Abstract
High blood pressure (BP) and type‐2 diabetes (T2DM) are forerunners of chronic kidney disease and left ventricular dysfunction. Home BP telemonitoring (HTM) and urinary peptidomic profiling (UPP) are technologies enabling risk stratification and personalized prevention. UPRIGHT‐HTM (NCT04299529) is an investigator‐initiated, multicenter, open‐label, randomized trial with blinded endpoint evaluation designed to assess the efficacy of HTM plus UPP (experimental group) over HTM alone (control group) in guiding treatment in asymptomatic patients, aged 55–75 years, with ≥5 cardiovascular risk factors. From screening onwards, HTM data can be freely accessed by all patients and their caregivers; UPP results are communicated early during follow‐up to patients and caregivers in the intervention group, but at trial closure in the control group. From May 2021 until January 2023, 235 patients were screened, of whom 53 were still progressing through the run‐in period and 144 were randomized. Both groups had similar characteristics, including average age (62.0 years) and the proportions of African Blacks (81.9%), White Europeans (16.7%), women 56.2%, home (31.2%), and office (50.0%) hypertension, T2DM (36.4%), micro‐albuminuria (29.4%), and ECG (9.7%) and echocardiographic (11.5%) left ventricular hypertrophy. Home and office BP were 128.8/79.2 mm Hg and 137.1/82.7 mm Hg, respectively, resulting in a prevalence of white‐coat, masked and sustained hypertension of 40.3%, 11.1%, and 25.7%. HTM persisted after randomization (48 681 readings up to 15 January 2023). In conclusion, results predominantly from low‐resource sub‐Saharan centers proved the feasibility of this multi‐ethnic trial. The COVID‐19 pandemic caused delays and differential recruitment rates across centers.
Keywords: chronic kidney disease, home blood pressure telemonitoring, hypertension, left ventricular function, risk factors, type‐2 diabetes mellitus
1. INTRODUCTION
The epidemiological transition is a global demographic change characterized by a longer life expectancy, but the number of years added to the human life comes at a cost of lower quality of life, that is, a greater number of years lived with disability. 1 This demographic change represents a huge social and economic challenge, in particular in sub‐Saharan Africa, which are transitioning from mortality dominated by communicable, maternal, neonatal, and nutritional causes to non‐communicable diseases. 1 Hypertension is the main driver of this shift in the disease burden, however, with vastly growing contributions of obesity and type‐2 diabetes. 2 , 3 The International Diabetes Federation predicts that the number of diabetic patients will rise from 24 million presently to 55 million by 2045. 4
The Urinary Proteomics Combined with Home Blood Pressure Telemonitoring for Health Care Reform (UPRIGHT‐HTM) is an investigator‐initiated 5‐year clinical trial, which is currently recruiting patients in Europe and sub‐Saharan Africa. The primary objective of UPRIGHT‐HTM is to demonstrate that risk profiling based on the urinary peptidome (UPP) administered on top of blood pressure telemonitoring at home (HTM) will generate better estimates of a person's likelihood to proceed from the presence of risk factors to symptomatic disease compared to HTM alone. 5 The hypothesis is that the increased awareness of disease‐specific risk, as provided by the UPP, will motivate patients and caregivers to address risk factors more persistently than they would do just based on HTM, resulting in benefits as captured by the primary composite endpoint consisting of intermediate target organ damage and fatal and nonfatal adverse health outcomes. This article is the first progress report on UPRIGHT‐HTM trial. Its objectives are to document the feasibility of running this patient‐centered multi‐ethnic trial, to describe initial progress, to summarize the characteristics of the first patients enrolled and randomized.
2. METHODS
UPRIGHT‐HTM (ClinicalTrials.gov Identifier: NCT04299529) complies with the Helsinki declaration 6 and the European General Data Protection Regulations. 7 The Ethics Committee of University Hospitals Leuven, Belgium (Belgian registration number, B3222020000276), functioning as the central Ethics Approval Board and the Institutional Review Boards of all participating clinical sites approved the clinical trial protocol. 5
2.1. Design
UPRIGHT‐HTM is an investigator‐initiated randomized clinical trial, comparing UPP combined with HTM (experimental group) with HTM alone (control group) in risk profiling, thereby providing guidance in starting or intensifying the management of cardiovascular risk factors. Active recruitment is currently ongoing in Europe (Belgium, Poland, Slovenia), Asia (Republic of China), and sub‐Saharan Africa (Nigeria and South‐Africa). UPRIGHT‐HTM starts with a run‐in period of variable length (2–5 weeks) during which the eligibility of patients is checked. After stratification for center and sex, eligible patients are randomized in a 1:1 proportion by stratum to the experimental group (HTM plus UPP) or control (HTM alone), using an automated randomized algorithm and permuted blocks. Patients leaving the planned 3‐year follow‐up prematurely are invited to take part in supervised follow‐up, during which the incidence of endpoints will be further monitored (Figure 1). The primary endpoint is a composite of new‐onset intermediate and “hard” cardiovascular and renal outcomes and the secondary endpoints consist of the components of the primary endpoint. 5 Endpoint validation will be done by the open study design blinded endpoint validation (PROBE) methodology. 8
FIGURE 1.
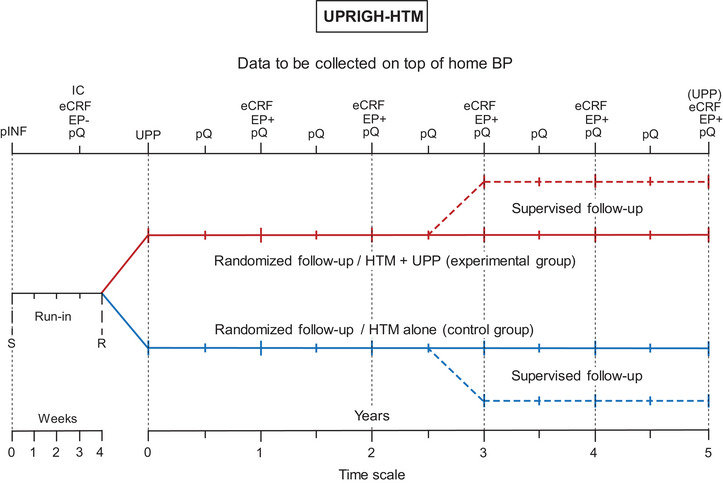
UPRIGHT‐HTM design. eCRF, electronic report forms completed by investigators; EP, absence (−) / incidence (+) of the components of the primary endpoint during the run‐in/follow‐up periods, respectively; IC, written informed consent; pINF, patients received the UPRIGHT‐HTM information sheet and were familiarized with operating the blood pressure monitoring devices; pQ, patient‐administered questionnaires, R, randomization after stratification for center and sex; S, initial screening; UPP, urinary proteomic profiling (mandatory prior to randomization—optional at the end of follow‐up). Reproduced from open‐access reference 5.
2.2. Selection of patients
Asymptomatic patients of either sex, aged 55−75 years can be enrolled, after written informed consent has been obtained. In addition to age, patients must have at least five additional guideline‐defined risk factors, preferably including hypertension, type‐2 diabetes mellitus, or both. Furthermore, patients must have an email address and internet access via smartphone and must be willing to engage in HTM for the duration of the study.
Study‐specific exclusion criteria include type‐1 diabetes, symptomatic renal or left ventricular dysfunction, chronic kidney disease stage‐3B or worse 9 defined as a glomerular filtration rate derived from serum creatinine 10 less than 45 mL/min/1.73 m2, a history of cardiovascular or renal disease within 1 year prior to enrolment, or any condition affecting the assessment of echocardiographic imaging of the left ventricle, such as a poor echocardiographic window, significant valvular heart disease, irregular heart rhythm. Exclusion criteria common to all clinical research include serious cardiovascular or noncardiovascular disease, cancer within 5 years prior to enrolment, suspected substance abuse, psychiatric illness, or participation in other studies. However, patients who experienced a cardiovascular or non‐cardiovascular event or a renal complication 1 year or longer before being considered for randomization qualify for entry if they fully recovered and are asymptomatic.
2.3. Data collection
UPRIGHT‐HTM is running as a patient‐centered study, mainly positioned at the patients’ homes and primary care practices or out‐patient clinics, using the CE‐certified web‐based and highly secured WiPaM platform (Wireless Patient Monitoring), developed by RDSM, Hasselt, Belgium (https://www.rdsm.eu).
2.3.1. Data collected by patients
For HTM, patients are using the validated 11 OMRON HEM 9210‐T monitors (Omron Healthcare Co., Ltd., Kyoto, Japan) fitted with a cuff that accommodates the range of adults upper‐arm circumferences. Patients are instructed to measure their blood pressure after 5 min rest in the sitting position preferably within 1 h after awakening, before breakfast and before taking any medication, if possible, daily. At randomization (and at 6‐monthly intervals during the study), patients complete in their native language the EQ‐5D quality of life questionnaire (https://euroqol.org) and the World Health Organization (G. Rose) questionnaire on chest pain (Figure 1). 12 The HTM and questionnaire data are collected via an android‐based smartphone application developed by RDSM.
2.3.2. Data collected by caregivers
Prior to randomization, caregivers collect informed written consent and complete the prerandomization eCRF showing that enrolled patients meet all eligibility criteria. Other information to be collected via the prerandomization eCRF includes: anthropometrics, use of medications by drug class, the patient's medical history, and the presence versus absence of components of the primary endpoint. Patients are referred to for echocardiography before or shortly after randomization (Figure 1). Venous blood samples are collected for hematological and biochemical measurements and the midmorning urine is sampled for the assessment of microalbuminuria (on at least two consecutive days) and UPP. All routine biological samples are processed by local certified laboratories. Low‐density lipoprotein (LDL) serum cholesterol is calculated from total serum cholesterol, high‐density lipoprotein (HDL) serum cholesterol and the serum triglyceride concentration by the Friedewald formula. 13 The glomerular filtration rate is computed from serum creatinine, using the Chronic Kidney Disease Epidemiology (CKD‐EPI) Collaboration formula. 10 Urine samples for UPP are kept deep‐frozen at −20°C until assayed at Mosaiques‐Diagnostics GmbH, Hannover, Germany. The UPP results fall outside the scope of this first UPRIGHT‐HTM progress report. ECGs are read by physicians and coded for voltage and repolarization changes indicative of left ventricular hypertrophy. 14
2.4. Echocardiography
A detailed description of the procedures for echocardiography has been published. 5 All centers used General Electric echocardiographic devices (Horton, Norway) interfaced with appropriate probes (Table S1). With patients in partial left decubitus and breathing normally, echocardiographic images are obtained together with a simultaneous ECG signal along the parasternal long and short axes and from the apical 4‐ and 2‐chamber long‐axis views. All recordings from each echocardiographic window must include at least five cardiac cycles and are digitally stored for off‐line analysis. M‐mode echocardiograms of the left ventricle are recorded from the parasternal long‐axis view under control of the 2‐dimensional image with the ultrasound beam positioned just below the mitral valve at the level of the posterior chordae tendineae. Using Tissue Doppler Imaging, the observers then record low‐velocity, high‐intensity myocardial signals at a high frame rate, while adjusting the imaging angle to ensure a parallel alignment of the ultrasound beam with the myocardial segment of interest. From the apical window, the sonographer places a 5‐mm3 Doppler sample at the septal, lateral, inferior or posterior sites of the mitral annulus, or at a combination of these sites.
The end‐diastolic left ventricular dimensions are used to calculate left ventricular mass by an anatomically validated formula. 15 Left ventricular mass index is left ventricular mass divided by body surface area. Left ventricular hypertrophy is a left ventricular mass index exceeding 110 g/m2 in women, and 125 g/m2 in men. Left atrial dimensions are measured in three orthogonal planes: the parasternal long, lateral, and superior‐to‐inferior axes. Left atrial volume is calculated using the prolate‐ellipsoid method and indexed to body surface area. Left atrial enlargement 16 is an atrial volume index exceeding 34 mL/m2. Left ventricular diastolic dysfunction is an abnormally low age‐specific transmitral E/A ratio, indicative of impaired relaxation, or a mildly‐to‐moderately elevated left ventricular filling pressure (E/e' > 8.5) with normal or decreased age‐specific E/A ratio in patients with an ejection fraction of 50% or higher. 17 , 18 Higher e’ and lower E/e’ on the Doppler imaging reflect faster early diastolic left ventricular relaxation and lower left ventricular filling pressure, respectively.
2.5. Statistical analysis
Data management and analysis were done using SAS version 9.4. From the WiPaM platform, completely anonymized data are directly imported into SAS at the Study Coordination Center. The central tendency (spread) of variables is represented by the arithmetic mean (SD) for normally distributed variables and by the median (interquartile range) for non‐normally distributed continuous variables. Categorical variables are presented as proportions. For the current analysis, which was restricted to baseline, group means and medians were compared by the unpaired t‐test and the Mann‐Whitney U test, respectively, while proportions were compared by a χ2 statistic or Fisher exact test, as appropriate according to the cell counts. Left ventricular diastolic dysfunction was correlated with the E/e’ ratio, using the Fisher Z transform. Statistical significance was a two‐sided α‐level of 0.05.
3. RESULTS
This report was compiled from data available on 15 January 2023. Across 20 centers, 235 patients had been screened, of which 20 did not meet the eligibility criteria, 10 had an incompatible mobile phone, and 4 defaulted for other reasons (Figure 2). Of the 201 patients enrolled and who went into the run‐in phase, 144 were randomized, while 53 were eligible, but still proceeding through the run‐in period. Furthermore, 1 patient withdrew consent and 3 did not adhere to HTM and were therefore not randomized.
FIGURE 2.
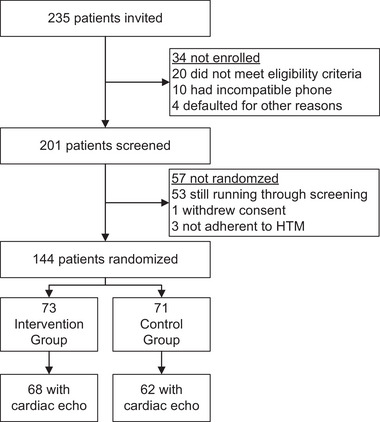
Flow Chart. HTM refers to home blood pressure telemonitoring.
3.1. Patients at screening
The characteristics of randomized and nonrandomized patients are summarized in Table 1. In addition to patients who were not enrolled (n = 34), non‐adherent to HTM (n = 3), or withdrew consent (n = 1), the nonrandomized patients (n = 91) also included 53 individuals, which were apparently eligible but who were still proceeding through the screening period. Randomized and nonrandomized patients had broadly similar characteristics. However, randomized compared with nonrandomized patients had higher body weight (85.7 vs. 80.2 kg; p = .021), higher body mass index (31.6 vs. 29.4 kg/m2; p = .010), and greater waist circumference (103.0 vs. 98.8 cm; p = .032). On the other hand, office heart rate (73.6 vs. 77.1 beats per minute; p = .035) was lower and office systolic/diastolic blood pressure (136.9/82.9 vs. 136.2/82.8 mm Hg; p ≥ .763) were similar in randomized compared with nonrandomized patients. In addition, there were no differences in the distributions of ethnicity and sex between the two groups. Of the randomized patients, 118 (81.9%) were Black Africans, 24 (16.7%) White Europeans, and 2 (1.4%) Chinese.
TABLE 1.
Characteristics of participants at screening.
| Characteristic | Not randomized | Randomized | p‐value |
|---|---|---|---|
| Number in group | 91 | 144 | |
| N° with characteristic (%) | |||
| Ethnicity | |||
| Chinese, n (%) | 3 (3.3) | 2 (1.4) | .636 |
| Whites, n (%) | 15 (16.5) | 24 (16.7) | |
| Black Africans, n (%) | 73 (80.2) | 118 (81.9) | |
| Igbo, n (%) | 30 (33) | 40 (27.8) | .202 |
| Yoruba, n (%) | 7 (7.7) | 20 (13.9) | |
| Hansa‐Fulani, n (%) | 7 (7.7) | 5 (3.5) | |
| Other Nigerian ethnicity, n (%) | 29 (31.9) | 53 (36.8) | |
| Female sex, n (%) | 48 (52.7) | 81 (56.2) | .696 |
| Current smoking, n (%) | 4 (4.8) | 3 (2.1) | .534 |
| Drinking alcohol, n (%) | 10 (11.9) | 15 (10.5) | >.999 |
| Mean characteristic (± SD) | |||
| Age, y | 62.1 (5.2) | 62.0 (5.4) | .839 |
| Body height, cm | 165.3 (8.4) | 164.8 (8.3) | .702 |
| Body weight, kg | 80.2 (15.5) | 85.7 (17.9) | .021 |
| Body mass index, kg/m2 | 29.4 (5.5) | 31.6 (6.40) | .010 |
| Waist circumference, cm | 98.8 (13.7) | 103.0 (13.6) | .032 |
| Office blood pressure | |||
| Systolic, mm Hg | 136.2 (19.4) | 137.0 (15.8) | .763 |
| Diastolic, mm Hg | 82.8 (10.8) | 82.8 (10.4) | .946 |
| Office heart rate, beats per minute | 77.1 (11.5) | 73.6 (12.0) | .035 |
Current smoking was inhaling tobacco smoke on a daily basis. Drinking alcohol was the occasional or daily consumption of ethanol containing beverages. Nonrandomized patients included 53 apparently eligible patients, who were still progressing through the screening period (Figure 1 ).
3.2. Patients at randomization
The median interval from screening to the prerandomization visit was 28 days (IQR: 21–42; 10th–90th percentile interval: 14–62). At randomization, patients randomized to the control and experimental group had similar anthropometric characteristics, blood pressure, renal function, biochemical measurements, and prevalence of hypertension and diabetes (Table 2). However, as shown in Tables 2 and S2, patients randomized to the experimental group (N = 67) compared with control (N = 66) had lower total serum cholesterol (190.2 vs. 209.9 mg/dL; p = .052), similar HDL cholesterol (52.6 vs. 48.2 mg/dL; p = .184), lower LDL cholesterol (116.6 vs. 141.5 md/dL; p = .005), and lower non‐HDL cholesterol (138.7 vs. 163.9 mg/dL; p = .005), resulting in a lower total‐to‐HDL serum cholesterol ratio (3.99 vs. 4.99; p = .009).
TABLE 2.
Main characteristics of randomized participants (starts).
| Control | Experimental | ||||
|---|---|---|---|---|---|
| Characteristic | N | Estimate | N | Estimate | p‐value |
| Ethnicity | |||||
| Chinese, n (%) | 71 | 1 (1.4) | 73 | 1 (1.4) | .911 |
| Whites, n (%) | 71 | 11 (15.5) | 73 | 13 (17.8) | |
| Black Africans, n (%) | 71 | 59 (83.1) | 73 | 59 (80.8) | |
| Female sex, n (%) | 71 | 42 (59.2) | 73 | 39 (53.4) | .600 |
| Current smoking, n (%) | 71 | 1 (1.4) | 73 | 2 (2.7) | >.999 |
| Drinking alcohol, n (%) | 71 | 8 (11.3) | 73 | 7 (9.6) | .955 |
| Home hypertension, n (%) | 71 | 29 (40.8) | 73 | 22 (30.1) | .242 |
| Office hypertension, n (%) | 71 | 34 (47.9) | 73 | 38 (52.1) | .739 |
| True normotension, n (%) | 71 | 30 (42.3) | 73 | 28 (38.4) | .759 |
| White‐coat hypertension, n (%) | 71 | 12 (16.9) | 73 | 23 (31.5) | .065 |
| Masked hypertension, n (%) | 71 | 7 (9.9) | 73 | 7 (9.6) | >.999 |
| Sustained hypertension, n (%) | 71 | 22 (31) | 73 | 15 (20.5) | .214 |
| Treated hypertension, n (%) | 71 | 69 (97.2) | 73 | 69 (94.5) | .702 |
| Type‐2 diabetes | 71 | 26 (36.6) | 73 | 25 (34.2) | .902 |
| Treated diabetes | 71 | 12 (16.9) | 73 | 15 (20.5) | .671 |
| Microalbuminuria, n (%) | 69 | 21 (30.4) | 70 | 20 (28.6) | .956 |
| Mean characteristic (± SD) | |||||
| Age, y | 71 | 61.7 (5.1) | 73 | 62.3 (5.6) | .525 |
| Body mass index, kg/m2 | 71 | 31.5 (5.8) | 73 | 31.7 (7.0) | .837 |
| Waist circumference, cm | 71 | 102.8 (13.5) | 73 | 103.1 (13.9) | .922 |
| Home systolic pressure, mm Hg | 71 | 130.6 (10.9) | 73 | 127.2 (10.0) | .053 |
| Home diastolic pressure, mm Hg | 71 | 80.0 (8.2) | 73 | 78.3 (7.1) | .194 |
| Home heart rate, beats per minute | 71 | 71.2 (10.4) | 73 | 72.7 (9.2) | .355 |
| Office systolic pressure, mm Hg | 71 | 138.0 (15.1) | 73 | 136.2 (14.4) | .461 |
| Office diastolic pressure, mm Hg | 71 | 83.7 (10.8) | 73 | 81.7 (9.5) | .237 |
| Office heart rate, beats per minute | 71 | 73.1 (12.2) | 73 | 75.4 (11.9) | .250 |
| Total serum cholesterol, mg/dL | 66 | 209.9 (65.4) | 67 | 190.2 (49.2) | .052 |
| Total‐to‐HDL serum cholesterol ratio | 66 | 4.99 (2.54) | 67 | 3.99 (1.60) | .009 |
| Serum creatinine, mg/dL | 63 | 0.93 (0.38) | 64 | 0.97 (0.26) | .492 |
| eGFR, mL/min/1.73 m2 | 63 | 79.5 (21.4) | 64 | 74.2 (18.3) | .133 |
Body mass index is weight (kg) divided by height squared (m2). Home blood pressure is the average of all available readings up to the prerandomization visit. Home hypertension is a self‐measured blood pressure of ≥135 mm Hg systolic or ≥85 mm Hg diastolic. Office hypertension is an office blood pressure of ≥140 mm Hg systolic or ≥90 mm Hg diastolic. If systolic and diastolic blood pressure were in different categories, patients were classified in the highest category. White‐coat hypertension is an elevated office blood pressure with a normal home blood pressure. Masked hypertension is a normal office blood pressure combined with an elevated home blood pressure. Home and office blood pressure were cross‐classified irrespective of antihypertensive drug treatment status. Treated diabetes refers to patients on antidiabetic drugs. Current smoking was inhaling tobacco smoke on a daily basis. Drinking alcohol was the occasional or daily consumption of ethanol containing beverages. Additional measurements are listed in Table S2.
Abbreviations: N, number of participants with data in group; HDL, high‐density lipoprotein; eGFR, glomerular filtration rate derived from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration formula.
In the control compared with the experimental group, there were no differences in the patients on diuretics (28 [39.4%] vs. 32 [43.8 %]; p = .616), β‐blockers (10 [14.1%] vs. 13 [17.8 %]; p = .651), angiotensin converting‐enzyme inhibitors (25 [35.2%] vs. 21 [29.6 %]; p = .476), angiotensin receptor blockers (35 [49.3%] vs. 30 [42.3 %]; p = .403), and calcium‐channel blockers (44 [62.0%] vs. 45 [61.6 %]; p > .999). Furthermore, in the control and experimental group, 38 (53.5%) versus 32 (43.8%) were on monotherapy with a single drug class (p = .317), whereas 22 (31.0%) versus 30 (41.1%) were on combination therapy (p = .228). The number of patients taking orally administered antidiabetic drugs was 12 (16.9%) in the control and 15 (20.8%) in the experimental group (p = .671) with metformin most commonly used: 12 (17.9%) versus 14 (19.2%); p = .830. In the control and experimental group, the patients using insulin or parenterally administered antidiabetic agents amounted to 1 (1.4 %) versus 0 (0%), respectively (p = .497). Finally, the number of patients taking lipid‐lowering drugs was 31 (43.7%) in the control and 35 (47.9%) in the experimental group (p = .620) with statins most frequently used: 31 (43.7%) versus 34 (46.6%); p = .741.
Analyses of the 144 randomized patients by sex (Table S3), revealed the expected differences in the body height (women vs. men; 159.9 vs. 171.0 cm; p < .001), home heart rate (73.7 vs. 69.7 bpm, p = .015), hemoglobin (12.8 vs. 14.2 g/dL; p < .001), hematocrit (38.6 vs. 42.2%; p < .001), the red blood cell count (4.51 vs. 4.82 million per mm3; p = .008)), and the serum creatinine concentration (0.88 vs. 1.04 mg/dL; p = .005).
3.3. Further analyses of blood pressure
The distributions of office and home blood pressure (average of all reading from screening to the prerandomization visit) did not significantly deviate from the normal distribution (p ≥ .150; Figure 3).
FIGURE 3.
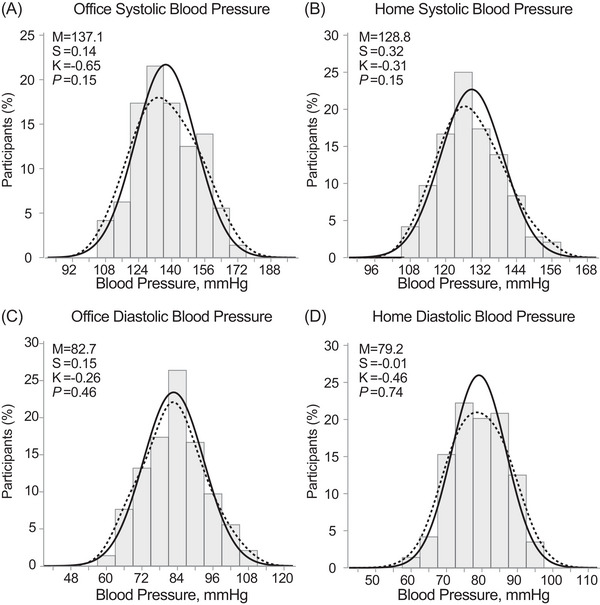
Distributions of the Office (A,C) and Home (B,D) systolic and diastolic blood pressure. The solid and dotted lines represent the normal and kernel density distributions. p‐values are for departure of the actually observed distribution from normality according to the Shapiro‐Wilk statistic. M indicates the arithmetic mean. Skewness (S) and kurtosis (K) were computed as the third and fourth moments about the mean divided by the cube of the standard deviation. The home blood pressure was the average of all readings from screening to randomization.
3.3.1. Cross‐classification of home and office blood pressure
Patients were cross‐classified according to their home and office blood pressure, irrespective of their antihypertensive treatment status. The thresholds applied were < 135/ < 85 versus ≥135/≥85 mm Hg for the home blood pressure and < 140/ < 90 versus ≥140/≥90 mm Hg for the office blood pressure. If systolic and diastolic blood pressure were in different categories, the highest level was used for categorization. The home blood pressure readings used for the cross‐classification were obtained from screening to the prerandomization visit. In the control compared with the experimental group, the prevalence of true normotension and white‐coat, masked and sustained hypertension amounted to 30 versus 28 (42.3 vs. 38.4%; p = .759), 12 versus 23 (16.9 vs. 31.5%; p = .065), 7 versus 9 (9.9 vs. 9.6%; p > .999), and to 22 versus 15 (31.0 vs. 20.5%; p = .214), respectively.
3.3.2. Persistence of home blood pressure monitoring
In the 144 randomized patients, the median time interval between the prerandomization visit and the last day of home blood pressure monitoring amounted to 315 days (IQR: 226–446 days; 10th–90th percentile interval: 167–539 days). The total number of home blood pressure readings in the 144 randomized patients after randomization amounted to 48 681. The median number of home blood pressure readings per center was 1554 (IQR: 274–7142), per patient 200 (IQR: 89–390), and per center and patient 199 (IQR: 134–233; Table S4). Figure S1 shows the distribution of home blood pressure readings as function of the time since the prerandomization visit. Figure S2 displays an exemplary recording of the home blood pressure in a Nigerian patient (Panel A) and the means with 95% confidence interval of all home blood pressure in randomized patients from the prerandomization visit to each patient's last reading up to 15 January 2023 (Panel B). Figure S3 shows the number of patients screened (Panel A), the number of patients randomized (Panel B), and the number of home blood pressure (Panel C) as a function of time. Figure S3 illustrates that the patients were adherent to the protocol requiring them to monitor their home blood pressure.
3.4. Questionnaire data
At the time of the prerandomization visit, 104 (72.2%) of 144 randomized patients had completed the quality‐of‐life form and 118 (81.9%) the WHO questionnaire. Self‐assessment of health relied on a visual analogue scale ranging from 0 (worst health possible) to 100 (best health possible). Self‐assessed scores of less than 40 were deemed incompatible with the eligibility criteria of the trial, leaving 101 questionnaires available for analysis. The median self‐assessed health score was 79 (N = 48; IQR: 72–90; 10th–90th percentile interval: 63–97) in the control group and 84 (N = 52; IQR: 71–90; 10th–90th percentile interval: 61–98) in the experimental group (p = .338 for the between‐group difference).
The presence of possible angina pectoris as picked up by the WHO questionnaire was defined as chest pain on exertion (walking fast or uphill) and disappearing after stopping the exercise. Of the patients who completed the questionnaire in the control (N = 60) and experimental group (N = 58), angina pectoris was suspected in 1 (1.7%) and 2 (3.4%) of patients (p > .509). However, none of the patients had overt angina pectoris according to the physician's supervising recruitment.
3.5. Left ventricular structure and function
The number of patients with ECG measurement was 63/71 (88.7%) in the control group versus 67/73 (91.8%) in the experimental group. All patients were in sinus rhythm. For 130 (90.3%) an echocardiographic examination was available around the time of randomization.
3.5.1. ECG measurements
The number of patients with ECG left ventricular hypertrophy was 9/63 (14.3%) versus 6/67 (9.0%) in the control versus experimental group, respectively (p = .415).
3.5.2. Echocardiographic measurements
The number of patients with echocardiographic studies was 62/71 (87.3%) in the control group and 68/73 (93.2%) in the experimental group. By and large, there were very few statistical between‐group differences in the echocardiographic measurements (Tables 3andS5) capturing left ventricular structure and left ventricular systolic and diastolic function. In the control compared with the experimental group (Tables 3 and S5), left ventricular ejection fraction was slightly higher (68.3 vs. 64.7%; p = .050), whereas the late diastolic peak velocity of the mitral annulus was lower in control patients (9.5 vs. 10.4 cm/s; p = .044). More importantly, left ventricular mass index (control vs. experimental; 90.5 vs. 89.1 g/m2; p = .787), left atrial volume index (27.9 vs. 26.6 g/m2; p = .840), E/e’ (8.46 vs. 7.86; p = .127), and the prevalence of echocardiographic left ventricular hypertrophy (9/62 [14.5%] vs. 8/68 [11.8%]; p = .838) and left ventricular diastolic dysfunction (19/61 [30.6%] vs. 23 [34.8%]; p = .751) were similar in the two randomized groups. The correlation coefficient of left ventricular diastolic dysfunction with the E/e’ ratio was 0.68 (p < .001).
TABLE 3.
ECG and echocardiographic measurements at randomization (starts).
| Control | Experimental | ||||
|---|---|---|---|---|---|
| Characteristic | N | Estimate | N | Estimate | p‐value |
| ECG measurements | |||||
| Sokolow‐Lyon index, mV | 60 | 2.29 (0.75) | 62 | 2.33 (0.74) | .775 |
| R‐wave in aVl, mV | 60 | 0.64 (0.32) | 61 | 0.60 (0.28) | .527 |
| Cornell product mV × ms | 57 | 116.8 (72.3) | 61 | 130.8 (66.6) | .277 |
| ECG LVH, n (%) | 60 | 9 (15.0) | 62 | 6 (9.7) | .596 |
| M‐mode LV structure | |||||
| Aortic root diameter, mm | 62 | 29.5 (4.2) | 68 | 29.7 (4.6) | .753 |
| Left atrial diameter, mm | 62 | 36.3 (6.5) | 64 | 36.3 (5.2) | .725 |
| Ratio of left atrial‐to‐aorta diameter | 62 | 1.27 (0.28) | 64 | 1.25 (0.25) | .705 |
| Interventricular septum, mm | 62 | 10.9 (2.07) | 68 | 11.1 (2.09) | .662 |
| Posterior wall thickness, mm | 62 | 10.6 (1.63) | 68 | 10.7 (1.77) | .840 |
| End‐diastolic diameter, mm | 62 | 43.9 (5.3) | 68 | 44.6 (7.8) | .521 |
| End systolic diameter, mm | 61 | 27.1 (5.2) | 68 | 28.7 (6.7) | .152 |
| Relative wall thickness | 62 | 49.9 (9.9) | 68 | 50.7 (13.5) | .721 |
| LV mass, g | 62 | 166.1 (46.2) | 68 | 173.4 (52.0) | .402 |
| LV mass index, g/m2 | 62 | 90.5 (30.9) | 68 | 89.1 (23.5) | .787 |
| Echocardiographic LVH, n (%) | 62 | 9 (14.5) | 68 | 8 (11.8) | .838 |
| LV systolic function | |||||
| Fractional shortening, % | 61 | 37.8 (8.4) | 68 | 35.4 (7.6) | .090 |
| Ejection fraction, % | 62 | 68.3 (11.1) | 68 | 64.7 (9.9) | .050 |
| Stroke volume, mL | 62 | 60.5 (19.9) | 68 | 60.5 (22.4) | .985 |
| Stroke volume index, mL/m2 | 62 | 33.1 (13.2) | 68 | 31.2 (10.9) | .368 |
| Cardiac output, L/min | 61 | 4.15 (1.49) | 66 | 4.30 (1.83) | .605 |
| LV diastolic function | |||||
| Isovolumetric relaxation time, ms | 58 | 104 (22) | 65 | 104 (30) | .974 |
| Transmitral E/A ratio | 62 | 0.99 (0.43) | 67 | 0.93 (0.28) | .371 |
| Mitral annular e’/a’ ratio | 62 | 0.90 (0.48) | 65 | 0.78 (0.30) | .102 |
| E/e’ ratio | 62 | 8.46 (1.93) | 66 | 7.86 (2.50) | .127 |
| LV diastolic dysfunction, N (%) | 62 | 19 (30.6) | 66 | 23 (34.8) | .751 |
Additional echocardiographic measurements are available in Table S5 .
Abbreviations: LV, left ventricular; LVH, left ventricular hypertrophy; N, number of participants with data in group.
Furthermore, in analyses of women compared with men (Table S6), the diameter of the aortic root (28.0 vs. 31.9 mm; p < .001), the end‐diastolic (42.6 vs. 46.6 mm; p < .001) and end‐systolic (26.5 vs. 29.9 mm; p = .002) left ventricular diameters, stroke volume (55.8 vs. 66.8 mL; p = .003) and stroke volume index (30.1 vs. 34.8 mL/m2; p = .027) were smaller in women than men.
3.6. Potential for future recruitment
Table S7 lists all UPRIGHT‐HTM recruiting sites, their initiation status, and the number of HTM devices that were or will be made available. Balanced against the number of randomized patients (N = 144), these results illustrate a great potential for further recruitment amounting to 581, that is, the difference between the number of devices in use by screened and randomized patients and the number of devices still unused.
4. DISCUSSION
UPRIGHT‐HTM is an investigator‐initiated multicenter clinical trial with as primary aim of demonstrating that risk profiling based on UPP administered on top of HTM will lead to more rigorous risk factor management and result in better outcomes. Collecting the self‐measured blood pressure and questionnaires via smartphone‐based applications is in line with the patient‐centered design of UPRIGHT‐HTM (Figure 1). This means that clinic visits are limited to the routine for follow‐up of hypertensive, diabetic or high‐risk patients at each recruitment site with no obligation to increase patient visits to accommodate trial procedures. Follow‐up visits are scheduled at annual intervals (Figure 1). This progress report shows that UPRIGHT‐HTM has been successfully deployed in 20 centers with a total of 192 patients either randomized (n = 144) or awaiting randomization (n = 53). Patients randomized into UPRIGHT‐HTM are apparently healthy without active or symptomatic cardiovascular or non‐cardiovascular disease. The 144 randomized patients had a median of 9 risk factors among women and 7 among men (Table S3). The prevalence of home hypertension, office hypertension and microalbuminuria were 31.2%, 50.0%, 29.4% respectively. Thus, in keeping with the concepts underlying the UPRIGHT‐HTM design, 5 the randomized patients in follow‐up were at high risk of developing target organ damage, in particular left ventricular diastolic dysfunction (LVDD) and chronic kidney disease (CKD), or experiencing “hard” cardiovascular endpoints, which will be blindly validated according to the PROBE design. 8 The high prevalence of these risk factors offers a variety of avenues, including lifestyle modifications and drug treatment using national and international guidelines, which caregivers can explore to improve the health of their patients. A glimpse into HTM data collected over 15 months reveals a blood pressure trajectory that was on average controlled (Figure S2).
The high‐risk profile of the UPRIGHT‐HTM patients (median number of risk factors on top of age in both sexes combined: 8 [IQR: 7–10] instead of 5) has implications for the power of the trial and required sample size. Indeed, the initial sample size calculations, 5 assumed a 20% rate of the primary composite endpoint in the control group and a 30% reduction of its rate in the experimental group with power and the 2‐sided α‐level set at 0.80 and 0.05, respectively. This would have required a total sample size of 1148 patients (574 per group). The higher risk profile than originally anticipated will likely result in a 30% instead of a 20% rate of the primary endpoint in the control group. A 30% reduction in the incidence of the primary endpoint in the experimental group, would require a total sample size of 686 patients (343 per group).
Given that the characteristics of non‐randomized and randomized patients were broadly similar, there was little selection bias during the screening period. The few differences between the control and experimental groups, for instance in the prevalence of white‐coat hypertension or serum lipids (Table 2), were due to between‐group differences in the recruitment rates at the Nigerian and European clinical centers. They are likely to disappear as recruitment continues and will therefore have no impact on the final results of the UPRIGHT‐HTM trial. Furthermore, randomized compared with non‐randomized patients had higher body weight, higher body mass index and greater waist circumference, perhaps because of the priority placed on hypertension and type‐2 diabetes mellitus, which combined were prevalent in no less than 60% of the randomized patients. The idea that the randomized patients were representative can further be justified by the internal validation based on the sex‐wise comparison of clinical characteristics. The observed sex difference in serum creatinine, hemoglobin, hematocrit, red blood cell count (Table S3) and a panel of structural and functional echocardiographic characteristics (Table S5) are typical of what exists in the general population. 19 , 20 , 21 , 22 , 23 , 24 The hematological sex differences are consistent with reports on Ugandans, Burkinabès, African Americans, Ethiopians, and citizens of the Central African Republic. 19 Serum creatinine is a product of muscle breakdown and is well‐known to be influenced by sex due to higher muscle mass in men than women. 20 Compared to women, men have greater left ventricular mass and left ventricular mass index, because of greater end‐diastolic diameter and posterior wall thickness (Table S5). This morphological phenomenon, which is also exemplified among athletes, is partly attributed to difference in body size. 21 , 22 , 23 , 24
Considered independently, the potential of HTM 25 , 26 , 27 , 28 , 29 and UPP 30 , 31 , 32 , 33 , 34 in the risk factor management is overwhelming. In keeping with the results of the HOMED‐BP trial (Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure), 25 long‐term use of HTM not only proved to be feasible, but also beneficial for blood pressure control (Figure S2). HTM overcomes treatment inertia and ensures that patients actively participate in the management of their risk profile along with their care providers. The evidence of the feasibility and potential benefit of HTM can be deduced from the number of home blood pressure records obtained (Figure S2) and the steady trajectory over 15 months of the monthly systolic and diastolic averages, which were less than 135 mm Hg systolic and 85 mm Hg diastolic. HTM is a powerful instrument in educating and empowering patients. 26 In a randomized clinical trial, involving 450 patients recruited at 59 primary care practices and followed up for 12 months, self‐monitoring and self‐titration of antihypertensive drugs lowered systolic blood pressure 8.8 mm Hg more than usual care based on office blood pressure measurement. 27 Moreover, self‐measurement of blood pressure increases adherence to antihypertensive drugs, 28 allows detecting symptoms that occur between clinic visits and reduces the number of clinic visits required for optimizing drug treatment. 29 However, self‐titration of medication was not considered as a practicable option in UPRIGHT‐HTM, because of the multi‐ethnic and multicultural settings of the study sites.
Both CKD and LVDD are associated with specific UPP profiles with high within‐individual reproducibility over a median follow‐up of 5 years as demonstrated for a UPP biomarker of accelerated aging in the Flemish Study on Environment, Genes and Health Outcomes. 35 The multidimensional marker CKD273 predicts progression of CKD earlier than micro‐albuminuria does. 33 HF1 is a marker of subclinical LVDD (stage‐2 heart failure). 31 Both CKD273 33 , 36 and HF1 31 have been extensively validated in longitudinal patient and population studies, and CKD273 also in the framework of the PRIORITY study. 32 Pharmacological treatment, including antihypertensive, lipid‐lowering, antidiabetic and antiplatelet drugs and even immunosuppressive drugs in transplant patients, 37 have no noticeable influence on the UPP or its association with study endpoints. As a working example, HF1 might be applied in clinical practice in asymptomatic high‐risk individuals. 30 In the presence of clinical risk factors for LVDD, in particular older age combined with overweight or abdominal obesity and hypertension (prevalence of 25.1% in European population studies, 30 HF1 might be used as a screening tool. 30 If its value is less than −0.350, managing risk factors over a 5‐year time span might be recommended. In contrast, if HF1 is −0.350 or higher, patient should not only have their risk factors addressed, but might be referred for echocardiography to confirm the presence of LVDD. 30 An added benefit of HF1 is that the marker predicts worsening of renal function 36 and the 5‐year incidence of cardiovascular and cardiac events. 38 In previous studies, 30 , 31 in line with other publications, 39 N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) did not add to the prediction of LVDD over and beyond classical risk factors. Moreover, HF1 in the presence of NT‐proBNP fully retained its prognostic value. 30 , 31 At the time of writing of this manuscript, urine samples of 106 Nigerian patients had already been collected and processed. The risk classifiers of LVDD and CKD that is, HF1 30 , 31 and CKD273 32 , 33 respectively, have already been communicated to the patients randomized to the experimental group and their caregivers. A full description of the over 20 000 peptides, of which 25% have been sequenced, thereby identifying the parental proteins and the molecular pathophysiological mechanisms underlying disease, will be presented in later UPRIGHT‐HTM reports. To our knowledge, there is no prior publication on the UPP of Blacks “born and living” in Africa. However, although the risk of left ventricular hypertrophy increases with blood pressure in Black Nigerians and Flemish Whites, the association is 3‐folds stronger in the former compared with latter. 14 Left ventricular remodeling does not occur without altering the extracellular matrices and this event is well known to be reflected by the UPP. 34
The COVID‐19 pandemic was a major obstacle in rolling out the trial procedures and to some extent explains the regional differences in patient recruitment. After the first patients had been enrolled in the Abuja center, a first interim analysis in February 2022 identified the need to debug the software running at the WiPaM website, a tedious process, which took several months. In some patients, poor internet connectivity and not handling the smartphone application in a correct way caused problems in acquiring the date and time of the home blood pressure readings. The problem was solved when these patients allowed the UPRIGHT‐HTM software to access the date and time on their android devices. When faced with poor access to the internet, they were encouraged to continue self‐monitoring of their blood pressure, because the software is designed to upload backlogged blood pressure records, whenever a better internet connection is available. Misinterpretation of the visual analogue scale, part of the EQ‐5D quality‐of‐life questionnaire, caused a loss of self‐assessed health status data. APPREMED, the sponsor of the UPRIGHT‐HTM trial organized two investigators’ meetings, the first held on April 7, 2022 via teleconference, while the second was a hybrid meeting that took place in Mechelen, Belgium from 1‐3 December 2022. During these meetings, progress reports were presented, experiences shared, and challenges addressed.
5. CONCLUSIONS
Deployment of UPRIGHT‐HTM in clinical settings proves to be feasible, especially in resource poor areas such as Nigeria. The control of the home blood pressure recorded at this stage underscores the importance of self‐measurement of blood pressure in hypertension management. Nigeria and other countries in the sub‐Saharan Africa are characterized by a population that is ethnically very diverse, hence their contribution to clinical trials is key to realizing evidence that is robust and generalizable. This calls for a need to focus more attention on why such countries remain underrepresented in global clinical trials. Finally, this first UPRIGHT‐HTM progress report should motivate all investigators to tap into the huge potential for further recruitment (Table S7).
AUTHOR CONTRIBUTIONS
Jan A. Staessen and Augustine N. Odili conceived the UPRIGHT‐HTM trial. Jan A. Staessen secured funding. Babangida S. Chori, De‐Wei An, Dries S. Martens, Yu‐Ling Yu and Jan A. Staessen contributed to the construction of the database, did the statistical analysis, take responsibility for the integrity of the data, and wrote the first draft of the manuscript. Natasza Gilis‐Malinowska, Godsent Isiguzo, Hao‐Min Cheng, Gontse Mokwatsi, Katarzyna Stolarz‐Skrzypek, Jana Brguljan‐Hitij, Augustine N. Odili and Jan A. Staessen are national or regional coordinators of the project. Peter Reyskens designed the eCRFs, wrote the Android application and maintained the WiPaM platform. All authors were involved in patient recruitment and follow‐up, commented on successive versions of the manuscript and approved the final version and its submission for publication.
CONFLICT OF INTEREST STATEMENT
HM is the cofounder and co‐owner of Mosaiques‐Diagnostics GmbH, Hannover, Germany. PR is an employee of RDSM NV, Hasselt, Belgium. All other authors declare no competing interests with the contents of the current article.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors gratefully acknowledge the enthusiasm of the patients enrolled in UPRIGHT‐HTM and the expert assistance of the consultants, residents and nursing staff supporting the trial at the clinical sites. The Alliance for the Promotion of Preventive Medicine is a not‐profit research institute (URL: www.appremed.org; Belgian registration number, 739849385), which received a non‐binding grant from OMRON Healthcare Co. Ltd., Kyoto, Japan. UPRIGHT‐HTM is a Top Z project supported by OMRON Healthcare Co. Ltd., Kyoto, Japan.
Chori BS, An D‐W, Martens DS, et al. Urinary proteomics combined with home blood pressure telemonitoring for health care reform trial—First progress report. J Clin Hypertens. 2023;25:521–533. 10.1111/jch.14664
Babangida S. Chori and De‐Wei An are joint first authors who contributed equally.
Augustine N. Odili and Jan A. Staessen are joint senior authors who contributed equally.
The UPRIGHT‐HTM Investigators are listed in reference 5.
DATA AVAILABILITY STATEMENT
The data of this progress report will not be made publicly available. However, once the final report on the UPRIGHT‐HTM Trial has been published, data sharing will become possible, provided that the request is submitted along with a justified research question and an analysis plan and on condition that the principal investigators approve sharing depersonalized data generated at their recruitment site. Request should be addressed to the Board of APPREMED via jan.staessen@appremed.org.
REFERENCES
- 1. GBD 2019 Investigators . Five insights from the Global Burden of Disease Study 2019. Lancet. 2020;396:1135‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keates AK, Mocumbi AO, Ntsekhe M, Sliwa K, Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nat Rev Cardiol. 2017;14:273‐293. [DOI] [PubMed] [Google Scholar]
- 3. Schutte AE, Botha S, Fourie CMT, et al. Recent advances in understanding hypertension development in sub‐Saharan Africa. J Hum Hypertens. 2017;31:491‐500. [DOI] [PubMed] [Google Scholar]
- 4. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global regional and country‐level diabetes prevalance estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thijs L, Asayama K, Maestre GE, et al. the UPRIGHT investigators calcupaa. Urinary proteomics combined with home blood pressure telemonitoring for health care reform trial: rational and protocol. Blood Press. 2021;30:269‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Medical Association . World medical association declaration of Helsinki. Ethical principles for medical research involving human subjects. J Am Med Assoc. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 7. Vlahou A, Hallinan D, Apweiler R, et al. Data sharing under the general data protection regulation: time to organize law and research ethics? Hypertension. 2021;77:1029‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end‐point (PROBE) study. A novel design for intervention trials. Blood Press. 1992;1:113‐119. [DOI] [PubMed] [Google Scholar]
- 9. KDIGO Board members . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1‐150. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alpert BS. Validation of the Omron HEM‐9210T by the ANSI/AAMI/ISO 81060‐2:2013 with two novel cuffs : wide‐range and extra‐large. Blood Press Monit. 2017;22:166‐168. [DOI] [PubMed] [Google Scholar]
- 12. Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1‐188. [PubMed] [Google Scholar]
- 13. Friedewald WT, Levy RI, Frederickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499‐502. [PubMed] [Google Scholar]
- 14. Odili A, Thijs L, Yang WY, et al. Office and home blood pressures as determinants of electrocardiographic left ventricular hypertropy among Black Nigerians compared with White Flemish. Am J Hypertens. 2017;30:1083‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450‐458. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233‐270. [DOI] [PubMed] [Google Scholar]
- 17. Kloch‐Badelek M, Kuznetsova T, Sakiewicz W, et al. Prevalence of left ventricular diastolic dysfunction in European populations based on cross‐validated diagnostic thresholds. Cardiovascular Ultrasound. 2012;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuznetsova T, Herbots L, López B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105‐112. [DOI] [PubMed] [Google Scholar]
- 19. Böhler T, Kynast‐Wolf G, Coulibaly B, Siè A, Kapaun A. Gender‐specific distribution of hematological parameters in adults living in Nouna, Burkino Faso. Open Hematol J. 2008;2:1874‐2769/08. [Google Scholar]
- 20. Hannemann A, Friedrich N, Dittmann K, et al. Age‐ and sex‐specific reference limits for creatinine, cystatin C and the estimated glomerular filtration rate. Clin Chem Lab Med. 2012;50:919‐926. [DOI] [PubMed] [Google Scholar]
- 21. St Pierre SR, Peirlinck M, Kuhl E. Sex matters: a comprehensive comparison of female and male hearts. Front Physiol. 2022;13:831179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rutkowski DR, Barton GP, Francois CJ, Aggarwal N, Roldan‐Alzate A. Sex differences in cardia flow dynamics of healthy volunteers. Radiol Cardiothor Imaging. 2020;2:e190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petitto M, Esposito R, Sorrentino R, et al. Sex‐specific echocardiographic reference values: the women's point of view. J Cardiovac Med. 2018;19:527‐535. [DOI] [PubMed] [Google Scholar]
- 24. Wooten SV, Moesti S, Chillibeck P, et al. Age‐and sex‐differences in cardiac characteristics determined in masters athletes. Front Physiol. 2020;11:630148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asayama K, Ohkubo T, Metoki H, et al. Hypertension objective treatment based on measurement by electrical devices of blood pressure (HOMED‐BP). Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self‐measured home blood pressure. Hypertens Res. 2012;35:1102‐1110. [DOI] [PubMed] [Google Scholar]
- 26. Evangelista LS, Lee JA, Moore AA, et al. Examining the effects of remote monitoring systems on activation, self‐care, and quality of life in older patients with chronic heart failure. J Cardiovasc Nurs. 2015;30:51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McManus RJ, Mant J, Haque MS, et al. Effect of self‐monitoring and medication self‐titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN‐SR randomized clinical trial. J Am Med Assoc. 2014;312:799‐808. [DOI] [PubMed] [Google Scholar]
- 28. Márquez‐Contreras E, Martell‐Claros N, Gil‐Guillén V, et al. Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM‐HTA study. J Hypertens. 2006;24:169‐175. [DOI] [PubMed] [Google Scholar]
- 29. Staessen JA, Thijs L, Ohkubo T, et al. Thirty years of research on diagnostic and therapeutic thresholds for the self‐measured blood pressure at home. Blood Press Monit. 2008;13:352‐365. [DOI] [PubMed] [Google Scholar]
- 30. Zhang ZY, Nkuipou‐Kenfack E, Yang WY, et al. Epidemiologic observations guiding clinical application of a urinary peptidomic marker of diastolic left ventricular dysfunction. J Am Soc Hypertens. 2018;12:438‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang ZY, Nkuipou‐Kenfack E, Staessen JA. Urinary peptidomic biomarker for personalized prevention and treatment of diastolic left ventricular dysfunction. Proteomics Clin Appl. 2019;13:e1800174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tofte N, Lindhardt M, Adamova K, et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo‐controlled trial. Lancet Diabetes Endocrinol. 2020;8:301‐312. [DOI] [PubMed] [Google Scholar]
- 33. Pontillo C, Zhang ZY, Schanstra JP, et al. Prediction of chronic kidney disease stage 3 by CKD273, a urinary proteomic biomarker. KI Reports. 2017;2:1066‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mavrogeorgis E, Mischak H, Latosinska A, et al. Collagen‐derived peptides in CKD: a link to fibrosis. Toxins. 2022;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martens DS, Thijs L, Latosinska A, et al. Staessen JA, the FLEMENGHO investigators. Urinary peptidomics to address age‐related disabilities: a prospective population study with replication in patients. Lancet Healthy Longevity. 2021;2:e690‐e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu YM, Thijs L, Liu YP, et al. The urinary proteome as correlate and predictor of renal function in a population study. Nephrol Dial Transplant. 2014;29:2260‐2268. [DOI] [PubMed] [Google Scholar]
- 37. Huang QF, Trenson S, Zhang ZY, et al. Biomarkers to assess right heart pressures in recipients of a heart transplant: a proof‐of‐concept study. Transplant Direct. 2018;4:e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang ZY, Thijs L, Petit T, et al. Urinary proteome and systolic blood pressure as predictors of 5‐year cardiovascular and cardiac outcomes in a general population. Hypertension. 2015;66:52‐60. [DOI] [PubMed] [Google Scholar]
- 39. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR. Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community‐based study. Circulation. 2004;109:3176‐3181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data of this progress report will not be made publicly available. However, once the final report on the UPRIGHT‐HTM Trial has been published, data sharing will become possible, provided that the request is submitted along with a justified research question and an analysis plan and on condition that the principal investigators approve sharing depersonalized data generated at their recruitment site. Request should be addressed to the Board of APPREMED via jan.staessen@appremed.org.


