Abstract
Background:
Gestational per- and polyfluoroalkyl substances (PFAS) exposure may be associated with adiposity and increased risk of obesity among children and adolescents. However, results from epidemiological studies evaluating these associations are inconsistent.
Objectives:
We estimated the associations of pregnancy PFAS concentrations with child body mass index (BMI) -scores and risk of overweight/obesity in eight U.S. cohorts.
Methods:
We used data from 1,391 mother–child pairs who enrolled in eight Environmental influences on Child Health Outcomes (ECHO) cohorts (enrolled: 1999–2019). We quantified concentrations of seven PFAS in maternal plasma or serum in pregnancy. We measured child weight and height between the ages of 2 and 5 y and calculated age- and sex-specific BMI -scores; 19.6% children had more than one BMI measurement. We estimated covariate-adjusted associations of individual PFAS and their mixture with child BMI -scores and risk of overweight/obesity using linear mixed models, modified Poisson regression models, and Bayesian approaches for mixtures. We explored whether child sex modified these associations.
Results:
We observed a pattern of subtle positive associations of PFAS concentrations in pregnancy with BMI -scores and risk of overweight/obesity. For instance, each doubling in perfluorohexane sulfonic acid concentrations was associated with higher BMI -scores (; 95% CI: 0.01, 0.12). Each doubling in perfluroundecanoic acid [; 95% CI: 1.04, 1.16] and -methyl perfluorooctane sulfonamido acetic acid (; 95% CI: 1.00, 1.12) was associated with increased risk of overweight/obesity, with some evidence of a monotonic dose–response relation. We observed weaker and more imprecise associations of the PFAS mixture with BMI or risk of overweight/obesity. Associations did not differ by child sex.
Discussion:
In eight U.S.-based prospective cohorts, gestational exposure to higher levels of PFAS were associated with slightly higher childhood BMI -score and risk of overweight or obesity. Future studies should examine associations of gestational exposure to PFAS with adiposity and related cardiometabolic consequences in older children. https://doi.org/10.1289/EHP11545
Introduction
Child obesity has reached epidemic levels in developed and developing countries.1 Children with overweight and obesity are more likely to manifest social and psychological disorders and are at higher risk for cardiovascular disease compared with children with normal weight.2,3 In addition, childhood obesity tracks into adulthood,4 and children with overweight and obesity are at increased risk of developing cardiometabolic diseases, musculoskeletal disorders, and cancers in adulthood, which may cause premature death and disability.2,3
Although the rise in childhood and adolescent obesity is widely attributed to the lack of physical activity and unhealthy diet, these factors do not fully explain the genesis and trends of the obesity epidemic.2,5,6 Fetal exposure to endocrine-disrupting chemicals (EDCs) or obesogens may predispose exposed individuals to higher adiposity and risk of obesity.7 Per- and polyfluoroalkyl substances (PFAS), a diverse group of fluorinated chemicals, are considered potential obesogens. PFAS have been used in a variety of consumer and industrial products, including food packaging, cleaning products, nonstick cookware, ski wax, fire-fighting foams, and processing aids for manufacturing fluoropolymers.8–10 PFAS may contribute to childhood obesity by inducing changes in DNA methylation11,12 and the metabolome13,14 or by activating peroxisome proliferator-activated receptors-alpha and -gamma () to alter lipid metabolism and adipocyte differentiation.15,16
Some prior studies have linked gestational PFAS exposure to preterm birth and lower birth weight,17,18 and alterations in child growth,19,20 whereas others have linked PFAS exposure to greater body mass index (BMI) or adiposity in children and adolescents.21–28 However, there are discrepant findings, with some studies reporting null or inverse associations.19,29–31 Prior meta-analysis and review studies have reported inconclusive associations between PFAS exposure and child obesity.32–34 Moreover, it is unclear whether associations of gestational PFAS exposure with children’s adiposity are attributable to individual PFAS or mixtures of PFAS, and whether associations are sex specific.26,35–37 Finally, most current studies are limited by their relatively small sample size.
Thus, we pooled data from eight cohorts participating in the Environmental influences on Child Health Outcomes (ECHO) Program to investigate the associations of gestational exposure to seven PFAS and their mixture with offspring BMI -scores and risk of overweight/obesity from 2 to 5 years of age and whether associations varied by child sex.
Methods
Study Participants
In this study, we included data from 1,391 ECHO mothers and their singleton births (Table S1). The ECHO Program is a large collaborative consortium that aims to understand a wide range of environmental exposures from conception through early childhood and their associated health outcomes in children and adolescents. The program includes a number of on-going individual pregnancy and birth cohorts in the United States.38 All participating cohorts followed the ECHO-wide Cohort Data Collection Protocol, and we used extant data that had been collected and shared by each individual cohort on the data platform.39,40 For the present analysis, we included participants with maternal serum or plasma PFAS who had at least one BMI measurement between 2 and 5 years of age. Of the 69 individual ECHO cohorts, 11 cohorts () had available data on gestational PFAS. After excluding children who had no data on BMI measurements between 2 and 5 years of age, there were 10 cohorts with information available (). We further excluded cohorts with a sample size of and mother–child pairs missing covariate information, leaving 1,391 mother-singleton pairs from 8 cohorts in our final data analysis (Figure 1). The 8 cohorts included in our study are Chemicals in Our Bodies (CiOB), Illinois Kids Development Studies (IKIDS), Project Viva, Healthy Start, New Hampshire Birth Cohort Study (NHBCS), Atlanta ECHO Cohort of Emory University. Pregnancy and EnvironmenT And Lifestyle Study (PETALS) and Rochester. The sociodemographic characteristics did not differ meaningfully between consented participants who were included and excluded participants from the same cohort (Table S2).
Figure 1.
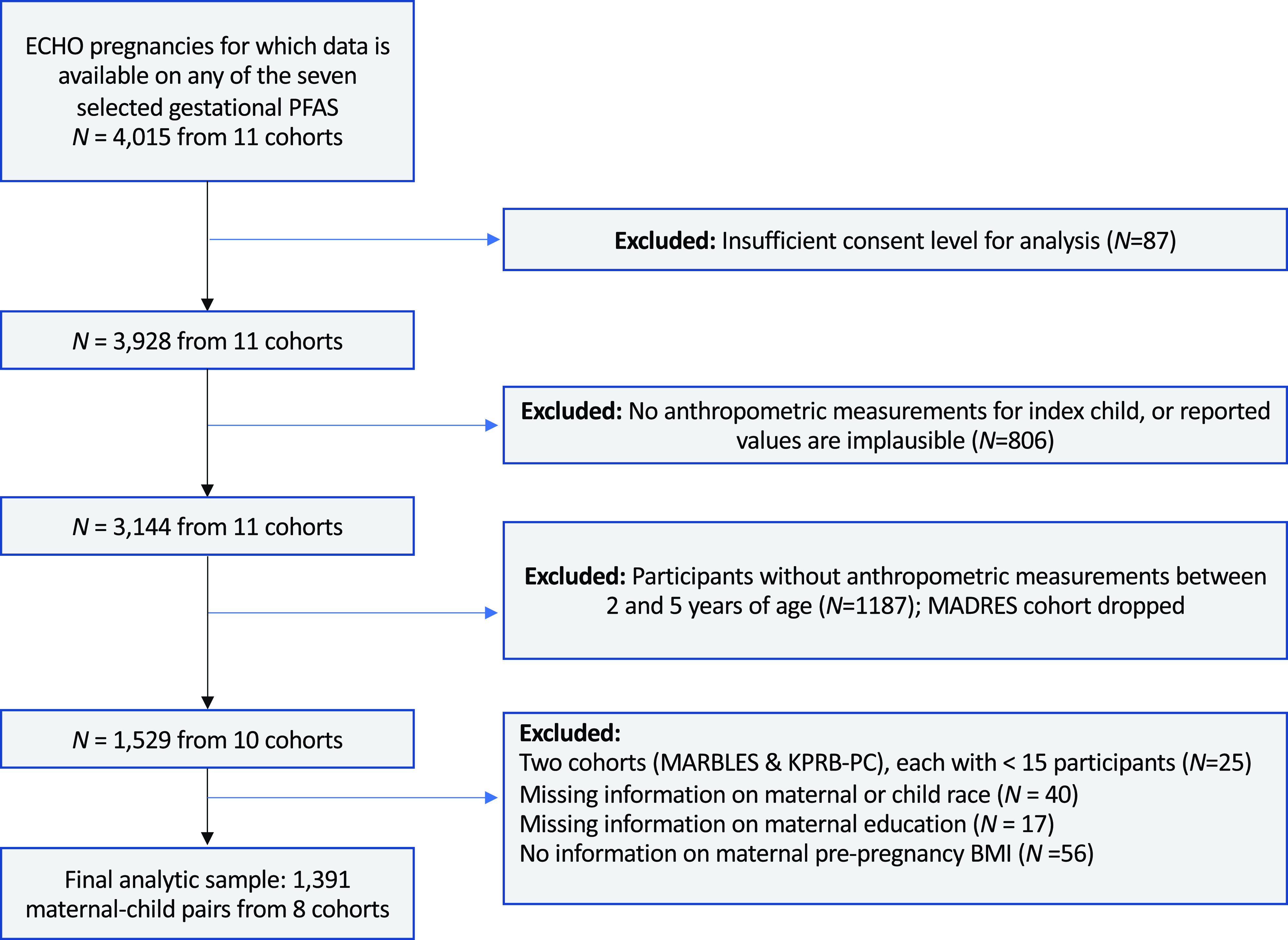
Flow chart for final analytic sample of 1,391 mother–child pairs from ECHO cohorts included in the analysis of PFAS concentrations and child adiposity. Note: BMI, body mass index; ECHO, Environmental influences on Child Health Outcomes; KPRB-PC, Kaiser Permanente Research Bank–Pregnancy Cohort; MADRES, Maternal and Developmental Risks from Environmental and Social Stressors; MARBLES, Markers of Autism Risk in Babies; PFAS, per- and polyfluoroalkyl substances.
The ECHO-wide Cohort Data Collection Protocol was approved by the central ECHO institutional review board (IRB) or by individual cohorts’ IRBs of record. Parents/guardians provided written informed consent for participation in individual cohorts and data sharing with ECHO.
PFAS Exposure Assessment
Maternal serum or plasma PFAS concentrations were measured in samples collected during pregnancy (Table S3). In these study participants, 39.2% had PFAS measured in the first trimester, 44.5% in the second trimester, and 16.3% in the third trimester. Three laboratories conducted these assays: California Department of Toxic Substances Control,41 Centers for Disease Control and Prevention (CDC),42,43 and the Wadsworth Human Health Exposure Analysis Resource (HHEAR) lab44 (Table S1). Although 14 separate PFAS analytes have been measured within these cohorts, we included individual PFAS if of participants in the eight cohorts had concentrations greater than the limit of detection (LOD) and if three or more cohorts had concentrations available for that individual PFAS (Tables S4 and S5). Thus, we included perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), perfluorohexane sulfonic acid (PFHxS), perfluorodecanoic acid (PFDA), perfluroundecanoic acid (PFUnDA), and -methyl perfluorooctane sulfonamido acetic acid (NMFOSAA) in our final analyses. PFAS such as -ethyl perfluorooctane sulfonamido acetic acid (EtFOSAA), perfluorobutane sulfonic acid (PFBS), PFDODA, perfluorohexanoic acid (PFHxA), perfluoropentanoic acid (PFPEA), and perfluorooctanesulfonamide (PFOSA) were not included in the analyses because most measurements were below the limit of detection or were not measured in at least three cohorts. In the case where a PFAS was not measured in a cohort, that cohort was excluded from the analysis, and hence the smaller sample size for PFUnDA.
For observations with concentrations below the LOD or the limit of quantification (LOQ; hereafter discussed as LOD for simplicity), we substituted the LOD divided by the square root of 2. Among 1,391 participants, 110 had PFAS measured more than once during pregnancy. Among these participants, if all concentrations were detectable, we averaged the observed measurements. If the PFAS concentration at one measurement was above the LOD and the other was below the LOD, the measurement above the LOD was used. When concentrations from all measurements were below the LOD, then the LOD divided by the square root of 2 was used. Because all PFAS concentrations were right-skewed, we them to reduce the influence of outliers in our analyses. Generally, we observed a strong Pearson’s correlation of PFAS concentrations during the first and second trimesters (, ) and second and third trimesters () except for NMFOSAA (, ) (Table S6).
Child BMI Measurements
The ECHO Data Analysis Center harmonized weight and height data from the eight ECHO cohorts (Table S7). Of the 1,391 child BMI measurements included in the analysis, 83% were direct measurements obtained by study staff at study visits, 7% were obtained via parent report, and 10% were obtained from medical records. Using weight and height collected between 2 and 5 years of age, we calculated age- and sex-standardized BMI -scores using the 2000 CDC growth reference for U.S. children.45 We examined continuous BMI -score and risk of overweight/obesity, defined as a BMI -score of th percentile for age and sex.45
Covariates
Covariate selection was informed by the literature on the association of each covariate with PFAS exposures and child adiposity,46 as well as by a directed acyclic graph (Figure S1). Covariates included child sex (male, female), highest level of education attained by the mother at time of pregnancy (less than high school, high school degree, General Educational Development or equivalent, some college, no degree, bachelor’s degree or higher), continuous maternal prepregnancy BMI, continuous maternal age at delivery, continuous age of the child at time of BMI measurement, maternal race (White, Black, others) and ethnicity (Hispanic, non-Hispanic). Other race includes Asian, multiple races, Indian or Alaska Native, or do not know. For the analysis, we created three categories for the assessment of race to avoid small cell sizes after adjustment for Hispanic ethnicity. When race information was missing for the mother (), we substituted information about the race of the child. In the context of our analysis, maternal race is a social construct and a proxy for structural and personal experiences of racism and discrimination.
Statistical Analyses
In the present study, we performed a complete case analysis. We calculated univariate statistics for PFAS concentrations, adiposity measures, and covariates within the eight cohorts. We also examined Pearson’s correlation of the analytes (PFOA, PFOS, PFHxS, PFNA, NMFOSAA, PFDA, and PFUnDA).
To evaluate associations between individual PFAS concentrations and continuous BMI -scores, we used multivariable linear mixed models to estimate the difference in BMI -score per doubling of PFAS concentrations with an unstructured correlation matrix. Then we used multivariable modified Poisson regression models with robust standard errors to estimate the risk of the child having overweight or obesity for each doubling in PFAS concentrations. All linear mixed and Poisson regression models were adjusted for covariates and included random intercepts for cohort and participant to account for within-cohort and within-participant clustering.
We examined whether associations between individual PFAS and outcomes were modified by sex by conducting sex-stratified analyses. In addition, we calculated -values for the difference in the association across sexes using previously described methods.47 To assess potential dose–response relationships, we estimated differences in BMI -score or relative risk (RR) of having overweight/obesity in the second through fifth quintiles of PFAS concentrations in reference to the first.
Because PFAS often co-occur in the environment, we estimated the potential effect of this mixture of PFAS on BMI -scores and risk of overweight/obesity using Bayesian approaches.48 This method used two model forms and two different Bayesian priors that allowed us to a) estimate the independent effects of individual PFAS adjusting for potential copollutant confounding by co-occurring PFAS using a shared mean Bayesian model and b) estimate the summed effect of PFAS using a Bayesian weighted sums approach. For independent effects, we applied a shared mean Bayesian model, otherwise referred to as a semi or empirical Bayesian model.49,50 This applied a prior assumption specified as , indicating that the estimated change per increase for each PFAS analyte, , may arise from the same distribution with a shared mean, m; the variance, s, is specified as weak to allow the data and model to inform the strength of this prior. For summed effects, the Bayesian weighted sums model48 estimates both a) the change in BMI- score or odds of being classified as overweight/obese for a simultaneous 1-unit increase in the log of all PFAS and b) the individual contribution of each PFAS to this mixture effect as percentages that sum to 1. This allows an understanding of which PFAS may contribute more or less to a mixture effect. The weights are subject to a Dirichlet prior, which restricts their values to a range from 0 to 1, always summing to 1 at any iteration of the Markov chain Monte Carlo (MCMC) simulation used to fit the Bayesian models. To assess convergence of models, we estimated effective sample sizes and conducted a Gelman-Rubin (or, R-hat) diagnostic test; the latter requires that we run multiple chains to ensure that results are not sensitive to starting values of the MCMC simulation; here, we used three chains. We required an rhat of 1.0 and an effective sample size of to ensure model convergence, and we excluded PFUnDA from the mixture analyses owing to the small effective sample size when it was included. The R package bws (available from https://cran.r-project.org/web/packages/bws) was used for estimation purposes.
In our study, we were not able to obtain model convergence using other mixture methods, such as Bayesian kernel machine regression or weighted quantile sum (WQS). Therefore, we used the Bayesian Weighted Sums approach that gives similar inference.48
We performed several sensitivity analyses to evaluate the robustness of our results. We adjusted for birth weight (continuous; in grams), maternal use of tobacco/nicotine products during pregnancy (yes, no), parity (continuous), gestational diabetes mellitus during the index pregnancy (yes, no), and child year of birth. Some of these variables were limited to sensitivity analyses because they may be causal intermediates (e.g., birth weight and gestational diabetes) or were missing on a substantial proportion of participants (specifically, 14.5% were missing parity and 10.1% missing information on tobacco/nicotine product use during pregnancy). We also ran all the models without additional adjustment of child age (BMI -scores were standardized by child age and sex). We performed sensitivity analysis restricted to participants with direct measures of BMI. We repeated our analysis using the World Health Organization (WHO) BMI cutoffs of to define overweight/obesity. We also examined associations stratified by the timing of exposure measure (first vs. second vs. third trimester) to determine whether pregnancy hemodynamics influenced our results.24 Finally, we conducted a leave-one-out cohort analysis to examine whether individual cohorts influenced our results.
We defined statistical significance as . We performed all the statistical analyses using R (version 4.0.5; R Development Core Team) and Stata (version 17.1; StataCorp).
Results
Of the 1,391 children from the eight ECHO cohorts, 51% were female, and 7% were born preterm (i.e., liveborn infant wk gestation) (Table 1). Mothers in our study were predominately non-Hispanic White (67%), nulliparous (54%), underweight or normal weight () before pregnancy (54%), and held a bachelor’s degree or higher (64%). Few women reported use of nicotine/tobacco products in pregnancy (5%). There were 272 (19.6%) children who had more than one BMI measurement, and 373 (21.0%) children were overweight or obese between 2 and 5 years of age (Table S8). The median [interquartile range (IQR)] of BMI -scores across all measurements was 0.22 ( to 0.89).
Table 1.
Characteristics of mother–child dyads in ECHO cohort analysis of PFAS concentrations and child adiposity (1999 and 2019, pairs).
| Characteristics | Healthy Start | Atlanta ECHO cohort of Emory University | PETALS | NHBCS | Rochester | Project Viva | IKIDS | CiOB | Total |
|---|---|---|---|---|---|---|---|---|---|
| Total number of BMI measurements per child | |||||||||
| Median (25th, 75th percentiles) | 1 (1–1) | 2 (1–2) | 2 (1–3) | 1 (1–1) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 2 (1–2) | 1 (1–1) |
| Range | 1–1 | 1–3 | 1–15 | 1–2 | 1–3 | 1–1 | 1–1 | 1–3 | 1–15 |
| Average age at BMI measurement per child (y) | |||||||||
| Median (25th, 75th percentiles) | 4.5 (4.3–4.6) | 2.6 (2.1–3.1) | 2.5 (2.4–2.9) | 3.9 (3.2–4.2) | 2.6 (2.2–3.1) | 3.1 (3.1–3.2) | 3.9 (3.9–3.9) | 2.3 (2.2–3.4) | 3.2 (2.9–4.00) |
| Range | 4.0–4.9 | 2.0–4.5 | 2.0–4.9 | 2.9–4.9 | 2.0–3.5 | 2.9–4.8 | 3.9–4.00 | 2.2–4.1 | 2.00–4.99 |
| Sex of the child [ (%)] | |||||||||
| Male | 162 (53.5) | 74 (47.7) | 41 (44.6) | 17 (60.7) | 55 (52.4) | 200 (47.2) | 37 (41.1) | 98 (50.5) | 684 (49.2) |
| Female | 141 (46.5) | 81 (52.3) | 51 (55.4) | 11 (39.3) | 50 (47.6) | 224 (52.8) | 53 (58.9) | 96 (49.5) | 707 (50.8) |
| Maternal race [ (%)] | |||||||||
| White | 235 (77.6) | 0 | 36 (39.1) | 28 (100.0) | 81 (77.1) | 348 (82.1) | () | () | 927 (66.6) |
| Black | 46 (15.2) | () | 6 (6.5) | 0 | 14 (13.3) | 42 (9.9) | () | () | 268 (19.3) |
| Othersa | 22 (7.2) | () | 50 (54.4) | 0 | 10 (9.5) | 34 (8.0) | 9 (10.0) | (34.0) | 196 (14.1) |
| Maternal Hispanic ethnicity [ (%)] | |||||||||
| No | 235 (77.6) | () | 66 (71.7) | () | 98 (93.3) | 394 (92.9) | () | 173 (89.2) | 1,233 (88.6) |
| Yes | 68 (22.4) | (3.2) | 26 (28.3) | () | 7 (6.7) | 30 (7.1) | () | 21 (10.8) | 158 (11.4) |
| Highest level of maternal education at pregnancy [ (%)] | |||||||||
| 41 (13.5) | 20 (12.9) | () | 0 | 0 | 5 (1.2) | 0 | () | 68 (4.9) | |
| Some high school or high school | 49 (16.2) | 61 (39.4) | () | () | 20 (19.0) | 22 (5.2) | 0 | () | 171 (12.3) |
| Some college or technical school | 74 (24.4) | 46 (29.7) | (32.6) | () | 15 (14.3) | 70 (16.5) | 9 (10.0) | () | 257 (18.5) |
| 139 (45.9) | 28 (18.1) | 49 (53.3) | () | 70 (66.7) | 327 (77.1) | 81 (90.0) | 178 (91.8) | 895 (64.3) | |
| Maternal age at delivery [y; (%)] | |||||||||
| 85 (28.1) | 70 (45.2) | 5 (5.4) | () | 7 (6.7) | 16 (3.8) | () | () | 187 (13.4) | |
| 25 to | 77 (25.4) | 46 (29.7) | 16 (17.4) | () | 27 (25.7) | 62 (14.6) | () | () | 269 (19.3) |
| 30 to | 86 (28.4) | 34 (21.9) | 38 (41.3) | () | 50 (47.6) | 182 (42.9) | 50 (55.6) | () | 530 (38.1) |
| 55 (18.1) | 5 (3.2) | 33 (35.9) | 10 (35.7) | 21 (20.0) | 164 (38.7) | 16 (17.8) | 101 (52.1) | 405 (29.1) | |
| Median (25th, 75th percentiles) | 29 (24–33) | 25 (22–30) | 33 (30–36) | 31.5 (28.5–36.5) | 31 (28–34) | 33 (31–36) | 32 (29–34) | 35 (33–37) | 32 (28–35) |
| Parity [ (%)] | |||||||||
| Nulliparous (0) | 164 (54.1) | 67 (43.2) | 40 (43.5) | 12 (42.8) | 38 (36.2) | 205 (48.4) | 45 (50.0) | 177 (91.2) | 748 (53.8) |
| Parous: 1 | 96 (31.7) | 41 (26.5) | 33 (35.9) | () | 46 (43.8) | 149 (35.1) | 29 (32.2) | () | 412 (29.6) |
| Parous: | 43 (14.2) | 47 (30.3) | 19 (20.6) | () | () | 70 (16.5) | 16 (17.8) | () | 224 (16.1) |
| Missing | 0 | 0 | 0 | 5 (17.9) | (4.8) | 0 | 0 | () | 7 (0.5) |
| Prepregnancy BMI [; (%)] | |||||||||
| Underweight/normal weight () | 148 (48.8) | 64 (41.3) | 31 (33.7) | 18 (64.3) | 49 (46.7) | 251 (59.2) | 43 (47.8) | 143 (73.7) | 747 (53.7) |
| Overweight (25 to ) | 94 (31.0) | 26 (16.8) | 30 (32.6) | () | 33 (31.4) | 113 (26.7) | 25 (27.8) | 41 (21.1) | 366 (26.3) |
| Obese () | 61 (20.1) | 65 (41.9) | 31 (33.7) | () | 23 (21.9) | 60 (14.1) | 22 (24.4) | 10 (5.2) | 278 (20.0) |
| Median (25th, 75th percentiles) | 25.1 (21.6–28.8) | 27.5 (22.6–34.6) | 27.5 (23.5–31.7) | 24.4 (21.5–28.2) | 25.4 (22.7–29.2) | 24 (21–27) | 25.4 (22.5–29.3) | 22.8 (21.1–25.5) | 24.2 (21.7–28.4) |
| Use of tobacco/nicotine products during pregnancy [ (%)] | |||||||||
| No | 282 (93.1) | 130 (83.9) | () | (82.1) | — | 406 (95.7) | (94.4) | 164 (84.5) | 1,186 (85.3) |
| Yes | 21 (6.9) | 25 (16.1) | () | () | — | 13 (3.1) | () | 0 | 65 (4.7) |
| Missing | 0 | 0 | 0 | 0 | 105 (100.0) | 5 (1.2) | 0 | 30 (15.5) | 140 (10.1) |
| Gestational diabetes [ (%)] | |||||||||
| No | 282 (93.1) | 147 (94.8) | 62 (67.4) | () | (95.2) | 394 (92.9) | 79 (87.8) | 150 (77.3) | 1,244 (89.5) |
| Yes | 14 (4.6) | 8 (5.2) | 30 (32.6) | () | 0 | 20 (4.7) | 11 (12.2) | 39 (20.1) | 123 (8.8) |
| Missing | 7 (2.3) | 0 | 0 | () | () | 10 (2.4) | 0 | 5 (2.6) | 24 (1.7) |
| Preterm birth (i.e., liveborn infant wk gestation) [ (%)] | |||||||||
| No | 292 (96.4) | 134 (86.4) | 83 (90.2) | (82.1) | 105 (100) | 397 (93.6) | 81 (90.0) | 180 (92.8) | 1,299 (93.4) |
| Yes | 11 (3.6) | 21 (13.6) | 9 (9.8) | () | 0 | 27 (6.4) | 9 (10.0) | 14 (7.2) | 92 (6.6) |
| Children’s years of birth | |||||||||
| Range | 2010–2014 | 2014–2017 | 2013–2017 | 2010–2013 | 2016–2019 | 1999–2003 | 2014–2016 | 2014–2019 | 1999–2019 |
| Childbirth weight (g) | |||||||||
| Median (25th, 75th percentiles) | 3,230 (2,930–3,565) | 3,118 (2,750–3,420) | 3,360 (3,120.5–3,665) | 3,500 (3,200–3,950) | 3,420 (3,130–3,790) | 3,546 (3,203–3,883) | 3,600 (3,232–3,856) | 3,402.5 (3,105–3,725) | 3,380 (3,061–3,725) |
| Range | 1,750–4,285 | 955–4,280 | 1,100–4,930 | 2,000–4,500 | 2,200–4,650 | 481–5,528 | 2,240–4,876 | 1,588–4,760 | 481–5,528 |
| Missing (%) | — | — | — | — | — | — | 13 (14.44) | — | 13 () |
| Child BMI -score | |||||||||
| Median (IQR) | 0.03 ( to 0.63) | ( to 0.81) | 0.18 ( to 0.88) | 0.24 ( to 0.87) | 0.25 ( to 0.76) | 0.52 ( to 1.11) | 0.40 ( to 1.01) | 0.17 ( to 0.80) | 0.22 ( to 0.89) |
Note: Symbols < or > were used to mask values in and around cells with to ensure nonidentifiability of participants. —, not applicable; BMI, Body mass index; CiOB, Chemicals in Our Bodies; ECHO, Environmental influences on Child Health Outcomes; IKIDS, Illinois Kids Development Studies; IQR, interquartile range; NHBCS, New Hampshire Birth Cohort Study; PETALS, Pregnancy and EnvironmenT and Lifestyle Study; PFAS, per- and polyfluoroalkyl substances.
We collected information on race and ethnicity using self-reported questionnaires. Among the 196 mothers in the other category, 120 (61.2%) identified as Asian, 59 (30.1%) identified as Multiple Races, and the remaining 17 (8.7%) identified as Indian or Alaska Native, Other race, or do not know. In addition, 28 (14.3%) identified as Hispanic. For the analysis, we created three categories for the assessment of race to avoid small cell sizes after adjustment for Hispanic ethnicity.
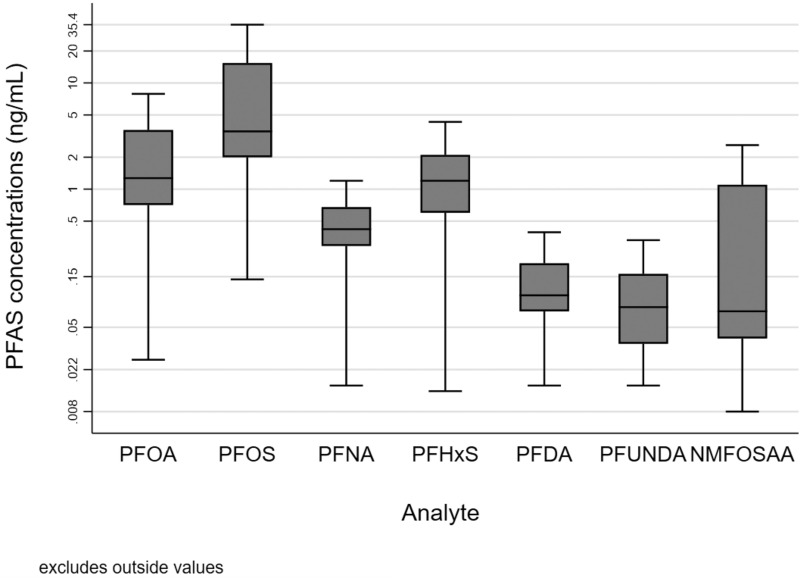
PFOA, PFOS, PFNA, and PFHxS were detected in blood samples collected from these pregnant women, whereas PFDA, PFUnDA, and NMFOSAA were detected in of maternal blood samples (Table S9). Concentrations of PFOA, PFOS, PFNA, and PFHxS were highest in Project Viva (1999–2003) and lowest in the Rochester (2016–2019) and CiOB cohorts (2014–2019) (Figure 2; Table S9). Pearson correlation coefficients between PFAS during pregnancy ranged from 0.06 (NMFOSAA–PFUnDA) to 0.87 (PFOA–PFOS) (Table S10). The correlation coefficients of PFAS ranged from 0.04 (PFHxS–PFUnDA) to 0.91 (PFOA–PFOS) in the first trimester, from (PFHxS–PFDA) to 0.74 (PFOA–PFNA) in the second trimester, and from 0.04 (NMFOSAA–PFUnDA) to 0.71 in the third trimester (PFOA–PFNA).
Figure 2.
Box-and-whisker plots of maternal PFAS concentrations during pregnancy among women in the ECHO cohorts (). Box plots show the median and interquartile ranges (IQRs) of the PFAS concentrations averaged across pregnancy for the analytic sample. Whiskers show the largest value within 1.5 times the IQR above the 75th percentile and the lowest value within 1.5 times the IQR below the 25th percentile. Numerical results of the medians and IQRs of each PFAS analyte (by cohort and overall) are provided in Table S9. Note: ECHO, Environmental influences on Child Health Outcomes; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFUnDA, perfluroundecanoic acid.
After adjustment for covariates, all seven PFAS analytes in pregnancy were associated with slightly higher BMI -scores at 2–5 years of age (Table 2). However, almost all 95% CIs included the null. Notably, PFHxS had the strongest association with BMI -scores at 2–5 years of age [; 95% confidence interval (CI): 0.01, 0.12]. Similar to associations we observed with continuous BMI -scores, PFAS concentrations were associated with increased risk of being overweight or obese at 2–5 years of age (Table 3). PFOS had the largest risk ratio (RR) (; 95% CI: 1.01, 1.24). Child sex did not modify the associations of gestational PFAS with child BMI -scores ( ) or risk of overweight/obesity ( ).
Table 2.
Adjusted difference in child BMI -scores from 2 to 5 years of age per doubling in PFAS concentrations during pregnancy: the ECHO cohorts.
| PFAS analytes () | Overall | Sex-specific results | |||||
|---|---|---|---|---|---|---|---|
| Males | Females | Sex-interaction -Value | |||||
| (95% CI) | (95% CI) | (95% CI) | |||||
| PFOA | 1,391 | 0.03 (, 0.08) | 684 | 0.04 (, 0.11) | 707 | 0.04 (, 0.11) | 1.000 |
| PFOS | 1,391 | 0.04 (, 0.09) | 684 | 0.06 (, 0.13) | 707 | 0.06 (, 0.13) | 1.000 |
| PFNA | 1,391 | 0.02 (, 0.08) | 684 | 0.02 (, 0.10) | 707 | 0.04 (, 0.12) | 0.744 |
| PFHxS | 1,391 | 0.07 (0.01, 0.12) | 684 | 0.08 (0.01, 0.15) | 707 | 0.06 (, 0.12) | 0.688 |
| PFDA | 1,352 | 0.00 (, 0.06) | 663 | 0.01 (, 0.08) | 689 | (, 0.07) | 0.847 |
| PFUnDA | 625 | 0.05 (, 0.11) | 301 | 0.06 (, 0.16) | 324 | 0.03 (, 0.12) | 0.656 |
| NMFOSAA | 1,352 | 0.03 (, 0.07) | 663 | 0.02 (, 0.08) | 689 | 0.04 (, 0.09) | 0.589 |
Note: Models were adjusted for sex of the child (2 categories), child age, maternal race (3 categories), maternal Hispanic ethnicity (2 categories), maternal age at delivery, highest level of maternal education at pregnancy (4 categories), and maternal prepregnancy BMI. Models included random intercepts for participant and cohort. BMI, body mass index; CI, confidence interval; ECHO, Environmental influences on Child Health Outcomes; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFUnDA, perfluroundecanoic acid.
Table 3.
Adjusted relative risk of overweight/obesity from 2 to 5 years of age per doubling in PFAS concentrations during pregnancy: the ECHO cohorts.
| PFAS analytes () | Overall | Sex-specific results | |||||
|---|---|---|---|---|---|---|---|
| Males | Females | Sex-interaction -Value | |||||
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |||||
| PFOA | 1,391 | 1.04 (0.93, 1.17) | 684 | 1.02 (0.85, 1.23) | 707 | 1.11 (1.01, 1.22) | 0.418 |
| PFOS | 1,391 | 1.12 (1.01, 1.24) | 684 | 1.17 (1.07, 1.28) | 707 | 1.13 (1.03, 1.23) | 0.588 |
| PFNA | 1,391 | 1.06 (0.95, 1.18) | 684 | 1.00 (0.82, 1.21) | 707 | 1.16 (1.07, 1.25) | 0.152 |
| PFHxS | 1,391 | 1.07 (0.98, 1.16) | 684 | 1.08 (0.95, 1.23) | 707 | 1.07 (0.99, 1.16) | 0.904 |
| PFDA | 1,352 | 1.00 (0.93, 1.08) | 663 | 1.00 (0.92, 1.08) | 689 | 1.03 (0.92, 1.16) | 0.682 |
| PFUnDA | 625 | 1.10 (1.04, 1.16) | 301 | 1.11 (0.90, 1.36) | 324 | 1.06 (0.88, 1.28) | 0.745 |
| NMFOSAA | 1,352 | 1.06 (1.00, 1.12) | 663 | 1.07 (0.87, 1.31) | 689 | 1.09 (1.02, 1.15) | 0.864 |
Note: Models were adjusted for sex of the child (2 categories), child age, maternal race (3 categories), maternal Hispanic ethnicity (2 categories), maternal age at delivery, highest level of maternal education at pregnancy (4 categories), and maternal prepregnancy BMI. Models included random intercepts for participant and cohort. BMI, body mass index; CI, confidence interval; ECHO, Environmental influences on Child Health Outcomes; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFUnDA, perfluroundecanoic acid; RR, relative risk.
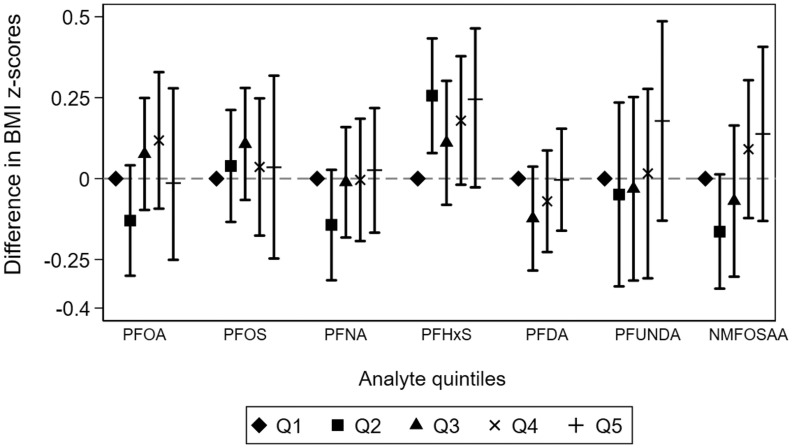
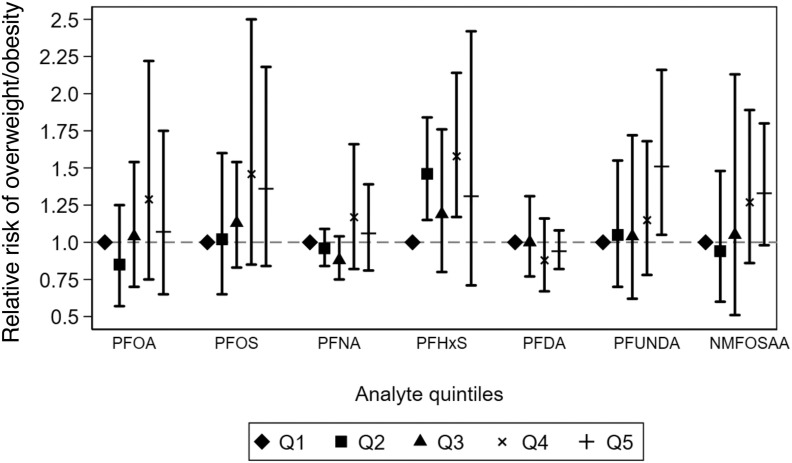
We did not observe compelling evidence of a monotonic dose–response association between any PFAS and BMI -scores (Figure 3; Table S11). However, it is worth noting that the range of PFAS concentrations in the first three quintiles were relatively narrow ( range in each quintile, Table S11). For PFHxS, child BMI -scores were on average 0.1–0.2 -scores higher across the second to fifth quintiles compared with the first. In contrast to continuous BMI -scores, there was some evidence suggesting monotonic associations of NMFOSAA and PFUnDA with the risk of child overweight/obesity (Figure 4; Table S12). Compared with children in the first quintile, those in the fifth quintile of NMFOSAA or PFUnDA concentrations had 33% (95% CI: 0.98, 1.80) and 51% (95% CI: 1.05, 2.16) higher risk of being overweight or obese.
Figure 3.
Adjusted difference in child BMI -score across quintiles of PFAS concentrations during pregnancy: the ECHO Cohorts. Models were adjusted for sex of the child (2 categories), child age, maternal race (3 categories), maternal Hispanic ethnicity (2 categories), maternal age at delivery, highest level of maternal education at pregnancy (4 categories), and maternal prepregnancy BMI. Models included random intercepts for participant and cohort. Cutoffs for quintiles and the full corresponding numerical results in this figure are presented in Table S11. Shapes show the reference, and point estimates for the difference in BMI- scores for each quintile of PFAS analyte. Error bars represent the lower and upper confidence intervals for each point estimate. Quintile 1 is the reference group for each analyte. Note: BMI, body mass index; ECHO, Environmental influences on Child Health Outcomes; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFUnDA, perfluroundecanoic acid; Q, quintile.
Figure 4.
Adjusted relative risk of child overweight/obesity across quintiles of PFAS concentrations during pregnancy: the ECHO Cohorts. Models were adjusted for sex of the child (2 categories), child age, maternal race (3 categories), maternal Hispanic ethnicity (2 categories), maternal age at delivery, highest level of maternal education at pregnancy (4 categories), and maternal prepregnancy BMI. Models included random intercepts for participant and cohort. Cutoffs for quintiles and the full corresponding numerical results in this figure are presented in Table S12. Shapes show the reference, and point estimates for the risk ratio of overweight/obesity for each quintile of PFAS analyte. Error bars represent the lower and upper confidence intervals for each point estimate. Quintile 1 is the reference group for each analyte. Note: BMI, body mass index; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFUnDA, perfluroundecanoic acid; Q, quintile.
When we estimated the individual effects of PFAS in a Bayesian mixture model accounting for copollutant confounding, we found that PFHxS and PFNA had the strongest positive association with BMI -score [ for ; 95% highest posterior density (HPD): to 0.11] and one of the strongest associations with risk of overweight/obesity [RR for (95% HPD: 0.85–1.47) and RR for (95% HPD: 0.83–1.64)]. However, there was little evidence of an association for the other PFAS (Tables 4 and 5). Consistent with the subtle or absent associations for individual PFAS, we observed a relatively weak and imprecise mixture effect of PFAS on child BMI -scores (; 95% HPD: to 0.12) or the risk of being overweight/obese (; 95% HPD: 0.75–1.94). The proportion that respective PFAS contributed to the summed effect estimate ranged from 14% to 23% for BMI -scores and from 15% to 19% for risk of overweight/obesity. Generally, the summed effect of the mixture on BMI -scores and risk of overweight/obesity was greater in males than females, but the estimates were considerably less precise than in analyses examining individual PFAS.
Table 4.
Bayesian weighted sums analysis of PFAS concentrations during pregnancy and child BMI -scores: the ECHO cohorts ().
| Overall | Males | Females | ||||
|---|---|---|---|---|---|---|
| Weighted sums | Difference or percentage | 95% HPD | Difference or percentage | 95% HPD | Difference or percentage | 95% HPD |
| Shared mean () | ||||||
| PFOA | ( to 0.05) | 0.00 | ( to 0.07) | ( to 0.06) | ||
| PFOS | ( to 0.05) | 0.00 | ( to 0.08) | ( to 0.05) | ||
| PFHxS | 0.04 | ( to 0.11) | 0.03 | ( to 0.11) | 0.04 | ( to 0.13) |
| PFNA | 0.01 | ( to 0.08) | 0.02 | ( to 0.11) | 0.00 | ( to 0.08) |
| PFDA | 0.00 | ( to 0.05) | ( to 0.05) | 0.01 | ( to 0.09) | |
| NMFOSAA | 0.01 | ( to 0.05) | 0.02 | ( to 0.08) | ( to 0.05) | |
| Summed effect () | 0.04 | ( to 0.12) | 0.06 | ( to 0.18) | 0.01 | ( to 0.12) |
| PFOA (%) | 15 | (0–42) | 15 | (0–41) | 16 | (0–44) |
| PFOS (%) | 15 | (0–41) | 16 | (0–43) | 17 | (0–45) |
| PFHxS (%) | 23 | (0–56) | 20 | (0–52) | 18 | (0–49) |
| PFNA (%) | 16 | (0–43) | 17 | (0–45) | 15 | (0–43) |
| PFDA (%) | 14 | (0–39) | 14 | (0–39) | 16 | (0–44) |
| NMFOSAA (%) | 16 | (0–42) | 17 | (0–44) | 17 | (0–45) |
Note: The summed mixture model estimates both the effect of PFAS as a mixture and the individual contribution of each PFAS to this mixture effect as percentages that sum to 1. Difference indicates a coefficient for difference in BMI per doubling in PFAS concentrations in pregnancy. Percentage indicates proportion that respective PFAS contributed to the summed effect estimate. BMI, body mass index; ECHO, Environmental influences on Child Health Outcomes; HPD, highest posterior density; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid.
Table 5.
Bayesian weighted sums analysis of PFAS concentrations during pregnancy and relative risk of overweight/obesity: the ECHO cohorts ().
| Overall | Males | Females | ||||
|---|---|---|---|---|---|---|
| Weighted sums | RR or percentage | 95% HPD | RR or percentage | 95% HPD | RR or percentage | 95% HPD |
| Shared mean (RR) | ||||||
| PFOA | 0.95 | (0.65–1.25) | 1.06 | (0.39–2.84) | 0.87 | (0.48–1.34) |
| PFOS | 0.97 | (0.70–1.30) | 0.92 | (0.29–2.12) | 0.92 | (0.53–1.50) |
| PFHxS | 1.09 | (0.85–1.47) | 1.25 | (0.60–2.94) | 1.06 | (0.71–1.75) |
| PFNA | 1.11 | (0.83–1.64) | 1.61 | (0.67–6.25) | 0.97 | (0.59–1.50) |
| PFDA | 1.01 | (0.79–1.29) | 1.07 | (0.45–2.22) | 1.03 | (0.69–1.62) |
| NMFOSAA | 1.08 | (0.87–1.39) | 1.36 | (0.75–2.99) | 1.00 | (0.69–1.51) |
| Summed effect (RR) | 1.18 | (0.75–1.94) | 2.25 | (0.65–8.74) | 0.82 | (0.36–1.95) |
| PFOA (%) | 15 | (0–42) | 15 | (0–42) | 18 | (0–47) |
| PFOS (%) | 15 | (0–41) | 14 | (0–39) | 17 | (0–47) |
| PFHxS (%) | 17 | (0–45) | 17 | (0–44) | 16 | (0–43) |
| PFNA (%) | 17 | (0–44) | 20 | (0–49) | 16 | (0–45) |
| PFDA (%) | 16 | (0–44) | 15 | (0–40) | 16 | (0–42) |
| NMFOSAA (%) | 19 | (0–48) | 19 | (0–47) | 17 | (0–46) |
Note: The summed mixture model estimates both the effect of PFAS as a mixture and the individual contribution of each PFAS to this mixture effect as percentages that sum to 1. Percentage indicates the proportion that respective PFAS contributed to the summed effect estimate. ECHO, Environmental influences on Child Health Outcomes; HPD, highest posterior density; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; RR, relative risk.
In sensitivity analyses, additional adjustment for birth weight, maternal use of nicotine/tobacco products, parity, gestational diabetes, or child year of birth did not meaningfully change the associations of gestational PFAS concentrations with child adiposity measures (Tables S13–S16). Adjusting for some of the additional confounders (e.g., parity) strengthened associations for some PFAS; however, these changes were subtle relative to the magnitude of our effect estimates. Removing child age from all the models did not change our results (Tables S17 and S18). When we repeated our analyses on the subset of participants who had direct BMI measurements, we observed immaterial changes in the associations between gestational PFAS and BMI -scores or the risk of being overweight/obese (Tables S19 and S20). Using WHO BMI cutoffs to define overweight/obesity did not meaningfully change our results (Tables S21 and S22). When examining results by trimester, we observed the strongest associations of PFOA, PFOS, and PFHxS concentrations with BMI -scores and risk of overweight/obesity when concentrations were measured in the first trimester compared with other trimesters (Tables S23–S28 and Figures S2 and S3).
When performing the leave-one-out analysis, for BMI -scores, we found that excluding the Healthy Start Study strengthened the associations for PFOS and PFOA, whereas excluding Project Viva attenuated the associations with NMFOSAA (Figure S4). For the risk of overweight/obesity outcome, excluding the Healthy Start Study strengthened associations for PFOA, PFNA, and PFDA, whereas excluding the CiOB Study strengthened the associations for PFOS, and excluding Project Viva attenuated the association for PFHxS (Figure S5).
Discussion
Taking advantage of data collected from 1,391 mother–child pairs in eight ECHO cohorts with children born between 1999 and 2019, we found a pattern of subtle positive associations of PFAS in maternal blood during pregnancy with BMI -scores and risk of overweight/obesity in the offspring at 2–5 years of age. These associations did not differ by child sex. We did not find consistent evidence of dose–response relationships between PFAS concentrations and childhood adiposity. There was a positive, but imprecise, association of the PFAS mixture with both BMI -scores and risk of overweight/obesity that was of a higher magnitude in males compared with females. These results also suggest that associations of some PFAS with BMI -scores and risk of overweight/obesity may be greater in cohorts measuring PFAS earlier in pregnancy, although it should be noted that we cannot disentangle cohort effects from sampling time. Finally, for both BMI -scores and risk of overweight/obesity, the results suggest some heterogeneity in these associations across the included cohorts, attenuating or strengthening the associations with different PFAS.
Concentrations of PFOA, PFOS, PFNA, and PFHxS during pregnancy in the ECHO pooled cohorts were similar to those of American women in the National Health and Nutrition Examination Survey (NHANES) 1999–2016,51 whereas PFDA, PFUnDA, and NMFOSAA concentrations in our study were slightly lower than those in the NHANES. Most cohorts in the present study had relatively low median PFAS concentrations, and the range of concentrations in the lowest quintiles was small (). Thus, this precluded us from examining dose–response relationships at higher PFAS concentrations and may have caused exposure misclassification at lower PFAS levels.
Our results are consistent with some previous studies showing that gestational exposure to certain PFAS (PFOA, PFOS, PFHxS, and PFNA) is associated with increased adiposity or risk of overweight/obesity in children21–23 or adolescents.25 Maternal concentrations of these PFAS in pregnancy or at delivery were associated with higher BMI in infants from Odense, Denmark,27 and in young children at 3–5 years of age from Uppsala County, Sweden,21 as well as higher BMI and odds of overweight/obesity in Norwegian and Swedish children at 5 years of age.22 Prior studies have reported that gestational PFAS concentrations were associated with greater BMI gains from 2 to 8 years of age,23 and a modest increase in the risk of obesity at 12 years of age from Cincinnati, Ohio.25 In addition, one study found that elevated PFOA concentrations were associated with accelerating BMI gains in mid-childhood and adolescence, and higher BMI at 12 years of age.52
In contrast, other studies have revealed that higher PFOA, PFOS, PFNA, or PFHxS concentrations in pregnancy were associated with lower BMI -scores in American infants19 and children at 6–8 years of age,26 lower BMI and risk of overweight in Danish children at 7 years of age,31 and lower adiposity among U.K. females at 9 years of age,29 whereas one prospective study in Spanish children reported no clear associations.30 The inconsistent results across studies could be attributed to the differences in the study design and population, the varying concentration ranges of PFAS in pregnancy, or the timing and assessments of children’s BMI.25
In addition to the four PFAS discussed above, we found slightly higher BMI -scores and risk of childhood overweight/obesity in relation to gestational exposure to PFDA, PFUnDA, and NMFOSAA. A limited number of epidemiological studies examining associations between these PFAS and child BMI reported inconsistent findings. Jensen et al. reported a positive association between gestational PFDA and BMI in Danish infants.27 Gyllenhammar et al.21 reported null associations of gestational PFUnDA or PFDA concentration with BMI at 3–5 years of age among Swedish children, whereas Bloom et al. found a positive association of pregnancy PFUnDA concentration, but an inverse association of pregnancy PFDA concentration with child adiposity among American children at 6–8 years of age.26 Future studies are needed to examine associations between these PFAS and child BMI or obesity to improve our understanding of the potential obsegenic effects of the PFAS that have not been extensively studied.
We observed little evidence suggesting that associations of individual PFAS with BMI differed by child sex. Some prior studies have examined the potential sex-specific associations between PFAS and BMI/adiposity, reporting mixed results.24,26,36,37 Specifically, gestational PFOA and PFNA concentrations were positively associated with adiposity in male infants, whereas inverse associations were observed for PFOS and PFHxS in female infants.37 Cord blood PFUnDA concentration was inversely associated with adiposity in females at 5 years of age, and cord blood PFNA was associated with greater adiposity among males.36 In contrast, gestational PFAS was associated with higher BMI at 7.7 years of age in females only.24 Similar to our study, a recent U.S. study found that associations between gestational PFAS and child adiposity in older children at 4–8 years of age did not vary by child sex.26
Our findings of positive associations of PFAS with BMI and risk of overweight/obesity are biologically plausible. Prior studies have shown that PFAS can readily pass through the placenta and move from the maternal to the fetal circulation, with PFHxS having the highest placental transfer rate and PFNA, PFDA, PFUnDA potentially having low placental transfer rates.53,54 PFAS structurally resemble free fatty acids and bind and activate peroxisome proliferator-activated receptor (PPAR) , affecting adipocyte programming and possibly causing adipogenesis, thereby increasing fat mass.15,16,55 In addition, PFAS may disrupt thyroid function and possibly decrease total or free circulating thyroxine (T4) levels and increase thyroid stimulating hormone (TSH) levels, which may increase adiposity.56–59 In addition, gestational exposures to PFAS are associated with DNA methylation differences in lipid homeostasis genes, neonatal cardiometabolic indicators and birth weight, all of which may predict cardiometabolic profiles in later life.11,12 Finally, gestational PFAS can alter the human serum metabolome to impact redox signaling and several fatty acid metabolism pathways.14
Comparing the effect size of PFAS with other risk factors of childhood obesity, our observed associations between gestational PFAS concentrations and risk of overweight/obesity were smaller. For instance, in one meta-analysis, maternal smoking in pregnancy was associated with 1.37 and 1.55 times the odds of child overweight and obesity, respectively.60 Another meta-analysis reported the OR for child overweight/obesity was 2.69 with maternal obesity.61 Despite the smaller effect estimates detected in the present study, the potential effects of PFAS at the population level could be large given the ubiquity of exposure and high prevalence of childhood overweight and obesity.
Our study has some limitations. The data were collected from prospective cohorts, which are subject to potential selection bias due to the risk of differential loss to follow up. However, the main characteristics did not differ meaningfully between included and excluded participants. In addition, some covariates and a small proportion of BMI measurements were collected via self-reported questionnaires, which could result in measurement error. However, restricting our analysis to participants who had direct measures of BMI did not influence the results. BMI is an indirect measurement of body fat and may introduce misclassification of child overweight/obesity. However, prior studies have shown that BMI was highly correlated with direct measures of adiposity, and the correlation is higher among children who are overweight or obese.62 Relatedly, our study was limited by the narrow range of PFAS concentrations in some cohorts and this may have resulted in exposure misclassification.
Furthermore, we cannot rule out the potential for residual confounding that may bias our results, which may be especially important given the relatively small effect estimates we observed. Diet (e.g., fish and shellfish consumption) has been associated with higher serum PFAS levels and is also a predictor of child growth and adiposity63 and, thus, may potentially confound PFAS–adiposity associations. However, we did not have data on maternal diet. Adjusting for dietary factors associated with higher PFAS and greater child BMI may bias our results toward the null. Physical activity may affect the associations between gestational PFAS exposure and adiposity in children.64 However, we were not able to adjust for maternal physical activity in our analysis. In addition, we did not adjust for breastfeeding duration in our study because we did not have information on breastfeeding, which could be a mediator for the associations between gestational PFAS and child BMI -scores.65 Finally, we did not measure the concentrations of albumin or glomerular filtration rate in pregnancy and were therefore unable to account for pregnancy-induced hemodynamic changes. However, when examining associations by the trimester of blood collection, we found that associations for some PFAS were strengthened for those measured in the first trimester compared with the third trimester. This may be because early pregnancy serum PFAS concentrations are not as strongly influenced by pregnancy hemodynamics as are concentrations measured later in pregnancy.66
Although we observed some heterogeneity in these associations, some of this variation may have arisen because we used data from eight cohorts who differed in terms of geographic location, calendar time of recruitment, methods of data collection, and PFAS concentrations. For instance, the exclusion of the Project Viva and Healthy Start cohorts had the largest influence on some results. The influence of Project Viva could be due to the higher PFAS concentrations in this cohort, relative to the other cohorts, as well as first trimester plasma collection. Although we observed some changes in our results when performing leave-one-out analysis, the overall associations were consistent and the changes were small relative to our effect estimates.
Strengths of our study include the large sample size and a diverse group of U.S. mothers and children. Another strength of this study is the application of Bayesian models to evaluate independent effects of individual PFAS in the mixture, the summed effect of PFAS, as well as the contribution of individual PFAS to the summed mixture effect. Finally, taking advantage of the large sample size, we were able to examine whether PFAS–adiposity associations differ by child sex.
Conclusions
Maternal gestational concentrations of some PFAS were associated with increased BMI -scores or the risk of overweight or obesity among children in these ECHO cohort studies. Future epidemiological studies are needed to evaluate the associations of PFAS concentrations in pregnancy with other adiposity-related health outcomes in children.
Supplementary Material
Acknowledgments
We are grateful to ECHO participants for the time they have given to our studies. We also thank our Child Health Outcomes (ECHO) Program colleagues, the medical, nursing and program staff, as well as the children and families participating in the ECHO cohorts. We acknowledge the contributions of the following ECHO Program collaborators: Coordinating Center—P.B. Smith, K.L. Newby, and D.K. Benjamin (Duke Clinical Research Institute, Durham, North Carolina); Data Analysis Center—L.P. Jacobson (Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland) and C.B. Parker (Research Triangle Institute, Durham, North Carolina); Human Health Exposure Analysis Resource—P. Parsons and K. Kurunthacalam (Wadsworth Center, Menands, New York) and T. Fennell, S. Sumner, and X. Du (RTI International, Research Triangle Park, North Carolina).
Research reported in this publication was supported by the ECHO Program, Office of the Director, National Institutes of Health (NIH), under awards U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), and UG3OD023272 (to S.S.), UH3OD023313 (S.CL.D.), UH3OD023275 (to M.R.K.), UH3OD023248 (to D.D.), UH3OD023289 (to A.F.), UH3OD023349 (to T.G.O.), UH3OD023286 (to E.O.), UH3OD023272 (to S.S. and T.J.W.). This work was supported by National Institute of Environmental Health Sciences grants R01ES019196 (to A.F.), P01ES022848 (to S.S.), P01ES022841 (to T.J.W.), R01HD034568 (to E.O.), R01ES030101 (to A.F.F.), RD83543301 (to T.J.W.), and RD83543401 (to S.S.) and by National Institute of General Medicine grant P20GM104416 (to M.R.K.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The data sets for this manuscript are not publicly available because, per the NIH-approved ECHO Data Sharing Policy, ECHO-wide data have not yet been made available to the public for review/analysis. Requests to access the data sets should be directed to the ECHO Data Analysis Center, ECHO-DAC@rti.org.
References
- 1.Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S. 2019. Childhood and adolescent obesity in the United States: a public health concern. Glob Pediatr Health 6:2333794X19891305, PMID: , 10.1177/2333794X19891305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Peterson KE. 2015. Maternal exposure to synthetic chemicals and obesity in the offspring: recent findings. Curr Environ Health Rep 2(4):339–347, PMID: , 10.1007/s40572-015-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA, et al. 2019. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med 17(1):212, PMID: , 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rundle AG, Factor-Litvak P, Suglia SF, Susser ES, Kezios KL, Lovasi GS, et al. 2020. Tracking of obesity in childhood into adulthood: effects on body mass index and fat mass index at age 50. Child Obes 16(3):226–233, PMID: , 10.1089/chi.2019.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grün F, Blumberg B. 2006. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147(suppl 6):S50–S55, PMID: , 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 6.Baillie-Hamilton PF. 2002. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 8(2):185–192, PMID: , 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 7.Nappi F, Barrea L, Di Somma C, Savanelli MC, Muscogiuri G, Orio F, et al. 2016. Endocrine aspects of environmental “obesogen” pollutants. Int J Environ Res Public Health 13(8):765, PMID: , 10.3390/ijerph13080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: , 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ATSDR (Agency for Toxic Substances and Disease Registry). 2018. Toxicological profile for Perfluoroalkyls. Atlanta, GA: ATSDR. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=1117&tid=237 [accessed 21 August 2021]. [PubMed] [Google Scholar]
- 10.OECD (Organisation for Economic Co-operation and Development). 2018. Toward a New Comprehensive Global Datbase of Per- and Polyfluoroalkyl Substances (PFAS): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFAS). ENV/JM/MONO(2018)7. Paris, France: OECD. https://fluoridealert.org/wp-content/uploads/endicott-legal.oecd-report.may-4-2018.pdf [accessed 21 August 2021]. [Google Scholar]
- 11.Starling AP, Liu C, Shen G, Yang IV, Kechris K, Borengasser SJ, et al. 2020. Prenatal exposure to per- and polyfluoroalkyl substances, umbilical cord blood DNA methylation, and cardio-metabolic indicators in newborns: the Healthy Start Study. Environ Health Perspect 128(12):127014, PMID: , 10.1289/EHP6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Eliot MN, Papandonatos GD, Kelsey KT, Fore R, Langevin S, et al. 2022. Gestational perfluoroalkyl substance exposure and DNA methylation at birth and 12 years of age: a longitudinal epigenome-wide association study. Environ Health Perspect 130(3):37005, PMID: , 10.1289/EHP10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore SC, Matthews CE, Sampson JN, Stolzenberg-Solomon RZ, Zheng W, Cai Q, et al. 2014. Human metabolic correlates of body mass index. Metabolomics 10(2):259–269, PMID: , 10.1007/s11306-013-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Li S, Cirillo PM, Krigbaum NY, Tran V, Jones DP, et al. 2019. Metabolome wide association study of serum poly and perfluoroalkyl substances (PFASs) in pregnancy and early postpartum. Reprod Toxicol 87:70–78, PMID: , 10.1016/j.reprotox.2019.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun JM. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13(3):161–173, PMID: , 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Ren XM, Wan B, Guo LH. 2014. Structure-dependent binding and activation of perfluorinated compounds on human peroxisome proliferator-activated receptor γ. Toxicol Appl Pharmacol 279(3):275–283, PMID: , 10.1016/j.taap.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Sagiv SK, Rifas-Shiman SL, Fleisch AF, Webster TF, Calafat AM, Ye X, et al. 2018. Early-pregnancy plasma concentrations of perfluoroalkyl substances and birth outcomes in Project Viva: confounded by pregnancy hemodynamics? Am J Epidemiol 187(4):793–802, PMID: , 10.1093/aje/kwx332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wikström S, Lin PI, Lindh CH, Shu H, Bornehag CG. 2020. Maternal serum levels of perfluoroalkyl substances in early pregnancy and offspring birth weight. Pediatr Res 87(6):1093–1099, PMID: , 10.1038/s41390-019-0720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoaff J, Papandonatos GD, Calafat AM, Chen A, Lanphear BP, Ehrlich S, et al. 2018. Prenatal exposure to perfluoroalkyl substances: infant birth weight and early life growth. Environ Epidemiol 2(2):e010, PMID: , 10.1097/EE9.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, et al. 2012. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect 120(10):1432–1437, PMID: , 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyllenhammar I, Diderholm B, Gustafsson J, Berger U, Ridefelt P, Benskin JP, et al. 2018. Perfluoroalkyl acid levels in first-time mothers in relation to offspring weight gain and growth. Environ Int 111:191–199, PMID: , 10.1016/j.envint.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Lauritzen HB, Larose TL, Øien T, Sandanger TM, Odland JØ, van de Bor M, et al. 2018. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: a prospective cohort study. Environ Health 17(1):9, PMID: , 10.1186/s12940-017-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. 2016. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: the HOME study. Obesity (Silver Spring) 24(1):231–237, PMID: , 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, et al. 2017. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect 125(3):467–473, PMID: , 10.1289/EHP246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Li N, Papandonatos GD, Calafat AM, Eaton CB, Kelsey KT, et al. 2020. Exposure to per- and polyfluoroalkyl substances and adiposity at age 12 years: evaluating periods of susceptibility. Environ Sci Technol 54(24):16039–16049, PMID: , 10.1021/acs.est.0c06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloom MS, Commodore S, Ferguson PL, Neelon B, Pearce JL, Baumer A, et al. 2022. Association between gestational PFAS exposure and children’s adiposity in a diverse population. Environ Res 203:111820, PMID: , 10.1016/j.envres.2021.111820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen RC, Andersen MS, Larsen PV, Glintborg D, Dalgård C, Timmermann CAG, et al. 2020. Prenatal exposures to perfluoroalkyl acids and associations with markers of adiposity and plasma lipids in infancy: an Odense Child Cohort study. Environ Health Perspect 128(7):77001, PMID: , 10.1289/EHP5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horikoshi T, Nishimura T, Nomura Y, Iwabuchi T, Itoh H, Takizawa T, et al. 2021. Umbilical cord serum concentrations of perfluorooctane sulfonate, perfluorooctanoic acid, and the body mass index changes from birth to 5 1/2 years of age. Sci Rep 11(1):19789, PMID: , 10.1038/s41598-021-99174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman TJ, Calafat AM, Holmes AK, Marcus M, Northstone K, Flanders WD, et al. 2017. Prenatal exposure to perfluoroalkyl substances and body fatness in girls. Child Obes 13(3):222–230, PMID: , 10.1089/chi.2016.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iñiguez C, Martinez D, et al. 2017. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA birth cohort study. Environ Health Perspect 125(9):097018, PMID: , 10.1289/EHP1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen CS, Fei C, Gamborg M, Nohr EA, Sørensen TIA, Olsen J. 2013. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am J Epidemiol 178(6):921–927, PMID: , 10.1093/aje/kwt057. [DOI] [PubMed] [Google Scholar]
- 32.Stratakis N, Rock S, La Merrill MA, Saez M, Robinson O, Fecht D, et al. 2022. Prenatal exposure to persistent organic pollutants and childhood obesity: a systematic review and meta-analysis of human studies. Obes Rev 23(suppl 1):e13383, PMID: , 10.1111/obr.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: , 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blake BE, Fenton SE. 2020. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: a review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 443:152565, PMID: , 10.1016/j.tox.2020.152565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian YP, Zeng XW, Bloom MS, Lin S, Wang SQ, Yim SHL, et al. 2019. Isomers of perfluoroalkyl substances and overweight status among Chinese by sex status: isomers of C8 Health Project in China. Environ Int 124:130–138, PMID: , 10.1016/j.envint.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Zhang X, Zhao Y, Lu W, Wu J, Zhao S, et al. 2019. Prenatal exposure to perfluorobutanesulfonic acid and childhood adiposity: a prospective birth cohort study in Shanghai, China. Chemosphere 226:17–23, PMID: , 10.1016/j.chemosphere.2019.03.095. [DOI] [PubMed] [Google Scholar]
- 37.Starling AP, Adgate JL, Hamman RF, Kechris K, Calafat AM, Dabelea D. 2019. Prenatal exposure to per- and polyfluoroalkyl substances and infant growth and adiposity: the Healthy Start Study. Environ Int 131:104983, PMID: , 10.1016/j.envint.2019.104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aris IM, Perng W, Dabelea D, Ganiban JM, Liu C, Marceau K, et al. 2022. Analysis of early-life growth and age at pubertal onset in US children. JAMA Netw Open 5(2):e2146873, PMID: , 10.1001/jamanetworkopen.2021.46873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaisdell CJ, Park C, Hanspal M, Roary M, Arteaga SS, Laessig S, et al. 2022. The NIH ECHO Program: investigating how early environmental influences affect child health. Pediatr Res 92(5):1215–1216, PMID: , 10.1038/s41390-021-01574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeWinn KZ, Caretta E, Davis A, Anderson AL, Oken E, program collaborators for Environmental Influences on Child Health Outcomes. 2022. SPR perspectives: Environmental influences on Child Health Outcomes (ECHO) Program: overcoming challenges to generate engaged, multidisciplinary science. Pediatr Res 92(5):1262–1269, PMID: , 10.1038/s41390-021-01598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eick SM, Enright EA, Geiger SD, Dzwilewski KLC, DeMicco E, Smith S, et al. 2021. Associations of maternal stress, prenatal exposure to per- and polyfluoroalkyl substances (PFAS), and demographic risk factors with birth outcomes and offspring neurodevelopment: an overview of the ECHO.CA.IL prospective birth cohorts. Int J Environ Res Public Health 18(2):742, PMID: , 10.3390/ijerph18020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218(15):2133–2137, PMID: , 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 43.Oh J, Bennett DH, Calafat AM, Tancredi D, Roa DL, Schmidt RJ, et al. 2021. Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ Int 147:106328, PMID: , 10.1016/j.envint.2020.106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honda M, Robinson M, Kannan K. 2018. A rapid method for the analysis of perfluorinated alkyl substances in serum by hybrid solid-phase extraction. Environ Chem 15(2):92–99, 10.1071/EN17192. [DOI] [Google Scholar]
- 45.CDC (Centers for Disease Control and Prevention). 2002. 2000 CDC Growth Charts for the United States: Methods and Development, Series 11, Number 246. Hyattsville, Md.: Department of Health and Humans, CDC, National Center for Health Statistics. https://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf [accessed 21 August 2021]. [Google Scholar]
- 46.Kingsley SL, Walker DI, Calafat AM, Chen A, Papandonatos GD, Xu Y, et al. 2019. Metabolomics of childhood exposure to perfluoroalkyl substances: a cross-sectional study. Metabolomics 15(7):95, PMID: , 10.1007/s11306-019-1560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckley JP, Doherty BT, Keil AP, Engel SM. 2017. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect 125(6):067013, PMID: , 10.1289/EHP334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamra GB, Maclehose RF, Croen L, Kauffman EM, Newschaffer C. 2021. Bayesian weighted sums: a flexible approach to estimate summed mixture effects. Int J Environ Res Public Health 18(4):1373, PMID: , 10.3390/ijerph18041373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacLehose RF, Hamra GB. 2014. Applications of Bayesian methods to epidemiologic research. Curr Epidemiol Rep 1(3):103–109, 10.1007/s40471-014-0019-z. [DOI] [Google Scholar]
- 50.Hamra G, MacLehose R, Richardson D. 2013. Markov chain Monte Carlo: an introduction for epidemiologists. Int J Epidemiol 42(2):627–634, PMID: , 10.1093/ije/dyt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin PID, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. 2021. Temporal trends of concentrations of per- and polyfluoroalkyl substances among adults with overweight and obesity in the United States: results from the Diabetes Prevention Program and NHANES. Environ Int 157:106789, PMID: , 10.1016/j.envint.2021.106789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun JM, Eliot M, Papandonatos GD, Buckley JP, Cecil KM, Kalkwarf HJ, et al. 2021. Gestational perfluoroalkyl substance exposure and body mass index trajectories over the first 12 years of life. Int J Obes (Lond) 45(1):25–35, PMID: , 10.1038/s41366-020-00717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, et al. 2019. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int 124:482–492, PMID: , 10.1016/j.envint.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Kang H, Kim HS, Yoon YS, Lee J, Kho Y, Lee J, et al. 2021. Placental transfer and composition of perfluoroalkyl substances (PFASs): a Korean birth panel of parent-infant triads. Toxics 9(7):168, PMID: , 10.3390/toxics9070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirk AB, Michelsen-Correa S, Rosen C, Martin CF, Blumberg B. 2021. PFAS and potential adverse effects on bone and adipose tissue through interactions with PPARγ. Endocrinology 162(12):bqab194, PMID: , 10.1210/endocr/bqab194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez-Espinosa MJ, Mondal D, Armstrong B, Bloom MS, Fletcher T. 2012. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ Health Perspect 120(7):1036–1041, PMID: , 10.1289/ehp.1104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winquist A, Steenland K. 2014. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology 25(2):255–264, PMID: , 10.1097/EDE.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 58.Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. 2010. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect 118(5):686–692, PMID: , 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim MJ, Moon S, Oh BC, Jung D, Ji K, Choi K, et al. 2018. Association between perfluoroalkyl substances exposure and thyroid function in adults: a meta-analysis. PLoS One 13(5):e0197244, PMID: , 10.1371/journal.pone.0197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rayfield S, Plugge E. 2017. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health 71(2):162–173, PMID: , 10.1136/jech-2016-207376. [DOI] [PubMed] [Google Scholar]
- 61.Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, et al. 2019. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med 16(6):e1002817, PMID: , 10.1371/journal.pmed.1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boeke CE, Oken E, Kleinman KP, Rifas-Shiman SL, Taveras EM, Gillman MW. 2013. Correlations among adiposity measures in school-aged children. BMC Pediatr 13:99, PMID: , 10.1186/1471-2431-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roth K, Imran Z, Liu W, Petriello MC. 2020. Diet as an exposure source and mediator of per- and polyfluoroalkyl substance (PFAS) toxicity. Front Toxicol 2:601149, PMID: , 10.3389/ftox.2020.601149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braun JM, Papandonatos GD, Li N, Sears CG, Buckley JP, Cecil KM, et al. 2022. Physical activity modifies the relation between gestational perfluorooctanoic acid exposure and adolescent cardiometabolic risk. Environ Res 214(pt 3):114021, PMID: , 10.1016/j.envres.2022.114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, et al. 2016. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 149:239–246, PMID: , 10.1016/j.envres.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nielsen C, Andersson Hall U, Lindh C, Ekström U, Xu Y, Li Y, et al. 2020. Pregnancy-induced changes in serum concentrations of perfluoroalkyl substances and the influence of kidney function. Environ Health 19(1):80, PMID: , 10.1186/s12940-020-00626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.