Abstract
Background
Fatigue is a commonly reported and severe symptom in primary brain tumor patients, but the exact occurrence in meningioma patients is unknown. This study aimed to determine the frequency and severity of fatigue in meningioma patients as well as associations between the level of fatigue and patient-, tumor-, and treatment-related factors.
Methods
In this multicenter cross-sectional study, meningioma patients completed questionnaires on fatigue (MFI-20), sleep (PSQI), anxiety and depression (HADS), tumor-related symptoms (MDASI-BT), and cognitive functioning (MOS-CFS). Multivariable regression models were used to evaluate the independent association between fatigue and each patient-, tumor-, and treatment-related factor separately, corrected for relevant confounders.
Results
Based on predetermined in- and exclusion criteria, 275 patients, on average 5.3 (SD = 2.0) year since diagnosis, were recruited. Most patients had undergone resection (92%). Meningioma patients reported higher scores on all fatigue subscales compared to normative data and 26% were classified as fatigued. Having experienced a complication due to resection (OR 3.6, 95% CI: 1.8–7.0), having received radiotherapy (OR 2.4, 95% CI: 1.2–4.8), a higher number of comorbidities (OR 1.6, 95% CI: 1.3–1.9) and lower educational level (low level as reference; high level OR 0.3, 95% CI: 0.2–0.7) were independently associated with more fatigue.
Conclusions
Fatigue is a frequent problem in meningioma patients even many years after treatment. Both patient- and treatment-related factors were determinants of fatigue, with the treatment-related factors being the most likely target for intervention in this patient population.
Keywords: central nervous system tumor, fatigue, long-term, meningioma, patient-reported outcome
Key Points.
Fatigue is a frequent problem in meningioma patients on the long term.
Important determinants of fatigue in meningioma patients are treatment-related.
Importance of the Study.
While some small studies have evaluated fatigue in meningioma patients, larger studies on this topic are lacking. Furthermore, little is known about the occurrence of fatigue in long-term meningioma patients as well as factors influencing fatigue in this particular patient population.In this study, we describe the frequency and severity of fatigue in meningioma patients on average > 5 year since diagnosis, showing that fatigue is a clinically relevant problem even many years after treatment. Moreover, we identified several determinants of fatigue in this patient population, including having experienced a complication due to resection, having received radiotherapy, a higher number of comorbidities and lower educational level. This information may help clinicians better understand the cause of fatigue in meningioma patients, and may help to identify possible targets of intervention which may result in reduced levels of fatigue. Ultimately, this may lead to an improved level of health-related quality of life.
Meningiomas are the most frequent primary central nervous system tumor, accounting for 38.3% of the cases.1 Meningiomas are classified according to the World Health Organization Classification of Tumors of the Central Nervous System into three grades: WHO grade 1 (benign), WHO grade 2 (atypical), and WHO grade 3 (anaplastic).2 Depending on their intracranial location, meningioma patients may present with focal neurological symptoms, seizures, motor deficits, cranial nerve deficits, cognitive deficits, personality changes, and/or psychosocial problems.3–5
Fatigue has been reported as the most common and severe symptom in patients with a primary brain tumor during the entire disease course.5–8 However, since these studies mainly included glioma patients, little is known about the occurrence and severity of fatigue in meningioma patients. A recent small study investigating fatigue in meningioma patients before and 1 year after surgery showed that patients reported high levels of fatigue9 and another small study found that meningioma patients assessed fatigue as the most important health-related quality of life (HRQoL) issue.10 These findings suggest that fatigue may be a relevant symptom in meningioma patients as well.
Fatigue can be caused by the emotional and physical consequences of the diagnosis11 and due to side effects of medication usage, such as antiepileptic drugs and corticosteroids.12 Furthermore, fatigue has many associations and can occur in a cluster of symptoms with sleep-wake disturbances, depression, anxiety, and cognitive symptoms.13–15 There are many theories for the development of fatigue in primary brain tumor patients. Cytokine dysregulation, particularly elevated levels of IL-1 and IL-6, can lead to a stimulation of the hypothalamic-pituitary-adrenal axis (HPA-axis), resulting in inflammatory-related fatigue and excessive daytime sleepiness.11,16 Moreover, radiotherapy can cause endocrine dysfunction and neuro-inflammation leading to aberrant cytokine and neurotransmitter production and hormone dysregulation, resulting in fatigue.6,17–19
While some small studies have evaluated the frequency of fatigue in meningioma patients, large studies on this topic are lacking, including data on the longer term and factors influencing fatigue. Increasing this knowledge may result in the identification of interventions targeting fatigue in this patient population, possibly resulting in an improved HRQoL. This study aimed to assess the frequency and severity of fatigue in meningioma patients, and to identify determinants of fatigue (etiological research), by determining the association between the overall level of fatigue and each patient-, tumor- and treatment-related factor.
Materials and Methods
Study Design and Patients
A cross-sectional study was performed in three large medical centers in the Netherlands; the Leiden University Medical Center (LUMC) in Leiden, the Haaglanden Medical Center (HMC) in The Hague, and the Erasmus Medical Center (EMC) in Rotterdam. Patients were eligible if they (1) were clinically (Magnetic Resonance Imaging) or histopathologically diagnosed with a meningioma WHO grade 1 or 2 between 2009 and 2017 (according to the WHO 2016 classification20), (2) were 18 year or older, and (3) had given written informed consent. Patients were excluded if they (1) were not fluent in Dutch or English, (2) had physical or mental conditions (as determined by the treating physician) interfering with the understanding and completion of patient-reported outcome measures (PROMs), and/or (3) were diagnosed with neurofibromatosis type II. All patients from the participating centers were approached for participation, irrespective of treatment history and moment in the disease trajectory. Approval for this study was obtained from the respective medical ethics committees (LUMC: P17.190, HMC: 2018-004, EMC: MEC-2018-1405).
Data Collection
Information on tumor- and treatment-related characteristics (ie, tumor size, time since treatment) were obtained from patients’ medical records. All tumor sizes were measured unidimensionally1 and classified, similar to previous studies in meningioma patients,21–26 by one researcher to ensure uniformity of measurement. Comorbidities were classified according to the Charlson Comorbidity Index (CCI),27 and complications of treatment were based on physicians’ descriptions in the medical charts and categorized independent of severity (yes/no variable). Information on sociodemographic characteristics were obtained or verified by telephone interview. In addition, patients completed PROMs on fatigue (MFI-20), sleep (PSQI), anxiety and depression (HADS), tumor-related symptoms (MDASI-BT), and subjective cognitive functioning (MOS-CFS). Detailed information regarding the questionnaires can be found in Supplementary Table 1.
Statistical Analysis
Sociodemographic and clinical characteristics, as well as scores on the questionnaires were described by means of descriptive statistics. The extent of response bias was assessed with a nonresponse analysis comparing selected baseline characteristics of participating and nonparticipating patients.
Using a similar cutoff as for the different dimensions of fatigue, fatigue was defined as a Z-score ≥ 1.5 below the general Dutch population28 on the MFI-20 summary score and a minimal clinically important difference of two points was used for each subscale as recommended by Purcell et al. (2010).29 This dichotomized outcome allowed to investigate determinants of clinically relevant fatigue. The MFI-20 summary score has shown to be a valid measure in people with fatiguing illness.30 Fatigue prevalence was calculated for the entire population as well as for several clinically relevant subgroups (ie, 0–5 year vs. >5 year since diagnosis and treatment modality), for which scores were compared using chi-square tests. Next, scores on the questionnaires were compared with normative data (i.e. general Dutch population28 for the MFI-20, general German population31 for the PSQI, low-grade glioma32 for the MDASI-BT, Medical Outcomes Study33 for the MOS-CFS, and the general Dutch population34 for the HADS) using a 2-tailed 1-sample t-test.
To identify determinants of fatigue, the association between the overall level of fatigue and each patient-, tumor- and treatment-related factor was determined using a multivariable logistic regression model. Each relevant sociodemographic and clinical characteristic was included as an independent variable in a separate model, corrected for its specific confounders. Confounders were selected prior to each analysis and were defined as associated with both the determinant and outcome, but not lying in the causal path between the determinant and outcome. The selection of confounders was based on available information in the literature and expert opinion. In case of high correlation between confounders, the most relevant and/or most practical variable to assess in clinical practice was selected, based on consensus between authors. Information regarding the selected confounders for each association can be found in Supplementary Table 2. Because of the exploratory nature, no adjustments were made for multiple comparisons.
All statistical analyses were performed with SPSS 27.0 for Windows and a P-value < 0.05 was considered statistically significant.
Results
Patient Characteristics
In total, 552 patients met the inclusion criteria and were approached for study participation of which 275 (49.8%) patients provided informed consent to participate. Patient characteristics are presented in Table 1. Patients were on average 61.9 (SD 11.5) year old and 5.3 (SD 2.0) year since diagnosis. The majority of patients was female (73%). Moreover, 40% of the patients had a tumor located at the skull base. Most patients had undergone resection (92%), 18% of the patients had received radiotherapy, and 6% of the patients had not undergone any treatment and were currently in a wait-and-scan trajectory. Patients were on average 4.3 (SD 2.0) year since treatment. More detailed patient characteristics are presented in Supplementary Table 3.
Table 1.
Sociodemographic and clinical characteristics
| Patient characteristics (n = 275) | No. | % |
|---|---|---|
| Sex | ||
| Male | 73 | 27 |
| Female | 202 | 73 |
| Age (years) (n = 275) | ||
| Mean (SD) | 61.9 (11.5) | |
| Time since diagnosis (years, n = 274) | ||
| Mean (SD) | 5.3 (2.0) | |
| 0–5 year | 116 | 42 |
| >5 year | 158 | 57 |
| Unknown | 1 | 0 |
| Total number of meningioma present during study participation | ||
| 0 (complete resection) | 150 | 55 |
| 1 | 91 | 33 |
| 2 | 13 | 5 |
| ≥3 | 9 | 3 |
| Unknown | 12 | 4 |
| Tumor location | ||
| Skull basea | 110 | 40 |
| Cerebral convexity | 72 | 26 |
| Falx | 39 | 14 |
| Multiple locations | 29 | 11 |
| Other | 24 | 9 |
| Unknown | 1 | 0 |
| Tumor lateralization | ||
| Left | 100 | 36 |
| Right | 109 | 40 |
| Midline | 43 | 16 |
| Multiple locations | 23 | 8 |
| Tumor size at the time of study participationb | ||
| No tumor (complete resection) | 150 | 55 |
| <40 mm | 102 | 37 |
| ≥40 mm | 9 | 3 |
| Unknown | 14 | 5 |
| Underwent resection | ||
| Yes | 254 | 92 |
| No | 21 | 8 |
| Underwent re-resection | ||
| Yes | 23 | 9 |
| No | 231 | 91 |
| Complications after resection | ||
| Yes | 74 | 29 |
| No | 179 | 70 |
| Unknown | 1 | 0 |
| Simpson grade most recent resection | ||
| I | 61 | 24 |
| II | 111 | 43 |
| III | 33 | 13 |
| IV | 34 | 13 |
| Unknown | 15 | 6 |
| WHO grade most recent resection | ||
| 1 | 216 | 85 |
| 2 | 27 | 11 |
| 3c | 1 | 0 |
| Unknown/not clear | 10 | 4 |
| Underwent radiotherapy | ||
| Yes | 50 | 18 |
| No | 225 | 82 |
| Most recent anti-tumor treatment | ||
| Resection | 214 | 78 |
| Radiotherapy | 44 | 16 |
| No treatment | 17 | 6 |
| Time since (most recent) treatment (years; n = 275) | ||
| Mean (SD) | 4.3 (2.0) | |
| KPS | ||
| <70 | 15 | 5 |
| ≥70 | 260 | 95 |
| Marital status | ||
| Married | 188 | 68 |
| Divorced | 25 | 9 |
| Single | 21 | 8 |
| Widow/widower | 19 | 7 |
| Living together with partner | 20 | 7 |
| Partner, not living together | 2 | 1 |
| Educational level | ||
| Low | 93 | 34 |
| Intermediate | 91 | 33 |
| High | 91 | 33 |
| Profession | ||
| Working | 102 | 37 |
| Not working but able to work | 18 | 7 |
| Not able to work | 37 | 13 |
| Retired | 117 | 43 |
| Unknown | 1 | 0 |
| Seizures in the past 3 months | ||
| 0 | 256 | 93 |
| ≥1 | 15 | 5 |
| Unknown | 4 | 1 |
| Total number of comorbidities | ||
| 0 | 116 | 42 |
| 1 | 60 | 22 |
| 2 | 43 | 16 |
| ≥3 | 56 | 20 |
No., number; n, number; SD, standard deviation; WHO, World Health Organization; KPS, Karnofsky Performance Status.
Percentages may not total 100% due to rounding.
a Skull base tumors were classified according to Al Mefty’s definition of skull base meningioma. 26
b A cutoff of 40 mm was used, similar to previous studies in meningioma patients. 21–26
c At the time of study inclusion WHO 2, however the patient was shortly operated on before filling in the questionnaires after which the pathology showed a WHO 3 meningioma.
Patients who were eligible but did not participate were on average older (64.7 vs. 61.9 year, P = 0.007) and more often did not receive any tumor treatment (13% vs. 6%, P = 0.008). No differences were found between eligible patients who did and did not participate regarding sex, time since diagnosis, tumor location and lateralization and time since treatment (see Supplementary Table 4 for patient characteristics of nonparticipants).
Fatigue Frequency and Severity
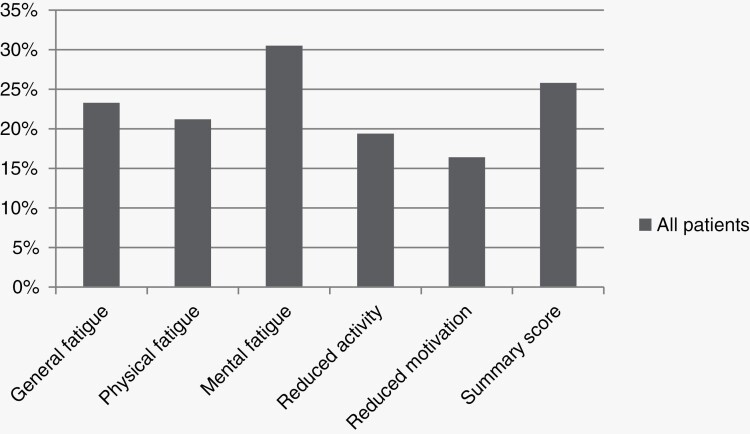
Patients’ mean score on the MFI-20 ranged between 9.9 and 11.9 for the different fatigue subscales (Table 2). Patients had a clinically relevant higher score on the general fatigue, physical fatigue and mental fatigue subscale and a significantly higher score on all fatigue subscales compared to the general Dutch population28 (mean difference range: 1.2–2.4, all P-values < 0.001). Twenty-six percent of the patients were classified as being fatigued (ie, score ≥ 1.5 SD below the general Dutch population on the MFI-20 summary score). Regarding the fatigue subscales, 31% of patients reported being mentally fatigued, 23% and 21% reported general fatigue and physical fatigue, respectively, whereas 16% of patients suffered from reduced motivation and 19% from reduced activity (Figure 1). Based on the results of the MDASI-BT, patients more often reported severe fatigue (25%) than moderate fatigue (17%).
Table 2.
Scores on the MFI-20, PSQI, MDASI-BT, MOS-CFS, HADS
| Measure | Mean (SD) | n= (%) | One sample t-test compared with normative data; mean difference (95% CI) |
|---|---|---|---|
| MFI-20 |
Clinically relevant fatigue
(Z-score ≥ 1.5) |
General Dutch population 28 | |
| General Fatigue (n = 275) | 11.9 (5.1) | 64 (23%) | 2.1 (1.5;2.7), P < 0.001 |
| Physical Fatigue (n = 273) | 10.9 (5.0) | 58 (21%) | 2.1 (1.5;2.6), P < 0.001 |
| Mental Fatigue (n = 275) | 10.7 (4.7) | 84 (31%) | 2.4 (1.9;3.0), P < 0.001 |
| Reduced Activity (n = 273) | 10.9 (4.9) | 53 (19%) | 1.6 (1.0;2.2), P < 0.001 |
| Reduced Motivation (n = 275) | 9.9 (4.4) | 45 (16%) | 1.2 (0.6;1.7), P < 0.001 |
| Summary Score (n = 271) | 54.4 (20.8) | 70 (26%) | 9.5 (7.0;11.9), P < 0.001 |
| PSQI | Severe difficulty | General German population 31 | |
| Subjective Sleep Quality (n = 275) | 1.0 (0.7) | 9 (3 %) | −0.1 (−0.2; −0.03), P = 0.011 |
| Sleep Latency (n = 270) | 1.1 (1.0) | 43 (16%) | 0.1 (0;0.2), P = 0.2 |
| Sleep Duration (n = 275) | 0.8 (0.8) | 15 (6%) | 0.2 (0.1;0.3), P < 0.001 |
| Habitual Sleep Efficiency (n = 266) | 0.9 (1.1) | 34 (13%) | 0.2 (0.1;0.4), P = 0.001 |
| Sleep Disturbances (n = 255) | 1.2 (0.6) | 4 (2%) | 0.6 (0.6;0.7), P < 0.001 |
| Use of Sleeping Medication (n = 275) | 0.4 (0.9) | 24 (9%) | 0.3 (0.2;0.4), P < 0.001 |
| Daytime Dysfunction (n = 273) | 0.9 (0.8) | 12 (4%) | 0.1 (0.1;0.2), P = 0.004 |
| Global Score (n = 240) | 6.3 (4.0) | 1.3 (0.7;1.8), P < 0.001 | |
| MDASI-BT | Symptom severity/interference | Glioma patients 32 | |
| Total Symptom Severity (n = 273) | 1.6 (1.5) | 8 (3 % moderate symptoms) 1 (0.4% severe symptoms) |
0.5 (0.4;0.7), P < 0.001 |
| Symptom Interference (n = 272) | 2.1 (2.4) | 30 (11% moderate interference) 17 (6% severe interference) |
1.5 (1.2;1.8), P < 0.001 |
| MOS-CFS (n = 273) | 77.4 (20.7) |
Normative data from the MOS Study
33
−3.5(−6.0; −1.1), P = 0.005 |
|
| HADS | Borderline/suspected disorder | General Dutch population 34 | |
| Anxiety (n = 273) | 5.1 (4.0) | 42 (15% borderline anxiety) 30 (11% suspected anxiety) |
1.2 (0.8;1.7), P < 0.001 |
| Depression (n = 273) | 4.4 (4.3) | 24 (9% borderline depression) 33 (12% suspected depression) |
0.7 (0.2;1.2), P = 0.008 |
n, number; SD, standard deviation: CI, confidence interval: MFI, Multidimensional Fatigue Inventory: PSQI, Pittsburgh Sleep Quality Index: MDASI-BT, MD Anderson Symptom Inventory—Brain Tumor Module; MOS-CFS, Medical Outcomes Study Cognitive Functioning Scale, HADS, Hospital Anxiety and Depression Scale.
Statistically significant differences are outlined in bold.
Figure 1.
Fatigue prevalence in all meningioma patients.
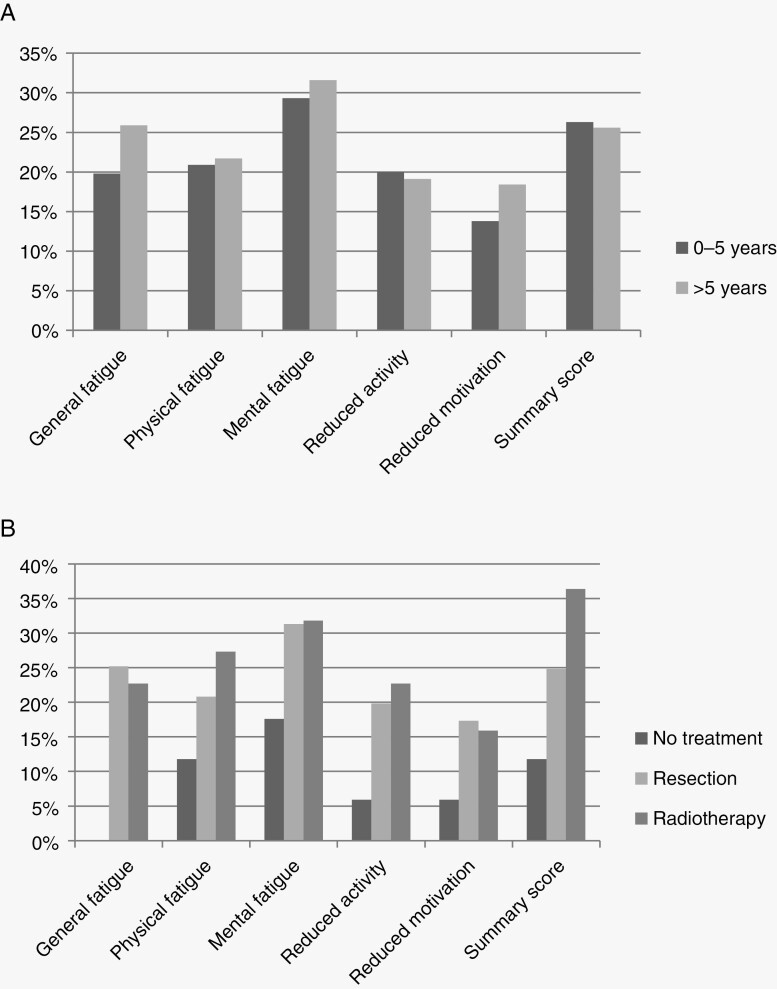
We compared fatigue prevalences between several relevant subgroups of patients. First, when comparing patients 0–5 year and > 5 year since diagnosis, no significant difference was found for fatigue prevalence (Figure 2A). Higher fatigue prevalences were seen in patients who had undergone treatment compared to patients who had not received any treatment, however these differences were not statistically significant (Figure 2B).
Figure 2.
(A) Fatigue prevalence in meningioma patients 0–5 year and > 5 year since diagnosis: No statistically significant differences between patients 0–5 year and > 5 year since diagnosis. (B) Fatigue prevalence in meningioma patients separately for most recent treatment modality: No statistically significant differences between the different treatment modalities.
Sleep, Tumor Symptom Severity and Interference, Symptoms of Anxiety and Depression and Cognitive Complaints
Patients’ mean scores on the PSQI, MDASI-BT, MOS-CFS, and HADS are presented in Table 2.
With respect to sleep (PSQI), patients reported the most amount of difficulty with sleep latency (16% severe difficulty), and the least amount of difficulty with sleep disturbances (2% severe difficulty) and sleep quality (3% severe difficulty). Patients had a significantly higher score on the PSQI global score compared to the general German population31 (mean difference 1.3, p < 0.001).
Based on the total symptom severity score of the MDASI-BT, 3% of the patients had moderate symptoms and 0.4% severe symptoms. Of note, a large proportion reported moderate to severe symptoms of fatigue (42%), disturbed sleep (32%), difficulty remembering things (29%), drowsiness (32%), a dry mouth (21%), sadness (23%), concentration problems (25%), vision problems (24%) and irritability (22%). Patients reported higher scores on the total symptom severity scale (mean difference 0.5, P < 0.001) and symptom interference scale (mean difference 1.5, P < 0.001) compared to glioma patients of which 63% had a low-grade glioma.32 Results for each individual symptom and their interference with daily living are presented in Supplementary Table 5.
With respect to self-reported cognitive functioning, patients reported the most difficulty with concentration (21% at least “a good bit of the time”) and forgetfulness (19% at least “a good bit of the time”) and reported the least amount of trouble with delayed reaction time (10% at least “a good bit of the time”). Patients reported lower scores on the MOS-CFS total score (mean difference –3.5, P = 0.005) compared with normative data from the Medical Outcomes Study.33
Based on the results of the HADS, 11% and 12%of the patients were suspected of having an anxiety disorder and depression (score ≥ 11), respectively. Patients reported a significantly higher score on the anxiety (mean difference 1.2, P < 0.001) and depression (mean difference 0.7, P = 0.008) subscale compared to the general Dutch population.34
Fatigue in Relation to Patient-, Tumor-, And Treatment-Related Factors
The results of the multivariable analyses (Table 3) showed that having experienced a complication due to resection (OR 3.6, 95% CI: 1.8–7.0, P < 0.001), having received radiotherapy (OR 2.4, 95% CI: 1.2–4.8, P = 0.019) and the number of comorbidities (OR 1.6, 95% CI: 1.3–1.9, P < 0.001) were independently associated with higher levels of fatigue. In contrast, patients with a high educational level reported lower levels of fatigue compared to patients with a low educational level (low level as reference; high level OR 0.3, 95% CI: 0.2–0.7, P = 0.005). No significant associations were found between other sociodemographic and clinical characteristics (sex, age, tumor location, tumor size, tumor resection, WHO grade, seizures) and the level of fatigue.
Table 3.
Determinants of fatigue as measured with the MFI-20 summary score
| Determinant | OR (95% CI) |
|---|---|
| Sex | 0.9 (0.5−1.7), P = 0.732 |
| Age | 1.0 (0.97−1.02), P = 0.576 |
| Educational level | |
| Low (reference) | – |
| Intermediate | 0.9 (0.5−1.7), P = 0.716 |
| High | 0.3 (0.2−0.7), P = 0.005 |
| Number of comorbidities | 1.6 (1.3−1.9), P < 0.001 |
| Tumor location | |
| Multiple locations (reference) | – |
| Cerebral convexity | 1.2 (0.4−3.1), P = 0.738 |
| Skull base | 0.6 (0.2−1.6), P = 0.331 |
| Falx | 1.0 (0.3−3.0), P = 0.988 |
| Other | 1.0 (0.3−3.5), P = 0.939 |
| Tumor size | |
| No tumor (complete resection) (reference) | – |
| <40 mm | 1.2 (0.6−2.3), P = 0.581 |
| ≥40 mm | 0.9 (0.2−4.8), P = 0.867 |
| WHO grade | |
| 1 (reference) | – |
| 2 | 2.2 (1.0−5.2), P = 0.064 |
| Unknown/not clear | 0.4 (0.1−3.7), P = 0.453 |
| Seizures in the past 3 months | 1.1 (0.9−1.5), P = 0.285 |
| Underwent resection | 1.7 (0.5−5.9), P = 0.373 |
| Underwent radiotherapy | 2.4 (1.2−4.8), P = 0.019 |
| Complications after resection | 3.6 (1.8−7.0), P < 0.001 |
OR, odds ratio, CI, confidence interval.
Statistically significant differences are outlined in bold.
Discussion
Meningioma patients were fatigued in 26% of cases and reported higher levels of fatigue compared to the general Dutch population.28 Most patients reported mental fatigue (31%) followed by general fatigue (23%). No significant differences in fatigue prevalence were found between patients who were 0–5 year and > 5 year since diagnosis. After adjusting for relevant sociodemographic and clinical variables, having experienced a complication due to resection, having received radiotherapy, a higher number of comorbidities, and lower educational level were independently associated with more fatigue.
To our knowledge, this is the first large cross-sectional study to show that fatigue is a clinically relevant problem in long-term meningioma patients (on average > 5 year since diagnosis). A previous study has shown that meningioma patients both shortly and > 2 year after surgery assess fatigue as the most important HRQoL issue.10 Similar to our study, a recent study investigating fatigue in meningioma patients before and 1 year after neurosurgery9 showed that meningioma patients reported higher levels of fatigue on all the subscales of the MFI-20 compared to data from the general population (current study: 16%–31% fatigued, van der Linden et al.9: 34%–43% fatigued preoperatively, 19%–49% fatigued postoperatively) and also mostly suffered from mental fatigue (current study: 31% mental fatigue, van der Linden et al.:9 43% mental fatigue preoperatively, 49% mental fatigue postoperatively). Moreover, levels of general fatigue, physical fatigue and mental fatigue did not decrease 1 year after surgery.9 These findings indicate that fatigue is a persistent and relevant problem in meningioma patients both shortly and also many years after completion of treatment.
In the present sample, patients were more often fatigued if they had undergone radiotherapy (OR 2.4, 95% CI: 1.2–4.8, P = 0.019). This finding is supported by current literature. A study investigating the side effects of adjuvant radiotherapy in meningioma patients who were on average 3.3 year since treatment found that patients who had received adjuvant radiotherapy reported lower scores on the SF-36 vitality subscale compared to patients who had only received surgery (mean difference 16.5, P = 0.039).35 Furthermore, fatigue during cranial irradiation is common36 and can significantly affect patients’ HRQoL.37 Lovely et al.7 reported that more than 80% of primary brain tumor patients suffer from fatigue during radiotherapy. Fatigue has been reported occurring as early as within the first week of radiotherapy38 and may even persist after completion of treatment.13 A possible explanation is that radiotherapy can cause endocrine dysfunction when the irradiated area encroaches upon the hypothalamus or pituitary gland.37,39 Moreover, radiotherapy can also cause neuro-inflammation leading to aberrant cytokine and neurotransmitter production and hormone dysregulation, resulting in fatigue.6,17–19 Since there was no significant difference in fatigue prevalence between patients who had received different treatment modalities (no treatment 12% fatigued; resection 25% fatigued; radiotherapy 36% fatigued) and since the number of patients in both the radiotherapy and wait-and-scan group were small, further research in larger patient samples is warranted.
In this study, having experienced a complication due to resection was strongly associated with a higher level of fatigue (OR 3.6, 95% CI: 1.8–7.0, P < 0.001). A study investigating perioperative fatigue in glioma patients had similar findings: having experienced a postoperative complication was strongly associated with high levels of fatigue (OR 7.1, 95% CI: 1.7–30.6, P = 0.008).40 A possible explanation is that patients who experience a complication after resection may simultaneously have other patient- and tumor-related factors (such as a larger tumor size, lower KPS, and more comorbidities41–43), which predisposes them for developing fatigue.13,44 However, previous studies in primary brain tumor patients13 and patients with other solitary tumors45–47 found no association between tumor size and the level of fatigue. Differences can also be explained by the fact that complications in our study could not reliably be categorized based on existing criteria.48 In addition, the fact that the degree of complications may vary between patients, and because patients may also develop several complications at once, the exact relationship between complications and fatigue remains unknown, warranting further research.
In this study, we also found that patients with a high educational level reported lower levels of fatigue compared to patients with a low educational level (low level as reference; high level OR 0.3, 95% CI: 0.2-0.7, P = 0.005). This is in contrast to a study in meningioma patients which found no association between educational level and fatigue.9 It is unknown whether these observed associations are of importance in meningioma patients. In general, a higher educational level and social status have been associated with less fatigue.49–56 An explanation is that patients with a high educational level may be better able to employ energy conservation techniques. Energy conservation techniques decrease the amount of energy required to complete a task by changing actions or the environment (ie, task delegation, time management),57 and if used correctly, can ultimately lead to lower levels of overall fatigue.57,58
The present study has several limitations. Due to the cross-sectional and exploratory nature of the study, we are unable to confirm a causal relationship between the examined patient-, tumor- and treatment-related factors and fatigue. Furthermore, because most patients had undergone resection and only a small number had undergone radiotherapy, the accuracy and interpretation of these variables may have been hampered. Likewise, our results are not generalizable to meningioma patients who have not received any treatment and are currently in a wait-and-scan trajectory due to small numbers, or to patients being treated in a peripheral setting since we only included patients from large medical centers who may have had more complex tumors (40% of the patients had a tumor located at the skull base). Similarly, our results are not generalizable to recently diagnosed meningioma patients since the number of patients who were recently diagnosed with a meningioma in our study were small (only 4.7% of the patients were 0–2 years after diagnosis, range 0.8–11 year). Nevertheless, other studies have shown that meningioma patients frequently report fatigue before and 1 year after neurosurgery9 and report fatigue to be the most important HRQoL issue both shortly and > 2 year after surgery,10 indicating that fatigue is a problem in both short- and long-term meningioma patients. Another limitation is the selection of confounding variables, which was based on the available literature and expert opinion, and may have been suboptimal. Moreover, although 1D measurements have shown to be comparable to volumetric measurements in adult glioma patients,1 volumetric measurements may be an appropriate alternative as they might better represent the probability of tissue damage/compression that may underly fatigue and other symptoms. Finally, response bias may have occurred due to patients declining study participation because they “felt too tired” or due to other health issues, likely resulting in an underestimation of fatigue. Although the nonresponse analysis showed participants and non-participants to be similar regarding measured relevant tumor- and treatment-related characteristics, there still may be some residual confounding due to unknown confounders, hampering generalizability of results. No patients were excluded because of physical or mental conditions interfering with the completion of questionnaires, but one patient was excluded because of lack of understanding of the Dutch or English language, hampering generalizability.
Conclusion and Future Perspectives
In conclusion, the results indicate that fatigue is a frequent problem in meningioma patients even many years after completion of treatment. More extensive research is warranted to determine the causal relation between the identified determinants of fatigue in the present study. Preferably, a large longitudinal study investigating fatigue before and after surgery, and possibly other treatments, should be conducted, thereby including the measurement of the identified factors from the present study and other possible relevant factors (such as inflammatory and endocrine markers). Such a study will enhance our understanding of the cause of fatigue in the meningioma patient population. Furthermore, randomized controlled trials may be performed to determine the effectiveness of both pharmacological and nonpharmacological interventions in the treatment of fatigue in meningioma patients as current evidence is lacking.6
Supplementary Material
Contributor Information
Kwong T Quach, Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands.
Linda Dirven, Department of Neurology, Leiden University Medical Center, Leiden, the Netherlands; Department of Neurology, Haaglanden Medical Center, the Hague, the Netherlands.
Aliede M Vingerhoed, Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands.
Jeroen de Bresser, Department of Radiology, Leiden University Medical Center, Leiden, the Netherlands.
Ruben Dammers, Department of Neurosurgery, Erasmus University Medical Center, Rotterdam, the Netherlands.
Eelke M Bos, Department of Neurosurgery, Erasmus University Medical Center, Rotterdam, the Netherlands.
Wouter A Moojen, Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurosurgery, Haaglanden Medical Center, the Hague, the Netherlands; Department of Neurosurgery, Haga Teaching Hospital, the Hague, the Netherlands.
Wilco C Peul, Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurosurgery, Haaglanden Medical Center, the Hague, the Netherlands.
Martin J B Taphoorn, Department of Neurology, Leiden University Medical Center, Leiden, the Netherlands; Department of Neurology, Haaglanden Medical Center, the Hague, the Netherlands.
Amir H Zamanipoor Najafabadi, Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands.
Wouter R van Furth, Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands.
Conflict of interest statement
None declared.
Funding
This study was supported by a personal MD/PhD-student grant of the Leiden University Medical Center. No specific funding was received for this project.
Author Contributions
L.D., W.R.v.F., and A.H.Z.N. designed the study. Data collection was performed by K.T.Q. and A.M.V., K.T.Q. performed data analysis with input from L.D. and A.H.Z.N., K.T.Q. wrote the first and successive versions of the manuscript. All authors contributed to the interpretation of the results, intellectual content, critical revisions to the drafts of the paper, and approved the final version. K.T.Q. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Ethical Approval
The study was approved by the medical ethical committees of all participating centers (LUMC: P17.190, HMC: 2018-004, EMC: MEC-2018-1405).
Informed Consent
All participants provided informed consent before study participation.
References
- 1. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS.. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Campbell B a., Jhamb A, Maguire JA, Toyota B, Ma R.. Meningiomas in 2009. Am J Clin Oncol. 2009;32(1):73–85. [DOI] [PubMed] [Google Scholar]
- 4. Xue H, Sveinsson O, Tomson T, Mathiesen T.. Intracranial meningiomas and seizures: a review of the literature. Acta Neurochir. 2015;157(9):1541–1548. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong TS, Vera-Bolanos E, Acquaye AA, Gilbert MR, Ladha H, Mendoza T.. The symptom burden of primary brain tumors: evidence for a core set of tumor-and treatment-related symptoms. Neuro Oncol. 2016;18(2):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Day J, Yust-Katz S, Cachia D, et al. Interventions for the management of fatigue in adults with a primary brain tumour. Cochrane Database Syst Rev. 2016;(4). doi: 10.1002/14651858.CD011376.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lovely MP, Miaskowski C, Dodd M.. Relationship between fatigue and quality of life in patients with glioblastoma multiformae. Oncol Nurs Forum. 1999;26(5):921–925. [PubMed] [Google Scholar]
- 8. Cahill JE, Lin L, LoBiondo-Wood G, et al. Personal health records, symptoms, uncertainty, and mood in brain tumor patients. Neuro-Oncol Pract. 2014;1(2):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Der Linden SD, Van Der Linden SD, et al. Prevalence and correlates of fatigue in patients with meningioma before and after surgery. Neuro-Oncol Pract. Published online 2020. doi: 10.1093/nop/npz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zamanipoor Najafabadi AH, Peeters MCM, Lobatto DJ, et al. Health-related quality of life of cranial WHO grade I meningioma patients: are current questionnaires relevant? Acta Neurochir. 2017;159(11):2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armstrong TS, Gilbert MR.. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;14:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siniscalchi A, Gallelli L, Russo E, De Sarro G.. A review on antiepileptic drugs-dependent fatigue: pathophysiological mechanisms and incidence. Eur J Pharmacol. Published online 2013. doi: 10.1016/j.ejphar.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong TS, Cron SG, Vera Bolanos E, Gilbert MR, Kang DH.. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715. [DOI] [PubMed] [Google Scholar]
- 14. Fox SW, Lyon D, Farace E.. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh. 2007;39(1):61–67. [DOI] [PubMed] [Google Scholar]
- 15. Pelletier G, Verhoef MJ, Khatri N, Hagen N.. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57(1):41–49. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong TS, Shade MY, Breton G, et al. Sleep-wake disturbance in patients with brain tumors. Neuro Oncol. 2017;19(3):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ballesteros-Zebadua P, Chavarria A, Angel Celis M, Paz C, Franco-Perez J.. Radiation-induced neuroinflammation and radiation somnolence syndrome. CNS Neurol Disord - Drug Targets. 2012;11(7):937–949. [DOI] [PubMed] [Google Scholar]
- 18. Scheff JD, Calvano SE, Lowry SF, Androulakis IP.. Modeling the influence of circadian rhythms on the acute inflammatory response. J Theor Biol. 2010;264(3):1068–1076. [DOI] [PubMed] [Google Scholar]
- 19. Hrushesky WJM, Grutsch J, Wood P, , et al. Circadian clock manipulation for cancer prevention and control and the relief of cancer symptoms. Integr Cancer Ther. 2009;8(4):387–397. [DOI] [PubMed] [Google Scholar]
- 20. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. Published online 2016. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 21. Couldwell WT, Fukushima T, Giannotta SL, Weiss MH.. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. Published online 1996. doi: 10.3171/jns.1996.84.1.0020. [DOI] [PubMed] [Google Scholar]
- 22. Otani N, Mori K, Wada K, Tomiyama A, Toyooka T, Takeuchi S.. Multistaged, multidirectional strategy for safe removal of large meningiomas in the pineal region. Neurosurg Focus. Published online 2018. doi: 10.3171/2017.12.FOCUS17602. [DOI] [PubMed] [Google Scholar]
- 23. Cheng CM, Chang CF, Ma HI, Chiang YH, McMenomey SO, Delashaw JB.. Modified orbitozygomatic craniotomy for large medial sphenoid wing meningiomas. J Clin Neurosci. Published online 2009. doi: 10.1016/j.jocn.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 24. Baussart B, Vanden Bulcke D, Villa C, Reina V, Gaillard S.. The Dural Dark-Side Approach for falcine and tentorial meningioma: a surgical series of five patients. Neurochirurgie. Published online 2021. doi: 10.1016/j.neuchi.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 25. Iwai Y, Yamanaka K, Shimohonji W, Ishibashi K.. Staged gamma knife radiosurgery for large skull base meningiomas. Cureus. Published online 2019. doi: 10.7759/cureus.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Del Maestro RF. Al-Mefty’s meningiomas. Second edition. 2011. Edited by Franco DeMonte, Michael W. McDermott, Ossama Al-Mefty. Published by Thieme Medical Publishers, Inc. 432 pages. C$210 approx. Can J Neurol Sci/ J Can des Sci Neurol. Published online 2013. doi: 10.1017/s0317167100017455. [DOI] [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. Published online 1987. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28. Kieffer JM, Starreveld DE, Boekhout A, Bleiker EM.. A questionable factor structure of the multidimensional fatigue inventory in the general Dutch population. J Clin Epidemiol. Published online 2021. doi: 10.1016/j.jclinepi.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 29. Purcell A, Fleming J, Bennett S, Burmeister B, Haines T.. Determining the minimal clinically important difference criteria for the multidimensional fatigue inventory in a radiotherapy population. Support Care Cancer. Published online 2010. doi: 10.1007/s00520-009-0653-z. [DOI] [PubMed] [Google Scholar]
- 30. Lin JMS, Brimmer DJ, Maloney EM, Nyarko E, BeLue R, Reeves WC.. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Popul Health Metr. Published online 2009. doi: 10.1186/1478-7954-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hinz A, Glaesmer H, Brähler E, et al. Sleep quality in the general population: psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Med. Published online 2017. doi: 10.1016/j.sleep.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 32. Kim SR, Shin YS, Kim JH, Choi M, Yoo SH.. Differences in type composition of symptom clusters as predictors of quality of life in patients with meningioma and glioma. World Neurosurg. 2017;98:50–59. doi: 10.1016/j.wneu.2016.10.085. [DOI] [PubMed] [Google Scholar]
- 33. Hays RD, Sherbourne CD, Mazel R.. User’s Manual for the Medical Outcomes Study (MOS) Core Measures of Health-Related Quality of Life. Santa Monica, CA: RAND Corporation; 1995. [Google Scholar]
- 34. Spinhoven P, Ormel J, Sloekers PPA, Kempen GIJM, Speckens AEM, Van Hemert AM.. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychol Med. Published online 1997. doi: 10.1017/S0033291796004382. [DOI] [PubMed] [Google Scholar]
- 35. van Nieuwenhuizen D, Klein M, Stalpers LJ, Leenstra S, Heimans JJ, Reijneveld JC.. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J Neurooncol. 2007;84(0167-594X (Print)):271–278. [DOI] [PubMed] [Google Scholar]
- 36. Jereczek-Fossa BA, Marsiglia HR, Orecchia R.. Radiotherapy-related fatigue. Crit Rev Oncol Hematol. Published online 2002. doi: 10.1016/S1040-8428(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 37. Page BR, Shaw EG, Lu L, et al. Phase II double-blind placebo-controlled randomized study of armodafinil for brain radiation-induced fatigue. Neuro Oncol. Published online 2015. doi: 10.1093/neuonc/nov084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drappatz J, Schiff D, Kesari S, Norden AD, Wen PY.. Medical management of brain tumor patients. Neurol Clin. Published online 2007. doi: 10.1016/j.ncl.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 39. Day J, Yust-Katz S, Cachia D, et al. Interventions for the management of fatigue in adults with aprimary brain tumour. Cochrane Database Syst Rev. 2016;(4). doi: 10.1002/14651858.CD011376.pub2.www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schei S, Solheim O, Jakola AS, Sagberg LM.. Perioperative fatigue in patients with diffuse glioma. J Neurooncol. Published online 2020. doi: 10.1007/s11060-020-03403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yasui M, Takemasa I, Miyake Y, et al. Tumor size as an independent risk factor for postoperative complications in laparoscopic low anterior resection for advanced rectal cancer: a multicenter Japanese study. Surg Laparosc Endosc Percutaneous Tech. Published online 2017. doi: 10.1097/SLE.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 42. Asano K, Nakano T, Takeda T, Ohkuma H.. Risk factors for postoperative systemic complications in elderly patients with brain tumors: clinical article. J Neurosurg. Published online 2009. doi: 10.3171/2008.10.17669. [DOI] [PubMed] [Google Scholar]
- 43. Brell M, Ibanez J, Caral L, Ferrer E.. Factors influencing surgical complications of intra-axial brain tumours. Acta Neurochir. 2000;142(7):739–750. http://www.ncbi.nlm.nih.gov/pubmed/10955668NS- [DOI] [PubMed] [Google Scholar]
- 44. Grøn KL, Ørnbjerg LM, Hetland ML, et al. The association of fatigue, comorbidity burden, disease activity, disability and gross domestic product in patients with rheumatoid arthritis. Results from 34 countries participating in the Quest-RA programme. Clin Exp Rheumatol. Published online 2014. [PubMed] [Google Scholar]
- 45. Hann DM, Jacobsen PB, Martin SC, Kronish LE, Azzarello LM, Fields KK.. Fatigue in women treated with bone marrow transplantation for breast cancer: a comparison with women with no history of cancer. Support Care Cancer. 1997;5(1):44–52. wos:A1997WH68300009 NS - [DOI] [PubMed] [Google Scholar]
- 46. Hann DM, Jacobsen P, Martin S, Azzarello L, Greenberg H.. Fatigue and quality of life following radiotherapy for breast cancer: a comparative study. J Clin Psychol Med Settings. Published online 1998. doi: 10.1023/A:1026249702250. [DOI] [Google Scholar]
- 47. Okuyama T, Akechi T, Kugaya A, et al. Factors correlated with fatigue in disease-free breast cancer patients: application of the Cancer Fatigue Scale. Support Care Cancer. 2000;8(3):215–222. wos:000086482300010 NS - [DOI] [PubMed] [Google Scholar]
- 48. Ibaez FAL, Hem S, Ajler P, et al. A new classification of complications in neurosurgery. World Neurosurg. Published online 2011. doi: 10.1016/j.wneu.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 49. Fuhrer R, Wessely S.. The epidemiology of fatigue and depression: a French primary-care study. Psychol Med. Published online 1995. doi: 10.1017/S0033291700037387. [DOI] [PubMed] [Google Scholar]
- 50. Hickie IB, Hooker AW, Hadzi-Pavlovic D, Bennett BK, Wilson AJ, Lloyd AR.. Fatigue in selected primary care settings: sociodemographic and psychiatric correlates. Med J Aust. Published online 1996. doi: 10.5694/j.1326-5377.1996.tb122199.x. [DOI] [PubMed] [Google Scholar]
- 51. Van Mens-Verhulst J, Bensing J.. Distinguishing between chronic and nonchronic fatigue, the role of gender and age. Soc Sci Med. Published online 1998. doi: 10.1016/S0277-9536(98)00116-6. [DOI] [PubMed] [Google Scholar]
- 52. Wessely S, Chalder T, Hirsch S, Wallace P, Wright D.. The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health. Published online 1997. doi: 10.2105/AJPH.87.9.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. David A, Pelosi A, McDonald E, et al. Tired, weak, or in need of rest: fatigue among general practice attenders. Br Med J. Published online 1990. doi: 10.1136/bmj.301.6762.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Van Mens-Verhulst J, Bensing JM.. Sex differences in persistent fatigue. Women Heal. Published online 1997. doi: 10.1300/J013v26n03_04. [DOI] [PubMed] [Google Scholar]
- 55. Watt T, Groenvold M, Bjorner JB, Noerholm V, Rasmussen NA, Bech P.. Fatigue in the Danish general population. Influence of sociodemographic factors and disease. J Epidemiol Community Health. Published online 2000. doi: 10.1136/jech.54.11.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Engberg I, Segerstedt J, Waller G, Wennberg P, Eliasson M.. Fatigue in the general population- associations to age, sex, socioeconomic status, physical activity, sitting time and self-rated health: the northern Sweden MONICA study 2014. BMC Public Health. Published online 2017. doi: 10.1186/s12889-017-4623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vatwani A, Margonis R.. Energy conservation techniques to decrease fatigue. Arch Phys Med Rehabil. Published online 2019. doi: 10.1016/j.apmr.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 58. O’Sullivan SB, Schmitz TJ, Fulk G.. Physical Rehabilitation. 6th ed. Philadelphia: F.A. Davis; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.