Abstract
Objective:
Interstitial lung disease (ILD) is a leading cause of mortality in rheumatoid arthritis (RA), particularly in those with the usual interstitial pneumonia subtype (RA-UIP). Serum antibodies to peptidylarginine deiminase type 4 (anti-PAD4), particularly a subset that cross-react with PAD3 (PAD3/4XR), have been associated with imaging evidence of ILD. We aimed to determine the specificity of anti-PAD4 antibodies in RA-ILD and to examine associations with markers of ILD severity.
Methods:
48 RA-ILD and 31 idiopathic pulmonary fibrosis (IPF) patients were identified from the National Jewish Health Biobank. RA-ILD subtype was defined by imaging pattern on high-resolution chest computed tomography (CT), and serum was tested for anti-PAD4 and anti-PAD3/4XR antibodies. Antibody prevalence, measures of ILD severity (% predicted forced vital capacity, FVC; % predicted diffusion capacity carbon monoxide, DLCO; quantitative CT fibrosis) and mortality were compared between groups.
Results:
Anti-PAD4 antibodies were present in 9/48 (19%) subjects with RA-ILD and no subjects with IPF. Within RA-ILD, anti-PAD4 antibodies were found almost exclusively in RA-UIP (89%). Within RA-UIP subjects, % predicted FVC was higher in anti-PAD4+ subjects, and this finding was most strongly associated with anti-PAD3/4XR antibodies. In addition, quantitative CT fibrosis score was lower in anti-PAD4+ RA-UIP subjects, including those with mono-reactive anti-PAD4 antibodies and anti-PAD3/4XR antibodies. Anti-PAD4+ RA-UIP subjects also exhibited decreased mortality.
Conclusion:
We demonstrate the presence of serum anti-PAD4 antibodies in a subset of patients with RA-UIP that were notably associated with better lung function, less fibrosis and decreased mortality.
Keywords: Rheumatoid Arthritis, ILD, IPF, biomarkers, PAD4
INTRODUCTION
Rheumatoid arthritis (RA) primarily manifests as inflammatory arthritis, but extra-articular manifestations are frequently observed. Of these, pulmonary disease is the most common, with RA-associated interstitial lung disease (RA-ILD) being present in 20–30% of patients.1 2 When present, RA-ILD is associated with significant morbidity and contributes to death in up to 10% of patients.3–5 The most common histopathologic pattern observed in RA-ILD is usual interstitial pneumonia (UIP) followed by nonspecific interstitial pneumonia (NSIP).6 The RA-UIP subtype is associated with a worse prognosis and higher mortality than RA-NSIP.4 7 8 RA-UIP shares similar risk factors and overall clinical course with idiopathic pulmonary fibrosis (IPF), another ILD with a UIP pattern, and suggests the potential for overlapping mechanisms of pathogenesis.9 10 Anti-fibrotic agents are now approved for both IPF and RA-ILD and can modestly slow the progression of disease yet more effective and better tolerated agents are needed. Identifying biomarkers associated with better or worse lung disease severity and mortality in RA-ILD or IPF could highlight important pathways involved in disease progression or stabilization leading to improved clinical care or novel therapeutic targets.
Peptidylarginine deiminase (PAD) enzymes catalyze the calcium-dependent post-translational deimination of arginine residues to citrulline. There are five forms of PAD enzymes, PAD1 through 4 and 6, with PAD2 and PAD4 having the strongest link with RA.11 In addition to generating citrullinated autoantigens associated with RA, PAD2 and PAD4 can also be antigenic targets. Anti-PAD4 antibodies have been identified in up to 40% of patients with RA,12–14 with higher prevalence associated with longer RA disease duration.15 16 Anti-PAD4 antibodies are associated with more erosive arthritis, but paradoxically, they may predict better responsivity to treatment escalation.17
A subset of anti-PAD4 antibodies can also cross react with PAD3 (anti-PAD3/4 XR) resulting in antibodies that lower the calcium threshold required for enzyme activation of PAD4, which leads to enhanced protein citrullination.18 These antibodies are identified in up to 50% of individuals with anti-PAD4 antibodies and are also associated with more erosive arthritis.16 18 This supports the prognostic utility of anti-PAD4 and anti-PAD3/4 XR antibodies for articular disease in RA, but their relevance to extra-articular manifestations such as RA-ILD is less clear. While some studies have reported an increased prevalence of ILD in serum anti-PAD4 or anti-PAD3/4 XR positive RA patients,12 19 these studies included RA patients without clinically significant RA-ILD, did not evaluate differences in RA-ILD subtype, and did not robustly evaluate associations with lung disease severity. Additionally, we recently found that anti-PAD4 and anti-PAD3/4 XR antibodies are present in the sputum of patients with RA and can alter PAD4 enzyme activity.20 This would further support the notion that anti-PAD4 antibodies can act directly in the lung, which could influence the pathogenesis of RA-related lung diseases such as RA-ILD. In this study, we sought to identify the specificity and associations with lung disease severity of serum anti-PAD4 antibodies in RA-ILD.
METHODS
Study population.
We selected all subjects with a diagnosis of RA-ILD from a larger biospecimen database at National Jewish Health of patients prospectively enrolled into a Specialized Center of Research (SCOR) under Institutional Review Board # HS-1603. All RA-ILD subjects included had been evaluated clinically by a board-certified rheumatologist and a pulmonologist specialized in ILD to confirm their diagnosis of RA and RA-ILD, respectively.21 Subgroups of RA-ILD were defined by imaging pattern on high-resolution computed tomography (HRCT) scan as either RA-UIP (including “definite” and “probable” UIP per current guidelines22) or RA-NSIP as determined by an expert thoracic radiologist. Patients with an HRCT pattern other than UIP or NSIP were excluded. We randomly selected 35 subjects with IPF from the same database. Upon chart review, four did not meet 2018 American Thoracic Society guidelines for the diagnosis of IPF.23 All serum samples were obtained between 2004 and 2012. The study was performed in the accordance with the Declaration of Helsinki. All subjects provided written informed consent to participate in the study.
Serum autoantibody testing.
Serum from all subjects was tested by ELISA for anti-citrullinated protein antibodies (ACPA) using anti-cyclic citrullinated peptide (CCP)3.1 (IgG/IgA, Inova Diagnostics, Inc. San Diego, CA, USA) and for rheumatoid factor (RF) using RF-IgA and RF-IgM (Inova). Positive cut-off levels were based on manufacturers recommendations. Serum anti-PAD4 antibodies were identified by immunoprecipitation of S35-labeled PAD4 protein as previously described.13 Anti-PAD4 positive sera were then evaluated for reactivity to in vitro transcribed and translated PAD3, as previously described.18 Immunoprecipitated proteins were separated by SDS-PAGE, visualized by radiography, and quantified by densitometry. A densitometry value >0.02 was considered positive. Within anti-PAD4 positive samples, sera that reacted to PAD4 but not PAD3 were defined as anti-PAD4 mono-reactive, and sera that reacted to both PAD4 and PAD3 were defined as anti-PAD3/4XR+.
Lung disease severity.
Pulmonary function tests (PFT) were used as one measure of lung disease severity and included % predicted forced vital capacity (%FVC) and % predicted diffusion capacity for carbon monoxide (%DLCO). PFTs included in this study were obtained during routine clinical care and were only used for analysis if collected within 3 months of serum collection. In RA-UIP subjects, another measure of lung disease severity included a quantitative measurement of lung fibrosis extent on HRCT. In a subgroup of subjects, fibrosis on HRCT was quantified using a data-driven texture analysis (DTA) fibrosis score as previously described.24–26 This approach applies machine learning to detect and quantify lung fibrosis (including reticular abnormalities, honeycombing and traction bronchiectasis) on HRCT images and expresses an extent score as the percentage of total lung volume involved. HRCT scans included in this study were obtained during routine clinical care and had imaging quality that was adequate for DTA analysis based on the following criteria: (1) axial slice thickness ≤1.25 mm and (2) axial slice spacing ≤10 mm. Adequate HRCT scans were performed a median (IQR) of 2 (0–39) months from the time of serum collection.
Mortality.
The date of death for all RA-ILD subjects was obtained from the Center for Disease Control and Prevention’s National Death Index, which can match research subjects to US death certificate records. Death certificates were queried through November 2019. Any subject without a US death certificate at the time of query was considered to be alive as of November 2019.
Statistical Analyses.
Clinical characteristics, prevalence of serum autoantibodies, measures of lung physiology and DTA fibrosis scores were compared using Chi-square, t-tests and Wilcoxon rank sum test, as appropriate. Kaplan Meier method with log-rank test was used to compare survival curves of the RA-UIP group based on anti-PAD4 positivity. Cox proportional hazards regression was used to examine the relationship between anti-PAD4 antibodies and mortality adjusted for GAP index, which is a composite score based on gender, age, and pulmonary physiology that has been shown to be a predictor of mortality in RA-ILD.27 All statistical analyses were performed using SPSS Statistics version 27. We considered p<0.05 to represent statistical significance.
RESULTS
Subject Demographics.
We identified 48 RA-ILD subjects and 31 IPF subjects with serum available from the NJH Biobank. Subject demographics are outlined in Table 1. Of the 48 RA-ILD subjects, 37 were characterized as RA-UIP and 11 were characterized as RA-NSIP based on HRCT review. There were significant differences in age across all groups with the IPF cohort being the oldest and RA-NSIP being the youngest. The IPF cohort had significantly less females (19%) compared to both the RA-UIP (49%) and RA-NSIP (55%) groups. There were no differences in smoking history or PFT measurements across all groups.
Table 1.
Characteristics, Pulmonary Function and Serum Antibody Testing in RA-ILD and IPF
| RA-ILD (n=48) | RA-UIP (n=37) | RA-NSIP (n=11) | IPF (n=31) | p-value* |
||||
|---|---|---|---|---|---|---|---|---|
| RA-ILD vs IPF | RA-UIP vs IPF | RA-NSIP vs IPF | RA-UIP vs NSIP | |||||
| Age | 62 ± 12 | 64 ± 11 | 56 ± 13 | 69 ± 8 | <0.01 | 0.03 | <0.01 | 0.05 |
| Female | 24 (50) | 18 (49) | 6 (55) | 6 (19) | <0.01 | 0.02 | 0.05 | NS |
| Ever Smoker** | 25 (53) | 20 (56) | 5 (45) | 21 (68) | NS | NS | NS | NS |
| Smoking pack years** | 13 ± 18 | 14 ± 19 | 10 ± 15 | 23 ± 27 | NS | NS | NS | NS |
| % predicted FVC** | 69 ± 20 | 69 ± 19 | 68 ± 23 | 67 ± 15 | NS | NS | NS | NS |
| % predicted DLCO** | 47 ± 18 | 45 ± 17 | 53 ± 22 | 44 ± 16 | NS | NS | NS | NS |
| Anti-CCP+ | 34 (71) | 26 (70) | 8 (11) | 10 (32) | <0.01 | <0.01 | 0.03 | NS |
| RF-IgA+ | 30 (63) | 24 (65) | 6 (55) | 6 (19) | <0.01 | <0.01 | 0.05 | NS |
| RF-IgM+ | 35 (73) | 29 (78) | 6 (55) | 0 (0) | <0.01 | <0.01 | <0.01 | NS |
| Anti-PAD4+ | 9 (19) | 8 (22) | 1 (9) | 0 (0) | 0.01 | <0.01 | NS | NS |
All values displayed as mean ± SD or N (%).
Based on Chi-square or t-test where appropriate, NS = not significant, p>0.05.
Clinical data was missing for: Ever smoking for 1 RA-UIP; pack years for 1 RA-NSIP, 4 RA-UIP, 3 IPF; FVC for 2 RA-UIP, 5 IPF; DLCO for 4 RA-UIP, 7 IPF.
Serum anti-CCP and RF autoantibodies.
Subjects with RA-ILD had a higher frequency of anti-CCP (71% vs 32%, p<0.01), RF-IgA (63% vs 19%, p<0.01) and RF-IgM (73% vs 0%, p<0.01) positivity compared to IPF subjects (Table 1). There were no significant differences in anti-CCP, RF-IgA and RF-IgM positivity between RA-UIP and RA-NSIP subjects.
Serum anti-PAD4 autoantibodies.
Nine out of 48 RA-ILD subjects were anti-PAD4+ compared to no subjects in the IPF group (19% vs 0%, p=0.01). Eight of the 9 (89%) RA-ILD subjects with anti-PAD4 antibodies had a UIP pattern on imaging. Four of the 8 anti-PAD4+ RA-UIP subjects were anti-PAD4 mono-reactive while the other 4 anti-PAD4+ subjects had anti-PAD3/4XR antibodies. There were no significant differences in age, sex or ever smoking history based on anti-PAD4 mono-reactive or anti-PAD3/4XR antibody positivity (Table 2). Anti-PAD4 mono-reactive RA-UIP patients had lower pack-years of smoking.
Table 2.
Differences in Characteristics and Lung Disease Severity Based on Serum anti-PAD4 and anti-PAD3/4XR Positivity in RA-UIP
| Anti-PAD4− (n=29) | Anti-PAD4+ (n=8) | Anti-PAD4+ mono-reactive (n=4) | Anti-PAD3/4XR+ (n=4) |
p-value* |
|||
|---|---|---|---|---|---|---|---|
| PAD4+ vs PAD4− | PAD4+ mono-reactive vs PAD4− | PAD3/4XR+ vs PAD4− | |||||
| Age | 64 ± 12 | 62 ± 7 | 64 ± 12 | 60 ± 6 | NS | NS | NS |
| Female | 13 (45) | 5 (63) | 50% | 75% | NS | NS | NS |
| Ever smoker** | 14 (50) | 6 (75) | 2 (50) | 4 (100) | NS | NS | NS |
| Smoking pack years** | 14 ± 20 | 14 ± 15 | 3 ± 5 | 23 ± 14 | NS | <0.05 | NS |
| Anti-CCP+ | 20 (69) | 6 (75) | 2 (50) | 4 (100) | Ns | NS | NS |
| RF-IgA+ | 18 (62) | 6 (75) | 3 (75) | 3 (75) | NS | NS | NS |
| RF-IgM+ | 21 (72) | 8 (100) | 4 (100) | 4 (100) | NS | NS | NS |
| % predicted FVC** | 65 ± 20 | 82 ± 12 | 78 ± 8 | 87 ± 16 | 0.02 | NS | 0.04 |
| % predicted DLCO** | 43 ± 17 | 51 ± 15 | 48 ± 12 | 54 ± 19 | NS | NS | NS |
| DTA fibrosis score | 40 ± 19 | 17 ± 8 | 16 ± 11 | 17 ± 7 | <0.01 | 0.02 | 0.03 |
All values displayed as mean ± SD or N (%).
Based on Chi-square or Kruskal-Wallis where appropriate, NS = not significant, p>0.05.
Clinical data was missing for: Ever smoking for 1 PAD4−; pack years for 3 PAD4−,1 PAD4+; FVC for 2 PAD4−; DLCO % 4 PAD4−
Anti-PAD4 antibodies and lung disease severity in RA-UIP.
In subjects with RA-UIP, anti-PAD4 positive subjects had a higher %FVC compared to anti-PAD4 negative subjects (82% vs 65%, p=0.02) (Table 2). Similar analyses were not performed in RA-NSIP subjects as there was only one anti-PAD4 positive subject. In addition, there was a significantly lower degree of lung fibrosis measured quantitatively in anti-PAD4 positive compared to anti-PAD4 negative RA-UIP subjects (median DTA fibrosis score, 14.6 vs 40.3, p<0.01, Table 2), a difference that persists when limiting analysis to only scans within 3 years of serum collection (13.1 vs 40.3, p=0.02). We compared markers of lung disease severity in anti-PAD4 positive RA-UIP subjects subgrouped by anti-PAD4 mono-reactive (n=4) or anti-PAD3/4XR (n=4) antibodies compared to anti-PAD4 negative RA-UIP subjects (n=29). The mean %FVC was lowest in anti-PAD4 negative subjects (65%), higher in anti-PAD4 mono-reactive subjects (78%) and highest in anti-PAD3/4XR+ subjects (87%) (Table 2). The %FVC was significantly different between anti-PAD4 negative and anti-PAD3/4XR positive subjects (p=0.04) (Figure 1, panel A). In a similar trend, mean %DLCO was lowest in anti-PAD4 negative subjects (43%), higher in anti-PAD4 mono-reactive (48%) and highest in anti-PAD3/4XR+ subjects (54%), although these differences were not statistically significant (Figure 1, panel B). Lastly, when using quantitative HRCT, there was a significantly lower degree of lung fibrosis in both the anti-PAD4 mono-reactive and anti-PAD3/4XR+ subjects compared to anti-PAD4 negative subjects (mean DTA fibrosis score, 16 vs. 17 vs. 40, respectively) (Figure 1, panel C).
Figure 1. Lung disease severity in anti-PAD4+ RA-UIP.
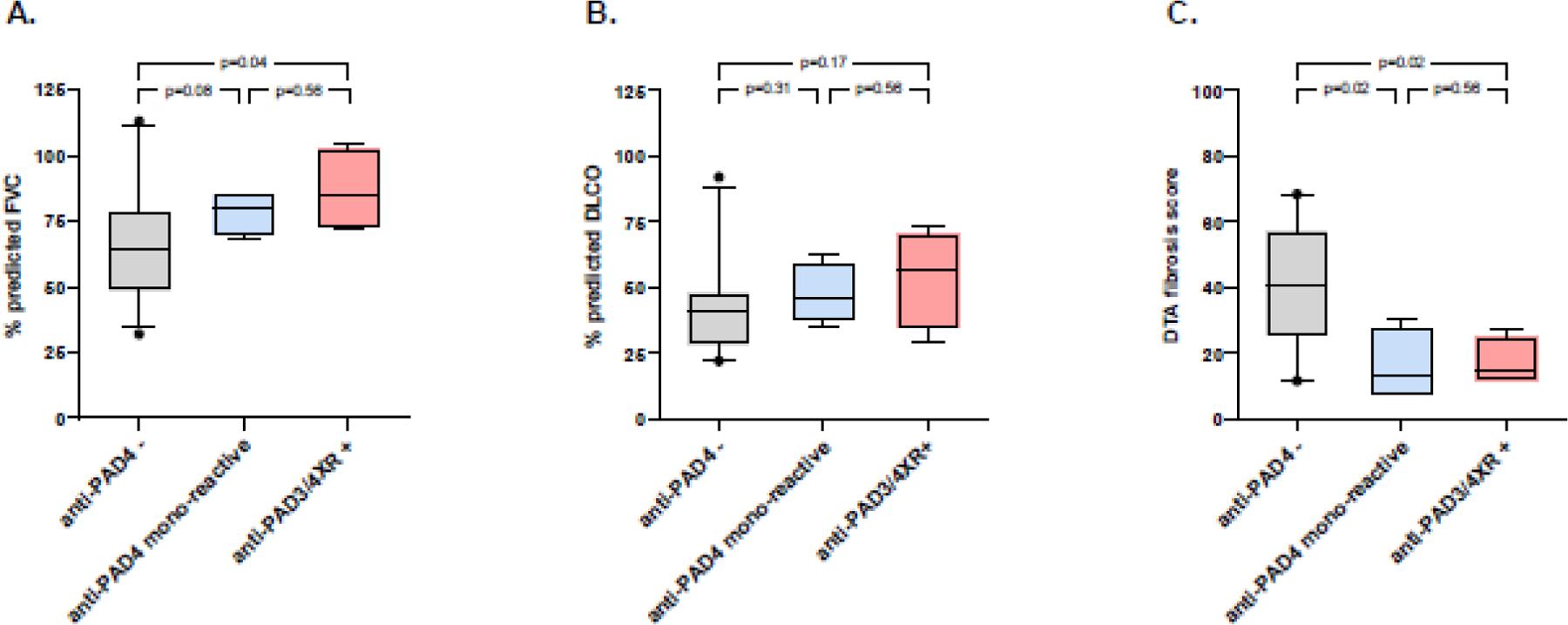
Box plots characterizing the % predicted FVC (panel A), % predicted DLCO (panel B), and DTA fibrosis score (panel C) for subjects with RA-UIP when stratified by anti-PAD antibody positivity (anti-PAD4-, n=29; anti-PAD4+ mono-reactive, n=4; anti-PAD3/4XR+, n=4). Lower and upper box boundaries designate interquartile range (25th to 75th percentile). Line inside box designates median. Lower and upper error lines designate 5th and 95th percentile, respectively, and black circles note designate points falling outside of this range. P-value based on Mann-Whitney test.
Anti-PAD4 antibodies and mortality in RA-UIP.
Survival from the time of serum collection through November 2019 was obtained for all RA-UIP subjects. The median survival was significantly longer in anti-PAD4 positive compared to anti-PAD4 negative RA-UIP subjects (10.6 years vs 4.8 years, p=0.02) (Figure 2). In a cox proportional hazards regression model adjusting for GAP index score (accounting for sex, age, and lung function), anti-PAD4 antibody positivity continued to be associated with lower mortality but was not statistically significant (HR:0.38, 95% CI 0.12 – 1.18, p=0.09). Moreover, there was a significant association between higher anti-PAD4 antibody levels and lower mortality (HR: 0.31, 95% CI 0.10 – 1.01, p=0.05) (Table 3). Of note, the median date of serum collection was similar between anti-PAD4 positive and anti-PAD4 negative RA-UIP subjects (June 2008 vs May 2008, respectively).
Figure 2. Survival curve in RA-UIP stratified by anti-PAD4 antibody positivity.
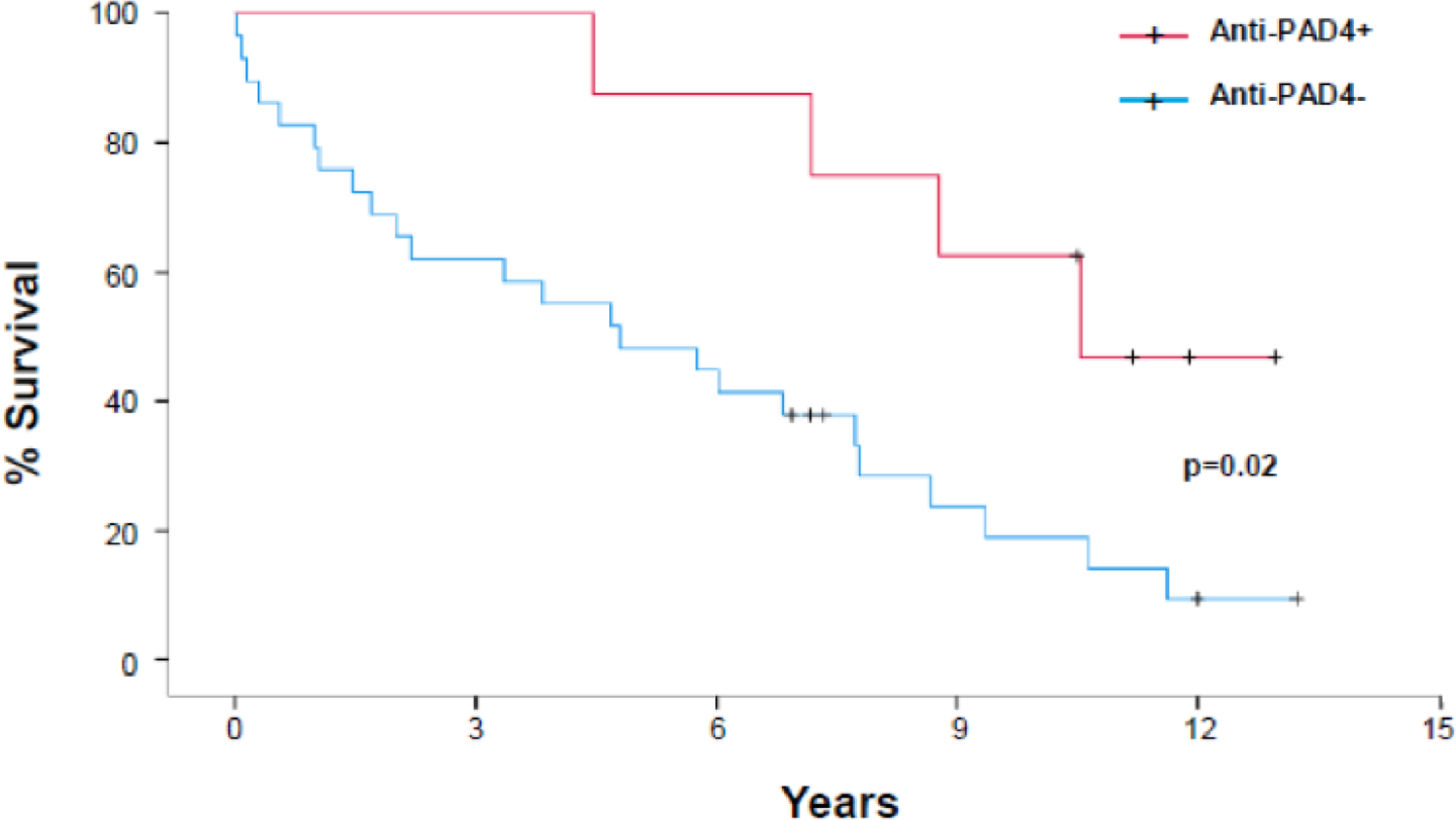
Kaplan Meier estimates for % survival of all RA-UIP subjects stratified by anti-PAD4 positivity (red line – positive, blue line – negative).
Table 3.
Cox Proportional Regression Model
|
Unadjusted |
Adjusted* |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Anti-PAD4 positive | 0.31 | 0.11 – 0.90 | 0.02 | 0.38 | 0.12 – 1.18 | 0.09 |
| Anti-PAD4 antibody levels | 0.34 | 0.11 – 1.08 | 0.07 | 0.31 | 0.10 – 1.01 | 0.05 |
| Anti-PAD3/4 antibody levels | 0.08 | 0.002 – 3.24 | 0.18 | 0.18 | 0.01 – 4.86 | 0.30 |
Model adjusted for GAP index (score based on sex, age, % predicted FVC, and % predicted DLCO
DISCUSSION
Our study found that serum anti-PAD4 antibodies were associated with better lung function, lower quantitative scores of fibrosis and improved survival in subjects with RA-UIP. To our knowledge, this is the first study characterizing lung disease severity associated with anti-PAD4 antibodies in specific subtypes of clinically apparent RA-ILD. Our findings suggest that anti-PAD4 antibodies in RA patients with ILD may modulate the pathogenesis of ILD leading to a less fibrotic phenotype with slower progression. Because RA-UIP is associated with more lung fibrosis and portends a worse prognosis compared to other forms of ILD in RA, such as RA-NSIP, the association between anti-PAD4 and outcomes in RA-UIP could help clinicians prognosticate for their patients and inform decisions on the initiation of therapy or referral for transplant. It could also facilitate cohort selection in future treatment trials in RA-ILD by enriching for those at higher risk of progression and death. Testing for anti-PAD4 opens the door to identify RA-specific mechanisms involved in the pathogenesis of lung fibrosis, including the potential for protective pathways that could lead to novel treatment approaches.
We also found that anti-PAD4 antibodies were specific (100%) for RA-ILD compared to IPF and were more common in RA-UIP compared to RA-NSIP. This finding suggests that despite the clinical overlap and possible overlapping mechanisms driving fibrosis in IPF and RA-UIP, anti-PAD4 antibodies may play a unique role in the pathogenesis of RA-UIP. Importantly, serum anti-PAD4 antibodies are not likely specific for ILD within an RA cohort. In fact, the prevalence of anti-PAD4 antibodies in our study was on the lower end of reported prevalence rates in RA cohorts.12 19 Of note, a recent study by Palterer and colleagues found that the presence of ILD was associated with lower anti-PAD4 antibody levels compared to RA patients without ILD.28 Given that our cohort only included RA patients with ILD, these findings could explain why we observed a somewhat lower prevalence of anti-PAD4 antibody positivity in our study. Our findings that anti-PAD4 antibodies were associated with less lung fibrosis in RA patients with ILD in combination with the findings from Palterer et al that anti-PAD4 antibody levels were lower in RA patients with ILD support future longitudinal studies of large RA cohorts that can determine whether anti-PAD4 antibodies have a protective role in the development of RA-ILD.
Our group recently published work that anti-PAD4 antibodies can be present and activate PAD4 enzyme function in the lung of RA patients.20 Future studies are needed to establish the specific mechanisms by which anti-PAD4 antibodies may influence the pathogenesis of ILD, such as increased citrullination of proteins in the lung or direct effects on lung-specific cells (i.e. airway epithelial cells or lung fibroblasts) or immunomodulatory cells recruited to the lungs. It is of interest that prior studies have consistently found anti-PAD4 antibodies, particularly the anti-PAD3/4XR subset, associated with more severe and erosive joint disease in RA16 17 19 yet we found these same antibodies associated with less severe lung disease in RA. These seemingly discrepant findings are likely highlighting the importance of site of action for anti-PAD4 antibodies. Different target cells and different effects of citrullinated proteins and inflammation between the joints and lungs could drive worsening disease at one site while driving less disease at another site.
Prior studies have described an association between the presence of serum anti-PAD4 antibodies and the presence of subclinical ILD in RA patients.12 19 Specifically, Giles and colleagues previously found that anti-PAD3/4XR antibodies were more prevalent in RA patients with radiographic evidence of ILD found on screening scans compared to those with normal CT imaging. In that study, antibody positivity was not associated with abnormalities of pulmonary physiology, suggesting that anti-PAD3/4XR antibodies were associated with early, mild ILD. If anti-PAD4 antibodies play a role in limiting the severity of RA-ILD, as suggested by our current study, this could potentially explain the increased frequency of anti-PAD3/4XR antibodies identified in RA patients with ILD that is mild and not associated with alterations in physiology. Longitudinal studies are needed in RA-ILD, including patients with subclinical disease, to better understand the association of anti-PAD4 antibodies and ILD progression over time.
While there were more ever smokers in RA-UIP anti-PAD4+ group compared to the RA-UIP anti-PAD4- subjects, this relationship was not significant. The relationship between smoking and anti-PAD4 antibodies remains poorly understood. Giles et al found that in patients with anti-PAD3/4XR antibodies, the probability of having radiographic evidence of ILD was significantly higher in subjects with prior smoke exposure compared to never smokers (93% vs 39%, p=0.03). Conversely, Cappelli et al found no association between smoking history and anti-PAD4 positivity in a cohort of 274 RA patients.29 Future studies are needed to better characterize this relationship.
There are several limitations to our study. First, our findings are based on a cross sectional analysis using a relatively small sample population from a single cohort. While we were able to obtain follow-up data on these subjects for mortality, future prospective longitudinal studies that include larger sample sizes are still needed to better understand associations of anti-PAD4 antibodies and disease progression. Second, our mortality outcome was all cause mortality, and we cannot definitively determine the contribution of the patent’s ILD on their mortality. Of note, our study evaluated mortality outcomes prior to the onset of the COVID-19 pandemic, which eliminated any confounding factors associated with mortality and COVID-19. Third, articular disease severity, such as erosive status or disease activity scores, were not available for this cohort, thus we were unable to correlate anti-PAD4 status with joint manifestations. Lastly, the current study was also limited to serum and future studies are needed to evaluate the presence and activity of anti-PAD4 antibodies within the lung in RA-ILD.
CONCLUSION
We demonstrate that serum anti-PAD4 antibodies were specific for RA-ILD when compared to IPF and in RA-UIP are associated with better lung function, less lung fibrosis and improved survival. These results support future studies that can further characterize the utility of anti-PAD4 antibodies as prognostic biomarkers in RA-ILD as well as mechanistic studies focused on enhancing our understanding of how these antibodies modulate lung disease progression.
FUNDING SOURCES
This work was supported by the National Institutes of Health [grant numbers AR066712 and AR07534]. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The funding sources had no involvement in the study design, data collection or analysis, writing of the manuscript or decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors disclose the following potential conflicts: ED and MKD have received research support from Pfizer Inc. on studies related to RA-related autoimmunity and ILD. ED has received research support from Bristol Myers Squibb and Celgene. SMH is an author on licensed patent no. 10,706,533, entitled “Systems and methods for automatic detection and quantification using dynamic feature classification”. ED is an author on licensed patent no. 8,975,033, entitled “Human autoantibodies specific for pad3 which are cross-reactive with pad4 and their use in the diagnosis and treatment of rheumatoid arthritis and related diseases”. All other authors have declared that no conflict of interest exists.
REFERENCES
- 1.Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997;156(2 Pt 1):528–35. doi: 10.1164/ajrccm.156.2.9609016 [published Online First: 1997/08/01] [DOI] [PubMed] [Google Scholar]
- 2.Habib HM, Eisa AA, Arafat WR, et al. Pulmonary involvement in early rheumatoid arthritis patients. Clin Rheumatol 2011;30(2):217–21. doi: 10.1007/s10067-010-1492-5 [published Online First: 2010/05/27] [DOI] [PubMed] [Google Scholar]
- 3.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183(3):372–8. doi: 10.1164/rccm.201004-0622OC [published Online First: 2010/09/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto MA, Rodriguez-Nieto MJ, Sanchez-Pernaute O, et al. Mortality rate in rheumatoid arthritis-related interstitial lung disease: the role of radiographic patterns. BMC Pulm Med 2021;21(1):205. doi: 10.1186/s12890-021-01569-5 [published Online First: 2021/07/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyldgaard C, Ellingsen T, Hilberg O, et al. Rheumatoid Arthritis-Associated Interstitial Lung Disease: Clinical Characteristics and Predictors of Mortality. Respiration 2019;98(5):455–60. doi: 10.1159/000502551 [published Online First: 2019/10/10] [DOI] [PubMed] [Google Scholar]
- 6.Kim EJ, Collard HR, King TE Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 2009;136(5):1397–405. doi: 10.1378/chest.09-0444 [published Online First: 2009/11/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35(6):1322–8. doi: 10.1183/09031936.00092309 [published Online First: 2009/12/10] [DOI] [PubMed] [Google Scholar]
- 8.Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016;47(2):588–96. doi: 10.1183/13993003.00357-2015 [published Online First: 20151119] [DOI] [PubMed] [Google Scholar]
- 9.Paulin F, Doyle TJ, Fletcher EA, et al. Rheumatoid Arthritis-Associated Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis: Shared Mechanistic and Phenotypic Traits Suggest Overlapping Disease Mechanisms. Rev Invest Clin 2015;67(5):280–6. [published Online First: 2015/12/24] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon JJ, Matson S, Kelmenson LB, et al. IgA Antibodies Directed Against Citrullinated Protein Antigens Are Elevated in Patients With Idiopathic Pulmonary Fibrosis. Chest 2020;157(6):1513–21. doi: 10.1016/j.chest.2019.12.005 [published Online First: 20191223] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witalison EE, Thompson PR, Hofseth LJ. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr Drug Targets 2015;16(7):700–10. doi: 10.2174/1389450116666150202160954 [published Online First: 2015/02/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Zhao Y, He J, et al. Prevalence and significance of anti-peptidylarginine deiminase 4 antibodies in rheumatoid arthritis. J Rheumatol 2008;35(6):969–74. [published Online First: 20080401] [PubMed] [Google Scholar]
- 13.Harris ML, Darrah E, Lam GK, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum 2008;58(7):1958–67. doi: 10.1002/art.23596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halvorsen EH, Pollmann S, Gilboe IM, et al. Serum IgG antibodies to peptidylarginine deiminase 4 in rheumatoid arthritis and associations with disease severity. Ann Rheum Dis 2008;67(3):414–7. doi: 10.1136/ard.2007.080267 [published Online First: 2007/11/17] [DOI] [PubMed] [Google Scholar]
- 15.Ferucci ED, Darrah E, Smolik I, et al. Prevalence of anti-peptidylarginine deiminase type 4 antibodies in rheumatoid arthritis and unaffected first-degree relatives in indigenous North American Populations. J Rheumatol 2013;40(9):1523–8. doi: 10.3899/jrheum.130293 [published Online First: 2013/08/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarro-Millan I, Darrah E, Westfall AO, et al. Association of anti-peptidyl arginine deiminase antibodies with radiographic severity of rheumatoid arthritis in African Americans. Arthritis Res Ther 2016;18(1):241. doi: 10.1186/s13075-016-1126-7 [published Online First: 2016/10/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darrah E, Yu F, Cappelli LC, et al. Association of Baseline Peptidylarginine Deiminase 4 Autoantibodies With Favorable Response to Treatment Escalation in Rheumatoid Arthritis. Arthritis Rheumatol 2019;71(5):696–702. doi: 10.1002/art.40791 [published Online First: 2018/12/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darrah E, Giles JT, Ols ML, et al. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci Transl Med 2013;5(186):186ra65. doi: 10.1126/scitranslmed.3005370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giles JT, Darrah E, Danoff S, et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS One 2014;9(6):e98794. doi: 10.1371/journal.pone.0098794 [published Online First: 20140605] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demoruelle MK, Wang H, Davis RL, et al. Anti-peptidylarginine deiminase-4 antibodies at mucosal sites can activate peptidylarginine deiminase-4 enzyme activity in rheumatoid arthritis. Arthritis Res Ther 2021;23(1):163. doi: 10.1186/s13075-021-02528-5 [published Online First: 2021/06/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62(9):2569–81. doi: 10.1002/art.27584 [published Online First: 2010/09/28] [DOI] [PubMed] [Google Scholar]
- 22.Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 2018;6(2):138–53. doi: 10.1016/S2213-2600(17)30433-2 [published Online First: 2017/11/21] [DOI] [PubMed] [Google Scholar]
- 23.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 24.Humphries SM, Swigris JJ, Brown KK, et al. Quantitative high-resolution computed tomography fibrosis score: performance characteristics in idiopathic pulmonary fibrosis. Eur Respir J 2018;52(3) doi: 10.1183/13993003.01384-2018 [published Online First: 2018/08/25] [DOI] [PubMed] [Google Scholar]
- 25.Humphries SM, Yagihashi K, Huckleberry J, et al. Idiopathic Pulmonary Fibrosis: Data-driven Textural Analysis of Extent of Fibrosis at Baseline and 15-Month Follow-up. Radiology 2017;285(1):270–78. doi: 10.1148/radiol.2017161177 [published Online First: 2017/05/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathai SK, Humphries S, Kropski JA, et al. MUC5B variant is associated with visually and quantitatively detected preclinical pulmonary fibrosis. Thorax 2019;74(12):1131–39. doi: 10.1136/thoraxjnl-2018-212430 [published Online First: 2019/09/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morisset J, Vittinghoff E, Lee BY, et al. The performance of the GAP model in patients with rheumatoid arthritis associated interstitial lung disease. Respir Med 2017;127:51–56. doi: 10.1016/j.rmed.2017.04.012 [published Online First: 2017/05/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palterer B, Vitiello G, Del Carria M, et al. Anti-protein arginine deiminase antibodies are distinctly associated with joint and lung involvement in rheumatoid arthritis. Rheumatology (Oxford) 2022. doi: 10.1093/rheumatology/keac667 [published Online First: 20221128] [DOI] [PubMed]
- 29.Cappelli LC, Konig MF, Gelber AC, et al. Smoking is not linked to the development of anti-peptidylarginine deiminase 4 autoantibodies in rheumatoid arthritis. Arthritis Res Ther 2018;20(1):59. doi: 10.1186/s13075-018-1533-z [published Online First: 20180323] [DOI] [PMC free article] [PubMed] [Google Scholar]


