Abstract
The objective of this study was to clarify the impact of adverse reactions on immune dynamics. We investigated the pattern of systemic adverse reactions after the second and third coronavirus disease 2019 (COVID-19) vaccinations and their relationship with immunoglobulin G against severe acute respiratory syndrome coronavirus 2 spike 1 protein titers, neutralizing antibody levels, peak cellular responses, and the rate of decrease after the third vaccination in a large-scale community-based cohort in Japan. Participants who received a third vaccination with BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (Moderna), had two blood samples, had not had COVID-19, and had information on adverse reactions after the second and third vaccinations (n = 2198) were enrolled. We collected data on sex, age, adverse reactions, comorbidities, and daily medicine using a questionnaire survey. Participants with many systemic adverse reactions after the second and third vaccinations had significantly higher humoral and cellular immunity in the peak phase. Participants with multiple systemic adverse reactions after the third vaccination had small changes in the geometric values of humoral immunity and had the largest geometric mean of cellar immunity in the decay phase. Systemic adverse reactions after the third vaccination helped achieve high peak values and maintain humoral and cellular immunity. This information may help promote uptake of a third vaccination, even among those who hesitate due to adverse reactions.
Subject terms: Immunology, Infectious diseases, Vaccines, Health services, Public health
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first confirmed in China in December 2019, has resulted in 672 million infections and 6.7 million deaths as of January 19, 20231. Vaccination is a key strategy for combating the COVID-19 pandemic. Messenger RNA (mRNA) vaccines mainly induce humoral and cellular immunity against COVID-19, preventing infection and severe disease2. Since July 2021, the third vaccination has been recommended due to waning vaccine effectiveness3–6. However, there are numerous challenges to address, including inequality in vaccine distribution7, waning effectiveness8, and adverse reactions after COVID-19 vaccination9. In addition, concerns about adverse reactions, including fever and fatigue, were essential factors in hesitation to receive the third vaccination10–12. Therefore, considering that the third vaccination is in progress worldwide, evaluating the short- and long-term effects of adverse reactions to COVID-19 vaccination is a crucial public health issue.
Several studies have been conducted on adverse reactions after COVID-19 vaccination. Adverse reactions can be influenced by age, sex, autoimmune disease, frequency of vaccine administration, injection technique, and use of immunosuppressants and non-steroidal anti-inflammatory drugs (NSAIDs)9,13. Notably, it has been suggested that by-products of type I interferons, which guide and promote the adaptive immune response of T and B cells, can cause adverse reactions14–16. Furthermore, some studies have reported that systemic adverse reactions correlate with humoral immunity17–21. However, others insist that humoral immunity does not correlate with adverse reactions22–24, and few studies have investigated the long-term effects of adverse reactions17,25. To the best of our knowledge, no studies have investigated the relationship between systemic adverse reactions after third vaccinations and the long-term dynamics of cellular immunity.
In Japan, more than 31 million infected patients and more than 63 thousand deaths have been reported as of January 19, 202326. In Japan, a third vaccination was provided in December 2021 to healthcare workers and residents of geriatric facilities. Adults aged 65 years or older and more than 85 million people (67.9%) had received a third vaccination by January 19, 202327. In Fukushima Prefecture, the Fukushima Vaccination Community Survey (FVCS), which is a series of antibody tests against COVID-19, has been conducted since the early phase of the pandemic28. The FVCS conducts antibody testing every 3 months, surveys adverse reactions after COVID-19 vaccination with approximately 2500 people under multi-sectional collaboration between local governments, universities, and hospitals, and reports the findings to the local population11,20,29–36. Therefore, this area is suitable for evaluating adverse reactions and immune dynamics, after third vaccinations, at the community level.
Although the third vaccination is in progress worldwide, information on the relationship between adverse reactions and humoral and cellular immunity after the third vaccination was limited. Therefore, this study aimed to clarify the impact of adverse reactions, particularly systemic adverse reactions, on immune dynamics. We investigated the pattern of systemic adverse reactions after the second and third vaccinations and their relationship with immunoglobulin G against spike 1 protein (IgG(S)) titers, neutralizing antibody (Nab) levels, peak cellular responses, and the rate of decrease after the third vaccination.
Results
Characteristics of participants and immunogenicity
Among the 2198 participants, the mean age, height, and weight were 53.5 ± 18.3 years, 161.4 ± 9.7 cm, and 61.8 ± 13.7 kg, respectively; and 1267 (57.6%) participants were female (Table 1). After the third vaccination, the median IgG(S) was 2221.4 AU/mL (interquartile range (IQR) 1318.8–3831.1) in the peak phase (T1: the median of the day from the third vaccination was 54 days) and decreased to 903.7 AU/mL (IQR 499.6–1685.4) in the decay phase (T2: the median of the day from the third vaccination was 145 days). The median Nab was 800 AU/mL (IQR 800.0–800.0) at T1 and decreased to 754.6 AU/mL (IQR 537.1–800.0) at T2. The median ELISpot was 11 (IQR 5–25) at T1 and 11 (IQR 5–25) at T2.
Table 1.
Basic characteristics of participants (N = 2198).
| n (%) | |
|---|---|
| Age, mean [SD] | 53.5 [18.3] |
| Female | 1267 (57.6) |
| Height, mean [SD] | 161.4 [9.7] |
| Weight, mean [SD] | 61.8 [13.7] |
| BMI | |
| Thin | 106 (4.8) |
| Normal | 1175 (53.5) |
| Overweight | 586 (26.6) |
| Alcohol | 963 (43.8) |
| Smoking | 411 (18.7) |
| Daily medications | |
| Steroids | 45 (2.1) |
| NSAIDs | 163 (7.5) |
| Acetaminophen | 52 (2.4) |
| Antihistamines | 135 (6.2) |
| Immunosuppression | 22 (1.0) |
| Biologics | 11 (0.5) |
| Anticancer drugs | 10 (0.5) |
| Comorbidities | |
| Hypertension | 607 (27.6) |
| Diabetes | 165 (7.5) |
| Asthma | 106 (4.8) |
| Anaphylactic shock | 18 (0.8) |
| Gout | 64 (2.9) |
| Dyslipidemia | 251 (11.4) |
| Rheumatoid arthritis | 35 (1.6) |
| Respiratory disease | 45 (2.0) |
| Cardiovascular disease | 169 (7.7) |
| Second vaccination type (Moderna) | 1 (0.1) |
| Adverse reactions after second vaccination | |
| Local pain | 1248 (56.8) |
| Fever | 610 (27.8) |
| Fatigue | 1091 (49.6) |
| Headache | 583 (26.5) |
| Muscle/joint pain | 677 (30.8) |
| Diarrhea | 49 (2.2) |
| Nausea | 85 (3.9) |
| Dizziness | 94 (4.3) |
| Third vaccination type (Moderna) | 730 (33.5) |
| Adverse reactions after third vaccination | |
| Local pain | 1358 (61.8) |
| Fever | 653 (29.7) |
| Fatigue | 1021 (46.4) |
| Headache | 634 (28.8) |
| Muscle/Joint pan | 698 (31.7) |
| Diarrhea | 54 (2.5) |
| Nausea | 92 (4.2) |
| Dizziness | 93 (4.2) |
| After second vaccination | |
| T1 | |
| IgG(S), median [IQR] | 315.4 [172.1–546.2] |
| Nab, median [IQR] | 91.0 [42.6–219.9] |
| T2 | |
| IgG(S), median [IQR] | 136.6 [80.4–225.4] |
| Nab, median [IQR] | 38.5 [21.3–82.0] |
| After third vaccination | |
| T1 | |
| IgG(S), median [IQR] | 2221.4 [1318.8–3831.1] |
| Nab, median [IQR] | 800.0 [800.0–800.0] |
| T spot (S), median [IQR] | 11 [5–25] |
| T2 | |
| IgG(S), median [IQR] | 903.7 [499.6–1685.4] |
| Nab, median [IQR] | 745.6 [537.1–800.0] |
| T spot (S), median [IQR] | 11 [5–25] |
Groups according to adverse reactions
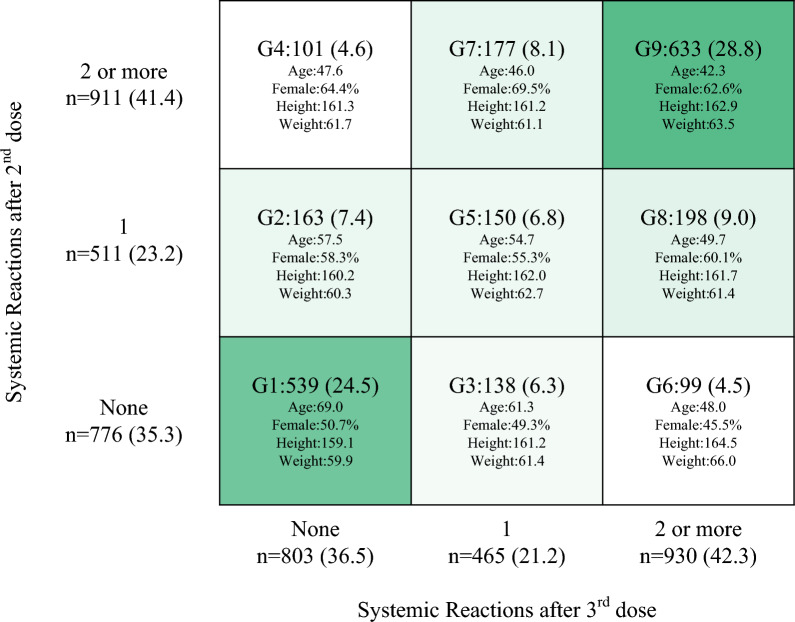
After the second and third vaccinations, the rates of local reactions (56.8% and 61.8% after the second and third vaccinations, respectively) and systemic reactions (64.6% and 63.5% after the second and third vaccinations, respectively) were similar. The most reported systemic adverse reactions were fatigue (49.6% and 46.4% after the second and third vaccinations, respectively), muscle/joint pain (30.8% and 31.7% after the second and third vaccinations, respectively), fever (27.8% and 29.7% after the second and third vaccinations, respectively), and headache (26.5% and 28.8% after the second and third vaccinations, respectively). Of the 776 (35.3%) patients without systemic symptoms after the second vaccination, 539 (24.5%, Group 1) had no systemic symptoms after the third vaccination, 138 (6.3%, Group 3) had one, and 99 (6.3%, Group 6) had two or more symptoms (Fig. 1). Of the 511 (23.2%) patients with one systemic symptom after the second vaccination, 163 (7.4%, Group 2) had no systemic symptoms after the third vaccination, 150 (6.8%, Group 5) had one, and 198 (9.0%, Group 8) had two or more. Of the 911 (41.4%) patients with two or more systemic symptoms after the second vaccination, 101 (4.6%, group 4) had no systemic symptoms after the third vaccination, 177 (8.1%, group 7) had one, and 633 (28.8%, group 9) had two or more.
Figure 1.
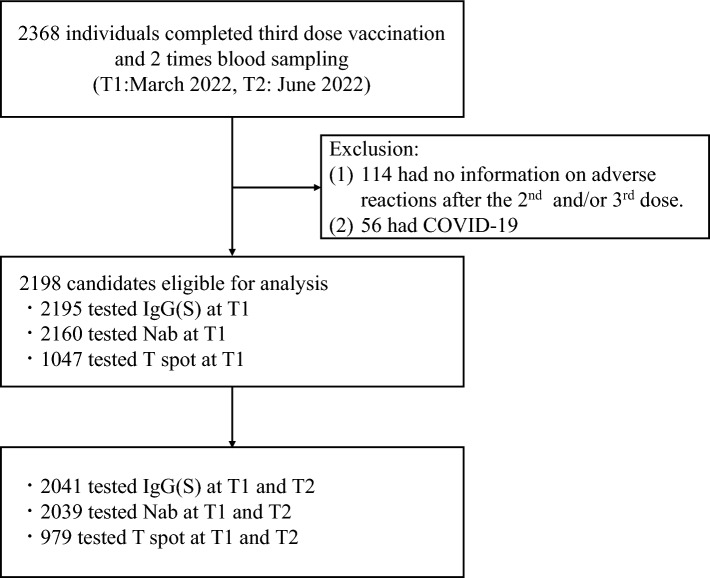
Eligible participants. Participants were included if they (i) completed the third COVID-19 mRNA vaccination (BNT162b2 or mRNA-1273) and (ii) completed blood sampling during the peak (T1: the median of the day from the third vaccination was 54 days) and decay phases (T2: the median of the day from the third vaccination was 145 days). We excluded participants without a record of adverse reactions after the second or third vaccination and those who had COVID-19 by T2. COVID-19 coronavirus disease 2019.
Factors associated with patterns of systemic adverse reactions
The results of multinomial logistics regression based on Group 1 revealed that Group 1 participants were significantly older than all groups (Table 2). Group 1 was significantly associated with males compared to the groups with an exceptionally high number of systemic reactions (Groups 7–9), the groups with one or multiple systemic reactions after the second vaccination only (Groups 2 and 4), and the group with one systemic reaction at each vaccination (Group 5). Furthermore, receiving mRNA-1273 (Moderna) as the third vaccination was significantly lower in Groups 2 and 4 and higher in Group 3. Smoking habits (Groups 4, 7, and 9), alcohol consumption (Group 7), asthma (Groups 5, 7, and 9), use of anticancer drugs (Group 4), and body mass index (BMI) (Group 8) were significantly different from those in Group 1.
Table 2.
Multinomial logistics regression analysis of differences among the groups.
| Group 1 (n = 539) | Group 2 (n = 163) | Group 3 (n = 138) | Group 4 (n = 101) | Group 5 (n = 150) | Group 6 (n = 99) | Group 7 (n = 177) | Group 8 (n = 198) | Group 9 (n = 633) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | |
| Female | 0.62 (0.18 to 1.07) | 0.006 | 0.19 (− 0.31 to 0.68) | 0.45 | 0.79 (0.22 to 1.36) | 0.007 | 0.57 (0.11 to 1.03) | 0.016 | 0.20 (− 0.36 to 0.76) | 0.48 | 1.27 (0.81 to 1.74) | < 0.001 | 0.93 (0.50 to 1.37) | < 0.001 | 1.12 (0.77 to 1.47) | 0.000 | |
| Age | − 0.04 (− 0.06 to − 0.03) | < 0.001 | − 0.03 (− 0.05 to − 0.01) | 0.001 | − 0.09 (− 0.11 to − 0.07) | < 0.001 | − 0.06 (− 0.07 to − 0.04) | < 0.001 | − 0.08 (− 0.10 to − 0.06) | < 0.001 | − 0.09 (− 0.11 to − 0.08) | < 0.001 | − 0.08 (− 0.10 to − 0.07) | < 0.001 | − 0.12 (− 0.13 to − 0.10) | 0.000 | |
| BMI | − 0.05 (− 0.1 to 0.01) | 0.104 | − 0.02 (− 0.08 to 0.04) | 0.47 | − 0.04 (− 0.11 to 0.02) | 0.21 | − 0.03 (− 0.09 to 0.02) | 0.24 | − 0.02 (− 0.09 to 0.04) | 0.49 | − 0.03 (− 0.09 to 0.02) | 0.22 | − 0.05 (− 0.11 to 0.00) | 0.044 | − 0.02 (− 0.06 to 0.02) | 0.26 | |
| Vaccine type (base = Pfizer) | − 0.7 (− 1.17 to − 0.23) | 0.003 | 0.57 (0.13 to 1.01) | 0.011 | − 0.75 (− 1.41 to − 0.09) | 0.025 | − 0.06 (− 0.5 to 0.38) | 0.79 | 0.33 (− 0.19 to 0.84) | 0.22 | − 0.31 (− 0.78 to 0.15) | 0.189 | 0.01 (− 0.41 to 0.42) | 0.97 | 0.15 (− 0.18 to 0.48) | 0.38 | |
| Smoking | − 0.35 (− 0.88 to 0.19) | 0.20 | − 0.39 (− 0.97 to 0.18) | 0.181 | − 0.73 (− 1.45 to 0.00) | 0.052 | − 0.03 (− 0.54 to 0.49) | 0.92 | − 0.58 (− 1.22 to 0.06) | 0.078 | − 0.69 (− 1.26 to − 0.13) | 0.033 | − 0.06 (− 0.54 to 0.42) | 0.80 | − 0.41 (− 0.80 to − 0.01) | 0.044 | |
| Alcohol | 0.24 (− 0.18 to 0.67) | 0.26 | 0.44 (− 0.04 to 0.91) | 0.072 | 0.18 (− 0.36 to 0.72) | 0.52 | 0.13 (− 0.31 to 0.57) | 0.56 | 0.09 (− 0.44 to 0.61) | 0.74 | 0.46 (0.04 to 0.89) | 0.033 | 0.22 (− 0.19 to 0.63) | 0.28 | 0.20 (− 0.13 to 0.53) | 0.24 | |
| Steroids | − 0.26 (− 1.6 to 1.07) | 0.70 | − 1.01 (− 3.12 to 1.10) | 0.35 | − 1.82 (− 4.48 to 0.84) | 0.180 | − 0.80 (− 2.27 to 0.68) | 0.29 | − 15.05 (− 2566.91 to 2536.81) | 0.99 | − 16.22 (− 1713.08 to 1680.64) | 0.99 | − 0.49 (− 1.88 to 0.90) | 0.49 | − 1.34 (− 2.70 to 0.01) | 0.053 | |
| Biologics | − 14.28 (− 3545.89 to 3517.33) | 0.99 | 0.80 (− 1.59 to 3.19) | 0.51 | 1.47 (− 1.04 to 3.99) | 0.25 | 0.17 (− 2.3 to 2.65) | 0.89 | − 13.94 (− 4233.31 to 4205.42) | 1.00 | 1.12 (− 1.36 to 3.6) | 0.26 | 0.91 (− − 1.09 to 2.90) | 0.37 | − 0.39 (− 2.86 to 2.09) | 0.76 | |
| Hypertension | − 0.06 (− 0.54 to 0.41) | 0.79 | − 0.10 (− 0.61 to 0.41) | 0.69 | − 0.07 (− 0.81 to 0.68) | 0.86 | 0.05 (− 0.46 to 0.56) | 0.85 | 0.52 (− 0.12 to 1.17) | 0.11 | 0.00 (− 0.57 to 0.57) | 0.96 | − 0.09 (− 0.60 to 0.43) | 0.75 | 0.01 (− 0.40 to 0.42) | 0.97 | |
| Immunosuppression | − 14.77 (− 2617.57 to 2588.02) | 0.99 | − 14.71 (− 3320.94 to 3291.52) | 0.99 | 0.63 (− 2.16 to 3.42) | 0.66 | 0.53 (− 1.34 to 2.39) | 0.58 | − 13.60 (− 3227.60 to 3200.39) | 0.99 | 1.24 (− 1.12 to 3.60) | 0.30 | 0.11 (− 1.9 to 2.11) | 0.92 | 0.27 (− 1.57 to 2.12) | 0.77 | |
| Anticancer drugs | 0.6 (− 1.79 to 2.99) | 0.63 | − 18.29 (− 25,723.2 to 25,686.61) | 1.00 | 2.36 (0.33 to 4.40) | 0.023 | 0.57 (− 1.76 to 2.90) | 0.63 | − 17.87 (− 28,652.33 to 28,616.60) | 1.00 | 1.23 (− 1.28 to 3.74) | 0.34 | − 18.36 (− 25,998.17 to 25,961.45) | 1.00 | 0.34 (− 2.07 to 2.75) | 0.78 | |
| Diabetes | − 0.07 (− 0.78 to 0.65) | 0.85 | − 0.17 (− 0.89 to 0.55) | 0.64 | − 0.05 (− 1.19 to 1.09) | 0.94 | 0.37 (− 0.31 to 1.04) | 0.29 | 0.48 (− 0.35 to 1.31) | 0.26 | − 0.63 (− 1.73 to 0.47) | 0.26 | 0.35 (− 0.37 to 1.07) | 0.40 | − 0.32 (− 0.98 to 0.34) | 0.34 | |
| Asthma | 0.23 (− 0.96 to 1.43) | 0.70 | 0.53 (− 0.66 to 1.72) | 0.38 | 1.02 (− 0.23 to 2.27) | 0.109 | 1.40 (0.47 to 2.33) | 0.003 | 0.61 (− 0.74 to 1.96) | 0.37 | 1.16 (0.15 to 2.17) | 0.024 | 0.58 (− 0.50 to 1.66) | 0.29 | 1 (0.15 to 1.86) | 0.021 | |
| Dyslipidemia | 0.29 (− 0.24 to 0.83) | 0.29 | 0.45 (− 0.11 to 1.01) | 0.118 | − 0.16 (− 1.08 to 0.77) | 0.74 | − 0.27 (− 0.93 to 0.39) | 0.43 | − 0.05 (− 0.84 to 0.74) | 0.90 | 0.02 (− 0.66 to 0.70) | 0.95 | 0.08 (− 0.53 to 0.69) | 0.79 | 0.41 (− 0.06 to 0.87) | 0.086 | |
Significant values are in bold.
Association between systemic adverse reactions and IgG titer against the S protein
We used multiple regression analysis to predict variables that affected IgG antibodies, Nab, and ELISpot at the peak after the third vaccination (T1) (Table 3). Significantly higher IgG(S) at T1 was associated with males, BMI, third vaccination type (Moderna), Groups 5–9, and smoking. Furthermore, fold changes in IgG(S) between T1 and T2 were larger in the groups with fewer systemic adverse reactions (Group 1: 0.36, Group 2: 0.42, and Group 3: 0.41), smaller in groups with multiple systemic adverse reactions after the third vaccination (Group 6: 0.52, Group 8: 0.47, Group 9: 0.46, and Groups 6, 8, and 9: 0.47), and multiple systemic adverse reactions only after the second vaccination (Group 4: 0.47) (Table 4 and Supplementary Table S4). The geometric mean of IgG(S) at T2 was the smallest in the group without systemic adverse reactions after the third vaccination (Group 1: 630.7 AU/mL, Group 2: 734.2 AU/mL, Group 4: 772.4 AU/mL, and Group 1, 2, and 4: 668.2 AU/mL).
Table 3.
Relationship between the groups and peak humoral or cellular immunity after the third vaccination.
| IgG(S) (N = 2195) | Nab (N = 2160) | T spot (N = 1047) | ||||
|---|---|---|---|---|---|---|
| B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | |
| Female | − 308.05 (− 451.06 to − 165.04) | < 0.001 | − 9.11 (− 22.33 to 4.12) | 0.177 | − 0.48 (− 2.92 to 1.95) | 0.70 |
| Age | − 4.64 (− 9.70 to 0.42) | 0.072 | − 0.85 (− 1.31 to − 0.38) | < 0.001 | − 0.17 (− 0.26 to − 0.08) | < 0.001 |
| BMI | 38.94 (22.44 to 55.44) | < 0.001 | 1.92 (0.40 to 3.45) | 0.013 | − 0.07 (− 0.34 to 0.2) | 0.60 |
| Vaccine type (Moderna) | 1054.17 (914.52 to 1193.81) | < 0.001 | 12.41 (− 0.51 to 25.33) | 0.06 | 1.87 (− 0.45 to 4.18) | 0.114 |
| Group 1 | ||||||
| Group 2 | 140.95 (− 121.13 to 403.04) | 0.29 | 21.89 (− 2.34 to 46.11) | 0.077 | 2.52 (− 1.88 to 6.92) | 0.26 |
| Group 3 | 194.46 (− 94.93 to 483.86) | 0.188 | 37.36 (10.61 to 64.11) | 0.006 | 1.83 (− 3.04 to 6.71) | 0.46 |
| Group 4 | 34.56 (− 309.03 to 378.15) | 0.84 | 2.17 (− 29.58 to 33.92) | 0.89 | 5.24 (− 0.74 to 11.22) | 0.086 |
| Group 5 | 322.89 (47.89 to 597.88) | 0.021 | 29.32 (3.90 to 54.74) | 0.024 | 7.73 (3.26 to 12.21) | 0.001 |
| Group 6 | 833.86 (501.17 to 1166.54) | < 0.001 | 36.52 (5.77 to 67.27) | 0.020 | 10.52 (4.40 to 16.64) | 0.001 |
| Group 7 | 374.92 (104.04 to 645.80) | 0.007 | 35.54 (10.5 to 60.58) | 0.005 | 8.00 (3.57 to 12.42) | < 0.001 |
| Group 8 | 785.7 (529.73 to 1041.66) | < 0.001 | 38.39 (14.67 to 62.11) | 0.002 | 15.19 (11.13 to 19.24) | < 0.001 |
| Group 9 | 667.32 (461.01 to 873.62) | < 0.001 | 37.04 (17.96 to 56.12) | < 0.001 | 12.78 (9.31 to 16.25) | < 0.001 |
| Interval between second and third vaccination | − 0.63 (− 4.09 to 2.82) | 0.72 | − 0.34 (− 0.66 to − 0.02) | 0.038 | 0.04 (− 0.03 to 0.10) | 0.26 |
| Smoking | − 220.45 (− 387.36 to − 53.53) | 0.01 | − 9.17 (− 24.62 to 6.28) | 0.25 | − 3.38 (− 6.25 to − 0.51) | 0.021 |
| Alcohol | − 86.54 (− 220.38 to 47.29) | 0.21 | 1.82 (− 10.56 to 14.20) | 0.77 | 1.79 (− 0.48 to 4.06) | 0.123 |
| Steroids | − 77.61 (− 615.21 to 459.99) | 0.78 | − 82.92 (− 132.59 to − 33.25) | 0.001 | 4.66 (− 3.16 to 12.48) | 0.24 |
| Biologicals | − 726.81 (− 1608.64 to 155.02) | 0.106 | − 139.8 (− 221.28 to − 58.32) | 0.001 | − 6.07 (− 20.72 to 8.59) | 0.42 |
| NSAIDs | − 17.76 (− 279.73 to 244.20) | 0.89 | − 41.96 (− 66.26 to − 17.67) | 0.001 | − 0.10 (− 4.38 to 4.18) | 0.96 |
| Immunosuppression | − 538.08 (− 1300.90 to 224.74) | 0.167 | − 79.86 (− 150.34 to − 9.38) | 0.026 | − 11.74 (− 23.17 to − 0.31) | 0.044 |
| Anticancer drugs | 171.09 (− 730.55 to 1072.74) | 0.71 | − 73.35 (− 156.65 to 9.96) | 0.084 | − 4.26 (− 19.54 to 11.01) | 0.58 |
| Hypertension | − 103.81 (− 275.6 to 67.99) | 0.24 | 1.31 (− 14.58 to 17.19) | 0.87 | 0.59 (− 2.19 to 3.37) | 0.68 |
| Diabetes | − 195.99 (− 445.94 to 53.96) | 0.124 | − 9.23 (− 32.33 to 13.87) | 0.43 | − 1.48 (− 5.46 to 2.50) | 0.47 |
| Asthma | − 108.20 (− 416.28 to 199.89) | 0.49 | − 1.66 (− 30.12 to 26.81) | 0.91 | − 0.74 (− 6.10 to 4.62) | 0.79 |
| Dyslipidemia | 147.64 (− 52.36 to 347.64) | 0.148 | 9.35 (− 9.13 to 27.83) | 0.32 | 0.76 (− 2.84 to 4.36) | 0.68 |
Significant values are in bold.
Table 4.
Reduction rates of the geometric mean (95% CI) of IgG antibodies, neutralizing antibodies, and T spot(S).
| Group (number of systemic adverse reactions after the second and third vaccinations) | T1 | T2 | T2/T1 |
|---|---|---|---|
| IgG(S) (N = 2041) | |||
| Group 1 (0 and 0) | 1739.0 (1623–1863.2) | 630.7 (579.9–686.1) | 0.36 |
| Group 2 (1 and 0) | 1743.3 (1549.2–1961.8) | 734.2 (637.8–845.2) | 0.42 |
| Group 3 (0 and 1) | 2079.9 (1806.5–2394.7) | 856.3 (746.2–982.7) | 0.41 |
| Group 4 (multiple and 0) | 1634.0 (1361.3–1961.2) | 772.4 (652.0–915.1) | 0.47 |
| Group 5 (1 and 1) | 2023.7 (1797.3–2278.6) | 854.0 (737.0–989.6) | 0.42 |
| Group 6 (0 and multiple) | 2595.8 (2214.3–3043.0) | 1356.5 (1120.6–1641.9) | 0.52 |
| Group 7 (multiple and 1) | 2045.7 (1850.4–2261.5) | 884.0 (786.4–993.6) | 0.43 |
| Group 8 (1 and multiple) | 2451.3 (2207.6–2721.9) | 1145.4 (1020.7–1285.4) | 0.47 |
| Group 9 (multiple and multiple) | 2436.1 (2305.7–2573.9) | 1130.4 (1055.8–1210.2) | 0.46 |
| Nab (N = 2039) | |||
| Group 1 (0 and 0) | 672.0 (641.3–704.2) | 413.0 (377.7–451.6) | 0.61 |
| Group 2 (1 and 0) | 723.2 (690.9–757.1) | 482.6 (430.1–541.5) | 0.67 |
| Group 3 (0 and 1) | 733.1 (673.7–797.7) | 555.7 (501.8–615.4) | 0.76 |
| Group 4 (multiple and 1) | 676.6 (606.4–754.9) | 488.8 (417.9–571.6) | 0.72 |
| Group 5 (1 and 1) | 726.2 (684.1–771.0) | 545.7 (486.7–612.0) | 0.75 |
| Group 6 (0 and multiple) | 733.9 (668.4–805.8) | 675.3 (616.1–740.2) | 0.92 |
| Group 7 (multiple and 1) | 745.5 (705.2–788.2) | 573.5 (524.0–627.8) | 0.77 |
| Group 8 (1 and multiple) | 744.8 (699.8–792.6) | 643.6 (597.6–693.3) | 0.86 |
| Group 9 (multiple and multiple) | 757.5 (737.9–777.6) | 619.4 (593.0–646.9) | 0.82 |
| T spot (N = 979) | |||
| Group 1 (0 and 0) | 6.4 (5.5–7.3) | 5.5 (4.8–6.3) | 0.86 |
| Group 2 (1 and 0) | 8.0 (6.1–10.4) | 6.8 (5.3–8.8) | 0.86 |
| Group 3 (0 and 1) | 9.1 (7.2–11.6) | 7.6 (6.0–9.7) | 0.84 |
| Group 4 (multiple and 1) | 11.5 (8.8–14.9) | 10.5 (7.6–14.7) | 0.92 |
| Group 5 (1 and 1) | 10.6 (8.1–14) | 8.9 (6.8–11.5) | 0.83 |
| Group 6 (0 and multiple) | 17.1 (12.4–23.6) | 12.0 (8.6–16.8) | 0.70 |
| Group 7 (multiple and 1) | 13.4 (10.8–16.6) | 11.9 (9.5–14.8) | 0.89 |
| Group 8 (1 and multiple) | 19.4 (16.2–23.1) | 15.6 (12.7–19.2) | 0.81 |
| Group 9 (multiple and multiple) | 17.9 (16.0–20.0) | 14.3 (12.6–16.4) | 0.80 |
Associations between adverse reaction and Nab
Significantly, higher Nab at T1 was associated with BMI in Groups 3 and 5–9. Conversely, significantly lower Nab at T1 was associated with age, the interval between the second and third vaccinations, steroids, biologicals, NSAIDs, and immunosuppression. In addition, fold changes in Nab were larger in the group without systemic adverse reactions after the third vaccination (Group 1: 0.61, Group 2: 0.67, Group 4: 0.72, and Groups 1, 2, and 4: 0.64). These groups had the lowest geometric mean of Nab at T2 (Group 1: 413.0 AU/mL, Group 2: 482.6 AU/mL, Group 4: 488.8 AU/mL, and Groups 1, 2, and 4: 435.9 AU/mL). Fold changes in Nab were smaller in the groups with multiple systemic adverse reactions after the third vaccination (Group 6: 0.92, Group 8: 0.86, Group 9: 0.82, and Groups 6, 8, and 9: 0.84).
Association between adverse reactions and cellar immunity
Peak ELISpot at T1 was significantly associated with age, Groups 5–9, and smoking habits. The geometric mean of ELISpot at T2 was the largest in the group with multiple systemic adverse reactions after the third vaccination (Group 6: 12.0, Group 8: 15.6, Group 9: 14.3, and Groups 6, 8, and 9: 14.4).
Discussion
We investigated the pattern of systemic adverse reactions after the second and third COVID-19 vaccinations and their short- and long-term relationship with IgG(S), Nab, and ELISpot to clarify the impact of adverse reactions on immune dynamics.
The pattern of systemic adverse reactions was associated with higher peak values of humoral and cellular immunity after the third vaccination. Groups with systemic adverse reactions in both the second and third vaccinations (Groups 5, 7, 8, and 9) and multiple systemic adverse reactions only after the third vaccination (Group 6) were associated with significantly higher IgG(S), Nab, and ELISpot in the peak phase (T1). The group with one systemic adverse reaction only after the third vaccination (Group 3) was associated only with peak values of Nab. The results for Groups 5–9 were consistent with previous reports that systemic adverse reactions were associated with higher antibody titers17–21. However, the results of Group 3 were consistent with reports that denied any association between adverse reactions and humoral immunity22–24. Immune dynamics after the third vaccination may differ depending on the experience and number of systemic adverse reactions.
Those who experienced multiple systemic adverse reactions after the third vaccination achieved high levels of humoral and cellular immunity, which was maintained over 3 months. Overall, 930 (42.3%) participants who experienced multiple systemic adverse reactions after the third vaccination (Groups 6, 8, and 9) had significantly higher peak values of humoral and cellular immunity, and the fold change in humoral immune levels between T1 and T2 was small. Notably, Groups 6, 8, and 9 had the largest geometric mean of cellar immunity at T2. Previous studies have reported an association between adverse reactions and the long-term kinetics of humoral immunity10. Studies examining the relationship between adverse reactions and the long-term dynamics of cellular immunity were limited. However, systemic adverse reactions were predominantly related to achieving and maintaining humoral and cellular immunity. Therefore, this information may help promote uptake of a third vaccination, even for those who hesitate to receive the vaccination because of concerns about adverse reactions.
The group without systemic symptoms (Group 1), who were significantly older than all the groups, had the lowest peak values and largest reduction in humoral immunity and could not induce cellular immunity. Group 1 included 539 (24.5%) participants, who were significantly older than all the groups and significantly more likely to be male than Groups 2, 7, 8, and 9. Older age was significantly associated with lower peaks of Nab and ELISpot after the third vaccination. The result that older participants and men had fewer adverse reactions was consistent with previous reports37–39. Previous studies have also reported that older patients had smaller antigen-specific memory B cell and antigen-specific memory T cell responses, and reduction of humoral immunity13,40–43. People who are less likely to have adverse reactions, including the elderly, may have less antibody and cytokine production by antigen-specific memory B and T cells. Therefore, they may need to continue infection control measures and discuss further vaccination. In addition, identifying those who are more likely to experience systemic adverse reactions and investigating their long-term effects may be crucial in preventing excessive adverse reactions.
This study had some limitations that should be considered when interpreting the results. First, we used the Wuhan strain of pseudo virus to measure humoral and cellular immunity. Therefore, we could not assess whether efficacy against the mutant strains differed among the groups, making it difficult to consider a fourth and subsequent vaccination. Further research is needed to determine the impact of adverse reactions and immune dynamics on the variant strains of SARS-CoV-2. Second, adverse reactions were self-reported and could not be evaluated based on the severity of each symptom or objective measures. Third, cellular immunity did not significantly change in any of the groups between T1 and T2, making it difficult to discuss the fold change. Despite these limitations, this study was the first to investigate systemic adverse reactions and their short- and long-term relationships to humoral and cellular immunity. They may need to continue infection control measures and discuss further vaccination.
In conclusion, systemic adverse reactions after the third vaccination were beneficial in achieving high peak values and maintaining humoral and cellular immunity. This information may help promote uptake of a third vaccination, even for those who hesitate to receive the vaccine because of concerns about adverse reactions. In contrast, the population without systemic symptoms was associated with older age, males, and vulnerability in acquiring humoral and cellular immunity. They may need to continue infection control measures and discuss further vaccination.
Methods
Study participants
This was an observational historical cohort study. The study participants were recruited from healthcare workers, government office staff, residents, and nursing home residents in Ishikawa Country, Soma City, and Minamisoma City in Fukushima Prefecture. The recruitment of participants, blood sampling, and questionnaire surveys were conducted in cooperation with hospital groups and municipalities in the central and Soso areas of Fukushima Prefecture. This area has been continually testing for antibodies in healthcare workers and residents since 2020 to identify their infection status and control infections.
Eligibility criteria
The inclusion criteria were as follows: (i) completed the third COVID-19 mRNA vaccination (BNT162b2 or mRNA-1273); (ii) completed blood sampling at T1 and T2. Overall, 2368 individuals met the criteria for (i) and (ii). We excluded (iii) 114 patients without a record of adverse reactions after the second or third vaccination and (iv) 56 patients who self-reported COVID-19 by June 2022 (T2). Finally, 2198 participants were included in the study. The ethics committees of Hirata Central Hospital (number 2021-0611-1) and Fukushima Medical University approved this study (number 2021-116). This study conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from all participants. This study was supported by the Japan Agency for Medical Research and Development and was conducted as part of the FVCS.
Study design
We collected information from participants on age, sex, weight, height, alcohol consumption, smoking habits, medications, comorbidities, second and third vaccination types, and adverse reactions after the second and third vaccinations from a questionnaire survey. Regarding adverse reactions, the patients were asked about local pain, fever, headache, muscle/joint pain, diarrhea, nausea, and dizziness. Of the 2198 individuals who met the study eligibility criteria, at T1, 2195, 2160, and 1047 completed measurements for IgG(S), Nab, and ELISpot, respectively; at both T1 and T2, 2041, 2039, and 979 completed measurements for IgG(S), Nab, and ELISpot, respectively (Fig. 2).
Figure 2.
Groups according to the number of systemic adverse reactions after the second and third vaccinations (N = 2199). We classified participants into nine groups according to the number of systemic adverse reactions (0, 1, or more than 2) after the second and third vaccinations. Systemic adverse reactions were defined as fatigue, headache, muscle/joint pain, diarrhea, nausea, and dizziness. Group numbers were defined so that the larger the number of systemic adverse reactions, the larger the group number; for example, G1 was for those without systemic adverse reactions after the second and third vaccinations, and G9 was for those with multiple systemic adverse reactions after both vaccinations, among others.
Cellular immune response
We evaluated cellular immune responses by ELISpot using T-spot COVID (Oxford Immunotec; UK). Blood samples were transferred from the hospital to LSI medicine within the blood sampling day; subsequently, all tests were performed in LSI medicine as per official guidelines. Effector T- cells generating interferon-gamma were counted as spots on the wells. The results were assessed by comparing the positive and negative control wells. The number of spots was counted up to 50; thus, more than 50 spots were shown as 50 and over. In addition, more than seven spots was evaluated as reactive, 5, 6, and 7 spots were evaluated as borderline, and fewer than five spots were evaluated as not reactive and complied following the official guidelines. The target antigen of ELISpot was the spike protein.
Serological assay
Serological assays for IgG(S) and Nab were performed using the chemiluminescence immunoassay with iFlash 3000 (YHLO Biotech, Shenzhen, China) and iFlash-2019-nCoV series (YHLO Biotech, Shenzhen, China) as reagents at Tokyo University. A chemiluminescence immunoassay is used to quantitatively determine humoral immunity in human serum using an enzyme and chemiluminescence. The cutoff value of each assay (IgG against the S protein, Nab) was 10 AU/mL, following the manufacturer’s official cutoff values44,45. The cutoff values were determined using the receiver operating characteristic curve method. For Nab, values > 800 AU/mL were not guaranteed to be accurate, according to the manufacturer’s instructions. Testing was performed according to the official guidelines. Quality evaluations were conducted daily before starting measurements.
Classification of participants
We classified the participants into nine groups according to the number of systemic adverse reactions (0, 1, or more than 2), after the second and third vaccinations, to investigate the effect of adverse reactions on immune dynamics (Table 5). Systemic adverse reactions were defined as fatigue, headache, muscle/joint pain, diarrhea, nausea, and dizziness. Group numbers were defined so that the larger the number of systemic adverse reactions, the larger the group number; for example, Group 1 was for those without systemic adverse reactions after the second and third vaccinations, and Group 9 was for those with two or more systemic adverse reactions in both vaccinations, among others.
Table 5.
Patterns of systemic adverse reactions.
| Group | Number of systemic adverse reactions | |
|---|---|---|
| After the second vaccination | After the third vaccination | |
| Group 1 | 0 | 0 |
| Group 2 | 1 | 0 |
| Group 3 | 0 | 1 |
| Group 4 | 2 ≥ | 0 |
| Group 5 | 1 | 1 |
| Group 6 | 0 | 2 ≥ |
| Group 7 | 2 ≥ | 1 |
| Group 8 | 1 | 2 ≥ |
| Group 9 | 2 ≥ | 2 ≥ |
Statistical analysis
We compared the participants’ characteristics using descriptive statistics according to the nine patterns of systemic adverse reactions after the second and third vaccinations. Based on the BMI of the participants, < 18.5 was defined as thin, 18.5–25 as normal, and ≥ 25 as overweight. Categorical variables (sex, BMI, alcohol, smoking, daily medicine, comorbidity, vaccine type, and adverse reaction) were summarized as frequencies, and continuous variables (age, height, and weight) were summarized as means and standard deviations. First, a multinomial logistics regression analysis was performed to determine whether participants’ backgrounds differed among the nine groups based on the group without systemic adverse reactions (Group 1). Independent variables included sex, age, BMI, vaccine type, smoking habits, alcohol consumption, medications, and comorbidity in Groups 2–9. Second, a multiple regression analysis was performed to examine the relationship between the peak of immunity after the third vaccination and the pattern of systemic adverse reactions. The dependent variables were the values of IgG(S), Nab, and ELISpot at T1, and the independent variables included sex, age, BMI, type of third vaccination, Groups 1–9, the interval between the second and third vaccinations, smoking habits, alcohol consumption, medications, and comorbidities. Third, the geometric means of IgG(S), Nab, and ELISpot for each group were reported at T1 and T2. The fold change between the two-time points was calculated for the nine groups. Fourth, the participants’ ages were divided by 10 years, and the percentage of those who experienced one, or more than two, systemic adverse reactions after the second and third vaccinations was calculated for each weight and BMI. An IgG(S) antibody titer over 5000 arbitrary units per milliliter (AU/mL) was defined as 5000 AU/mL and Nab over 800 AU/mL was defined as 800 AU/mL for all analyses. Statistical significance was set at p < 0.05. All statistical analyses were performed using STATA/IC (version 15; Lightstone, DL, College Station, TX, USA) and Python (version 3.7.12).
Supplementary Information
Acknowledgements
We would like to thank all the staff of Fukushima Medical University, Seireikai health care group, Hirata Village office, Soma City office, Soma Central Hospital, Soma General Hospital, Minamisoma City office, Minamisoma City Medical Association, Minamisoma Municipal General Hospital, and the Medical Governance Institute, who contributed significantly to the accomplishment of this research, especially Ms. Yuka Harada, Ms. Serina Noji, Mr. Masahiko Nihei, Mr. Hideo Sato, Ms. Rie Yanai, Ms. Yasuko Suzuki, Ms. Keiko Abe, Dr. Hidekiyo Tachiya, Mr. Kouki Nakatsuka, Dr. Ryuzaburo Shineha, Ms. Miki Sato, Dr. Masahiko Sato, Mr. Naoharu Tadano, Mr. Kazuo Momma, Mr. Shu-ichi Mori, Ms. Saori Yoshisato, Ms. Katsuko Onoda, Mr. Satoshi Kowata, Mr. Masatsugu Tanaki, Ms. Xujin Zhu, Ms. Tomoyo Nishimura, Ms. Moe Kawashima, Ms. Yuna Uchi, Mr. Taiga Uchiyama, Mr. Kenmei Kitazawa, Mr. Yuta Tani, and Mr. Sota Sugiura. This study was supported by AMED under Grant Number JP21nf0101638, and by Medical & Biological Laboratories Co., Ltd. and Shenzhen YHLO Biotech Co., Ltd., the distributor and manufacturer of the antibody measurement system (iFlash 3000). This study was also supported by grants from Kowa Co. and the Research Center for Advanced Science and Technology at the University of Tokyo.
Author contributions
Conceptualization of the study: Y. Ko., T. Ka., Y.N., F.O., M. Ta., T. Ko., M.Ts. Data collection: Y. Ko., Y.S., H.S., Y.N., F.O., C.Y., T.Z., N.I., M.Ts. Investigation: T. Ka., K. Tat., Y. Ka., A.N., T. Ko. Data analysis and interpretation: M.Y., Y. Ko., M.Ts. Drafting of the article: M.Y., Y. Ko. Critical revision of the article: Y.N., M.W., K.Tak., M.Ts. Final approval of the version to be published: all authors.
Data availability
The data that supports the findings of this study are available from the corresponding author. However, restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data are, however, available upon reasonable request to the corresponding author and with permission from Fukushima Medical University School of Medicine.
Competing interests
Kaneko is employed by Medical & Biological Laboratories, Co., (MBL, Tokyo, Japan). MBL imported the testing material used in this study. Kaneko participated in the testing process; however, he did was not involved in the study design and analysis. Kobashi and Tsubokura received a research grant from the Pfizer Health Research Foundation for research not associated with this work. The remaining authors declare no potential conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-36429-1.
References
- 1.Johns Hopkins University of Medicine. COVID-19 Dashboard by the Center for Systems Science and Engineering (CCCE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html (2022).
- 2.Lee KM, Lin SJ, Wu CJ, Kuo RL. Race with virus evolution: The development and application of mRNA vaccines against SARS-CoV-2. Biomed. J. 2023 doi: 10.1016/j.bj.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-On YM, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta RK, Topol EJ. COVID-19 vaccine breakthrough infections. Science. 2021;374:1561–1562. doi: 10.1126/science.abl8487. [DOI] [PubMed] [Google Scholar]
- 5.Feikin DR, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atmar RL, et al. Homologous and heterologous Covid-19 booster vaccinations. N. Engl. J. Med. 2022;386:1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayati M, Noroozi R, Ghanbari-Jahromi M, Jalali FS. Inequality in the distribution of Covid-19 vaccine: A systematic review. Int. J. Equity Health. 2022;21:122. doi: 10.1186/s12939-022-01729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferdinands JM, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance—VISION network, 10 States, August 2021-January 2022. MMWR Morb. Mortal Wkly. Rep. 2022;71:255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanis P, et al. First COVID-19 Booster Dose in the general population: A systematic review and meta-analysis of willingness and its predictors. Vaccines. 2022;10:1097. doi: 10.3390/vaccines10071097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida M, et al. Factors associated with COVID-19 vaccine booster hesitancy: A retrospective cohort study, Fukushima vaccination community survey. Vaccines. 2022;10:515. doi: 10.3390/vaccines10040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyce MC, et al. From trial to practice: incidence and severity of COVID-19 vaccine side effects in a medically at-risk and vaccine-hesitant community. BMC Public Health. 2022;22:2351. doi: 10.1186/s12889-022-14824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collier DA, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprent J, King C. COVID-19 vaccine side effects: The positives about feeling bad. Sci. Immunol. 2021;6:eabj9256. doi: 10.1126/sciimmunol.abj9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasilache AM, Qian H, Blomqvist A. Immune challenge by intraperitoneal administration of lipopolysaccharide directs gene expression in distinct blood–brain barrier cells toward enhanced prostaglandin E(2) signaling. Brain. Behav. Immun. 2015;48:31–41. doi: 10.1016/j.bbi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naaber P, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy I, et al. Correlation between adverse events and antibody titers among healthcare workers vaccinated with BNT162b2 mRNA COVID-19 vaccine. Vaccines. 2022;10:1220. doi: 10.3390/vaccines10081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koike R, et al. Systemic adverse effects induced by the BNT162b2 vaccine are associated with higher antibody titers from 3 to 6 months after vaccination. Vaccines. 2022;10:451. doi: 10.3390/vaccines10030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobashi Y, et al. Factors associated with anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antibody titer and neutralizing activity among healthcare workers following vaccination with the BNT162b2 vaccine. PLoS ONE. 2022;17:e0269917. doi: 10.1371/journal.pone.0269917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tani N, et al. Relation of fever intensity and antipyretic use with specific antibody response after two doses of the BNT162b2 mRNA vaccine. Vaccine. 2022;40:2062–2067. doi: 10.1016/j.vaccine.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi M, Higa Y, Esaki A, Nabeshima Y, Nakazono A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLoS ONE. 2021;16:e0257668. doi: 10.1371/journal.pone.0257668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Held J, et al. Reactogenicity correlates only weakly with humoral immunogenicity after COVID-19 vaccination with BNT162b2 mRNA (Comirnaty(®)) Vaccines. 2021;9:1063. doi: 10.3390/vaccines9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang YH, et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J. Intern. Med. 2021;36:1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto N, et al. Examining the association between vaccine reactogenicity and antibody titer dynamics after the third dose of BNT162b2 vaccine using a mixed-effects model. J. Infect. Chemother. 2023;29:39–42. doi: 10.1016/j.jiac.2022.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Japan Broadcasting Corporation. Number of infected people and deaths in Japan. https://www3.nhk.or.jp/news/special/coronavirus/data-all/ (2023).
- 27.Nikkei inc. COVID-19 Vaccination Status in Japan Charted. https://vdata.nikkei.com/newsgraphics/coronavirus-japan-vaccine-status/ (2023).
- 28.Kobashi Y, et al. Maturing of public-private-people partnership (4P): Lessons from 4P for triple disaster and subsequently COVID-19 pandemic in Fukushima. J. Glob. Health. 2022;12:03028. doi: 10.7189/jogh.12.03028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobashi Y, et al. Waning of humoral immunity and the influencing factors after BNT162b2 vaccination: A cohort study with a latent growth curve model in Fukushima. Vaccines. 2022;10:2007. doi: 10.3390/vaccines10122007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobashi Y, et al. Humoral immunity after second dose of BNT162b2 vaccine in Japanese communities: an observational cross-sectional study. Fukushima vaccination community survey. Sci. Rep. 2022;12:18929. doi: 10.1038/s41598-022-21797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao T, et al. Humoral response to SARS-CoV-2 vaccination in haemodialysis patients and a matched cohort. BMJ Open. 2022;12:e065741. doi: 10.1136/bmjopen-2022-065741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida M, et al. Time course of adverse reactions following BNT162b2 vaccination in healthy and allergic disease individuals aged 5–11 years and comparison with individuals aged 12–15 years: An observational and historical cohort study. Eur. J. Pediatr. 2023;182:123–133. doi: 10.1007/s00431-022-04643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobashi Y, et al. Peak IgG antibody titers against SARS-CoV-2 spike protein following immunization with the Pfizer/BioNTech BNT162b2 vaccine. Fukushima J. Med. Sci. 2022;68:67–70. doi: 10.5387/fms.2021-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobashi Y, et al. Seroprevalence of SARS-CoV-2 antibodies among hospital staff in rural Central Fukushima, Japan: A historical cohort study. Int. Immunopharmacol. 2021;98:107884. doi: 10.1016/j.intimp.2021.107884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobashi Y, et al. The difference between IgM and IgG antibody prevalence in different serological assays for COVID-19; lessons from the examination of healthcare workers. Int. Immunopharmacol. 2021;92:107360. doi: 10.1016/j.intimp.2020.107360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawashima M, et al. Antibody and T-Cell responses against SARS-CoV-2 after booster vaccination in patients on dialysis: A prospective observational study. Vaccines. 2023;11:260. doi: 10.3390/vaccines11020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munro APS, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urakawa R, Isomura ET, Matsunaga K, Kubota K. Young age, female sex, and no comorbidities are risk factors for adverse reactions after the third dose of BNT162b2 COVID-19 vaccine against SARS-CoV-2: A prospective cohort study in Japan. Vaccines. 2022;10:1357. doi: 10.3390/vaccines10081357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Notarte KI, et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: A systematic review. Crit. Rev. Clin. Lab. Sci. 2022;59:373–390. doi: 10.1080/10408363.2022.2038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goel RR, et al. Distinct antibody and memory B cell responses in SARS-CoV2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar NP, et al. BCG vaccination induces enhanced frequencies of memory T cells and altered plasma levels of common γc cytokines in elderly individuals. PLoS ONE. 2021;16:e0258743. doi: 10.1371/journal.pone.0258743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroemer M, et al. COVID-19 patients display distinct SARS-CoV-2 specific T-cell responses according to disease severity. J. Infect. 2021;82:282–327. doi: 10.1016/j.jinf.2020.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauvau G, Goriely S. Memory CD8+ T cells: orchestrators and key players of innate immunity? PLoS Pathog. 2016;12:e1005722. doi: 10.1371/journal.ppat.1005722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenzhen YHLO Biotech Co., Ltd iFlash-SARS-CoV-2 IgG-S. (2020).
- 45.Shenzhen YHLO Biotech Co., Ltd iFlash-2019-nCoV NAb. (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author. However, restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data are, however, available upon reasonable request to the corresponding author and with permission from Fukushima Medical University School of Medicine.