Abstract
Objectives:
The purpose of this study was to measure sexually transmitted infection (STI) testing among Medicaid enrollees initiating preexposure prophylaxis (PrEP) to prevent human immunodeficiency virus. Secondary data are in the form of Medicaid enrollment and claims data in six states in the US South.
Methods:
Research partnerships in six states in the US South developed a distributed research network to accomplish study aims. Each state identified all first-time PrEP users in fiscal year 2017–2018 (combined N = 990) and measured the presence of STI testing for chlamydia, syphilis, and gonorrhea through 2019. Each state calculated the percentage of individuals with at least one STI test during 3-, 6-, and 12-month follow-up periods.
Results:
The proportion of first-time PrEP users that received an STI test varied by state: 37% to 67% of all of the individuals in each state who initiated PrEP received a test within the first 6 months of PrEP treatment and 50% to 77% received a test within the first 12 months.
Conclusions:
Although the Centers for Disease Control and Prevention recommends STI testing at least every 6 months for PrEP users, our analysis of Medicaid data suggests that STI testing occurs less frequently than recommended in populations at elevated risk of syphilis, gonorrhea, and chlamydia.
Keywords: delivery of health care, health services research, HIV, Medicaid, sexually transmitted infections
Continuing the decades-long progress toward ending the human immunodeficiency virus (HIV) epidemic, the United States experienced an 8% decline in new HIV diagnoses since 2015, with 34,800 new HIV infections in 2019.1 According to the latest data from the Centers for Disease Control and Prevention (CDC), 1.2 million people in the United States aged 13 and older were living with HIV at the end of 2019.1 The HIV National Strategic Plan recently set a target to reduce new infections by 90% by 2030.2 A key strategy in achieving this goal is expanding and improving the implementation of preventive interventions, with a focus on preexposure prophylaxis (PrEP). In 2019, the US Preventive Services Task Force recommended offering PrEP to individuals who are at high risk of HIV acquisition.3,4
Meanwhile, the United States is facing an epidemic of bacterial sexually transmitted infections (STIs). Rates of chlamydia, gonorrhea, and syphilis have risen considerably in recent years—by 20%, 50%, and 70%, respectively, from 2015 to 2019—with significant racial and ethnic disparities persisting.5 Increasing rates of syphilis among women led to the highest number of congenital syphilis cases since 1995 in 2019.5 STIs also greatly increase the susceptibility and transmission of HIV.6 Identifying at-risk individuals and ensuring that they receive routine STI testing is an effective method for reducing the public health burden of bacterial STIs.
Because HIV and STI risk is strongly associated through common risk factors, including risky sexual behaviors, PrEP users are an important population to reach with STI testing services.7 To identify and treat STIs among PrEP users, the CDC’s 2017 clinical practice guidelines for PrEP recommended STI testing for all adults at PrEP initiation and during regular follow-up visits.8 In 2021, the CDC released updated STI treatment guidelines that reiterate the 2017 PrEP treatment guidelines, recommending STI screening for individuals taking PrEP every 3 to 6 months. The importance of including STI services in PrEP care is underscored by the phenomenon of risk compensation.9,10 Regardless of whether and where risk compensation is occurring, PrEP guidelines that result in better STI detection and treatment could offset increases in risky sexual behavior.9 A simulation study found that an increase in STI testing and treatment would decrease the overall burden of bacterial STIs making PrEP rollout and STI control synergistic in populations with low base rates of STI testing.11,12
HIV and Bacterial STIs in the Southern United States
The highest HIV infection rate is currently found in the US South, with 15.2 new HIV cases per 100,000 population in 2019, compared with 11.1/100,000 nationwide. The high overall rate of HIV transmission in the US South is mirrored by high rates of transmission among several key populations of interest for HIV prevention. For example, although approximately 38% of the total US population is concentrated in the South, the proportion of 2019 HIV diagnoses that occurred in the South was 55% for females and 51% for men who have sex with men.1
Residents of the US South also are at heightened risk for bacterial STI infections. Counties in the US South are at least four times as likely compared with counties in other regions to be a hotspot for chlamydia, gonorrhea, or syphilis.13 Interestingly, the racial/ethnic composition of counties also can increase the risk of a county being identified as a hotspot. This is because the risk of bacterial STI is elevated for people who are non-Hispanic Black, American Indian/Alaska Native, or Hispanic because of the underlying social contexts, which include greater exposure to poverty, discrimination, incarceration, and residential segregation.5,14 The same racial and ethnic disparities are observed for HIV risk in all regions of the United States, and these differences intersect with other HIV risk factors such as number of sexual partners.1 Given a shared set of key risk factors for both HIV and STI, targeting STI testing to those taking PrEP may be a powerful strategy for prevention.
Medicaid is the largest payer for HIV treatment services, and all 50 states cover PrEP in their Medicaid programs.15,16 Because states differ in Medicaid policy, there may be considerable variation in the implementation of public health measures that are intended to decrease HIV and bacterial STI infection rates, such as increasing PrEP availability or improving STI surveillance. The ratio of PrEP use to new HIV diagnoses is known as the PrEP-to-need ratio, with a lower ratio indicating more unmet need. In 2017, the ratio in the South was 1.5 compared with the highest rate in the Northeast of 4.7.17,18 As of 2021, only 12 states had not adopted Medicaid expansion and 8 were in the US South, making the South the region with the lowest uptake of Medicaid expansion.19
Present Study
There are no studies examining STI testing among Medicaid enrollees using PrEP focused solely on the US South. Furthermore, few studies examine PrEP initiation in the US South more generally. Multistate studies using a distributed research network (DRN) approach present an opportunity to provide these needed data. Because of the high prevalence of HIV in the South, the present study leveraged a DRN in six southern states (Georgia, Kentucky, Louisiana, North Carolina, South Carolina, and Tennessee). The aim of the study was to estimate the rate of PrEP initiation among Medicaid enrollees and to measure the percentage of first-time PrEP users with at least one STI test (syphilis or chlamydia/gonorrhea) by 3, 6, and 12 months following PrEP initiation, among Medicaid beneficiaries, overall and among specific demographic subgroups, between 2017 and 2019.
Methods
DRN Approach
We developed a study design and structure modeled after the Medicaid Outcomes Distributed Research Network multistate study of opioid use disorder treatment and outcomes among Medicaid beneficiaries.20,21 Six state-university partnerships for the STI Testing Among Medicaid Patients (STAMP) study were selected from members participating in the AcademyHealth State-University Partnership Learning Network and/or Medicaid Outcomes Distributed Research Network. These state-university partnerships comprise state Medicaid agency partners and university researchers who provide data analytic support to inform Medicaid policy and cost–benefit analyses. One state served as the coordinating center to pilot analytic plans and collect aggregate results from states. Another external organization AcademyHealth facilitated processes related to data use agreements, contracting, and dissemination of findings. This study was reviewed and approved by the institutional review boards at the six participating universities.
Study Sample
The study sampling frame consisted of Medicaid beneficiaries enrolled between July 1, 2017 and June 30, 2018 in 6 southern states. The study criteria excluded anyone dually enrolled in Medicare and Medicaid or outside of the 16 to 64 years age range at the start of the study (July 1, 2017). We then applied a PrEP algorithm used in prior studies to Medicaid claims to identify individuals prescribed PrEP between July 1, 2017 and June 30, 2018.12,18 Following this algorithm, we excluded those who had an HIV diagnosis or treatment or a hepatitis B diagnosis or treatment before the first prescription of PrEP. Those with a PrEP prescription for 30 or fewer days also were assumed to be receiving treatment, or postexposure prophylaxis, and were excluded. To identify first-time PrEP users, we also excluded anyone who received a PrEP prescription up to 1 year before the index prescription. To ensure that individuals were enrolled in Medicaid during the follow-up period, and therefore detectable in our data for an STI test, individuals needed to be enrolled continuously during the follow-up period after index prescription, with up a 30-day maximum gap. For example, for the 3-month follow-up period, we required at least 60 days of Medicaid enrollment during that period. Because there is a trade-off between sample size and Medicaid enrollment restriction, we conducted a sensitivity test of the 12-month follow-up period using samples that required either 150 days enrollment or 335 days enrollment.
Measures
STI tests were identified in Medicaid reimbursement claims using Current Procedural Technology codes to identify testing for syphilis (86592, 86593, and 86780) and chlamydia/gonorrhea (87590, 87591, 87592, 87850, 86631, 86632, 87110, 87270, 87320, 87490, 87491, 87810, and 87800). We used the date of service for each screening claim to identify when the test occurred relative to the index date for the PrEP prescription.
The results were calculated separately for Medicaid enrollees using a hierarchy of four eligibility groups determined at the first enrollment during the study period: children (16–19 years), disabled adults, Medicaid expansion adults, and nondisabled, nonexpansion adults. The other subgroups identified were age at the beginning of the study period (categorized as 16–19, 20–24, 25–29, 30–34, 35–39, and 40–64 years), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other), sex (male or female), and geographic area (urban or rural) based on enrollee ZIP code. Between-state differences in subgroup composition reflect the demographic differences of the states as well as differences in Medicaid eligibility policies. Raw counts of fewer than 11 individuals are suppressed per study protocol.
Statistical Analysis
We first calculated the rate of PrEP initiation in each of the defined Medicaid populations. Then, we calculated the proportion of PrEP initiators with any STI test by 3, 6, and 12 months following the first prescription and separately for syphilis and for chlamydia/gonorrhea combined because of the use of a combined chlamydia/gonorrhea screening test. Because the Medicaid claims data represent a census of all Medicaid beneficiaries in participating states, we did not conduct statistical tests for comparisons because any differences observed represent true differences at the population level.
Results
Description of PrEP Users
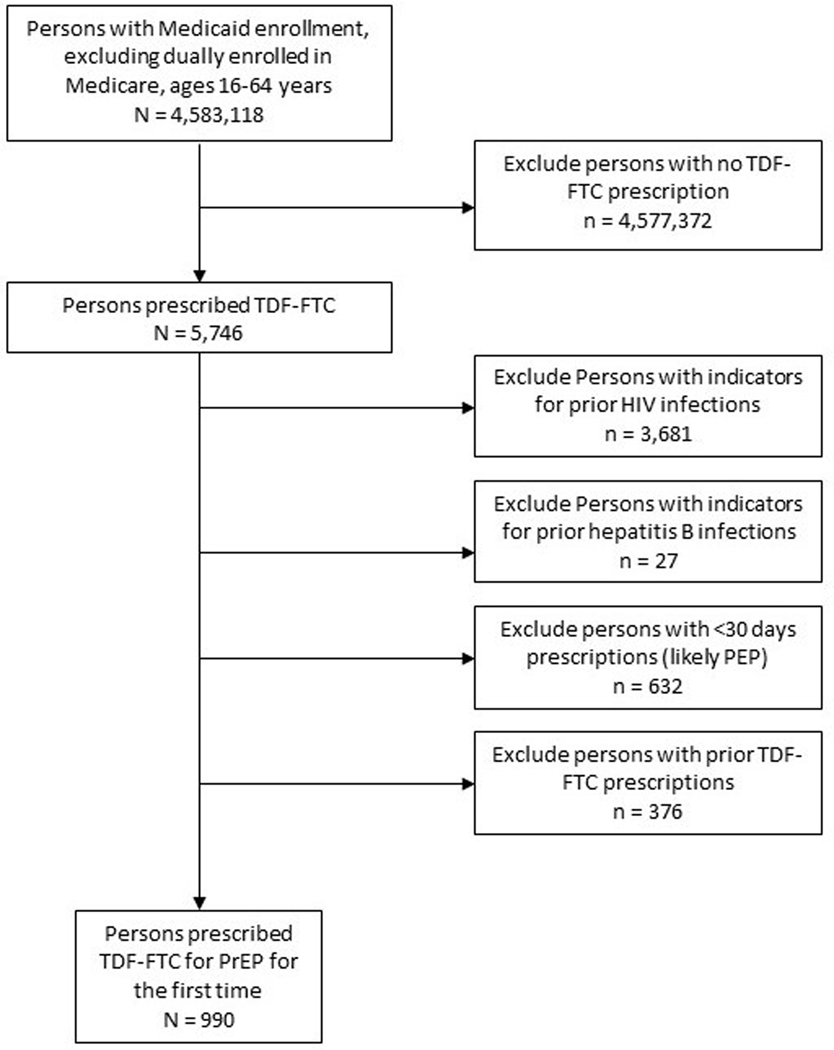
The Figure describes the results of applying the PrEP algorithm to the Medicaid population in the six study states. A total of 5746 individuals were identified who were prescribed tenofovir disoproxil fumarate with emtricitabine. After applying exclusion criteria, we identified a combined total of 990 individuals with a first-time PrEP prescription, yielding a rate of 22 first-time PrEP users per 100,000 Medicaid enrollees in these 6 states. There were, however, notable differences between the states in the sample, with PrEP use ranging from 9.1/100,000 to 51.3/100,000 Medicaid enrollees. Table 1 describes the first-time PrEP user sample in each of the states, including information about the Medicaid expansion status of each state. The demographic characteristics of PrEP users differed by state, with inconsistent patterns of age, race/ethnicity, and living area.
Table 1.
Description of PrEP study cohort by state
| Medicaid expansion state | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| State A | State B | State C | State D | State E | State F | ||||||||
|
| |||||||||||||
| No | Yes | Yes | No | No | No | ||||||||
|
|
|||||||||||||
| Characteristic | N | % | N | % | N | % | N | N | % | N | % | ||
| Age group, y | |||||||||||||
| 16–19 | 28 | 20.4 | <11 | — | 37 | 8.4 | 56 | 31.1 | 11 | 14.7 | 16 | 20.5 | |
| 20–24 | 16 | 11.7 | 12 | 15.0 | 68 | 15.5 | 24 | 13.3 | <11 | — | 14 | 17.9 | |
| 25–29 | 23 | 16.8 | 14 | 17.5 | 111 | 25.2 | 22 | 12.2 | 17 | 22.7 | 12 | 15.4 | |
| 30–34 | 27 | 19.7 | 13 | 16.3 | 58 | 13.2 | 21 | 11.6 | <11 | — | <11 | — | |
| 35–39 | 12 | 8.8 | 13 | 16.3 | 50 | 11.4 | 15 | 8.3 | 12 | 16.0 | <11 | — | |
| 40–64 | 31 | 22.6 | 24 | 30.0 | 116 | 26.4 | 42 | 23.3 | 16 | 21.3 | 22 | 28.2 | |
| Race/ethnicity | |||||||||||||
| Non-Hispanic White | 32 | 23.4 | 47 | 58.8 | 148 | 33.6 | 72 | 40.0 | 26 | 34.7 | 22 | 28.2 | |
| Non-Hispanic Black | 75 | 54.7 | <11 | — | 137 | 31.1 | 83 | 46.1 | 40 | 53.3 | 23 | 29.5 | |
| Hispanic | <11 | — | <11 | — | 27 | 6.1 | <11 | — | <11 | — | <11 | — | |
| Other | 30 | 21.9 | 23 | 28.7 | 128 | 29.1 | 20 | 11.1 | <11 | — | 33 | 42.3 | |
| Sex | |||||||||||||
| Female | 100 | 73.0 | 9 | 11.3 | 98 | 22.3 | 117 | 65.0 | 54 | 72.0 | 35 | 44.9 | |
| Male | 37 | 27.0 | 71 | 88.8 | 342 | 77.7 | 63 | 35.0 | 21 | 28.0 | 43 | 55.1 | |
| Eligibility group | |||||||||||||
| Children, 16–19 y | 28 | 20.4 | <11 | — | 37 | 8.4 | 56 | 31.1 | 11 | 14.7 | 16 | 20.5 | |
| Disabled adults | 46 | 33.6 | <11 | — | 32 | 7.3 | 48 | 26.6 | 21 | 28.0 | 32 | 41.0 | |
| Medicaid expansion adults | 0 | 0.0 | 70 | 87.5 | 344 | 78.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Nondisabled, nonexpansion adults | 63 | 46.0 | <11 | — | 27 | 6.1 | 70 | 38.8 | 43 | 57.3 | 30 | 38.5 | |
| Living area | |||||||||||||
| Urban | 114 | 83.2 | 58 | 72.5 | 399 | 90.7 | 144 | 80.0 | 57 | 76.0 | 65 | 83.3 | |
| Rural | 21 | 15.3 | 22 | 27.5 | 38 | 8.6 | 36 | 20.0 | 17 | 22.7 | <11 | — | |
| Total PrEP, n | 137 | — | 80 | — | 440 | — | 180 | — | 75 | — | 78 | — | |
| Total PrEP/100,000 Medicaid enrollees | 32.2 | — | 9.1 | — | 51.3 | — | 18.2 | — | 11.0 | — | 10.5 | — | |
PrEP, preexposure prophylaxis.
STI Testing
Table 2 and Table 3 presents the results for STI testing during the 12-month follow-up period. Supplementary Tables 1 and 2 AcademyHealth provide testing results for each state by type (syphilis, chlamydia/gonorrhea) and follow-up period (3, 6, and 12 months). The state proportion of PrEP users with any STI test by 12 months following the index PrEP prescription ranged from 49.5% to 77.3%. The proportion with any STI test by 3 months ranged from 20.6% to 49.2% and by 6 months ranged from 37.4% to 66.5% (Supplemental Table 1). In five of the six states, the proportion of PrEP users with a chlamydia/gonorrhea test was higher than the proportion with a syphilis test at each follow-up time point.
Table 2.
Proportion of first-time PrEP users with any STI test, a syphilis test, or a chlamydia and/or gonorrhea test within first 12 mo (states A, B, C)
| State A | State B | State C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Characteristic | Any STI, % | Syphilis, % | Chlamydia or gonorrhea, % | Any STI, % | Syphilis, % | Chlamydia or gonorrhea, % | Any STI, % | Syphilis, % | Chlamydia or gonorrhea, % | |
| Age group, y | ||||||||||
| 16–19 | 47.1 | 29.4 | 47.1 | — | — | — | 81.8 | 69.7 | 81.8 | |
| 20–24 | 58.3 | 33.3 | 41.7 | 77.8 | 55.6 | 66.7 | 75.5 | 71.7 | 73.6 | |
| 25–29 | 52.6 | 42.1 | 42.1 | 55.6 | 33.3 | 55.6 | 84.1 | 83.0 | 84.1 | |
| 30–34 | 66.7 | 62.5 | 50.0 | 54.5 | 45.5 | 45.5 | 84.1 | 72.7 | 75.0 | |
| 35–39 | 55.6 | 33.3 | 44.4 | 44.4 | 44.4 | 44.4 | 75.0 | 72.5 | 67.5 | |
| 40–64 | 26.9 | 15.4 | 19.2 | 63.6 | 54.5 | 54.5 | 68.4 | 61.1 | 60.0 | |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 42.9 | 38.1 | 38.1 | 50.0 | 42.1 | 42.1 | 73.2 | 68.8 | 66.1 | |
| Non-Hispanic Black | 55.2 | 39.7 | 39.7 | — | — | — | 78.5 | 70.7 | 77.6 | |
| Hispanic | — | — | — | — | — | — | 90.5 | 90.5 | 90.5 | |
| Other | 42.9 | 28.6 | 39.3 | 70.6 | 41.2 | 70.6 | 77.9 | 72.1 | 71.2 | |
| Sex | ||||||||||
| Female | 50.6 | 35.8 | 40.7 | 75.0 | 62.5 | 62.5 | 83.9 | 74.7 | 80.5 | |
| Male | 46.2 | 38.5 | 34.6 | 58.9 | 44.6 | 53.6 | 75.2 | 70.7 | 70.3 | |
| Eligibility group | ||||||||||
| Children, 16–19 y | 47.1 | 29.4 | 47.1 | — | — | — | 81.8 | 69.7 | 81.8 | |
| Disabled adults | 43.2 | 31.8 | 36.4 | — | — | — | 70.4 | 63.0 | 63.0 | |
| Medicaid expansion adults | — | — | — | 59.3 | 48.1 | 53.7 | 77.8 | 73.0 | 72.6 | |
| Nondisabled, nonexpansion adults | 56.5 | 43.5 | 39.1 | — | — | — | 73.9 | 69.6 | 73.9 | |
| Living area | ||||||||||
| Urban | 49.4 | 39.1 | 37.9 | 68.1 | 53.2 | 63.8 | 78.0 | 73.3 | 73.6 | |
| Rural | 44.4 | 27.8 | 38.9 | 41.2 | 29.4 | 29.4 | 68.8 | 53.1 | 65.6 | |
| Total | 49.5 | 36.4 | 39.3 | 60.9 | 46.9 | 54.7 | 77.3 | 71.7 | 72.8 | |
PrEP, preexposure prophylaxis; STI, sexually transmitted infection.
Table 3.
Proportion of first-time PrEP users with any STI test, a syphilis test, or a chlamydia and/or gonorrhea test within first 12 mo (states D, E, F)
| State D | State E | State F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Characteristic | Any STI, % | Syphilis, % | Chlamydia or gonorrhea, % | Any STI, % | Syphilis, % | Chlamydia or gonorrhea, % | Any STI, % | Syphilis, % | Chlamydia or gonorrhea, % | |
| Age group, y | ||||||||||
| 16–19 | 58.9 | 53.6 | 50.0 | 54.5 | 45.5 | 54.5 | 37.5 | 25.0 | 37.5 | |
| 20–24 | 54.2 | 45.8 | 45.8 | — | — | — | 57.1 | 50.0 | 57.1 | |
| 25–29 | 50.0 | 45.5 | 27.3 | 41.2 | 29.4 | 41.2 | 75.0 | 50.0 | 66.7 | |
| 30–34 | 76.2 | 66.7 | 61.9 | — | — | — | — | — | — | |
| 35–39 | 53.3 | 26.7 | 53.3 | 66.7 | 50.0 | 58.3 | — | — | — | |
| 40–64 | 57.1 | 45.2 | 45.2 | 43.8 | 37.5 | 31.3 | 59.1 | 54.5 | 50.0 | |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 50.0 | 41.7 | 38.9 | 53.8 | 38.5 | 53.8 | 54.5 | 45.5 | 54.5 | |
| Non-Hispanic Black | 62.7 | 54.2 | 49.4 | 50.0 | 40.0 | 42.5 | 69.6 | 47.8 | 65.2 | |
| Hispanic | — | — | — | — | — | — | — | — | — | |
| Other | 65.0 | 50.0 | 60.0 | — | — | — | 45.5 | 42.4 | 39.4 | |
| Sex | ||||||||||
| Female | 61.5 | 51.3 | 47.9 | 57.4 | 44.4 | 51.9 | 71.4 | 57.1 | 65.7 | |
| Male | 52.4 | 44.4 | 46.0 | 38.1 | 33.3 | 38.1 | 41.9 | 34.9 | 39.5 | |
| Eligibility group | ||||||||||
| Children, 16–19 y | 58.9 | 53.6 | 50.0 | 54.5 | 45.5 | 54.5 | 37.5 | 25.0 | 37.5 | |
| Disabled adults | 66.7 | 52.1 | 62.5 | 52.4 | 38.1 | 42.9 | 65.6 | 56.3 | 62.5 | |
| AMedicaid expansion adults | — | — | — | — | — | — | — | — | — | |
| Nondisabled, nonexpansion adults | 54.3 | 45.7 | 37.1 | 51.2 | 41.9 | 48.8 | 53.3 | 43.3 | 46.7 | |
| Living area | ||||||||||
| Urban | 60.4 | 50.7 | 47.9 | 49.1 | 38.6 | 47.4 | 58.5 | 49.2 | 53.8 | |
| Rural | 50.0 | 41.7 | 44.4 | 64.7 | 52.9 | 52.9 | — | — | — | |
| Total | 58.3 | 48.9 | 47.2 | 52.0 | 41.3 | 48.0 | 55.0 | 45.0 | 51.0 | |
PrEP, preexposure prophylaxis; STI, sexually transmitted infection.
In Table 3, row Medicaid expansion adults, should the entire row be “—” (there were no data and no — in the State E section as originally received)?
Subgroup Differences
In all states, STI testing was higher among non-Hispanic Black enrollees relative to non-Hispanic White enrollees. Testing proportions and total testing rates were consistently lower for males (range 38.1%–75.2%) compared with females (range 50.6%–83.9%), with a few exceptions by state, type of test, and follow-up period. Testing overall tended to increase with age and leveled off at the 25- to 29- and 30- to 34-year-old groups before decreasing in the 40- to 64-year-old groups. In all states, testing was higher among PrEP users in urban areas relative to rural areas.
Discussion
The purpose of this study was to examine the prevalence of STI testing following PrEP initiation in six states in the US South. Using claims data, we estimated a rate of 22 first-time PrEP users per 100,000 Medicaid enrollees. This rate is similar to the 21/100,000 PrEP users estimated in the total population of the South estimated using 2017 national health data.18 Of 990 PrEP users identified using Medicaid claims, at least half received at least one STI test during the 12 months following the index PrEP prescription; however, there was considerable variation between states in the proportion of PrEP users tested within 3, 6, or 12 months. In the best-performing expansion state, 66.5% of PrEP users were tested within 6 months and 77.3% were tested within 12 months. In contrast, in the nonexpansion state with the lowest testing, only 37.4% and 49.5% of PrEP users were tested within 6 or 12 months, respectively. Overall, within the first 12 months, expansion states tested 60.9% to 77.3% of PrEP users and nonexpansion states tested 49.5% to 58.3% of PrEP users. Given that the CDC recommends STI testing every 6 months, these findings suggest that STI testing occurs less frequently than what is recommended. The relatively low proportion of Medicaid enrollees receiving a test within 12 months of initiating PrEP suggests that there is substantial room for improving STI testing for at-risk populations, particularly for states that have not expanded Medicaid.
Although states differed considerably in the rate of PrEP initiation, the rate did not appear to be unassociated with expansion status among the states examined in this study. This finding was unexpected because the impact of Medicaid expansion on PrEP access was recently demonstrated with a modeling study, which estimated that Medicaid expansion since 2010 has more than doubled the PrEP-to-need ratio18 and added on average approximately 8 additional PrEP users per 100,000 population per year, a finding consistent with other studies highlighting the importance of insurance access and type.22,23,24 Differences may be seen in overall PrEP use and initiation, however. PrEP uptake among Medicaid enrollees is lower than among private insurance populations. A recent study using national claims data found that only 12% of all PrEP patients’ payment method was Medicaid; however, 45% of individuals living with HIV were insured by Medicaid, indicating that far more individuals with Medicaid coverage could have benefited from PrEP.25 As such, it is possible that both expansion and nonexpansion states face similar challenges to engaging at-risk individuals with PrEP. In nonexpansion states, which are clustered in the US South, many young people have access only to family planning Medicaid, which is a missed opportunity for men to access sexual health services.
The demographic characteristics of PrEP initiators and PrEP initiators who received STI testing varied between states, particularly between expansion and nonexpansion states. For states that expanded Medicaid (Parts B and C), 77.7% to 88.8% of PrEP users were male, compared with 27.0% to 55% of PrEP users in nonexpansion states. In addition, expansion states conducted STI screening for a greater proportion of male PrEP recipients (58.9% to 75.2%) than nonexpansion states (38.1% to 52.4%). This finding has larger implications for the role Medicaid plays in preventing HIV and bacterial STI infection among men who have sex with men.11 Although females were more likely than males to receive STI testing in every state, expansion states also had higher STI testing for female PrEP initiators, compared with nonexpansion states. Differences also were noted in the association between Medicaid expansion and STI testing by race/ethnicity, with the proportion of PrEP initiators tested moderately higher among non-Hispanic White enrollees (50.0% to 73.2% in expansion states, 42.9% to 54.5% in nonexpansion states) compared with non-Hispanic Black enrollees (78.5% to 88.9% in expansion states, 50.0% to 69.6% in nonexpansion states). As such, Medicaid expansion appears to be a valuable policy tool for providing PrEP and/or STI screening to people with key risk factors for HIV and bacterial STI infection.1,5,26
These current analyses follow findings from similar studies. Previous work conducted by Pearson and colleagues found deficiencies in STI testing among HIV-positive Medicaid enrollees in 22 states, indicating that Medicaid programs could benefit from further quality improvement initiatives focused on STI testing to prevent the spread of HIV.27 In addition, studies using national data of commercially insured individuals taking PrEP found shortcomings in receiving recommended STI testing pointing to opportunities for the improvement in quality of care of all individuals taking PrEP.28 Our findings further support the idea that more work is needed to improve STI testing among Medicaid enrollees who are taking PrEP.
Public Health Implications
People who initiated PrEP within the examined states varied in both their demographic characteristics, including gender and racial/ethnic composition, and the extent to which they were tested for bacterial STIs within 3, 6, and 12 months of initiation. Although in all of the states testing was well below the recommended universal STI screening for all PrEP users, the observed differences between the states appear to be linked to Medicaid expansion status. The present study, however, was descriptive; additional research is needed to establish whether Medicaid expansion contributed to greater PrEP initiation or STI testing among key HIV risk groups.
Limitations and Future Research
First, the sample was drawn from a selection of states within the US South, a region at particularly high risk for HIV and STI and with a relatively low uptake of PrEP and Medicaid expansion. The findings may not generalize to other states in the US South or other non-Medicaid populations within these states. Second, the focus on Medicaid recipients may obscure the statewide rate of PrEP use and STI testing, particularly for states without Medicaid expansion. Local public health programs and STI clinics may provide this type of care without billing Medicaid, and thus some Medicaid enrollees may have received STI testing that was not captured in our data. Future studies could extend the present study by examining a nationwide population of Medicaid enrollees to gain a broader view of national- and state-level trends in PrEP use and compliance with the CDC STI testing guidelines in the Medicaid program. In addition, examining the effect of required copayments for some Medicaid programs on continued PrEP adherence would be beneficial in both Medicaid expansion and nonexpansion states.
Conclusions
The present study examined bacterial STI testing among Medicaid beneficiaries within states at high risk of HIV and bacterial STI infection. State-level differences in the utilization of PrEP among at-risk groups and the rate of STI testing among those initiating PrEP highlight the heterogeneity of STI prevention efforts within the United States. Policy differences between states, such as Medicaid expansion, may influence how Medicaid plays a role in addressing HIV and bacterial STI.
Supplementary Material
Fig.
Algorithm for identifying PrEP users combined for all states. HIV, human immunodeficiency virus; PEP, postexposure prophylaxis; PrEP, preexposure prophylaxis; TDF-FTC, tenofovir disoproxil fumarate/emtricitabine.
Key Points.
Overall, this sample of six states in the US South had screening rates below the universal access required in current clinical guidelines.
States in this study varied widely in sexually transmitted infection screening rates, likely reflecting important state differences in populations accessing preexposure prophylaxis, as well as differences in Medicaid policy.
This is the first study to examine sexually transmitted infection screening among preexposure prophylaxis initiators in the Medicaid program in a large geographic region of the US South using a novel research approach.
Acknowledgment
We are indebted to the state Medicaid agency partners who provided access to the data for the study and ongoing consultation.
This publication was supported by the Centers for Disease Control and Prevention (CDC) of the US Department of Health and Human Services (HHS) as part of a financial assistance award totaling $471,017.00, with 100% funded by CDC/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the US government.
S.K., L. Hammerslag, and A.L.-D.F. have received compensation from the CDC Foundation. C.G.G. has received compensation from the Agency for Healthcare Research and Quality, the US Food and Drug Administration, Merck, the National Institutes of Health, Pfizer, Sanofi, and Syneos Health. The remaining authors did not report any financial relationships or conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://sma.org/smj).
References
- 1.Centers for Disease Control and Prevention. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas 2019. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-32/index.html. Published May 2021. Accessed February 26, 2023.
- 2.US Department of Health and Human Services. HIV national strategic plan for the United States: a roadmap to end the epidemic 2021–2025. https://files.hiv.gov/s3fs-public/HIV-National-Strategic-Plan-2021-2025.pdf. Published 2021. Accessed February 26, 2023.
- 3.US Preventive Services Task Force; Owens DK, Davidson KW, et al. Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement. JAMA 2019;321:2203–2213. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Evans C, Hoverman A, et al. Preexposure prophylaxis for the prevention of HIV infection: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019;321:2214–2230. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2019. https://www.cdc.gov/nchhstp/newsroom/2021/2019-std-surveillance-report-press-release.html. Published 2021. Accessed February 26, 2023.
- 6.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2:33–42. [DOI] [PubMed] [Google Scholar]
- 7.Wasserheit J. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 1992;19:61–77. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention; US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. Accessed February 26, 2023. [Google Scholar]
- 9.Quaife M, MacGregor L, Ong JJ, et al. Risk compensation and STI incidence in PrEP programmes. Lancet HIV 2020;7:e222–e223. [DOI] [PubMed] [Google Scholar]
- 10.Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018;67:676–686. [DOI] [PubMed] [Google Scholar]
- 11.Ramchandani MS, Golden MR. Confronting rising STIs in the era of PrEP and treatment as prevention. Curr HIV/AIDS Rep 2019;16:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenness SM, Weiss KM, Goodreau SM, et al. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who Have sex with men: a modeling study. Clin Infect Dis 2017;65:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang BA, Pearson WS, Owusu-Edusei K. Correlates of county-level nonviral sexually transmitted infection hot spots in the US: application of hot spot analysis and spatial logistic regression. Ann Epidemiol 2017;27:231–237. [DOI] [PubMed] [Google Scholar]
- 14.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis 2005;191(Suppl 1):S115–S122. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser Family Foundation. Medicaid’s role for women. https://firstfocus.org/wp-content/uploads/2019/11/Fact-Sheet-Medicaids-Role-for-Women.pdf. Published March 2019. Accessed February 26, 2023.
- 16.Kay ES, Pinto RM. Is insurance a barrier to HIV preexposure prophylaxis? Clarifying the issue. Am J Public Health 2020;110:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan PS, Mena L, Elopre L, et al. Implementation strategies to increase PrEP uptake in the South. Curr HIV/AIDS Rep 2019;16:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol 2018;28:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser Family Foundation. Status of state Medicaid expansion decisions: interactive map. https://www.kff.org/medicaid/issue-brief/status-of-state-medicaid-expansion-decisions-interactive-map. Published February 16, 2023. Accessed February 26, 2023.
- 20.Adams L, Kennedy S, Allen L, et al. Innovative solutions for state Medicaid programs to leverage their data, build their analytic capacity, and create evidence-based policy. EGEMS (Wash DC) 2019;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medicaid Outcomes Distributed Research Network (MODRN); Brown E, Schutze M, et al. Use of medications for treatment of opioid use disorder among US Medicaid enrollees in 11 states, 2014–2018. JAMA 2021;326:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan PA, Seiler N, Chu CT. Leveraging Medicaid to enhance preexposure prophylaxis implementation efforts and ending the HIV epidemic. Am J Public Health 2020;110:65–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Mendoza MCB, Huang Ya-Lin A, et al. Uptake of HIV preexposure prophylaxis among commercially insured persons—United States, 2010–2014. Clin Infect Dis 2017;64:144–149. [DOI] [PubMed] [Google Scholar]
- 24.Karletsos D, Stoecker C. Impact of Medicaid expansion on PrEP utilization in the US: 2012–2018. AIDS Behav 2021;25:1103–1111. [DOI] [PubMed] [Google Scholar]
- 25.Chan SS, Chappel AR, Maddox KEJ, et al. Pre-exposure prophylaxis for preventing acquisition of HIV: a cross-sectional study of patients, prescribers, uptake, and spending in the United States, 2015–2016. PLoS Med 2020;17:e1003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol 2018;28:850–857.e9. [DOI] [PubMed] [Google Scholar]
- 27.Pearson WS, Tao G, Gift TL. Improvement still needed in sexually transmitted disease testing among HIV-positive Medicaid enrollees. Sex Transm Dis 2018;45:49. [DOI] [PubMed] [Google Scholar]
- 28.Tao G, Pearson WS, Sullivan JM, et al. STI testing and prevalence before and after PrEP initiation among males aged ≥18 years in US private settings. Sex Transm Dis 2021;48:515–520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.