Abstract
An appropriate therapeutic option for pial arteriovenous fistula (PAVF) can vary according to the angioarchtecture of the lesion. We present a case of adult infratentorial PAVF treated by transarterial coil embolization. A 26-year-old man was referred to our institution for an asymptomatic intracranial vascular lesion. Cerebral angiograms revealed PAVF fed by three arteries in the right cerebellomedullary cistern. The feeding arteries were accurately identified by three-dimensional rotational angiography and were successfully embolized using coils while normal arterial flow was preserved. This case report suggests that stepwise transarterial coil embolization can cure PAVF under detailed evaluation of its angioarchitecture.
Keywords: pial arteriovenous fistula, vascular malformation, endovascular treatment
Introduction
Pial arteriovenous fistula (PAVF) is a vascular disorder of the brain, that directly connects arteries and veins without the nidal component.1,2) The best treatment modality for each PAVF case varies according to its location and angioarchitecture.3) Little is known regarding the safety and effectiveness of the endovascular treatment for PAVF because of its rarity.4) Here, we report a case of PAVF successfully treated with transarterial coil embolization. We also reviewed the literature of infratentorial PAVF.
Case Report
A 26-year-old man with a history of hepatoblastoma and cryptorchidism underwent head magnetic resonance imaging (MRI) because of dizziness. There were no demonstrable cranial nerve deficits. He had no family history or hereditary disease. Dizziness improved spontaneously, but MRI showed a cluster of dilated vessels occupying the right cerebellomedullary cistern (Fig. 1a). Six-vessel cerebral angiography revealed a high-flow arteriovenous shunt within the right cerebellomedullary cistern (Fig. 1b-e). The shunt was supplied by the arteries branching from the right vertebral artery (VA) and the right posterior inferior cerebellar artery (PICA). The feeding arteries anastomosed each other, and they flowed into multiple drainage routes that drained into bilateral internal jugular veins and the right superior petrosal sinus. Varix formed on the drainage route behind the medulla oblongata. There was no nidal component and dural supply. On the basis of these findings, the patient was diagnosed with a PAVF. We suggested transarterial embolization to prevent future development.
Fig. 1.
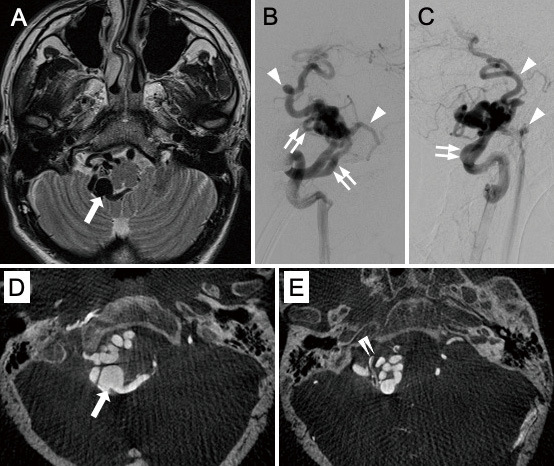
T2-weighted magnetic resonance imaging (A) shows dilated and tortuous vessels in the right cerebellomedullary cistern. The varix (arrow) is observed behind the medulla oblongata. Anterioposterior view (B) and lateral view (C) of the right vertebral angiogram revealed pial arteriovenous fistula. Arrowheads indicate the early filling of veins, and double arrows indicate feeders branched from the vertebral artery and posterior inferior cerebellar artery. Axial views of slab MIP images (D and E) of the right vertebral angiogram show the detailed structure of the lesion. Note that vessels are distinguishable from each other and that it is easy to chase their connections. Double arrowheads indicate the right posterior inferior cerebellar artery.
While the patient is under general anesthesia, a guiding catheter was positioned within the right VA. Before the embolization procedure, a balloon microcatheter (Scepter C; MicroVention, Tustin, CA, USA) was threaded into each feeder, and superselective angiography was performed with balloon occlusion. Arterial supplies to normal nervous tissues were not observed from each feeding artery. Then, all three supplying arteries were occluded in turn using detachable coils through two microcatheters. After two of the feeders from the VA were occluded, the feeding artery that branched from the PICA was occluded just proximal to the varix, which was estimated as the shunt point. Immediate occlusion of the fistula that preserved the VA and the PICA flow was achieved. The postoperative course was uneventful, and the patient did not present any neurological deficits following treatment. The follow-up angiography performed 9 months after treatment showed no residual shunt (Fig. 2).
Fig. 2.
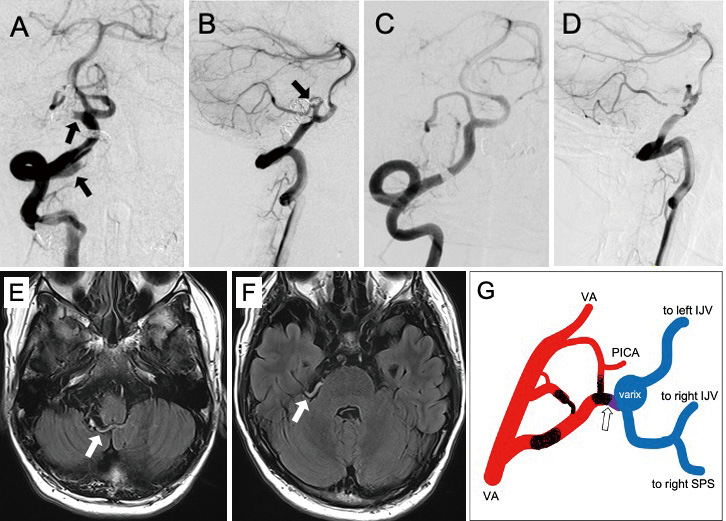
Anterioposterior view (A) and lateral view (B) of the right vertebral angiogram immediately after coil embolization. The right vertebral angiogram 9 months after treatment (C and D) showed no residual sunt. Note that the origins of each feeder (arrows in A and B) are no longer shown in C and D. Fluid attenuated inversion recovery images 3 months after treatment (E and F) show obstructed varix and veins (arrows).
G: A simplified schematic drawing of the angioarchitecture. The open arrow indicates the fistula. The sites trapped by coils are also depicted. IJV, internal jugular vein; PICA, posterior inferior cerebellar artery; SPS, superior petrosal sinus; VA, vertebral artery
Discussion
PAVF was once recognized as a subtype of arteriovenous malformation (AVM), but it is currently considered a distinct entity from AVM and dural AVF in terms of angioarchitecture and clinical course. It is important to differentiate PAVF and other shunt disorders because the therapeutic goal of these vascular diseases can be different.5) PAVF is composed of a single arterial feeder or multiple arterial feeders in direct connection to venous drainage without intervening nidal vessels.6) The cause of PAVF in infants and children is often congenital,7) whereas PAVF in adults is usually caused by an acquired factor, such as trauma or venous thrombosis.8,9) In our case, there was no family history or possible cause of the shunt.
We searched PubMed using the keyword “pial arteriovenous fistula” and “non-galenic arteriovenous fistula” from the inception of the databases in December 2022. We excluded articles without clear descriptions regarding angiographic finding, treatment choice, and outcome. We also excluded galenic PAVF, secondary PAVF, cases with dural arterial supply, and untreated cases. Among 266 PAVF cases reported since 1977, 35 lesions (13.2%) were located in the posterior cranial fossa.4,10-33) Those cases and the present case were reviewed (Table 1).
Table 1.
Literature review of treated infratentorial pial arteriovenous fistula
| Reference | Age | Sex | Presentation | Location | Feeder | Varix | Treatment | Complication | GOS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vinuela et al.10) | 24 | M | focal neurological sign | CMC | PICA | + | E, | detachable balloon | transient lateral medullary syndrome | 5 |
| Vinuela et al.11) | 4 | M | retardation, seizure, macrocephaly | CMC | PICA | + | S, | clip | − | 5 |
| Smith et al.12) | 46 | M | focal neurological sign | CPC | SCA | − | E, | coil | − | 4 |
| Garcia-Monaco et al.13) | 11 | F | headache, focal neurological sign | CMC | PICA | + | E, | NBCA | − | 5 |
| Morimoto et al.14) | 38 | M | focal neurological sign | CMC | VA | − | S, | cauterization | − | 5 |
| Coubes et al.15) | 13 | F | focal neurological sign | CMC | VA | + | E, | NBCA | − | 5 |
| Hoh et al.16) | 57 | M | hemorrhage | NA | VA | + | S, | clip | transient dysphonia and dysphagia | 5 |
| 12 | M | asymptomatic | NA | SCA | + | E, | coil | − | 5 | |
| Oya et al.17) | 60 | M | hemorrhage | CPC | AICA | + | S, | cauterization | transient facial nerve palsy | 5 |
| Yoshida et al.18) | 1 | M | macrocephaly | PMC | BA | + | E, | NBCA | − | 1 |
| 1 | F | CHF, retardation, focal neurological sign | PMC | BA | + | E, | NBCA | − | 1 | |
| 6 | M | headache, macrocephaly | CMC | PICA | + | E, | NBCA | − | 5 | |
| 3 | F | headache, focal neurological sign | PMC | SCA | − | E, | coil | − | 5 | |
| Limaye et al.19) | 28 | M | headache | PMC | SCA | + | E, | NBCA | − | 5 |
| Passacantilli et al.20) | 54 | F | hemorrhage | convexity | SCA, PICA | − | S, | clip | surgical site infection | 5 |
| Izzo et al.21) | 0 | F | macrocephaly | CMC | VA | + | E, | coil | − | 5 |
| Masuoka et al.22) | 51 | F | hydrocephalus | convexity | AICA | + | S, | resection | − | 5 |
| Lv et al.23) | 9 | F | headache | PMC | BA | + | E, | coil | − | 5 |
| 17 | M | focal neurological sign | CMC | PICA | + | E, | coil | − | 5 | |
| Guimaraens et al.24) | 2 | M | focal neurological sign | CMC | PICA | + | E, | coil | − | 5 |
| Newman et al.25) | 6 | M | hydrocephalus, focal neurological sign | CPC | VA | + | E, | Onyx | − | 5 |
| M | hemorrhage | CPC | VA | NA | E, | Onyx | − | 4 | ||
| Guerra et al.26) | 0 | M | macrocephaly | CPC | VA | + | E, | coil | − | 5 |
| Requejo et al.27) | 12 | M | retardation | CMC | PICA | NA | E, | coil | − | 4 |
| 8 | F | headache | PMC | SCA | NA | E, | coil, NBCA | − | 5 | |
| 0 | F | CHF | CMC | VA | − | E, | coil | − | 1 | |
| 10 | M | retardation | CPC | VA | + | E, | coil | − | 4 | |
| Kanai et al.28) | 73 | M | focal neurological sign | convexity | SCA | + | E+S, | coil, resection | − | 5 |
| Lylyk et al.29) | 27 | M | hydrocephalus | PMC | SCA | + | E, | coil, NBCA | − | 5 |
| Ye et al.30) | 21 | M | hemorrhage | NA | PICA | + | E, | NBCA | − | 5 |
| Akamatsu et al.31) | 4 | M | hemorrhage | convexity | SCA | + | E, | NBCA | − | 5 |
| Wang et al.32) | 65 | M | focal neurological sign | convexity | PICA | + | E, | coil, Onyx | − | 5 |
| Yan et al.33) | 1 | F | macrocephaly, hydrocephalus, retardation | convexity | SCA, PICA | + | E, | coil, Onyx | subdural effusion | 5 |
| Jin et al.4) | 3 | M | seizure | convexity | VA | + | E, | coil | − | 5 |
| 8 | M | headache | convexity | VA | + | E, | coil | − | 5 | |
| Present case | 26 | M | asymptomatic | CMC | VA, PICA | + | E, | coil | − | 5 |
AICA, anterior inferior cerebellar artery; BA, basilar artery; CHF, congestive heart failure; CMC, cerebellomedullary cistern; CPC, cerebellopontine cistern; E, endovascular; NA, not available; NBCA, n-butyl-2-cyanoacrylate; PICA, posterior inferior cerebellar artery; PMC, perimesencephalic cistern; S, surgical; SCA, superior cerebellar artery; VA, vertebral artery
A summary of the reviewed cases is shown in Table 2. Of the total of 36 patients whose infratentorial PAVF was treated, the most common presentation symptom was focal neurological deficit (12 cases, 33.3%), which might be caused by venous congestion or the mass effect of dilated vessels. Hemorrhage was observed in six patients, and only two patients had an asymptomatic lesion. Thirteen patients (36.1%) received treatment in their adulthood. Few lesions (9 cases, 25.0%) were fed by multiple arteries. A total of 29 lesions received endovascular treatment, of which 14 were occluded only with detachable coils and other 14 were occluded with liquid embolic agents. Most cases (33 cases, 91.7%) achieved a favorable outcome (Glasgow Outcome Scale 4 or 5).
Table 2.
Summary of clinical characteristics in reported pial arteriovenous fistula
| Presentation | |||
| (One patient may have multiple symptoms) | |||
| Focal neurological deficit | 12 | ||
| Headache | 7 | ||
| Symptom due to heamorrhage | 6 | ||
| Macrocephaly | 6 | ||
| Retardation | 5 | ||
| Hydrocephalus | 4 | ||
| Seizure | 2 | ||
| Asymptomatic | 2 | ||
| Location of the shunt | |||
| Cerebellomedullary cistern | 12 | ||
| Cerebellar convexity | 8 | ||
| Perimesencephalic cistern | 7 | ||
| Cerebellopontine cistern | 6 | ||
| NA | 3 | ||
| Feeder | |||
| (One patient may have multiple feeders) | |||
| VA | 12 | ||
| PICA | 12 | ||
| SCA | 10 | ||
| BA | 3 | ||
| AICA | 2 | ||
| Patient with multiple feeders | 9 | ||
| Varix | |||
| + | 29 | ||
| − | 5 | ||
| NA | 2 | ||
| Treatment | |||
| Surgical | 6 | ||
| Endovascular | Coil only | 14 | |
| NBCA | 10 | ||
| Onyx | 4 | ||
| Balloon | 1 | ||
| Combined (Surgical+Endovascular) | 1 | ||
| Glasgow Outcome Scale | |||
| 5 | 29 | ||
| 4 | 4 | ||
| 1 | 3 | ||
AICA, anterior inferior cerebellar artery; BA, basilar artery; NA, not available; NBCA, n-butyl-2-cyanoacrylate; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery; VA, vertebral artery
Whether asymptomatic PAVF should be treated or not is controversial because a natural history of PAVF and risk of bleeding remains unclear because of its rarity. Yu et al. presented that a natural history of PAVF is unfavorable, especially in patients with multiple feeding arteries and high blood flow.34) Moreover, PAVF in adults can cause high-flow occlusive venopathy in a major sinus, resulting in the onset of symptoms.35) The present case also had deep venous drainage, which can cause cerebral venous congestion. Treatment for asymptomatic PAVF with the factors mentioned above might be reasonable.
The therapeutic options for PAVF include surgery, endovascular treatment, and their combination. For all options, the key procedure for radical cure is the interruption of feeders close to the fistula with the preservation of the venous drainage route.28) Total resection of the lesion is unnecessary. As a previous review of the literature revealed that surgical treatment offers a higher occlusion rate than that of endovascular treatment,6,16) surgery provides greater benefit for PAVF in areas of easy surgical access. By contrast, endovascular treatment is favorable when the lesion is deep-seated, surrounded by critical structures, or obscured by a dilated vein. For infratentorial complex PAVF like our case, the endovascular procedure is preferred to obliterate the shunt point precisely and safely. The currently mainstream embolic materials are liquid embolic agents.23,34) They are useful particularly for PVAF with multiple feeders or fistulas as they can penetrate over the shunt point into the drainers or other feeders.36) However, owing to the high-flow perfusion of PAVF, the distal migration of liquid agents is likely to occur.5) Partial or total occlusion of the draining veins without reducing the arterial flow can lead to intra- or postoperative rupture.36) Liquid embolic agents also have a risk of back-streaming to the parent vessel, especially when the feeding artery is a short branch of the parent artery. For these reasons, we chose detachable coils as embolic materials in the present case. Owing to the prior flow reduction by the occlusion of the proximal feeders, stable placement of the coils just proximal to the shunt point was achieved.
For the safe and complete interruption of a shunt, pretreatment selective angiography and three-dimensional rotational angiography are helpful in observing accurate angioarchitectural characteristics. Several reports in the cerebrovascular field showed the usefulness of slab maximum intensity projection (MIP) images derived from rotational angiography data.37,38) This can be applied to understanding the angioarchitecture of PAVF. PAVF does not have nidus, and its affected vessels are often dilated. For these reasons, it is relatively easy in PAVF, compared with other shunt disorders, to chase and comprehend vascular connections by examining slab MIP images.
Conclusions
The present case suggests that transarterial coil embolization is an effective treatment modality for adult infratentorial PAVF. Understanding the detailed angioarchitecture is necessary for optimal treatment planning.
Patient Consent
The patient has consented to the submission of the case report to the journal.
Conflicts of Interest Disclosure
All authors declare no competing interests.
References
- 1). Tomlinson FH, Rufenacht DA, Sundt TM Jr., Nichols DA, Fode NC: Arteriovenous fistulas of the brain and the spinal cord. J Neurosurg 79: 16-27, 1993 [DOI] [PubMed] [Google Scholar]
- 2). Halbach VV, Higashida RT, Hieshima GB, Hardin CW, Dowd CF, Barnwell SL: Transarterial occlusion of solitary intracerebral arteriovenous fistulas. AJNR Am J Neuroradiol 10: 747-752, 1989 [PMC free article] [PubMed] [Google Scholar]
- 3). Itami H, Sugiu K, Tokunaga K, Ono S, Onoda K, Date I: [Endovascular treatment of adult pial arteriovenous fistula]. No Shinkei Geka 35: 599-605, 2007[Japanese] [PubMed] [Google Scholar]
- 4). Jin H, Meng X, Quan J, Lu Y, Li Y: Role of endovascular embolisation for curative treatment of intracranial non-Galenic pial arteriovenous fistula. Stroke Vasc Neurol 6: 260-266, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Wang YC, Wong HF, Yeh YS: Intracranial pial arteriovenous fistulas with single-vein drainage. Report of three cases and review of the literature. J Neurosurg 100: 201-205, 2004 [DOI] [PubMed] [Google Scholar]
- 6). Yang WH, Lu MS, Cheng YK, Wang TC: Pial arteriovenous fistula: a review of literature. Br J Neurosurg 25: 580-585, 2011 [DOI] [PubMed] [Google Scholar]
- 7). Paramasivam S, Toma N, Niimi Y, Berenstein A: Development, clinical presentation and endovascular management of congenital intracranial pial arteriovenous fistulas. J Neurointerv Surg 5: 184-190, 2013 [DOI] [PubMed] [Google Scholar]
- 8). Nomura S, Ishikawa O, Tanaka K, Otani R, Miura K, Maeda K: Pial arteriovenous fistula caused by trauma: a case report. Neurol Med Chir (Tokyo) 55: 856-858, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Vilela P, Terbrugge K, Willinsky R: Association of distinct intracranial pial and dural arteriovenous shunts. Neuroradiology 43: 770-777, 2001 [DOI] [PubMed] [Google Scholar]
- 10). Vinuela F, Fox AJ, Kan S, Drake CG: Balloon occlusion of a spontaneous fistula of the posterior inferior cerebellar artery. Case report. J Neurosurg 58: 287-290, 1983 [DOI] [PubMed] [Google Scholar]
- 11). Vinuela F, Drake CG, Fox AJ, Pelz DM: Giant intracranial varices secondary to high-flow arteriovenous fistulae. J Neurosurg 66: 198-203, 1987 [DOI] [PubMed] [Google Scholar]
- 12). Smith MD, Russell EJ, Levy R, Crowell RM: Transcatheter obliteration of a cerebellar arteriovenous fistula with platinum coils. AJNR Am J Neuroradiol 11: 1199-1202, 1990 [PMC free article] [PubMed] [Google Scholar]
- 13). Garcia-Monaco R, Taylor W, Rodesch G, et al. : Pial arteriovenous fistula in children as presenting manifestation of Rendu-Osler-Weber disease. Neuroradiology 37: 60-64, 1995 [DOI] [PubMed] [Google Scholar]
- 14). Morimoto T, Yamada T, Hashimoto H, Tokunaga H, Tsunoda S, Sakaki T: Direct approach to intracranial vertebral arteriovenous fistula. Case report. Acta Neurochir (Wien) 137: 98-101, 1995 [DOI] [PubMed] [Google Scholar]
- 15). Coubes P, Humbertclaude V, Rodesch G, Lasjaunias P, Echenne B, Frerebeau P: Total endovascular occlusion of a giant direct arteriovenous fistula in the posterior fossa in a case of Rendu-Osler-Weber disease. Childs Nerv Syst 12: 785-788, 1996 [DOI] [PubMed] [Google Scholar]
- 16). Hoh BL, Putman CM, Budzik RF, Ogilvy CS: Surgical and endovascular flow disconnection of intracranial pial single-channel arteriovenous fistulae. Neurosurgery 49: 1351-1363; discussion 1363-1354, 2001 [DOI] [PubMed] [Google Scholar]
- 17). Oya S, Shigeno T, Kumai J, Matsui M: [A case of pial single-channel cerebral arteriovenous fistula]. No Shinkei Geka 32: 67-72, 2004[Japanese] [PubMed] [Google Scholar]
- 18). Yoshida Y, Weon YC, Sachet M, et al. : Posterior cranial fossa single-hole arteriovenous fistulae in children: 14 consecutive cases. Neuroradiology 46: 474-481, 2004 [DOI] [PubMed] [Google Scholar]
- 19). Limaye US, Siddhartha W, Shrivastav M, Anand S, Ghatge S: Endovascular management of intracranial pial arterio-venous fistulas. Neurol India 52: 87-90, 2004 [PubMed] [Google Scholar]
- 20). Passacantilli E, Pichierri A, Guidetti G, Santoro A, Delfini R: Surgical treatment of pial cerebellar arteriovenous fistulas with aneurysm of the main feeding artery. Surg Neurol 65: 90-94, 2006 [DOI] [PubMed] [Google Scholar]
- 21). Izzo R, Alvaro Diano A, Lavanga A, Vassallo P, Muto M: Posterior fossa arteriovenous pial fistula: diagnostic and endovascular therapeutic features. A case report. Neuroradiol J 19: 783-786, 2007 [DOI] [PubMed] [Google Scholar]
- 22). Masuoka J, Sakata S, Maeda K, Matsushima T: Intracranial pial single-channel arteriovenous fistula presenting with significant brain edema. J Neurosurg 109: 497-501, 2008 [DOI] [PubMed] [Google Scholar]
- 23). Lv X, Jiang C, Li Y, Yang X, Wu Z: Clinical outcomes of endovascular treatment for intracranial pial arteriovenous fistulas. World Neurosurg 73: 385-390, 2010 [DOI] [PubMed] [Google Scholar]
- 24). Guimaraens L, Casasco A, Sola T, Cuellar H, Miralbes S, Cambra FJ: Endovascular treatment of a pial arteriovenous fistula of a posteroinferior cerebellar artery with a double origin. J Neurointerv Surg 3: 233-236, 2011 [DOI] [PubMed] [Google Scholar]
- 25). Newman CB, Hu YC, McDougall CG, Albuquerque FC: Balloon-assisted Onyx embolization of cerebral single-channel pial arteriovenous fistulas. J Neurosurg Pediatr 7: 637-642, 2011 [DOI] [PubMed] [Google Scholar]
- 26). Guerra LR, Barbosa Lde A, Barbosa LG, Pimentel Dde P, Leite FI: Pial arteriovenous fistula in the posterior fossa. Arq Neuropsiquiatr 69: 718-719, 2011 [DOI] [PubMed] [Google Scholar]
- 27). Requejo F, Jaimovich R, Marelli J, Zuccaro G: Intracranial pial fistulas in pediatric population. Clinical features and treatment modalities. Childs Nerv Syst 31: 1509-1514, 2015 [DOI] [PubMed] [Google Scholar]
- 28). Kanai R, Shinoda J, Akatsuka S: Infratentorial pial arteriovenous fistula in the elderly. J Stroke Cerebrovasc Dis 24: e307-309, 2015 [DOI] [PubMed] [Google Scholar]
- 29). Lylyk P, Chudyk J, Bleise C, Serna Candel C, Aguilar Perez M, Henkes H: Endovascular occlusion of pial arteriovenous macrofistulae, using pCANvas1 and adenosine-induced asystole to control nBCA injection. Interv Neuroradiol 23: 644-649, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Ye M, Zhang P: Transarterial balloon-assisted glue embolization of pial arteriovenous fistulas. World Neurosurg 115: e761-e767, 2018 [DOI] [PubMed] [Google Scholar]
- 31). Akamatsu Y, Hayashi T, Sato K, Karibe H, Kameyama M, Tominaga T: Bilateral Upper Cerebellar Hemorrhage Due to Pial Arteriovenous Fistula and Its Pathophysiological Insight. World Neurosurg 115: 388-392, 2018 [DOI] [PubMed] [Google Scholar]
- 32). Wang S, Mo J, Gai S, Ou C, Chen Y: Trigeminal neuralgia associated with cerebellar pial arteriovenous fistula: A case report. Medicine (Baltimore) 99: e18873, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Yan WT, Li XZ, Yan CX, Liu JC: Typical subdural contrast effusion secondary to endovascular treatment of a pediatric pial arteriovenous fistula. Interv Neuroradiol 27: 31-36, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Yu J, Shi L, Lv X, Wu Z, Yang H: Intracranial non-galenic pial arteriovenous fistula: a review of the literature. Interv Neuroradiol 22: 557-568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Song JK, Patel AB, Duckwiler GR, et al. : Adult pial arteriovenous fistula and superior sagittal sinus stenosis: angiographic evidence for high-flow venopathy at an atypical location. Case report. J Neurosurg 96: 792-795, 2002 [DOI] [PubMed] [Google Scholar]
- 36). da Silva Martins WC, de Albuquerque LA, de Souza Filho CB, Dellaretti M, de Sousa AA: Surgical treatment of the intracranial pial arteriovenous fistula. Surg Neurol Int 6: 102, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Aadland TD, Thielen KR, Kaufmann TJ, et al. : 3D C-arm conebeam CT angiography as an adjunct in the precise anatomic characterization of spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 31: 476-480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Hiramatsu M, Sugiu K, Hishikawa T, et al. : Detailed arterial anatomy and its anastomoses of the sphenoid ridge and olfactory groove meningiomas with special reference to the recurrent branches from the ophthalmic artery. AJNR Am J Neuroradiol 41: 2082-2087, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


