Abstract
A recent publication by Barreto and colleagues showed that SARS-CoV-2 directly triggers hyperglycemia by infecting hepatocytes and inducing phosphoenolpyruvate carboxykinase (PEPCK)-dependent gluconeogenesis. Here, we discuss the biological importance of these findings, including the hepatic tropism of SARS-CoV-2. We also comment on the clinical implications of the bidirectional connection between COVID-19 and noncommunicable diseases.
Keywords: SARS-CoV-2, liver, glucose metabolism
Main text
Modern societies suffer chronic, highly prevalent, endemic diseases, known as noncommunicable diseases, which include cardiovascular diseases, cancers, chronic respiratory diseases, and diabetes. Similarly, the 21st century is witnessing significant health challenges, including emerging infectious diseases, zoonosis, and new contagious diseases.
The coronavirus 2019 (COVID-19) pandemic has unmasked a new paradigm: the crosstalk between epidemics worsens the clinical scenario. We have learned that host risk factors, including lifestyle, obesity/overweight and other comorbidities, genetic predisposition, underlying immune-related conditions, and even demographic differences among the affected individuals, impact the course of COVID-19. Nevertheless, we still do not know the underlying molecular mechanisms and potential causative links between infections (viral) and noncommunicable diseases. This includes the connection between COVID-19 and noncommunicable disease onsets and the exacerbation by COVID-19 of existing noncommunicable diseases.
A recent publication by Barreto and colleagues showed that severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) directly triggers hyperglycemia by infecting hepatocytes [1]. The authors used retrospective clinical and laboratory data to assess the pattern of blood glucose changes following hospital admission and during the disease course, including surrogate markers of endocrine pancreas indemnity. The authors also used postmortem liver biopsies to assess the presence of SARS-CoV-2 and its host receptors in hepatocytes. Finally, the authors exposed human hepatocytes to the ancestral SARS-CoV-2 strain and variants of concern. They found that liver cells are susceptible to viral infection, as indicated by the presence of the spike protein within the hepatocytes. Most importantly, the authors provided data showing that infection of hepatocytes is a crucial cause of hyperglycemia in patients with COVID-19 by inducing PEPCK-dependent gluconeogenesis.
Is SARS-CoV-2 on the list of hepatotropic viruses?
The lung and respiratory tract are the main target sites of SARS-CoV-2 replication. However, there is robust evidence indicating SARS-CoV-2 hepatic tropism. For example, single cell transcriptomic analysis revealed that hepatocytes express receptors commonly used by SARS-CoV-2 to enter human cells, including angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRS22) [2]. More recently, the assessment of autopsy specimens of patients who died from COVID-19 shed light on the concept that SARS-CoV-2 can efficiently replicate in the liver [3].
Barreto and coworkers provide evidence that SARS-CoV-2 can infect, replicate, and produce infectious viral particles in primary human hepatocytes. Nevertheless, the authors could not find proof of cytopathic changes in the liver [1], which is consistent with autopsy findings in patients with fatal COVID-19 [4]. Although Barreto et al. provide robust evidence that SARS-CoV-2 hepatocyte entry depends on ACE2 and heat shock protein family A (Hsp70) member 5 (GRP78), an intriguing aspect is that the simultaneous inhibition of both targets diminished the effects of individual receptor blockades [1]. It appears plausible that there are other players cooperating to internalize the virus. Likewise, SARS-CoV-2 may use diverse cell entry mechanisms, including other liver cell types, such as cholangiocytes [2].
The hepatic tropism of SARS-CoV-2 may have other clinical consequences, such as the possibility of chronic liver infection. In connection with this, mouse coronavirus (MHV), which diverges by ~1000 nucleotides from the first SARS-CoV-2, causes chronic hepatitis in mice.
Glucometabolic control by SARS-CoV-2 in the liver: why gluconeogenesis instead of glycolysis?
Hepatic glucose production is regulated by gluconeogenesis and glycolysis. The strategies used by viruses to derive energy from the host cells vary according to the virus and the tissue or cell types. For example, SARS-CoV-2 induces glycolysis in monocytes [5], which might explain the increased lactate dehydrogenase (LDH) levels observed in patients with severe COVID-19 outcomes. Hepatotropic viruses, such as hepatitis C virus, do not present consistent mechanisms, because both gluconeogenesis and glycolysis have been reported. Gluconeogenesis, which enables glucose synthesis from lactate and amino acids, such as alanine, is restricted to liver and kidney cells. This involves a series of transamination reactions and the activity of critical enzymes, including PEPCK and the catalytic subunit glucose-6-phosphatase (G-6-Pase). Intriguingly, Barreto et al. found that infected hepatocytes increase glucose production via stimulation of PEPCK activity without changes in gene transcription.
Molecular signatures explaining gluconeogenesis modulation by SARS-CoV-2: is there a single truth?
Other potential molecular mechanisms may explain glucose metabolic reprogramming by SARS-CoV-2 (Figure 1 ). For example, Mercado-Gomez et al. reported that the binding of the SARS-CoV-2 spike protein alters mitochondrial activity and glucose homeostasis in human primary hepatocytes [6].
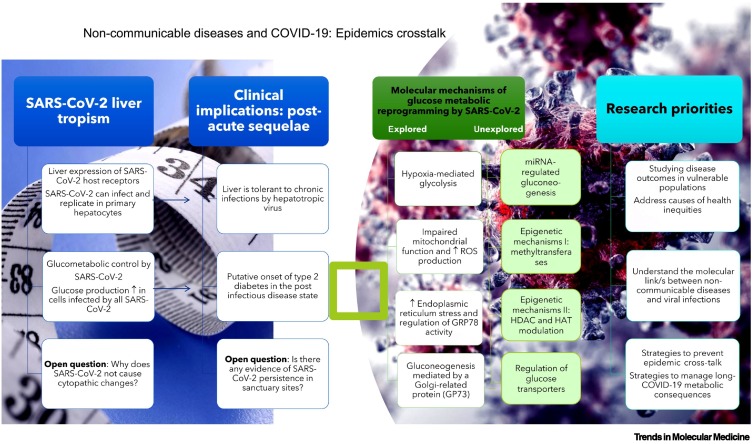
Figure 1.
Noncommunicable diseases and coronavirus 2019 (COVID-19): epidemic cross-talk.
The figure illustrates the intricate relationship between non-communicable diseases and COVID-19. It summarizes key messages, including the hepatic tropism of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV2) and glucometabolic control and how these aspects may impact the COVID-19 postacute sequelae. In addition, the figure lists novel explanations of glucose metabolic reprogramming by SARS-CoV2 and provides potential research priorities. Abbreviations: GP73, Golgi membrane protein 1; GRP78, heat shock protein family A (Hsp70) member 5; HAT, histone acetyltransferase; HDAC, histone deacetylase; ROS, reactive oxygen species.
There are provocative, although poorly explored strategies, including virus-regulated miRNA enhancing of metabolic enzymes. First, SARS-CoV-2-miRNAs may mimic host miRNAs by sharing their seeds [7]. Then, the virus alters specific metabolic enzymes of the Krebs cycle (aconitase; ACO1) or branched amino acid degradation (branched-chain amino acid transaminase 1; BCAT1) [8]. Finally, all these metabolic processes are favored by a milieu of virus-induced lysosomal and proteasomal protein degradation [7].
Other epigenetic mechanisms involving methyltransferases and histone deacetylases, which are target promiscuous, may also explain virus-mediated glucose production by host-infected cells. Furthermore, a central regulation of PEPCK activity is acetylation/deacetylation [9].
Postacute sequelae clinical implications
The manipulation of hepatic metabolism by SARS-CoV-2 may have severe clinical implications in patients with long COVID-19. For instance, there is evidence of patients developing type 2 diabetes mellitus (T2DM) during the postacute sequelae state of COVID-19. A meta-analysis of nine studies showed that the incidence of T2DM after COVID-19 was 15.53 (7.91–25.64) per 1000 person-years with a significant relative risk (RR) of 1.62 compared with the control group [10]. The factors predisposing to this condition are not entirely clear. However, it is thought that patients with severe COVID-19 are more prone to develop T2DM compared with patients with mild disease [10].
Why do patients develop T2DM 4–6 months after acute infection with SARS-CoV-2? One potential explanation is the presence of viral persistence and chronic infection by SARS-CoV-2. In this regard, the liver and the gut might be tissues associated with persistent SARS-CoV-2 infection. The liver is immune tolerant to hepatotropic viruses, including hepatitis B and C, which cause chronic infection. By contrast, the gut may be considered an immune-privileged site for SARS-CoV-2, where the virus may evade immune system surveillance. Another putative explanation is that SARS-CoV2 induces long-lasting epigenetics alterations of the metabolism, and that T2DM develops in those individuals with scarce pancreatic reserves to manage glucose overload.
In conclusion, the bi-directional association between noncommunicable and viral diseases, including COVID-19, offers multiple perspectives on outstanding questions (Figure 1).
Acknowledgments
Acknowledgments
The funding information is as follows: PICT 2018-889, PICT 2019-0528, PICT 2018-00620, PICT 2020-799 (Agencia Nacional de Promoción Científica y Tecnológica, FONCyT).
Author contributions
Both authors contributed equally to the conception, literature review, drafting, critical revision, editing, and final approval of this paper.
Declaration of interests
No interests are declared.
References
- 1.Barreto E.A., et al. COVID-19-related hyperglycemia is associated with infection of hepatocytes and stimulation of gluconeogenesis. Proc. Natl. Acad. Sci. U. S. A. 2023;120 doi: 10.1073/pnas.2217119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirola C.J., Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanner N., et al. Molecular consequences of SARS-CoV-2 liver tropism. Nat. Metab. 2022;4:310–319. doi: 10.1038/s42255-022-00552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanley B., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Codo A.C., et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 2020;32:498–499. doi: 10.1016/j.cmet.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercado-Gomez M., et al. The spike of SARS-CoV-2 promotes metabolic rewiring in hepatocytes. Commun. Biol. 2022;5:827. doi: 10.1038/s42003-022-03789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diambra L., et al. Single cell gene expression profiling of nasal ciliated cells reveals distinctive biological processes related to epigenetic mechanisms in patients with severe COVID-19. Comput. Biol. Med. 2022;148 doi: 10.1016/j.compbiomed.2022.105895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng F., et al. Viral microRNAs encoded by nucleocapsid gene of SARS-CoV-2 are detected during infection, and targeting metabolic pathways in host cells. Cells. 2021;10:1762. doi: 10.3390/cells10071762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong Y., et al. Regulation of glycolysis and gluconeogenesis by acetylation of PKM and PEPCK. Cold Spring Harb. Symp. Quant. Biol. 2011;76:285–289. doi: 10.1101/sqb.2011.76.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T., et al. Risk for newly diagnosed diabetes after COVID-19: a systematic review and meta-analysis. BMC Med. 2022;20:444. doi: 10.1186/s12916-022-02656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]