Abstract
During 2007–2019, the percentage of HIV Outpatient Study participants reporting anal or vaginal condomless sex in the past 6 months ranged from a low of 17% among heterosexual males to 59% for men who have sex with men (MSM). MSM reported having had condomless sex more frequently than heterosexual males and females and were the only group in which an increase in condomless sex was observed during the study period (from 39 to 59%). Although persons with undetectable HIV viral load have effectively no risk of transmitting HIV sexually (U = U), there is still the potential risk of transmission or acquisition of other sexually transmitted infections (STIs) when engaging in condomless sex. Continuing education about risks of HIV and STI transmission as well as ongoing screening for and treatment of STIs, retention in HIV treatment, and support for sexual health are critical components of care for people living with HIV.
Keywords: HIV, Risk behavior, Longitudinal, Condomless sex
Introduction
Approximately 80% of annual HIV infections are transmitted by individuals not in care [1]. Improved levels of HIV viral suppression and reductions in anal/vaginal condomless sex among people with HIV (PWH) may substantially decrease the rates of transmission for HIV [2, 3] and other sexually transmitted infections (STIs) [4], contributing to the national goal for reducing new HIV infections outlined in the federal “Ending the HIV Epidemic” initiative and the National HIV/AIDS Strategy [1, 5–7]. Reported rates of condomless sex among PWH, including gay, bisexual and other men who have sex with men (MSM) with HIV, vary widely depending on the populations assessed, specific practices, and time frame for the data recorded, anywhere from 13 to 66% in some studies [8–11]. The findings from the HPTN052 study [12] and subsequent research [13] affirmed HIV treatment as prevention: PWH on antiretroviral therapy (ART) with an undetectable viral load have virtually no risk of sexually transmitting HIV, also referred to as U = U or undetectable equals untransmittable [7]. Across the age spectrum, sexually active people [14, 15] who have a detectable HIV viral load, however, remain at risk of transmitting HIV to their sexual partners if engaging in condomless sex, including rare instances of superinfection [16, 17]. HIV prevention efforts now also include biomedical-prevention strategies such as post-exposure prophylaxis (PEP) and pre-exposure prophylaxis (PrEP) [18–20] which are recommended for persons without HIV who are unable to use condoms or who use them inconsistently.
The characteristics and risk factors for engaging in condomless sex have been extensively studied among people without HIV. However, there are limited data exploring condomless sex behaviors and their changes over time among PWH who are under regular medical care, especially women and heterosexual men. Identifying and characterizing factors that may be associated with increases or decreases in condomless sex among PWH may lead to the development and implementation of improved interventions for reducing HIV and STI transmission [1, 21]. Using data from the HIV Outpatient Study (HOPS), we sought to address this gap in the literature and (1) determine if there were significant changes in self-reported condomless sex behaviors over time among sexually active PWH under regular medical care; and (2) explore patient- and treatment-related factors associated with these changes.
Methods
The HIV Outpatient Study (HOPS)
The HOPS is an ongoing prospective observational cohort study of adults 18 years and older diagnosed with HIV, receiving care at eight participating HIV clinics (university-based, public and private) in six U.S. cities (Chicago, IL; Denver, CO; Stony Brook, NY; Philadelphia, PA; Tampa, FL; and Washington, DC) since 1993 [22]. The HOPS is an open cohort: patients may enter the study at any point after a diagnosis of HIV infection regardless of treatment history and may leave the study at any point for a variety of reasons such as patient request or loss to follow-up [23]. Through December 2019, the HOPS had collected information on over 11,250 patients from more than 584,000 clinical encounters. Patient data, including sociodemographic characteristics, diagnoses, ART use and other treatments, and laboratory values (including CD4+ lymphocyte cell counts/mm3 [CD4 count] and plasma HIV RNA levels (viral load, HIV VL) are abstracted from medical charts and entered into an electronic database by trained medical record abstractors with backgrounds in nursing or other healthcare-related fields. Since 2007, the HOPS has employed an optional supplemental survey for patients to also gather self-reported information on sociodemographic and behavioral measures, including sexual behaviors, substance use and adherence to ART. The survey is conducted via telephone or web-based audio-computer assisted self-interview (ACASI). All data are reviewed for quality and analyzed centrally.
Ethics
Since its inception, the HOPS protocol has been reviewed and approved annually by CDC’s (Atlanta, GA, USA), Cerner Corporation’s (Kansas City, MO, USA) and each local site’s institutional review board. All participants provided written informed consent. The study protocol conforms to the regulations of the U.S. Department of Health and Human Services for the protection of human subjects in research.
Study Population
We analyzed medical records data from HOPS participants who received HIV care at eight U.S. HIV clinics during March 2007 through December 2019. Participants who completed at least one ACASI and reported sexual activity beyond hugging or kissing in at least one ACASI were included in the analysis. The start of observation was considered to be the date of the first ACASI taken, hereafter referred to as the baseline date. Study observation was discontinued after the date of the last ACASI completed by each participant. A total of 2193 participants were included in this study, contributing a total of 5214 completed ACASI observations.
Measurements and Definitions
Baseline demographic variables included age at survey date, sex at birth, race/ethnicity (non-Hispanic/Latino Black or African American [Black], non-Hispanic/Latino white, Hispanic/Latino or other/unknown race/ethnicity), and HIV transmission risk (MSM, heterosexual males and females). Updated variables in the modeling analyses included CD4 count and HIV VL as well as type of insurance from the medical records, and from the ACASI survey the following items: sexual activity in the past 6 months, recreational drug use, cannabis use, alcohol use, and cigarette smoking. CD4 count was categorized as < 200, 200–349, 350–499 and ≥ 500 cells/mm3. Plasma HIV VL was characterized as undetectable or detectable, where undetectable was a VL test result less than the lower limit of detectability for the particular test used. Years elapsed since ACASI was first instituted in the HOPS (March 15, 2007) was also used as an explanatory measure, which enabled us to evaluate temporal trends in sexual behavior over time. Primary endpoint of interest was having reported anal or vaginal condomless sex during the past 6 months on an ACASI survey. Secondary endpoints of interest were anal or vaginal condomless sex with partners of HIV-negative or unknown HIV status during the past 6 months.
Statistical Analyses
Descriptive summaries of the data were generated for single-time observations per subject at baseline. Categorical and continuous measures were compared using the likelihood ratio chi-square test, and Wilcoxon rank-sum test, respectively. Expected cell counts for this test were verified to be at least five for at least 80% of cells for each of these tests. Generalized linear mixed model analyses (GLMMs) were used to evaluate longitudinal trends. To account for repeated measures, we used random intercept terms that estimated a best linear unbiased predictor for each subject for these models. Our model building procedure was as follows. Initial univariable GLMMs were estimated, adjusted for observation time. Next, full multivariable models were generated including all factors from univariable models. The final multivariable models were then created by removing all factors with P values > 0.05 when adjusted for other factors in the full multivariable model step. Because of our primary interest in studying temporal trends, time was also included in each model regardless of statistical significance. Separate models were estimated for MSM, heterosexual males, and females due to substantial observed differences in descriptive plots of our endpoints over time. Based on the descriptive plots, we explored trends of the annual percentages of condomless sex overall and condomless sex with partners of negative or unknown HIV status during 2008–2011 and 2011–2019, as during 2011, the percentage of MSM reporting condomless sex was at a nadir. These time windows corresponded to the period before and after the release of the results of HPTN052 study which opened the era of HIV treatment as prevention [12, 23]. For these explorations we estimated slopes using simple linear regression with the descriptive percentages (unadjusted) as the response. Due to the smaller number of ACASIs completed during 2007, the 1st year they were offered, 2007 was not included in these linear regressions. We used SAS version 9.4 (SAS Institute, Cary, NC) for our analyses. Results with P values < 0.05 were considered statistically significant.
Results
Among the 11,253 participants enrolled in HOPS as of December 31, 2019 (Fig. 1), we identified 2856 participants who completed one or more ACASI assessments and had at least one VL test result recorded. Of these, 2193 reported sexual activity beyond hugging or kissing in at least one ACASI and were included in the analysis. Of these participants, 65% (N = 1429) were MSM, 16% (N = 352) were heterosexual males, and 19% (N = 428) were female (Table 1). Overall, the median age at first assessment was 45 years (interquartile range [IQR] 39–52 years), 54.5% (N = 1195) were non-Hispanic/Latino white, 30.8% (N = 675) non-Hispanic/Latino Black, 11.3% (N = 248) Hispanic/Latino and 3.4% (N = 75) were persons of other or unknown race/ethnicity. Median follow-up for those who completed > 1 ACASI was 4.6 years (IQR 2.6–6.4 years). In terms of the number of surveys completed during 2007–2019, 864 (39%) participants contributed only one ACASI, 498 (23%) contributed two, 301 (14%) contributed three, 199 (9%) contributed four, and 331 (15%) contributed five or more ACASIs.
Fig. 1.
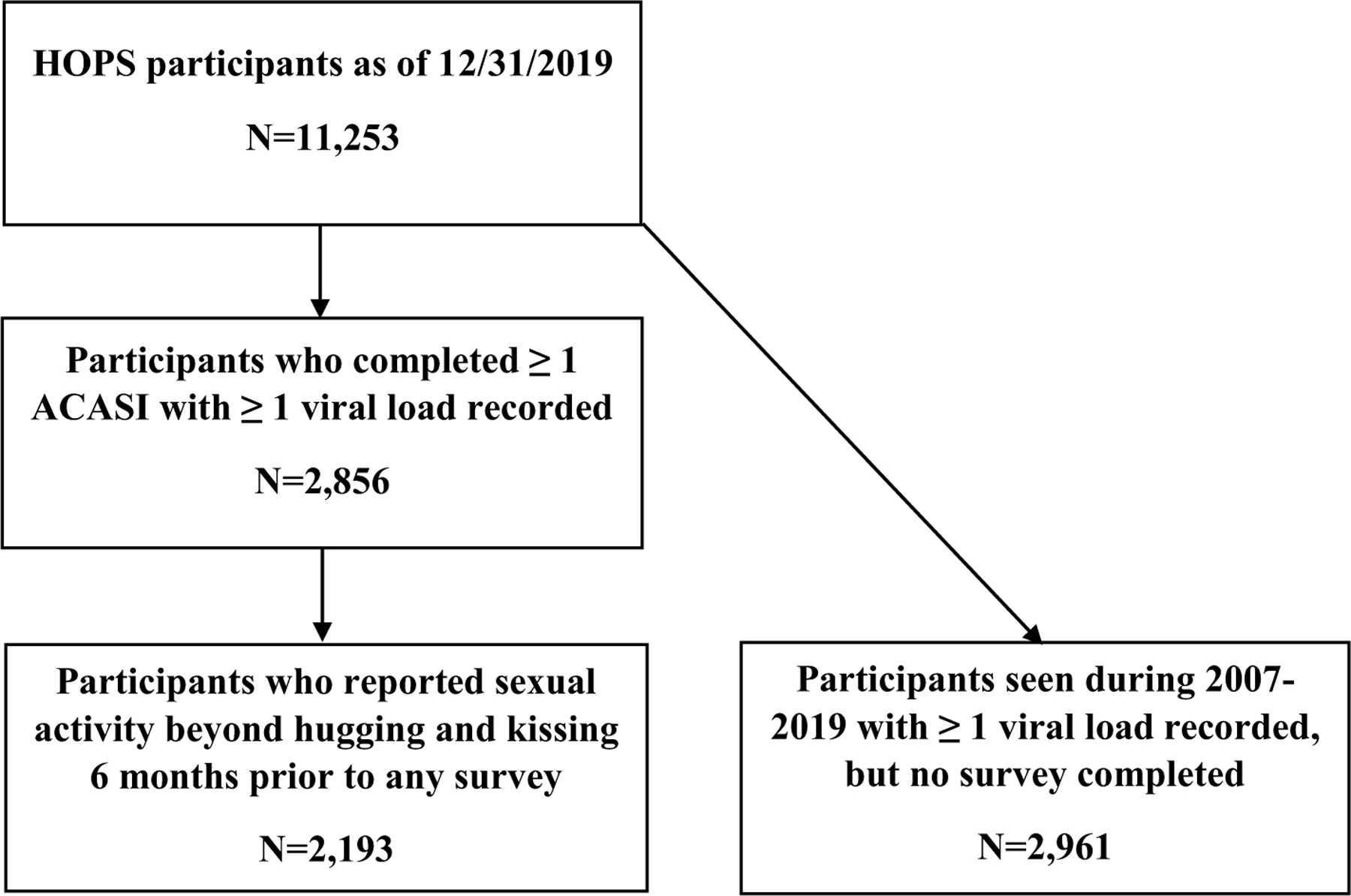
Selection steps outlining patients included in the current analysis, HIV Outpatient Study 2000–2019, USA, (N = 2193)
Table 1.
Characteristics at first assessment among patients with one or more Audio Computer Assisted Self Interview, HIV Outpatient Study, USA, 2007–2019 (N = 2193)
| Patient characteristics at first ACASI completed: N (%) or median (IQR) |
MSM N = 1429 (65.2%) |
Heterosexual males N = 352 (16.0%) |
Females N = 412 (18.8%) |
P value | Chi-square‡ | All participants N = 2193 (100.0%) |
|---|---|---|---|---|---|---|
| Year range first ACASI completed | < 0.001 | 25.5 | ||||
| 2007–2010 | 889 (62.2) | 196 (55.7) | 240 (58.3) | 1325 (60.4) | ||
| 2011–2014 | 326 (22.8) | 114 (32.4) | 131 (31.8) | 571 (26.1) | ||
| 2015–2019 | 214 (15.0) | 42 (11.9) | 41 (10.0) | 297 (13.5) | ||
| Age group (years) at first ACASI | < 0.001 | 27.4 | ||||
| ≤ 29 | 120 (8.4) | 19 (5.4) | 30 (7.3) | 169 (7.7) | ||
| 30–39 | 279 (19.5) | 49 (13.9) | 102 (24.8) | 430 (19.6) | ||
| 40–49 | 552 (38.6) | 145 (41.2) | 173 (42.0) | 870 (39.7) | ||
| ≥ 50 | 478 (33.5) | 139 (39.5) | 107 (26.0) | 724 (33.0) | ||
| Median age (IQR) at first ACASI | 45 (39–52) | 48 (42–54) | 43 (38–50) | < 0.001 | 30.5 | 45 (39–52) |
| Race/ethnicity | < 0.001 | 439.2 | ||||
| White, non-Hispanic/Latino | 998 (69.8) | 96 (27.3) | 101 (24.5) | 1195 (54.5) | ||
| Black, non-Hispanic/Latino | 251 (17.6) | 179 (50.9) | 245 (59.5) | 675 (30.8) | ||
| Hispanic/Latino | 134 (9.4) | 60 (17.0) | 54 (13.1) | 248 (11.3) | ||
| Other/unknown | 46 (3.2) | 17 (4.8) | 12 (2.9) | 75 (3.4) | ||
| Insurance payer at first ACASI | < 0.001 | 324.9 | ||||
| Private | 989 (69.2) | 122 (34.7) | 106 (25.7) | 1217 (55.5) | ||
| Public/None | 440 (30.8) | 230 (65.3) | 306 (74.3) | 976 (44.5) | ||
| CD4 cell count (cells/mm3)* | < 0.001 | 70.9 | ||||
| < 200 | 86 (6.0) | 45 (12.8) | 55 (13.3) | 186 (8.5) | ||
| 200–349 | 179 (12.5) | 75 (21.3) | 65 (15.8) | 319 (14.5) | ||
| 350–499 | 284 (19.9) | 69 (19.6) | 58 (14.1) | 411 (18.7) | ||
| ≥ 500 | 835 (58.4) | 143 (40.6) | 217 (52.7) | 1195 (54.5) | ||
| Unknown/Missing | 45 (3.2) | 20 (5.7) | 17 (4.1) | 82 (3.7) | ||
| HIV RNA viral load* | < 0.001 | 39.3 | ||||
| Undetectable | 1063 (74.4) | 228 (64.8) | 246 (59.7) | 1537 (70.1) | ||
| Detectable | 319 (22.3) | 105 (29.8) | 149 (36.2) | 573 (26.1) | ||
| Missing | 47 (3.3) | 19 (5.4) | 17 (4.1) | 83 (3.8) | ||
| Any sex, prior 6 months | < 0.001 | 27.4 | ||||
| Yes | 1329 (93.0) | 304 (86.4) | 353 (85.7) | 1986 (90.6) | ||
| No | 100 (7.0) | 48 (13.6) | 59 (14.3) | 207 (9.4) | ||
| Recreational drug use, past 6 months† | ||||||
| Poppers | 462 (32.3) | 23 (6.5) | 5 (1.2) | < 0.001 | 307.3 | 490 (22.3) |
| Injection drug use | 46 (3.2) | 8 (2.3) | 6 (1.5) | 0.10 | 4.5 | 60 (2.7) |
| Heroin | 7 (0.5) | 15 (4.3) | 9 (2.2) | < 0.001 | 26.5 | 31 (1.4) |
| Methamphetamine | 125 (8.7) | 10 (2.8) | 3 (0.7) | < 0.001 | 56.2 | 138 (6.3) |
| Club drugs | 86 (6.0) | 5 (1.4) | 3 (0.7) | < 0.001 | 38.0 | 94 (4.3) |
| Cocaine | 113 (7.9) | 31 (8.8) | 37 (9.0) | 0.72 | 0.6 | 181 (8.3) |
| Erectile dysfunction drugs, past 6 months (whether prescribed or not) | < 0.001 | 196.9 | ||||
| Yes | 398 (27.9) | 73 (20.7) | 4 (1.0) | 475 (21.7) | ||
| No | 1031 (72.2) | 279 (79.3) | 408 (99.0) | |||
| Cannabis use, past 6 months† | < 0.001 | 14.9 | ||||
| Yes | 479 (33.5) | 101 (28.7) | 99 (24.0) | 679 (31.0) | ||
| No | 950 (66.5) | 251 (71.3) | 313 (76.0) | 1514 (69.0) | ||
| Alcohol use, prior 30 days | < 0.001 | 117.4 | ||||
| Yes | 1113 (77.9) | 212 (60.2) | 215 (52.2) | 1540 (70.2) | ||
| No | 316 (22.1) | 140 (39.8) | 197 (47.8) | 653 (29.8) | ||
| Current cigarette smoker | < 0.001 | 94.7 | ||||
| Yes | 316 (22.1) | 145 (41.2) | 177 (43.0) | 638 (29.1) | ||
| No | 1113 (77.9) | 207 (58.8) | 235 (57.0) | 1555 (70.9) |
P values are for the comparison of the three groups: MSM, heterosexual males, and females. Specific recreational substances use only show those who answered “yes” to their use. Other responses not shown would include those who answered “no” or did not answer the question regarding the specific substance in question
ACASI audio-computer assisted self-interview; ART antiretroviral therapy; IQR interquartile range; MSM men who have sex with men; PWID persons who inject drugs
Closest CD4 or viral load measurement within 365 days of date of ACASI survey, obtained from the medical records
During the 6 months preceding date of ACASI survey
Test statistic is likelihood ratio chi-square for categorical variables, and Kruskal–Wallis chi-square for continuous variables
Participants who did not complete any ACASIs (n = 2961) and were thus excluded from the analyses, were more likely to be > 40 years of age, not have MSM HIV risk, not be of white non-Hispanic/Latino race/ethnicity, not be privately insured, have detectable HIV viral load, and become inactive or deceased during the study period compared with those who completed one or more than one ACASI (see Supplemental Table 1).
In terms of the characteristics of the three demographic subsets of participants we studied (Table 1), compared with heterosexual males and females, MSM were more likely to have completed the assessment during 2007–2010, be white, non-Hispanic/Latino persons, be privately insured, have CD4 count ≥ 500 cells/mm3, have undetectable viral load, have had any sex beyond hugging and kissing, used poppers, methamphetamine, club drugs, erectile dysfunction drugs (EDD), cannabis or alcohol, but less likely to use heroin or be a current smoker.
Trends in Condomless Sex Over Time
The percentage of ACASI surveys indicating anal or vaginal condomless sex in the past 6 months by HIV transmission risk group and calendar year ranged from a low of 17% among heterosexual males in 2012 to 59% for MSM in 2017 (Fig. 2a). MSM reported having had condomless sex more frequently than heterosexual males and females, and that trend held over time. Additionally, the levels of condomless sex among females and heterosexual males tended to be similar over time. Using general linear modeling to look at years 2008–2011 and 2011–2019, only among MSM did the percentages of those engaging in either condomless sex overall or condomless sex with partners of negative or unknown HIV status, change over time. During 2008–2011, the estimated rate ratios for MSM were 0.83 per calendar year (95% confidence interval [CI] 0.74–0.93) and 0.77 (CI 0.69–0.87) per calendar year for engaging in condomless sex overall or condomless sex with partners of negative or unknown HIV status, respectively, indicating a temporal decrease in these activities during this period. In contrast, during 2011–2019, the estimated rate ratios for MSM were 1.53 per calendar year (CI 1.26–1.86) and 1.65 (CI 1.34–2.03) for engaging in condomless sex overall or condomless sex with partners of negative or unknown HIV status, respectively, indicating an increase in these activities during this period (Fig. 2a, b).
Fig. 2.
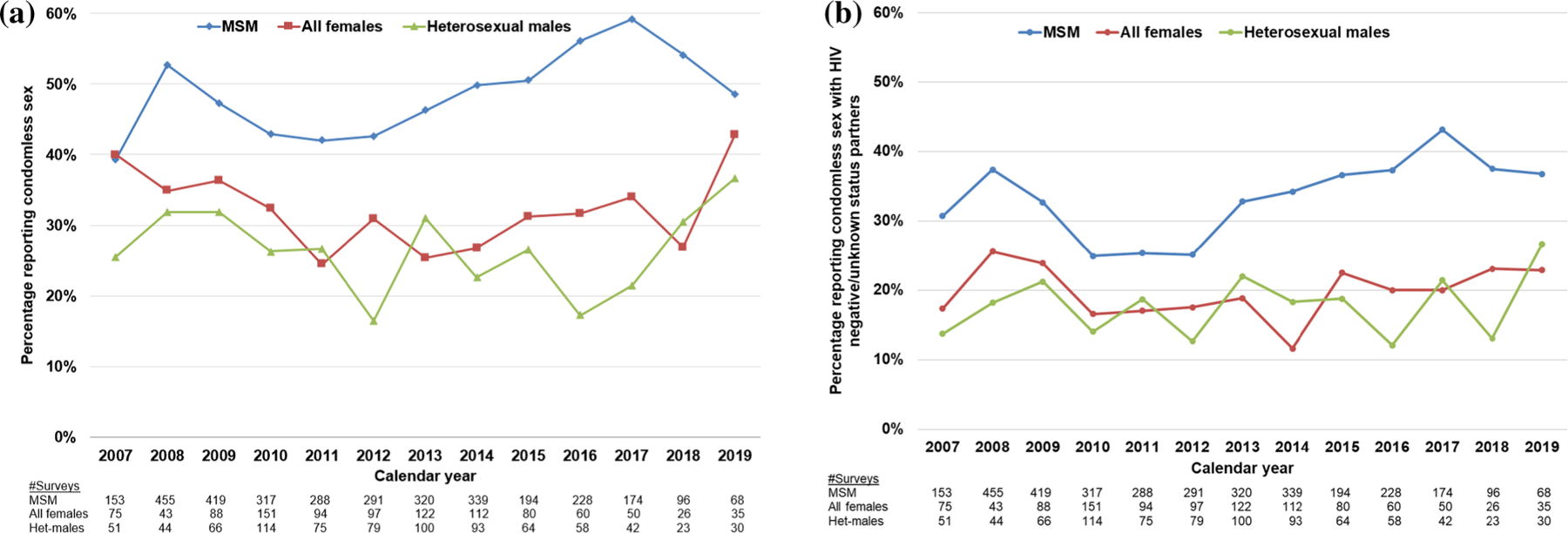
Reported frequency of condomless anal or vaginal sex by sociodemographic group and calendar year with a partners of any HIV status and b partners of HIV negative/unknown status, HIV Outpatient Study, USA, 2007–2019 (N = 2193). a Linear regressions of the frequency of condomless sex by MSM during 2008–2011 and 2011–2019 were the following: 0.83 (95% CI 0.74–0.93, P = 0.001), and 1.53 (95% CI 1.26–1.86, P < 0.001), respectively. Similar linear regression results for heterosexual males and all females were not statistically significant. Het heterosexual; MSM men who have sex with men. b Linear regressions of the frequency of condomless sex with partners of negative or unknown HIV status by MSM during 2008–2011 and 2011–2019 were the following: 0.77 (95% CI 0.69–0.87, P < 0.001), and 1.65 (95% CI 1.34–2.13, P < 0.001), respectively. Similar linear regression results for heterosexual males and all females were not statistically significant
Among 1429 MSM who completed two or more ACASI surveys, 370 (26%) reported having had condomless sex in the past 6 months on all of their surveys, 743 (52%) reported not having had condomless sex on all surveys, and 316 (22%) had mixed responses. Among 352 heterosexual males who had two or more ACASI surveys, 39 (11%) reported having had condomless sex in the past 6 months on all of their surveys, 248 (70%) reported not having had condomless sex on all surveys, and 65 (18%) had mixed responses. Among 412 female participants who had two or more ACASI surveys, 51 (12%) reported having had condomless sex in the past 6 months on all of their surveys, 277 (67%) reported not having had condomless sex on all surveys, and 84 (20%) had mixed responses.
Correlates of Condomless Sex in Longitudinal Analyses
In the final multivariable GLMM analysis, among 1429 MSM who completed a total of 3342 surveys (Table 2), the overall frequency of reporting condomless sex increased over time (odds ratio [OR] 1.06 per year, 95% CI 1.03–1.09). Younger MSM had substantially higher odds of having had condomless sex than those aged 50 and older (Table 2). Other independent correlates of condomless sex included having private rather than public insurance (OR 1.74), using cocaine (OR 2.42), poppers (OR 3.41), methamphetamine (OR 2.99), EDD (OR 2.49), injecting drugs (OR 2.43), and using alcohol (OR 1.34). MSM with CD4 counts in all strata < 500 cells/mm3 were less likely to have had condomless sex than those with CD4 ≥ 500 cells/mm3 (Table 2). As compared with non-Hispanic/Latino white MSM, those of Hispanic/Latino race/ethnicity were less likely to have had condomless sex (OR 0.59), and those of non-Hispanic/Latino Black MSM did not differ in their odds of engaging in that behavior after adjusting for other covariates.
Table 2.
Repeated measures GLMM analyses of factors associated with condomless sex among MSM (N = 1429 participants and 3342 ACASIs), HIV Outpatient Study, USA, 2007–2019
| Participant characteristics | Univariable OR (95% CI) | Full multivariable OR (95% CI) | Final multivariable OR (95% CI) | |
|---|---|---|---|---|
| Age, years | ||||
| ≤ 29 | 3.40 (2.18–5.29)** | 11.85 (5.79–24.24)** | 5.36 (3.29–8.73)** | |
| 30–39 | 2.70 (2.01–3.65)** | 5.33 (3.31–8.58)** | 3.38 (2.43–4.70)** | |
| 40–49 | 2.10 (1.67–2.63)** | 3.24 (2.31–4.54)** | 2.32 (1.82–2.96)** | |
| ≥ 50 | Referent | Referent | Referent | |
| Race/ethnicity | ||||
| White, non-Hispanic/Latino | Referent | Referent | Referent | |
| Black, non-Hispanic/Latino | 0.69 (0.51–0.93)* | 0.76 (0.47–1.25) | 0.86 (0.62–1.20) | |
| Hispanic/Latino | 0.67 (0.46–0.98)* | 0.48 (0.26–0.86)* | 0.59 (0.39–0.88)* | |
| Other/Unknown | 1.23 (0.64–2.35) | 1.17 (0.44–3.11) | 1.09 (0.55–2.13) | |
| Insurance | ||||
| Private | 2.12 (1.67–2.68)** | 2.46 (1.65–3.65)** | 1.74 (1.35–2.26)** | |
| Public/none | Referent | Referent | Referent | |
| CD4 cell count (cells/mm3) | ||||
| < 200 | 0.50 (0.32–0.78)* | 0.48 (0.24–0.96)* | 0.60 (0.37–0.98)* | |
| 200–349 | 0.50 (0.37–0.69)** | 0.38 (0.24–0.61)** | 0.52 (0.37–0.72)** | |
| 350–499 | 0.77 (0.60–0.98)* | 0.64 (0.45–0.91)* | 0.73 (0.56–0.95)* | |
| ≥ 500 | Referent | Referent | Referent | |
| Unknown/Missing | 0.97 (0.47–1.98) | 2.21 (0.23–21.21) | 0.98 (0.45–2.13) | |
| HIV RNA viral load (copies/mL) | ||||
| Undetectable | 1.08 (0.86–1.35) | 1.00 (0.72–1.40) | ||
| Detectable | Referent | Referent | ||
| Unknown/Missing | 1.11 (0.53–2.32) | 0.41 (0.04–4.01) | ||
| Cocaine | 3.49 (2.29–5.30)** | 3.54 (1.88–6.68)** | 2.42 (1.53–3.83)** | |
| Poppers | 12.38 (8.56–17.91)** | 6.05 (4.22–8.67)** | 3.41 (2.66–4.37)** | |
| Injection drug use | 5.84 (2.98–11.45)** | 3.46 (1.20–9.97)* | 2.43 (1.09–5.44)* | |
| Methamphetamine | 8.20 (5.19–12.94)** | 4.73 (2.30–9.76)** | 2.99 (1.78–5.01)** | |
| Club drugs | 6.44 (3.62–11.45)** | 1.76 (0.75–4.15) | ||
| Erectile dysfunction drugs | 2.87 (2.30–3.58)** | 3.62 (2.58–5.07)** | 2.49 (1.96–3.18)** | |
| Cannabis use | 1.77 (1.43–2.18)** | 1.45 (1.05–2.00)* | 1.24 (0.99–1.56) | |
| Alcohol use | 1.71 (1.37–2.15)** | 1.54 (1.11–2.15)* | 1.34 (1.05–1.70)* | |
| Current cigarette smoker | 1.28 (0.99–1.64) | 1.35 (0.92–1.97) | ||
| Time since 3/15/2007, per year | 1.08 (1.03–1.12)** | 1.06 (1.03–1.09)** | ||
CI confidence interval; GLMM generalized linear mixed model analyses; MSM men who have sex with men; OR odds ratio
Indicates P-value < 0.05 and
indicates P-value < 0.001
Among 353 heterosexual males who completed a total of 844 surveys (Table 3), engagement in condomless sex did not change over time (OR 1.03 per year; 95% CI 0.97–1.10). Those who were more likely to engage in condomless sex included participants who were 30–39 (OR 2.32) and 40–49 (OR 1.66) years old as compared with older, and those reporting use of cocaine (OR 2.75), poppers (OR 3.90), or EDD (OR 2.71). Heterosexual men of Black race (OR 0.51) were less likely to report engaging in condomless sex over time than heterosexual white men.
Table 3.
Repeated measures GLMM analyses for heterosexual males, with outcome being condomless-sex (N = 353 participants and 844 ACASIs), HIV Outpatient Study, USA, 2007–2019
| Participant Characteristics | Univariable OR (95% CI) | Full multivariable OR (95% CI) | Final multivariable OR (95% CI) |
|---|---|---|---|
| Age, years | |||
| ≤ 29 | 1.78 (0.60–5.25) | 2.62 (0.57–12.01) | 2.55 (0.86–7.54) |
| 30–39 | 1.72 (0.87–3.39) | 3.08 (1.16–8.18)* | 2.32 (1.16–4.63)* |
| 40–49 | 1.59 (1.00–2.52)* | 2.07 (1.08–3.98)* | 1.66 (1.03–2.67)* |
| ≥ 50 | Referent | Referent | Referent |
| Race/Ethnicity | |||
| White, non-Hispanic/Latino | Referent | Referent | Referent |
| Black, non-Hispanic/Latino | 0.48 (0.28–0.81)** | 0.39 (0.17–0.91)* | 0.51 (0.29–0.87)* |
| Hispanic/Latino | 0.67 (0.34–1.34) | 0.64 (0.22–1.84) | 0.68 (0.34–1.37) |
| Other/Unknown | 0.88 (0.29–2.63) | 0.79 (0.15–4.23) | 0.71 (0.23–2.24) |
| Insurance | |||
| Private | 1.30 (0.81–2.10) | 0.83 (0.38–1.83) | |
| Public/None | Referent | Referent | |
| CD4 cell count (cells/mm3) | |||
| < 200 | 0.63 (0.29–1.39) | 0.53 (0.17–1.63) | |
| 200–349 | 0.82 (0.47–1.45) | 0.70 (0.32–1.54) | |
| 350–499 | 1.03 (0.62–1.72) | 1.09 (0.55–2.18) | |
| ≥ 500 | referent | referent | |
| Unknown/Missing | 0.58 (0.18–1.90) | † | |
| HIV RNA viral load (copies/mL) | |||
| Undetectable | 0.95 (0.61–1.47) | 0.91 (0.50–1.68) | |
| Detectable | referent | ||
| Unknown/Missing | 0.65 (0.19–2.22) | † | |
| Cocaine | 3.59 (1.21–10.63)* | 3.39 (1.12–10.31)* | 2.75 (1.27–5.96)* |
| Poppers | 4.75 (2.15–10.50)** | 5.03 (1.63–15.53)* | 3.90 (1.72–8.84)* |
| Injection drug use | 1.30 (0.38–4.52) | 1.09 (0.17–7.17) | |
| Methamphetamine | 3.99 (1.10–14.53)* | 1.73 (0.20–15.25) | |
| Club drugs | 8.24 (1.05–64.91)* | 1.12 (0.08–16.30) | |
| Erectile dysfunction drugs | 4.47 (2.22–9.00)** | 3.66 (1.84–7.26)** | 2.71 (1.64–4.46)** |
| Cannabis use | 1.76 (1.13–2.74)* | 1.69 (0.87–3.28) | |
| Alcohol use | 1.57 (1.03–2.38)* | 1.33 (0.72–2.44) | |
| Current cigarette smoker | 0.82 (0.53–1.26) | 0.68 (0.36–1.26) | |
| Time since 3/15/2007, per year | 1.03 (0.94–1.12) | 1.03 (0.97–1.10) |
CI confidence interval; GLMM generalized linear mixed model analyses; OR odds ratio
Indicates P-value < 0.05 and
indicates P-value < 0.001
Participant numbers too small to calculate ORs and CIs in the full multivariable analyses
For 411 females who completed a total of 1028 surveys (Table 4), engagement in condomless sex did not change over time (OR 0.99 per year; 95% CI 0.94–1.04). Those who were more likely to engage in condomless sex over the period of observation included females who were ≤ 29 years of age (OR 3.72), 30–39 (OR 2.42) and 40–49 (OR 1.68) as compared with older, and those reporting use of cannabis (OR 1.67) or alcohol (OR 1.79).
Table 4.
Repeated measures GLMM analyses for females, with outcome being condomless-sex (N = 411 participants and 1028 ACASIs)
| Univariable OR (95% CI) | Full multivariable OR (95% CI) | Final multivariable OR (95% CI) | |
|---|---|---|---|
| Age, years | |||
| ≤ 29 | 4.64 (2.07–10.37)** | 7.67 (2.42–24.33)** | 3.72 (1.65–8.39)* |
| 30–39 | 2.93 (1.72–4.98)** | 3.19 (1.53–6.68)* | 2.42 (1.42–4.14)* |
| 40–49 | 1.93 (1.25–2.98)* | 1.91 (1.06–3.44)* | 1.68 (1.08–2.61)* |
| ≥ 50 | Referent | Referent | Referent |
| Race/Ethnicity | |||
| White, non-Hispanic/Latino | Referent | ||
| Black, non-Hispanic/Latino | 0.96 (0.61–1.51) | 0.99 (0.51–1.90) | |
| Hispanic/Latino | 1.02 (0.53–1.99) | 1.29 (0.50–3.32) | |
| Other/Unknown | 1.04 (0.31–3.51) | 1.04 (0.20–5.49) | |
| Insurance | |||
| Private | 1.44 (0.94–2.21) | 1.50 (0.78–2.85) | |
| Public/None | Referent | Referent | |
| CD4 cell count (cells/mm3) | |||
| < 200 | 0.70 (0.38–1.30) | 0.60 (0.26–1.39) | |
| 200–349 | 0.98 (0.60–1.61) | 1.06 (0.55–2.02) | |
| 350–499 | 0.83 (0.50–1.36) | 0.75 (0.39–1.43) | |
| ≥ 500 | Referent | Referent | |
| Unknown/Missing | 1.40 (0.48–4.13) | 0.97 (0.05–19.79) | |
| HIV RNA viral load (copies/mL) | |||
| Undetectable | 1.14 (0.79–1.63) | 1.21 (0.74–1.98) | |
| Detectable | Referent | ||
| Unknown/Missing | 1.64 (0.54–5.00) | 1.05 (0.05–23.72) | |
| Cocaine | 1.82 (0.74–4.48) | 1.75 (0.67–4.58) | |
| Poppers | 4.87 (0.68–35.02) | 0.99 (0.04–22.10) | |
| Injection drug use | 3.30 (1.01–10.78)* | 4.30 (0.80–23.17) | |
| Methamphetamine | 12.23 (1.03–144.79)* | 16.93 (0.55–525.28) | |
| Club drugs | † | ||
| Erectile dysfunction drugs | 22.62 (0.73–698.83) | 19.46 (0.46–833.36) | |
| Cannabis use | 2.09 (1.39–3.13)** | 2.00 (1.15–3.50)* | 1.67 (1.11–2.52)* |
| Alcohol use | 2.11 (1.50–2.98)** | 2.30 (1.43–3.69)** | 1.79 (1.26–2.54)* |
| Current cigarette smoker | 0.80 (0.56–1.15) | 0.66 (0.39–1.10) | |
| Time since 3/15/2007, per year | 0.97 (0.91–1.05) | 0.99 (0.94–1.04) |
HIV Outpatient Study, USA, 2007–2019
CI confidence interval; GLMM generalized linear mixed model analyses; OR odds ratio
Indicates P-value < 0.05 and
indicates P-value < 0.001
Participant numbers too small to calculate ORs and CIs in the univariable analyses
Secondary Analyses
In analyses with the outcome of condomless anal or vaginal sex with negative/unknown HIV status partners, similar trends over time were observed for the three demographic subsets, except that the frequency of condomless sex was lower (Fig. 2b). In the GLMMs for MSM, age < 50 years, having either private insurance, CD4 cell count ≥ 500 cells/mm3, using cocaine, poppers, methamphetamine, or EDD were associated with having condomless sex with negative/unknown HIV status partners, and such sexual encounters increased during the observation (data not shown). Viral load was not associated with this outcome. For females, only cocaine use was associated with this outcome; for heterosexual males, use of cocaine, poppers, methamphetamine, and EDD was associated with this outcome and such sexual encounters increased during observation (data not shown).
Similar sensitivity analyses were performed with age group and race/ethnicity included in the models (regardless of statistical significance) to adjust for any residual confounding, with the outcome being condomless sex with negative/unknown HIV status partners (data not shown). The results from the models for MSM and heterosexual men were qualitatively similar to those from the main analyses and independent correlates remained the same. For female participants, being of Hispanic/Latina or other/unknown race/ethnicity was associated with the outcome, but such sexual encounters did not increase during observation.
Discussion
In this prospective cohort of adult PWH in care, the frequency of reported anal or vaginal condomless sex in the past 6 months ranged from 31 to 59% among MSM and it ranged from 13 to 45% for heterosexual females and males. MSM reported having had condomless sex more frequently than heterosexual males and females, and that trend held over time. Modest increases in the frequency of condomless sex were observed for MSM, coinciding with the growing evidence for HIV treatment as prevention, but such increases were not seen for females or heterosexual males. In this report, we were specifically interested in the frequency and correlates of self-reported condom use among sexually active adults; consequently about one-quarter of HOPS participants without any reported sexual activity in the previous 6 months were excluded from the analysis, but could be considered in a future focused investigation around sexual health.
Other published studies have reported variable rates of self-reported condomless sex among study participants depending on the time frame and definitions for ascertaining sex. A large cross-sectional multicenter survey of HIV positive MSM in the UK seen at HIV clinics in 2011–2012 reported 38% of its respondents had engaged in any condomless sex in the 3 months prior to survey response [24]. A systematic literature review of 37 studies published from 2004 to 2015 examining trends in sexual risk behaviors found a decreasing trend in condom use among MSM over time including condomless sex with negative or unknown status partners [25]. In an analysis of CDC’s National HIV Behavioral Surveillance data, among 5371 HIV-positive MSM, there were increases in discordant condomless sex (15–19%, P < 0.001). [26].
We identified several factors associated with changes in condom use behavior over time. Younger HOPS participants under the age of 50 years were more likely to have condomless sex compared with their older counterparts, a trend which has also been reported elsewhere [27, 28]. This finding may be due to older participants having fewer sexual partners, being more cautious than their younger counterparts, or other reasons. Recreational substance use was associated with increased condomless activity over time, consistent with reports from earlier studies [29]. Substance use may provide individuals with a method to cope with their HIV infection or it may be used to reduce inhibitions or enhance sexual pleasure. Secondary analyses indicated that among those who had condomless sex with partners of HIV-negative or unknown status, the frequency of reported condomless sex increased over time for males, both MSM and heterosexual; increasing trends were noted after the release of HPTN052 study results which affirmed the role of HIV treatment for prevention. However, in these secondary analyses, we did not detect an association between condomless sex and having an undetectable VL.
Our results should be interpreted in light of some caveats and limitations. The data collected via the ACASI survey are from a convenience sample of volunteer participants from the larger HOPS cohort. Although the survey is offered to participants on an annual basis, persons may elect to not participate or delay participation. Therefore, the study is subject to some bias due to missing data if that missingness is informative, and potentially with respect to both the estimates of the frequency of condomless sex and the trends over time. Although the ACASI method has been established as a good way of capturing quality data containing sensitive information [30–32], the survey non-participation (see Fig. 1) and social desirability bias may still affect the reporting of certain behaviors. We did not collect individual partner-level information using ACASI, and instead queried about the total number of partners during the period of observation and whether or not the HOPS study participant engaged (in aggregate) in specific behaviors with sexual partners who were HIV-negative, HIV-positive or of an unknown HIV status in the past 6 months. We were unable ascertain if condomless sex acts were protected with the use of PrEP by HIV-negative partners. With the brief survey and medical records data in the HOPS, we also did not have information on psychosocial and structural factors that may have contributed to condomless sex, including stigma, exchanging sex for money or drugs and the like [33, 34]. Finally, because nearly half of the HOPS cohort participants are white non-Hispanic/Latino MSM, findings may be less robust for other racial/ethnic groups and not be generalizable to other PWH (i.e., people not in routine HIV medical care). Despite the limitations noted, the strength of this study lies in the demonstrated ability to link clinical (i.e., HIV viral load and CD4 cell count) and behavioral data, and the consistent longitudinal data collection which enables us to explore the longer-term changes in sexual risk behavior. Identifying and characterizing changes in and correlates of condomless sexual activity among PWH may lead to further development and implementation of more tailored HIV prevention interventions addressing factors such as reducing condomless sex with HIV-negative or unknown status partners [35–37], limiting the number of sexual partners [38], or reducing substance use [39].
In conclusion, throughout their observation in this cohort, sexually active persons in HIV care continued to report engaging in condomless anal and vaginal sex, including with partners of HIV-negative or unknown status. The frequency of reported condomless sex increased among MSM but appeared stable for heterosexual males and females over time. Although persons with undetectable HIV viral load have effectively no risk of sexually transmitting HIV to others, there is still the potential risk of transmission or acquisition of other STIs [40]; effective interventions are available to assist persons living with HIV to link to care and achieve and maintain viral suppression thus protecting their health and that of their sexual partners [1]. Coordinated services and programming by clinical, community and federal and local health department partners, that support comprehensive care and sexual health for persons with HIV, will be instrumental for ending the HIV epidemic in the United States [1, 5, 6].
Supplementary Material
Acknowledgements
The HIV Outpatient Study (HOPS) Investigators include the following persons and sites: Kate Buchacz, Marcus D. Durham, Jun Li, Division of HIV/AIDS Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, GA; Cheryl Akridge, Stacey Purinton, Selom Agbobil-Nuwoaty, Kalliope Chagaris, Kimberly Carlson, Qingjiang Hou, Carl Armon, Linda Battalora, Jonathan Mahnken, Cerner Corporation, Kansas City, MO; Frank J. Palella, Conor Daniel Flaherty, Feinberg School of Medicine, Northwestern University, Chicago, IL; Cynthia Firnhaber, Barbara Widick, Rosa Franklin, Billie Thomas, Vivent Health, Denver, CO; Douglas J. Ward, Linda Kirkman, DuPont Circle Physicians Group, Washington, DC; Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, Jane Esteves, State University of New York (SUNY), Stony Brook, NY; Ellen M. Tedaldi, Ramona A. Christian, Faye Ruley, Dania Beadle, Princess Davenport, Lewis Katz School of Medicine at Temple University, Philadelphia, PA; Richard M. Novak, Andrea Wendrow, Stockton Mayer, University of Illinois at Chicago, Chicago, IL; Cynthia Mayer, Karen Maroney, Mark Waggoner, Kimberly Braden, Anicette Richardson, Michelle Orzechowski, St. Joseph’s Hospital Comprehensive Research Institute, Tampa, FL.
Funding
This study was funded by Centers for Disease Control and Prevention (Contract Nos. 200–2001-00133, 200–2006-18797, 200–2011-41872, 200–2015-63931, and 75D30120C08752).
Footnotes
Declarations
Disclaimers The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10461-022-03655-z.
Conflict of interest The authors do not have any associations that may pose a conflict of interest.
References
- 1.Centers for Disease Control and Prevention. Effective interventions. Available at: https://www.cdc.gov/hiv/effective-interventions/treat/index.html. Accessed 8 Jan 2022.
- 2.Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019;393(10189):2428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durham MD, Hart R, Buchacz K, et al. Antiretroviral nonadherence and condomless sex in the HIV Outpatient Study, USA, 2007–2014. Int J STD AIDS 2018;29(2):147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021;70(4):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services. What is “Ending the HIV Epidemic: A plan for America?” Available at: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview. Accessed 10 Feb 2022.
- 6.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019;321(9):844–5. [DOI] [PubMed] [Google Scholar]
- 7.The White House. National HIV/AIDS strategy for the United States 2022–2025 Available at: https://www.hiv.gov/. Accessed 11 Feb 2022.
- 8.Cox J, Beauchemin J, Allard R. HIV status of sexual partners is more important than antiretroviral treatment related perceptions for risk taking by HIV positive MSM in Montreal, Canada. Sex Transm Infect 2004;80(6):518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halkitis PN, Parsons JT. Intentional unsafe sex (barebacking) among HIV-positive gay men who seek sexual partners on the Internet. AIDS Care 2003;15(3):367–78. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta S, Tie Y, Bradley H, et al. Characteristics of sexual partnerships among men with diagnosed HIV who have sex with men, United States and Puerto Rico-2015–2019. J Acquir Immune Defic Syndr 2020;84(5):443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Kudon H, Mulatu MS, Song W, Heitgerd J, Rao S. Trends in condomless sex among MSM who participated in CDC-funded HIV risk-reduction interventions in the United States, 2012–2017. J Public Health Manag Pract 2020. 10.1097/PHH.0000000000001143. [DOI] [PubMed]
- 12.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316(2):171–81. [DOI] [PubMed] [Google Scholar]
- 14.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV infection and older Americans: the public health perspective. Am J Public Health 2012;102(8):1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hebberecht L, Vancoillie L, Schauvliege M, et al. Frequency of occurrence of HIV-1 dual infection in a Belgian MSM population. PLoS ONE 2018;13(4):e0195679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casado C, Pernas M, Rava M, et al. High-risk sexual practices contribute to HIV-1 double infection among men who have sex with men in Madrid. AIDS Res Hum Retrovir 2020;36(11):896–904. [DOI] [PubMed] [Google Scholar]
- 18.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387(10013):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol 2018;28(12):850–7.e9. [DOI] [PubMed] [Google Scholar]
- 20.Huang YA, Tao G, Smith DK, Hoover KW. Persistence with HIV preexposure prophylaxis in the United States, 2012–2017. Clin Infect Dis 2020. 10.1093/cid/ciaa037. [DOI] [PubMed]
- 21.Du P, Crook T, Whitener C, Albright P, Greenawalt D, Zurlo J. HIV transmission risk behaviors among people living with HIV/AIDS: the need to integrate HIV prevention interventions and public health strategies into HIV care. J Public Health Manag Pract 2015;21(2):E1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338(13):853–60. [DOI] [PubMed] [Google Scholar]
- 23.Moorman AC, Holmberg SD, Marlowe SI, et al. Changing conditions and treatments in a dynamic cohort of ambulatory HIV patients: the HIV outpatient study (HOPS). Ann Epidemiol 1999;9(6):349–57. [DOI] [PubMed] [Google Scholar]
- 24.Daskalopoulou M, Rodger AJ, Phillips AN, et al. Condomless sex in HIV-diagnosed men who have sex with men in the UK: prevalence, correlates, and implications for HIV transmission. Sex Transm Infect 2017;93(8):590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess KL, Crepaz N, Rose C, Purcell D, Paz-Bailey G. Trends in sexual behavior among men who have sex with men (MSM) in high-income countries, 1990–2013: a systematic review. AIDS Behav 2017;21(10):2811–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paz-Bailey G, Mendoza MC, Finlayson T, Wejnert C, Le B, Rose C, Raymond HF, Prejean J, NHBS Study Group. Trends in condom use among MSM in the United States: the role of antiretroviral therapy and seroadaptive strategies. AIDS (London, England) 2016;30(12):1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durham MD, Buchacz K, Richardson J, et al. Sexual risk behavior and viremia among men who have sex with men in the HIV Outpatient Study, United States, 2007–2010. J Acquir Immune Defic Syndr 2013;63(3):372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arentoft A, Van Dyk K, Thames AD, Thaler NS, Sayegh P, Hinkin CH. HIV-transmission-related risk behavior in HIV+ African American men: exploring biological, psychological, cognitive, and social factors. J HIV AIDS Soc Serv 2016;15(3):299–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hightow-Weidman L, Muessig K, Egger JR, LeGrand S, Platt A. Predictors of condomless anal intercourse in young HIV-positive men who have sex with men with detectable viral loads. J Adolesc Health 2020;66(6):672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kane JC, Bolton P, Murray SM, et al. Psychometric evaluation of HIV risk behavior assessments using Audio Computer Assisted Self-Interviewing (ACASI) among orphans and vulnerable children in Zambia. AIDS Care 2018;30(2):160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam MM, Topp L, Conigrave KM, et al. The reliability of sensitive information provided by injecting drug users in a clinical setting: clinician-administered versus audio computer-assisted self-interviewing (ACASI). AIDS Care 2012;24(12):1496–503. [DOI] [PubMed] [Google Scholar]
- 32.Estes LJ, Lloyd LE, Teti M, et al. Perceptions of audio computer-assisted self-interviewing (ACASI) among women in an HIV-positive prevention program. PLoS ONE 2010;5(2):e9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffries WL, Greene KM, Paz-Bailey G, et al. Determinants of HIV incidence disparities among young and older men who have sex with men in the United States. AIDS Behav 2018;22(7):2199–213. [DOI] [PubMed] [Google Scholar]
- 34.Whiteman A, Baugher A, Sionean C. Assessing self-reported discrimination among men who have sex with men (MSM). AIDS 2021;35(1):141–6. [DOI] [PubMed] [Google Scholar]
- 35.Cruess DG, Burnham KE, Finitsis DJ, et al. A randomized clinical trial of a brief internet-based group intervention to reduce sexual transmission risk behavior among HIV-positive gay and bisexual men. Ann Behav Med 2018;52(2):116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalichman SC, Cherry C, Kalichman MO, et al. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. Am J Public Health 2011;101(3):531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurth AE, Spielberg F, Cleland CM, et al. Computerized counseling reduces HIV-1 viral load and sexual transmission risk: findings from a randomized controlled trial. J Acquir Immune Defic Syndr 2014;65(5):611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Bassel N, Gilbert L, Goddard-Eckrich D, et al. Effectiveness of a couple-based HIV and sexually transmitted infection prevention intervention for men in community supervision programs and their female sexual partners: a randomized clinical trial. JAMA Netw Open 2019;2(3):e191139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermanstyne KA, Shoptaw S, Cunningham WE. Associations of types of substances with condomless sex in vulnerable people living with HIV/AIDS. J HIV/AIDS Soc Serv 2018;17(2):118–26. [Google Scholar]
- 40.van den Berg JJ, Zaller ND, Gillani FS, et al. Longitudinal HIV transmission risk profiles among men who have sex with men living with HIV in the SUN study. Am J Mens Health 2019;13(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


