Abstract
Background:
By 2050, one in six people globally will be 65 or older. Confusion and delirium are significant complications after burn injury, especially in the elderly population. The etiology is still unknown, however complications may be driven by pro-inflammatory activation of astrocytes within the hippocampus (HPC) after burn injury. Reduced levels of phosphorylated cyclic-AMP response binding element (pCREB), caused by elevated systemic pro-inflammatory cytokines, could lead to cognitive decline and memory impairment.
Methods:
To examine the effects of remote injury on neuroinflammation in advanced age, young and aged mice were subjected to a 15% total body surface area scald burn or sham injury, and euthanized after 24 hours. Expression of ccl2 and tnfa were measured by qPCR in the whole brain and HPC. Astrocyte activation was measured by immunofluorescence within the HPC. pCREB was measured by immunohistochemistry in the dentate gyrus.
Results:
We saw an 80-fold increase in ccl2 and a 30-fold elevation in tnfa after injury in the whole brain of aged mice compared to young groups and aged sham mice (p<0.05 and p<0.05, respectively). Additionally, there was a 30-fold increase in ccl2 within isolated HPC of aged injured mice when compared to sham injured animals (p<0.05). When investigating specific HPC regions, immunofluorescence staining showed a >20% rise in glial fibrillary acidic protein (GFAP) positive astrocytes within the cornu ammonis 3 (CA3) of aged injured mice when compared to all other groups (p<0.05). Lastly, we observed a >20% decrease in pCREB staining by immunohistochemistry in the dentate gyrus of aged mice compared to young regardless of injury (p<0.05).
Conclusions:
These novel data suggest that remote injury in aged, but not young, mice is associated with neuroinflammation and astrocyte activation within the HPC. These factors, paired with an age related reduction in pCREB, could help explain the increased cognitive decline seen in burn patients of advanced age. To our knowledge, we are the first group to examine the impact of advanced age combined with burn injury on inflammation and astrocyte activation within the brain.
Keywords: Brain, traumatic injury, inflamm-aging, dentate gyrus, cytokine, chemokine, advanced age
1. Introduction:
The global population is aging, as the numbers of people aged >65 years of age has risen steadily since 2010. In the United States alone, the aged population accounts for 16.5% of all residents, amounting to 54 million people1. Burns amongst the aged population only represent 16.9% of all cases reported from 2009 to 2018, but the injuries are more fatal, with 52% of these patients succumbing to their injuries compared to just 14% of patients between 20–60 years of age2. Not only is advanced age an independent risk factor for burn injury, it also contributes to heightened morbidity, mortality3, and neurocognitive complications4 following insult. Additionally, when compared to male burn patients, females with equivalent burns have shown higher levels of mortality5, 6, lower mental health status7, and increased psychological distress7.
The neurocognitive decline observed after burn injury involves multiple factors. Cutaneous burn injury triggers the release of cytokines, chemokines and danger-associated molecular patterns (DAMPs) from the injured tissue, contributing to a systemic “cytokine storm,” which promotes systemic inflammation8. Burn-induced systemic inflammation contributes to neurocognitive impairment4, 9 in the aged population due to increases in neuroinflammation10, 11, but the mechanisms behind this are still unknown. In the absence of burn or other insult, the blood-brain barrier (BBB) restricts access to the brain to pro-inflammatory cytokines and other potentially harmful factors12. Although the exact mechanisms remain elusive, heightened levels of IL-6 and IL-1β in the circulation and the brain of mice after burn injury have been linked to disruption of the BBB13. Burn injury also impairs the integrity of the BBB by reducing protein expression of tight junctional proteins in the cerebral endothelium and increasing transcytosis13. Disruption in the ability of the BBB to act as a barrier leads to increased pro-inflammatory cytokines and chemokines, such as CC motif chemokine ligand 2 (CCL2) and tumor-necrosis factor alpha (TNFα), within the brain14, which contributes to neuroinflammation-induced neurological decline, confusion, delirium in patients11. The incidence of confusion, delirium, or memory loss after burn injury implicates damage to the HPC, which plays a vital role in learning, memory formation and recall15, 16.
Burn related systemic inflammation activates microglia in young mice14. Microglia are the tissue resident macrophages of the brain and are maintained in a homeostatic state by the cytokine milieu of the brain17. Microglia can be activated18 by illness or injury to secrete IL-1α and TNFα, which induce the activation of reactive astrocytes19. Prior to activation, astrocytes play a vital role in maintaining the blood-brain barrier, as well as ionic and bioenergetics homeostasis20–22. These active astrocytes change their phenotype into reactive, glial fibrillary acidic protein positive (GFAP+) astrocytes11, 20 that can induce neuronal death through the secretion of a yet to be identified soluble toxin19, 23. Importantly, the presence of reactive astrocytes is an early feature of Alzheimer’s Disease24, 25, and may contribute to the progression of Alzheimer’s Disease through impaired clearance of ameloid beta plaques26.
Taken together, systemic inflammation caused by age27, injury, or illness28 activates astrocytes, which can contribute to excessive inflammation and neuronal damage. The dentate gyrus (DG) is the region of the HPC that is tasked with compiling and encoding sensory input to begin encoding into memories29. The activation of cyclic adenosine monophosphate response element binding-protein (CREB) by phosphorylation to form pCREB in the brain is necessary for a host of functions, including neuronal proliferation, cell differentiation, and neuronal survival30. In the DG, pCREB has a well-studied role in the formation of memories31, 32 and is reduced in memory related neurocognitive disorders, such as Alzheimer’s Disease33. If burn injury in aged patients triggers neuroinflammation and reactive astrocytes in the HPC, the region of the brain responsible for the encoding, formation, and recall of memories, it may explain the neurocognitive decline, confusion, and delirium seen in this patient cohort.
Using our well-documented, clinically relevant model of scald burn injury, we conducted a comparative study of neuroinflammation in young and aged mice. Scald burn was selected due to its prevalence, accounting for approximately 34% of all burn injuries requiring medical attention in the United States2, compared to chemical burns, which account for 3%2. Importantly, scald burn injury induces a cytokine storm8 that is similar to that seen in other traumatic injuries34. Here, we sought to examine the effects of advanced age and burn injury on pCREB, reactive astrocytes and expression of pro-inflammatory cytokines and chemokines in the brain, and, specifically, the HPC. We hypothesized that increased age would exacerbate neuroinflammation in the HPC and would lead increased activation of reactive astrocytes.
2. Materials and Methods
2.1. Mice and murine model of scald injury
Young (4–5 months of age, 35 year old human equivalent) and aged (18–22 months of age, 65 year old human equivalent) female BALB/cBy and C57BL/6 mice were obtained from the National Institute of Aging (NIA) Colony (Charles River Laboratories, Wilmington, MA). C57BL/6 mice were used for pCREB visualization, BALB/cBy mice were used for all other experiments. Mice were housed in sterile conditions in the University of Colorado Anschutz Medical Campus vivarium. All protocols were approved by the University of Colorado Denver Anschutz Medical Campus Institutional Animal Care and Use Committee prior to conducting experiments. We utilized our well-established clinically relevant murine model of burn injury as previously described35–37. Briefly, aged and young mice, weighing approximately 25–30 g, were randomly divided into two groups (burn or sham). Higher numbers of mice were included in burn groups because of increased mortality, especially among aged mice. Mice were anesthetized (25 mg/kg of ketamine and 2.5 mg/kg of xylazine with constant isoflurane) (Webster Veterinary) and dorsa were shaved. Anesthetized mice were subjected to a 12–15% total body surface area (TBSA) scald burn injury by using a template and exposing the shaved dorsum to a 92–93°C water bath for 8 seconds, resulting in an insensate full-thickness skin injury. Sham-injured animals were exposed to room temperature water. Animals received 1mL of resuscitation fluids immediately post burn in addition to pain medication (1.0 mg/kg buprenorphine-SR LAB, Zoo Pharm). Animals received an additional volume of resuscitation fluids equal to 10 μL/g per mouse the following morning. At 24 hours after burn, a time point at which we have measured appreciable markers of inflammation in circulation35 and multiple organ systems37–41, mice were euthanized using CO2 followed by exsanguination.
2.2. RNA extraction and quantitative RT-PCR.
Whole brains or isolated cortex and HPC were lysed in TRIzol (cat#15596018, Life Technologies) and RNA was extracted using chloroform (cat#C2432, Sigma-Aldrich) and isopropanol (cat#BP2618–1, Thermo-Fisher) precipitation. cDNA was then synthesized using iScript kit according to manufacturer protocol (Bio-Rad) as previously described38. Quantitative RT-PCR was performed using TaqMan probes ccl2(Mm00441242_m1), il1b(Mm00434226_m1), il6(Mm00446190_m1), il10(Mm01288386_m1), il22(Mm01226722_g1), tnf(Mm00443258_m1) (Applied Biosystems) and TaqMan Universal PCR Mastermix (cat#4364340, Applied Biosystems) run on a QuantStudio 3 Real-Time PCR System (Applied Biosystems). Gene expression was quantified using the ΔΔCt algorithm42 with gapdh as the endogenous control (cat#4352339E, Applied Biosystems).
2.3. Immunohistochemistry and Immunofluorescence
Mice were transcardially perfused with PBS under isoflurane anesthesia43 prior to brain collection. Brains were fixed in 4%-paraformaldehyde (cat#47340, Thermo-Fisher) and cut into 50μm coronal sections using a Leica SM2010R freezing microtome (Leica Biosystems). For immunohistochemistry, sections were fixed with acetone prior to antigen retrieval (cat#H-3301, Vector), quenching of endogenous peroxidase activity, and blocked with animal-free blocking solution (cat#15019L, Cell Signaling). pCREB was visualized by incubation with 1:500 dilution of primary antibody (#ab32096, Abcam), 1:1000 dilution of HRP-conjugated secondary antibody (cat#7074s, Cell Signaling), 1:10000 dilution of SA-HRP (cat#3999s, Cell Signaling), and then incubated with 3,3′-Diaminobenzidine according to manufacturer’s protocol (cat#8059, Cell Signaling). For immunofluorescence44, 45, free-floating sections were washed, blocked, and incubated with primary antibody against GFAP (cat#Z0334, Agilent) overnight at 4oC.The next day sections were incubated with fluorescent conjugated secondary antibody (cat#712–545-150, Jackson ImmunoResearch) and Hoechst (cat#5117, Tocris) prior to being mounted and imaged using a Zeiss microscope. Image analysis was performed by obtaining pixel intensity using ImageJ software. Each image underwent auto-thresholding with identical parameters for each image. Mean pixel intensity was measured for each positive cell. The mean pixel intensities for each cell were then averaged across each image, resulting in an average mean pixel intensity for every image. %GFAP pixel intensity within the CA3 region was determined by pixel intensity of GFAP within the CA3 region divided by the total image pixel intensity of GFAP, multiplied by 100 to receive a percentage. All immunohistochemical analysis, including selection of sections, fields of study, cells, and quantitation were performed by an investigator blinded to the experimental groups that the samples originated from.
3. Results
3.1. Post-burn neuroinflammation is exacerbated in aged compared to young mice
Increased neuroinflammation has been reported independently in age11, 15, 16, 46 and burn injury4, 9, 10, 13, 47 in humans and rodent models. Here, we assessed whether there is heightened neuroinflammation in the brains of aged mice, relative to young mice, subjected to cutaneous burn injury. In order to measure inflammatory responses in the brain, whole brains were collected from young and aged sham- and burn-injured mice and mRNA levels of ccl2 and tnfa were measured. Our results show no difference in the expression of both ccl2 and tnfa after burn injury in young mice (Fig. 1). Additionally, we observed no change in both ccl2 and tnfa in aged sham-injured mice when compared to young sham-injured mice. Importantly, there was a significant increase in the expression of ccl2 in aged burn-injured mouse brains when compared to young sham, young burn, and aged sham mice (80.8-, 15.4-, and 21.6-fold higher, respectively [p<0.05]). Expression of tnfa in the brains of aged burn-injured mice was similarly heightened when compared to all other groups (30.2-, 10.5-, and 7.5-fold, respectively [p<0.05]). Interestingly, we did not see changes in expression of other pro-inflammatory cytokines that were tested, namely interleukin-1β (il1b) or interleukin-6 (il6). Lastly we failed to detect differences in expression of anti-inflammatory cytokines interleukin-10 (il10) or interleukin-22 (il22) in any experimental group (data not shown).
Figure 1. Dramatic elevation in markers of neuroinflammation in whole brains of aged burn-injured mice relative to other groups.
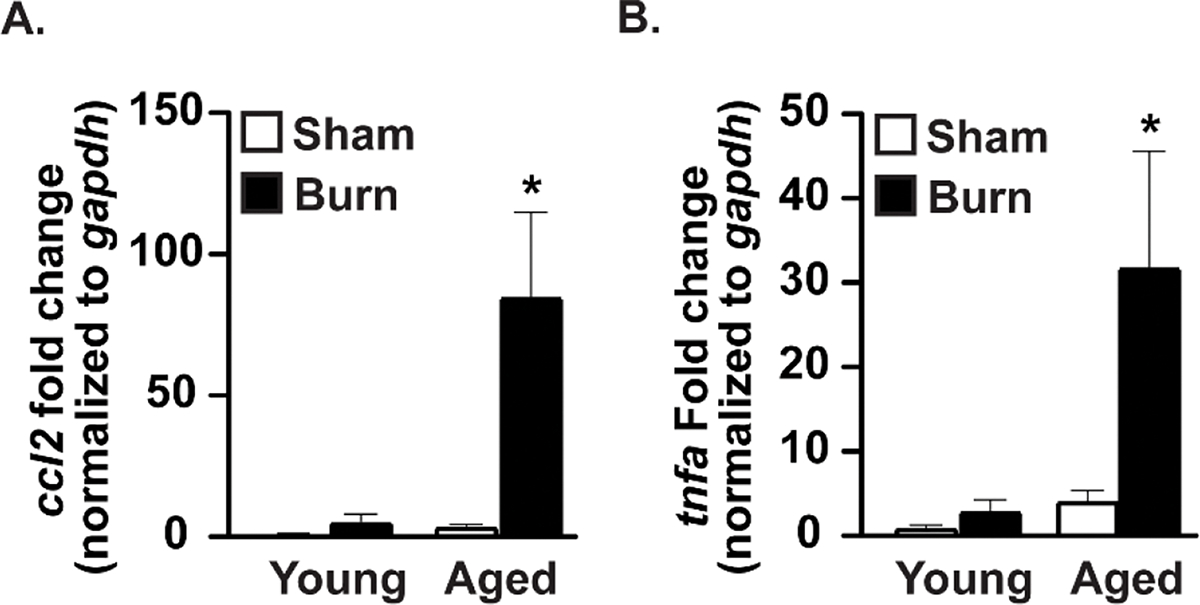
Brains were collected after sham or burn injury. mRNA expression of (a) ccl2 and (b) tnfa across all groups are shown as mean fold-change relative to gapdh ± Standard Error of the Mean (SEM). *p<0.05 compared to all other groups by one-way Analysis of Variance (ANOVA), Tukey’s multiple comparison test. Graphs shown are from a single representative of two independent experiments. n = 6 (sham) and 9 (burn) mice per group for ccl2, n=3 (sham) and 6 (burn) mice per group for tnfa in this representative experiment.
3.2. Inflammatory response in the hippocampus is heightened in aged mice
The HPC is vital for integrating sensory input into memories, and the encoding and recall of those memories48. Since memory deficits, confusion, and delirium can occur in the aged population after burn injury, we decided to assess levels of expression markers of inflammation in isolated HPC of young and aged mice. Here, we measured ccl2 expression in the isolated HPC and cortex of mice after burn injury (Fig. 2). There was no difference in ccl2 within the HPC when comparing young sham-injured mice to either young burn-injured or aged sham-injured mice. However, there was a marked elevation in ccl2 expression in the HPC of aged burn-injured mice, which was 30-fold above that of young sham-injured (p<0.05 from all groups). Within the cortex, a small increase in ccl2 was noted in both young and aged animals after burn injury, but this increase was not statistically significant. These results reveal that burn injury in aged animals can increase markers of inflammation, which we found to be specifically located within the HPC.
Figure 2. ccl2 expression is elevated in the hippocampus, but not the cortex, of age burn-injured mice.
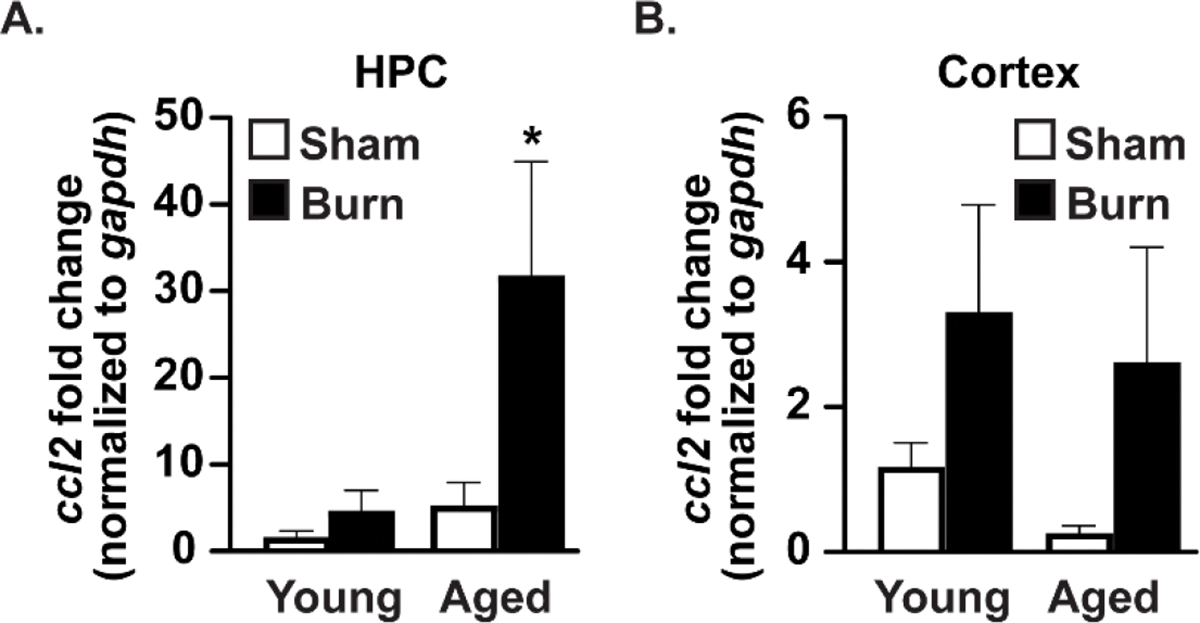
mRNA expression of ccl2 in the (A) hippocampus and (B) cortex by RT-qPCR after burn injury across all groups shown as mean fold-change relative to gapdh ±SEM. *p<0.05 compared to all other groups by one-way ANOVA, Tukey’s multiple comparison test. Graphs shown are from a single representative of two independent experiments. n=3 (sham) and 5 (burn) mice per group in this representative experiment.
3.2. Increased glial fibrillary acidic protein (GFAP) positive astrocytes in the cornu ammonis 3 (CA3) region of the hippocampus in aged burn-injured mice
We next sought to measure whether aged burn-injured mice had increased amounts of activated reactive astrocytes in their HPC by utilizing immunofluorescence relative to comparably treated younger mice (Fig. 3). The total amount of GFAP+ signal, normalized to total Hoechst, did not change significantly between groups (Fig. 3B), although we noted a trend toward an elevation in GFAP in the young burn group. Quantitation of the amount of GFAP+ signal specifically in the CA3 region of the HPC (Fig. 3A,C) of aged burn-injured mice, revealed a 23.7%, 51.2%, and 21.4% rise in signal over young sham, young burn, and aged sham mice respectively (p<0.05). The CA3 region assists in new memory encoding through pattern separation49. Additionally, the CA3 region is responsible for spatial rapid one-trial learning, pattern completion, and spatial short-term memory50–52. Reactive astrocytes within this region may damage neuronal cells and lead to neurocognitive decline. Interestingly, the percentage of GFAP+ cells within the young burn group fell to 13.4% of total GFAP, perhaps as a consequence of the observed trend towards an increase in GFAP+ reactive astrocytes within the rest of the HPC while being mostly excluded from the CA3 region.
Figure 3. Increased GFAP+ reactive astrocytes in the CA3 region of the hippocampus of aged burn-injured mice.
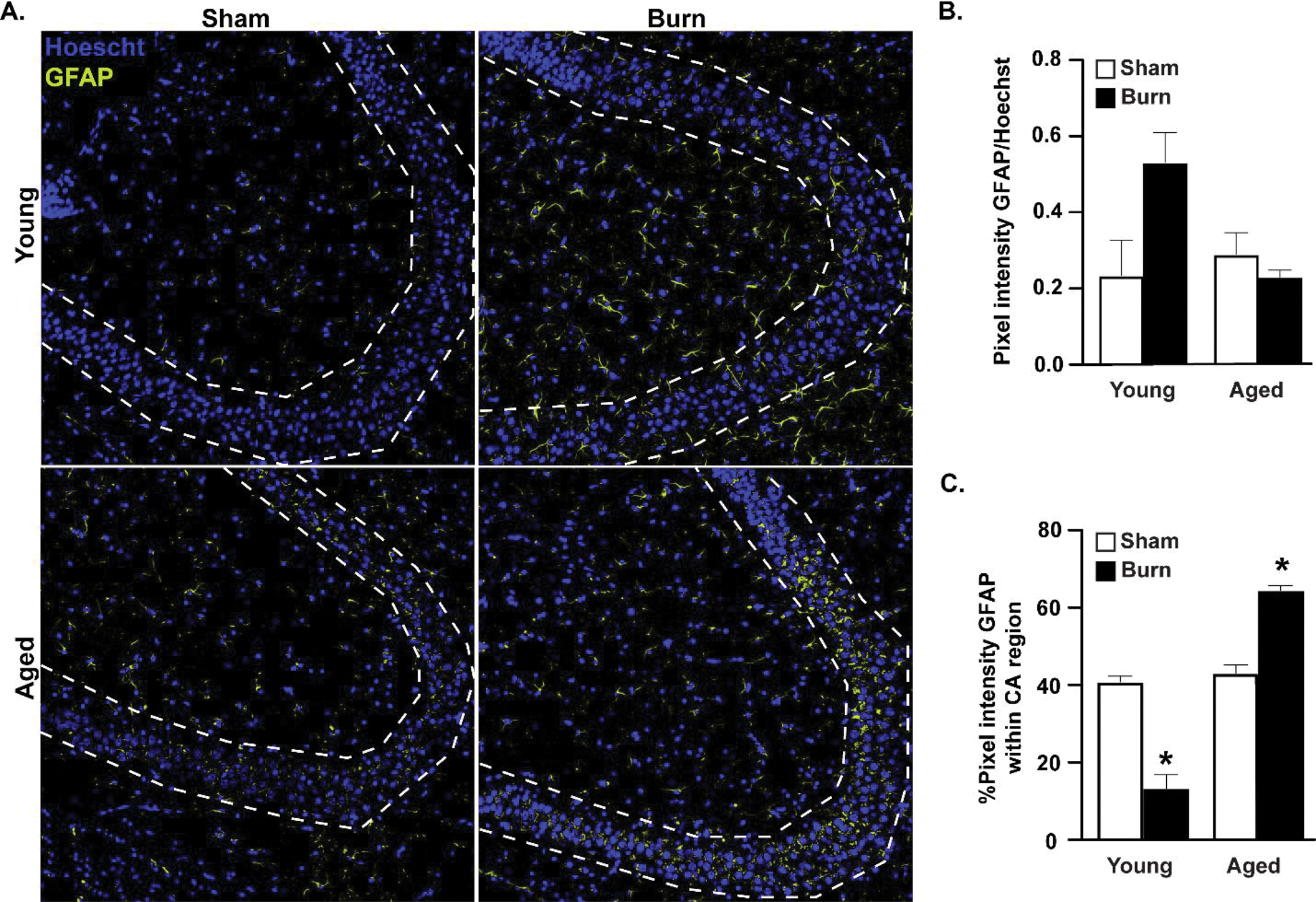
(A) GFAP+ reactive astrocytes identified by immunofluorescence in perfused brains after burn injury. In aged burn-injured mice, GFAP+ cells increase their localization to the CA3 region (outlined in dotted line). Representative IF shown, n=3–4 mice per group, images shown from a representative of two independent experiments. (B) GFAP+ cells analyzed with ImageJ and presented as mean pixel density±SEM, normalized to Hoechst. n=3(sham)-4(burn) mice per group, experiment shown is a representative of two independent experiments. (C) Percentage of total GFAP+ signal that is within the CA3 (dotted lines) region reported as mean percentage±SEM. *p<0.05 from all other groups by ANOVA, Tukey’s post-hoc test. Graphs shown are from a single representative of two independent experiments. n=3 (sham) and 4 (burn) mice per group in this representative experiment.
3.4. Reduction in phospho-cyclic adenosine monophosphate response element binding-protein (pCREB) in dentate gyrus of aged mice
We sought to examine whether pCREB in the dentate gyrus was altered by age and/or burn injury (Fig. 4). The dentate gyrus is one of few brain sites where neurogenesis can occur in adult mammals53, and this process is dependent on the presence of pCREB within its granular neurons54. We found that pCREB was reduced by 21.2% in the aged sham-injured mice and 26.5% in aged burn-injured animals, when compared to young sham-injured mice. However, there was no effect of burn on pCREB expression within the dentate gyrus, signifying that age is the primary determining factor in pCREB expression (Fig. 4B).
Figure 4. pCREB is reduced in aged mouse dentate gyrus regardless of burn injury.
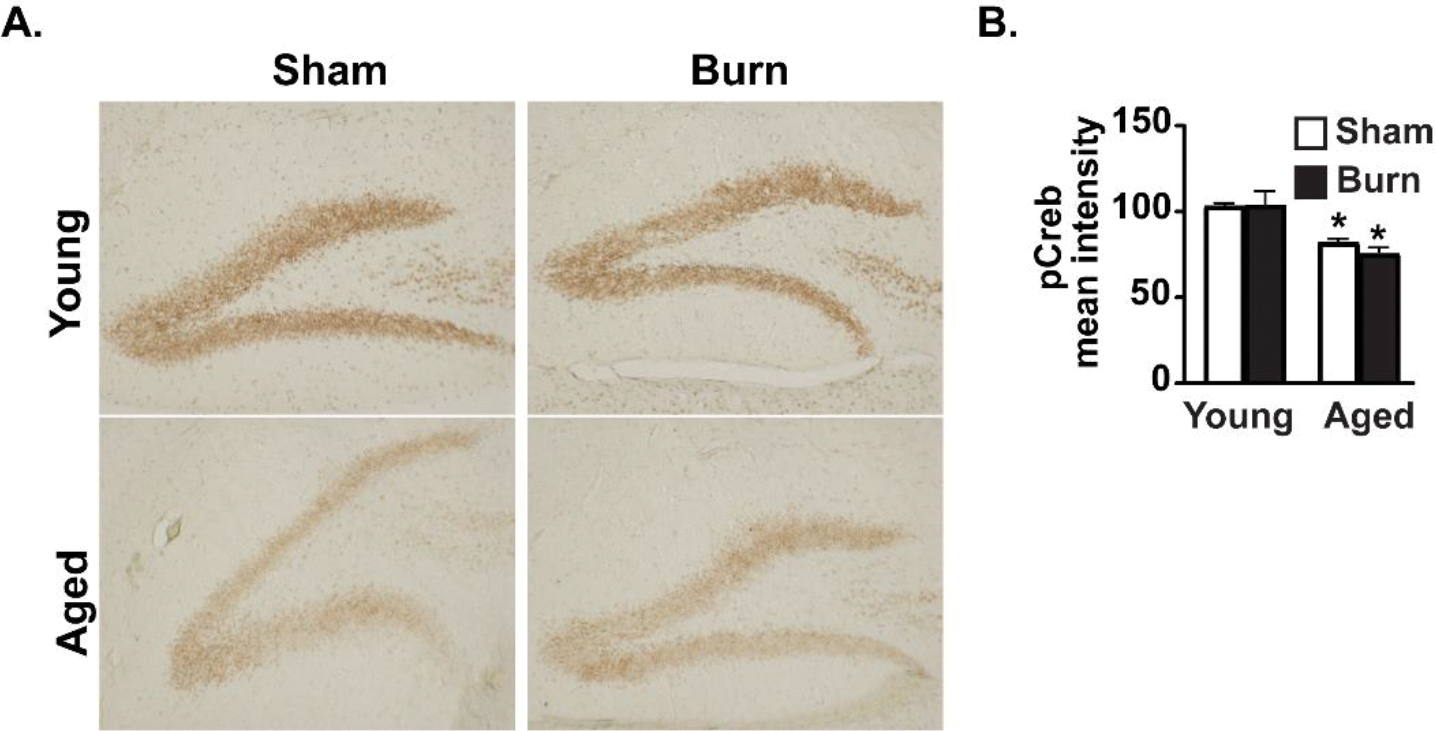
(A) pCREB was measured by immunohistochemistry 3,3′-Diaminobenzidine staining in all groups after burn injury. (B) Quantification indicates that age, but not burn injury, reduces pCREB staining. pCREB mean intensity analyzed using ImageJ and reported as mean percentage ± SEM. *p<0.05 from young groups by ANOVA, Tukey’s post-hoc test. Graphs and histology shown are from a single representative of two independent experiments. n=3 (sham) and 4 (burn) mice per group in this representative experiment.
4. Discussion
Burn patients of advanced age carry a higher risk of post-burn complications, including neurological decline4, 9, 10, which is associated with worse post-burn prognosis when compared to younger individuals who sustain comparable injuries2, 3, 55. While it is known that both increased age15, 16, 27 and burn injury4, 8–10, 13, 47 are associated with heightened neurological complications, the mechanisms behind burn-related neurological decline in advanced age are not well understood. The aim of this study was to determine whether there are differential responses in astrocyte activation and markers of inflammation in the brain of young and aged mice after remote burn injury using our well-documented, clinically relevant murine model of scald burn injury35, 36, 39–41, 56–62. We investigated whether burn injury differentially activated astrocytes in the brains of young and aged mice. We found that burn injury in older mice is associated with heightened neuroinflammation when compared to young burn-injured mice. This was achieved by documenting the levels of mRNA expression of ccl2 and tnfa in whole-brain tissue (Fig. 1) and ccl2 expression within the HPC (Fig. 2). CCL2 is a known monocyte chemoattractant63 that is produced by neurons after injury and contributes to microglial activation64. TNFα is a highly pleotropic cytokine that contributes to neuro-cognitive decline65. In addition to the direct effects of CCL2 on microglial activation, increased levels of neuroinflammation in the brain can give rise to activated microglia,66 which in turn secrete pro-inflammatory cytokines that activate reactive astrocytes19, causing further neurodegeneration. After burn injury, CCL2, TNFα, and a number of other pro-inflammatory cytokines and chemokines are elevated systemically and can impair the BBB13. The mechanisms behind neuroinflammation after burn injury remain elusive in young animals, and evidence demonstrating enhanced neuroinflammation in aged animals, prior to this study, has been lacking. However, leakiness at the BBB may induce neuroinflammation through less restricted entry of systemic pro-inflammatory cytokines14 and bacterial products such as LPS18 into the brain, which are able to activate reactive astrocytes.
To further investigate this neuroinflammatory phenotype, we isolated the HPC, which is vital for memory formation and recall48. We found that ccl2 was significantly increased within the HPC of aged burn-injured mice when compared to young mice (Fig. 2). While it is known that age15 or traumatic brain injury67 alone can result in damage to the HPC, this study is the first to interrogate the intersection of age and remote burn injury in this brain region. This region specific increase in neuroinflammation may lead to increased neurodegeneration within the HPC, directly damaging the region associated with memory formation and recall, and thus causing the confusion, delirium, and memory deficits observed in burn patients of advanced age68.
Using immunofluorescence, we visualized GFAP+ astrocytes within the HPC and observed that there was an increase in activated reactive astrocytes within the CA3 region of aged burn-injured mice when compared to young and sham groups (Fig. 3). The CA3 region of the HPC is directly connected to the dentate gyrus and assists in new memory encoding through pattern separation49. Additionally, the CA3 region is responsible for spatial rapid one-trial learning, pattern completion, and spatial short-term memory50–52. Given our data, reactive astrocytes in the CA3 region of older patients who sustain a burn injury may be contributing to site-specific neurodegeneration, thereby causing deficits in short term memory loss and confusion/delirium. To our knowledge, there is no evidence of CA3 specific damage after burn injury, in either young or aged mice. The CA3 region has been shown to experience more neuronal loss than the CA1 region after models of traumatic brain injury67, 69. Previous literature has suggested that this disparity may be due to differential levels of physical tissue strain experienced in these regions during injury70, 71. However, since traumatic brain injury induces neuroinflammation69, our data would suggest that the differences in damage may be due to differential activation of reactive astrocytes within different regions of the HPC, especially in aged individuals. Further, traumatic brain injury has been shown to sensitize astrocytes to a secondary insult72, such as burn injury, which could lead to further CA3 region damage and neurological consequences. This damage to the hippocampal CA3 region, combined with the observed age related deficit in pCREB in the dentate gyrus (Fig. 4), may explain the neurological complications in burn patients of advanced age. Together, our novel studies increase our understanding of the effects of aging and burn injury on neuroinflammation. Future work based on this study will examine the mechanistic underpinnings of this neuroinflammation and ultimately identify therapeutic interventions that will target the specific pathways that contribute to clinical neuro-cognitive decline.
Further study will be required to determine why reactive astrocytes are found more often within the CA3 region of aged mice than young after burn injury. Perhaps the region specific increase in ccl2 expression is of sufficient magnitude to trigger microglia activation64 and subsequent activation of reactive astrocytes19. Increased ccl2 may also lead to recruitment of blood monocytes61, 63, which could, in turn, be activated in the brain by cleaved gelsolin47, perpetuating the pro-inflammatory state. Unraveling the mechanisms responsible for excessive neuroinflammation and activation of reactive astrocytes after burn injury in aged mice will allow treatments to be developed to specifically target these pathways for therapeutic benefit. Finally, understanding these mechanisms will extend beyond just burn-injury to other forms of trauma and lead to a broader understanding and better treatment of trauma in the aged population.
Acknowledgements
The authors wish to acknowledge Lauren Giesy, BS and Kevin Najarro, MS for helping to perform experiments for this manuscript and Manisha Patel, PhD, for reading the manuscript and offering insights.
Funding
This work was supported in part by NIH R01 AG018859 (EJK), R35 GM131831 (EJK), K08 GM134185 (JPI), VA I01BX004335 (EJK), and Institutional Training grant T32 AG000279 (KM).
Footnotes
Declaration of competing interest
None.
Credit authorship contribution statement
T.W.: Conceptualization, methodology, formal analysis, investigation, writing – original draft, writing – review & editing, visualization. R.H.M., J.P.I., and N.Q.: writing – review & editing. E.J.K.: conceptualization and writing – review & editing.
References
- 1.Bureau U Annual estimates of the resident population by single year of age and sex for the United States: April 1, 2010 to July 1, 2017. Accessed 2018-04-11. [Online]. Available: https://www.census.gov/data, 2016.
- 2.Association AB. National Burn Repository 2019 Update, Report of Data from 2009–2018. American Burn Association: Chicago, IL, USA: 2019. [Google Scholar]
- 3.Pham TN, Kramer CB, Wang J, Rivara FP, Heimbach DM, Gibran NS, Klein MB. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. Journal of burn care & research 2009; 30(1): 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esechie A, Bhardwaj A, Masel T, Raji M. Neurocognitive sequela of burn injury in the elderly. Journal of Clinical Neuroscience 2019; 59: 1–5. [DOI] [PubMed] [Google Scholar]
- 5.Karimi K, Faraklas I, Lewis G, Ha D, Walker B, Zhai Y, Graves G, Dissanaike S. Increased mortality in women: sex differences in burn outcomes. Burns Trauma 2017; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams FN, Strassle PD, Knowlin L, Napravnik S, van Duin D, Charles A, Nizamani R, Jones SW, Cairns BA. Sex-Based Differences in Inpatient Burn Mortality. World J Surg 2019; 43(12): 3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasiak J, Lee SJ, Paul E, Shen A, Tan H, Cleland H, Gabbe B. Female patients display poorer burn-specific quality of life 12 months after a burn injury. Injury 2017; 48(1): 87–93. [DOI] [PubMed] [Google Scholar]
- 8.Comish PB, Carlson D, Kang R, Tang D. Damage-Associated Molecular Patterns and the Systemic Immune Consequences of Severe Thermal Injury. J Immunol 2020; 205(5): 1189–1197. [DOI] [PubMed] [Google Scholar]
- 9.Holmes EG, Jones SW, Laughon SL. A Retrospective Analysis of Neurocognitive Impairment in Older Patients With Burn Injuries. Psychosomatics 2017; 58(4): 386–394. [DOI] [PubMed] [Google Scholar]
- 10.Flierl MA, Stahel PF, Touban BM, Beauchamp KM, Morgan SJ, Smith WR, Ipaktchi KR. Bench-to-bedside review: Burn-induced cerebral inflammation--a neglected entity? Crit Care 2009; 13(3): 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Valdés HE, Martínez-Coria H. The Role of Neuroinflammation in Age-Related Dementias. Rev Invest Clin 2016; 68(1): 40–48. [PubMed] [Google Scholar]
- 12.Ehrlich P Das sauerstoff-bedurfnis des organismus. Eine Farbenanalytische Studie 1885. [Google Scholar]
- 13.Yang J, Ma K, Zhang C, Liu Y, Liang F, Hu W, Bian X, Yang S, Fu X. Burns Impair Blood-Brain Barrier and Mesenchymal Stem Cells Can Reverse the Process in Mice. Front Immunol 2020; 11: 578879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation 2015; 12: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans AK, Park HH, Saw NL, Singhal K, Ogawa G, Leib RD, Shamloo M. Age-related neuroinflammation and pathology in the locus coeruleus and hippocampus: beta-adrenergic antagonists exacerbate impairment of learning and memory in aged mice. Neurobiol Aging 2021; 106: 241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrientos RM, Frank MG, Watkins LR, Maier SF. Aging-related changes in neuroimmune-endocrine function: Implications for hippocampal-dependent cognition. Hormones and Behavior 2012; 62(3): 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nature neuroscience 2014; 17(1): 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss DW, Bates TE. Activation of murine microglial cell lines by lipopolysaccharide and interferon-gamma causes NO-mediated decreases in mitochondrial and cellular function. Eur J Neurosci 2001; 13(3): 529–538. [DOI] [PubMed] [Google Scholar]
- 19.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017; 541(7638): 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiwaji Z, Hardingham GE. Good, bad, and neglectful: Astrocyte changes in neurodegenerative disease. Free Radical Biology and Medicine 2022; 182: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. Journal of neurochemistry 2001; 77(6): 1601–1610. [DOI] [PubMed] [Google Scholar]
- 22.Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. Journal of Neuroscience 1996; 16(8): 2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A. Reactive astrocyte nomenclature, definitions, and future directions. Nature neuroscience 2021; 24(3): 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter SF, Herholz K, Rosa-Neto P, Pellerin L, Nordberg A, Zimmer ER. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol Med 2019; 25(2): 77–95. [DOI] [PubMed] [Google Scholar]
- 25.Acosta C, Anderson HD, Anderson CM. Astrocyte dysfunction in Alzheimer disease. J Neurosci Res 2017; 95(12): 2430–2447. [DOI] [PubMed] [Google Scholar]
- 26.Jain P, Wadhwa PK, Jadhav HR. Reactive Astrogliosis: Role in Alzheimer’s Disease. CNS Neurol Disord Drug Targets 2015; 14(7): 872–879. [DOI] [PubMed] [Google Scholar]
- 27.Dahan L, Rampon C, Florian C. Age-related memory decline, dysfunction of the hippocampus and therapeutic opportunities. Prog Neuropsychopharmacol Biol Psychiatry 2020; 102: 109943. [DOI] [PubMed] [Google Scholar]
- 28.Chesnokova V, Pechnick RN, Wawrowsky K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun 2016; 58: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesner RP. An analysis of the dentate gyrus function. Behav Brain Res 2013; 254: 1–7. [DOI] [PubMed] [Google Scholar]
- 30.Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends in Neurosciences 2005; 28(8): 436–445. [DOI] [PubMed] [Google Scholar]
- 31.Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain 2012; 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 1994; 79(1): 49–58. [DOI] [PubMed] [Google Scholar]
- 33.Bartolotti N, Bennett DA, Lazarov O. Reduced pCREB in Alzheimer’s disease prefrontal cortex is reflected in peripheral blood mononuclear cells. Mol Psychiatry 2016; 21(9): 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nature Immunology 2018; 19(4): 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacs EJ, Grabowski KA, Duffner LA, Plackett TP, Gregory MS. Survival and cell mediated immunity after burn injury in aged mice. Journal of the American Aging Association 2002; 25(1): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plackett TP, Schilling ME, Faunce DE, Choudhry MA, Witte PL, Kovacs EJ. Aging enhances lymphocyte cytokine defects after injury. The FASEB journal 2003; 17(6): 688–689. [DOI] [PubMed] [Google Scholar]
- 37.Najarro KM, Boe DM, Walrath TM, Mullen JE, Paul MT, Frankel JH, Hulsebus HJ, Idrovo JP, McMahan RH, Kovacs EJ. Advanced age exacerbates intestinal epithelial permeability after burn injury in mice. Exp Gerontol 2022; 158: 111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen MM, Zahs A, Brown MM, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. An alteration of the gut-liver axis drives pulmonary inflammation after intoxication and burn injury in mice. Am J Physiol Gastrointest Liver Physiol 2014; 307(7): G711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choudhry MA, Plackett TP, Schilling EM, Faunce DE, Gamelli RL, Kovacs EJ. Advanced age negatively influences mesenteric lymph node T cell responses after burn injury. Immunology letters 2003; 86(2): 177–182. [DOI] [PubMed] [Google Scholar]
- 40.Gomez CR, Nomellini V, Baila H, Oshima K, Kovacs EJ. Comparison of the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury: scald injury versus ip LPS administration. Shock (Augusta, Ga) 2009; 31(2): 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. Journal of leukocyte biology 2008; 83(6): 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zahs A, Bird MD, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. American Journal of Physiology-Gastrointestinal and Liver Physiology 2012; 303(6): G705–G712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalmers NE, Yonchek J, Steklac KE, Ramsey M, Bayer KU, Herson PS, Quillinan N. Calcium/Calmodulin-Dependent Kinase (CaMKII) Inhibition Protects Against Purkinje Cell Damage Following CA/CPR in Mice. Mol Neurobiol 2020; 57(1): 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orfila JE, Grewal H, Dietz RM, Strnad F, Shimizu T, Moreno M, Schroeder C, Yonchek J, Rodgers KM, Dingman A, Bernard TJ, Quillinan N, Macklin WB, Traystman RJ, Herson PS. Delayed inhibition of tonic inhibition enhances functional recovery following experimental ischemic stroke. J Cereb Blood Flow Metab 2019; 39(6): 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quillinan N, Deng G, Shimizu K, Cruz-Torres I, Schroeder C, Traystman RJ, Herson PS. Long-term depression in Purkinje neurons is persistently impaired following cardiac arrest and cardiopulmonary resuscitation in mice. J Cereb Blood Flow Metab 2017; 37(8): 3053–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation 2008; 15(4–6): 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang QH, Li JC, Dong N, Tang LM, Zhu XM, Sheng ZY, Yao YM. Burn injury induces gelsolin expression and cleavage in the brain of mice. Neuroscience 2013; 228: 60–72. [DOI] [PubMed] [Google Scholar]
- 48.Opitz B Memory function and the hippocampus. Front Neurol Neurosci 2014; 34: 51–59. [DOI] [PubMed] [Google Scholar]
- 49.Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem 2007; 14(11): 745–757. [DOI] [PubMed] [Google Scholar]
- 50.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology 2006; 79(1): 1–48. [DOI] [PubMed] [Google Scholar]
- 51.Rolls ET, Kesner RP. Pattern separation and pattern completion in the hippocampal system. Introduction to the Special Issue. Neurobiology of Learning and Memory 2016; 129: 1–3. [DOI] [PubMed] [Google Scholar]
- 52.Rolls ET. An attractor network in the hippocampus: theory and neurophysiology. Learn Mem 2007; 14(11): 714–731. [DOI] [PubMed] [Google Scholar]
- 53.Koehl M, Abrous DN. A new chapter in the field of memory: adult hippocampal neurogenesis. Eur J Neurosci 2011; 33(6): 1101–1114. [DOI] [PubMed] [Google Scholar]
- 54.Pinnock SB, Blake AM, Platt NJ, Herbert J. The roles of BDNF, pCREB and Wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PLoS One 2010; 5(10): e13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeschke MG, Pinto R, Costford SR, Amini-Nik S. Threshold age and burn size associated with poor outcomes in the elderly after burn injury. Burns 2016; 42(2): 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheatley EG, Curtis BJ, Hulsebus HJ, Boe DM, Najarro K, Ir D, Robertson CE, Choudhry MA, Frank DN, Kovacs EJ. Advanced age impairs intestinal antimicrobial peptide response and worsens fecal microbiome dysbiosis following burn injury in mice. Shock (Augusta, Ga) 2020; 53(1): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frankel JH, Boe DM, Albright JM, O’Halloran EB, Carter SR, Davis CS, Ramirez L, Burnham EL, Gamelli RL, Afshar M. Age-related immune responses after burn and inhalation injury are associated with altered clinical outcomes. Experimental gerontology 2018; 105: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomez CR, Karavitis J, Palmer JL, Faunce DE, Ramirez L, Nomellini V, Kovacs EJ. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators of inflammation 2010; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kovacs EJ, Duffner LA, Plackett TP. Immunosuppression after injury in aged mice is associated with a TH1–TH2 shift, which can be restored by estrogen treatment. Mechanisms of ageing and development 2004; 125(2): 121–123. [DOI] [PubMed] [Google Scholar]
- 60.Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. Journal of leukocyte biology 2004; 76(1): 36–41. [DOI] [PubMed] [Google Scholar]
- 61.Shallo H, Plackett TP, Heinrich SA, Kovacs EJ. Monocyte chemoattractant protein-1 (MCP-1) and macrophage infiltration into the skin after burn injury in aged mice. Burns 2003; 29(7): 641–647. [DOI] [PubMed] [Google Scholar]
- 62.Nomellini V, Gomez CR, Gamelli RL, Kovacs EJ. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock (Augusta, Ga) 2009; 31(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol 1999; 72: 7–29. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Tan J, Jiang X, Qian W, Yang T, Sun X, Chen Z, Zhu Q. Neuron-derived CCL2 contributes to microglia activation and neurological decline in hepatic encephalopathy. Biol Res 2017; 50(1): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clark IA, Alleva LM, Vissel B. The roles of TNF in brain dysfunction and disease. Pharmacol Ther 2010; 128(3): 519–548. [DOI] [PubMed] [Google Scholar]
- 66.D Skaper S, Facci L, Giusti P. Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 2014; 13(10): 1654–1666. [DOI] [PubMed] [Google Scholar]
- 67.Colicos MA, Dixon CE, Dash PK. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res 1996; 739(1–2): 111–119. [DOI] [PubMed] [Google Scholar]
- 68.Purohit M, Goldstein R, Nadler D, Mathews K, Slocum C, Gerrard P, DiVita MA, Ryan CM, Zafonte R, Kowalske K, Schneider JC. Cognition in patients with burn injury in the inpatient rehabilitation population. Arch Phys Med Rehabil 2014; 95(7): 1342–1349. [DOI] [PubMed] [Google Scholar]
- 69.Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. J Neurotrauma 2003; 20(10): 929–941. [DOI] [PubMed] [Google Scholar]
- 70.Cater HL, Sundstrom LE, Morrison B, 3rd. Temporal development of hippocampal cell death is dependent on tissue strain but not strain rate. J Biomech 2006; 39(15): 2810–2818. [DOI] [PubMed] [Google Scholar]
- 71.Mao H, Elkin BS, Genthikatti VV, Morrison B 3rd, Yang KH. Why is CA3 more vulnerable than CA1 in experimental models of controlled cortical impact-induced brain injury? J Neurotrauma 2013; 30(17): 1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015; 96: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


