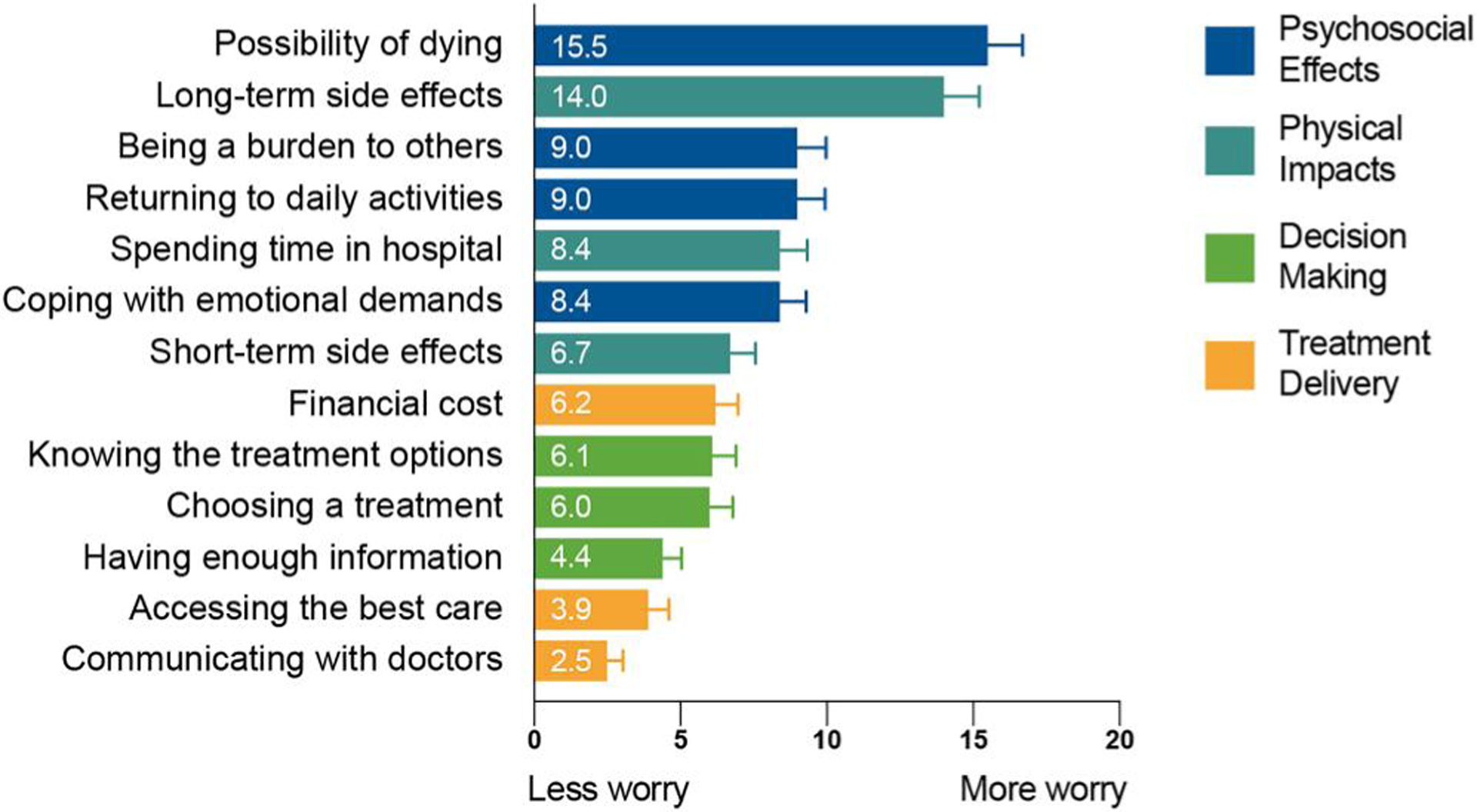
Standardized best–worst scaling scores of the worries of patients with acute myeloid leukemia, by domain (n = 832 patients)
The worries of patients in the caregiver-reported sample were correlated to those in the patient-reported sample (Spearman’s ρ = 0.89). These patients had the same prioritization of the top two and bottom-most items (Figure 3). They had more worry about the possibility of dying, spending time in the hospital, and knowing about all the treatment options (all p ≤ 0.01). They worried less about the long-term side effects and the overall financial cost (both p < 0.001).
FIGURE 3.
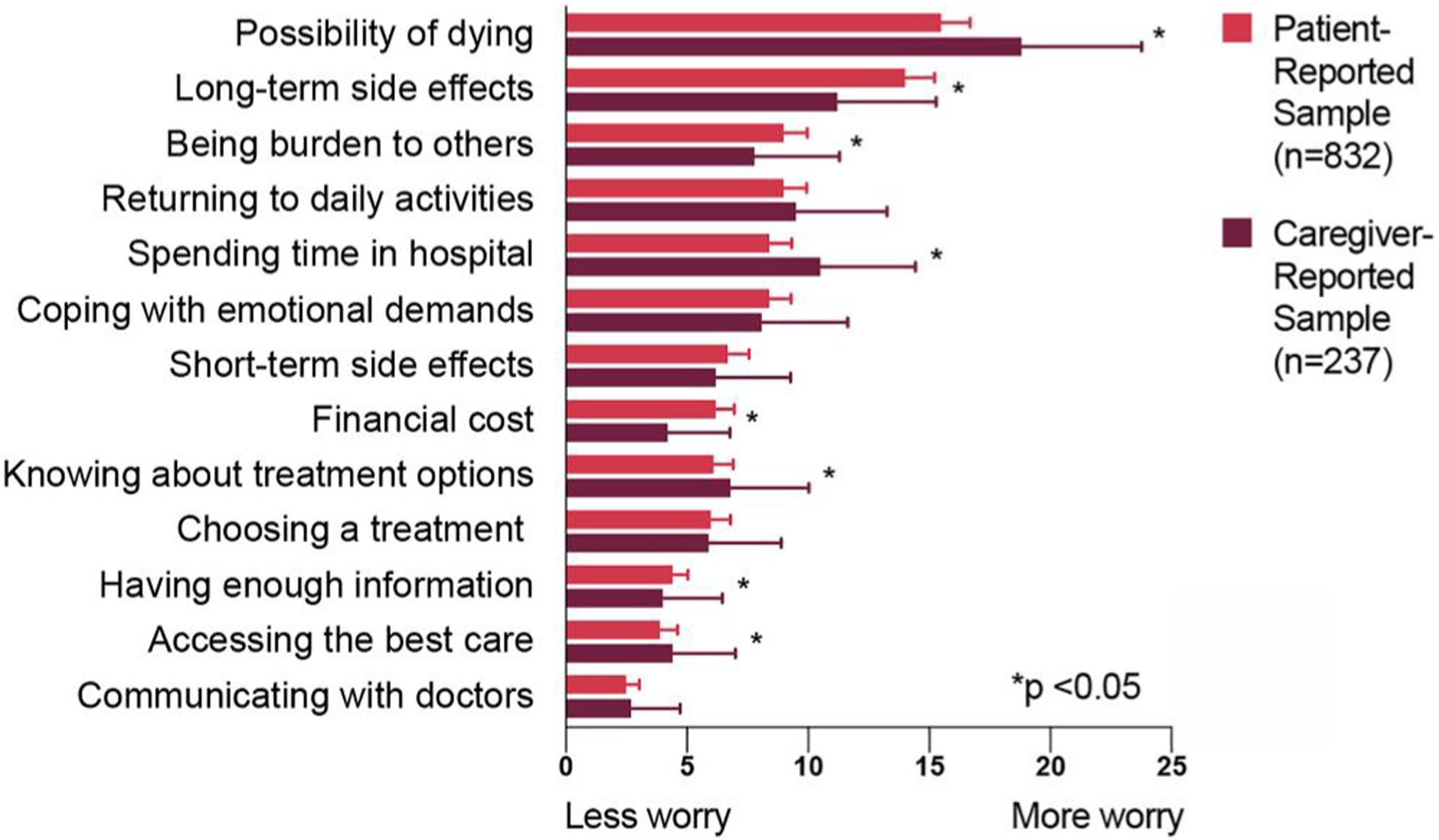
Comparison of standardized best–worst scaling scores of AML patient worries between the patient-reported sample and the caregiver-reported sample (n = 1069). AML, acute myeloid leukemia
On subgroup analysis combining the patient- and caregiver-reported samples, patients who were not in remission had significantly different worries than those in remission (p < 0.001). Notably, they also were most worried about the possibility of dying though to a greater degree (17.4 vs. 16.0, p = 0.045). They worried relatively more about spending time in the hospital (10.7 vs. 8.56, p < 0.001) and knowing the treatment options (7.73 vs. 6.05, p < 0.001). They were less worried about financial costs, and short- and long-term side effects (all p < 0.001).
The prioritization of the top two worries and bottom four worries were identical between long-term survivors (diagnosed over 5 years ago) and those diagnosed more recently. Patients who received an allogeneic HCT were more worried about long-term side effects and returning to daily activities (both p < 0.001).
Non-white patients (n = 132) were more worried about knowing the treatment options and less about short-term side effects, returning to daily activities, and spending time in the hospital (all p ≤ 0.01), although the prioritization of the top two and bottom three worries were identical.
Generally, patients and caregivers found the survey easy to understand (78% and 71%, respectively). Caregivers felt that the survey was more difficult to answer than patients (I found it easy to answer all questions: 64% vs. 54%, p = 0.01).
4 |. DISCUSSION
The diagnosis of leukemia is devastating to patients, bringing with it “incomprehensible shock” and profound psychological distress.26 In addition to acute distress, patients face many unpleasant future outcomes. Most AML patients will die of their disease; most survivors do not return to baseline function following treatment and the majority have chronic long-term side effects.4,27,28 These outcomes, among others such as increasing financial costs and extended time in the hospital, contribute to the worries of patients.20 Understanding and quantifying these worries allows the prioritization of appropriate supportive care and exposes unmet needs.
This is the largest study to date aimed at eliciting and prioritizing the worries of AML patients with over 1000 patients represented, many of whom are long-term survivors and are likely cured. An innovative, iterative community-centered approach was used to engage stakeholders in the development of the BWS instrument.20 As opposed to Likert rankings, which have been well-described to be prone to bias,29,30 BWS allows for increased efficiency in data collection and produces results that allow for meaningful comparison between items.22,24,31 BWS scores were highly correlated with Likert ratings supporting the validity of the method.
Here, we show that patients have two primary worries: death and suffering long-term side effects. These worries far exceed all other items, being roughly 30% more worrisome than the next item (Figure 2). Patients found these items more than twice as worrisome as any item within the domains of care delivery and decision-making including worries about the financial burden of AML.
The FDA strongly advocates for collecting patient experience data from proxies when patients cannot complete assessments.32 We collected the worries of AML patients who were too ill to participate or had passed away through the perspective of their caregivers. While recognizing this introduces a level of concern about the validity of the proxy report, we believed that including the experience of these patients was essential because of the high morbidity and mortality of AML. Failing to capture data from caregivers would exclude a critical demographic of patients and overemphasize the survivor perspective. Clinical features of these patients, many (40%) of whom were deceased, were explicably different than the patient-reported population; many had advanced disease—40% were not in remission and 22% received palliative care (as compared to 3% and 4% respectively in the patient-reported sample). Despite such differences, the worries of these patients were highly correlated to those reported by patients themselves, providing prima facie evidence of content validity of the caregiver report and demonstrating that certain patient worries are shared throughout the disease experience (Figure 3). The stronger worry about spending time in the hospital and less worry about the financial costs could be attributed to changes in worries over the course of the disease. As patients near the end of their life, it is reasonable that they would care less about financial costs and more about spending time at home.33 It is not possible, however, to determine the extent to which the proxy report itself contributed to these differences. Future studies including caregiver/patient pairs could directly measure differences between informants.
4.1 |. Clinical implications
The fact that patients reported worrying about the long-term side effects of treatment nearly as much as dying highlights an important unmet need in our care of AML patients. Mitigating long-term side effects has traditionally not been a primary consideration when choosing induction treatment regimens for adult patients because survival is fundamentally dependent upon achieving remission. “Fit” patients receive intensive induction, and the potential for long-term side effects is considered an unavoidable risk worth taking.34 An unprecedented number of recent drug approvals in AML will allow more consideration to be given to the expected long-term side effects of therapy.35 Serious thought is warranted about how to incorporate the long-term side effect profile of new therapies into shared decision-making (and FDA approvals and drug development) as the potential for long-term symptom burden for newer agents is currently unknown.36 Effective interventions to mitigate the collateral damage from therapy, especially maintaining physical and psychological function, would be significant advances.37,38
Patients worried most about the possibility of dying from their disease. This was reported by patients across demographics and across disease states including those post-HCT. Although largely expected, this result nevertheless raises important questions around how this worry should be addressed. The “existential” nature of this worry cause it to fall outside the traditional purview of the treating oncologist. This may be to the great detriment of patients, however, as the oncologist is the primary coordinator of care for patients. What is left unaddressed by the oncologist likely remains unaddressed by the healthcare system. Consideration should be given to routinely leverage resources such as chaplains or local clergy, palliative care, or psychiatry to engage patients in their greatest worry. Future studies should explore the worries of patients at different disease stages with repeated sampling to guide supportive care.
Overall, patients had relatively stronger worries about items within the two domains of physical impacts and psychosocial effects. Treatment delivery and decision-making were relatively less worrisome. In interpreting the results, it is important to note that BWS allows prioritization among items that may each be valued. Thus, treatment delivery and decision-making may be highly important to patients, but comparatively not as important as, for example, survival or side effects. Therefore, results should not diminish the importance of treatment delivery or decision-making. In fact, recent publications have demonstrated the critical need for improvements in these domains in AML.10,11,39
Patients’ worries should be distinguished from patients’ preferences for outcomes. Worry is often conceived as the emotional response associated with perceived vulnerability about the future. Quantifying preferences, in contrast, requires capturing patients’ risk perception—often conceptualized and measured as if it were a cognitive, rational process—of how they would prefer to trade-off future potential risks and benefits.13–15 This process is critically informed by the emotional feelings of worry about potential outcomes, though conceptually is a distinct, secondary process. Parsing the emotional and rational in real patients is far more complex than making these distinctions. Although assessing patient preferences is arguably the most appropriate way to guide patient-centered care, substantial methodological and practical challenges remain to quantify patient preferences at the time of treatment decision-making.21 Capturing worries, values, or goals may be appropriate surrogates, though further studies are required to demonstrate the relationship between these important domains. Such research is critical to guide clinicians and researchers to know how, and when, patient experience data should be captured to adequately inform patient-centered care and PFDD. Ongoing research into the implementation of stated-preference methods, such as BWS, into the care of individual patients is crucially needed. Individual preference data could inform shared decision-making and the development of personalized decision aids, both of which would be high-quality interventions to improve patient-centered care.
4.2 |. Study limitations
The community-centered multistakeholder development process of the instrument identified the most important worries of AML patients, though not all worries could be included.20 Our recruitment strategy of convenience sampling resulted in a large sample, though a low response rate and a somewhat homogenous population. The sample is younger and less racially diverse than the general AML population. Although this study expands the literature demonstrating differences in worry by race,16 insufficient numbers of individual minority populations limits adequate representation of their experiences. Therefore, application of the data to these populations is speculative. Future studies should intentionally enhance recruitment for underrepresented populations such as racial/ethnic minorities, uninsured and older patients, and those with cognitive impairment. We did not capture income or socioeconomic status. Variation in worry based on these factors, especially about financial costs, may exist and should be explored in future studies. By design, we recruited mostly AML survivors who had experience with the disease, treatment, and long-term side effects. This likely resulted in some selection and recall biases in our data, and future prospective studies are warranted to validate these results. Finally, although stated-preference methods are efficient at establishing statistical differences, these may not represent meaningful differences to patients or clinicians.
5 |. CONCLUSION
The care we deliver to patients is fundamentally effective only to the degree it accomplishes outcomes that are valued by patients. Understanding what matters most to the patient, therefore, is foundational to delivering effective care. This large convenience sample exposes the priorities of AML patients and illustrates areas of unmet need and opportunities for advancing patient-centered care. Worries about dying and enduring long-term side effects are most important to patients and should be among the top priorities to be addressed.
Supplementary Material
ACKNOWLEDGMENTS
This data was presented in part to the FDA on 30 April 2018 and at the American Society of Hematology Annual Conference on 3 December 2018. This work was supported by the Leukemia & Lymphoma Society (J. F. P. Bridges) and through the National Research Service Award Post-Doctoral Traineeship from the Agency for Healthcare Research and Quality (Grant no. 5T32 HS000032-28) (D. R. Richardson).
Funding information
Leukemia and Lymphoma Society; Agency for Healthcare Research and Quality
Footnotes
CONFLICT OF INTERESTS
B. O’Donoghue is employed by the Leukemia & Lymphoma Society. J. F. P. Bridges reports grant support from the Leukemia & Lymphoma Society. The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study was reviewed and approved as a nonhuman subjects research activity by the institutional review board at the Johns Hopkins Bloomberg School of Public Health (#00007200).
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. [DOI] [PubMed] [Google Scholar]
- 2.Acute Myeloid Leukemia. Cancer Stat Facts. Acute Myeloid Leukemia Statistics; 2019. https://seer.cancer.gov/statfacts/html/amyl.html. Accessed November 18, 2018.
- 3.Buckley SA, Jimenez-Sahagun D, Othus M, Walter RB, Lee SJ. Quality of life from the perspective of the patient with acute myeloid leukemia. Cancer. 2018;124(1):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crossnohere NL, Richardson DR, Reinhart C, et al. Side effects from acute myeloid leukemia treatment: results from a national survey. Curr Med Res Opin. 2019:5(11):1965–1970. [DOI] [PubMed] [Google Scholar]
- 5.Johnson FR, Zhou M. Patient preferences in regulatory benefit-risk assessments: a US perspective. Value Health. 2016;19(6):741–745. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services Food and Drug Administration. Prescription drug user fee amendments. https://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/.Accessed December 17, 2018.
- 7.Institute of Medicine (US). Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. http://www.ncbi.nlm.nih.gov/books/NBK222274/. Accessed August 23, 2018. [PubMed] [Google Scholar]
- 8.Levit LA, Balogh E, Nass SJ, Ganz P. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 9.Winer ES, Stone RM. Novel therapy in acute myeloid leukemia (AML): moving toward targeted approaches. Ther Adv Hematol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeBlanc TW, Fish LJ, Bloom CT, et al. Patient experiences of acute myeloid leukemia: a qualitative study about diagnosis, illness understanding, and treatment decision-making. Psychooncology. 2017;26(12):2063–2068. [DOI] [PubMed] [Google Scholar]
- 11.El-Jawahri A, Nelson-Lowe M, VanDusen H, et al. Patient-clinician discordance in perceptions of treatment risks and benefits in older patients with acute myeloid leukemia. Oncologist. 2019;24(2):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efficace F, Gaidano G, Lo-Coco F. Patient-reported outcomes in hematology: is it time to focus more on them in clinical trials and hematology practice? Blood. 2017;130(7):859–866. [DOI] [PubMed] [Google Scholar]
- 13.Worry Sjöberg L. and risk perception. Risk Anal. 1998;18(1):85–93. [DOI] [PubMed] [Google Scholar]
- 14.Zajac LE, Klein WMP, McCaul KD. Absolute and comparative risk perceptions as predictors of cancer worry: moderating effects of gender and psychological distress. J Health Commun. 2006; 11(suppl 1):37–49. [DOI] [PubMed] [Google Scholar]
- 15.McCaul K, Canevello A, Mathwig J, Klein W. Risk communication and worry about breast cancer. Psychol Health Med. 2003;8(4): 379–389. [DOI] [PubMed] [Google Scholar]
- 16.Martin MY, Fouad MN, Oster RA, et al. What do cancer patients worry about when making decisions about treatment? Variation across racial/ethnic groups. Support Care Cancer. 2014;22(1): 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips KM, McGinty HL, Gonzalez BD, et al. Factors associated with breast cancer worry 3 years after completion of adjuvant treatment. Psychooncology. 2013;22(4):936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo J, Smith BD, Estey E, Voyard E, Donoghue BO, Bridges JFP. Developing an instrument to assess patient preferences for benefits and risks of treating acute myeloid leukemia to promote patient-focused drug development. Curr Med Res Opin. 2018;34(12):2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen EM, Benz HL, Tsai J-H, Bridges JF. Identifying and prioritizing concerns associated with prosthetic devices for use in a benefit-risk assessment: a mixed-methods approach. Expet Rev Med Dev. 2018;15(5):385–398. [DOI] [PubMed] [Google Scholar]
- 20.Bridges J, Oakes A, Reinhart CA, Voyard E, O’Donoghue B. Developing and piloting an instrument to prioritize the worries of patients with acute myeloid leukemia. Patient Prefer Adherence. 2018;12:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson DR, Crossnohere NL, Seo J, et al. Age at diagnosis and patient preferences for treatment outcomes in AML: a discrete choice experiment to explore meaningful benefits. Cancer Epidemiol Biomark Prev. 2020;29(5):942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn TN, Louviere JJ, Peters TJ, Coast J. Best–worst scaling: what it can do for health care research and how to do it. J Health Econ. 2007;26(1):171–189. [DOI] [PubMed] [Google Scholar]
- 23.McFadden D Conditional logit analysis of qualitative choice behavior. In: Zarembka P, ed. Frontiers in Econometrics. New York: Academic Press; 1973:105–142. [Google Scholar]
- 24.Cheung KL, Wijnen BFM, Hollin IL, et al. Using best–worst scaling to investigate preferences in health care. Pharmacoeconomics. 2016;34(12):1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawtooth Software Inc. The MaxDiff system technical paper. 2013;21. [Google Scholar]
- 26.Nissim R, Zimmermann C, Minden M, et al. Abducted by the illness: a qualitative study of traumatic stress in individuals with acute leukemia. Leuk Res. 2013;37(5):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korol EE, Wang S, Johnston K, Ravandi-Kashani F, Levis M, van Nooten F. Health-related quality of life of patients with acute myeloid leukemia: a systematic literature review. Oncol Ther. 2017;5(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redaelli A, Stephens JM, Brandt S, Botteman MF, Pashos CL. Short- and long-term effects of acute myeloid leukemia on patient health-related quality of life. Cancer Treat Rev. 2004;30(1):103–117. [DOI] [PubMed] [Google Scholar]
- 29.Blaikie N Analyzing Quantitative Data. London: SAGE Publications Ltd; 2003. http://methods.sagepub.com/book/analyzing-quantitative-data. Accessed July 15, 2013. [Google Scholar]
- 30.Douven I A Bayesian perspective on Likert scales and central tendency. Psychon Bull Rev. 2018;25(3):1203–1211. [DOI] [PubMed] [Google Scholar]
- 31.Lee JA, Soutar G, Louviere J. The best-worst scaling approach: an alternative to Schwartz’s values survey. J Pers Assess. 2008;90(4):335–347. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services Food and Drug Administration. Patient-Focused Drug Development: Collecting Comprehensive and Representative Input; 2018. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm610442.pdf. Accessed March 19, 2019.
- 33.Anderson WG, Chase R, Pantilat SZ, Tulsky JA, Auerbach AD. Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93(10):1267–1291. [DOI] [PubMed] [Google Scholar]
- 36.Williams LA, Yucel E, Cortes JE, Cleeland CS. Measuring symptoms as a critical component of drug development and evaluation in hematological diseases. Clin Invest. 2013;3(12):1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghodraty-Jabloo V, Alibhai SMH, Breunis H, Puts MTE. Keep your mind off negative things: coping with long-term effects of acute myeloid leukemia (AML). Support Care Cancer. 2016;24(5):2035–2045. [DOI] [PubMed] [Google Scholar]
- 38.Storey S, Gray TF, Bryant AL. Comorbidity, physical function, and quality of life in older adults with acute myeloid leukemia. Curr Geriatr Rep. 2017;6(4):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loh KP, Abdallah M, Kadambi S, et al. Treatment decision-making in acute myeloid leukemia: a qualitative study of older adults and community oncologists. Leuk Lymphoma. 2021;62(2):387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


