Abstract
1-Aryl-substituted bicyclo[1.1.1]pentanes (BCPs) are an important class of BCP derivatives with widespread application in drug development. Most syntheses of these materials require multiple chemical steps via BCP electrophiles or nucleophiles derived from [1.1.1]propellane. Although one-step, multicomponent radical cross-coupling reactions could provide a more sustainable and rapid route to access diverse heteroarylated BCPs, current approaches are limited to tertiary alkyl radicals, leading to a decrease in their practical value. In this study, a conceptually different approach enabled by a radical multicomponent heteroarylation of [1.1.1]propellane to access functionalized heteroarylated BCPs is described. Importantly, this protocol is compatible with primary-, secondary-, and tertiary aliphatic radicals, as well as various fluoroalkyl radical sources, thus enabling rapid library generation of sought-after BCP derivatives for drug development.
Keywords: Photocatalysis, Heteroarylation, Propellanes, Bicyclo[1.1.1]pentanes, Multicomponent
Graphical Abstract
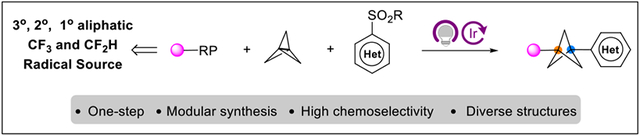
We herein report a conceptually different approach to access diverse arylated bicyclo[1.1.1]pentanes (BCPs). A wide variety of alkyl radicals derived from alkyltrifluoroborates and alkylsilicates were successfully incorporated to form a range of synthetically valuable 1,3-difunctionalized heteroarylated BCPs. Moreover, more electron-deficient •CF2H or •CF3 radicals were also found to be amenable, affording fluoroalkyl-substituted heteroaryl BCPs
Introduction
Biaryl scaffolds are ubiquitous in natural products and drug molecules, exhibiting diverse and interesting biological activities.[1] To date, Pd-catalyzed C(sp2)–C(sp2) coupling reactions using readily available coupling partners to access these biaryls are well-established,[2] meeting the synthetic needs of pharmaceutical chemists. However, investigating sp3-rich aryl isosteres in drug development programs has become an important focus of drug research,[3] which creates new challenges for synthetic chemists. Of particular interest is the bicyclo[1.1.1]pentane (BCP) motif, because of its well-known use as a bioisostere for para-substituted arenes to improve the pharmacokinetic profile of pharmaceutical candidates while providing similar levels of potency.[4] In this context, aryl-substituted BCP derivatives, as bioisosteres of biaryl scaffolds, are found in numerous pharmaceutically-relevant molecules (Figure 1A). Although the construction of arylated BCPs has been investigated by many research groups, most methods rely on the stepwise C─C bond formation via the initial functionalization of [1.1.1]propellane to the corresponding electrophile or nucleophile followed by a second transition metal-catalyzed cross-coupling step (Figure 1B).[5,6]
Figure 1.
A. Examples of (het)arylated BCP cores appearing in bioactive compounds. B. Conventional strategies toward (het)arylated BCP derivatives. C. One-step, multi-component access to (het)arylated BCP derivatives. D. This work: A general and practical route to functionalized heteroarylated BCPs.
In contrast to these multi-step transformations that have been employed to access diverse arylated BCPs with varying levels of synthetic utility, new strategic designs that would enable one-step access to complex BCP derivatives would be of major value to the pharmaceutical industry.[7] In this vein, our group disclosed a study on three-component dicarbofunctionalization of [1.1.1]propellane to form the corresponding arylated BCPs under dual nickel/photoredox catalysis (Figure 1C).[8a] Meanwhile, the Gutierrez group also developed a three-component reaction involving [1.1.1]propellane to form a range of 1,3-difunctionalized arylated BCPs under iron catalysis (Figure 1C).[8b] Despite the advances, both protocols are limited to tertiary radicals because secondary radicals react with the metal catalyst directly, resulting in a known, two-component coupling that omits the bicyclopentane skeletons. Very recently, our group developed transition metal-free methods for the divergent preparation of functionalized BCP alkylamines.[9] Based on this, we wondered whether it may be possible to develop a multicomponent reaction of [1.1.1]propellane to access arylated BCPs without the aid of transition metal-catalyzed coupling, which would overcome current limitations of using only tertiary radicals. Herein, a conceptually different approach to access diverse arylated BCPs is presented that is compatible with tertiary-, secondary-, and primary alkyl radicals (Figure 1D). Moreover, the strategy can be extended to •CF2H and •CF3 radicals to provide a sustainable route to access fluoroalkyl-substituted arylated BCPs.
Results and discussion
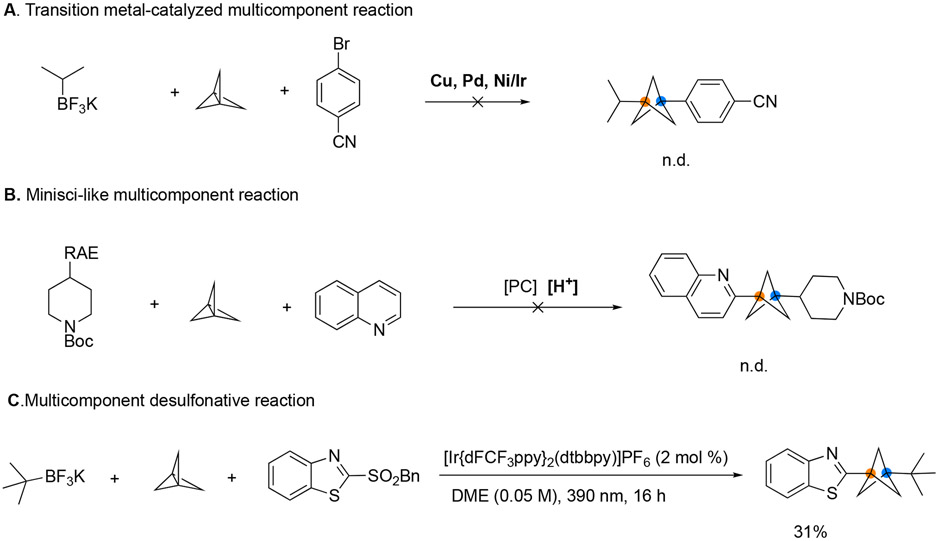
To achieve a general multicomponent reaction involving [1.1.1]propellane that is compatible with various radical sources, investigations were begun by screening different nickel catalysts to alter the chemoselectivity of the developed reaction.[8a] After extensive trials, two-component products were shown to form as the major product, and the chemoselectivity of the reaction was not affected by the nature of the nickel catalyst (Figure 2A). Additionally, multicomponent products were not observed at all when using other catalysts based on palladium or copper complexes (Figure 2A). The participation of transition metal catalysts was postulated to complicate the entire catalytic cycle, making it difficult to control the chemoselectivity of this multicomponent transformation.
Figure 2.
Proposed multicomponent reaction of [1.1.1]propellane to construct arylated BCPs A. Transition metal-catalyzed multicomponent reaction. B. Minisci-like multicomponent reaction. C. Multicomponent desulfonative reaction.
To circumvent the use of transition metal catalysts, we wondered whether it might be possible to develop a Minisci-type multi-component reaction with incorporation of [1.1.1]propellane to construct heteroarylated BCPs, particularly in view of the success of the reported two-component reaction (Figure 2B).[6e-6f] However, the Minisci multicomponent reaction of [1.1.1]propellane did not afford any desired products after many trials. Inspired by our recent work,[9] we envisaged that heteroaryl sulfones could serve as a suitable radical acceptor in multicomponent reactions involving [1.1.1]propellane to access BCP-heteroaryls. Obviously, the challenge of this transformation lies in that photoredox-derived alkyl radicals are prone to react with highly active heteroaryl sulfones directly, resulting in two-component products, and the efficiency of the reaction of the resulting BCP radical with heteroaryl sulfones is unknown.[10] When a heteroaryl sulfone was first studied in this context, the corresponding heteroarylated BCP product was obtained in 31% yield, and more excitingly, the two-component product was not observed at all (Figure 2C).
Gratifyingly, through extensive evaluation of a range of reaction parameters, we found that in the presence of [Ir(dFCF3ppy)2dtbbpy]PF6 and K2CO3, irradiation of the reaction mixture with a 390 nm LED light at 25 °C afforded the desired heteroarylated BCP product in 86% yield (entry 1). Select results of these optimization studies are provided in Table 1. As expected, control studies demonstrated that both photocatalyst and light were essential for this transformation (entries 6, 7). Notably, similar results can be obtained using the methylsulfonyl group as a leaving group (entry 9)
Table 1.
Optimization of Reaction Conditions

| ||
|---|---|---|
| Entry | Deviation from std Conditions | NMR yield (%) |
| 1 | none | 86 |
| 2 | no base | 45 |
| 3 | 0.01 M | 78 |
| 4 | 0.025 M | 84 |
| 5 | Blue LEDs instead of Kessil lamp | 46 |
| 6 | No [Ir] catalyst | 0 |
| 7 | No light | 0 |
| 8 | DME instead of EtOAc | 78 |
| 9 | methylsulfonyl instead of benzylsulfonyl | 82 |
Optimization of reaction conditions: 1 (0.15 mmol), 2 (0.3 mmol), 3 (0.10 mmol) under purple Kessil irradiation (λ max=390 nm) for 16 h at rt; NMR yield was calculated using 1,3,5-trimethoxybenzene as an internal standard (IS) from the crude mixture.
With suitable conditions established, the scope of the multicomponent process was evaluated. The initial focus was on examining alkyl radical sources. A wide variety of tertiary-, secondary-, and even primary alkyl radical precursors were competent despite their dramatically different electrophilicities and steric hindrance (Figure 3). Notably, the two-component product was not observed in any of the examples tested. A wide array of functionalized radical precursors could be used under the mild and redox-neutral conditions, leading to products such as nitrile 5, alkene 6, ketone 8, amide 15, and ester 17. Additionally, alkyltrifluoroborates possessing various ring sizes reacted smoothly to afford coupled products 7, 10, 11, and 14. A reaction was also performed on 3 mmol scale to access 13 with almost unchanged yield, demonstrating the practicality of the developed protocol. In contrast, primary alkyltrifluoroborates were not suitable substrates under standard conditions, presumably because of their high oxidation potentials (1.8 V vs. SCE).[10a] After screening different alkyl radical sources, alkylsilicates with lower oxidation potentials (0.75 V vs. SCE) provided a solution to this problem.[11] Indeed, experiments demonstrated that primary alkylsilicates were viable under standard conditions, affording the corresponding 1,3-dicarbofunctionalized heteroarylated BCPs 21-24. Unfortunately, however, tertiary- and secondary alkylsilicates were ineffective alkyl radical precursors under standard conditions (see Supporting Information).
Figure 3.
Scope of various alkyl radical sources. Reaction conditions: 2-(benzylsulfonyl)benzo[d]thiazole (0.20 mmol, 1.0 equiv), [1.1.1]propellane (0.60 mmol, 3.0 equiv), alkyltrifluoroborates (0.30 mmol, 1.5 equiv), or alkylsilicates (0.30 mmol, 1.5 equiv) [Ir(dFCF3ppy)2dtbbpy]PF6 (2 mol %, 0.004 mmol), K2CO3 (0.4 mmol, 2.0 equiv), EtOAc (0.05 M), irradiating with purple Kessil irradiation (λ max = 390 nm) for 16 h at rt; a Reaction performed on 3 mmol scale of (benzylsulfonyl)benzo[d]thiazole; b Using 2-(methylsulfonyl)benzo[d]thiazole instead of (benzylsulfonyl)benzo[d]thiazole.
We next turned our attention to evaluating the scope of heteroaryl sulfones (Figure 4). N-Heteroarenes rank among the most prominent structural motifs in pharmaceuticals, and over 84% of FDA-approved drugs contain at least one nitrogen, with 60% containing nitrogen heterocycles.[12] Gratifyingly, a range of nitrogen heterocycles were incorporated within the BCP structure in this transformation. For example, a variety of benzothiazole- (25-29), benzoxazole- (31), and benzimidazole- (33) derived scaffolds were installed in good yields. Furthermore, five-membered heteroaromatic rings that incorporate three heteroatoms were utilized, as highlighted by the coupling of 1,3,4-oxadiazole 30. Importantly, the success of the reaction with bromo-substituted benzothiazole 27, which was not compatible in conventional transition metal-catalyzed cross-coupling reactions, provided an important complement to current methods. Encouraged by this result, we moved on to explore the scope of six-membered heterocycles. Pyrimidine substrates with different substitution patterns were found to be viable (34-38). Notably, pyridine was also incorporated into this protocol when using the more electrophilic trifluoromethanesulfonyl unit as a leaving group (39). In addition to the above heterocycles, an alkenylsulfone turned out to be a viable substrate, yielding product 40 with moderate yield.
Figure 4.
Scope of various heteroaryl sulfones. Reaction conditions: heteroaryl sulfone (0.20 mmol, 1.0 equiv), [1.1.1]propellane(0.60 mmol, 3.0 equiv), alkyltrifluoroborates (0.30 mmol, 1.5 equiv), [Ir(dFCF3ppy)2dtbbpy]PF6 (2 mol %, 0.004 mmol), K2CO3 (0.4 mmol, 2.0 equiv), EtOAc (0.05 M), irradiating with purple Kessil irradiation (λ max = 390 nm) for 16 h at rt; a R1 = Bn; b R1 = Ph; c R1 = Me; d R1 = CF3; e using DME instead of EtOAc.
Considering the significance of fluoroalkyl-containing molecules within the pharmaceutical industry and the emergence of fluoroalkyl-substituted BCP derivatives in recent medicinal chemistry patents,[13][14] the development of a method to synthesize fluoroalkyl-substituted, heteroarylated BCPs incorporating these components efficiently is of great importance.[15] The readily available and easy-to-handle fluoroalkylsulfinate salts have proven to be very effective fluoroalkyl radical precursors in this regard.[9] Encouraged by the previous results, the compatibility of fluoroalkyl radical sources was next explored (Figure 5). Pleasingly, a range of five- and six-membered heteroaryl sulfones were competent partners in this reaction, affording difluoromethyl-substituted, heteroarylated BCPs (41-48). Moreover, a difluoroethyl radical source was also incorporated in this multicomponent transformation to generate 49, along with a trifluoromethyl radical precursor, the latter affording trifluoromethyl-substituted BCPs (50-52) in acceptable yields. Although the reaction efficiency is not ideal in these transformation, in the initial stage of drug discovery, where diverse analogues need to be obtained rapidly for screening, this method may be broadly applicable.
Figure 5.
Scope of various fluoroalkylsulfinate salts. Reaction conditions: heteroaryl sulfone (0.20 mmol, 1.0 equiv), [1.1.1]propellane (0.60 mmol, 3.0 equiv), fluoroalkylsulfinate salts (0.30 mmol, 1.5 equiv), [Ir(dFCF3ppy)2dtbbpy]PF6 (2 mol %, 0.004 mmol), K2CO3 (0.4 mmol, 2.0 equiv), EtOAc (0.05 M), irradiating with purple Kessil irradiation (λ max = 390 nm) for 36 h at rt; a R1 = Bn; b R1 = Me.
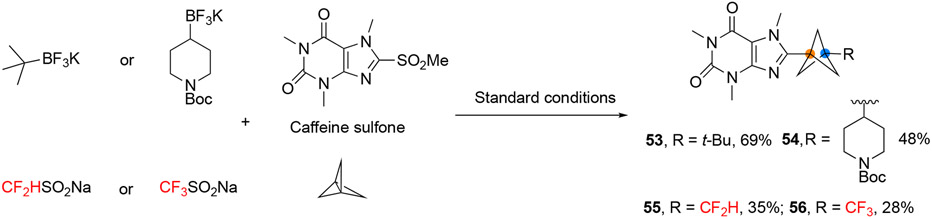
To illustrate the advantages of the developed method over traditional two-component cross-coupling approaches in rapid library generation of (het)arylated BCP derivatives, we endeavored to perform a unified, divergent synthesis of BCP analogues. Employing the developed methods, various functionalized BCP structures were rapidly introduced into caffeine by changing the radical source, which is challenging for established methods (Figure 6).
Figure 6.
Modular modification of caffeine
Interestingly, heteroarylated BCP 57 was often observed as a byproduct when the range of primary alkyltrifluoroborates was evaluated, indicating that the heteroaryl sulfones were themselves substrates for difunctionalization of [1.1.1]propellane. Thus, in the absence of alkyltrifluoroborate, the reaction of 2-(benzylsulfonyl)benzo[d]thiazole with [1.1.1]propellane proceeds smoothly to afford the heteroarylated BCP product 57, providing a unique approach to access BCP derivatives (Figure 7A).
Figure 7.
A. Dicarbofunctionalization of [1.1.1]propellane; B. Radical ring-opening reaction; C. Radical-trapping experiments.
Regarding the reaction mechanism, a series of control experiments was conducted. The reaction of the alkyltrifluoroborate generated from verbenone under the standard conditions afforded ring-opened product 58 (Figure 7B). TEMPO trapping experiments showed that the reaction was completely suppressed, and only TEMPO adduct 59 derived from the radical precursors were observed (Figure 7C). Notably, when α-(trifluoromethyl)-styrene was added to the standard reaction as radical scavenger, multicomponent product 60 was isolated as the major product (Figure 7C).
Based on previous reports and the mechanistic findings described herein,[10] a possible catalytic cycle is depicted (Figure 8). Radical V is presumably generated from favorable single-electron oxidation of alkyltrifluoroborate or fluoroalkylsulfinate reagents with excited photocatalyst II. Subsequently, radical V undergoes radical addition to [1.1.1]propellane, leading to BCP radical VI. At this juncture, two scenarios can be suggested that lead to the observed products. In the first, addition of this neutral radical species VI to a highly electrophilic heteroarene via radical addition provides the radical complex VII, which may readily undergo single-electron reduction to the anion, followed by loss of an anionic leaving group (SO2R−) to provide the final BCP product. Alternatively, radical species VI may add to the heteroaryl sulfone with eventual homolytic loss of sulfonyl radical. The reduced photocatalyst then reduces the sulfonyl radical to the anion. Either of these reduction steps would simultaneously complete the photoredox cycle. Stern-Volmer plots and quantum yield measurements support this mechanism (see Supporting Information).
Figure 8.
Proposed mechanism
In summary, a general and straightforward method to prepare functionalized, heteroarylated BCP derivatives has been established. A wide variety of alkyl radicals derived from alkyltrifluoroborates and alkylsilicates were successfully incorporated to form a range of synthetically valuable 1,3-difunctionalized heteroarylated BCPs. Moreover, more electron-deficient •CF2H or •CF3 radicals were also found to be amenable, affording fluoroalkyl-substituted heteroaryl BCPs. Overall, this modular method, which enables a rapid buildup of BCP-containing molecular complexity from simple building blocks, is expected to stimulate further research endeavors for applications in medicinal chemistry.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful for the financial support from NIH General Medical Sciences (R35 GM 131680 to G.M.) and the National Science Foundation (CHE-1952583 to G.M.). An SIOC fellowship to W.H. from the Shanghai Institute of Organic Chemistry (CAS) is also gratefully acknowledged. Financial support for this research was also provided in part by AbbVie. The NSF Major Research Instrumentation Program (award NSF CHE-1827457), the NIH supplements awards 3R01GM118510-03S1 and 3R01GM087605-06S1, as well as the Vagelos Institute for Energy Science and Technology, supported the purchase of the NMRs used in this study. The authors thank Dr. Charles W. Ross, III (University of Pennsylvania) for obtaining HRMS data. We acknowledge Kessil Lighting for lights used in this study.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- [1].a) Jana A, Ravichandiran V, Swain SP, New J. Chem, 2021, 45, 17753–17771. [Google Scholar]; b) Belama N, Meanwell NA, J. Med. Chem, 2014, 57, 5057–5071. [DOI] [PubMed] [Google Scholar]
- [2].a) Brown GD, Boström JJ, J. Med. Chem 2016, 59, 4443–4458. [DOI] [PubMed] [Google Scholar]; b) Han FS, Chem. Soc. Rev, 2013, 42, 5270–5298. [DOI] [PubMed] [Google Scholar]
- [3].a) Bauer MR, Di Fruscia P, Lucas SCC, Michaelides IN, Nelson JE, Storer RI, Whitehurst BC, RSC Med. Chem 2021,12, 448–471. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lovering F, Bikker J, Humblet C, J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; c) Mykhailiuk PK, Org. Biomol. Chem 2019, 17, 2839–2849. [DOI] [PubMed] [Google Scholar]; d) Lovering F, MedChemComm. 2013, 4, 515–519. [Google Scholar]
- [4].a) Stepan AF, Subrama-nyam C, Efremov IV, Dutra JK, O’Sullivan TJ, DiRico KJ, McDonald WS, Won A, Dorff PH, Nolan CE, Becker SL, Pustilnik LR, Riddell DR, Kauffman GW, Kormos BL, Zhang L, Lu Y, Capetta SH, Green ME, Karki K, Sibley E, Atchison KP, Hallgren AJ, Oborski CE, Robshaw AE, Sneed B, O’Donnell CJ, J. Med. Chem 2012, 55, 3414–3424. [DOI] [PubMed] [Google Scholar]; b) Westphal MV, Wolfstadter BT, Plancher JM, Gatfield J, Carreira EM, ChemMedChem 2015, 10, 461–469. [DOI] [PubMed] [Google Scholar]; c) Measom ND, Down KD, Hirst DJ, Jamieson C, Manas ES, Patel VK, Somers DO, ACS Med. Chem. Lett 2017, 8, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Methods reported to forge arylated BCP products via cross-coupling from BCP nucleophiles: Messner M, Kozhushkov SI, de Meijere A, Eur. J. Org. Chem, 2000, 1137–1155. [Google Scholar]; b) Rehm JDD, Ziemer B, Szeimies G, Eur. J. Org. Chem, 2001, 1049–1052. [Google Scholar]; c) Makarov IS, Brocklehurst CE, Karaghiosoff K, Koch G, Knochel P, Angew. Chem., Int. Ed, 2017, 56, 12774–12777. [DOI] [PubMed] [Google Scholar]; d) Schwarzer K, Zipse H, Karaghiosoff K, Knochel P, Angew. Chem. Int. Ed, 2020, 59, 20235–20241. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Kondo M, Kanazawa J, Ichikawa T, Shimokawa T, Nagashima Y, Miyamoto K, Uchiyama M, Angew. Chem. Int. Ed, 2020, 59, 1970–1974. [DOI] [PubMed] [Google Scholar]; f) Shelp RA, Ciro A, Pu Y, Merchant RR, Hughes JME, Walsh PJ, Chem. Sci 2021, 12, 7066–7072. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Vanheyst MD, Qi J, Roecker AJ, Hughes JME, Cheng L, Zhao Z, Yin J, Org. Lett, 2020, 22, 1648–1654. [DOI] [PubMed] [Google Scholar]
- [6].a) Methods reported to forge arylated BCP products from BCP electrophiles: Toriyama F, Cornella J, Wimmer L, Chen TG, Dixon DD, Creech G, Baran PS, J. Am. Chem. Soc 2016, 138, 11132–11135. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Salgueiro DC, Chi BK, Guzei IA, García-Reynaga P, Weix DJ, Angew. Chem. Int. Ed 2022, 61, e2022056. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Polites VC, Badir SO, Keess S, Jolit A, Molander GA, Org. Lett 2021, 23, 4828–483. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nugent J, Shire B, Caputo D, Pickford H, Nightingale F, Houlsby I, Mousseau J, Anderson EA, Angew. Chem. Int. Ed 2020, 59, 11866–11870. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Mousseau JJ, Perry MA, Bundesmann MW, Chinigo GM, Choi C, Gallego G, Hicklin RW, Hoy S, Limburg DC, Sach NW, Zhang Y ACS Catal. 2022, 12, 600–606. [Google Scholar]; f) Sharique M, Majhi J, Dhungana RK, Kammer LM, Krumb M, Lipp A, Romero E, Molander GA Chem. Sci 2022, 13, 5701–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Kanazawa J, Maeda K, Uchiyama M, J. Am. Chem. Soc 2017, 139, 17791–17794. [DOI] [PubMed] [Google Scholar]; b) Zhang X, Smith RT, Le C, McCarver SJ, Shireman BT, Carruthers NI, MacMillan DWC, Nature 2020, 580, 220–226; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Dong W, Yen-Pon E, Li L, Bhattacharjee A, Jolit A, Molander GA, Nat. Chem 2022, 14, 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Huang W, Keess S, Molander GA, Chem. Sci 2022, 13, 11936–11942. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Kim H, Ruffoni A, Al-Faiyz YSS, Sheikh NS, Leonori D, Angew. Chem. Int. Ed, 2020, 59, 8225–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Huang W, Keess S, Molander GA, J. Am. Chem. Soc 2022, 144, 12961–12969. [DOI] [PubMed] [Google Scholar]; b) Rentería-Gómez A, Lee W, Yin S, Davis M, Gogoi AR, Gutierrez O, ACS Catal. 2022, 12, 11547–11556. [Google Scholar]
- [9].Huang W, Zheng Y, Keess S, Molander GA, J. Am. Chem. Soc 2023, 145, 5363–5369. [DOI] [PubMed] [Google Scholar]
- [10].a) Wang ZJ, Zheng S, Matsui JK, Lu Z, Molander GA, Chem. Sci 2019, 10, 4389–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Prier CK, MacMillan DWC, Chem. Sci 2014, 5, 4173–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kamijo S, Kamijo K, Murafuji T, J. Org. Chem 2017, 82, 2664–2671. [DOI] [PubMed] [Google Scholar]
- [11].a) Jouffroy M, Primer DN, Molander GA, J. Am. Chem. Soc 2016, 138, 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Corcé V, Chamoreau L-M, Derat E, Goddard JP, Ollivier C, Fensterbank L, Angew. Chem. Int. Ed 2015, 54, 11414–11418. [DOI] [PubMed] [Google Scholar]; c) Zheng S, Primer DN, Molander GA, ACS Catal. 2017, 7, 7957–7961. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Phelan JP, Lang SB, Sim J, Berritt S, Peat AJ, Billings K, Fan L, Molander GA, J. Am. Chem. Soc 2019, 141, 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Welsch ME, Snyder SA, Stockwell BR, Curr. Opin. Chem. Biol, 2010, 14, 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Vitaku E, Smith DT, Njardarson JT, J. Med. Chem, 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- [13].a) Purser S, Moore PR, Swallow S, Gouverneur V, Chem. Soc. Rev 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]; b) Hagmann WK, J. Med. Chem 2008, 51, 4359–4369. [DOI] [PubMed] [Google Scholar]; c) Müller K, Faeh C, Diederich F, Science, 2007, 317, 1881–1886. [DOI] [PubMed] [Google Scholar]
- [14].a) Hopkins CP, Pinchman JR, Bunker KD, Slee DH, Boren BC, Preparation of substituted bicyclo[1.1.1]pentyl compounds as analgesics (Zeno Royalties & Milestones LLC; ) WO2018213140 A1 2018-11–22.; b) Pinchman JR, Huang PQ, Bunker KD, Sit RK, Samatar AA, Preparation of benzamide compounds as antitumor and anti-HIV agents (Zeno Royalties & Milestones LLC; ) WO2019139907 A1 2019-07–18.; c) Pinchman JR, Bunker KD, Huang PQ, Bcl-2 protein inhibitors and application as antitumor agents (Recurium Ip holdings LLC; ) WO2021222114 A1 2021-11–04.
- [15].a) Shin S, Lee S, Choi W, Kim N, Hong S, Angew. Chem. Int. Ed, 2021, 60, 7873–7879. [DOI] [PubMed] [Google Scholar]; b) Zhu J, Guo Y, Zhang Y, Li W, Zhang P, Xu J, Green Chem., 2023, DOI: 10.1039/D2GC04521D. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.