Abstract
Background
In Europe, Serbia occupies a high position in antibiotic utilization and antimicrobial resistance (AMR).
Aim
The aim was to analyse utilization trends of meropenem, ceftazidime, aminoglycosides, piperacillin/tazobactam and fluoroquinolones (2006–2020), and the reported AMR in Pseudomonas aeruginosa (2013–2020) in Serbia and to compare with data from eight European countries (2015–2020).
Method
Joinpoint regression was used to analyse antibiotic utilization data (2006–2020) and the reported AMR in Pseudomonas aeruginosa (2013–2020). Data sources were relevant national and international institutions. Antibiotic utilization and AMR in Pseudomonas aeruginosa data in Serbia were compared with eight European countries.
Results
There was a significantly increased trend for ceftazidime utilization and reported resistance in Pseudomonas aeruginosa, Serbia (p < 0.05) (2018–2020). For ceftazidime, piperacillin/tazobactam, and fluoroquinolones resistances in Pseudomonas aeruginosa an increased trend was observed, Serbia (2013–2020). A decrease in both the utilization of aminoglycosides, Serbia (p < 0.05) (2006–2018) and contemporaneous Pseudomonas aeruginosa resistance (p > 0.05) was detected. Fluoroquinolone utilization (2015–2020) was highest in Serbia compared to Netherlands and Finland, 310 and 305% higher, similar compared to Romania, and 2% less compared to Montenegro. Aminoglycosides (2015–2020) were 2550 and 783% more used in Serbia compared to Finland and Netherlands, and 38% less regarding Montenegro. The highest percentage of Pseudomonas aeruginosa resistance was in Romania and Serbia (2015–2020).
Conclusion
The use of piperacillin/tazobactam, ceftazidime and fluoroquinolones should be carefully monitored in clinical practice due to increased Pseudomonas aeruginosa resistance. The level of utilization and AMR in Pseudomonas aeruginosa is still high in Serbia compared to other European countries.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11096-023-01603-y.
Keywords: Antibiotic utilization, Antimicrobial resistance, Pseudomonas aeruginosa, Serbia
Impact statements
In Serbia, the use of antibiotics is still high compared to the other European countries, even though decreasing or stabilising utilization trends have been noted for most of the studied antibiotics. There is a clear need to evaluate antibiotic use and rationalize use.
In Serbia, increased Pseudomonas aeruginosa resistance has been shown for piperacillin/tazobactam, ceftazidime and fluoroquinolones, and therefore, the use of these antibiotics should be carefully monitored in clinical practice due to increased resistance.
Ceftazidime use increased significantly during a 3-year period (2018-2020) in Serbia, therefore, its usage in clinical practice should be carefully monitored and optimized.
Introduction
Antimicrobial resistance (AMR), one of the main consequences of irrational antimicrobial use, is the emerging threat of modern medicine and a recognised public health problem affecting morbidity, mortality and costs [1, 2]. In the European Union (EU) and European Economic Area (EEA), in 2015, infections caused by antibiotic-resistant bacteria accounted for an estimated 33,110 deaths [3].
Pseudomonas aeruginosa is one of nine pathogens under Central Asian and European Surveillance of Antimicrobial Resistance (CAESAR) surveillance, being a common cause of invasive infections [4]. It can easily develop resistance to antimicrobials commonly used in the treatment of Pseudomonas aeruginosa infections such as piperacillin/tazobactam, ceftazidime, carbapenems, fluoroquinolones or aminoglycosides [5]. Thus, when AMR develops, therapeutic options may be severely limited.
In the framework of implementing the European Strategic Action Plan on Antibiotic Resistance, World Health Organization (WHO) Europe has established compatible networks for monitoring antibiotic consumption (AMC) and bacterial resistance to antibiotics (CAESAR) for countries that are not members of the EU [6]. Serbia is the part of the CAESAR network of national AMR surveillance systems [4]. The system for the registration and monitoring of AMR consists of the National Reference Laboratory for Registration and Monitoring of Bacterial Strains Resistance to Antimicrobial Agents and the national network of 24 clinical laboratories, established on a voluntary basis [7]. The European Surveillance of Antimicrobial Consumption Network (ESAC-Net) is an EU/ EEA-wide network of national surveillance systems, coordinated by the European Centre for Disease Prevention and Control (ECDC) and covering all EU/EEA countries, providing European reference data on AMC [8].
According to the data on the utilization of antibiotics, in the last decade Serbia occupied an unenviably high position among European countries, with a high level of AMR present in all species of bacteria tested in Serbia [9, 10]. In recent years, multidrug resistance proportions exceeding 50% were reported in Serbia for Pseudomonas aeruginosa, a common cause of infection in hospitalized patients [4]. A potential cause of Serbia’s high ranking for antibiotic utilization and AMR is that antibiotics are being prescribed for conditions in which they provide no benefit [11]. Moreover, recent studies indicate that the practice of self-medication in Serbia includes not only over-the-counter (OTC) medicines, but also antibiotics [12–14]. In that regard, the results of the study performed in Novi Sad, the second largest city in Serbia, demonstrated the presence of antibiotics obtained without a prescription in a large share of households [15].
The ECDC introduced the European Antibiotic Awareness Day (EAAD), a European health initiative and annual event in 2008 to support EU/EEA countries in their efforts to increase prudent use of antibiotics [16]. Since November 2015, the Serbian Ministry of Health has joined the global efforts to combat AMR and to ensure the prudent use of antibiotics [9]. One of its activities for raising awareness about the seriousness of irrational use of antibiotics and AMR is conducting a National Campaign for the Rational Use of Antibiotics [9].
Along with the above mentioned measures, continued monitoring of antibiotic use and its resistance is necessary in order to reveal the effects of activities, as exemplified above, and to suggest further actions and strategies. A comparative analysis of secular trends of antibiotic use and its resistance could inform the evaluation of evidence based practice and research outcomes.
Aim
The aim of this study was to describe and analyse trends utilization of meropenem, ceftazidime, aminoglycosides, piperacillin/tazobactam and fluoroquinolones, between 2006 and 2020, and the reported AMR in Pseudomonas aeruginosa between 2013 and 2020 in Serbia, and to compare antibiotic utilization and AMR in Pseudomonas aeruginosa in Serbia with eight European countries.
Ethics approval
This study did not require the medical ethics committee’s approval because it was based on publicly available aggregate data, both on the use of antibiotics and Pseudomonas aeruginosa resistance.
Method
Data sources
For assessing trends in antibiotic utilization of meropenem, ceftazidime, aminoglycosides, fluoroquinolones and piperacillin/tazobactam, data were extracted from the relevant annual databases on turnover of medicines provided by the Medicines and Medical Devices Agency of Serbia (MMDAS), for the period 2006–2020 in the form of annual reports [17]. These databases were based on the WHO ATC/DDD (Anatomical Therapeutic Chemical/ Defined Daily Dose) methodology [18]. As stated in the MMDAS reports, data on turnover or sales of drugs for human use were obtained from the marketing authorization holders for wholesale medicines. Accordingly, wholesale data were used for the analysis, and not consumption data [17].
The above-mentioned antibiotics were selected in line with the study aim; as those under surveillance for detecting Pseudomonas aeruginosa resistance in Serbia in line with the CAESAR manual [19]. In addition, they belong to a group of critically important antimicrobials according to the WHO [20].
The following data were selected and extracted from the reports on turnover of medicine: (i) ATC codes of the medicines of interest in line with ATC Index for 2021: meropenem (J01DH02), ceftazidime (J01DD02), aminoglycosides (J01G), fluoroquinolones (J01MA) and piperacillin/tazobactam (J01CR05), (ii) DDDs per 1000 inhabitants per day (DID) per each ATC code of interest.
The DDD represents a unit of measurement that is an international compromise of the assumed average maintenance dose per day for a drug used for its main indication in adults [18]. The utilization of this measure enabled us to create a database for measuring and comparing antibiotic use.
Using national reference laboratory reports and CAESAR data, the AMR percentages for Pseudomonas aeruginosa isolates (2013–2020) were extracted and analysed [4, 21–28].
The publications used for this study contain only aggregate data, both on the use of antibiotics and on Pseudomonas aeruginosa resistance.
Trend analysis of antibiotic usage and reported Pseudomonas aeruginosa resistance was carried out for this time period since the data for above mentioned timeline were available.
Finally, to place the Serbian trends in a broader European context, antibiotic utilization and AMR in Pseudomonas aeruginosa data in Serbia were compared with eight European countries which data sources were relevant national and international institutions (national medicines agencies, ECDC and CAESAR) [4, 22–33]. They are Croatia, Romania and Bulgaria [28–32] and Montenegro [4, 22–24, 33], countries in the region, as well as Finland [28, 30–32], a Scandinavian country with developed practice of rational antibiotic use, and Greece and Spain (countries with the highest levels of antibiotics utilisation in EU) and Netherlands (country with the lowest level of antibiotics utilisation in EU) [28, 30–32].
Statistical analysis
Using Joinpoint software, version 4.7.0.0 (National Cancer Institute, Bethesda, United States), a regression analysis of data on DIDs was performed, as listed in the ATC Index for 2021 [34] on the use of meropenem (J01DH02), ceftazidime (J01DD02), aminoglycosides (J01G) and fluoroquinolones (J01MA) between 2006 and 2020, and piperacillin/tazobactam (J01CR05) between 2007 and 2020. The DID for piperacillin/tazobactam in 2006 was 0 which was the reason why the trend for this antibiotic was analysed in the 14-year study period. The reported AMR percentages in Pseudomonas aeruginosa (2013–2020) isolates were analysed with the same Joinpoint methodology.
This method identifies the year(s) when a trend change in DIDs occurs over the study period, it calculates the annual percentage change (APC) for each trend segment and the corresponding 95% confidence interval (CI), and it also estimates the average annual percentage change (AAPC) in the whole studied period. The APC is tested to determine whether a difference exists from the null hypothesis of no change (0%). APC equals to AAPC when it is constant and there are no join points (i.e., no changes in trend). Otherwise, the whole period is segmented by the points with trend change [35]. To avoid statistical anomalies, a join point segment must contain at least 3 observed data points, and no join point segment can begin or end less than 3 data points from the beginning or end of the data series [34]. In the final model, each join point informs a statistically significant change in trends (increase or decrease).
The graphs and tables showing annual trends in differences between antibiotics utilisation rates and AMR rates in the first and last years were also created using Joinpoint software. Two joinpoint model results were presented for antibiotic utilisation and one joinpoint model results were presented for AMR.
Results
Antimicrobial utilization rates over time
Different utilization trends were recorded across all studied antimicrobial groups during observed period, from 2006 to 2020: the ceftazidime utilization rate increased the most between 2006 and 2020, by more than 900% (p < 0.05), while the DID for meropenem and fluoroquinolones increased by 100%. On the other hand, the utilization rate for aminoglycosides decreased by 63.3% (p < 0.05) (Appendix 1, Figs. 1, 2, 3, 4 and 5).
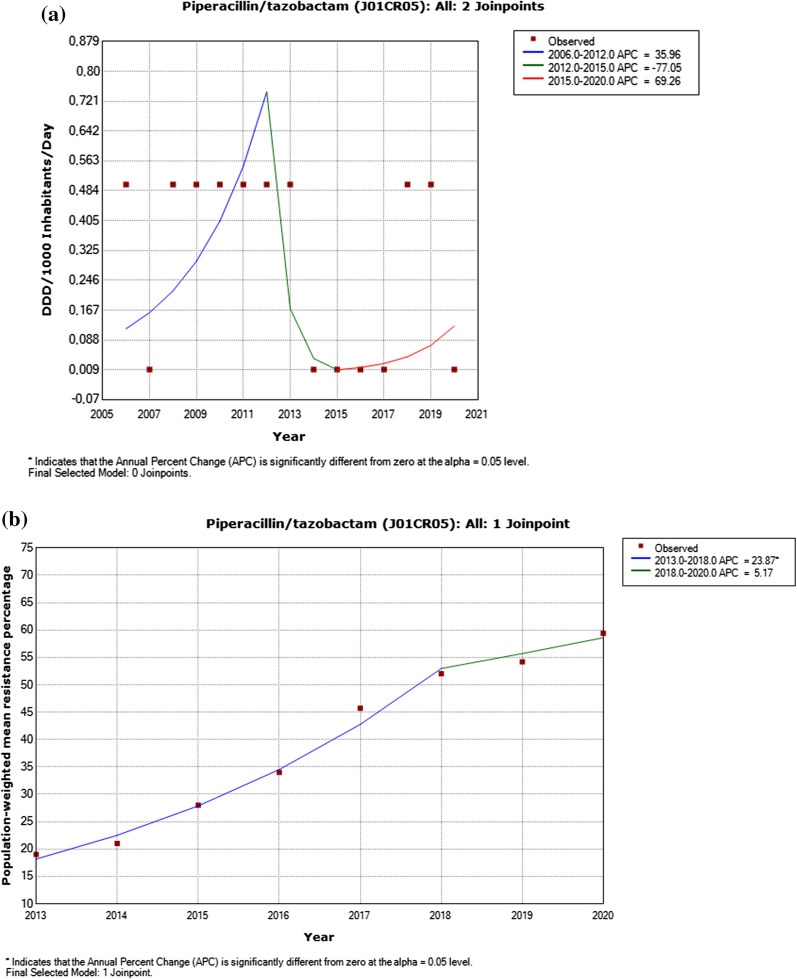
Fig. 1.
a Utilization of piperacillin/tazobactam, including detected trend segments, among Serbian population, 2007–2020. DDD Defined daily doses. b Piperacillin/tazobactam resistance in Pseudomonas aeruginosa, including detected trend segments, 2013–2020
Fig. 2.
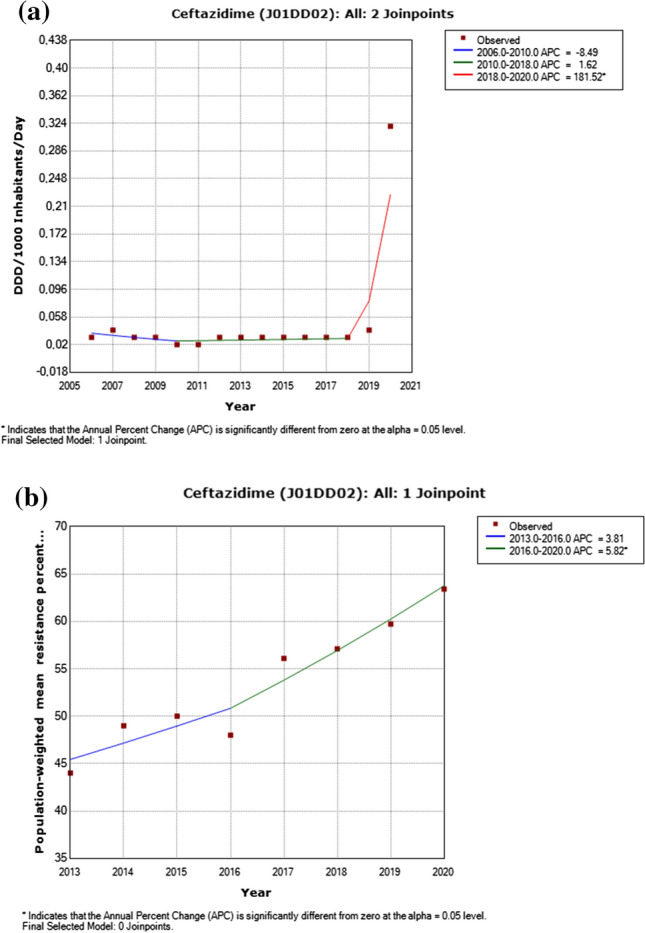
a Utilization of ceftazidime, including detected trend segments, among Serbian population, 2006–2020. DDD Defined daily dose. b Ceftazidime resistance in Pseudomonas aeruginosa, including detected trend segments, 2013–2020
Fig. 3.
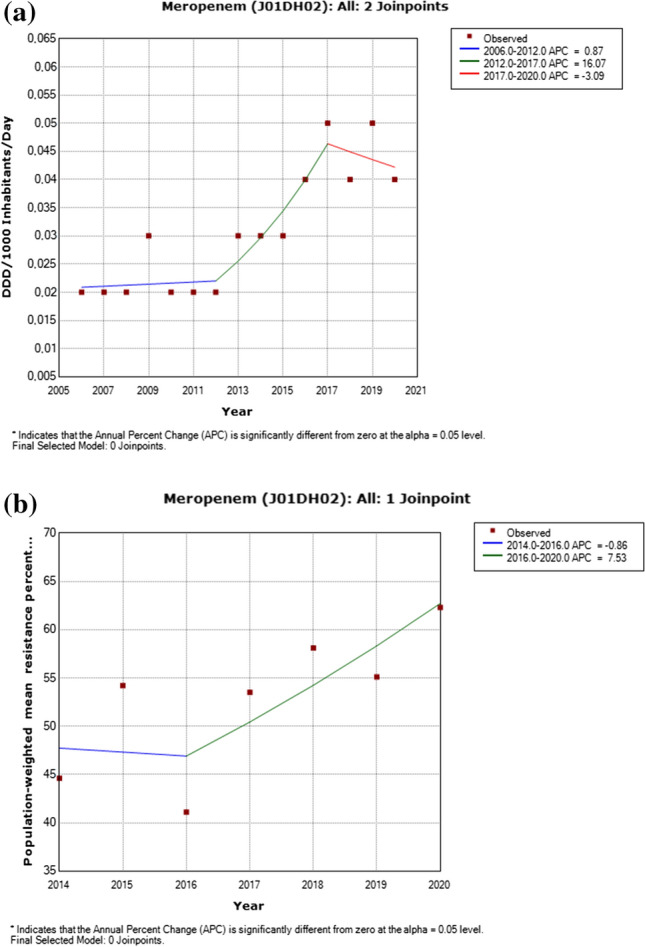
a Utilization of meropenem, including detected trend segments, Serbian population weighted means, 2006–2020. DDD Defined daily doses. b Meropenem resistance in Pseudomonas aeruginosa, including detected trend segments, 2014–2020
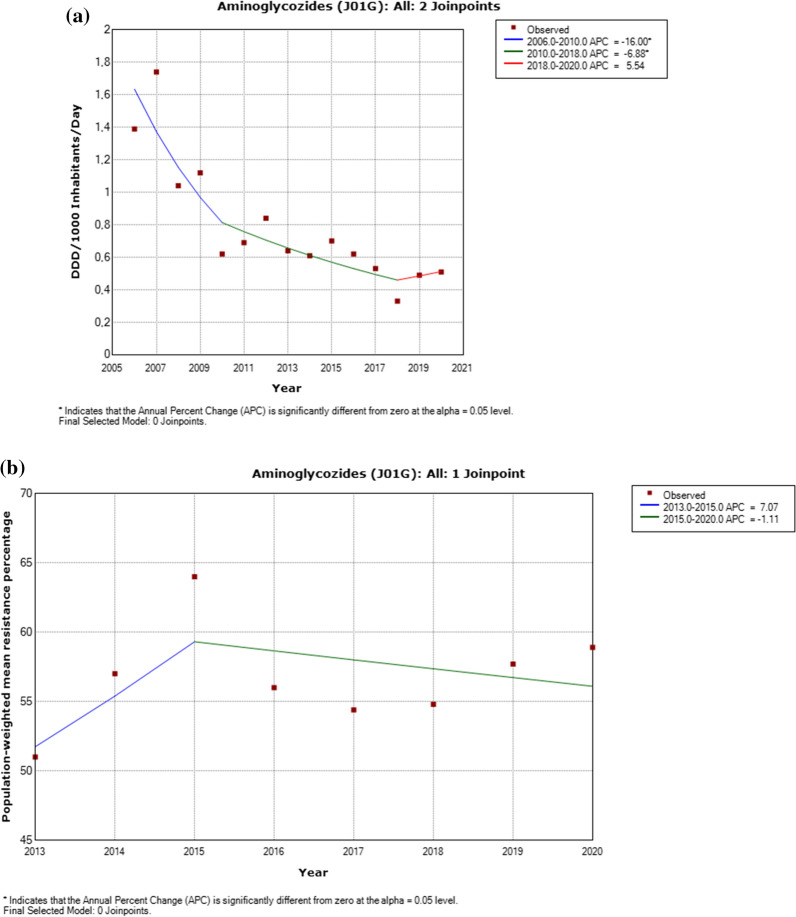
Fig. 4.
a Utilization of aminoglycosides, including detected trend segments, Serbian population weighted means, 2006–2020. DDD Defined daily doses. b Aminoglycosides resistance in Pseudomonas aeruginosa, including detected trend segments, 2013–2020
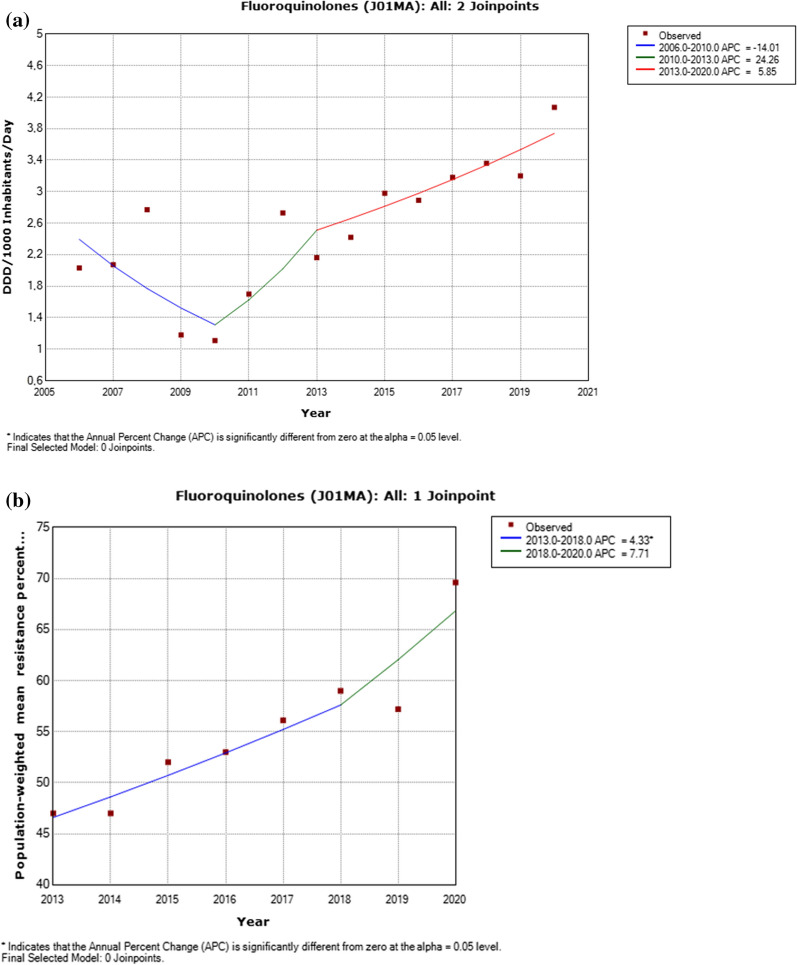
Fig. 5.
a Utilization of fluoroquinolones, including detected trend segments, Serbian population weighted means, 2006–2020. DDD defined daily doses. b. Fluoroquinolones resistance in Pseudomonas aeruginosa, including detected trend segments, 2013–2020
Trends of antimicrobial utilization
For antimicrobial utilization, the Joinpoint model identified three separate trend segments for all investigated antimicrobial groups (Appendix 2, Figs. 1, 2, 3, 4 and 5).
The trend segments of the earliest years, starting between 2006 and 2010 or 2012 depending on the antimicrobial group, all showed stable or decreasing trends, while segments for the most recent years showed more variation (Appendix 2, Figs. 1, 2, 3, 4 and 5).
For utilization of ceftazidime (Appendix 2, Fig. 2), the most recent segment showed statistically significant increasing trends. For utilization of piperacillin/tazobactame (Appendix 2, Fig. 1), aminoglycosides (Appendix 2, Fig. 4), and fluoroquinolones (Appendix 2, Fig. 5), the most recent segments showed stable trends.
Trends in antimicrobial resistance percentages
The Joinpoint model identified two separate trend segments for Pseudomonas aeruginosa resistance (Appendix 3, Fig. 1, 2, 3, 4 and 5). For ceftazidime, piperacillin/tazobactam, and fluoroquinolones resistances in Pseudomonas aeruginosa an increased trend was observed during the whole period (Appendix 3, Figs. 1, 2 and 5). By contrast, for meropenem and aminoglycosides the resistance rates of Pseudomonas aeruginosa were stable (Appendix 3, Figs. 3, 4).
In the first trend segment, from 2013 to 2015 or 2016 or 2018, depending on medicine, for piperacillin/tazobactam and fluoroquinolones an increased resistance trend was found, and for all other antimicrobials a stable resistance trend was detected (Appendix 3, Fig. 1–5).
In the second trend segment, a stable trend was noticed for all antimicrobials’ resistances except for ceftazidime resistance that showed an increased trend (Appendix 3, Figs. 1, 2, 3, 4 and 5).
Antimicrobial utilization and Pseudomonas aeruginosa resistance trends
There was a significantly increased trend for ceftazidime utilization and reported resistance in Pseudomonas aeruginosa (p < 0.05) in the period between 2018 and 2020 (Appendix 2–3, Fig. 2). A decrease in utilization of aminoglycosides during the period 2006–2018 (p < 0.05) was detected yet a contemporaneous decrease in resistance among Pseudomonas aeruginosa isolates was not statistically significant (Appendix 2–3, Fig. 4). A nonsignificant increased trend for fluoroquinolones use and reported resistance in Pseudomonas aeruginosa (p < 0.05) was identified from 2013 to 2018 (Appendix 2–3, Fig. 5).
Comparison of antimicrobial utilization between selected European countries
Utilization of fluoroquinolones expressed in DID as an average value for the 6-year period of time, 2015–2020 (for Montenegro the data was available only until 2018), was 310, 305 and 89% higher in Serbia compared to Netherlands, Finland and Croatia, respectively, and 2% less compared to Montenegro (Serbia: 3.28 DID, Netherlands: 0.8 DID, Finland: 0.81 DID, Croatia: 1.74 DID, Montenegro: 3.35 DID) (Appendix 4). Additionally, the trend difference for fluoroquinolones was lowest compared to Romania, and was just 2% higher in Serbia, (Serbia: 3.28 DID, Romania: 3.23 DID). The trend difference was even greater for aminoglycosides, which were 2550 and 783% more used in Serbia compared to Finland and Netherlands, 2015–2020, respectively, and 38% less with regard to Montenegro (Serbia: 0.53 DID, Finland: 0.02 DID, Netherlands: 0.06 DID, Montenegro: 0.86 DID) (Appendix 4).
Comparison of Pseudomonas aeruginosa resistance trends between selected European countries
Regarding piperacillin-tazobactam, ceftazidime, carbapenem, aminoglycosides and fluoroquinolones resistance in Pseudomonas aeruginosa, the highest percentages were identified for Romania and Serbia, the average value for the 6-year period, 2015–2020, were 50.2 and 45.3%, 51.0 and 55.5%, 56 and 54.4%, 51.4 and 59.7%, 54.5 and 58.4%, respectively (Appendix 5). On the other hand, Netherlands and Finland had the lowest percentages of piperacillin-tazobactam, ceftazidime, carbapenem, aminoglycosides and fluoroquinolones resistance in Pseudomonas aeruginosa, 5.8 and 7%, 3.4 and 5.4%, 4.4 and 5.3%, 2.3 and 1.5%, 8.2 and 9.9%, respectively (Appendix 5).
Discussion
Statement of key findings
To our knowledge, this is the first study to present antibiotic utilization trends and the Pseudomonas aeruginosa resistance over a 15-year period at a national level in Serbia. Our results show different patterns of fluctuating utilization trends for antibiotics and contemporaneous variation in the level of resistance in Pseudomonas aeruginosa against a background of high levels of utilisation and of resistance compared to selected European countries.
Our results revealed a significantly increased trend for ceftazidime in a 3-year period (2018–2020) in Serbia. Additionally, the ceftazidime utilization rate has increased tremendously by more than 900% in 2020 compared to 2006. This might be explained by the beginning of Coronavirus disease 2019 (COVID-19) pandemic in 2020. However, further studies are needed to investigate the reasons for this increased trend. According to the study conducted in a tertiary hospital in Serbia from March 2020 to the end of 2021 cephalosporins were one of the most frequently used antibiotics (29.6%) for COVID-19, although inappropriate overprescribing could be the primary reason for such high rates as it remains the primary drug of choice for empirical treatment of community acquired pneumonia [36].
The decreasing trend in utilization of aminoglycosides described in this study for the last 15 years could be an encouraging signal of the positive effects of local antimicrobial stewardship initiatives in Serbia. On the other hand, the analysis for Serbia shows the increasing trends in piperacillin/tazobactam, ceftazidime and fluoroquinolones resistances in Pseudomonas aeruginosa. Observed increasing trends are consistent with recent studies conducted in Serbia [37, 38] and around the world [39–41]. Accordingly, the ceftazidime, piperacillin/tazobactam and fluoroquinolones resistances in Pseudomonas aeruginosa present a threat worldwide. Usage in clinical practice and therapy of Pseudomonas aeruginosa must be restricted and carefully observed.
The antimicrobial resistance percentages in the Serbian population were compared to the EU/EEA resistance data. In 2017, the highest EU/EEA population-weighted mean resistance percentage to Pseudomonas aeruginosa was reported for fluoroquinolones (20.3%), as well as in Serbia, but with more than double values (56.1%) [42]. Significantly higher values are observed also for remaining antibiotics, piperacillin ± tazobactam (18.3% in EU/EEA population vs. 45.7% in Serbia), carbapenems (17.4% in EU/EEA population vs. 53.5% in Serbia), ceftazidime (14.7% in EU/EEA population vs. 56.1% in Serbia) and aminoglycosides (13.2% in EU/EEA population vs. 54.4% in Serbia) [42]. During the studied period, our results show an increasing trend regarding Pseudomonas aeruginosa resistance, with statistical significance for three out of five studied antibiotics, piperacilin/tazobactam, ceftazidime and fluoroquinolones. On the contrary, for all antimicrobial groups under regular surveillance, the EU/EEA population-weighted mean percentage decreased significantly between 2014 and 2017 [42]. Additionally, the ECDC statement from 2015 is that 13.7% of Pseudomonas aeruginosa isolates were resistant to at least three antimicrobial groups and 5.5% to all five antimicrobial groups under surveillance European Antimicrobial Resistance Surveillance Network (EARS-Net) [43]. Moreover, high resistance and antibiotics overuse were recorded in a 10-year study in a hospital setting in Serbia [44]. The consequences of these increasing resistance trends could be serious, as Pseudomonas aeruginosa bloodstream infections, and pose a substantial threat regarding morbidity and mortality.
Strengths and weaknesses
Our results can be generalized at the population level. The resistance source network has broad geographical and population coverage and includes various types of hospitals. Additionally, the antibiotics sales data are based on the whole country. Moreover, the long study duration provides a comprehensive insight into the trend changes over the years. Importantly, the publications used as a data source were prepared in line with the guidelines of the WHO for the processing of data on trade and monitoring of indicators of medicine use [18].
Our study has several limitations, mainly related to data availability and comprehensiveness. The wholesale data used only allows associations to be suggested. The representative nature of the resistance data is limited by the over-representation of patients with hospital-acquired infections. However, these data were comparable since two sources (national and CAESAR reports) were used for data extraction. On the other hand, antibiotic utilisation data comes from a single source of harmonised data on Serbian antibiotic sales provided by wholesalers. Accordingly, it is worth considering that wholesale data can present overestimated results in comparison to consumption data due to some amount of antibiotics still unused by patients, either in wholesalers, pharmacies, or in patients’ homes. Additionally, data on medicines utilisation used in this study represent overall drug usage, regardless of diagnosis or microbial. Accordingly, a total antibiotic usage was analysed and not only the consumption in treatment of Pseudomonas aeruginosa. However, the antimicrobial groups included in this study were selected as they are of relevance to treat Pseudomonas aeruginosa in line with the National recommendation for antibiotic proscribing [9]. Additionally, comparison of trends of antibiotic consumption and reported Pseudomonas aeruginosa resistance can suggest potential associations even in the cases when a total antibiotic utilisation was analysed and not only consumption data in treatment of certain infection. Such methodology has been already implemented in similar studies [45]. The antibiotic use and resistance data were only available in annualised forms, which might have obscured more complex trends and therefore, more detailed analysis was not possible. Therefore, detailed and indication specific antibiotic utilization data for the Serbian market would be important and useful for getting a broad and clearer picture of antibiotic utilization.
Conclusion
In conclusion, this study has highlighted the importance of antibiotic utilization rates and resistant rates monitoring to hypothesize potential causal relations. Even though decreasing or stabilising utilization trends have been noted for most of the studied antibiotics for Serbia, the level of utilization is still high compared to the other European countries. It would be useful for future research to examine the trends in antibiotic utilization and the AMR by regions of country, by hospital and community settings and by expense coverage from the national health insurance fund, to inform further actions to be well-tailored to the actual demands.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all individuals who have supported this work and supply data on antibiotic turnover and AMR for the analysis (Medicines and medical devices agency of Serbia, Institute of public health of Vojvodina and World Health Organization). The authors are grateful to Stephanie Corrigan, native English speaker, for proof reading the manuscript.
Funding
This study was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia through Grant Agreement with University of Belgrade- Faculty of Pharmacy No: 451-03-68/2022-14/200161.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jinks T, Lee N, Sharland M, et al. A time for action: antimicrobial resistance needs global response. Bull World Health Organ. 2016;94(8):558–558A. doi: 10.2471/BLT.16.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakovljevic M, Lazarevic M, Milovanovic O, et al. The new and old Europe: east-west split in pharmaceutical spending. Front Pharmacol. 2016;7:18. doi: 10.3389/fphar.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Central Asian and Eastern European surveillance of antimicrobial resistance. CAESAR Annual report, 2019. https://www.euro.who.int/__data/assets/pdf_file/0003/418863/53373-WHO-CAESAR-annual-report-2019.pdf. Accessed 21 Dec 2021.
- 5.Ruiz-Garbajosa P, Cantón R. Epidemiology of antibiotic resistance in Pseudomonas aeruginosa Implications for empiric and definitive therapy. Rev Esp Quimioter. 2017;30(Suppl 1):8–12. [PubMed] [Google Scholar]
- 6.World Health Organization . Republic of Serbia: National antibiotic resistance control programme for the period 2019–2021. Switzerland: World Health Organization; 2019. [Google Scholar]
- 7.Medic D, Bozic Cvijan B, Bajcetic M. Impact of antibiotic consumption on antimicrobial resistance to invasive hospital pathogens. Antibiotics. 2023;12:259. doi: 10.3390/antibiotics12020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European centre for disease prevention and control (ECDC). TESSy, The European surveillance system – antimicrobial consumption (AMC) reporting protocol 2023 – European surveillance of antimicrobial consumption network (ESAC-Net) surveillance data for 2022. Stockholm: ECDC; 2023. Available from: https://www.ecdc.europa.eu/en/publications-data/esac-net-reporting-protocol-2023. Accessed 15 Dec 2021.
- 9.Working group for the development of a national guide to good clinical practice for the rational use of antibiotics, Ministry of health of Republic of Serbia. National guide to good clinical practice for the rational use of antibiotics. Belgrade; 2018. https://www.zdravlje.gov.rs/view_file.php?file_id=527&cache=sr. Accessed 15 Dec 2021.
- 10.Tomas A, Pavlović N, Stilinović N, et al. Increase and change in the pattern of antibiotic use in Serbia (2010–2019) Antibiotics. 2021;10:397. doi: 10.3390/antibiotics10040397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krantz EM, Zier J, Stohs E, et al. Antibiotic prescribing and respiratory viral testing for acute upper respiratory infections among adult patients at an ambulatory cancer center. Clin Infect Dis. 2020;70(7):1421–1428. doi: 10.1093/cid/ciz409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukovic JA, Miletic V, Pekmezovic T, et al. Self-medication practices and risk factors for self-medication among medical students in Belgrade, Serbia. PLoS ONE. 2014;9(12):e114644. doi: 10.1371/journal.pone.0114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tripković K, Nešković A, Janković J, et al. Predictors of self-medication in Serbian adult population: cross-sectional study. Int J Clin Pharm. 2018;40(3):627–634. doi: 10.1007/s11096-018-0624-x. [DOI] [PubMed] [Google Scholar]
- 14.Kusturica MP, Tomic Z, Bukumiric Z, et al. Home pharmacies in Serbia: an insight into self-medication practice. Int J Clin Pharm. 2015;37(2):373–378. doi: 10.1007/s11096-015-0071-x. [DOI] [PubMed] [Google Scholar]
- 15.Tomas A, Paut Kusturica M, Tomić Z, et al. Self-medication with antibiotics in Serbian households: a case for action? Int J Clin Pharm. 2017;39:507–513. doi: 10.1007/s11096-017-0461-3. [DOI] [PubMed] [Google Scholar]
- 16.Earnshaw S, Mancarella G, Mendez A, et al. European antibiotic awareness day technical advisory committee. European antibiotic awareness day collaborative group european antibiotic awareness day: a five-year perspective of Europe-wide actions to promote prudent use of antibiotics. Euro Surveill. 2014;19(41):20928. doi: 10.2807/1560-7917.es2014.19.41.20928. [DOI] [PubMed] [Google Scholar]
- 17.Reports on turnover and use of medicines of the Medicines and medical devices agency of Serbia. https://www.alims.gov.rs/ciril/o-agenciji/publikacije/. Accessed 17 Aug 2022.
- 18.World Health Organization. WHO collaborating centre for drug statistics methodology, guidelines for ATC classification and DDD assignment 2023. Oslo, Norway, 2022. https://www.whocc.no/filearchive/publications/2023_guidelines_web.pdf. Accessed 16 Mar 2023.
- 19.World Health Organization. Central Asian and Eastern European surveillance of antimicrobial resistance. CAESAR manual, version 3, 2019. https://apps.who.int/iris/bitstream/handle/10665/346572/WHO-EURO-2019-3583-43342-60804-eng.pdf?sequence=1&isAllowed=y. Accessed 22 Dec 2021.
- 20.World Health Organization (WHO). WHO access, watch, reserve (AWaRe) classification of antibiotics for evaluation and monitoring of use, 2021. Geneva: WHO; 2021 (WHO/MHP/HPS/EML/2021.04). Available from: https://www.who.int/publications/i/item/2021-aware-classification. Accessed 16 Mar 2023.
- 21.Reports on the resistance of invasive bacterial isolates to antimicrobial drugs of Institute of public health of Vojvodina. http://www.izjzv.org.rs/. Accessed 26 Jul 2022.
- 22.World Health Organization. Central Asian and Eastern European surveillance of antimicrobial resistance. Annual report. 2018. https://apps.who.int/iris/handle/10665/324806. Accessed 3 Aug 2022.
- 23.World Health Organization. Central Asian and Eastern European surveillance of antimicrobial resistance. Annual report. 2020. https://apps.who.int/iris/handle/10665/345873. Accessed 3 Aug 2022.
- 24.WHO Regional office for Europe/European centre for disease prevention and control. Antimicrobial resistance surveillance in Europe 2022 – 2020 data. https://www.who.int/europe/publications/i/item/9789289056687. Accessed 3 Aug 2022.
- 25.World Health Organization. Central Asian and Eastern European surveillance of antimicrobial resistance. Annual report. 2017. https://apps.who.int/iris/bitstream/handle/10665/342131/9789289052993-eng.pdf?sequence=1&isAllowed=y. Accessed 3 Aug 2022.
- 26.World Health Organization. Central Asian and Eastern European surveillance of antimicrobial resistance. Annual report. 2016. https://apps.who.int/iris/bitstream/handle/10665/344085/9789289052252-eng.pdf?sequence=2&isAllowed=y. Accessed 3 Aug 2022.
- 27.World Health Organization. Central Asian and Eastern European surveillance of antimicrobial resistance. Annual report. 2014. https://apps.who.int/iris/bitstream/handle/10665/344413/9789289051088-eng.pdf?sequence=1&isAllowed=y. Accessed 3 Aug 2022.
- 28.Antimicrobial resistance dashboard. Copenhagen: WHO regional office for Europe; 2022 https://worldhealthorg.shinyapps.io/WHO-AMR-Dashboard-main/. Accessed 15 Mar 2023.
- 29.Reports on turnover and use of medicines of the agency for medicinal products and medical devices of Croatia. https://www.halmed.hr/Promet-proizvodnja-i-inspekcija/Promet/Potrosnja-lijekova/Izvjesca-o-prometu-lijekova/. Accessed 17 Aug 2022.
- 30.European centre for disease prevention and control. Latest surveillance data on antimicrobial consumption. Antimicrobial consumption dashboard (ESAC-Net). AMC | European Centre for Disease Prevention and Control. https://qap.ecdc.europa.eu/public/extensions/AMC2_Dashboard/AMC2_Dashboard.html#eu-consumption-tab. Accessed 8 Mar 2023.
- 31.European centre for disease prevention and control. Antimicrobial resistance in the EU/EEA (EARS-Net) - annual Epidemiological report 2019. Country summaries - antimicrobial resistance in the EU/EEA 2019. Stockholm: ECDC; 2020. https://www.ecdc.europa.eu/sites/default/files/documents/Country%20summaries-AER-EARS-Net%20202019.pdf. Accessed 15 Mar 2023.
- 32.European centre for disease prevention and control. Antimicrobial resistance in the EU/EEA (EARS-Net) - annual epidemiological report 2020. Country summaries - antimicrobial resistance in the EU/EEA 2020. Stockholm: ECDC; 2022. https://www.ecdc.europa.eu/sites/default/files/documents/AER_EARS-Net%202020%20country-summaries.pdf. Accessed 15 Mar 2023.
- 33.Reports on turnover and use of medicines of Institute for medicines and medical devices of Montenegro. https://www.cinmed.me/. Accessed 17 Aug 2022.
- 34.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z.Erratum.In:StatMed2001Feb28;20(4):655. [DOI] [PubMed] [Google Scholar]
- 35.Dragomirescu I, Llorca J, Gómez IA, et al. A join point regression analysis of trends in mortality due to osteoporosis in Spain. Sci Rep. 2019;9(1):4264. doi: 10.1038/s41598-019-40806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Despotović A, Barać A, Cucanić T, et al. Antibiotic (Mis) use in COVID-19 patients before and after admission to a tertiary hospital in Serbia. Antibiotics. 2022;11:847. doi: 10.3390/antibiotics11070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zivanovic V, Bukarica LG, Scepanovic R, et al. Differences in antimicrobial consumption, prescribing and isolation rate of multidrug resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii on surgical and medical wards. PLoS ONE. 2017;12(5):e0175689. doi: 10.1371/journal.pone.0175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djordjevic ZM, Folic MM, Jankovic SM. Previous antibiotic exposure and antimicrobial resistance patterns of Acinetobacter spp and Pseudomonas aeruginosa isolated from patients with nosocomial infections. Balkan Med J. 2017;34:527–533. doi: 10.4274/balkanmedj.2016.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiner LM, Fridkin SK, Aponte-Torres Z, et al. Vital signs: preventing antibiotic-resistant infections in hospitals - United States, 2014. Am J Transp. 2016;16(7):2224–2230. doi: 10.1111/ajt.13893. [DOI] [PubMed] [Google Scholar]
- 40.Mancini A, Verdini D, La Vigna G, et al. Retrospective analysis of nosocomial infections in an Italian tertiary care hospital. New Microbiol. 2016;39(3):197–205. [PubMed] [Google Scholar]
- 41.Goel N, Wattal C, Oberoi JK, et al. Trend analysis of antimicrobial consumption and development of resistance in non-fermenters in a tertiary care hospital in Delhi. India J Antimicrob Chemother. 2011;66(7):1625–1630. doi: 10.1093/jac/dkr167. [DOI] [PubMed] [Google Scholar]
- 42.European centre for disease prevention and control. Surveillance of antimicrobial resistance in Europe – annual report of the European antimicrobial resistance surveillance network (EARS-Net) 2017. Stockholm: ECDC; 2018. https://www.ecdc.europa.eu/sites/default/files/documents/AMR%202017_Cover%2BInner-web_v3.pdf. Accessed 20 Dec 2021.
- 43.European centre for disease prevention and control. Antimicrobial resistance surveillance in Europe 2015. Annual report of the European antimicrobial resistance surveillance network (EARS-Net). Stockholm: ECDC; 2017. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf. Accessed 20 Dec 2021.
- 44.Mladenovic-Antic S, Kocic B, Velickovic-Radovanovic R, et al. Correlation between antimicrobial consumption and antimicrobial resistance of Pseudomonas aeruginosa in a hospital setting: a 10-year study. J Clin Pharm Ther. 2016;41(5):532–537. doi: 10.1111/jcpt.12432. [DOI] [PubMed] [Google Scholar]
- 45.Peñalva G, Högberg LD, Weist K, et al. Decreasing and stabilising trends of antimicrobial consumption and resistance in Escherichia coli and Klebsiella pneumoniae in segmented regression analysis, European Union/European Economic Area, 2001 to 2018. Euro Surveill. 2019;24(46):1900656. doi: 10.2807/1560-7917.ES.2019.24.46.1900656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.