Abstract
Smoking cessation treatments that are easily accessible and deliver intervention content at vulnerable moments (e.g., high negative affect) have great potential to impact tobacco abstinence. The current study examined the feasibility and acceptability of a multi-component Just-In-Time Adaptive Intervention (JITAI) for smoking cessation. Daily smokers interested in quitting were consented to participate in a 6-week cessation study. Visit 1 occurred 4 days pre-quit, Visit 2 was on the quit day, Visit 3 occurred 3 days post-quit, Visit 4 was 10 days post-quit, and Visit 5 was 28 days post-quit. During the first 2 weeks (Visits 1–4), the JITAI delivered brief mindfulness/motivational strategies via smartphone in real-time based on negative affect or smoking behavior detected by wearable sensors. Participants also attended 5 in-person visits, where brief cessation counseling (Visits 1–4) and nicotine replacement therapy (Visits 2–5) were provided. Outcomes were feasibility and acceptability; biochemically-confirmed abstinence was also measured. Participants (N = 43) were 58.1 % female (AgeMean = 49.1, mean cigarettes per day = 15.4). Retention through follow-up was high (83.7 %). For participants with available data (n = 38), 24 (63 %) met the benchmark for sensor wearing, among whom 16 (67 %) completed at least 60 % of strategies. Perceived ease of wearing sensors (Mean = 5.1 out of 6) and treatment satisfaction (Mean = 3.6 out of 4) were high. Biochemically-confirmed abstinence was 34 % at Visit 4 and 21 % at Visit 5. Overall, the feasibility of this novel multi-component intervention for smoking cessation was mixed but acceptability was high. Future studies with improved technology will decrease participant burden and better detect key intervention moments.
Keywords: Mindfulness, Just-in-time adaptive intervention, Smoking cessation, mHealth, Micro-randomized trial
1. Introduction
Globally, tobacco use is responsible for >8 million deaths per year (World Health Organization, 2021) and the smoking prevalence ranges from 6.5 % to 32.6 % in 2020 (Dai et al., 2022). In particular, cigarette smoking remains the leading preventable cause of morbidity and mortality in the United States (US Department of Health and Human Services, 2014). In particular, individuals of low socioeconomic status (SES) are disproportionately affected by cigarette smoking given chronic high stress (Cambron et al., 2019) and limited access to cessation resources (Boland et al., 2017; Boland et al., 2019; Copeland et al., 2010). Focused smoking cessation interventions that target stress and alleviate barriers to access should be further explored and tested.
Just-in-Time Adaptive Interventions (JITAIs) may alleviate such barriers. JITAIs provide intervention content at specific moments that are most relevant to an individual (Nahum-Shani et al., 2018; Naughton, 2017). JITAIs can be delivered at the moment in which an individual is vulnerable to lapse or relapse of smoking while they are perhaps unaware of such heightened vulnerability (Nahum-Shani et al., 2018). In particular, continuous passive assessment of participants’ behaviors via wearables sensors (e.g., physiological sensors) to detect vulnerable moments could be beneficial because treatment content can be delivered with reduced burden (Nahum-Shani et al., 2018). For example, to detect prolonged sedentary behavior or a moment of smoking and then to intervene, wrist sensors can be used. The combination of wrist sensors and chest band can be also used to detect smoking behavior and/or physiological stress states (Nakajima et al., 2020). However, to date, published JITAI studies that use wearable sensors either have been used only for monitoring purposes (e.g., Klasnja et al., 2019) or are in the data collection phase (Battalio et al., 2021; Horvath et al., 2021; Kizakevich et al., 2018; Nahum-Shani et al., 2021). JITAI studies using other types of sensors have been mostly limited to the detection of geolocations (see a review, Perski et al., 2021). Together, the emerging literature suggests that JITAIs using wearable sensors for smoking cessation would not be only beneficial regarding abstinence via detection and delivery of intervention content at vulnerable moments, but may also reduce treatment barriers (e.g., access to in-person treatment, time and monetary cost to attend in-person treatment, challenges associated with using strategies learned when most needed in the real-world).
Mindfulness is one potential intervention approach that may enhance tobacco abstinence by focusing on key mechanisms in the relapse process by leveraging a JITAI. Theoretically, mindfulness disrupts the automatic response to craving and rewarding stimuli (e.g., cigarette cues, Breslin et al., 2002) that signal relief of withdrawal or negative affect, by targeting, thereby weakening, the associative learning process between aversive affective states (e.g., negative affect), craving, and smoking (Brewer et al., 2013), leading to a reduced likelihood of lapse. Indeed, daily smokers in a traditional mindfulness-based intervention (MBI), compared to active control, demonstrated favorable cessation outcomes (e.g., maintenance of abstinence at follow-ups, Brewer et al., 2011; Davis et al., 2014). Notably, traditional MBIs alter smoking-related cognitive-affective processes such as perceived sense of control (Spears et al., 2017), self-efficacy in managing negative affect (Spears et al., 2017), and stress reactivity at the neuronal level (Kober et al., 2017). Recent evidence shows that the delivery of static mindfulness content through a smartphone app may work in a similar way as traditional in-person MBIs, that is, weakening the link between craving and smoking (Garrison et al., 2020). Although the literature has identified several momentary risk factors of smoking lapse (e.g., craving, affect, self-efficacy, expectancies, presence of other smokers, and cigarette availability), stress remains one prominent risk factor of smoking lapse (Cambron et al., 2019; Potter et al., 2021). Given mindfulness targets transdiagnostic psychological vulnerabilities (Kraemer et al., 2020), a mindfulness-based smoking cessation intervention could benefit individuals in managing chronic stress (Adams et al., 2015).
Mindfulness-based JITAIs may reduce negative affect and automatic response (e.g., craving, smoking behaviors) in real-time by increasing the salience of precipitants through purposeful attentional engagement with the present moment (Bishop et al., 2004). Although the research on JITAIs in the context of smoking cessation is in its infancy, JITAIs appear to positively impact abstinence (Businelle et al., 2016), and reduce stress, urge to smoke (Hébert et al., 2018), and likelihood of momentary lapse (Huh et al., 2021). Despite great potential, a JITAI utilizing mindfulness with wearable sensors has not been evaluated.
Prior to fully testing a JITAI for efficacy on distal outcomes (e.g., abstinence), it is ideal to first determine its impact on proximal outcomes (Nahum-Shani et al., 2018). Utilizing a micro-randomized trial (MRT) design, the current study piloted a JITAI, delivered via smartphone during a quit attempt among daily smokers (Hernandez et al., 2021). Specifically, the MRT design allowed key moments to be randomized to either receive or not brief mindfulness/motivational strategies in real-time (i.e., JITAI component). AutoSense (Ertin et al., 2011; Hovsepian et al., 2015; Saleheen et al., 2015), a suite of wearable sensors that detects physiological responses (i.e., electrocardiography, respiration) and arm movements, was used to trigger the JITAI component. This paper specifically reports findings that address the following research questions: (1) Will a multi-component JITAI be feasible for smoking cessation? and (2) Will daily smokers find the JITAI acceptable and helpful? Per guidelines on reporting outcomes from feasibility studies with a small sample size (Tickle-Degnen, 2013), descriptive information on the preliminary outcome of biochemically-confirmed tobacco abstinence is provided.
2. Method
2.1. Study design
The current study was a 6-week, single-arm, non-randomized pilot study that used an MRT design to randomize intervention content at key moments within each participant. There were 5 in-person visits following an orientation session (Table 1). Specifically, Visit 1 occurred 4 days prior to the Quit Date (QD-4), Visit 2 was on the QD, Visit 3 was 3 days after the QD (QD + 3), Visit 4 was 10 days after the QD (QD + 10), and Visit 5 was 28 days after the QD (QD + 28). Four visits (i.e., Visits 1–4) occurred during the first 2 weeks. During the visits, participants were provided brief smoking cessation counseling and nicotine replacement therapy and completed self-reports and biochemical verification of smoking status. The JITAI and ecological momentary assessment (EMA) items were also provided daily during the first 2 weeks. During Visit 5, participants completed self-reports and biochemical verification of smoking status. The CONSORT checklist for a pilot/feasibility trial was completed and guided the study design and reporting (Supplementary Material 1).
Table 1.
Participant flow.
| Study Component | Days Orientation Session | QD-4 Visit 1 | QD Visit 2 | QD + 3 Visit 3 | QD + 10 Visit 4 | QD + 28 Visit 5 |
|---|---|---|---|---|---|---|
| Informed Consent | Initial informed consent for collection of eligibility data | Informed consent to participate in study | ||||
| Measures/ Procedures | CO measurement, pregnancy test, semi-structured interview | CO measurement, self-report measures, taught to wear/use the sensors/smartphone | CO measurement, self-report measures | CO measurement, self-report measures | CO measurement, self-report measures, intervention feedback | CO measurement, self-report measures |
| EMAs sent | X | X | X | X | ||
| Sensors worn | X | X | X | X | ||
| JITAIs | X | X | X | X | ||
| Brief Counseling | X | X | X | X | Provided additional resources on smoking cessation |
Note. CO = Carbon Monoxide. EMA = Ecological Momentary Assessment. JITAI = Just-In-Time Adaptive Intervention. QD = Quit Date. All visits were conducted in-person.
2.2. Participants
Daily smokers were recruited through community and online advertisements, and via a participant database at the Tobacco Research and Intervention Program at Moffitt Cancer Center. The sample size of 43 was determined based on extant feasibility studies (Bricker et al., 2010; Gwaltney et al., 2008) and the guidelines on the sample size determination for feasibility studies (Hertzog, 2008). We accounted for 30 % drop out. The initial phone screen assessed eligibility based on the following inclusion criteria: (1) ≥18 years old, (2) ≥3 cigarettes per day (CPD) in the past year, (3) verification of smoking status via carbon monoxide (CO ≥ 6 ppm), (4) motivated to quit in the next 30 days, (5) valid home address and functioning phone number, and (6) able to read, write, and speak in English. Exclusion criteria were: (1) contraindication for nicotine patch use, (2) current psychosis via a semi-structured interview (Sheehan et al., 1998), (3) having implanted cardiac rhythm devices, (4) other tobacco use with the exception of e-cigarettes, (5) current/planning pregnancy or lactation, (6) physical condition that limits use of wearable sensors or good readings of physiological measures, (7) current smoking cessation efforts, (8) a household member enrolled in the study, and (9) no prior experience using a smartphone. The data were collected at the Tobacco Research and Intervention Program at Moffitt Cancer Center. Recruitment started January 2019 and ended September 2020. The study protocol was approved by the institution’s institutional review board (Protocol # 19002) and registered in clinicaltrial.gov (NCT03404596).
2.3. Measures
2.3.1. Baseline characteristics
Participants completed self-report measures at each visit. Visit 1 measures reported in this paper include demographic information, a Tobacco History Questionnaire, Heaviness of Smoking Index (Heatherton et al., 1989), and two questionnaires developed for the current study: (1) prior experience using smartphones and wearable devices and (2) prior experience with mindfulness meditation practices.
2.3.2. Wearable sensors
The wearable sensor suite consisted of AutoSense (Ertin et al., 2011; Hovsepian et al., 2015; Saleheen et al., 2015), a noncommercial sensor suite, which included 4 sensors (a chest band/box with two electrodes to assess electrocardiography and respiration, and two wrist sensors to assess arm movement) and a smartphone (Samsung Galaxy S5) loaned to participants during the study (Hernandez et al., 2021). Negative affect (NA) was identified by physiological data - electrocardiogram and respiration data - and was categorized as low vs high based on previous work (Hovsepian et al., 2015). Specifically, AutoSense’s algorithm – cStress – utilizes a “physiological signature” that was trained using several emotionally, cognitively, and physically challenging laboratory stressors. Subsequent field testing demonstrated that the cStress physiological signature yielded 72 % accuracy in predicting self-reported NA. Thus, AutoSense was designed to detect physiological stress that was validated based on self-reported NA. Smoking behavior (i.e., smoking or not) was identified by respiration data and wrist movements and was categorized as no smoking vs smoking based on prior work showing 97 % accuracy in detecting smoking behavior (Saleheen et al., 2015). Participants were instructed to wear the sensors daily for up to 12 h over 2 weeks for the continuous assessment of NA and smoking.
2.3.3. Ecological momentary assessment (EMA) measures
EMAs collected data on state cognitive-affective constructs (e.g., mindfulness, affect, self-efficacy) and smoking behaviors. In the current paper, we only report the EMA completion rate. The protocol paper presents the full description of the EMA measures (Hernandez et al., 2021).
2.3.4. Feasibility
Retention and Adherence.
Retention was measured by attendance at in-person visits. Adherence included the wearing of sensors, completion of strategies and ecological momentary assessments (EMAs), and nicotine patch use.
Ease of Technology Use.
The System Usability Scale (SUS; Lewis & Sauro, 2009) assessed learnability and usability of the system at Visit 4 (Cronbach’s α = 0.77 in the current sample). A second measure assessing feasibility of technology developed for the study assessed the relative difficulty of using the smartphone and wearable sensors at the beginning (measured retrospectively at Visit 4) vs the end of the intervention (1 = very difficult, 6 = very easy), as well as the number of days it took to be comfortable wearing the sensors.
2.3.5. Acceptability
Acceptability was measured with the Client Satisfaction Questionnaire (CSQ; Larsen et al., 1979) at Visit 4 (α = 0.93 in the current sample). A questionnaire on the utility of mindfulness strategies (1 = not very useful, 6 = very useful) was developed for the study and administered at Visit 4, and a follow-up questionnaire on the use of mindfulness strategies at Visit 5. Mindfulness strategy ratings were collected following each strategy on a 5-star scale (1 star = did not like, 5 stars = liked a lot). The frequency of using on-demand mindfulness content was explored as part of acceptability. Participants’ feedback on the treatment was collected at Visit 4 using open-ended questions developed by the study team. Items queried the most and least favorite parts of treatment, barriers to using mindfulness, and general suggestions for improvement of the treatment.
2.3.6. Tobacco abstinence
Smoking status since the last visit was collected via the Timeline Followback (Sobell & Sobell, 1992) at Visits 2–5 and was verified via CO level with less than 6 ppm considered abstinent. The primary smoking outcome was biochemically-confirmed, seven-day point prevalence abstinence (PPA) at the end of treatment and follow-up visit.
2.4. Study procedures
2.4.1. Study Visits
The protocol paper (Hernandez et al., 2021) provides a full, detailed description of the study procedures. The study protocol and materials can be accessed by contacting the last author. Table 1 presents the participant flow through the study. In short, during the in-person orientation session, biochemical verification of smoking status was confirmed via CO level. Individuals who were deemed eligible by study staff at the orientation session scheduled a total of 5 in-person visits over 6 weeks. Four in-person visits occurred during the first 2 weeks following the in-person orientation session. Given the JITAI lasted for 2 weeks, participants wore the sensors during that time window to receive strategies from the phone. Participants were compensated for completing the in-person assessments ($10 at orientation, $30 for each of the Visits 1–3, $50 at Visits 4 and 5) and for completing EMAs/sensor wearing ($1.25 if they had worn the sensors at least 60 % of the time since the last EMA or $0.50 if not). Participants could earn up to $157.50 in total. See Supplementary Material 2 for modified COVID-19 procedures.
2.4.2. Just-in-Time Adaptive intervention (JITAI) and ecological momentary assessments (EMAs)
Below is a summary of the JITAI, along with the delivery rules for the JITAIs and EMAs. See the protocol paper (Hernandez et al., 2021) for further details.
JITAI Content.
Mindfulness and motivation-based strategies were delivered via the mCerebrum study app (Hossain et al., 2017) on the smartphone over the course of 2-weeks, from Visits 1 to 4. Mindfulness-based strategies were randomly drawn from a pool of 76 potential strategies; motivational messages were randomly drawn from a pool of 40 potential messages. Mindfulness-based strategies prompted participants to practice brief mindfulness strategies lasting 1–3 min and included content from 5 topic areas: breath, thoughts, sensations, acceptance/nonjudging, and craving. Motivation-based messages were included, based on previous research indicating the perceived helpfulness and appeal of such messages by smokers attempting to quit (Businelle et al., 2016; Hartzler et al., 2016; Yingst et al., 2018). These were brief statements encouraging participants to quit smoking/maintain abstinence. Participants could also self-initiate the strategies on demand via a button on the smartphone.
JITAI and EMA Delivery.
For the JITAI delivery, the day was organized into six, 2-hour blocks. The start time of the day varied per individual depending on when they began wearing the sensors. The six, 2-hour blocks were fixed once the day began. JITAIs were randomized to either be delivered or not based on key moments detected during continuous data collection via the wearable sensors (i.e., NA using the cStress algorithm, Hovsepian et al., 2015; smoking behavior [i.e., smoking or not] using the puffMarker algorithm, Saleheen et al., 2015). In other words, JITAIs were delivered contingent upon the detection of the key moments (i.e., NA, smoking behavior) via the sensors. Participants could receive up to 12 strategies per day. Completion of a mindfulness/motivational strategy was indicated based on the participants’ response to an item on the app that asked whether they completed it or not.
There were two types of EMAs: (1) Random EMAs delivered within 3, 4-hour blocks (up to 3 per day) and (2) EMAs that followed the randomization of JITAIs (i.e., prompted or unprompted). To limit burden, we only sent EMAs following randomization about 50 % of the time. EMAs did not have any role in triggering the JITAI. Regardless of the EMA type, all EMAs presented the same set of questions, which took less than 5 min to complete (Hernandez et al., 2021).
2.4.3. Smoking cessation counseling and nicotine patch
Brief smoking cessation counseling was consistent with the Treating Tobacco Use and Dependence Clinical Practice Guideline (Fiore et al., May 2008). Counseling was provided by a clinical psychology graduate student, a clinical social worker, and two clinical psychologists. Visits 1–3 also provided a brief introduction to mindfulness and played a 10-minute audio recording of a mindfulness meditation. The nicotine patch was provided from Visits 2 to 5, with participants receiving a total of 6 weeks of the patch (if smoking ≤10 CPD, 14 mg and if smoking >10 CPD, 21 mg).
2.5. Analytic plan
A priori benchmarks for feasibility were (Hernandez et al., 2021): ≥75 % retention through follow-up; ≥70 % of participants wearing the equipment at least 8 days, and of those participants, ≥60 % of strategies completed (i.e., adherent participants) and ≥ 70 % of EMAs completed; and ≥ 68 SUS total score. Acceptability was determined by ≥ 3 CSQ score. Descriptive analyses (e.g., mean, SDs, proportion) were conducted on the study variables including demographics and measures of feasibility and acceptability, as well as EMA completion rate and biochemically-verified abstinence.
We utilized a benchmark of ≥ 70 % of participants wearing the equipment for at least 8 days for several reasons. We expected that participants might be less adherent to wearing sensors over weekends or while they were becoming familiar with the equipment. Potential equipment failures and time to troubleshoot were also considered. Given these factors, we determined that the 8-day cutoff (i.e., the majority of the 14-day period) would be reasonable. Similarly, we decided that 70 % of participants would indicate that the majority wore the equipment and were engaged in the study.
Adherent participants were those who wore at least 2 sensors continuously for 120 min per day on at least 8 days. These participants wore the sensor suite not only for the majority of the 2 weeks, but also long enough within a day to presumably receive one strategy. Strategy completion was determined by participants’ clicking a button ‘completed’ on the phone screen.
3. Results
3.1. Sample characteristics
Table 2 presents demographic (58.1 % female; 16 % Hispanic/Latinx; 28 % Black/African American), smoking characteristics, and previous experience with technology and mindfulness practices. Results of a comparison of baseline characteristics between adherent and nonadherent participants is presented in Supplementary Material 3.
Table 2.
Sample characteristics at baseline. (N = 43).
| Variable | Mean (SD) or N (%) |
|---|---|
| Age | 49.07 (13.38) |
| Gender: Female | 25 (58.1 %) |
| Ethnicity: Hispanic/Latinx * | 7 (16.3 %) |
| Race | |
| White* | 31 (72.1 %) |
| Black/African American | 12 (27.9 %) |
| Marital Status | |
| Single | 16 (37.2 %) |
| Married/Living with significant other | 16 (37.2 %) |
| Other | 11 (25.6 %) |
| Education | |
| Less than high school | 4 (9.3 %) |
| High school diploma | 4 (9.3 %) |
| GED | 7 (16.3 %) |
| Some college/Undergraduate degree | 24 (55.8 %) |
| Graduate degree | 3 (7.0 %) |
| Annual Household Income: Under $30,000 | 24 (55.8 %) |
| Smoking Characteristics | |
| Cigarettes Per Day | 15.41 (7.62) |
| First Cigarette within 5 Minutes of Waking (n, %) | 13 (30.2 %) |
| Heaviness of Smoking Index (HSI) (M, SD) | 2.84 (1.33) |
| Previous Experience with Technology | |
| Using smartphone 5 or more years (n, %) | 34 (79.1 %) |
| Comfortable using a smartphone (1–6 point scale; M, SD) | 5.38 (1.10) |
| Wearable device (yes; n, %) | 13 (30.2 %) |
| Previous Experience with Mindfulness Meditation | |
| Current use | 4 (9.3 %) |
| Used in past, but not currently | 11 (25.6 %) |
| Never use | 26 (60.5 %) |
Note.
Due to missing ethnicity (n = 1) and race data (n = 1) at Visit 1, phone screen data was used for these individuals.
3.2. Feasibility
3.2.1. Retention
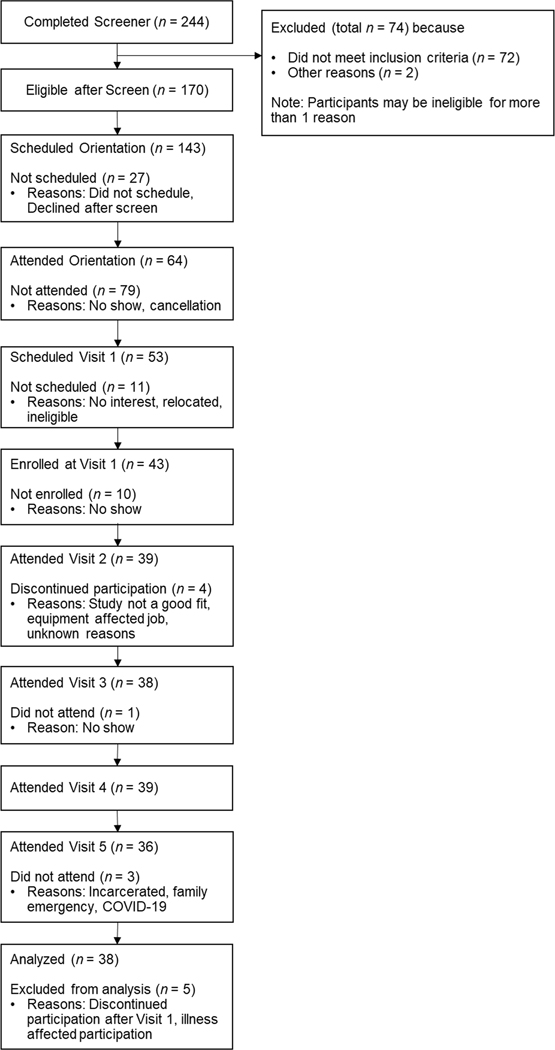
Fig. 1 presents the full CONSORT. Sixty-four daily smokers were deemed eligible per the phone screen and attended the orientation session. Of those, 43 consented to participate and attended Visit 1, among whom, 91 % completed Visit 2, 88 % completed Visit 3, 91 % completed Visit 4, and 84 % completed Visit 5, which met our benchmark criterion for retention. Between Visits 1 and 2, four participants dropped and one participant became ill and was unable to adhere to the treatment. These five participants were excluded from further analyses.
Fig. 1.
CONSORT diagram.
3.2.2. Adherence
Among the 38 participants, 24 participants (63 %) wore the sensors for at least 8 of the 14 days, and of those 24 participants, 16 (67 %) completed at least 60 % of the delivered strategies, indicating the adherence in our sample was slightly below our benchmark criterion. Table 3 presents results from the 38 participants and the subset of 16 with high treatment adherence.
Table 3.
Feasibility outcomes.
| Feasibility | N = 38 | n = 16§ |
|---|---|---|
| Adherence (n, %) | ||
| Visit completed | Visit 1: 43 Visit 2: 39 (90.7 %) Visit 3: 38 (88.4 %) Visit 4: 39 (90.7 %) Visit 5: 36 (83.7 %) |
– |
| Mindfulness/Motivational Strategies (completed / prompted) | ||
| Overall across conditions | 990/1480 (66.9 %) | 665/837 (79.5 %) |
| Negative Affect - High | 17/31 (54.8 %) | 10/19 (52.6 %) |
| Negative Affect – Low | 522/787 (66.3 %) | 328/416 (78.8 %) |
| Smoking - Yes | 4/5 (80.0 %) | 4/4 (100.0 %) |
| Smoking - No | 447/657 (68.0 %) | 323/398 (81.2 %) |
| On-Demand feature (M, SD) ¶ | 43.25 (123.9) | 61 (143.5) |
| EMAs (completed / prompted) | ||
| Random EMAs | 582/1129 (51.6 %) | 363/537 (67.6 %) |
| EMAs following strategies - Overall | 522/597 (87.4 %) | 346/387 (89.4 %) |
| Negative Affect – High | 8/8 (100.0 %) | 3/3 (100.0 %) |
| Negative Affect – Low | 267/309 (86.4 %) | 166/190 (87.4 %) |
| Smoking – Yes | 3/3 (100.0 %) | 3/3 (100.0 %) |
| Smoking – No | 244/277 (88.1 %) | 174/191 (91.1 %) |
| EMAs not following strategies - Overall | 566/894 (63.3 %) | 356/491 (72.5 %) |
| Negative Affect – High | 7/10 (70.0 %) | 3/3 (100.0 %) |
| Negative Affect – Low | 301/494 (60.9 %) | 190/263 (72.2 %) |
| Smoking – Yes | 2/2 (100.0 %) | 2/2 (100.0 %) |
| Smoking – No | 256/388 (66.0 %) | 161/223 (72.2 %) |
| Number of Days Wearing Sensors (M, SD) | 8.84 (4.52) | 12.44 (2.19) |
| Use of Nicotine Patch (n, %) | ||
| Visit 2 | 28 (73.7 %) | 12 (75.0 %) |
| Visit 3 | 34 (89.5 %) | 15 (93.8 %) |
| Visit 4 | 32 (84.2 %) | 14 (87.5 %) |
| Visit 5 | 23 (60.5 %) | 11 (68.8 %) |
| System Usability Scale (M, SD) + | 63.23 (17.01) | 66.39 (16.49) |
|
Feasibility of Technology Survey (1–6 scale; M, SD)
Difficulty using the smartphone: | ||
| Beginning of the study | 4.82 (1.59) | 5.31 (1.40) |
| Last day of using the smartphone | 4.87 (1.65) | 5.44 (1.09) |
| Difficulty using the wearable sensors: | ||
| Beginning of the study | 4.53 (1.57) | 4.69 (1.62) |
| Last day of wearing the sensors | 5.08 (1.40) | 5.12 (1.20) |
| No. days to be comfortable wearing sensors (M, SD; n and % never felt comfortable) | 2.08 (2.48); n = 8 (21.1 %) | 2.81 (3.37); n = 2 (12.5 %) |
Note.
Adherent participants are those who wore the sensors for at least 8 of the 14 days and completed at least 60 % of the delivered strategies.
Among n = 32 who used the on-demand feature at least once.
n = 14 (total sample) and n = 7 (adherent participants) were missing due to accidentally leaving the questionnaire out of the battery.
The algorithms of cStress and puffMarker did not accurately detect high NA and smoking, respectively. Specifically, the algorithm for cStress was too stringent and detected few high NA moments. For puffMarker, there was a mismatch of the orientation parameters within the wrist-worn inertial sensors, resulting in very few smoking episodes being detected. The puffMarker algorithm was developed with a specific orientation of a chip on the circuit board that resulted in a particular orientation of the x, y, and z axes of the accelerometer with respect to the wrist and gravity. However, when a newer version of the circuit board was used for the current study, the original mounting was not used, causing a different orientation of the x, y, and z axes of the accelerometer with respect to the wrist and gravity. Because the puffMarker algorithm was expecting a certain axis of the accelerometer to show change when the hand was raised to the mouth against gravity, it failed to pick up this motion as the monitored axis was no longer the one experiencing large change against gravity when the hand was raised to the mouth.
Regarding strategy completion, participants completed 67 % of the prompted strategies with 55 % and 66 % completion rate from the high and low NA conditions, respectively, and 80 % and 68 % completion rate from the smoking and no smoking conditions. For on-demand strategies, six (16 %) participants never used it, 20 (53 %) used it less than 10 times, eight (21 %) used it 10–29 times, and four (11 %) used it frequently (i.e., 79, 106, 489, and 526 times). Among those with high treatment adherence, the completion rate of the prompted strategies was generally higher: 80 % overall, 53 % for high NA, 79 % for low NA, 100 % for smoking, and 81 % for no smoking.
Overall the random EMA completion rate was 52 %. Regarding EMAs deployed following strategies, the completion rate was 87 % overall. The completion rate of EMAs without prompted strategies was 63 % overall. For those with high treatment adherence, the completion rate of random EMAs was higher (68 %). For EMAs following strategies, adherent participants completed 89 % overall. For the EMAs without prompted strategies, the overall completion rate was 73 %. Taken together, the completion rate of EMAs generally met our benchmark criterion although the random EMA completion rate was relatively low among both all participants and adherent participants. Table 3 provides an additional breakdown of EMAs by NA and smoking behavior.
3.2.3. In-Person measures
The mean total score of the SUS (M = 63.23, SD = 17.01) indicated that the system was generally easy to use and learn although the mean total score was below our a priori benchmark (i.e., ≥68). Participants indicated that the smartphone application was fairly easy at both pre- and post-treatment with nonsignificant change (t(37) = −0.19, p = .90), whereas there was a significant decrease in perceived difficulty in using the sensors over time (t(37) = −2.95, p = .01). Overall, participants reported taking 2 days to feel comfortable wearing the sensors, with 8 (21 %) participants reporting never feeling comfortable.
3.3. Acceptability
Table 4 presents acceptability results. At end of treatment, participants reported a high CSQ score (M = 3.61, SD = 0.49). Participants found delivered strategies helpful for managing both craving and stress, indicating that they would likely continue using the learned strategies in the future. At follow-up, 29 (76 %) of the entire sample, and 15 (94 %) of adherent participants continued using the mindfulness strategies. Participants rated strategies 883 times with 80 % rated as a four or five in low NA and no smoking conditions (M = 4.30, SD = 0.96 for low NA and M = 4.27, SD = 0.99 for no smoking) and 75 % rated as a four or five in the high NA and smoking conditions (M = 4.25, SD = 1.13 and M = 3.25, SD = 2.06). On-demand mindfulness content was rated a four or five 87 % of the time (M = 4.59, SD = 0.81).
Table 4.
Acceptability outcomes.
| Acceptability | N = 38 | n = 16§ |
|---|---|---|
| Client Satisfaction Questionnaire (1–4 scale; M, SD) | 3.61 (0.49) | 3.66 (0.52) |
| Utility of Mindfulness Strategies (1–6 scale; M, SD) | ||
| Helpful for craving | 4.68 (1.36) | 4.88 (1.41) |
| Helpful for stress | 4.55 (1.35) | 4.81 (1.47) |
| Likelihood of continuing to use strategies | 5.11 (1.27) | 5.25 (0.77) |
| Continued Use of Mindfulness Skills between V4 and V5 among those who completed V5 (n, %) | 29 (76.3 %) | 15 (93.8 %) |
Note.
Adherent participants are those who wore the sensors for at least 8 of the 14 days and completed at least 60 % of the delivered strategies.
Participant feedback on the intervention indicated that the number of mindfulness strategies sent each day and the variety of mindfulness strategies were “just right.” Participants also said that the mindfulness strategies helped them step back and pause when experiencing craving. Qualitative feedback on most/least favorite parts of the intervention, barriers, and areas for further improvement are reported in Supplementary Table 1.
3.4. Smoking outcomes
Biochemically-confirmed, seven-day PPA was 34 % (n = 13) among treatment completers at the end of treatment and 21 % (n = 8) at follow-up. Among treatment adherent participants (n = 16), abstinence was 38 % (n = 6) at the end of treatment and 25 % (n = 4) at follow-up. Among non-adherent participants (n = 22), abstinence was 32 % (n = 7) at the end of treatment and 22 % (n = 4) at follow-up. Although overall abstinence was descriptively higher among the adherent group, these differences did not reach significance.
4. Discussion
The current study examined the feasibility and acceptability of a multi-component JITAI for smoking cessation. Findings indicated that the feasibility of the treatment was somewhat mixed, whereas acceptability was high. Due to the sensors not appropriately detecting high NA and smoking moments, the feasibility of the current intervention in the high NA and smoking conditions was lower than expected. Among the subset of treatment adherent participants, feasibility and acceptability were higher. Exploratory unplanned analyses revealed that biochemically-confirmed tobacco abstinence at end of treatment and follow-up was slightly higher among those with greater treatment adherence, although statistically nonsignificant.
Feasibility was partially supported by both the high retention rate and perceived ease of technology. However, the percentage of participants wearing sensors (63 %) was lower than our benchmark criterion (70 %), which could be attributable to the technical issues encountered with the software. It is also possible that participants felt comfortable overall with the logistics of the technology (e.g., navigating the smartphone application, how to wear sensors) while simply not wanting to wear the sensors for an extended period of time. Regarding the strategies, the completion rate at times of low NA was higher than during high NA, suggesting lower intervention engagement during high NA. However, given the very few high NA episodes detected, these instances may not be representative of true high NA. Pertaining to EMAs, among adherent participants, EMA completion following JITAI randomization met our benchmark criterion, whereas the completion of random EMAs did not. These observations vary somewhat from the high completion of random EMAs observed in the previous EMA literature (e.g., >75 %), although the design of the current study is quite different (Schüz et al., 2013; Vinci et al., 2017). In the current study, participants may have been more likely to complete an EMA post-strategy due to being near/on the phone, whereas it is easier to miss random EMAs or those not linked to a strategy. Participants could complete more EMAs within a day in this study than is typical in other tobacco studies (e.g., 4–5 per day, Schüz et al., 2013; Shiyko et al., 2013; Vinci et al., 2017), so it is possible that participants reached a ceiling for completion. Regarding the ease of using the equipment, participants reported that the smartphone and sensors were easy to use and that their comfort level increased as the study progressed. Although overall usability of the technology (i.e., wearable sensors, smartphone application) as indexed by SUS was slightly below our benchmark (≥68), it is not surprising due to the complexity of the equipment. However, 14 participants’ SUS data were not collected, which should be considered when interpreting this score in light of the high level of perceived ease of using equipment at the end of treatment.
Acceptability was supported by high satisfaction reported on the CSQ, meeting our benchmark criterion (CSQ ≥ 3). Additionally, the mindfulness strategies were perceived as helpful for managing craving and stress at the end of treatment, which coincides with the high likability ratings of mindfulness strategies as collected in real-time. Along with high acceptability, participants’ feedback reflected theoretically proposed mechanisms of an MBI (i.e., a disruption of the automatic response to craving, Breslin et al., 2002; Brewer et al., 2013) and that the mindfulness strategies were delivered at the right time, when most needed.
Tobacco abstinence in the current study is comparable to previous mHealth smoking cessation JITAI studies, despite differences in study designs and intervention content. For example, JITAI studies that delivered tailored motivational messages to smokers of low SES reported biochemically verified 31 % abstinence 2-weeks post-quit (Businelle et al., 2016) and 22 % at 4-weeks post-quit (Businelle et al., 2016; Hébert et al., 2020).
Several limitations of the current study should be noted. First, there was no comparison condition, as comparison conditions are not typically employed in MRTs and proximal outcomes are priority. Second, technology-related issues combined with the complexity of equipment impacted the data quality. One of the most prominent issues was that the sensors rarely detected instances of high NA and smoking. Prior to a full launch of the study, 6 participants completed pilot testing to identify technology-related issues. Although issues with the detection of smoking behavior were identified and fixed, this did not prevent other issues from arising during the trial itself. Our experience reflects common challenges likely encountered in other studies utilizing sensing technology (Carpenter et al., 2020). In particular, despite the established accuracy of AutoSense used in our study, detecting relatively rare events (e.g., high NA) accurately is still a challenge when using sensors (e.g., Epstein et al., 2020; Nakajima et al., 2020). Participant adherence was also a challenge. Despite frequent monitoring of participants’ activities via a study dashboard and ongoing troubleshooting efforts, there were occasions when reasons for equipment failure were not identifiable. These technology issues, combined with other indicators of feasibility reported above, led us to qualify this treatment as “generally feasible.”
Given both the significant novelty and notable limitations of the current study, recommendations for future research are warranted. First, a pre-treatment acclimation period that allows participants to become familiar with sensors might aid in enhancing adherence for those without prior sensor experience, given our observation that adherent participants reported more prior experience with wearable sensors than nonadherent participants. Further, monitoring data quality should be considered as part of the pre-treatment acclimation period to allow for the early detection of any equipment failure and relevant troubleshooting. Second, as reflected in participants’ feedback, future studies would benefit from modifying the app (e.g., adding audio-recorded mindfulness strategies/meditations) and/or logistics of JITAI delivery. Third, improvement in sensing technology is warranted to reduce participant burden and enhance adherence (e.g., using only wrist sensors to detect high NA and smoking, (Skinner et al., 2019; Vinci et al., 2018)). We speculate that both the lack of a pre-treatment acclimation period and the equipment issues contributed to lower adherence among our participants. Future studies should consider these issues to improve adherence and to identify an ideal adherence rate. Fourth, as much as possible, the research team should anticipate and plan for software updates that could interrupt data collection. Fifth, despite prior validation of AutoSense (Hovsepian et al., 2015), future research may benefit from additional validation that more closely aligns with the NA/stress construct targeted for a given population and treatment.
The current study adds evidence to the growing body of mHealth literature in that brief mindfulness/motivational strategies delivered in the context of a JITAI are generally feasible and highly acceptable. Given the theoretical emphasis of mindfulness on cultivating awareness of the present moment and ongoing internal/external status, combined with our acceptability findings and open-ended feedback from participants, we believe that mindfulness-based JITAIs have substantial theoretical and practical implications for smoking cessation and that research in this area should further explore mindfulness moving forward. Sensor-based interventions for individuals motivated to quit smoking hold a promising future, given how quickly new technologies are evolving that will enable better detection of human behavior.
Supplementary Material
Acknowledgements
The findings reported in the current manuscript were partially presented at the annual conference of the 2021 Society for Research on Nicotine and Tobacco.
Role of funding Sources.
This work was supported by the National Institute on Minority Health and Health Disparities at the National Institutes of Health (R00MD010468), the National Institute of Biomedical Imaging and Bioengineering (U54EB020404) through funds provided by the trans-National Institutes of Health Big Data-to-Knowledge (BD2K) initiative and P41EB028242, and the Biostatistics and Bioinformatics Shared Resources at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (P30-CA076292: PI: J.L. Cleveland). MJY was supported by the National Cancer Institute training grant at the National Institutes of Health (T32CA090314). Funding sources noted above had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Clinical Trial Registration Details:
The current study design is registered in clinicaltrials.gov (NCT03404596): see https://clinicaltrials.gov/ct2/show/NCT03404596
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.addbeh.2022.107467.
Data availability
Data will be made available on request.
References
- Adams CE, Cano MA, Heppner WL, Stewart DW, Correa-Fernández V, Vidrine JI, Li Y, Cinciripini PM, Ahluwalia JS, & Wetter DW (2015). Testing a moderated mediation model of mindfulness, psychosocial stress, and alcohol use among African American smokers. Mindfulness, 6(2), 315–325. 10.1007/s12671-013-0263-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battalio SL, Conroy DE, Dempsey W, Liao P, Menictas M, Murphy S, Nahum-Shani I, Qian T, Kumar S, & Spring B. (2021). Sense2Stop: A micro-randomized trial using wearable sensors to optimize a just-in-time-adaptive stress management intervention for smoking relapse prevention. Contemporary Clinical Trials, 109, Article 106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, & Velting D. (2004). Mindfulness: A proposed operational definition. Clinical Psychology, 11(3), 230–241. 10.1093/clipsy/bph077 [DOI] [Google Scholar]
- Boland VC, Mattick RP, McRobbie H, Siahpush M, & Courtney RJ (2017). “I’m not strong enough; I’m not good enough. I can’t do this, I’m failing”: A qualitative study of low-socioeconomic status smokers’ experiences with accessing cessation support and the role for alternative technology-based support. International Journal of Equity Health, 16(1), 1–11. 10.1186/s12939-017-0689-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland VC, Mattick RP, Siahpush M, Barker D, Doran CM, Martire KA, Bonevski B, McRobbie H, Borland R, & Farrell M. (2019). Factors associated with Quitline and pharmacotherapy utilisation among low-socioeconomic status smokers. Addictive Behaviors, 89, 113–120. 10.1016/j.addbeh.2018.09.029 [DOI] [PubMed] [Google Scholar]
- Breslin FC, Zack M, & McMain S. (2002). An information-processing analysis of mindfulness: Implications for relapse prevention in the treatment of substance abuse. Clinical Psychology, 9(3), 275–299. 10.1093/clipsy.9.3.275 [DOI] [Google Scholar]
- Brewer JA, Elwafi HM, & Davis JH (2013). Craving to quit: Psychological models and neurobiological mechanisms of mindfulness training as treatment for addictions. Psychology of Addictive Behaviors, 27(2), 366–379. 10.1037/a0028490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, Minnix-Cotton CA, Byrne SA, Kober H, & Weinstein AJ (2011). Mindfulness training for smoking cessation: Results from a randomized controlled trial. Drug and Alcohol Dependence, 119(1–2), 72–80. 10.1016/j.drugalcdep.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Mann SL, Marek PM, Liu J, & Peterson AV (2010). Telephone-delivered acceptance and commitment therapy for adult smoking cessation: A feasibility study. Nicotine & Tobacco Research, 12(4), 454–458. [DOI] [PubMed] [Google Scholar]
- Businelle MS, Ma P, Kendzor DE, Frank SG, Vidrine DJ, & Wetter DW (2016). An ecological momentary intervention for smoking cessation: Evaluation of feasibility and effectiveness. Journal of Medicines Internet Research, 18(12), Article e321. 10.2196/jmir.6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambron C, Haslam AK, Baucom BR, Lam C, Vinci C, Cinciripini P, Li L, & Wetter DW (2019). Momentary precipitants connecting stress and smoking lapse during a quit attempt. Health Psychology, 38(12), 1049–1058. 10.1037/hea0000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter SM, Menictas M, Nahum-Shani I, Wetter DW, & Murphy SA (2020). Developments in Mobile Health Just-in-Time Adaptive Interventions for Addiction Science. Current addiction reports, 7(3), 280–290. 10.1007/s40429-020-00322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland AL, Businelle MS, Stewart DW, Patterson SM, Rash CJ, & Carney CE (2010). Identifying barriers to entering smoking cessation treatment among socioeconomically disadvantaged smokers. Journal of Smoking Cessation, 5(2), 164–171. 10.1375/jsc.5.2.164 [DOI] [Google Scholar]
- Dai X, Gakidou E, & Lopez AD (2022). Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tobacco Control, 31(2), 129–137. [DOI] [PubMed] [Google Scholar]
- Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, & Baker TB (2014). Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Substance Use & Misuse, 49(5), 571–585. 10.3109/10826084.2013.770025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Tyburski M, Kowalczyk WJ, Burgess-Hull AJ, Phillips KA, Curtis BL, & Preston KL (2020). Prediction of stress and drug craving ninety minutes in the future with passively collected GPS data. npj Digital Medicine, 3(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertin E, Stohs N, Kumar S, Raij A, Al’Absi M, & Shah S. (2011). AutoSense: Unobtrusively wearable sensor suite for inferring the onset, causality, and consequences of stress in the field. Proceedings of the 9th ACM Conference on Embedded Networked Sensor Systems. [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, . . . Wewers ME (May 2008). Treating tobacco use and dependence: 2008 update. In Clinical Practice Guideline. US Department of Health and Human Services. Public Health Service. https://www.ncbi.nlm.nih.gov/books/NBK63952/. [Google Scholar]
- Garrison KA, Pal P, O’Malley SS, Pittman BP, Gueorguieva R, Rojiani R, Scheinost D, Dallery J, & Brewer JA (2020). Craving to quit: A randomized controlled trial of smartphone app–based mindfulness training for smoking cessation. Nicotine & Tobacco Research, 22(3), 324–331. 10.1093/ntr/nty126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Bartolomei R, Colby SM, & Kahler CW (2008). Ecological momentary assessment of adolescent smoking cessation: A feasibility study. Nicotine & Tobacco Research, 10(7), 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler AL, BlueSpruce J, Catz SL, & McClure JB (2016). Prioritizing the mHealth design space: A mixed-methods analysis of smokers’ perspectives. JMIR Mhealth Uhealth, 4(3), Article e95. 10.2196/mhealth.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, & Robinson J. (1989). Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction, 84(7), 791–800. 10.1111/j.1360-0443.1989.tb03059.x [DOI] [PubMed] [Google Scholar]
- Hébert ET, Ra CK, Alexander AC, Helt A, Moisiuc R, Kendzor DE, Vidrine DJ, Funk-Lawler RK, & Businelle MS (2020). A mobile Just-in-Time Adaptive Intervention for smoking cessation: Pilot randomized controlled trial. Journal of Medicine Internet Research, 22(3), Article e16907. 10.2196/16907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert ET, Stevens EM, Frank SG, Kendzor DE, Wetter DW, Zvolensky MJ, Buckner JD, & Businelle MS (2018). An ecological momentary intervention for smoking cessation: The associations of just-in-time, tailored messages with lapse risk factors. Addictive Behaviors, 78, 30–35. 10.1016/j.addbeh.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Wetter DW, Kumar S, Sutton S, & Vinci C. (2021). Smoking cessation using wearable sensors: Protocol for a microrandomized trial. JMIR Research Protocols, 10(2), Article e22877. 10.2196/22877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog MA (2008). Considerations in determining sample size for pilot studies. Research in Nursing & Health, 31(2), 180–191. 10.1002/nur.20247 [DOI] [PubMed] [Google Scholar]
- Horvath M, Grutman A, O’Malley SS, Gueorguieva R, Khan N, Brewer JA, & Garrison KA (2021). Smartband-Based Automatic Smoking Detection and Real-time Mindfulness Intervention: Protocol for a Feasibility Trial. JMIR Research Protocols, 10(11), Article e32521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain SM, Hnat T, Saleheen N, Nasrin NJ, Noor J, Ho BJ, Condie T, Srivastava M, & Kumar S. (2017, November). mCerebrum: A mobile sensing software platform for development and validation of digital biomarkers and interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovsepian K, Al’Absi M, Ertin E, Kamarck T, Nakajima M, & Kumar S. (2015). cStress: towards a gold standard for continuous stress assessment in the mobile environment. Proceedings of the 2015 ACM international joint conference on pervasive and ubiquitous computing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J, Cerrada CJ, Dzubur E, Dunton GF, Spruijt-Metz D, & Leventhal AM (2021). Effect of a mobile just-in-time implementation intention intervention on momentary smoking lapses in smoking cessation attempts among Asian American young adults. Translational Behaviours Medicines, 11(1), 216–225. 10.1093/tbm/ibz183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizakevich PN, Eckhoff R, Brown J, Tueller SJ, Weimer B, Bell S, Weeks A, Hourani LL, Spira JL, & King LA (2018). PHIT for duty, a mobile application for stress reduction, sleep improvement, and alcohol moderation. Military Medicine, 183(suppl_1), 353–363. [DOI] [PubMed] [Google Scholar]
- Klasnja P, Smith S, Seewald NJ, Lee A, Hall K, Luers B, Hekler EB, & Murphy SA (2019). Efficacy of contextually tailored suggestions for physical activity: A micro-randomized optimization trial of HeartSteps. Annals of Behavioral Medicine, 53(6), 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Brewer JA, Height KL, & Sinha R. (2017). Neural stress reactivity relates to smoking outcomes and differentiates between mindfulness and cognitive-behavioral treatments. NeuroImage, 151, 4–13. 10.1016/j.neuroimage.2016.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KM, Luberto CM, Hall DL, Ngo LH, & Yeh GY (2020). A systematic review and meta-analysis of mindfulness-and acceptance-based interventions for affect intolerance/sensitivity. Behaviour Research and Therapy, 103746. 10.1016/j.brat.2020.103746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen DL, Attkisson CC, Hargreaves WA, & Nguyen TD (1979). Assessment of client/patient satisfaction: Development of a general scale. Eval Program Plann, 2(3), 197–207. 10.1016/0149-7189(79)90094-6 [DOI] [PubMed] [Google Scholar]
- Lewis J, & Sauro J. (2009). The factor structure of the System UsabilityScale Proceedings of the 1st International Conference on Human Centered Design: Held as Part of HCI International 2009, San Diego, CA. [Google Scholar]
- Nahum-Shani I, Potter LN, Lam CY, Yap J, Moreno A, Stoffel R, Wu Z, Wan N, Dempsey W, & Kumar S. (2021). The mobile assistance for regulating smoking (MARS) micro-randomized trial design protocol. Contemporary clinical trials, 110, Article 106513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, & Murphy SA (2018). Just-in-time adaptive interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine, 52(6), 446–462. 10.1007/s12160-016-9830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Lemieux AM, Fiecas M, Chatterjee S, Sarker H, Saleheen N, Ertin E, Kumar S, & Al’Absi M. (2020). Using novel mobile sensors to assess stress and smoking lapse. International Journal of Psychophysiology, 158, 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton F. (2017). Delivering “Just-In-Time” smoking cessation support via mobile phones: Current knowledge and future directions. Nicotine & Tobacco Research, 19 (3), 379–383. 10.1093/ntr/ntw143 [DOI] [PubMed] [Google Scholar]
- Perski O, Hébert ET, Naughton F, Hekler EB, Brown J, & Businelle MS (2021). Technology-mediated just-in-time adaptive interventions (JITAIs) to reduce harmful substance use: A systematic review. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter LN, Haaland BA, Lam CY, Cambron C, Schlechter CR, Cinciripini PM, & Wetter DW (2021). A time-varying model of the dynamics of smoking lapse. Health Psychology, 40(1), 40–50. 10.1037/hea0001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleheen N, Ali AA, Hossain SM, Sarker H, Chatterjee S, Marlin B, Ertin E, Al’Absi M, & Kumar S. (2015). puffMarker: a multi-sensor approach for pinpointing the timing of first lapse in smoking cessation. Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing. [PMC free article] [PubMed] [Google Scholar]
- Schüz N, Walters JA, Frandsen M, Bower J, & Ferguson SG (2013). Compliance with an EMA monitoring protocol and its relationship with participant and smoking characteristics. Nicotine & Tobacco Research, 16(Suppl_2), S88–S92. 10.1093/ntr/ntt142 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Shiyko M, Naab P, Shiffman S, & Li R. (2013). Modeling complexity of EMA data: Time-varying lagged effects of negative affect on smoking urges for subgroups of nicotine addiction. Nicotine & Tobacco Research, 16(Suppl_2), S144–S150. 10.1093/ntr/ntt109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner AL, Stone CJ, Doughty H, & Munafò MR (2019). StopWatch: The preliminary evaluation of a smartwatch-based system for passive detection of cigarette smoking. Nicotine & Tobacco Research, 21(2), 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back. In Measuring Alcohol Consumption (pp. 41–72). Humana Press. [Google Scholar]
- Spears CA, Hedeker D, Li L, Wu C, Anderson NK, Houchins SC, Vinci C, Hoover DS, Vidrine JI, & Cinciripini PM (2017). Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. Journal of Consulting and Clinical Psychology, 85 (11), 1029–1040. 10.1037/ccp0000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle-Degnen L. (2013). Nuts and bolts of conducting feasibility studies. American Journal of Occupational Therapy, 67(2), 171–176. 10.5014/ajot.2013.006270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2014). The health consequences of smoking—50 years of progress: a report of the Surgeon General (US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Issue. [Google Scholar]
- Vinci C, Haslam A, Lam CY, Kumar S, & Wetter DW (2018). The use of ambulatory assessment in smoking cessation. Addictive Behaviors, 83, 18–24. 10.1016/j.addbeh.2018.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci C, Li L, Wu C, Lam CY, Guo L, Correa-Fernández V, Spears CA, Hoover DS, Etcheverry PE, & Wetter DW (2017). The association of positive emotion and first smoking lapse: An ecological momentary assessment study. Health Psychology, 36(11), 1038–1046. 10.1037/hea0000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2021). Tobacco. https://www.who.int/news-room/fact-sheets/detail/tobacco.
- Yingst JM, Veldheer S, Hrabovsky S, Hammett E, Nicholson J, Berg A, & Foulds J. (2018). Pilot randomized trial of an automated smoking cessation intervention via mobile phone text messages as an adjunct to varenicline in primary care. Journal of Health Communications, 23(4), 370–378. 10.1080/10810730.2018.1453890 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.