Graphical abstract
Dear Editor,
Policies for deployment of SARS-CoV2 vaccines depends on detailed knowledge of immunity after natural infection. With hybrid immunity in the global population, after the combination of infection and vaccination, it is no longer possible to characterize infection induced immunity. Recently Shaw and colleagues conducted a head-to-head comparison of different heterologous mRNA and ChAdOx1 COVID vaccination schedules finding similar humoral and cellular responses six months after vaccination irrespective of regime.1 They recommended easing logistical challenges by using heterologous vaccine regimes in future pandemics.
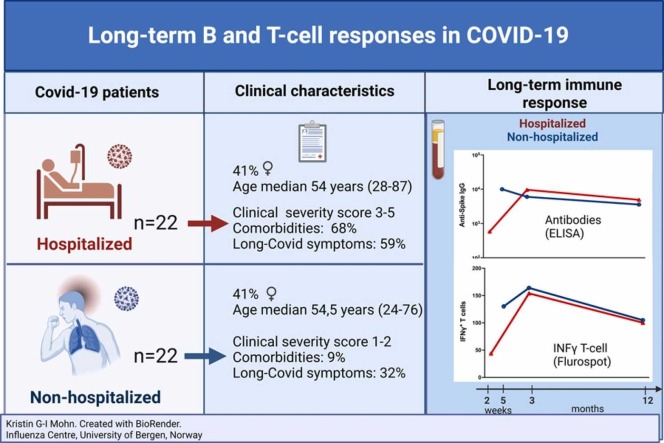
We compared long-term humoral and cellular immune responses in age and gender matched SARS-CoV-2 infected hospitalized(n=22) and non-hospitalized(n=22) patients during the Wuhan-like wave, prior to available treatment or vaccines with a 12-15 month (m) follow-up (Supplementary Table 1). Patients (41% female, aged 24–87years) were classified according to disease severity, based on the need for oxygen treatment and respiratory failure.2
Detailed immunological analyses were conducted of humoral (Spike-specific SARS-CoV-2 IgG, memory B-cells (MBC)) and T-cell responses. Despite less severe disease, non-hospitalized patients achieved comparable long-term MBC and T-cellular responses for >1 year, although often higher in patients requiring oxygen. In both groups, spike-specific IgG peaked at 3-9 weeks(w), and declined over time, remaining detectable at 12-15 m. Significantly more hospitalized patients had comorbidities (68%vs9%) and reported persisting post COVID condition symptoms 12-15 m post-infection (59%vs32%, p<0.001) (Supplementary Table 1).
Strain-specific antibodies decrease within six months of vaccination, limiting their protection and necessitating boosters in risk groups.3 However, we found patients still had detectable levels after 12-15 m, although the highest antibody levels declined most rapidly, similarly to the antibody decay seen after heterologous vaccination in the Com-COV2 study.1
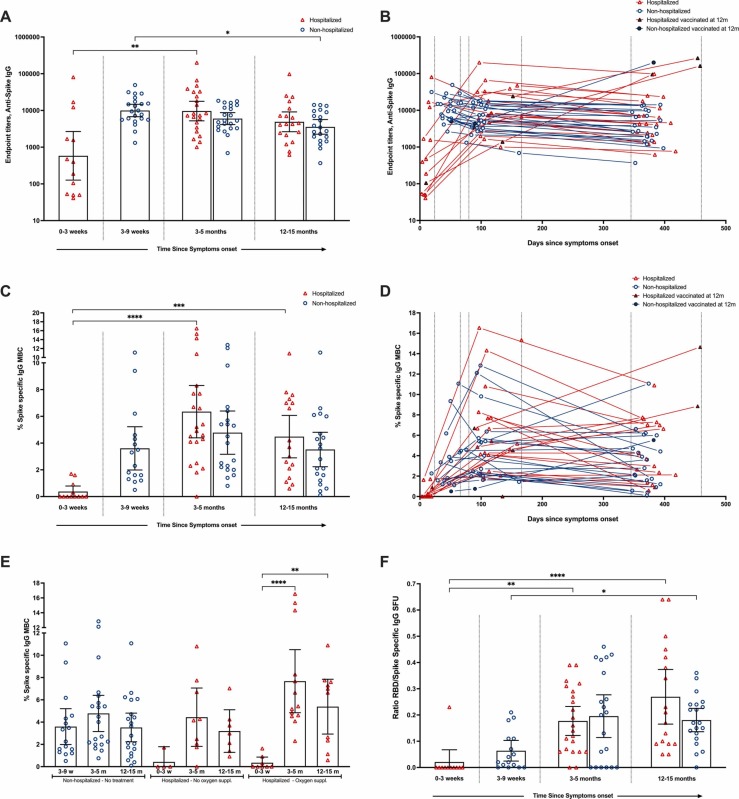
In the hospitalized cohort, there was a significant increase in IgG titers from 0-3w peaking at 3-5 m (GMT 9632, p<0.01) ( Fig. 1A), declining by 49% at 12-15 months (ns)(GMT 4899). In the non-hospitalized cohort, IgG was highest at 3-9w (GMT 5999), maintained at 3-5 m, followed by a significant decrease (40%) at 12-15 m(GMT 3571)(Fig. 1A). Overall, we found no significant difference in the magnitude of spike-specific IgGs between patient groups, or by severity score, although higher titers were observed in hospitalized patients receiving oxygen (Figs. 1A,1B,1E).
Fig. 1.
Frequencies of SARS CoV-2 Spike specific IgG antibody and IgG Memory B cell (MBC) responses 12-15 months post-infection: Longitudinal comparison of the SARS-CoV-2 spike IgG titers between hospitalized and non-hospitalized patients is shown at 0-3 weeks (hospitalized only), 3-9 weeks (non-hospitalized only), 3-5 months and 12 months post symptoms onset (A). Each symbol represents individual antibody titers. The horizontal bars show the geometric mean of the antibody titers ± 95% CI. The kinetics of the individual titers of SARS-CoV-2 spike IgG antibody titers since symptom onset (PSO) (days) (B). Triangles represent hospitalized cases and circles represent non-hospitalized cases. Closed triangles and circles indicate cases that were vaccinated against SARS-CoV-2 prior to sampling at 12 months and are not included in the analysis at this timepoint (B,D). Comparison of the SARS-CoV-2 spike IgG MBC response between non-hospitalized and hospitalized patients is shown (C). Results are presented as percentage of spike specific IgG MBC out of total IgG MBC. The kinetics of the individual MBC responses from symptom onset PSO (days)(D). Comparison of the frequency of MBC according to disease severity (non-hospitalized and hospitalized with/without oxygen supplementation during acute phase)(E). Ratio of RBD specific IgG MBC versus spike specific IgG MBC (F). The horizontal bars show the mean of the MBC frequencies ± 95% CI (C,E,F). A nonparametric Kruskal–Wallis multiple comparisons test was used to compare the different timepoints within each cohort (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
In the acute phase, spike-specific IgG MBCs were not detected in 67% hospitalized patients, indicating that antibodies mainly originated from naïve B-cells, not cross-reactive coronavirus MBCs(Fig. 1C+D). MBCs then increased significantly, peaking at 3-5 m(p<0.0001) for both groups, and were maintained at 12-15 m significantly higher than the acute phase for the hospitalized group(p<0.001)(Fig. 1C).4 The frequencies of spike-specific MBC decreased most in hospitalized cases indicating immune contraction moving to a resting state over time. The magnitude and kinetics of the MBC response at 3-5 m and 12-15 m was similar in the two groups, irrespective of disease severity(Fig. 1C+1E). Nevertheless, hospitalized patients requiring oxygen had the highest IgG MBC responses, with the most significant increase between 0-3w and 3-5 m(p<0.0001) (Fig. 1D+1E). There was heterogeneity of responses within both hospitalized and non-hospitalized groups (Fig. 1D). Interestingly RBD-specific MBC frequencies increased relative to spike-specific MBCs up to 12-15 months in both hospitalized and non-hospitalized individuals (Fig. 1F), perhaps suggesting antigen persistence, prolonged activity in germinal centers, and affinity maturation. Supporting this, antibody maturation favors epitopes overlapping the ACE2 binding site on the RBD.5 Interestingly, four hospitalized patients showed immune activation with detectable levels of spike-specific IgG MBC at admission, >10 days from symptom onset suggesting rapid, early memory responses. A less likely explanation is pre-existing cross-reactive MBC from earlier coronavirus infections.
Patients with severe and fatal COVID-19 may have impaired germinal centers, associated with extrafollicular B-cell responses, high magnitudes of antibody secreting cells, and neutralizing antibodies.6 Consistent with our results, hospitalized COVID-19 patients showed higher antibody and MBC responses compared to milder infections.4, 7 However, our findings of robust and long-lived MBC responses after infection in non-hospitalized patients supports findings indicating that lymph node function is either maintained or restored, at least in survivors with less severe disease.8 This long-lived immunity may protect against severe disease upon reinfection from SARS-CoV-2 variants.
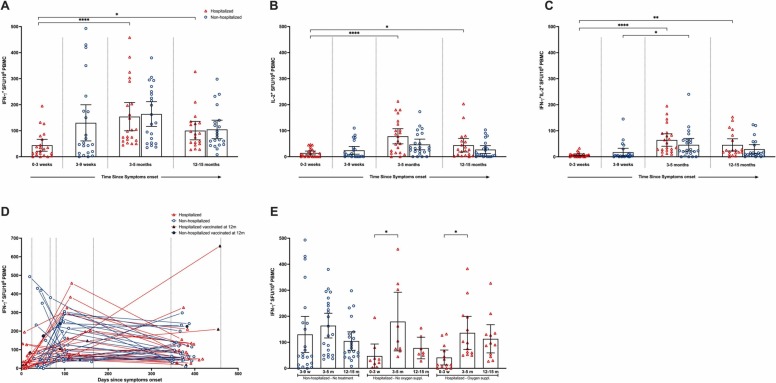
The grade of COVID-19 severity may influence the function of central and effector memory T-cells.4 In non-hospitalized patients the frequencies of single cytokine (IFN-γ/IL-2) and double positive IFN-γ+IL-2+ producing T-cell peaked at 3-5 m and were maintained at 12-15 m( Fig. 2A+D). There was a significant increase in IFN-γ+IL-2+ producing T-cells from 3-9w to 3-5 m, not observed for single IFN-γ/ IL-2 cytokine producing T-cells (Fig. 2A-C).
Fig. 2.
SARS CoV-2 specific T-cell cytokine responses 12-15 months post-infection. T-cell immune responses were evaluated by measuring the number of SARS-CoV-2 specific IFN-γ (A), IL-2 (B) and IFN-γ/ IL-2 (C) secreting T-cells after infection. The triangles represent hospitalized cases and circles represent non-hospitalized cases. Each symbol represents one individual and the combined number of spot forming units (SFU) per 1×106cells) after stimulation with virus spike antigen (spike, non-spike (nucleocapsid and matrix peptides). For the individual responses the time from symptom onset is indicated (D). Closed triangles and circles represent cases that were vaccinated against SARS-CoV-2 prior to sampling at 12 months, not included in the analysis at this timepoint. Comparison of the frequency of T-cell responses according to disease severity (non-hospitalized and hospitalized with/without oxygen supplementation during acute phase)(E). Disease severity is divided into three scores: 1) non- hospitalized (scores 1-2), 2) hospitalized cases with no oxygen supplementation (scores 3-4) and 3) hospitalized cases with oxygen supplementation (score 5). The horizontal bars show the mean of the SFU ± 95% confidence interval (CI). Statistical differences between hospitalized and non-hospitalized subjects were determined by the nonparametric Kruskal–Wallis multiple comparisons test (* = P<0.05, ** = P<0.01, ***P<0.001, ****P<0.0001).
In contrast, in hospitalized patients, the frequencies of all three T-cell subsets increased significantly from 0-3w to 3-5 m(p<0.001), remaining significantly higher at 12-15 m(p<0.05)(Fig. 2A-C). Although in the acute phase, only low numbers of double positive IFN-γ+IL-2+ T-cells were found(Fig. 2A-C). Overall, no statistically significant differences of T-cell frequencies were observed by disease severity (Fig. 2A-C), or by oxygen treatment(Fig. 2E). As expected, T-cells were boosted in patients(n=4) vaccinated prior to sampling at 12 m(Fig. 2D, excluded from other analyses).
The cytokines IL-2 and IFN-γ are specific biomarkers of SARS-CoV-2 T-cell responses supporting our earlier findings of T-cell responses at 6 months which were significantly stronger in hospitalized than non-hospitalized patients.9 However, our 15 m data show similar T-cell frequencies independently of disease severity. The quality of the T cell response, with increased IL-2 and decreased IFN-γ production in hospitalized patients, may indicate higher levels of central memory T cells relative to effector memory T cells in hospitalized compared to non-hospitalized patients.10 Of importance, frequencies of IFN-γ T-cells for both hospitalized and non-hospitalized patients at 12-15 m were equivalent to the most superior heterologous booster regime (80 and 79 SFCs/106 PBMC) found by Shaw and colleagues.1
The strength of our study is the longitudinal follow-up of two matched, unvaccinated cohorts, with differing disease severity following natural infection. Our findings of durable SARS-CoV-2 antibodies, MBC and T-cellular protective immune responses more than one-year post- infection, contribute to detailed knowledge of long-term memory responses, and are important for developing future vaccination strategies. Similar kinetics of durable B and T cell respiratory responses were found irrespective of disease severity between non-hospitalised and hospitalised which were comparable to heterologous vaccination schedules.
Funding
This study received funding from the Trond Mohn Stiftelse (grant no. TMS2020TMT05), Research Council of Norway (grant no 312780), Western Norway Regional Health Authority (grant number, F-11628 and F-12621and Clinical research grant nr F-12626), South-Eastern Norway Regional Health Authority (grant no 2018084 and 39550), Haukeland University Hospital, University of Bergen, Oslo University Hospital, University of Oslo, the Ministry of Health and Care Services, Norway; and the Oslo research group has received a philanthropic donation from Vivaldi Invest A/S owned by Jon Stephenson von Tetzchner. The Influenza Centre, Bergen is funded by the Norwegian Research Council Globvac (R.J.C., grant no. 284930); the European Union (R.J.C., grant nos. EU IMI115672, FLUCOP, H2020 874866 INCENTIVE and H2020 101037867 Vaccelerate) and the Faculty of Medicine, University of Bergen, Norway. The funders had no role in study design, data collection, or decision to publishthis article. The authors declare no conflict of interest.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kristin G-I Mohn has received honoraria from Takeda and IQVIA. All other authors declare no competing interests
Acknowledgments
We thank the staff at Bergen Municipality Emergency clinic, the Emergency Care Clinic and infectious diseases department at Haukeland university Hospital, the Influenza Centre at the University of Bergen, and the Departments of Infectious Diseases and Medical Microbiology at Oslo University Hospital, Ullevål for inclusion and follow-up of the patients, blood sampling, and biobank processing. We wish to thank all staff at the Influenza Centre for logistical and practical help conducting the study. Constructs required to produce the purified SARS-CoV-2 (Wuhan-Hu-1 isolate) receptor binding domain (RBD) and spike proteins were kindly provided by Professor Krammer from the Ican School of Medicine, Mount Sinai, New York, USA. Finally, we thank Professor Nina Langeland for valuable scientific input to the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jinf.2023.05.023.
Appendix A.
Dagrunn Waag Linchausen, Nina Ertesvåg, Anders Madsen, Håkon Amdam
1Influenza Centre
2Department of Clinical Science, University of Bergen, N-5021 Bergen, Norway
Beathe Kiland Granerud, Elisabeth Toverud Landaas
7Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, 0315 Oslo, Norway
9Department of Microbiology, Oslo University Hospital, 0424 Oslo, Norway.
Appendix B. Supplementary material
Supplementary material
References
- 1.Shaw R.H., Greenland M., Stuart A.S.V., et al. Persistence of immune response in heterologous COVID vaccination schedules in the Com-COV2 study - A single-blind, randomised trial incorporating mRNA, viral-vector and protein-adjuvant vaccines. The Journal of infection. 2023 doi: 10.1016/j.jinf.2023.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the Treatment of Covid-19 - Final Report. The New England journal of medicine. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin E.G., Lustig Y., Cohen C., et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. The New England journal of medicine. 2021;385 doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021:371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z., Muecksch F., Schaefer-Babajew D., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff M.C., Ramonell R.P., Nguyen D.C., et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nature immunology. 2020;21:1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long Q.X., Liu B.Z., Deng H.J., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature medicine. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 8.Ogega C.O., Skinner N.E., Blair P.W., et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease. The Journal of clinical investigation. 2021:131. doi: 10.1172/JCI145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohn K.G., Bredholt G., Zhou F., et al. Durable T-cellular and humoral responses in SARS-CoV-2 hospitalized and community patients. PloS one. 2022;17 doi: 10.1371/journal.pone.0261979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material