Abstract
Background:
After subarachnoid hemorrhage (SAH), early brain injury (EBI) and delayed cerebral ischemia (DCI) lead to poor outcomes. Discovery of biomarkers indicative of disease severity and predictive of DCI is important. We tested whether leucine-rich alpha-2-glycoprotein 1 (LRG1) is a marker of severity, DCI, and functional outcomes after SAH.
Methods:
We performed untargeted proteomics using mass spectrometry in plasma samples collected at < 48 h of SAH in two independent discovery cohorts (n = 27 and n = 45) and identified LRG1 as a biomarker for DCI. To validate our findings, we used enzyme-linked immunosorbent assay and confirmed this finding in an internal validation cohort of plasma from 72 study participants with SAH (22 DCI and 50 non-DCI). Further, we investigated the relationship between LRG1 and markers of EBI, DCI, and poor functional outcomes (quantified by the modified Rankin Scale). We also measured cerebrospinal fluid (CSF) levels of LRG1 and investigated its relationship to EBI, DCI, and clinical outcomes.
Results:
Untargeted proteomics revealed higher plasma LRG1 levels across EBI severity and DCI in both discovery cohorts. In the validation cohort, the levels of LRG1 were higher in the DCI group compared with the non-DCI group (mean (SD): 95 [44] vs. 72 [38] pg/ml, p < 0.05, Student’s t-test) and in study participants who proceeded to have poor functional outcomes (84 [39.3] vs. 72 [43.2] pg/ml, p < 0.05). Elevated plasma LRG1 levels were also associated with markers of EBI. However, CSF levels of LRG1 were not associated with EBI severity or the occurrence of DCI.
Conclusions:
Plasma LRG1 is a biomarker for EBI, DCI, and functional outcomes after SAH. Further studies to elucidate the role of LRG1 in the pathophysiology of SAH are needed.
Keywords: Subarachnoid hemorrhage, Early brain injury, Cerebral edema, Delayed cerebral ischemia, Leucine-rich alpha-2-glycoprotein 1, Proteomics
Introduction
The lack of clinical biomarkers has hindered clinical management and research in subarachnoid hemorrhage (SAH). Delayed cerebral ischemia (DCI) is an important secondary complication after SAH and is characterized by worsening neurological status and development of new cerebral infarcts, affecting up to 30% of individuals with SAH [1] and contributing to worse outcomes [2]. Early brain injury (EBI) refers to the injury processes that occur within the first 72 h of SAH [2–4]. The lack of widely validated biomarkers in SAH is an unmet need in SAH [5]. Previously, systemic and cerebrospinal fluid (CSF) proteins have both been investigated in the context of predictive markers [6–11] and as mechanistic indicators of disease processes [12, 13]. However, there is a dearth of validated clinical markers in SAH [14, 15]. The National Institutes of Health’s ’Common Data Elements’ recommendations on biomarkers in SAH reviewed 54 clinical SAH studies and listed 33 biomarkers related to various pathophysiological processes, including cell death and recovery, inflammation, and vascular, genetic, and extracellular processes. However, none of the biomarkers have been validated for inclusion as a “core” recommendation by the committee. The primary goal of this study is to identify predictive markers of DCI after SAH [5]. We undertook an unbiased interrogation (via mass spectrometry) of clinical SAH plasma samples in discovery cohorts. Because mass spectrometry simultaneously investigates a large number of proteins, there is a high probability of false positives. To minimize false positives, we performed mass spectrometry in two independent discovery cohorts and found that leucine-rich alpha-2-glycoprotein 1 (LRG1) was associated with disease severity and clinical outcomes in both the discovery cohorts. To confirm our findings, we used enzyme-linked immunosorbent assay (ELISA) to measure both plasma and CSF levels of LRG1 in an SAH validation cohort with matched controls. We further investigated whether the levels of LRG1 were associated with clinical and radiographical EBI severity and functional outcomes. We also tested whether LRG1 improved the prediction of DCI and functional outcomes. Functionally, it has been shown that LRG1 modulates the endothelial transforming growth factor-β (TGFβ) signaling pathway [16]. We measured two markers in the TGFβ pathway (TGFβ1 and TGFβ2) and investigated it with LRG1 and the clinical parameters in SAH.
Methods
Study Population and Patient Criteria
This is a prospective study of patients with SAH admitted to the neuroscience intensive care unit at the Memorial Herman Hospital-Texas Medical Center, Houston, Texas. Inclusion criteria were age > 18 years and a spontaneous aneurysmal SAH diagnosed by computed tomography (CT) within 24 h of ictus. Exclusion criteria were nonaneurysmal SAH due to trauma, arteriovenous malformation, and mycotic aneurysms. Because comorbidities can affect baseline inflammation, we excluded patients with comorbidities such as autoimmune disease and history of malignancy.
Discovery Cohort
We undertook an unbiased investigation of plasma proteins by mass-spectrometry-based proteomic analysis (Supplemental Material) in discovery cohorts. To minimize type 1 errors (false positives), we repeated the mass spectrometry analysis in two independent discovery cohorts (cohort 1 and cohort 2; Supplemental Table 1) and only included proteins that were significantly different in both the cohorts for subsequent analysis. Cohort 1 included study participants admitted from July 2013 to March 2015, and cohort 2 included study participants admitted from December 2017 to December 2019. Within each discovery cohort, we used stratified sampling to maintain a similar proportion of study participants who developed DCI and study participants who did not develop DCI (non-DCI). In addition, to minimize the effect of confounders in the process of biomarker discovery, study participants across the DCI and non-DCI groups in both cohorts were stratified for known confounders, including age, sex, prehospital morbidities (including hypertension, hyperlipidemia, and diabetes) and admission risk factor for DCI (including the Hunt–Hess score). Plasma was obtained within 48 h of admission. The expression levels of the proteins were measures as the exponentially modified protein abundance index (EmPAI) [17].
Validation Cohort
To validate the findings from the mass spectrometry analysis in the discovery cohorts, we used ELISA to measure LRG1 levels in plasma and CSF in a validation SAH cohort. There were 72 consequentially sampled plasma samples from study participants with SAH admitted between October 2016 and July 2018 at our institution who met the inclusion criteria. Plasma samples from seven nonneurological study participants were used as controls (patients who were enrolled at the University of Texas Physician Cardiology clinic). Plasma samples were processed using standard protocols (Supplemental Material). CSF SAH samples (n = 63) were collected from patients who had a ventriculostomy drain or a lumbar puncture (Supplemental Material). CSF control samples (n = 8) were collected from patients who required surgical decompression for trigeminal neuralgia. These samples were collected at the time of surgical decompression and were processed using standard protocols. Age and sex were matched across the control cohort and SAH cohort.
Measurement of LRG1
To measure plasma and CSF levels of LRG1, the LRG1 ELISA kit (catalog No: 27769, IBL Co., Ltd) was used according to the manufacturer’s recommendations. Experiments were conducted blinded to clinical information (including DCI, EBI status, and functional outcomes status). See the Supplemental Material for the protocol used. A separate multiplex panel (Catalog No: TGFBMAG-64 K-03, MilliporeSigma) was used to measure the levels of TGFβ1 and TGFβ2. The cytokine expression levels are measured in pg/mL. See the Supplemental Material for the protocol to measure the TGFs.
Clinical and Radiographic EBI Parameters
EBI describes a wide range of clinical and pathophysiological manifestations that occur within 72 h after SAH. EBI quantification is an ongoing topic of research in SAH. For this study, we used two clinical and two radiographic measures of EBI. Clinically, EBI was quantified using the World Federation of Neurological Surgeons (WFNS) [18] and Hunt–Hess scores [19] (Supplemental Material). Study participants were dichotomized as having low clinical EBI (Hunt–Hess score ≤ 3 and WFNS score ≤ 3) and high clinical EBI (Hunt–Hess score ≥ 4 and WFNS score ≥ 4). Radiographically, EBI was quantified using the dichotomous global cerebral edema (GCE) score (either 0 or 1 denoting absence or presence of edema) [20] (Supplemental Material) and the subarachnoid hemorrhage early brain edema score (SEBES) [3] (Supplemental Material). Both the GCE score and SEBES were graded on the CT scan that is typically obtained from all patients with SAH at the time of admission. An SEBES of ≤ 2 was considered low-grade EBI, and an SEBES ≥ 3 was considered high-grade EBI. Both the GCE score and SEBES were adjudicated by two independent observers, at least one being an attending neurointensivist. Another radiographic score, the modified Fisher score (mFS), an established risk factor for DCI, was also adjudicated on all patients.
DCI and Functional Outcomes
DCI (Supplemental Material) was assessed daily and prospectively adjudicated during weekly research meetings that included a neurocritical care attending physician. Functional outcomes at discharge and at 3 months post discharge were quantified by the 0–6 modified Rankin score (mRS) (Supplemental Material). Good clinical outcomes were defined as an mRS ≤ 3, and poor clinical outcomes were defined as an mRS ≥ 4. The mFS and the intraventricular hemorrhage score [21] were calculated.
Statistical Analysis
Descriptive statistics were calculated for demographic variables and protein levels in DCI and non-DCI study participants. To describe differences in demographics, the χ2 test, Fisher’s exact test, Student’s t-test, and the Mann–Whitney U-test were used as appropriate. The Mann–Whitney U-test was used to test for differences in protein levels across different groups. Proteins that showed a trendy (p < 0.1) toward a difference for outcomes in each cohort were chosen for further analysis. In the validation cohort, a p value of ≤ 0.05 was considered statistically significant (two tailed). The expression levels are reported as mean (standard deviation). The logistic regression method was used, and predictive performance was measured using area under the curve (AUC) analysis. Receiver operating characteristic (ROC) curves were computed, and the AUC of each model was obtained. The ROC curves were compared using the De-Long method. All statistical analyses were performed using open-source software packages in R (v3.1.3) and MedCalc for Windows, version 15.0 (MedCalc Software, Ostend, Belgium).
Standard Protocol Approvals and Registrations and Patient Consents
The study was conducted with the approval of the institutional review board (IRB number HSC-MS-17–0776, HSC-MS-12–0637 and HSC-MH-17–0452). Written informed consent was obtained from the patient or surrogate.
Results
Discovery Cohorts
There were two independent cohorts: cohort 1 had 27 study participants and cohort 2 had 45 study participants. Each cohort had a similar proportion of DCI vs. non-DCI study participants, and the study participants were matched between DCI and non-DCI status. The patient characteristics of the cohorts (including age, comorbidities, and clinical outcomes) are shown in Supplemental Table 1. The demographics across DCI vs. non-DCI in the discovery cohorts are shown in Supplemental Table 2. Mass spectrometry identified 2,228 proteins in cohort 1 and cohort 2 in which the emPAI values were available for at least one study participant. We rejected proteins that were undetectable for more than 30% of the study participants. After rejection, 135 and 122 proteins were retained in cohort 1 and cohort 2, respectively, for further analysis. LRG1 levels were elevated across EBI severity, DCI, and functional outcomes in both discovery cohorts (Fig. 1).
Fig. 1.
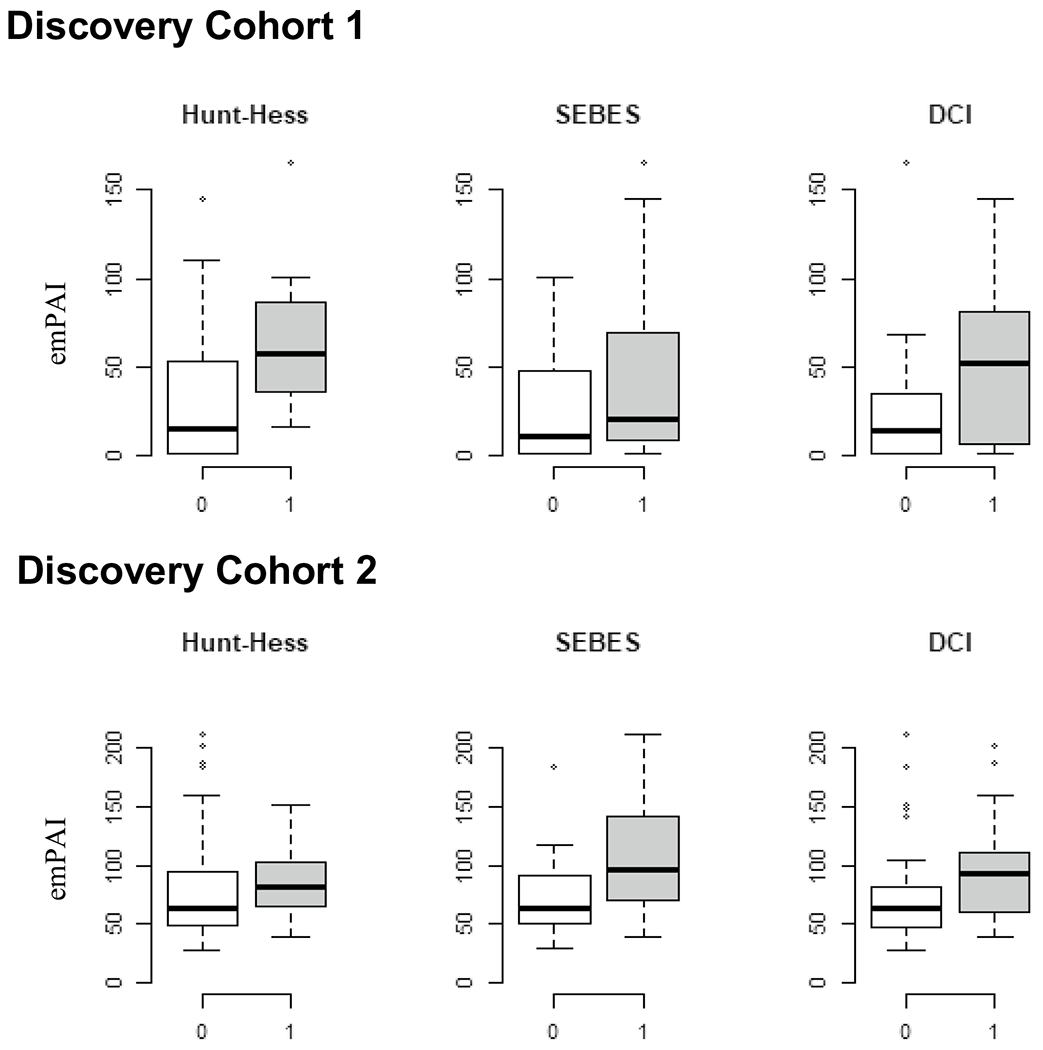
LRG1 levels in the discovery cohorts. Differences in subarachnoid hemorrhage (SAH) plasma leucine-rich alpha-2-glycoprotein 1 (LRG1) levels across early brain injury (EBI) severity and delayed cerebral ischemia (DCI). The levels of plasma LRG1 were significantly higher in study participants with higher grade of clinical severity and subarachnoid hemorrhage early brain edema scores (SEBES) and DCI. emPAI exponentially modified protein abundance index
Validation Cohort Patient Characteristics
During the enrollment period, 99 study participants consented and had a plasma sample at < 48 h. Seventy-two study participants met the inclusion criteria. The median age of study participants with SAH was 53 (interquartile range 45–63), and 74% were female. Seventy-nine percent had hypertension, and 19% had diabetes. There were no significant differences in age, sex, and past medical history across study participants with SAH and controls (Table 1). There were significantly more Hispanic study participants in the SAH cohort compared with controls. All results discussed subsequently pertain to the validation cohort.
Table 1.
Differences in characteristics between SAH and control validation cohorts
| Validation cohorts |
|||
|---|---|---|---|
| SAH (n = 72) | Control (n = 7) | p | |
| Demographics | |||
|
| |||
| Age, median (IQR) | 53 (45–63) | 64 (56–65) | 0.53 |
|
| |||
| Female, n (%) | 54 (74) | 4 (57) | 0.37 |
|
| |||
| Ethnicity, n (%) | |||
| Hispanic | 36 (50) | 0 (0) | 0.014 |
| non-Hispanic | 36 (50) | 7 (100) | |
| Race,n (%) | |||
| Black | 15 (21) | 0 (0) | 0.3 |
| White | 53 (74) | 7 (100) | 0.18 |
| Others | 4 (5) | 0 (0) | 1 |
| Past medical history, n (%) | |||
|
| |||
| Hypertension | 57 (79) | 4 (57) | 0.19 |
|
| |||
| Diabetes mellitus | 14 (19) | 1 (14) | 0.19 |
|
| |||
| Clinical parameters | |||
|
| |||
| Fisher score, median (IQR) | 3 (3–3) | ||
|
| |||
| mFS score 3, n (%) | 50 (69) | ||
|
| |||
| EBI parameters | |||
|
| |||
| WFNS score, median (IQR) | 3 (2–5) | ||
|
| |||
| Hunt–Hess score, median (IQR) | 3 (3–4) | ||
|
| |||
| GCE, n (%) | 17 (23) | ||
|
| |||
| SEBES, median (IQR) | 2 (1–3) | ||
|
| |||
| Clinical end points and outcomes | |||
|
| |||
| DCI, n (%) | 22 (30) | ||
|
| |||
| mRS, median (IQR) | 4 (3–4) | ||
|
| |||
| mRS at 3 months, median (IQR) | 3 (2–4) | ||
DCI Delayed cerebral ischemia, EBI Early brain injury, GCE Global cerebral edema, IQR Interquartile range, mFS Modified Fisher score, mRS Modified rankin score, SAH Subarachnoid hemorrhage, SEBES Subarachnoid hemorrhage early brain edema score, WFNS World federation of neurological surgeons
LRG1 Levels were Higher in Plasma and Not in CSF
Plasma LRG1 levels were significantly higher in participants with SAH compared with controls (mean [SD]: 79 [41] vs. 32 [9], p < 0.001). However, CSF LRG1 levels were not significantly higher in participants with SAH compared with controls (1.4 [1.14] vs. 1.2 [0.94], not significant) (Fig. 2).
Fig. 2.
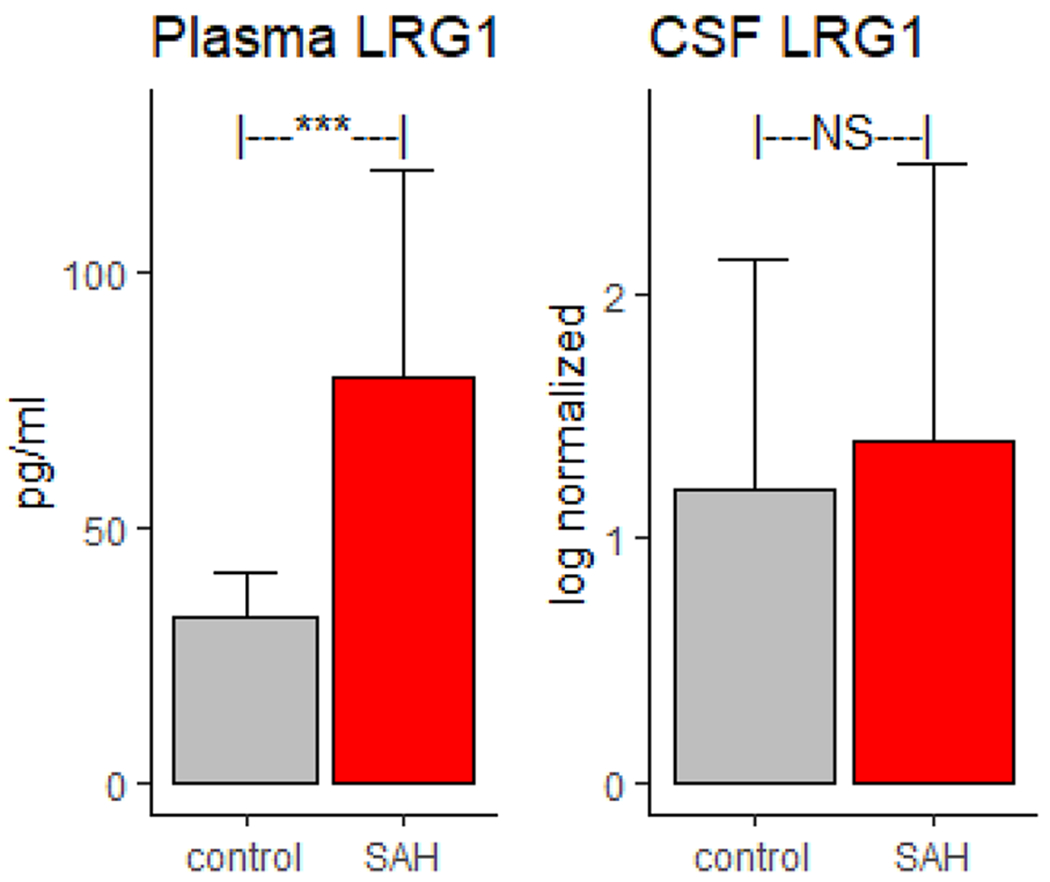
Plasma and cerebrospinal fluid (CSF) levels of leucine-rich alpha-2-glycoprotein 1 (LRG1) in study participants with subarachnoid hemorrhage (SAH) and controls. Plasma LRG1 levels are significantly (p < 0.001) higher in study participants with SAH compared with cardiac controls. CSF levels (log-normalized) of LRG1 levels are not significantly higher compared with those in TGN controls
LRG1 Levels were Higher in High-Grade EBI
Plasma LRG1 levels were higher in both clinical and radiographic measures of EBI (Fig. 3). Across clinical severity, plasma LRG1 levels were significantly higher in study participants with WFNS scores ≥ 4 (n = 36) compared with those with WFNS scores ≤ 3 (n = 36) (91.5 [43] vs. 67 [35], p < 0.01) and in study participants with Hunt–Hess scores ≥ 4 (n = 29) compared with those with Hunt–Hess scores ≤ 3 (n = 43) (92.7 [47.7] vs. 70.1 [33.5], p < 0.001). Across radiographical measures of EBI, plasma LRG1 levels were significantly higher in study participants with SEBES ≥ 3 (n = 24) compared with those with SEBES ≤ 2 (n = 48) (99 [52.7] vs. 70.1 [33.5], p < 0.05) and in study participants with GCE (n = 17) compared with those without GCE (n = 55) (105 [61] vs. 71 [28.6], p < 0.05). In a multivariable logistic regression model, plasma LRG1 was independently associated with clinical severity (after adjusting for age and sex), high-grade SEBES (after adjusting for age and Hunt–Hess score), and GCE (after adjusting for age and Hunt–Hess score).
Fig. 3.
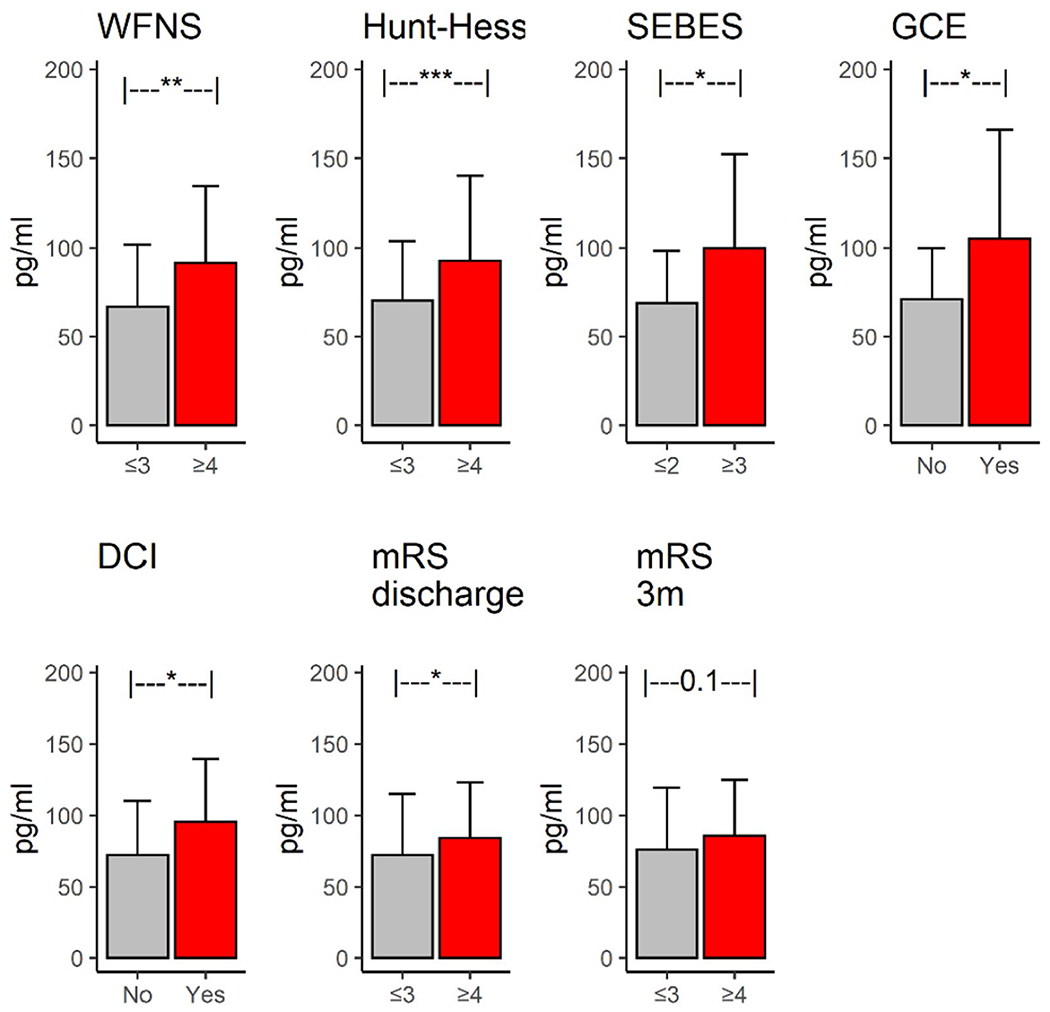
Plasma leucine-rich alpha-2-glycoprotein 1 (LRG1) levels across early brain injury (EBI), delayed cerebral ischemia (DCI), and outcomes. Plasma LRG1 levels were significantly elevated across markers of EBI (Hunt–Hess score, subarachnoid hemorrhage early brain edema score [SEBES], and global cerebral edema [GCE]), DCI, and functional outcomes (at discharge and at 3 months post discharge). mRS modified Rankin score, WFNS World Federation of Neurological Surgeons
LRG1 Levels were Higher in DCI and Poor Functional Outcomes
Plasma LRG1 levels were significantly higher in study participants who proceeded to develop DCI (95 [44] vs. 72 [38], p < 0.05) (Fig. 3: DCI). In a multivariable model, plasma LRG1 was found to be independently associated with DCI after adjusting for the Hunt–Hess score, the mFS, and sex (Table 2). The multivariable model (with Hunt–Hess score, mFS, and sex as independent variables) that included plasma LRG1 significantly improved the prediction of DCI by 25% compared to a model that only included the standard risk factors of Hunt–Hess score and mFS (AUC: 0.73 vs. 0.58, p < 0.01, De-Long test) (Fig. 4: DCI).
Table 2.
LRG1 levels across EBI, DCI, and functional outcomes
| SAH cohort | ||||||
|---|---|---|---|---|---|---|
| DCI (n = 22) | non-DCI (n = 50) | p value | mRS ≤ 3 (n = 29) | mRS ≥ 4 (n = 43) | p value | |
| Demographics | ||||||
| Age, median (IQR) | 42 (45–60) | 52 (46–63) | 0.41 | 48 (45–59) | 57 (45–64) | 0.1 |
| Female, n (%) | 17 (77) | 37 (74) | 1 | 22 | 32 | 1 |
| Ethnicity, n (%) | ||||||
| Hispanic | 12 | 24 | 0.79 | 14 | 22 | 1 |
| non-Hispanic | 10 | 26 | 15 | 11 | ||
| Race, n (%) | ||||||
| Black | 3 (13) | 12 (24) | 0.52 | 7 | 8 | 0.5 |
| White | 19 (86) | 34 (68) | 0.14 | 22 | 31 | 0.79 |
| Others | 0 | 4 (8) | 0.3 | 0 | 4 | 0.14 |
| Past medical history, n (%) | ||||||
| Hypertension | 18 (82) | 39 (80) | 1 | 23 | 34 | 1 |
| Diabetes mellitus | 4 (18) | 10 (20) | 1 | 5 | 9 | 0.76 |
| LRG1, pg/ml, mean (SD) | 95 (44) | 72 (38) | 0.01 a | 72 (43) | 84 (38) | 0.06 b |
| Clinical parameters | ||||||
| Fisher score, median (IQR) | 3 (3–3) | 3 (3–3) | 1 | 3 (3–3) | 3 (3–3) | 1 |
| mFS score 3, n (%) | 16 (72) | 34 (68) | 0.9 | 19 | 31 | 0.73 |
| EBI parameters | ||||||
| WFNS score, median (IQR) | 4 (2–5) | 2 (2–4.75) | 0.3 | 2 (1–2) | 4 (1–5) | < 0.01 |
| Hunt–Hess score, median (IQR) | 3.5 (3–4) | 3 (3–4) | 0.25 | 3 (2–3) | 4 (3–5) | < 0.01 |
| GCE, n (%) | 6 (27) | 11 (22) | 0.85 | 9 | 8 | 0.34 |
| SEBES, median (IQR) | 2 (2–3.75) | 2 (0–3) | 0.06 | 2 (0–4) | 2 (1–3) | 0.9 |
| End points and outcomes | ||||||
| DCI, n (%) | na | na | na | 8 | 14 | 0.85 |
| mRS at discharge, median (IQR) | 4 (3–4) | 4 (3–4) | 0.7 | na | na | na |
| mRS at 3 months, median (IQR) | 4 (3–4) | 3 (2–4) | 0.18 | 2 (1.25–3) | 4 (3–5) | < 0.01 |
DCI Delayed cerebral ischemia, EBI Early brain injury, GCE Global cerebral edema, IQR Interquartile range, LRG1 Leucine-rich alpha-2-glycoprotein 1, mFS Modified Fisher score, mRS Modified Rankin score, na, XXX, SAH Subarachnoid hemorrhage, SEBES Subarachnoid hemorrhage early brain edema score, WFNS World Federation of Neurological Surgeons
Significant after adjusting for Hunt–Hess score, mFS, and sex
Significant after adjusting for age and Hunt–Hess score
Fig. 4.
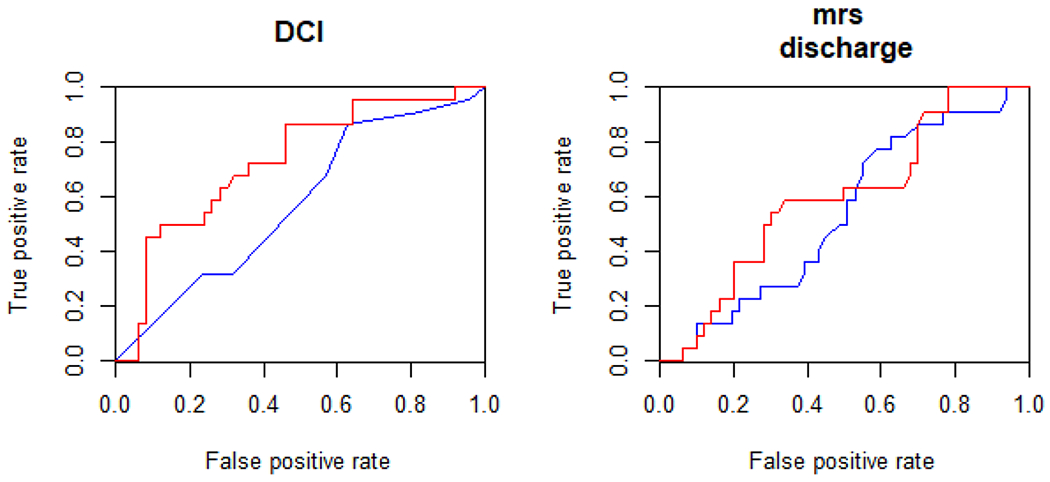
Plasma leucine-rich alpha-2-glycoprotein 1 (LRG1) improves prediction of delayed cerebral ischemia (DCI) and functional outcomes. LRG1 significantly improved the prediction of DCI (left) and functional outcomes at discharge (right). mRS modified Rankin score
Plasma LRG1 levels were near significantly higher in study participants who proceeded to have poor functional outcomes (n = 43) compared with those who proceeded to have good outcomes (n = 29) at hospital discharge (84 [39.3] vs. 72 [43.2], p < 0.05) (Fig. 3: mRS discharge). It was found to be an independent predictor of functional outcome after adjusting for age and admission Hunt–Hess score (Table 2). To test whether plasma LRG1 was independently associated with functional outcomes, we adjusted for age, Hunt–Hess score, and DCI in a multivariate mode. We found that plasma LRG1 was not significantly associated with functional outcomes. Plasma LRG1 significantly improved the prediction of discharge outcomes by 10.6% compared to the standard risk factors of age and Hunt–Hess score (AUC: 0.83 vs. 0.75, p < 0.01, De-Long test) (Fig. 4: mRS discharge). Though plasma LRG1 levels were higher in study participants with poor 3-month functional outcomes compared with those with good outcomes, the effect was not statistically significant when controlled for other factors.
Furthermore, we also tested two models based on ’the subarachnoid haemorrhage international trialists’ multinational study [22]—one baseline model (model 1: with age, WFNS score, preexisting hypertension, and plasma LRG1 as independent variables) and another model (model 2: with age, WFNS score, preexisting hypertension, mFS, location, and plasma LRG1 as independent variables)—and tested whether plasma LRG1 was independently associated with DCI and mRS at discharge (dependent variables). We found that plasma LRG1 was independent associated with DCI in model 1 and model 2. However, although age and WFNS score were independently associated with mRS at discharge, plasma LRG1 was not independently associated with functional outcomes in both the models.
TGFβ1 and TGFβ2
Plasma levels of both TGFβ1 and TGFβ2 were significantly higher in study participants with SAH compared with controls (6,843 [7,424] vs. 2,026 [1,051] pg/mL [p < 0.01] and 425 [422] vs. 117 [78] pg/mL [p < 0.01], respectively). In study participants with SAH, TGFβ1 and TGFβ2 levels were not associated with EBI markers, DCI, or poor functional outcomes. Furthermore, plasma LRG1 levels were not significantly correlated with either TGFβ1 (r = − 0.12, p = 0.3) or TGFβ2 (r = − 0.06, p = 0.6).
Discussion
We undertook an unbiased proteomic approach to investigate plasma proteins predictive of DCI after SAH. We investigated whether plasma levels of LRG1 at < 48 h after SAH are predictive of DCI. We investigated whether LRG1 was associated with EBI and functional outcomes after SAH. Because LRG1 is known to functionally modulate the TGFβ pathway, we investigated the levels of TGFβ1 and TGFβ2. The main findings are as follows: (1) Plasma LRG1 levels were higher in study participants with SAH compared with controls, although CSF LRG1 levels were not significantly higher in study participants with SAH than in controls. (2) Plasma LRG1 levels were associated with markers of EBI, DCI, and functional outcomes after clinical SAH. (3) Plasma LRG1 levels improved the prediction of DCI and functional outcomes. (4) TGFβ markers were elevated after SAH but were not associated with SAH clinical end points or outcomes.
LRG1 is a conserved member of the leucine-rich repeat family of proteins and a secreted glycoprotein and can be upregulated in acute-phase response [23]. It is involved in a variety of biological processes, including cell proliferation, angiogenesis (via modulation of the endothelial TGFβ signaling pathway) [16], apoptosis, mobility, and adhesion [24, 25]. We have identified plasma LRG1 as a biomarker of SAH as plasma LRG1 levels elevated at < 48 h after SAH.
LRG1 is Associated with EBI
LRG1 levels were higher in study participants with high-grade clinical EBI and high-grade radiographic EBI (Fig. 3). LRG1 was independently associated with all parameters of EBI (including the Hunt–Hess score, the SEBES, and GCE). Experimental and clinical studies have investigated the role of LRG1 in other acute neurological diseases. A study in ischemic stroke reported that LRG1 levels were proportional to the infarction volume, stroke severity, and prognosis in stroke patients with supratentorial infarction [16]. An experimental study showed that LRG1 expression was increased in ischemic rat brain immediately after MCAO and persisted for to up to 14 days after stroke [26]. Additionally, LRG1 has been shown to mediate activation of the TGFβ pathway [16]. TGFβ activation promotes basement membrane fibrosis and alters CSF flow dynamics, a pathway that potentially links LRG1 and brain edema [27]. LRG1 is expressed in neutrophil progenitor cells and is upregulated during neutrophilic differentiation [28], and neutrophils have been implicated as the main cellular driver of EBI after SAH [29].
LRG1 Levels are Higher in DCI and Functional Outcomes
DCI is the most preventable contributor of secondary injuries after SAH [30]. Although DCI is multifactorial, cerebrovascular pathology after aneurysm rupture and microvascular dysfunction [31] is implicated in the mechanisms leading to DCI [32]. Although the presence of vasospasm can be identified by angiography or transcranial Doppler, microcirculatory dysfunction cannot be detected easily [33]. Microcirculatory disturbances can cause disruption of cerebral autoregulation, neurovascular coupling, and blood–brain barrier function [34–36]. LRG1 has recently been proposed as a mechanism for vascular dysfunction that can drive disease pathology. It has been implicated in diseases in which there is a loss of vascular stability and in the abnormal formation of blood vessels by interfering with the TGFβ signaling network [16]. The inhibition of LRG1 activity has been shown to reduce neovascularization and reduce vascular leakage [37]. Studies examining the role of LRG1 and the TGFβ signaling pathway in SAH pathophysiology are needed.
LRG1 also improved the prognostication of DCI and functional outcomes (Fig. 4). We measured LRG1 at < 48 h of SAH, and clinical symptoms of DCI occurred 4–21 days after SAH (median of 7 days after SAH). LRG1-related dysfunction is prodromal to DCI symptomology, suggesting that this is a suitable time window for therapies targeting DCI. Also, because LRG1 improves the prediction of DCI and functional outcomes, LRG1 could serve as an important DCI prognostic marker in the management of clinical SAH. As a biomarker for DCI, it will allow for biomarker-stratified clinical trials in which only patients who are at high risk for DCI will be targeted, maximizing the therapeutic potential. Studies have correlated LRG1 with long-term cognitive deficits and neurodegeneration in other neurologic disease processes [38, 39].
LRG1 and TGFβ Pathway
The LRG1 level is known to be elevated in the brain [40] and systemically [41], and ours is the first study to identify it in SAH. Although TGF1β and TGF2β (proteins involved in the TGFβ signaling pathway) levels were higher in study participants with SAH compared with controls, we found no association between the TGFβ proteins and EBI, DCI, and clinical outcomes. We tested whether there was an association between LRG1 and the TBFβ proteins and did not observe a strong association between LRG1 and either TGFβ1 or TGFβ2. However, the lack of an association between plasma levels do not necessarily have implications on their functions or in their contributions in SAH pathophysiology. Future studies are required to understand the mechanisms of LRG1 and the TGFβ pathway in SAH.
Limitations
This study is a single-center observational study and has a relatively small number of enrolled study participants. This can be a possible source of confounders and bias. Second, although we excluded study participants with conditions (including suspicion of infection) at admission, a modest number of study participants developed infections. Infection is an issue that involves patient cohorts admitted in the intensive care unit; however, we believe that it has a negligible effect on inflammatory status in study participants in our cohort. Third, although biomarker discovery studies that involve high throughput screening can interrogate a large number of molecules, there is a risk of identifying proteins that are false positives. To limit the number of false positives, we repeated the mass spectrometry experiment in two different discovery pilot cohorts. Furthermore, in another independent validation cohort, we undertook ELISA to confirm our findings. In future studies, we plan to externally validate the findings of this study in a larger cohort and undertake a thorough investigation to describe performance of the marker, including sensitivity and specificity analyses for the outcome of interest. Fourth, in our discovery phase, because of the small sample sizes, we used stratified random sampling to maintain a similar DCI/non-DCI ratio and minimize the effects of confounders. Though a “consecutive sampling” strategy (which we employed for the validation phase) would have been ideal, we believe that this approach was optimal for the discovery phase. Furthermore, a strength of our design is that the three cohorts (the two discovery phase cohorts and the one validation cohort) are from different time periods (separated by years in between), and the consistent replication of our findings in multiple cohorts over a span of years strengthens the generalizability and reproducibility of our findings. Fifth, we only analyzed LRG1 data with respect to CT radiographic scores at the time of admission and not subsequent time points. In our institution, CT scans at admission are available for all patients with SAH; however, follow-up CT scans are not consistently available for all patients because follow-up CT scans are usually available by physician discretion. Furthermore, because some study participants with severe disease die sooner and study participants with very mild disease are discharged sooner (or CT scans are not ordered for them), the study participants for whom the follow-up CT scans are available are not representative of the SAH population.
Conclusions
We have identified plasma LRG1 as a new biomarker in SAH. Levels of this marker were associated with EBI, DCI, and functional outcomes. CSF levels of LRG1 were not higher in study participants with SAH and were not associated with EBI, DCI, or outcomes. Our findings suggest that LRG1 could be a new systemic marker in SAH.
Supplementary Material
Source of support
Research reported in this manuscript was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award 1R61NS119640-01A1 and by the Clinical and Translational Proteomics Service Center at The University of Texas Health Science Center at Houston.
Footnotes
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1007/s12028-022-01652-7.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The authors confirm adherence to the ethical guidelines and have conducted this study under the approval of the institutional review board.
References
- 1.Origins of the Concept of Vasospasm | Stroke [online]. Accessed at: 10.1161/STROKEAHA.114.006498. Accessed April 9, 2021. [DOI]
- 2.Cahill WJ, Calvert JH, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–53. [DOI] [PubMed] [Google Scholar]
- 3.Ahn S-H, Savarraj JP, Pervez M, et al. The Subarachnoid Hemorrhage Early Brain Edema Score Predicts Delayed Cerebral Ischemia and Clinical Outcomes. Neurosurgery [online serial]. Accessed at: 10.1093/neuros/nyx364/3930954. Accessed May 31, 2018. [DOI] [PubMed]
- 4.Savarraj J, Parsha K, Hergenroeder G, et al. Early brain injury associated with systemic inflammation after subarachnoid hemorrhage. Neurocrit Care. 2018;28:203–11. [DOI] [PubMed] [Google Scholar]
- 5.Chou SH-Y, Macdonald RL, Keller E, et al. Biospecimens and molecular and cellular biomarkers in aneurysmal subarachnoid hemorrhage studies: common data elements and standard reporting recommendations. Neurocrit Care. 2019;30:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci. 2016;17:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMahon CJ, Hopkins S, Vail A, et al. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J Neurointerv Surg. 2013;5:512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr KR, Zuckerman SL, Mocco J. Inflammation, Cerebral Vasospasm, and Evolving Theories of Delayed Cerebral Ischemia. Neurol Res Int [online serial]. 2013;2013. Accessed at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3766617/. Accessed October 29, 2017. [DOI] [PMC free article] [PubMed]
- 9.Savarraj JPJ, Parsha K, Hergenroeder GW, et al. Systematic model of peripheral inflammation after subarachnoid hemorrhage. Neurology. 2017;88:1535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savarraj JP, McGuire MF, Parsha K, et al. Disruption of thrombo-inflammatory response and activation of a distinct cytokine cluster after subarachnoid hemorrhage. Cytokine. 2018;111:334–41. [DOI] [PubMed] [Google Scholar]
- 11.Ahn S-H, Savarraj JPJ, Parsha K, et al. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J Neuroinflammation. 2019;16:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osuka K, Suzuki Y, Tanazawa T, et al. Interleukin-6 and development of vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien). 2022;140:943–51. [DOI] [PubMed] [Google Scholar]
- 13.Provencio JJ. Inflammation in subarachnoid hemorrhage and delayed deterioration associated with vasospasm: a review. Acta Neurochir Suppl. 2013;115:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Przybycien-Szymanska MM, Ashley WW. Biomarker discovery in cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:1453–64. [DOI] [PubMed] [Google Scholar]
- 15.Lad SP, Hegen H, Gupta G, Deisenhammer F, Steinberg GK. Proteomic biomarker discovery in cerebrospinal fluid for cerebral vasospasm following subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2012;21:30–41. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Abraham S, McKenzie JAG, et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature. 2013;499:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides… - PubMed - NCBI [online]. Accessed at: https://www.ncbi.nlm.nih.gov/pubmed/15958392. Accessed February 13, 2020. [DOI] [PubMed]
- 18.Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988;68:985–986. [DOI] [PubMed] [Google Scholar]
- 19.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20. [DOI] [PubMed] [Google Scholar]
- 20.Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–32. [DOI] [PubMed] [Google Scholar]
- 21.Hallevi H, Dar NS, Barreto AD, et al. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Crit Care Med. 2009;37:969–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaja BNR, Saposnik G, Lingsma HF, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. 2018;360: j5745. [DOI] [PubMed] [Google Scholar]
- 23.Shirai R, Hirano F, Ohkura N, Ikeda K, Inoue S. Up-regulation of the expression of leucine-rich alpha(2)-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem Biophys Res Commun. 2009;382:776–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11:725–32. [DOI] [PubMed] [Google Scholar]
- 25.Wong CC-L, Tse AP-W, Huang Y-P et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014;60:1645–58. [DOI] [PubMed] [Google Scholar]
- 26.Meng H, Song Y, Zhu J, et al. LRG1 promotes angiogenesis through upregulating the TGF-β1 pathway in ischemic rat brain. Mole Med Report Spandidos Publicat. 2016;14:5535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howe MD, Furr JW, Munshi Y, et al. Transforming growth factor-β promotes basement membrane fibrosis, alters perivascular cerebrospinal fluid distribution, and worsens neurological recovery in the aged brain after stroke. Geroscience. 2019;41:543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donnell LC, Druhan LJ, Avalos BR. Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol. 2002;72:478–85. [PubMed] [Google Scholar]
- 29.Chou SH-Y, Feske SK, Simmons SL, et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res. 2011;2:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergouwen MDI, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5. [DOI] [PubMed] [Google Scholar]
- 31.Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19:50. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H, Kanamaru H, Kawakita F, Asada R, Fujimoto M, Shiba M. 2020 Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol Histopathol. 2020. Sep 30;36(2):143–58. [DOI] [PubMed] [Google Scholar]
- 33.Budohoski KP, Guilfoyle M, Helmy A, et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiat. 2014;85:1343–53. [DOI] [PubMed] [Google Scholar]
- 34.Beaumont A, Clarke M, Whittle IR. The effects of malignant glioma on the EEG and seizure thresholds: an experimental study. Acta Neurochir (Wien). 1996;138:370–81. [DOI] [PubMed] [Google Scholar]
- 35.Sabri M, Ai J, Lakovic K, Macdonald RL. Mechanisms of microthrombosis and microcirculatory constriction after experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:185–92. [DOI] [PubMed] [Google Scholar]
- 36.Sehba FA, Friedrich V. Cerebral microvasculature is an early target of subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:199–205. [DOI] [PubMed] [Google Scholar]
- 37.Kallenberg D, Tripathi V, Javaid F, et al. A Humanized Antibody against LRG1 that Inhibits Angiogenesis and Reduces Retinal Vascular Leakage. Epub 2020 Jul 25. Accessed at: https://europepmc.org/article/ppr/ppr192310. Accessed April 9, 2021.
- 38.Akiba C, Nakajima M, Miyajima M, et al. Leucine-rich ɑ2-glycoprotein overexpression in the brain contributes to memory impairment. Neurobiol Aging. 2017;60:11–9. [DOI] [PubMed] [Google Scholar]
- 39.Miyajima M, Nakajima M, Motoi Y, et al. Leucine-rich ɑ2-glycoprotein is a novel biomarker of neurodegenerative disease in human cerebrospinal fluid and causes neurodegeneration in mouse cerebral cortex. PLoS ONE. 2013;8: e74453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong PF, Sakai Y, Torisu H, et al. Leucine-rich alpha-2 glycoprotein in the cerebrospinal fluid is a potential inflammatory biomarker for meningitis. J Neurol Sci. 2018;392:51–5. [DOI] [PubMed] [Google Scholar]
- 41.Pek SLT, Cheng AKS, Lin MX, et al. Association of circulating proinflammatory marker, leucine-rich-ɑ2-glycoprotein (LRG1), following metabolic/bariatric surgery. Diabetes Metab Res Rev. 2018;34: e3029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


