Abstract
Background Research is needed to identify how clinical decision support (CDS) systems can support communication about and engagement with tobacco use treatment in pediatric settings for parents who smoke. We developed a CDS system that identifies parents who smoke, delivers motivational messages to start treatment, connects parents to treatment, and supports pediatrician–parent discussion.
Objective The objective of this study is to assess the performance of this system in clinical practice, including receipt of motivational messages and tobacco use treatment acceptance rates.
Methods The system was evaluated at one large pediatric practice through a single-arm pilot study from June to November 2021. We collected data on the performance of the CDS system for all parents. Additionally, we surveyed a sample of parents immediately after the clinical encounter who used the system and reported smoking. Measures were: (1) the parent remembered the motivational message, (2) the pediatrician reinforced the message, and (3) treatment acceptance rates. Treatments included nicotine replacement therapy, quitline referral (phone counseling), and/or SmokefreeTXT referral (text message counseling). We described survey response rates overall and with 95% confidence intervals (CIs).
Results During the entire study period, 8,488 parents completed use of the CDS: 9.3% ( n = 786) reported smoking and 48.2% ( n = 379) accepted at least one treatment. A total of 102 parents who smoke who used the system were approached to survey 100 parents (98% response rate). Most parents self-identified as female (84%), aged 25 to 34 years (56%), and Black/African American (94%), and had children with Medicaid insurance (95%). Of parents surveyed, 54% accepted at least one treatment option. Most parents recalled the motivational message (79%; 95% CI: 71–87%), and 31% (95% CI: 19–44%) reported that the pediatrician reinforced the motivational message.
Conclusion A CDS system to support parental tobacco use treatment in pediatric primary care enhanced motivational messaging about smoking cessation and evidence-based treatment initiation.
Keywords: clinical decision support systems, smoking cessation, secondhand smoke, human-centered design, pediatrics
Background and Significance
The health toll of smoking is severe, 1 and there is no safe level of secondhand smoke (SHS) exposure. 2 SHS exposure among children increases the risk of sudden infant death syndrome, chronic respiratory diseases, such as asthma, and lung cancer in adulthood. 2 More than 40% of children in the United States are regularly exposed to the harms of SHS, most often by a parent. 1 3 Parents who quit smoking can increase their own life expectancy, 4 eliminate the majority of their children's SHS exposure, 1 and decrease the risk of their children becoming adult smokers. 5 Pediatric clinicians can protect children from SHS exposure by promoting evidence-based tobacco use treatment for parents who smoke. 6 7 Evidence-based smoking cessation treatments, however, are rarely delivered in pediatric settings. 8 Major barriers to wider adoption include a lack of methods to support effective pediatrician–parent communication regarding tobacco use treatment and mechanisms to systematize, consistently deliver, and scale effective intervention delivery. 8 9 10 This article describes preliminary evaluation of an innovative clinical decision support (CDS) system embedded within the electronic health record (EHR) to address these barriers.
In pediatric settings, tobacco use treatment messages to parents that emphasize the impact of smoking on their child may increase acceptance of cessation treatment. 11 12 Prior research suggests that messages to parents about their smoking should focus on child health rather than, for example, the financial impact of smoking. 11 Incorporating foundational elements of motivational interviewing, framing conversations to address concerns unique to parents, can increase engagement and intrinsic motivation as well as perceived clinician empathy. 13 14 These motivational messages may improve conversations around parent smoking, potentially increasing treatment engagement.
CDS systems may help deliver messages that promote parent–provider communication while facilitating treatment engagement. For instance, in adult health care settings, CDS systems can support evidence-based clinical conversations between physicians and patients about diabetes medications 15 and support self-management. 16 Additionally, CDS systems can improve the delivery of clinical interventions for tobacco use in adult settings. 17 18 19 20 21 Further, other communication modalities geared toward adults, including internet-based or app-based services, may promote smoking cessation. 22 23 24 25 In pediatric settings, CDS systems have shown potential to support screening for SHS exposure and facilitate the connection to and delivery of evidence-based tobacco treatments. 26 27 28 29 30 31 These systems, however, have not been widely integrated into pediatric settings due to critical pediatric EHR workflow limitations, especially the inability to account for the family aspect of child health care. 32 33 EHR vendors often develop technological services for the physician–adult patient dyad and are unable to connect family relationships, making it difficult to leverage existing technologies to support care for parents through pediatric settings. 34 For CDS systems to be effective in addressing parent tobacco use in pediatric settings, the interface needs to be responsive to the needs of the child's health care team and family as a whole, supporting communication with and treatment delivery to all household members. 35
Objective
We developed a CDS system, using human-centered design (HCD) processes, 36 37 38 39 40 41 that identifies parents and other household members who smoke, delivers motivational messages to start treatment, connects to evidence-based tobacco treatment, and supports further pediatrician–parent discussion about treatment, as needed. 42 We sought to evaluate if the CDS system led to the communication of messages that were reliably delivered and remembered by parents. Thus, our objective was to assess the performance of this system in clinical practice, including receipt of motivational messages and tobacco use treatment acceptance rates.
Methods
Overall Design
We developed the CDS system 42 and tested it through a single-arm intervention study. We collected data on the performance of the CDS system for all parents. Additionally, we surveyed a sample of parents who smoke and used the CDS system to estimate the following measures: (1) if the parent remembered the key motivational message, (2) if the pediatric clinician reinforced the message, and (3) parent tobacco use treatment acceptance rates.
Study Setting, Participants, and Recruitment
This study took place at one high-volume outpatient primary care pediatric practice (∼30,000 pediatric patients) from Children's Hospital of Philadelphia (CHOP's) Pediatric Research Consortium, a highly productive practice-based research network. 43 The study clinic is located in West Philadelphia, serving a patient population with high rates of adult smoking (25–30%) 44 and high rates of Medicaid insurance. Participating pediatric clinicians at the clinic include all physicians, resident physicians, and nurse practitioners. For the survey, the inclusion criteria were parents/caregivers (hereafter referred to as parents) who self-identified as using any combustible tobacco products (i.e., cigarettes, cigars, Black and mild cigars, or hookah) through the CDS system, aged ≥ 18 years, present at their child's preventive visit (well-childcare), and able to communicate in English. Responses to the CDS system, available within the child's medical record, were reviewed to identify potentially eligible parent participants. When research staff were onsite (∼3 d/wk based on staff availability), all parents were approached after their visit with the pediatric clinician to complete a brief survey about the office visit on a tablet or laptop with the assistance of the research assistant. We received a waiver of informed consent for survey completion and chart review. The study was approved by the CHOP Institutional Review Board.
Intervention: Clinical Decision Support System
We developed the CDS system using an HCD process that engaged pediatricians, clinical staff, and parents who smoke in a series of iterative, scenario-based usability testing sessions that captured a range of objective and subjective metrics on CDS system usability, utility, and intent to use. Participants from each cohort were presented with scenario-based, high-fidelity mockups of system components and then provided input related to their role in using the CDS system. 42 This work was performed by a multidisciplinary team with expertise in pediatric primary care, smoking cessation, CDS, HCD and human factor methods, and software engineering. 27 29 36 37 38 39 40 41 An overarching theme of the design review sessions across all cohorts was automating key functions as much as possible. Further, all cohorts supported directly screening parents for tobacco use and automatically directing and connecting them to treatment. These approaches were suggested to overcome critical pediatric EHR workflow limitations that made it difficult to both document and connect the parents of patients to tobacco use treatment. 42
The CDS system has parent- and clinician-facing components ( Fig. 1 ). The parent-facing portion of the system is a survey that supports directly screening parents for smoking both in the office on a tablet or a mobile device and before the pediatric patient's visit via a patient portal, to decrease the burden of in-office completion. This survey is sent to the parent once a year at a scheduled preventive care visit. If the parent indicates they are a smoker on the survey, the parent portion of the system displays a simple and succinct message designed to motivate treatment engagement ( Fig. 2 ). This message, developed based on concepts from behavioral economics, emphasized to parents the impact of smoking on their child's health. 11 12 Next, the parent is presented three treatment options for them to consider: (1) nicotine replacement therapy (NRT), (2) quitline, and/or (3) SmokefreeTXT. The quitline offers telephone-based counseling and is an effective and scalable program that helps smokers quit. 45 SmokefreeTXT, also shown to be effective in helping smokers quit, 46 sends automated text messages that provide adults who smoke with encouragement, advice, and tips to quit smoking. Connection or referral to each of the treatment options is streamlined, meaning NRT is delivered to the parent's home (via a pharmacy delivery company) and the quitline and SmokefreeTXT programs proactively contact parents to begin treatment. See the Appendix for the full parent-facing system, including screening items and treatment workflows.
Fig. 1.
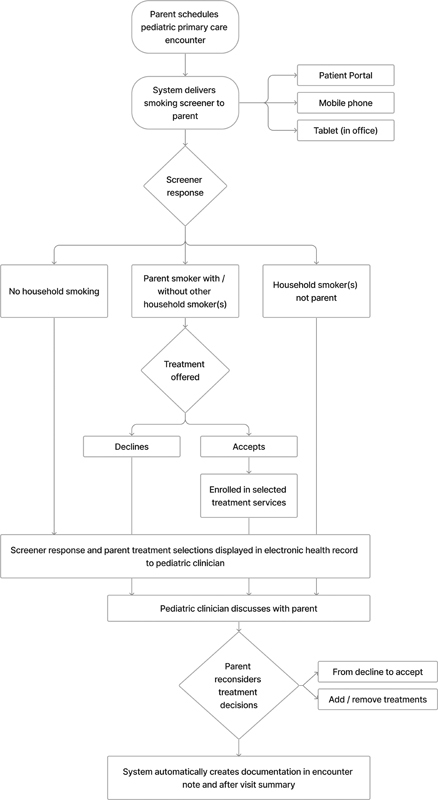
Intervention flow diagram: an automated system that identifies parents who smoke, delivers motivational messages to start treatment, connects to treatment, and supports pediatrician–parent discussion.
Fig. 2.
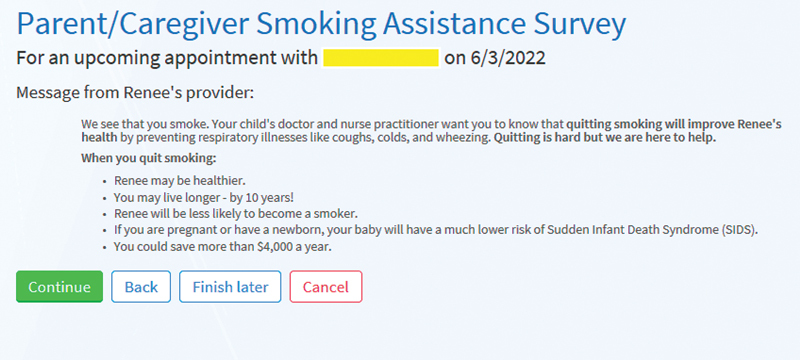
Parent clinical decision support system screen: motivational message for parents who smoke.
The pediatric clinician is notified of the parent's and other family members' smoking status and parent treatment selections within the clinician portion of the system ( Fig. 3 ). The system includes information to help the clinician in what is often perceived as a difficult conversation about smoking, 47 with simple guidance on key conversation prompts that motivate treatment, including the key messages presented to parents in the parent portion of the CDS system.
Fig. 3.
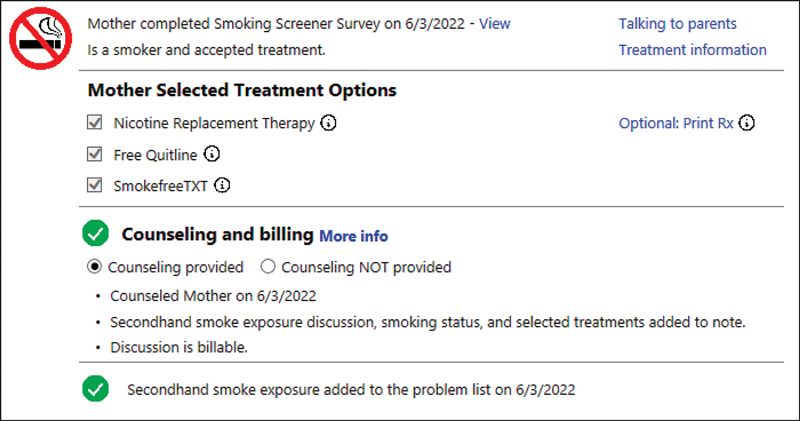
Pediatric clinician clinical decision support system screen: main view
Study Procedures
Parents who used the CDS system and self-identified as smokers were surveyed immediately after the clinical encounter by research staff. The brief survey asked if the parent remembered the motivational message included in the CDS system (“quitting smoking will improve your child's health by preventing respiratory illnesses like coughs, colds, and wheezing”), whether or not the pediatrician asked about their responses in the CDS system, and if the pediatrician communicated the same motivational message, as suggested by the pediatrician-facing portion of the CDS system. Additionally, to account for potential social desirability bias, the survey asked the parent if they remember content that was not actually included in the CDS system. Parent age, gender, race/ethnicity, as well as patient/child age and medical insurance status were also collected via the survey to help best understand the representativeness of the population surveyed. The CDS system data for the surveyed parents were collected and analyzed.
Measures and Analysis
We collected data on the performance of the CDS system for all parents, including the number of parents who completed the CDS system, rates of parent reported smoking and, for those that reported smoking, acceptance of tobacco use treatments. For the after-visit survey, the main measures included if the parent remembered the key motivational message, if the pediatrician reinforced the message, and treatment acceptance rates (collected via the CDS system). Parent responses to the survey were described overall and presented with 95% confidence intervals (CI). We calculated that surveying a sample of 100 parents would be feasible based on the availability of research assistants. Further, we estimated that a message recall rate of 75% was a reasonable target and that our proposed sample would provide a tight 95% CI for whether or not these messages were recalled by the parents (e.g., message recall rate 75%; 95% CI: 73–77%).
Results
During the entire study period and including parents surveyed about the encounter and those not surveyed, 10,679 surveys were sent to parents and 8,488 parents completed the parent-facing component of the system (response rate: 79.4%). Of parents who completed the system, 9.3% ( n = 786) reported smoking and 48.2% ( n = 379) accepted at least one evidence-based treatment.
We surveyed 100 parents who smoke at one large pediatric clinic from June to November 2021. A total of 102 parents who smoke were approached (98% response rate). Most parents self-identified as female (84%), aged 25 to 34 years (56%), and Black/African American (94%), and had children with Medicaid insurance (95%). See Table 1 for demographics.
Table 1. Demographics.
| N = 100 | N (%) |
|---|---|
| Parent age (y) | |
| 18–24 | 3 (3%) |
| 25–34 | 56 (56%) |
| 35–44 | 33 (33%) |
| 45–54 | 8 (8%) |
| Parent gender | |
| Man | 16 (16%) |
| Woman | 84 (84%) |
| Parent race | |
| White | 2 (2%) |
| Black or African American | 94 (94%) |
| Asian | 1 (1%) |
| American Indian or Alaska Native | 1 (1%) |
| Other | 3 (3%) |
| Missing | 1 (1%) |
| Parent ethnicity | |
| Hispanic or Latino | 1 (1%) |
| Not Hispanic or Latino | 99 (99%) |
| Child health insurance | |
| Medicaid | 95 (95%) |
| Private | 5 (5%) |
| Child age | |
| 0–6 mo | 11 (11%) |
| 7 mo–2 y | 35 (35%) |
| 3–5 y | 13 (13%) |
| 6–10 y | 22 (22%) |
| 11–14 y | 14 (14%) |
| 15 y and older | 5 (5%) |
Of the 100 parents surveyed, 54% accepted at least one evidence-based tobacco use treatment: 49% SmokefreeTXT, 40% quitline, and/or 33% NRT. Most parents reported remembering reading the motivational message about how quitting smoking will improve their child's health (79%; 95% CI: 71–87%). Additionally, almost all parents correctly identified the negative control questions as messages that were not actually included in the parent CDS system. Meaning, for example, most parents correctly reported not remembering a message about how to best clean their clothes after smoking (92%; 95% CI: 87–97%). Slightly over half of parents reported that, during the visit, the pediatric clinician asked about their responses in the CDS system (53%; 95% CI: 43–63%). Approximately one-third of all parents surveyed (31%; 95% CI: 19–44%) reported the pediatrician used the suggested motivational message with them during the encounter ( Table 2 ) .
Table 2. Parent survey responses.
| Response | N (%); 95% confidence interval [CI] |
|---|---|
| Question about motivational message included in the CDS system | |
| Do you remember a message, in the survey you filled out, that talked about how quitting smoking will improve your child's health? N = 100 | |
| Yes | 79 (79%); 71–87% |
| No | 21 (21%); 13–29% |
| Control questions (this content was not included in the CDS system) | |
| Do you remember a message, in the survey you filled out, about how to best clean your clothes after smoking? N = 100 | |
| Yes | 8 (8%); 3–13% |
| No | 92 (92%); 87–97% |
| Do you remember a message, in the survey you filled out, about the harms of roll your own tobacco? N = 100 | |
| Yes | 15 (15%); 8–22% |
| No | 85 (85%); 78–92% |
| Questions about pediatrician portion of the visit | |
| As part of your visit, did the pediatrician ask you about the smoking survey you filled out? N = 100 | |
| Yes | 53 (53%); 43–63% |
| No | 47 (47%); 37–57% |
| Of parents who reported that the pediatrician asked about the survey ( N = 53) | |
| Do you remember the pediatrician saying that “quitting smoking will improve your child's health by preventing respiratory illnesses like coughs, colds, and wheezing?” a | |
| Yes | 31 (58%); 45–72% |
| No | 22 (42%); 28–55% |
Percentages reported for the 53-parent sample who reported that the pediatrician asked about the survey.
Discussion
A CDS system to support parental tobacco use treatment in pediatric primary care successfully triggered evidence-based tobacco cessation messaging to parents. Half of parents accepted evidence-based tobacco treatment. Of parents surveyed, most remembered the motivational message. Further, the system influenced the pediatrician–parent conversation, with approximately one-third of parents reporting that the pediatrician reinforced the motivational message with them during the clinical conversation.
This preliminary study fills an important gap in our understanding of how to leverage CDS systems to reliably deliver a message to families about tobacco cessation that they remember and that is commonly reinforced by clinicians. To enhance cessation rates, it is critical for health care providers to consistently identify smokers, advise them to quit, and offer evidence-based cessation treatments. 48 According to surveys of current smokers, of those who attempted to quit smoking in the past year, 31.2% used counseling and/or medication. 49 According to the National Ambulatory Medical Care Survey (based on a national sample of visits for adults > 18 y old to ambulatory medical care services in the United States), of individuals who currently smoke and who visited a physician in the past year, 20.9% received physician tobacco counseling during the visit, and only 7.6% received a prescription or an order for a medication associated with tobacco use treatment. 50 Our results suggest our system performed better than this patient-reported data. By developing a CDS system that supports pediatrician–parent communication and systematizes key processes related to tobacco treatment, our approach streamlines clinical workflows to support tobacco use treatment engagement.
Our study has several limitations. First, we do not yet have data on the CDS system's impact on parent smoking cessation rates. Our preliminary evidence suggests that parents remembered the key recommendations about quitting smoking, recalled pediatric clinicians provided evidence-based counseling, and accepted evidence-based tobacco treatment. All of these activities are strong predictors of tobacco cessation. 48 Second, we limited tobacco use treatment pharmacotherapy to NRT. Practice concerns limit pediatrician willingness to prescribe varenicline or bupropion. As comfort levels change, pediatrician prescribing may change. In the interim, counseling via connection to the quitline combined with NRT use is effective in helping smokers quit, with meta-analyses estimated quit rates (95% CI) of 28.1% (24.5–32.0). 13 Third, while we sought a representative sample of parents who smoke for the survey, the present sample was not representative of all parents in our health system and may not be representative of all parents who attend pediatrics visits with their children. The sample is heavily weighted to parents who are female, African American, and whose children have Medicaid insurance. Fourth, parent responses to the surveys may not reflect all the potential aspects of the clinical conversation around tobacco use and may be prone to social desirability bias. We attempted to mitigate this potential bias with control questions for content not presented in the CDS system. Finally, the CDS system to support parent tobacco use treatment was limited to a single institution as part of a research effort and no comparison group was included.
Conclusions
A CDS system to support parental tobacco use treatment in pediatric primary care enhanced motivational messaging about smoking cessation and treatment initiation. This preliminary work justifies evaluating the impact of the system on rates of smoking cessation. A randomized control trial is underway evaluating the CDS system's effectiveness in helping parent smokers quit ( https://clinicaltrials.gov/ct2/show/NCT04974736 ).
Clinical Relevance Statement
This article describes preliminary evaluation of an innovative CDS system embedded within the EHR to identify parents who smoke, deliver motivational messages to start treatment, connect to evidence-based treatment, and support further pediatrician–parent discussion about treatment. We assessed the performance of this system in clinical practice, including receipt of motivational messages and tobacco use treatment acceptance rates, through use of parent surveys and data available from the CDS system. For parents surveyed, most recalled the motivational message, 31% reported the pediatrician reinforced the motivational message and, overall, 54% of parents accepted at least one treatment option.
Multiple-Choice Questions
-
One of the key features of HCD approaches are:
A formatting system for displaying material within the EHR
Information present at the right time in the workflow
Active user involvement in the development process
A stochastic analysis describing a sequence of possible events
Correct Answer: The correct answer is option c. Human-centered design approaches, guided by usability experts, involves an analysis of the work environment, active user involvement in the development process, iterative systems development, and testing systems in real-word settings
-
The CDS system to help parents quit smoking described in this study has which of the following features:
Screening for parent tobacco use
Providing motivational messages to start treatment
Connecting to evidence-based tobacco use treatments
All of the above
Correct Answer: The correct answer is option d. The CDS system described in this study identifies parents and other household members who smoke, delivers motivational messages to start treatment, connects to evidence-based tobacco treatment, and supports further pediatrician–parent discussion about treatment.
Acknowledgments
We thank the parents, pediatric clinicians, and clinical staff for their contribution to this study.
Funding Statement
Funding This study was supported by the U.S. Department of Health and Human Services; the National Institutes of Health; the National Cancer Institute (K08 CA226390-01).
Conflict of Interest None declared.
Protection of Human and Animal Subjects
The study was performed in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects and was reviewed by the CHOP Institutional Review Board.
References
- 1.U.S. Department of Health and Human Services The health consequences of smoking—50 years of progress: a report of the surgeon general, 2014. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Accessed February 11, 2016 at:http://www.surgeongeneral.gov/library/reports/50-years-of-progress/
- 2.Office on Smoking and Health (US) The health consequences of involuntary exposure to tobacco smoke: a report of the surgeon generalCenters for Disease Control and Prevention (US); 2006. Accessed February 11, 2016 at:http://www.ncbi.nlm.nih.gov/books/NBK44324/ [PubMed]
- 3.Tsai J, Homa D M, Gentzke A S. Exposure to secondhand smoke among nonsmokers - United States, 1988-2014. MMWR Morb Mortal Wkly Rep. 2018;67(48):1342–1346. doi: 10.15585/mmwr.mm6748a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(04):341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 5.den Exter Blokland E AW, Engels R CME, Hale W W, III, Meeus W, Willemsen M C. Lifetime parental smoking history and cessation and early adolescent smoking behavior. Prev Med. 2004;38(03):359–368. doi: 10.1016/j.ypmed.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Rosen L J, Noach M B, Winickoff J P, Hovell M F. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. 2012;129(01):141–152. doi: 10.1542/peds.2010-3209. [DOI] [PubMed] [Google Scholar]
- 7.Section on Tobacco Control . Farber H J, Walley S C, Groner J A, Nelson K E. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136(05):1008–1017. doi: 10.1542/peds.2015-3108. [DOI] [PubMed] [Google Scholar]
- 8.Winickoff J P, Nabi-Burza E, Chang Y. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics. 2013;132(01):109–117. doi: 10.1542/peds.2012-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly J B, Mackenzie L J, Freund M, Wolfenden L, Roseby R, Wiggers J H. Interventions by health care professionals who provide routine child health care to reduce tobacco smoke exposure in children: a review and meta-analysis. JAMA Pediatr. 2016;170(02):138–147. doi: 10.1001/jamapediatrics.2015.3342. [DOI] [PubMed] [Google Scholar]
- 10.Behbod B, Sharma M, Baxi R, Roseby R, Webster P. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2018;1(01):CD001746. doi: 10.1002/14651858.CD001746.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenssen B P, Kelly M K, Faerber J. Parent preferences for pediatric clinician messaging to promote smoking cessation treatment. Pediatrics. 2020;146(01):e20193901. doi: 10.1542/peds.2019-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenssen B P, Kelly M K, Faerber J. Pediatrician delivered smoking cessation messages for parents: a latent class approach to behavioral phenotyping. Acad Pediatr. 2021;21(01):129–138. doi: 10.1016/j.acap.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore M C, Jaén C R, Baker T B. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. [Google Scholar]
- 14.Section on Tobacco Control . Farber H J, Groner J, Walley S, Nelson K. Protecting children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136(05):e1439–e1467. doi: 10.1542/peds.2015-3110. [DOI] [PubMed] [Google Scholar]
- 15.Montori V M, Breslin M, Maleska M, Weymiller A J. Creating a conversation: insights from the development of a decision aid. PLoS Med. 2007;4(08):e233. doi: 10.1371/journal.pmed.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez W, Hackstadt A J, Hickson G B. The My Diabetes Care Patient Portal Intervention: usability and pre-post assessment. Appl Clin Inform. 2021;12(03):539–550. doi: 10.1055/s-0041-1730324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database Syst Rev. 2014;2014(12):CD008743. doi: 10.1002/14651858.CD008743.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidrine J I, Shete S, Li Y. The Ask-Advise-Connect approach for smokers in a safety net healthcare system: a group-randomized trial. Am J Prev Med. 2013;45(06):737–741. doi: 10.1016/j.amepre.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hood-Medland E A, Stewart S L, Nguyen H. Health system implementation of a tobacco quitline eReferral. Appl Clin Inform. 2019;10(04):735–742. doi: 10.1055/s-0039-1697593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiore M, Adsit R, Zehner M.An electronic health record-based interoperable eReferral system to enhance smoking Quitline treatment in primary care J Am Med Inform Assoc 201926(8-9):778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake L A, Suresh K, Chrastil H, Lewis C L, Altman R L. Improving tobacco cessation rates using inline clinical decision support. Appl Clin Inform. 2022;13(05):1116–1122. doi: 10.1055/a-1961-9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor G MJ, Dalili M N, Semwal M, Civljak M, Sheikh A, Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev. 2017;9(09):CD007078. doi: 10.1002/14651858.CD007078.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilaplana J, Solsona F, Abella F, Cuadrado J, Alves R, Mateo J. S-PC: an e-treatment application for management of smoke-quitting patients. Comput Methods Programs Biomed. 2014;115(01):33–45. doi: 10.1016/j.cmpb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen Thanh V, Guignard R, Lancrenon S. Effectiveness of a fully automated internet-based smoking cessation program: a randomized controlled trial (STAMP) Nicotine Tob Res. 2019;21(02):163–172. doi: 10.1093/ntr/nty016. [DOI] [PubMed] [Google Scholar]
- 25.Pifarré M, Carrera A, Vilaplana J. TControl: a mobile app to follow up tobacco-quitting patients. Comput Methods Programs Biomed. 2017;142:81–89. doi: 10.1016/j.cmpb.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Sharifi M, Adams W G, Winickoff J P, Guo J, Reid M, Boynton-Jarrett R. Enhancing the electronic health record to increase counseling and quit-line referral for parents who smoke. Acad Pediatr. 2014;14(05):478–484. doi: 10.1016/j.acap.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Jenssen B P, Bryant-Stephens T, Leone F T, Grundmeier R W, Fiks A G. Clinical decision support tool for parental tobacco treatment in primary care. Pediatrics. 2016;137(05):e20154185. doi: 10.1542/peds.2015-4185. [DOI] [PubMed] [Google Scholar]
- 28.Jenssen B P, Shelov E D, Bonafide C P, Bernstein S L, Fiks A G, Bryant-Stephens T. Clinical decision support tool for parental tobacco treatment in hospitalized children. Appl Clin Inform. 2016;7(02):399–411. doi: 10.4338/aci-2015-12-ra-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenssen B P, Muthu N, Kelly M K. Parent eReferral to tobacco quitline: a pragmatic randomized trial in pediatric primary care. Am J Prev Med. 2019;57(01):32–40. doi: 10.1016/j.amepre.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabi-Burza E, Drehmer J E, Hipple Walters B. Treating parents for tobacco use in the pediatric setting: the clinical effort against secondhand smoke exposure cluster randomized clinical trial. JAMA Pediatr. 2019;173(10):931–939. doi: 10.1001/jamapediatrics.2019.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheffers-van Schayck T, Hipple Walters B, Otten R, Kleinjan M. Implementation of a proactive referral tool for child healthcare professionals to encourage and facilitate parental smoking cessation in the Netherlands: a mixed-methods study. BMC Health Serv Res. 2021;21(01):973. doi: 10.1186/s12913-021-06969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Committee on Adolescence ; Council on Clinical and Information Technology . Blythe M J, Del Beccaro M A. Standards for health information technology to ensure adolescent privacy. Pediatrics. 2012;130(05):987–990. doi: 10.1542/peds.2012-2580. [DOI] [PubMed] [Google Scholar]
- 33.Council on Clinical Information Technology ; Committee on Medical Liability and Risk Management ; Section on Telehealth Care . Webber E C, Brick D, Scibilia J P, Dehnel P. Electronic communication of the health record and information with pediatric patients and their guardians. Pediatrics. 2019;144(01):e20191359. doi: 10.1542/peds.2019-1359. [DOI] [PubMed] [Google Scholar]
- 34.Jenssen B P, Thayer J, Nekrasova E, Grundmeier R W, Fiks A G. Innovation in the pediatric electronic health record to realize a more effective platform. Curr Probl Pediatr Adolesc Health Care. 2022;52(01):101109. doi: 10.1016/j.cppeds.2021.101109. [DOI] [PubMed] [Google Scholar]
- 35.Karsh B-T. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Clinical practice improvement and redesign: how change in workflow can be supported by clinical decision support. AHRQ Publication No. 09-0054-EF. [Google Scholar]
- 36.Gulliksen J, Göransson B, Boivie I, Blomkvist S, Persson J, Cajander Å. Key principles for user-centred systems design. Behav Inf Technol. 2003;22(06):397–409. [Google Scholar]
- 37.Belden J L, Wegier P, Patel J. Designing a medication timeline for patients and physicians. J Am Med Inform Assoc. 2019;26(02):95–105. doi: 10.1093/jamia/ocy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curran R L, Kukhareva P V, Taft T. Integrated displays to improve chronic disease management in ambulatory care: a SMART on FHIR application informed by mixed-methods user testing. J Am Med Inform Assoc. 2020;27(08):1225–1234. doi: 10.1093/jamia/ocaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ISO 9241-210:2019. In: Ergonomics of human-system interaction-Part 210: human-centred design for interactive systems. 2nd ed.2019https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/75/77520.html
- 40.Nielsen J. New York: Academic Press; 1993. Usability Engineering. [Google Scholar]
- 41.Kushniruk A W, Patel V L. Cognitive and usability engineering methods for the evaluation of clinical information systems. J Biomed Inform. 2004;37(01):56–76. doi: 10.1016/j.jbi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Jenssen B P, Karavite D J, Kelleher S. Electronic health record-embedded, behavioral science-informed system for smoking cessation for the parents of pediatric patients. Appl Clin Inform. 2022;13(02):504–515. doi: 10.1055/s-0042-1748148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiks A G, Grundmeier R W, Margolis B. Comparative effectiveness research using the electronic medical record: an emerging area of investigation in pediatric primary care. J Pediatr. 2012;160(05):719–724. doi: 10.1016/j.jpeds.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philadelphia Department of Public Health . Tobacco use in Philadelphia. Chart. 2021;6(08):1–7. [Google Scholar]
- 45.Matkin W, Ordóñez-Mena J M, Hartmann-Boyce J. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2019;5(05):CD002850. doi: 10.1002/14651858.CD002850.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y, Dobson R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst Rev. 2019;10(10):CD006611. doi: 10.1002/14651858.CD006611.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez-Stable E J, Juarez-Reyes M, Kaplan C, Fuentes-Afflick E, Gildengorin V, Millstein S. Counseling smoking parents of young children: comparison of pediatricians and family physicians. Arch Pediatr Adolesc Med. 2001;155(01):25–31. doi: 10.1001/archpedi.155.1.25. [DOI] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services . Washington (DC): U.S. Department of Health and Human Services; 2020. Smoking cessation: a report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- 49.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults - United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. doi: 10.15585/mmwr.mm6552a1. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC) . Jamal A, Dube S R, Malarcher A M, Shaw L, Engstrom M C. Tobacco use screening and counseling during physician office visits among adults–National Ambulatory Medical Care Survey and National Health Interview Survey, United States, 2005-2009. MMWR Suppl. 2012;61(02):38–45. [PubMed] [Google Scholar]


