Abstract
Introduction
Proton pump inhibitor (PPI) use is increasing in the general population. Chronic PPI use can lead to hypergastrinemia, which has been purported to increase the risk of developing colorectal cancer (CRC). Several studies have failed to report any association between PPI use and the risk of CRC. However, little is known about the effect of PPI use on CRC survival. In this retrospective analysis, we studied the effect of PPI use on CRC survival in a racially diverse population.
Methods
Data were abstracted for 1050 consecutive patients diagnosed with CRC from January 2007 to December 2020. The Kaplan-Meier curve was created to study the effect of PPI exposure compared to no exposure on overall survival (OS). Univariate and multivariate analyses were performed to investigate predictors of survival.
Results
Complete data were available for 750 patients with CRC, 52.5% were males, 22.7% were Whites, 60.1% were Asians, and 17.2% were Pacific Islanders. A total of 25.6% of patients had a history of PPI use. Moreover, 79.2% had hypertension, 68.8% had hyperlipidemia, 38.0% had diabetes mellitus, and 30.2% had kidney disease.
There was no difference in median OS among PPI users compared to non-users, p value=0.4. Age, grade, and stage were predictors of inferior OS. No significant association was noticed with gender, race, comorbidities, or treatment with chemotherapy.
Conclusion
In this retrospective analysis of a racially diverse population of CRC patients, we found that PPI use was not associated with worse OS. Until high-quality prospective data are available, physicians should not stop PPIs that are clinically indicated.
Keywords: hypergastrinemia, overall survival, mortality, colon cancer, proton pump inhibitor
Introduction
Proton pump inhibitors (PPI) are used to manage various gastrointestinal diagnoses such as peptic ulcer disease and gastroesophageal reflux disease (GERD) [1,2]. The usage of PPI is increasing in the general population [2]. Chronic PPI use can lead to hypergastrinemia, which has been purported to increase the risk of colorectal cancer (CRC) development as serum gastrin can promote replication of both benign and malignant colonic epithelial cells [3-6]. Furthermore, animal studies have shown the progression of colon adenomas in the setting of hypergastrinemia [7]. On the other hand, pantoprazole has been shown in vitro to inhibit T-cell-originated protein kinase (TOPK), which is highly expressed in CRC cells and can promote tumorigenesis and progression, while another study showed similar anti-TOPK activity in vitro and in vivo with mice using ilaprazole [8,9].
Multiple observational studies and meta-analyses conducted across different populations have failed to report any association between long-term PPI and the risk of CRC [10-18]. However, one retrospective analysis studied the effect of PPI use on CRC survival and suggested a potential adverse effect of PPI use on the overall survival (OS) of CRC patients [19]. This study was limited by the small number of CRC patients using PPI. Moreover, the racial make-up of the study participants was not described, thus it is unknown if these results would be generalizable to a wider population. The primary objective of this study was to evaluate the impact of PPI use on survival among a large, racially diverse cohort of CRC patients.
An abstract of this study was presented as a poster at the American College of Gastroenterology 2021 Annual Scientific Meeting, Las Vegas, October 22-27, 2021.
A preprint of this study was published online on Research Square.
Materials and methods
Patients and data collection
A retrospective analysis was performed on data gathered from the Queen’s Medical Center (QMC), Honolulu, Hawaii, Oncology Data Registry (ODR). QMC is the largest hospital in Hawaii and treats 40-50% of all colon cancer patients in the state. ODR was established in 1960 as part of the Hawaii Tumor Registry and has been contributing data to the Surveillance, Epidemiology, and End Results (SEER) program since 1973. All patients diagnosed with colorectal adenocarcinoma between January 1, 2007, and December 30, 2020, were eligible.
Institutional Review Board (IRB) approval was obtained from QMC for the conduct of this study; IRB approval number RA-2020-013. Data on patient demographics, clinicopathologic characteristics, and survival were collected from medical records. Race was self-reported by the patient and documented in the ODR. Patients were categorized into three racial groups: Asian (Korean, Chinese, Japanese, Filipino, Asian Indian (Indian and Pakistani), Southeast Asian (Thai, Vietnamese, Cambodian, and Laotian), and other Asian), Pacific Islander (Native Hawaiian, Samoan, Tongan, Micronesian, Marshallese, Fijian, Chamorro, and other Pacific Islander), and White. Patients of other races or unknown races only made up 1.2% of our study population and were excluded from the analysis.
Statistics
The primary objective was to study the potential effect of PPI use on OS among patients diagnosed and treated for CRC. Nonparametric descriptive statistics were used to evaluate characteristics of standard demographic, clinical, and tumor data. A two-sided p < 0.05 was considered statistically significant. OS was calculated by the Kaplan-Meier method and univariate comparisons between groups were carried out by using the log-rank test. Cox proportional hazards regression models for survival were built to obtain hazard ratios (HR) and 95% confidence intervals (CI) adjusting for age, gender, race, histologic grade, stage, surgery, chemotherapy, PPI use, and associated comorbidities. Statistical analyses and survival graphics were performed with R 4.0.3 (The R Foundation for Statistical Computing).
Results
Demographic and clinical characteristics
In this study, a cohort of 1050 patients with CRC was identified, and 750 patients were included after excluding patients who did not have complete data (n=293) had multifocal lesions or unclear tumor location (n=6), and had unknown ethnicity (n=1) (Figure 1).
Figure 1. Flow chart of patients after applying inclusion and exclusion criteria.
Table 1 shows details about the patient’s demographic and clinical characteristics (Table 1). There were 394 males (52.5%) and 356 females (47.4%). The median patient age was 66 (range, 28-100). We identified 192 patients (25%) who were using PPI, 71 of whom (37%) used PPI for over a year. There was no significant difference in race, tumor location, grade, and stage among patients with a history of PPI use compared to no use. There were more patients with hypertension (HTN), hyperlipidemia (HLD), diabetes mellitus (DM), and chronic kidney disease (CKD) among those with a history of PPI use. All patients received surgery and 48% (360) received chemotherapy.
Table 1. Baseline demographic and clinical characteristics of patients with colorectal cancer.
BMI: body mass index; HTN: hypertension; HLD: hyperlipidemia; CKD: chronic kidney disease; PPI: proton pump inhibitor
| Variable | No PPI use (n=558) | PPI use (n=192) | P-value |
| Age | 66 | 67 | 0.314 |
| Sex | 0.286 | ||
| Male | 300 (53.8%) | 94 (49.0%) | |
| Female | 258 (46.2%) | 98 (51.0%) | |
| Race | 0.211 | ||
| White | 118 (21.1%) | 52 (27.1%) | |
| Asian | 340 (60.9%) | 111 (57.8%) | |
| Native Hawaiian and other pacific islanders | 100 (17.9%) | 29 (15.1%) | |
| Tumor location | 0.680 | ||
| Right colon | 163 (29.2%) | 58 (30.2%) | |
| Left colon | 184 (33.0%) | 56 (29.2%) | |
| Transverse colon | 43 (7.7%) | 13 (6.8%) | |
| Rectum | 168 (30.1%) | 65 (33.9%) | |
| Lymph node involvement | 0.900 | ||
| No | 309 (55.4%) | 108 (56.2%) | |
| Yes | 249 (44.6%) | 84 (43.8%) | |
| Grade | 0.774 | ||
| Grade 1 + 2 | 469 (84.1%) | 159 (82.8%) | |
| Grade 3 + 4 | 89 (15.9%) | 33 (17.2%) | |
| Stage | 0.214 | ||
| Stage I | 137 (24.6%) | 40 (20.8%) | |
| Stage II | 151 (27.1%) | 67 (34.9%) | |
| Stage III | 208 (37.3%) | 67 (34.9%) | |
| Stage IV | 62 (11.1%) | 18 (9.4%) | |
| Surgery | |||
| Yes | 558 (100%) | 192 (100%) | |
| Chemotherapy | 0.656 | ||
| No | 287 (51.4%) | 103 (53.6%) | |
| Yes | 271 (48.6%) | 89 (46.4%) | |
| BMI | 25.8 | 25.6 | 0.880 |
| HTN | < 0.001 | ||
| No | 236 (42.3%) | 40 (20.8%) | |
| Yes | 322 (57.7%) | 152 (79.2%) | |
| HLD | <0.001 | ||
| No | 268 (48.0%) | 60 (31.2%) | |
| Yes | 290 (52.0%) | 132 (68.8%) | |
| DM | <0.001 | ||
| No | 424 (76.0%) | 119 (62.0%) | |
| Yes | 134 (24.0%) | 73 (38.0%) | |
| CKD | <0.001 | ||
| No | 509 (91.2%) | 134 (69.8%) | |
| Yes | 49 (8.8%) | 58 (30.2%) | HLD: |
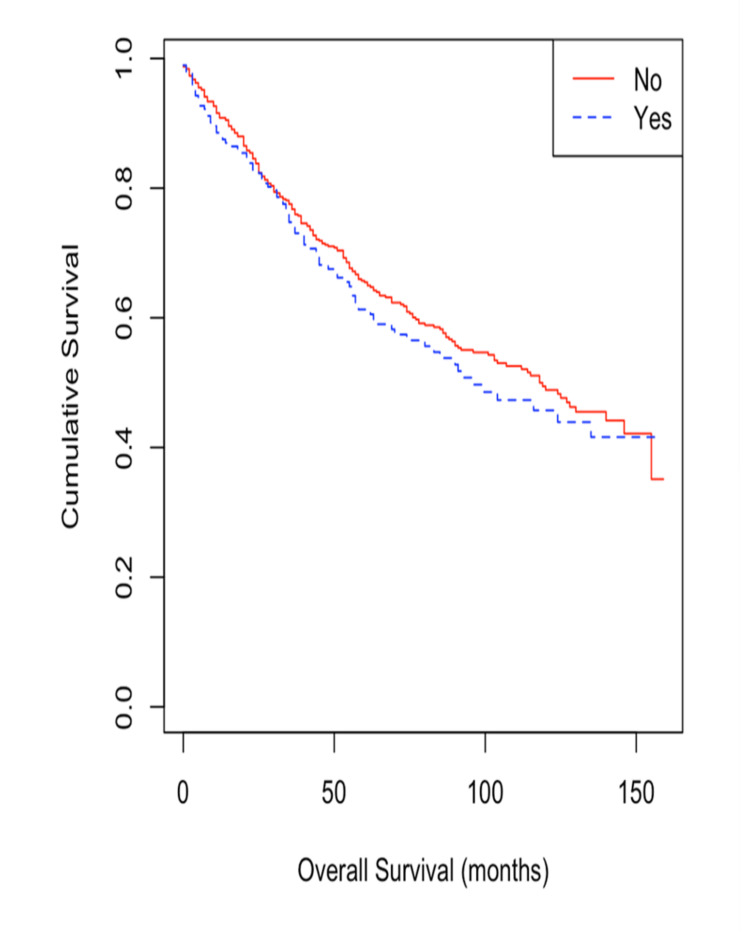
OS in patients with PPI users compared to non-users
The cohort was divided according to PPI use into two distinct groups and evaluated for OS using Kaplan-Meier curves. Patients with a history of PPI use demonstrated shorter median survival time compared with patients without a history of PPI use (96 months vs 118 months) (Figure 2), however, this difference was not significant (p-value=0.4). Even after adjustment for demographic factors, tumor characteristics, and comorbidities, the difference in median OS between PPI users and non-users remained non-significant (Table 2).
Table 2. Univariate and multivariate Cox regression model for potential predictors of overall survival in colorectal cancer patients.
DM: diabetes mellitus; HTN: hypertension; HLD: hyperlipidemia; CKD: chronic kidney disease; PPI: proton pump inhibitor
| Univariate | Multivariate | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Sex (1=men, 2=women) | 0.889 (0.7142 to 1.107) | 0.293 | ||
| Age (continuous) | 1.035 (1.026 to 1.044) | < 0.001 | 1.039 (1.029 to 1.048) | < 0.001 |
| Race (reference = white) | ||||
| Asian | 0.947 (0.728 to 1.232) | 0.684 | ||
| Native Hawaiian or Other Pacific Islander | 0.937 (0.661 to 1.330) | 0.716 | ||
| Grade (reference = G1-2) | ||||
| G3-4 | 1.765 (1.349 to 2.308) | < 0.001 | 1.471 (1.122 to 1.928) | 0.008 |
| Stage | 1.713 (1.509 to 1.943) | < 0.001 | 2.134 (1.806 to 2.520) | < 0.001 |
| Chemotherapy (reference = no) | 0.995 (0.800 to 1.238) | 0.965 | ||
| PPI | 1.111 (0.869 to 1.420) | 0.402 | 1.095 (0.846 to 1.418) | 0.482 |
| HTN | 1.133 (0.902 to 1.424) | 0.284 | ||
| HLD | 0.913 (0.734 to 1.137) | 0.416 | ||
| DM | 1.028 (0.806 to 1.311) | 0.822 | ||
| CKD | 1.456 (1.098 to 1.931) | 0.009 | 1.167 (0.867 to 1.572) | 0.273 |
Figure 2. Kaplan-Meier survival curve for CRC patients with PPI exposure vs no exposure.
CRC: colorectal cancer; PPI: proton pump inhibitor
Predictors of OS in CRC patients
On both univariate and multivariate analysis, age, grade, and stage of the tumor were all negative predictors of OS. However, CKD was a negative predictor of OS on univariate analysis only. Conversely, gender, race, treatment with chemotherapy, HTN, HLD, and DM did not have a significant effect on OS (Table 2).
Discussion
This study did not demonstrate an association between PPI use and median OS in patients with CRC. This is the second retrospective analysis conducted to examine this association, but unlike that conducted by Graham et al., our study featured considerably more patients (117 vs 192) who were using PPI [19]. While Graham et al. only reported about 9% of their patients were using a PPI, our study showed over 25% were exposed to a PPI, 37% of which used the PPI for over a year. This likely more closely represents the general population given the increased use of PPI [2]. In addition, fewer patients in our study had an advanced CRC stage, with around 47% having stage III or IV as compared to 62% of patients in the Graham et al. study. This difference is relevant, as a more advanced disease would significantly impact OS and may explain the observed association between PPI use and OS in Graham et al. study.
Our study showed no difference in demographic characteristics among patients who used PPI compared to non-users. Although there was a higher incidence of HTN, HLD, DM, and CKD among patients with a history of PPI use, there were no differences in histologic grade, stage, or location between the two groups. The absence of an association between PPI use and known negative prognostic factors supports our conclusion that PPI use does not impact OS among CRC patients.
Graham et al. did not describe the racial makeup of their study population, but it is likely that our study had a higher proportion of Asian patients given that they comprised 60% of the study population [19]. It has been widely noted that the incidence and mortality rates of CRC are lowest in Asians and Pacific Islanders in the United States, though more recent studies suggest that both the incidence and mortality of CRC are rising in Asia [20,21]. Moreover, Asians, such as Japanese and Chinese, have a higher proportion of poor metabolites relative to Caucasian and African populations, therefore, strong gastric acid suppression can be obtained even with low doses of PPI [22]. However, even after adjusting for race, there was no PPI impact on OS in our study. Our unique study population is racially diverse which enhances the generalizability of the results.
Our study has several limitations, in particular, due to the retrospective nature of the analysis. Due to this research method, all data were extracted from patient charts, though every effort was made to limit errors at each step. Due to the nature of chart review data, it is also not certain that the patients listed as taking PPIs were compliant with the medications or were using the medication intermittently. Moreover, it is extremely hard to determine the intake duration accurately. In addition, the measured outcome in our study is OS rather than cancer-specific survival. Cancer-specific survival would be an important endpoint to study given the purported physiologic interaction between PPI use and CRC progression. Furthermore, all the patients in the study, after data refinement, underwent surgery, and this explains the relatively low percentage of patients with advanced stages (stages III and IV). On the other hand, our study has a large sample size, as well as a multi-racial population which is unique and helps in generalizing the results on the general population.
Conclusions
In a large retrospective study of racially diverse CRC patients, our study did not demonstrate an association between PPI use and CRC OS. Future studies would ideally be prospective, collect data about PPI history prior to and after diagnosis of CRC, and include a measure of cancer-specific survival in the outcomes analysis. We would suggest that, until high-quality prospective data are available, oncologists and other physicians not stop PPIs that are otherwise clinically indicated.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. The Queen's Medical Center Institutional Review Board (IRB), Honolulu, HI issued approval RA-2020-013. This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of the Queen’s Medical Center approved this study.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Acid suppression: optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Wolfe M, Sachs G. Gastroenterology. 2000;118:9–31. doi: 10.1016/s0016-5085(00)70004-7. [DOI] [PubMed] [Google Scholar]
- 2.Persistence and adherence to proton pump inhibitors in daily clinical practice. Van Soest EM, Siersema PD, Dieleman JP, Sturkenboom MC, Kuipers EJ. Aliment Pharmacol Ther. 2006;24:377–385. doi: 10.1111/j.1365-2036.2006.02982.x. [DOI] [PubMed] [Google Scholar]
- 3.Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Watson SA, Morris TM, McWilliams DF, Harris J, Evans S, Smith A, Clarke PA. Br J Cancer. 2002;87:567–573. doi: 10.1038/sj.bjc.6600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elevated gastrin levels in patients with colon cancer or adenomatous polyps. Smith JP, Wood JG, Solomon TE. Dig Dis Sci. 1989;34:171–174. doi: 10.1007/BF01536047. [DOI] [PubMed] [Google Scholar]
- 5.Postprandial hypergastrinaemia in patients with colorectal cancer. Wong K, Beardshall K, Waters CM, Calam J, Poston GJ. Gut. 1991;32:1352–1354. doi: 10.1136/gut.32.11.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A review of epidemiological studies on cancer in relation to the use of anti-ulcer drugs. La Vecchia C, Tavani A. Eur J Cancer Prev. 2002;11:117–123. doi: 10.1097/00008469-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hypergastrinemia promotes adenoma progression in the APC(Min-/+) mouse model of familial adenomatous polyposis. Watson SA, Smith AM. https://pubmed.ncbi.nlm.nih.gov/11212260/ Cancer Res. 2001;61:625–631. [PubMed] [Google Scholar]
- 8.Pantoprazole, an FDA-approved proton-pump inhibitor, suppresses colorectal cancer growth by targeting T-cell-originated protein kinase. Zeng X, Liu L, Zheng M, et al. Oncotarget. 2016;7:22460–22473. doi: 10.18632/oncotarget.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proton pump inhibitor ilaprazole suppresses cancer growth by targeting T-cell-originated protein kinase. Zheng M, Luan S, Gao S, et al. Oncotarget. 2017;8:39143–39153. doi: 10.18632/oncotarget.16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colorectal cancer risk in relation to use of acid suppressive medications. Chubak J, Boudreau DM, Rulyak SJ, Mandelson MT. Pharmacoepidemiol Drug Saf. 2009;18:540–544. doi: 10.1002/pds.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proton pump inhibitor use and risk of gastric, colorectal, liver, and pancreatic cancers in a community-based population. Lee JK, Merchant SA, Schneider JL, Jensen CD, Fireman BH, Quesenberry CP, Corley DA. Am J Gastroenterol. 2020;115:706–715. doi: 10.14309/ajg.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 12.Proton pump inhibitor use and risk of colorectal cancer: a population-based, case-control study. Robertson DJ, Larsson H, Friis S, Pedersen L, Baron JA, Sørensen HT. Gastroenterology. 2007;133:755–760. doi: 10.1053/j.gastro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Effect of proton pump inhibitors on colorectal cancer. Sasaki T, Mori S, Kishi S, et al. Int J Mol Sci. 2020;21:3877. doi: 10.3390/ijms21113877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colorectal neoplasia in veterans is associated with Barrett's esophagus but not with proton-pump inhibitor or aspirin/NSAID use. Siersema PD, Yu S, Sahbaie P, Steyerberg EW, Simpson PW, Kuipers EJ, Triadafilopoulos G. Gastrointest Endosc. 2006;63:581–586. doi: 10.1016/j.gie.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Proton pump inhibitors and the risk of colorectal cancer. van Soest EM, van Rossum LG, Dieleman JP, van Oijen MG, Siersema PD, Sturkenboom MC, Kuipers EJ. Am J Gastroenterol. 2008;103:966–973. doi: 10.1111/j.1572-0241.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 16.Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Yang YX, Hennessy S, Propert K, Hwang WT, Sedarat A, Lewis JD. Gastroenterology. 2007;133:748–754. doi: 10.1053/j.gastro.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Use of proton pump inhibitor and risk of colorectal cancer: a meta-analysis of observational studies. Ahn JS, Park SM, Eom CS, Kim S, Myung SK. Korean J Fam Med. 2012;33:272–279. doi: 10.4082/kjfm.2012.33.5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proton pump inhibitors and the risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Ma T, Wu M, Jia S, Yang L. Int J Colorectal Dis. 2020;35:2157–2169. doi: 10.1007/s00384-020-03717-5. [DOI] [PubMed] [Google Scholar]
- 19.A retrospective analysis of the role of proton pump inhibitors in colorectal cancer disease survival. Graham C, Orr C, Bricks CS, Hopman WM, Hammad N, Ramjeesingh R. Curr Oncol. 2016;23:0–8. doi: 10.3747/co.23.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The rise of colorectal cancer in Asia: epidemiology, screening, and management. Onyoh EF, Hsu WF, Chang LC, Lee YC, Wu MS, Chiu HM. Curr Gastroenterol Rep. 2019;21:36. doi: 10.1007/s11894-019-0703-8. [DOI] [PubMed] [Google Scholar]
- 21.Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. J Clin Oncol. 2018;36:25–33. doi: 10.1200/JCO.2017.74.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effects of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of proton pump inhibitors. Zhang HJ, Zhang XH, Liu J, et al. Pharmacol Res. 2020;152:104606. doi: 10.1016/j.phrs.2019.104606. [DOI] [PubMed] [Google Scholar]