Abstract
This study aimed to assess the relationship between dietary sodium/potassium intake and cognition in elderly individuals with hypertension. We designed a cross‐sectional study based on the 2011–2014 National Health and Nutrition Examination Survey (NHANES) 2011–2014. A multivariable‐logistic regression analysis was performed to analyze the relationship between sodium/potassium intake and cognitive impairment. Restricted cubic spline (RCS) based on regression analysis to assess the nonlinear dose‐response relationship between dietary sodium intake and cognitive performance. Out of the 2276 participants included in this study, 1670 patients had hypertension. Compared with the lowest quartile of dietary sodium intake, the lowest weighted odds ratio of cognitive impairment in DSST was observed in Q4 (OR = 0.45, 0.29–0.70), and a similar trend was observed in AFT (OR = 0.34, 0.18–0.65). After adjusting the covariates, the lowest weighted multivariable‐adjusted OR of cognitive impairment in DSST were also observed in Q4 (OR = 0.47, 0.26‐0.84) compared with the lowest quartile of dietary sodium intake. The RCS results showed that dietary sodium intake was U‐shaped and associated with the risk of cognitive impairment in the DSST (Pnon–linearity = 0.0067). In addition, no significant association was observed between dietary potassium intake and different dimensions of cognitive performance. In conclusion, excessively high and low low dietary sodium were associated with impairment of specific processing speed, sustained attention, and working memory for elderly patients with hypertension in the United States. However, no association was observed between dietary potassium intake and cognition.
Keywords: cognition, dietary sodium, hypertension
1. INTRODUCTION
Salt is a widely used condiment worldwide, and sodium chloride is its main component. Sodium is an essential macronutrient that maintains fluid balance and cellular homeostasis 1 and is involved in nerve conduction 2 in the human body. However, a high level of dietary sodium intake is considered to be associated with hypertension, a major risk factor for cardiovascular disease (CVD). 3 A high level of dietary sodium intake is also a primary dietary risk for death and disability‐adjusted life‐years in China, Japan, and Thailand. 4 In 2017, the estimated global mean salt intake was 6 g/day, greatly exceeding optimal levels. 4
Properly managing sodium intake is a highly effective way to significantly reduce the risk of cardiovascular disease, and dietary sodium intake has been considered a common modifiable risk factor for hypertension. 5 , 6 , 7 Additionally, potassium‐containing salts have been used as substitutes for sodium. 8 , 9 , 10 Many studies have shown that high potassium intake may lower blood pressure and CVD risk partly, 11 , 12 , 13 and high potassium intake may mitigate the negative impacts of high sodium intake. 14
Cognitive impairment is linked to cardiovascular risk factors in the general population, 15 and diet is considered an important factor in cognitive function. 16 , 17 , 18 It is suggested that dietary sodium may influence cognition through cerebrovascular and cerebral blood flow. 19 , 20 Animal experiments have shown that tau hyperphosphorylation can be induced by high levels of dietary sodium, followed by cognitive impairment 21 ; nonetheless, their findings have remained controversial. A prospective cohort study has reported that sodium and potassium intake were not associated with cognitive impairment. 22 Another cross‐sectional study has shown that lower sodium intake was associated with an increased risk of cognitive impairment in older adults. 23
We have hypothesized that both excessively high and low levels of dietary sodium intake may increase the risk of cognitive impairment. Accordingly, this study was performed to evaluate the association between sodium intake and cognitive impairment in older individuals with hypertension. In this study, we aimed to establish a continuous dose‐response relationship between sodium and cognitive impairment and assess the relationship between dietary potassium intake and cognition.
2. METHODS
2.1. Study design
The National Health and Nutrition Examination Surveys (NHANES) is a program of studies designed to assess the health and nutritional status of adults and children in the United States. The program aims to select a representative sample of approximately 10,000 individuals every 2 years on a national level. The survey includes information about demographic, socioeconomic, dietary, and health‐related questions, as well as physical examinations comprising medical, dental, and physiological measurements and laboratory tests.
2.2. Study population
Data were extracted from the 2011−2012 and 2013−2014 NHANES database. The participants with complete demographic data, smoking and alcohol use information, Hemal biochemistry data, and cognitive data were included in this study. All the participants included in this study were aged 60 years or older.
The protocols of NHANES were approved by the National Center for Health Statistics Research Ethics Review Board. Written informed consent was obtained from all participants. According to the policy of our local Research Ethics Committee, the published available data does not need a secondary review.
2.3. Diagnosis of hypertension
Participants were considered hypertensive if they met the following criteria: (1) they had been told by a doctor or other health professional that they had hypertension (also called high blood pressure), (2) self‐reported antihypertensive drug use, and (3) had a high blood pressure measurement (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg). The NHANES consists of three consecutive blood pressure measurements and an additional measurement if required.
2.4. Cognitive tests
Cognition was assessed using a questionnaire that included (1) word learning and recall modules from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD), 24 (2) the Animal Fluency test (AFT), 25 and (3) the Digit Symbol Substitution Test (DSST) 26 in NHANES. The CERAD test assesses the immediate and delayed learning abilities for new verbal information. The test consisted of three consecutive learning trials and a delayed‐recall trial. We calculated the sum of the three learning and recall trial scores as the final score of the CERAD test. The AFT was used to examine the executive function, and the DSST was used to assess processing speed, sustained attention, and working memory. We also extracted the scores of the AFT and DSST in the NHANES as indices of cognition.
However, there is no acknowledged threshold for the DSST, CERAD, and AFT to distinguish cognitive impairment. Age was considered a significant confounding factor for performance on cognitive tests. Therefore, the participants were stratified according to age: 60−65 years, 66−70 years, 71−75 years, and ≥76 years. We used the 25th percentile of the score according to age stratification as the threshold for identifying cognitive impairment.
2.5. Dietary sodium intake
It is considered that 24‐h urine sodium excretion is the gold standard for monitoring sodium intake. However, this method is complicated. Previous studies have confirmed that dietary recall has a significant dose‐response association with estimated 24‐h urine sodium excretion, 27 , 28 and it is another main method for accessing salt intake. 29 In this study, dietary sodium intake was extracted from 24‐h dietary recall interviews in the NHANES database and expressed in milligrams. The U.S. Department of Agriculture Food and Nutrient Database for Dietary Studies was used to process the total daily nutrient intake of food and beverages.
2.6. Covariates
We also included other variables of interest as covariates consisting of age, sex (male and female), race (Non‐Hispanic White, Mexican American, Non‐Hispanic Black, Other Hispanic, and Other Race), education (less than 9th grade, 9−11th grade, high school graduate; some college or AA degree, college graduate or above), body mass index (BMI), SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and use of antihypertensive drugs.
2.7. Statistical analysis
All statistical analyses in this study were performed using R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria https://www.r‐project.org/). Survey package version 4.1‐1 and NHANES R package version 0.9.3.8 were used to analyze complex survey samples. The raw data are shown in Supplementary file 1. Continuous variables in this study are expressed as mean (standard error, SE), while categorical variables are expressed as frequencies (percentages). Dietary sodium intake was grouped into quantiles from lowest (first quantile, Q1) to highest (fifth quantile, Q5). We compared the covariates of participants with and without cognitive impairment. Student's t‐test was used to compare continuous variables, while the Rao‐Scott chi‐square test was used to compare categorical variables. Logistic regression models were used to analyze the relationship between sodium intake and cognitive impairment. Model 1 was established using a binary logistic regression. Model 2 was adjusted for age and sex. Model 3 was adjusted for age, sex, race, education, BMI, SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and antihypertensive drug use. Additionally, we performed a restricted cubic spline (RCS) to assess the nonlinear dose‐response relationship between dietary sodium intake and cognitive performance after adjustment for all covariates.
3. RESULTS
3.1. Characteristics of study population
In this study, 19,931 participants were included in the NHANES from 2011 to 2014. After excluding participants with incomplete data, 2276 participants were included in this study, of which 1670 patients had hypertension (Figure 1).
FIGURE 1.
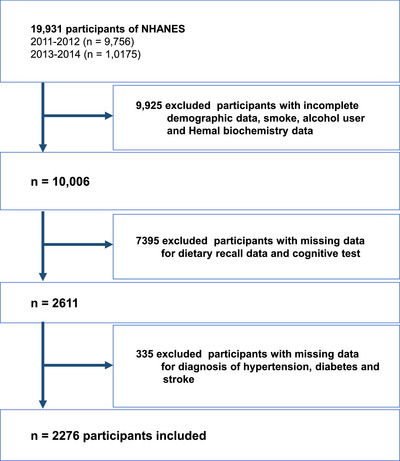
Flow diagram of study identification, screening, eligibility assessment, and inclusion.
The mean dietary sodium intake of all participants was 3183.31 mg/day, which was similar to that of participants with hypertension (3159.13 mg/day). Dietary sodium intake was grouped into quantiles from lowest (first quantile, Q1) to highest (fifth quantile, Q5). Table 1 shows the clinical characteristics of the participants with hypertension according to dietary sodium intake quintiles. The results indicate that there were significant differences between different dietary sodium intake levels in the distribution of age, sex, race, education, dietary energy, dietary potassium, creatinine, alcohol use, diabetes, and stroke among patients with hypertension. Moreover, there were significant differences between different dietary potassium intake levels in the distribution of sex, race, education, digit symbol score test, animal fluency score, dietary energy, dietary sodium, serum potassium, serum sodium, alcohol use, and smoking among patients with hypertension (Table S1). Tables S2–S3 show the clinical characteristics of all participants.
TABLE 1.
Characteristics of the study population with hypertension.
| Total daily dietary sodium (mg/day) | |||||||
|---|---|---|---|---|---|---|---|
| Total N = 1610 | Q1 (286−2076) N = 333 | Q2 (2076−2604) N = 334 | Q3 (2604−3226) N = 328 | Q4 (3226−3944.5) N = 316 | Q5 (3944.5−8765) N = 299 | p‐value | |
| Age (years) a | 69.79 (0.33) | 71.68 (0.72) | 70.39 (0.68) | 69.85 (0.61) | 69.98 (0.48) | 67.74 (0.59) | <0.001 |
| Sex, n (%) b | <0.0001 | ||||||
| Female | 845 (52.48) | 241 (79.71) | 220 (74.11) | 165 (51.78) | 134 (47.02) | 85 (23.05) | |
| Male | 765 (47.52) | 92 (20.29) | 114 (25.89) | 163 (48.22) | 182 (52.98) | 214 (76.95) | |
| Race/ethnicity, n (%) b | 0.01 | ||||||
| Non‐Hispanic White | 789 (49.01) | 136 (68.44) | 167 (79.79) | 168 (76.79) | 169 (78.86) | 149 (83.50) | |
| Mexican American | 128 (7.95) | 32 (5.80) | 22 (2.14) | 27 (3.20) | 21 (3.56) | 26 (2.90) | |
| Non‐Hispanic Black | 419 (26.02) | 93 (13.40) | 92 (10.69) | 81 (9.50) | 73 (8.84) | 80 (7.96) | |
| Other Hispanic | 153 (9.5) | 49 (6.83) | 39 (5.00) | 24 (3.82) | 25 (2.85) | 16 (1.35) | |
| Other Race | 121 (7.52) | 23 (5.53) | 14 (2.39) | 28 (6.69) | 28 (5.89) | 28 (4.29) | |
| Education, n (%) b | 0.02 | ||||||
| Less than 9th grade | 160 (9.94) | 53 (11.22) | 29 (5.32) | 32 (5.34) | 25 (4.79) | 21 (4.48) | |
| 9−11th grade | 234 (14.53) | 61 (11.39) | 50 (11.50) | 52 (11.56) | 34 (8.04) | 37 (9.47) | |
| High school graduate/GED or equivalent | 411 (25.53) | 86 (34.12) | 92 (22.69) | 82 (24.58) | 76 (20.76) | 75 (23.19) | |
| Some college or AA degree | 468 (29.07) | 88 (28.59) | 110 (37.04) | 83 (24.80) | 105 (38.13) | 82 (25.77) | |
| College graduate or above | 337 (20.93) | 45 (14.68) | 53 (23.45) | 79 (33.72) | 76 (28.27) | 84 (37.09) | |
| BMI (kg·m2) a | 29.93 (0.32) | 29.27 (0.55) | 29.35 (0.61) | 30.04 (0.67) | 29.45 (0.38) | 31.18 (0.77) | 0.2 |
| Digit symbol score test a | 51.08 (0.86) | 46.30 (1.53) | 50.44 (1.40) | 50.96 (1.35) | 53.64 (1.69) | 52.94 (1.11) | 0.01 |
| Animal fluency score total a | 17.54 (0.25) | 16.13 (0.45) | 17.22 (0.51) | 17.45 (0.51) | 18.35 (0.46) | 18.18 (0.66) | 0.04 |
| CERAD score a | 25.57 (0.41) | 24.59 (0.56) | 25.20 (0.80) | 25.42 (0.38) | 25.83 (0.57) | 26.47 (0.62) | 0.07 |
| SBP (mmHg) a | 135.35 (0.71) | 137.53 (1.36) | 135.11 (2.12) | 135.95 (1.32) | 136.44 (1.41) | 132.56 (1.32) | 0.05 |
| DBP (mmHg) a | 68.73 (0.54) | 68.15 (1.13) | 68.95 (0.72) | 68.86 (1.09) | 68.07 (1.34) | 69.42 (0.98) | 0.92 |
| Dietary energy (kcal) a | 1867.68 (28.62) | 1125.52 (16.23) | 1532.68 (29.67) | 1810.88 (19.35) | 2043.87 (30.00) | 2567.95 (60.07) | <0.0001 |
| Dietary sodium (mg) a | 3159.13 (63.96) | 1635.79 (27.00) | 2343.73 (11.08) | 2929.88 (15.52) | 3531.09 (17.82) | 4787.03 (80.07) | <0.0001 |
| Dietary potassium (mg) a | 2628.31 (36.71) | 1719.78 (45.64) | 2317.90 (69.58) | 2591.03 (49.39) | 2844.93 (65.21) | 3375.95 (87.91) | <0.0001 |
| Creatinine (umol/L) a | 88.73 (0.99) | 88.47 (3.62) | 84.23 (1.78) | 89.01 (1.74) | 88.42 (2.79) | 92.65 (2.38) | 0.14 |
| Serum potassium (mmol/L) a | 4.07 (0.02) | 4.01 (0.05) | 4.04 (0.03) | 4.08 (0.02) | 4.07 (0.03) | 4.11 (0.06) | 0.36 |
| Serum sodium (mmol/L) a | 139.40 (0.14) | 139.28 (0.25) | 139.37 (0.25) | 139.24 (0.17) | 139.57 (0.21) | 139.51 (0.30) | 0.66 |
| Alcohol user, n (%) b | 0.01 | ||||||
| Yes | 913 (56.71) | 155 (49.19) | 189 (63.30) | 193 (67.99) | 176 (63.65) | 200 (70.52) | |
| No | 697 (43.29) | 178 (50.81) | 145 (36.70) | 135 (32.01) | 140 (36.35) | 99 (29.48) | |
| Smoke, n (%) b | 0.45 | ||||||
| Yes | 184 (11.43) | 40 (11.00) | 37 (7.73) | 36 (6.53) | 27 (7.96) | 44 (7.76) | |
| No | 1426 (88.57) | 293 (89.00) | 297 (92.27) | 292 (93.47) | 289 (92.04) | 255 (92.24) | |
| Diabetes, n (%) b | 0.04 | ||||||
| Yes | 597 (37.08) | 145 (35.92) | 117 (23.10) | 129 (38.58) | 108 (28.29) | 98 (28.55) | |
| No | 1013 (62.92) | 188 (64.08) | 217 (76.90) | 199 (61.42) | 208 (71.71) | 201 (71.45) | |
| Stroke, n (%) b | 0.21 | ||||||
| Yes | 130 (8.07) | 39 (10.71) | 18 (4.32) | 25 (8.67) | 27 (8.74) | 21 (5.29) | |
| No | 1480 (91.93) | 294 (89.29) | 316 (95.68) | 303 (91.33) | 289 (91.26) | 278 (94.71) | |
| Antihypertensive drug user, n (%) b | 0.73 | ||||||
| Yes | 311 (19.32) | 68 (20.89) | 66 (19.97) | 65 (18.34) | 53 (15.99) | 59 (16.74) | |
| No | 1299 (80.68) | 265 (79.11) | 268 (80.03) | 263 (81.66) | 263 (84.01) | 240 (83.26) | |
Abbreviations: BMI, body mass index; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Values shown are mean (standard error).
Values shown are numbers (weighted percentage).
3.2. Dietary sodium intake and cognition
A logistic regression model was used to assess the association between dietary sodium intake and cognitive performance in all participants (Table 2). Compared with the lowest quartile of dietary sodium intake, the lowest weighted odds ratios of cognitive impairment in DSST were observed in Q4 (OR = 0.42, 0.31−0.57), and a similar trend was observed in AFT (OR = 0.43, 0.26−0.73). Q3 showed the lowest odds ratios (OR = 0.64, 0.42−0.96) of cognitive impairment in CERAD compared with Q1. After adjusting for age, sex, race, education, BMI, SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and use of antihypertensive drugs, the lowest weighted multivariable‐adjusted OR of cognitive impairment in the DSST was also observed in Q4 (OR = 0.47, 0.26−0.84) compared with the lowest quartile of dietary sodium intake.
TABLE 2.
Weighted odds ratios (95% confidence interval) of cognitive impairment by quartiles of sodium intake in all participants.
| DSST | |||||||
|---|---|---|---|---|---|---|---|
| Dietary sodium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (286−2076) | 224 (32.77) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (2076−2604) | 171 (21.35) | 0.56 (0.37−0.84) | 0.01 | 0.51 (0.34−0.77) | 0.002 | 0.60 (0.30−1.23) | 0.13 |
| Q3 (2604−3226) | 146 (21.00) | 0.55 (0.39−0.77) | 0.001 | 0.46 (0.32−0.68) | <0.001 | 0.68 (0.33−1.39) | 0.24 |
| Q4 (3226−3944.5) | 144 (17.01) | 0.42 (0.31−0.57) | <0.0001 | 0.34 (0.23−0.48) | <0.0001 | 0.47 (0.26−0.84) | 0.02 |
| Q5 (3944.5−8765) | 164 (23.94) | 0.65 (0.44−0.95) | 0.03 | 0.45 (0.29−0.69) | <0.001 | 0.75 (0.32−1.74) | 0.44 |
| AFT | |||||||
|---|---|---|---|---|---|---|---|
| Dietary sodium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (286−2076) | 178 (30.54) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (2076−2604) | 147 (23.62) | 0.70 (0.42,1.17) | 0.17 | 0.69 (0.40,1.19) | 0.17 | 0.93 (0.43,2.03) | 0.83 |
| Q3 (2604−3226) | 129 (23.91) | 0.71 (0.44,1.15) | 0.16 | 0.71 (0.42,1.20) | 0.19 | 1.05 (0.46,2.38) | 0.89 |
| Q4 (3226−3944.5) | 109 (16.05) | 0.43 (0.26,0.73) | 0.003 | 0.42 (0.24,0.74) | 0.004 | 0.69 (0.28,1.70) | 0.35 |
| Q5 (3944.5−8765) | 106 (17.68) | 0.49 (0.31,0.78) | 0.004 | 0.47 (0.28,0.79) | 0.01 | 0.92 (0.40,2.15) | 0.82 |
| CERAD test | |||||||
|---|---|---|---|---|---|---|---|
| Dietary sodium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (286−2076) | 166 (30.06) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (2076−2604) | 136 (28.00) | 0.90 (0.58−1.42) | 0.65 | 0.82 (0.51−1.33) | 0.4 | 1.06 (0.59−1.91) | 0.83 |
| Q3 (2604−3226) | 131 (21.44) | 0.64 (0.42−0.96) | 0.03 | 0.53 (0.31−0.89) | 0.02 | 0.69 (0.34−1.41) | 0.25 |
| CERAD test | |||||||
|---|---|---|---|---|---|---|---|
| Dietary sodium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q4 (3226−3944.5) | 127 (23.09) | 0.70 (0.46−1.06) | 0.09 | 0.55 (0.36−0.83) | 0.01 | 0.80 (0.41−1.58) | 0.45 |
| Q5 (3944.5−8765) | 132 (24.11) | 0.74 (0.47−1.16) | 0.18 | 0.51 (0.28−0.91) | 0.02 | 0.82 (0.39−1.73) | 0.53 |
Abbreviations: AFT, Animal fluency score total; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; DSST, Digit Symbol Score Test.
Model 1 calculated by binary logistic regression; Model 2 adjusted for age and sex Model 3 was adjusted for age, sex, race, education, BMI, SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and antihypertensive drug use.
Additionally, we used a logistic regression model in participants with hypertension (Table 3). Compared with the lowest quartile of dietary sodium intake, the lowest weighted odds ratio of cognitive impairment in DSST was observed in Q4 (OR = 0.45, 0.29−0.70), and a similar trend was observed in AFT (OR = 0.34, 0.18−0.65). After adjusting the covariates, the lowest weighted multivariable‐adjusted OR of cognitive impairment in DSST was also observed in Q4 (OR = 0.47, 0.26−0.84) compared with the lowest quartile of dietary sodium intake.
TABLE 3.
Weighted odds ratios (95% confidence interval) of cognitive impairment by quartiles of sodium intake in participants with hypertension.
| DSST | |||||||
|---|---|---|---|---|---|---|---|
| Dietary sodium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (286−2076) | 174 (36.20) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (2076−2604) | 132 (23.22) | 0.53 (0.35−0.81) | 0.005 | 0.52 (0.34−0.78) | 0.003 | 0.67 (0.33– 1.40) | 0.23 |
| Q3 (2604−3226) | 117 (23.65) | 0.55 (0.38−0.79) | 0.002 | 0.47 (0.32−0.70) | <0.001 | 0.62 (0.29– 1.30) | 0.17 |
| Q4 (3226−3944.5) | 111 (20.38) | 0.45 (0.29−0.70) | <0.001 | 0.38 (0.25−0.58) | <0.0001 | 0.47 (0.25– 0.86) | 0.02 |
| Q5 (3944.5−8765) | 112 (23.59) | 0.54 (0.34−0.86) | 0.01 | 0.41 (0.25−0.69) | 0.001 | 0.54 (0.18– 1.64) | 0.23 |
| AFT | |||||||
|---|---|---|---|---|---|---|---|
| Dietary sodium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (286−2076) | 146 (36.14) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (2076−2604) | 113 (25.83) | 0.62 (0.35,1.09) | 0.09 | 0.64 (0.35,1.15) | 0.13 | 0.87 (0.39,1.91) | 0.67 |
| Q3 (2604−3226) | 103 (28.64) | 0.71 (0.43,1.17) | 0.17 | 0.73 (0.43,1.23) | 0.23 | 0.97 (0.40,2.36) | 0.94 |
| Q4 (3226−3944.5) | 80 (16.20) | 0.34 (0.18,0.65) | 0.002 | 0.34 (0.18,0.67) | 0.003 | 0.47 (0.16,1.33) | 0.13 |
| Q5 (3944.5−8765) | 72 (20.11) | 0.44 (0.26,0.77) | 0.01 | 0.47 (0.28,0.81) | 0.01 | 0.77 (0.28,2.17) | 0.56 |
| CERAD test | |||||||
|---|---|---|---|---|---|---|---|
| Dietary sodium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (286−2076) | 130 (34.84) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (2076−2604) | 98 (32.34) | 0.89 (0.54−1.48) | 0.65 | 0.91 (0.55−1.49) | 0.69 | 1.13 (0.62−2.04) | 0.63 |
| Q3 (2604−3226) | 106 (27.03) | 0.69 (0.44−1.09) | 0.11 | 0.59 (0.34−1.03) | 0.06 | 0.72 (0.34−1.51) | 0.31 |
| CERAD test | |||||||
|---|---|---|---|---|---|---|---|
| Dietary sodium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q4 (3226−3944.5) | 88 (26.06) | 0.66 (0.39−1.12) | 0.12 | 0.53 (0.32−0.87) | 0.01 | 0.69 (0.34−1.42) | 0.26 |
| Q5 (3944.5−8765) | 88 (27.58) | 0.71 (0.43−1.18) | 0.18 | 0.54 (0.31−0.95) | 0.03 | 0.78 (0.38−1.58) | 0.42 |
Abbreviations: AFT, Animal fluency score total; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; DSST, Digit Symbol Score Test.
Model 1 calculated by binary logistic regression; Model 2 adjusted for age and sex Model 3 was adjusted for age, sex, race, education, BMI, SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and antihypertensive drug use.
Furthermore, RCS was examined to explore the nonlinear relationship between dietary sodium intake and the risk of cognitive impairment (Figure 2a). We found that dietary sodium intake was U‐shaped and associated with the risk of cognitive impairment (determined by the DSST) (P non–linearity = 0.0067). Figure 2b shows the relationship between dietary sodium intake and DSST score (P non–linearity = 0.0067).
FIGURE 2.
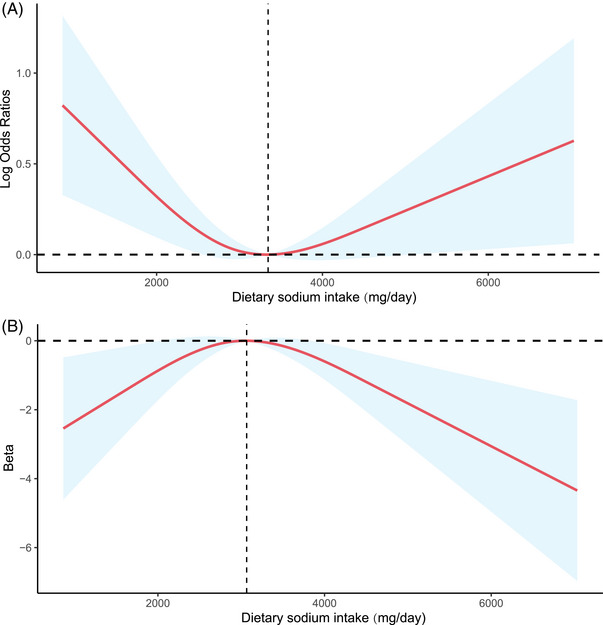
(A) Relationship between sodium intake and risk of cognitive impairment (determined by DSST). The model was based on logistic regression models and adjusted for age, sex, race, education, BMI, SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and antihypertensive drug use. (B) Relationship between sodium intake and DSST score. The model was based on liner regression models and adjusted for age, sex, race, education, BMI, SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and antihypertensive drug use.
3.3. Dietary potassium intake and cognition
A logistic regression model was used to assess the association between dietary potassium intake and cognitive performance in all the participants (Table 4). Compared with the lowest quartile of dietary potassium intake, the crude OR of Q5 was (OR = 0.42, 0.26−0.67) for DSST, and Q3 was (OR = 0.40, 0.21−0.77) for AFT. After adjusting for age, sex, race, education, BMI, SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and use of antihypertensive drugs, no significant association was observed between dietary potassium intake and the different dimensions of cognitive performance.
TABLE 4.
Weighted odds ratios (95% confidence interval) of cognitive impairment by quartiles of potassium intake in participants with hypertension.
| DSST | |||||||
|---|---|---|---|---|---|---|---|
| Dietary potassium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (108.5−1762) | 177 (37.87) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (1762−2235) | 137 (24.35) | 0.53 (0.33−0.85) | 0.01 | 0.52 (0.31−0.84) | 0.01 | 0.70 (0.38– 1.31) | 0.22 |
| Q3 (2235−2690) | 126 (25.50) | 0.56 (0.38−0.84) | 0.01 | 0.52 (0.35−0.78) | 0.003 | 0.87 (0.44– 1.72) | 0.64 |
| Q4 (2690−3280) | 106 (21.05) | 0.44 (0.27−0.70) | 0.001 | 0.35 (0.21−0.57) | <0.001 | 0.70 (0.30– 1.62) | 0.34 |
| Q5 (3280−22666) | 100 (20.20) | 0.42 (0.26−0.67) | <0.001 | 0.33 (0.20−0.56) | <0.001 | 0.70 (0.24– 2.00) | 0.43 |
| AFT | |||||||
|---|---|---|---|---|---|---|---|
| Dietary potassium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (108.5−1762) | 151 (35.59) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (1762−2235) | 124 (29.80) | 0.77 (0.49,1.20) | 0.24 | 0.83 (0.55,1.26) | 0.38 | 1.23 (0.70,2.14) | 0.4 |
| Q3 (2235−2690) | 90 (21.45) | 0.49 (0.32,0.76) | 0.002 | 0.50 (0.33,0.76) | 0.002 | 0.79 (0.45,1.39) | 0.35 |
| Q4 (2690−3280) | 79 (18.04) | 0.40 (0.21,0.77) | 0.01 | 0.39 (0.21,0.71) | 0.003 | 0.77 (0.32,1.83) | 0.48 |
| Q5 (3280−22666) | 70 (22.04) | 0.51 (0.26,1.00) | 0.05 | 0.51 (0.27,0.98) | 0.04 | 1.19 (0.45,3.14) | 0.67 |
| CERAD test | |||||||
|---|---|---|---|---|---|---|---|
| Dietary potassium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q1 (108.5−1762) | 118 (32.66) | 1.00 (Ref.) | – | 1.00 (Ref.) | – | 1.00 (Ref.) | – |
| Q2 (1762−2235) | 115 (30.21) | 0.89 (0.49−1.63) | 0.7 | 0.97 (0.53−1.79) | 0.92 | 1.24 (0.62−2.49) | 0.47 |
| Q3 (2235−2690) | 100 (27.57) | 0.78 (0.47−1.30) | 0.33 | 0.74 (0.43−1.28) | 0.27 | 1.00 (0.54−1.85) | 0.99 |
| CERAD test | |||||||
|---|---|---|---|---|---|---|---|
| Dietary potassium (mg/day) | Case, n (%) | Model 1 * | P‐value | Model 2 * | P‐value | Model 3 * | P‐value |
| Q4 (2690−3280) | 86 (26.21) | 0.73 (0.40−1.32) | 0.29 | 0.56 (0.30−1.04) | 0.07 | 0.87 (0.40−1.91) | 0.69 |
| Q5 (3280−22666) | 91 (30.56) | 0.91 (0.52−1.58) | 0.72 | 0.72 (0.40−1.30) | 0.27 | 1.31 (0.74−2.32) | 0.29 |
Abbreviations: AFT, Animal fluency score total; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; DSST, Digit Symbol Score Test.
Model 1 calculated by binary logistic regression; Model 2 adjusted for age and sex Model 3 was adjusted for age, sex, race, education, BMI, SBP, DBP, total energy intake, dietary potassium intake, serum sodium, creatinine, smoking, alcohol use, diabetes, stroke, and antihypertensive drug use.
Furthermore, RCS was examined to explore the nonlinear relationship between dietary potassium intake and different dimensions of cognitive performance (Figure S1a–c). We found that there were no nonlinear relationships between dietary potassium intake and cognition (DSST score: P non–linearity = 0.1401; AFT: P non–linearity = 0.1055; CERAD test: P non–linearity = 0.0815).
4. DISCUSSION
A previous study showed that high dietary sodium intake might influence cognitive performance. 30 Additionally, dietary sodium is a risk factor for hypertension. 31 , 32 In this study, we combined data from NHANES 2011 to 2012 and 2013−2014 and involved 2276 Americans (1,670 participants with hypertension) aged ≥60 years. After adjusting for all confounding factors, the associations between dietary sodium intake and cognitive performance were significant in participants with or without hypertension, and a parabolic‐shaped dose–response relationship was also detected. In the United States, excessively high or low dietary sodium levels were associated with impaired processing speed, sustained attention, and working memory for hypertension in the older patients. Dietary sodium intake was unrelated to learning ability or executive function in this study. Here, we also analyzed the relationship between dietary potassium intake and cognitive performance. We found that potassium intake did not modify the risk of cognitive decline in the older patients with hypertension.
A dietary sodium intake of less than 2300 mg/day is recommended for the general population and 1500 mg/day for certain groups at risk, including individuals above 51 years of age. 33 In mice, excess dietary salt results in reduced cerebral blood flow, endothelial function, and cognitive impairment. 20 Another animal study showed that dietary salt could disrupt the tricarboxylic acid cycle 34 and lead to tau hyperphosphorylation, a significant biomarker of Alzheimer's pathology. 21 In mice, the researchers observed that excess dietary salt promotes plasma interleukin‐17 production by proinflammatory cytokine interleukin‐17, which promotes endothelial dysfunction and cognitive impairment. 19
There is no doubt that a reduction in dietary sodium has beneficial cardiovascular effects. 35 , 36 , 37 However, the relationship between dietary sodium intake and cognitive impairment remains unclear. Anna et al. 38 examined the effects of hypertension and dietary salt intake on cerebrovascular disease and found that dietary salt intake positively correlated with white‐matter hyperintensity (WMH) volume. Stephen et al. 39 also found that patients who did not reduce their salt intake in the long term were more likely to have lacunar strokes, lacunes, microbleeds, and severe WMH. In contrast, a recent dietary study based on community‐dwelling older adults found that sodium/potassium intake was not associated with micro‐ or macro‐structural brain magnetic resonance imaging (MRI) indices. 22 A cross‐sectional study involving 925 community participants (74.5 ± 8.7 years) showed that lower sodium intake was associated with worse cognitive function. 23 The results of our study differ from those of the research mentioned above. This could be because of the different cognitive tests used in these studies. It is worth mentioning that a cross‐sectional study observed that lower serum sodium (126−140 mmol/L) is associated with both prevalent cognitive impairment and cognitive decline in community‐dwelling older men, 40 which implies that a lack of sodium has a harmful effect on cognition.
The relationship between dietary potassium intake and cognitive impairment has been controversial in previous studies. The Hisayama Study, 41 a prospective cohort study, showed that a higher self‐reported dietary intake of potassium could reduce the risk of dementia in the general Japanese population. Another cohort study based on community‐dwelling older adults showed that potassium intake was not associated with a decline in cognitive function. 22 We also observed that dietary potassium intake was not associated with cognitive impairments.
Our study has some limitations that should be acknowledged. First, because this was a cross‐sectional study, it was difficult to determine a causal relationship between dietary sodium intake and cognitive performance. Furthermore, the assessment of cognitive performance is complicated and cognitive function is best assessed using multiple methods. However, only the DSST score was associated with dietary sodium intake in this study, which limited our ability to examine the association between sodium intake and cognition. Finally, 24‐h dietary recall is used as a simple and effective method in epidemiological studies, but it may not capture long‐term dietary exposures and result in recall bias, 42 and it is an indirect method to evaluate dietary intake compared with 24‐h urine sodium excretion. Therefore, the results of this study need to be interpreted with caution, and more large prospective studies are needed to validate these conclusions further.
In conclusion, both excessively high and low dietary sodium levels were associated with cognitive impairment, specifically in processing speed, sustained attention, and working memory, for hypertension in the older patients in the United States. Therefore, in addition to avoiding high‐sodium dietary patterns, older patients with hypertension should also avoid extremely low sodium intake to prevent cognitive impairment. However, we did not observe an association between dietary potassium intake and cognition. Further large‐scale prospective studies are still needed to clarify the effect of dietary sodium/potassium on cognition.
AUTHOR CONTRIBUTIONS
Jing Yu conceived and designed the study. Chengkun Kou and Xu Zhao contributed to data collection and analysis, and wrote the manuscript. Xin Fan, Xin Lin and Qiongying Wang contributed to study design, data analysis, and manuscript revision. Chengkun Kou and Xu Zhao contributed equally to this work.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
We would like to thank the Laboratory of the Department of Cardiology, Lanzhou University Second Hospital, for their kind technical support. We would like to thank Editage (www.editage.cn) for the English language editing. This study was supported by the National Natural Science Foundation of China (NSFC 81960086, 82160089), Gansu Province Health Research Project (GSWSKY2017‐02), and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2021‐MS‐A13, CY2021‐QN‐A11). This study was also supported by the Special Fund Project for Doctoral Training of Lanzhou University Second Hospital (YJS‐BD‐24), International science and technology cooperation base (PR0124002) and Gansu Province Education Science and Technology Innovation Project (2022‐B042).
Kou C, Zhao X, Fan X, Lin X, Wang Q, Yu J. Dietary sodium/potassium intake and cognitive impairment in older patients with hypertension: Data from NHANES 2011–2014. J Clin Hypertens. 2023;25:534–544. 10.1111/jch.14667
DATA AVAILABILITY STATEMENT
The original data in this study are openly available in National Center for Health Statistics (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). And there is no additional data available.
REFERENCES
- 1. He FJ, Tan M, Ma Y, MacGregor GA. Salt reduction to prevent hypertension and cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(6):632‐647. [DOI] [PubMed] [Google Scholar]
- 2. Babcock MC, Robinson AT, Migdal KU, et al. Reducing dietary sodium to 1000 mg per day reduces neurovascular transduction without stimulating sympathetic outflow. Hypertension. 2019;73(3):587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2017 Diet . Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393(10184):1958‐1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang JG, Zhang W, Li Y, Liu L. Hypertension in China: epidemiology and treatment initiatives [published online ahead of print, 2023 Jan 11]. Nat Rev Cardiol. 2023. doi: 10.1038/s41569-022-00829-z [DOI] [PubMed] [Google Scholar]
- 6. Titze J, Luft FC. Speculations on salt and the genesis of arterial hypertension. Kidney Int. 2017;91(6):1324‐1335. [DOI] [PubMed] [Google Scholar]
- 7. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta‐analysis of randomised trials. BMJ. 2020;368:m315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brand A, Visser ME, Schoonees A, Naude CE. Replacing salt with low‐sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8(8):CD015207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin X, Paige E, Tian M, et al. The proportion of dietary salt replaced with potassium‐enriched salt in the SSaSS: implications for scale‐up. Hypertension. 2023; 80(5):956‐965. [DOI] [PubMed] [Google Scholar]
- 10. Aburto NJ, Hanson S, Gutierrez H, et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie Y, Qi H, Peng W, et al. Higher potassium intake and lower sodium intake may help in reducing CVD risk by lowering salt sensitivity of blood pressure in the Han Chinese population. Nutrients. 2022;14(20):4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marklund M, Tullu F, Raj TS, et al. Estimated benefits and risks of using a reduced‐sodium, potassium‐enriched salt substitute in india: a modeling study. Hypertension. 2022;79(10):2188‐2198. [DOI] [PubMed] [Google Scholar]
- 13. Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium‐to‐potassium ratio in the reduction of blood pressure: a meta‐analysis of randomized controlled trials. J Hypertens. 2015;33(8):1509‐1520. [DOI] [PubMed] [Google Scholar]
- 14. Marklund M, Singh G, Greer R, et al. Estimated population wide benefits and risks in China of lowering sodium through potassium enriched salt substitution: modelling study. BMJ. 2020;369:m824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Z, Wang H, Edwards D, et al. Association of blood lipids, atherosclerosis and statin use with dementia and cognitive impairment after stroke: a systematic review and meta‐analysis. Ageing Res Rev. 2020;57:100962. [DOI] [PubMed] [Google Scholar]
- 16. Milte CM, Ball K, Crawford D, McNaughton SA. Diet quality and cognitive function in mid‐aged and older men and women. Bmc Geriatr. 2019;19(1):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen X, Liu Z, Sachdev PS, et al. Dietary patterns and cognitive health in older adults: findings from the Sydney memory and ageing study. J Nutr Health Aging. 2021;25(2):255‐262. [DOI] [PubMed] [Google Scholar]
- 18. Ozawa H, Miyazawa T, Miyazawa T. Effects of dietary food components on cognitive functions in older adults. Nutrients. 2021;13(8):2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306(20):2229‐2238. [DOI] [PubMed] [Google Scholar]
- 20. Faraco G, Brea D, Garcia‐Bonilla L, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut‐initiated TH17 response. Nat Neurosci. 2018;21(2):240‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faraco G, Hochrainer K, Segarra SG, et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature. 2019;574(7780):686‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nowak KL, Fried L, Jovanovich A, et al. Dietary sodium/potassium intake does not affect cognitive function or brain imaging indices. Am J Nephrol. 2018;47(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rush TM, Kritz‐Silverstein D, Laughlin GA, et al. Association between dietary sodium intake and cognitive function in older adults. J Nutr Health Aging. 2017;21(3):276‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 25. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; 2006. [Google Scholar]
- 26. Wechsler D. WAIS‐III and WMS‐III Technical Manual. Psychological Corporation; 1997. [Google Scholar]
- 27. Lee H, Cho HJ, Bae E, et al. Not salt taste perception but self‐reported salt eating habit predicts actual salt intake. J Korean Med Sci. 2014;29 Suppl 2(Suppl 2):S91‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ikehara S, Iso H, Date C, et al. Salt preference and mortality from stroke and coronary heart disease for Japanese men and women: the JACC study. Prev Med. 2012;54(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 29. Li Y, Huang Z, Jin C, et al. Longitudinal change of perceived salt intake and stroke risk in a Chinese population. Stroke. 2018;49(6):1332‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santisteban MM, Iadecola C. Hypertension, dietary salt and cognitive impairment. J Cereb Blood Flow Metab. 2018;38(12):2112‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Little R, Murali SK, Poulsen SB, et al. Dissociation of sodium‐chloride cotransporter expression and blood pressure during chronic high dietary potassium supplementation. JCI Insight. 2023:e156437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao D, Sun H, Li H, Li C, Zhou B. A prediction model for the impact of environmental and genetic factors on cardiovascular events: development in a salt substitutes population. J Transl Med. 2023;21(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haring B, Wu C, Coker LH, et al. Hypertension, dietary sodium, and cognitive decline: results from the Women's Health Initiative Memory Study. Am J Hypertens. 2016;29(2):202‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuan M, Wang Y, Wen J, et al. Dietary salt disrupts tricarboxylic acid cycle and induces tau hyperphosphorylation and synapse dysfunction during aging. Aging Dis. 2022;13(5):1532‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hunter RW, Dhaun N, Bailey MA. The impact of excessive salt intake on human health. Nat Rev Nephrol. 2022;18(5):321‐335. [DOI] [PubMed] [Google Scholar]
- 36. Jaques DA, Wuerzner G, Ponte B. Sodium intake as a cardiovascular risk factor: a narrative review. Nutrients. 2021;13(9):3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rossitto G, Delles C. Mechanisms of sodium‐mediated injury in cardiovascular disease: old play, new scripts. Febs J. 2022;289(23):7260‐7273. [DOI] [PubMed] [Google Scholar]
- 38. Heye AK, Thrippleton MJ, Chappell FM, et al. Blood pressure and sodium: association with MRI markers in cerebral small vessel disease. J Cereb Blood Flow Metab. 2016;36(1):264‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makin S, Mubki GF, Doubal FN, et al. Small vessel disease and dietary salt intake: cross‐sectional study and systematic review. J Stroke Cerebrovasc Dis. 2017;26(12):3020‐3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nowak KL, Yaffe K, Orwoll ES, et al. Serum sodium and cognition in older community‐dwelling men. Clin J Am Soc Nephrol. 2018;13(3):366‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ozawa M, Ninomiya T, Ohara T, et al. Self‐reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: the Hisayama Study. J Am Geriatr Soc. 2012;60(8):1515‐1520. [DOI] [PubMed] [Google Scholar]
- 42. Peeri NC, Egan KM, Chai W, Tao MH. Association of magnesium intake and vitamin D status with cognitive function in older adults: an analysis of US National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Eur J Nutr. 2021;60(1):465‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The original data in this study are openly available in National Center for Health Statistics (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). And there is no additional data available.


