Abstract
Enduring patterns of epigenomic and transcriptional plasticity within the mesolimbic dopamine system contribute importantly to persistent behavioral adaptations that characterize substance use disorders (SUD). While drug addiction has long been thought of as a disorder of dopamine (DA) neurotransmission, therapeutic interventions targeting receptor mediated DA-signaling have not yet resulted in efficacious treatments. Our laboratory recently identified a non-canonical, neurotransmission-independent signaling moiety for DA in brain, termed dopaminylation, whereby DA itself acts as a donor source for the establishment of post-translational modifications (PTM) on substrate proteins (e.g., histone H3 at glutamine 5; H3Q5dop). In our previous studies, we demonstrated that H3Q5dop plays a critical role in the regulation of neuronal transcription and, when perturbed within monoaminergic neurons of the ventral tegmental area (VTA), critically contribute to pathological states, including relapse vulnerability to both psychostimulants (e.g., cocaine) and opiates (e.g., heroin). Importantly, H3Q5dop is also observed throughout the mesolimbic DA reward pathway (e.g., in nucleus accumbens/NAc and medial prefrontal cortex/mPFC, which receive DA input from VTA). As such, we investigated whether H3Q5dop may similarly be altered in its expression in response to drugs of abuse in these non-dopamine-producing regions. In rats undergoing extended abstinence from cocaine self-administration (SA), we observed both acute and prolonged accumulation of H3Q5dop in NAc, but not mPFC. Attenuation of H3Q5dop in NAc during drug abstinence reduced cocaine-seeking and affected cocaine-induced gene expression programs associated with altered dopamine signaling and neuronal function. These findings thus establish H3Q5dop in NAc, but not mPFC, as an important mediator of cocaine-induced behavioral and transcriptional plasticity during extended cocaine abstinence.
Introduction
Substance use disorder (SUD) represents a chronic, relapsing disease characterized by compulsive drug-taking and -seeking behaviors despite negative consequences (DSM-V, APA 2022). Cocaine is one of the most commonly abused recreational drugs, with ~20 million users worldwide, and an approximate doubling of cocaine-related deaths since 2016 (2016–2019) (UNDOC, World Drug Report 2021), highlighting an urgent need to develop more effective treatment strategies. Despite considerable efforts from the field to identify the precise molecular underpinnings of cocaine use disorder (CUD), no pharmacotherapeutics are currently approved to treat this pervasive affliction.
While drug addiction has long been thought of as a disorder of dopamine (DA) neurotransmission, therapeutic interventions targeting receptor mediated DA-signaling have not yet resulted in efficacious therapeutics. Our laboratory recently discovered a novel signaling moiety for DA, whereby DA not only acts as a neurotransmitter, but also as a chemical donor for a novel post-translational modification (PTM) on select substrate proteins (e.g., histone H3 at glutamine 5; H3Q5dop) (Lepack et al., 2020). This finding followed work from our group and others, which identified that serotonin, another monoamine neurotransmitter, can also be covalently attached to H3Q5, a mark that functions to stabilize permissive transcription in neural cells (Farrelly et al., 2019; Lukasak et al., 2022; Zhao et al., 2021a; Zhao et al., 2021b). Specifically, with respect to H3 dopaminylation, we found that H3Q5dop within DAergic neurons of the ventral tegmental area (VTA) contributes to relapse vulnerability during extended abstinence from both psychostimulants (e.g., cocaine) (Lepack et al., 2020) and opiates (e.g., heroin) (Fulton et al., 2022). However, the extent to which H3Q5dop mediates drug-induced responses within non-DAergic reward-related brain structures has remained unclear.
The nucleus accumbens (NAc), located within the ventral striatum, is a key brain reward region that receives dense DAergic innervation from VTA and plays critical roles in the relapsing nature of CUD (Lee and Dong, 2011; Teague and Nestler, 2022). Numerous studies have demonstrated functional alterations within NAc following cocaine use, including perturbations to dopamine and glutamate signaling dynamics and related downstream receptor-mediated molecular cascades, as potential causative factors in the precipitation of cocaine relapse vulnerability (Loweth et al., 2014; Parsons et al., 1991). Despite this large body of work implicating aberrations in DAergic signaling in the pathophysiology of CUD, dopamine receptor agonism/antagonism strategies for the treatment of this disorder have not yet led to efficacious therapeutics (Kampman, 2019). Furthermore, such studies have provided little clarity regarding the underlying molecular mechanisms responsible for the persistence of drug-induced phenotypes. In the past decade, however, emerging evidence has implicated chromatin regulatory processes as direct mediators of persistent drug-induced maladaptive transcriptional plasticity in NAc that may help to explain the prolonged nature of drug abuse related phenotypes (Stewart et al., 2020; Teague and Nestler, 2022; Werner et al., 2019). In particular, numerous histone modifications have been shown to be either dysregulated in response to cocaine, or have been causally linked to various aspects of drug-taking and -seeking behaviors (Maze et al., 2010; Stewart et al., 2020).
In addition to NAc, the medial prefrontal cortex (mPFC) also receives robust innervation from VTA, with alterations in these inputs in response to cocaine also being causally linked to relapse-related behaviors. For example, repeated cocaine exposures have been shown to decrease the basal activity of mPFC while also increasing membrane excitability within this brain region (Dong et al., 2005; Sun and Rebec, 2006; Suska et al., 2013). However, much less is known regarding the specific contributions of aberrant transcriptional plasticity in mPFC to cocaine-induced phenotypes, particularly with respect to relapse vulnerability during periods of withdrawal. Notable studies, for example, have shown that alterations in CREB (cAMP response element-binding protein) phosphorylation in mPFC impact the expression of BDNF (brain-derived neurotrophic factor) and cocaine reward, and contribute to alterations in MeCP2 and HDAC expression. Such phenomena, in turn, contribute to cocaine-taking and appear to enhance motivation to seek the drug (Deschatrettes et al., 2013; Sadri-Vakili et al., 2010). What little work has been done with respect to investigating roles for mPFC in the precipitation of relapse vulnerability has, however, implicated dynamic changes in the expression of histone modifying enzymes and/or chromatin regulatory events in the pathophysiology of CUD (Rogge and Wood, 2013).
Interestingly, despite neither of these reward-related projection regions containing DA synthesizing neurons, we found that H3Q5dop is indeed expressed in both NAc and mPFC (results that mimicked what was found earlier with H3 serotonylation, a mark that displays broad patterns of expression throughout the brain) (Farrelly et al., 2019). As such, in this study we sought to examine whether H3Q5dop within these projection regions may similarly display alterations in the mark following volitional cocaine self-administration (SA), and if so, whether such phenomena may contribute functionally to the development of relapse vulnerability-like behavior during prolonged drug withdrawal. We demonstrated that H3Q5dop in NAc, but not mPFC, plays important roles in cocaine-induced transcriptional plasticity. In rats undergoing abstinence from cocaine SA, we observed acute and prolonged accumulation of H3Q5dop in NAc, but not mPFC. Attenuation of H3Q5dop in NAc during drug abstinence reduced cocaine-seeking and perturbed cocaine-induced gene expression programs. Our findings thus establish H3Q5dop in NAc, but not mPFC, as an important mediator of cocaine-induced behavioral and transcriptional plasticity.
Materials and Methods
Animals
All procedures were done in accordance with NIH guidelines and the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai. Male Sprague-Dawley rats (Charles River Laboratories; 300–325 g) were pair housed and maintained on a 12 h reverse light/dark cycle with ad libitum food and water.
Jugular Catheterization
Surgical jugular catheter implantation was performed as previously described (Lepack et al., 2020). Animals were anesthetized via 3% isoflurane in oxygen for the entire surgical procedure. Small incisions were made in clean-shaven areas on the back and chest above the jugular vein. Catheters were passed subcutaneously from the back to the chest, and a small incision was made in the jugular vein where the catheter was inserted and subsequently secured to the jugular vein. For 3 days following surgery, back and chest incisions were closed via suture and treated with topical antibiotics and an anti-inflammatory analgesic (meloxicam; 1mg/kg, s.c.). Animals were flushed daily with hepain + Baytril (1mg/ml; 0.1 mg/ml) to maintain catheter patency and to reduce bacterial infections.
Cocaine Self-Administration (SA)
Animals were food restricted (20 g per day; 3–4 days) and trained to respond to the ‘active’ lever on a fixed ratio (FR) schedule 1 of reinforcement for food pellets (1 hr sessions per day) prior to jugular catheterization. Following meeting threshold requirements (~100 pellets per session), animals were implanted with jugular catheters and given 5 days to recover before cocaine SA. The day prior to cocaine SA, catheter patency was tested with 1 mg methohexital sodium (Brevital; Sigma-Aldrich, St. Louis, MO). Rats then trained to respond for an IV infusion of cocaine hydrochloride diluted in 0.09% sterile saline on the ‘active’ lever at 1mg/kg/infusion (0.1 ml injection volume delivered over 4 s), with a 20 s timeout in between each infusion, indicated by a cue light located directly above the ‘active’ lever. Responding on the lever during this period was recorded without consequence. Rodents were trained on this paradigm at a FR 1 (2 days), FR3 (1 day) and then FR5 (2 days; 1 hr sessions). After meeting the threshold at FR5 (~15 rewards total), rats were given extended access (6 hr sessions, 0.5mg/kg/infusion) to cocaine for 10 days. A separate group of rats were put through the same paradigm but were only given saline infusions (0.9%) (nonreinforcement controls). For molecular analyses, cocaine vs. saline animals were euthanized immediately (abstinence day/AD0), 24 hr (AD1) or 30 days (AD30) after the last infusion, and NAc and mPFC tissues punches were immediately dissected and flash frozen.
For yoked experiments, animals were catheterized and received the same amount and frequency of infusions (saline or 1 mg/kg/infusion for 5 days, 0.5mg/kg/infusion for following 10 days). For food SA, animals were trained similarly to that of cocaine rats to respond to an ‘active’ lever at a FR1-FR5 for food pellets before being switched to an extended access paradigm. Rats in the food SA group were kept at ~80% body weight throughout the experiment. For all molecular analyses using yoked/food controls, rats were euthanized 30 days post the last infusion/food reward.
Lentiviral Constructs
Lentiviral constructs were generated as previously described (Lepack et al., 2020). H3.3 constructs [wildtype (WT) vs. (Q5A)-Flag-HA] were cloned into a pCDH-RFP vector via PCR and restriction digestion. Plasmids were validated by sanger sequencing at GENEWIZ. pCDH-RFP-H3.3WT and pCDH-RFP-H3.3Q5A plasmids were then shipped to Cyagen Biosciences for lentiviral packaging at high titer.
Viral Infusion
The day following the last session of cocaine SA (day 11), animals were anaesthetized via ketamine/xylazine (80/6 mg/kg) i.p., positioned in a stereotaxic frame (Kopf instruments) and 1μl of viral construct was infused bilaterally into NAc or mPFC using the following coordinates; NAc core: anterior-posterior (AP) +1.8, medial-lateral (ML) +/−2.1, dorsal-ventral (DV) −7.5; mPFC AP +3, ML +/−0.5, DV −4.5. Rats received meloxicam (1 mg/kg) s.c. and topical antibiotic treatments for 3 days following surgery. Behavioral testing commenced 29 days after surgery to allow for maximal expression of the viral constructs and a prolonged forced abstinence period.
Cocaine-seeking
Following injection intra-NAc or mPFC with lenti-H3.3WT or lenti-H3.3Q5A, rats were left undisturbed in home cages for 29 days before testing at AD30. Following the forced abstinence period, rats were reintroduced to the operant chamber and allowed to respond to the ‘active’ vs. ‘inactive’ lever for 1 hr. receiving only the cue-light associated with the reward, but no reward administration.
Western Blotting and Antibodies
NAc and mPFC tissues were collected from rats (2 mm punches) and immediately frozen. Punches were homogenized in Buffer A using a dounce homogenizer, containing 10mM HEPES (pH 7.9), 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, and 1mM EDTA, and 1X protease inhibitor cocktail. Following dounce homogenization, Triton-100X was added to a final concentration of 0.1%, and the samples were incubated on ice for 30 min before being centrifuged for 5 min at 1300g at 4° C. The supernatant (cytosolic fraction) was discarded. pellets (nuclear fraction) were resuspended with Buffer A to wash, and then spun in the centrifuge for 5 min at 1300g at 4° C. The supernatant was discarded and pellets were sonicated in sample buffer (0.3M sucrose, 1% SDS, 5mM HEPES and 1X protease inhibitor cocktail).
Protein concentrations were first determined via the DC protein assay kit (BioRad). Nuclear lysates (15 – 20 μg) were loaded onto 4–12% NuPage BisTris polyacrylamide gels (Invitrogen) for electrophoresis and then proteins were transferred to nitrocellulose membranes. Nitrocellulose membranes were blocked in 5% milk in PBS + 0.1% Tween 20 (PBST) for 1 hr followed by incubation with primary antibodies for 24 hr, or in the case of the anti-H3Q5dop antibody 48 hrs, at 4°C. The following antibodies were used: rabbit anti-H3Q5dop (1:200, Millipore ABE2588) – extensively validated in our previous publication (Lepack et al., 2020) –, and rabbit anti-H3 (1:50,000, Abcam ab1791). The next day, membranes were washed in 1X PBST 3 times for 10 min and incubated for 1 hr with horseradish peroxidase conjugated anti-rabbit (BioRad 170–6515) secondary antibodies (1:10,000; 1:50,000 for anti-H3 antibody, BioRad) in 5% milk/PBST at RT. This was followed by 6 final washes in PBST for 10 min each and bands were detected using enhanced chemiluminescence (ECL; Millipore). Densitometry was used to quantify protein bands using Image J Software (NIH), and proteins were normalized to total H3.
RNA-sequencing
Animals were exposed to extended access SA for 10 days and then injected bilaterally intra-NAc or intra-PFC with lenti-H3.3 WT or lenti-H3.3 Q5A the following day (day 11). Animals were put through forced abstinence for 29 more days, behaviorally assessed for cue-seeking on AD30, and then euthanized 48 hr later (to avoid molecular confounds introduced during active cue-seeking) with their virally transduced brains flash frozen. Brains were later sectioned at 150 μm on a cryostat, and RFP was illuminated using a NIGHTSEA flashlight to validate appropriate viral targeting, to qualitatively monitor viral transduction efficiency (which appeared equal in both NAc and mPFC) and to microdissect virally infected tissues. NAc/mPFC tissue punches were homogenized in Trizol (Thermo Fisher), and RNA was isolated on RNeasy Microcolumns (Qiagen) following manufacturer’s instructions. RNA concentrations were assessed using a nanodrop spectrophotometer and RNA 260/280 and 260/230 ratios were confirmed at >1.8. Following RNA purifications, RNA-seq libraries were prepared according to Illumina protocols and sequenced on an Illumina HiSeq 4000 (or equivalent) sequencer.
Following sequencing, raw reads from rat NAc/mPFC were mapped to the rat genome (v. rn6) using HISAT2v2.1.0. Aligned reads were quantified using featureCounts against Ensembl v90 annotation. Differential expression analysis was performed using the DESeq2 package (v1.6.3), with differentially expressed genes defined at an FDR cutoff of 0.1. Pathway analysis was performed using Enrichr with the PANTHER and Biological Process Gene Ontology databases to assess pathway enrichment for differentially expressed genes(Chen et al., 2013; Kuleshov et al., 2016; Xie et al., 2021).
Statistical Analysis
For cocaine SA acquisition, a two-way, repeated measures ANOVA was performed with subsequent Bonferroni post hoc analyses comparing to day 1 to monitor escalation of cocaine intake. Western blots and seeking behaviors were analyzed using two-tailed Student’s t-tests. Note, that for all western blotting experiments presented in Figure 1, blots were run comparing only two treatment groups (e.g., volitional cocaine vs. saline SA) at a single time point (e.g., AD1) per blot, and as such, we cannot statistically compare H3Q5dop levels across AD time points or across treatment types (e.g., volitional cocaine SA vs. yoked cocaine). Significance was determined at p<0.05. All data are represented as mean ± SEM.
Figure 1. H3Q5dop accumulates in NAc, but not mPFC, following abstinence from cocaine SA.
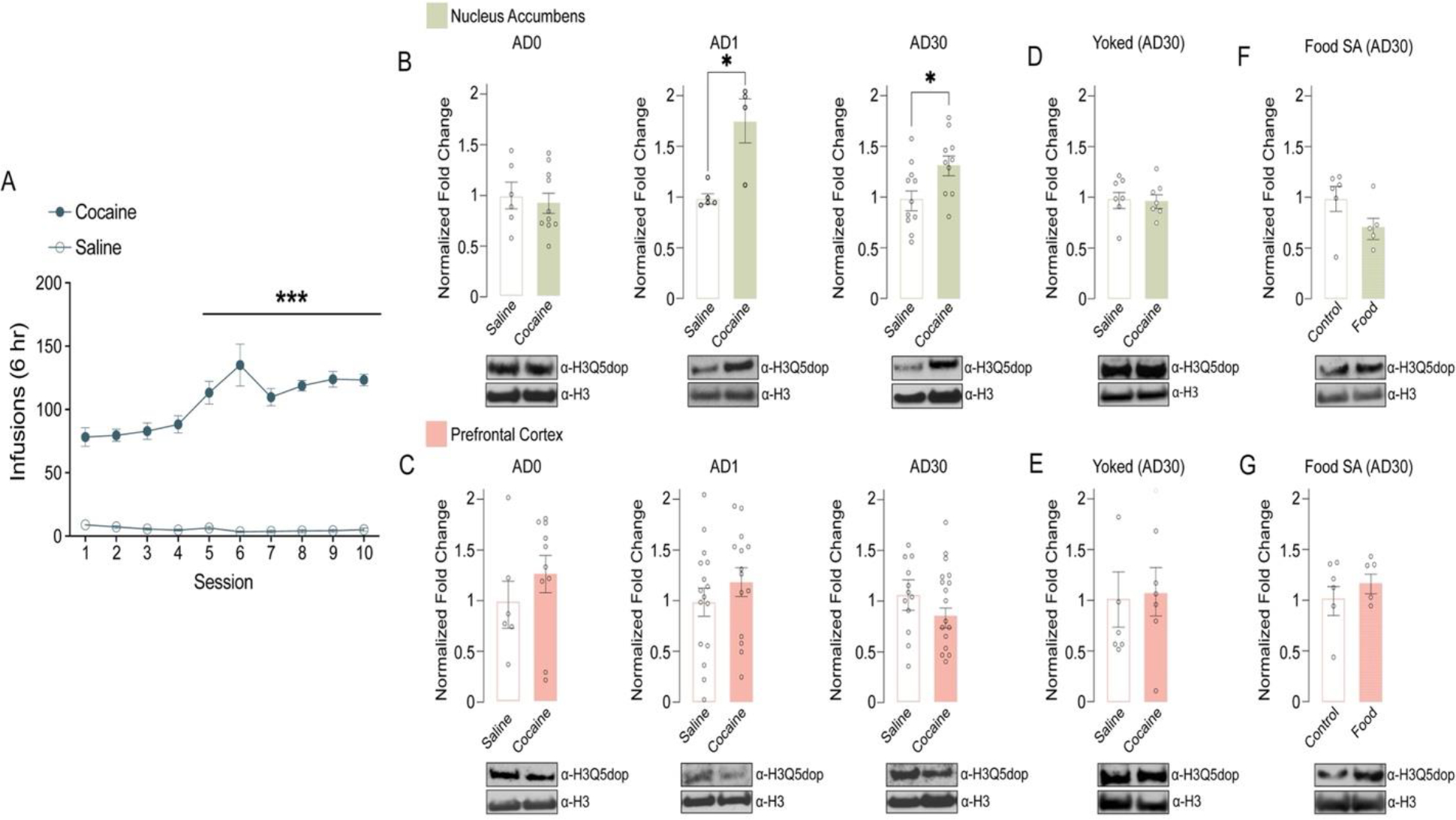
(A) Number of infusions earned in daily 6-hour test sessions (FR5) for rats self-administering saline (non-reinforcement controls) or cocaine (0.5 mg/kg/infusion). Extended access, cocaine vs. saline: two-way repeated measures ANOVA, with Bonferroni post hoc analysis vs. day 1, ***p<0.001 (n = 21/group). Analysis of H3Q5dop in (B) NAc (n = 4–11/group/time point) or (C) mPFC (n = 6–17/group/time point) (0 vs. 1 vs. 30 days of abstinence) from rats with extended access to cocaine vs. saline. Unpaired Student’s t-tests per AD time point, *p<0.05. Western blot analysis of H3Q5dop levels in (D) NAc (n = 7/group) or (E) mPFC (n = 6/group) (AD30) from yoked extended access cocaine vs. saline rats (p>0.05). Western blot analysis of H3Q5dop levels in (F) NAc (n = 5–6/group) or (G) mPFC (n = 5–6/group) (AD30) from extended access food (natural reinforcer) vs. non-reinforcement control rats (p>0.05). Total H3 was used as a loading control for all measurements of H3Q5dop, and bar plots represent normalized fold changes comparing cocaine vs. saline or food SA vs. control. Data presented as average ± SEM.
Results
H3 dopaminylation levels accumulate in NAc, but not mPFC, following both acute and prolonged abstinence from cocaine
Given that we previously observed dynamic accumulation of H3Q5dop in VTA following prolonged, but not acute, abstinence from volitional cocaine SA (Lepack et al., 2020), we first sought to examine whether H3Q5dop is restricted to dopamine producing brain regions, such as VTA and substantia nigra par compacta (SNc), or if it is more broadly observed throughout the brain, including within regions that receive innervation by dopamine synthesizing neurons. Following western blotting for H3Q5dop across multiple brain structures (e.g., VTA, SNc, NAc, mPFC, amygdala, etc.), we found that the mark is indeed not restricted to DA producing brain nuclei, but rather displays ubiquitous expression across all of the brain regions examined (Supplementary Figure 1), consistent with other H3 monoaminylation marks. Given its broad distribution patterns, along with a preponderance of previous evidence implicating altered dopamine signaling in NAc and mPFC in the precipitation of cocaine-related phenotypes, we next sought to examine whether H3Q5dop itself in NAc and/or mPFC may similarly display dynamic patterns of regulation following chronic cocaine exposures. To do so, we utilized an extended access (6-hour), fixed-ratio (FR) 5 schedule of reinforcement cocaine SA paradigm (Figure 1A) that characteristically results in an escalation of drug intake across sessions (Edwards and Koob, 2013) to assess potential changes in H3Q5dop in both NAc and mPFC following acute vs. prolonged periods of drug abstinence (e.g., 0, 1, or 30 days post the last infusion). As our previous studies of H3Q5dop in VTA showed no alterations in the mark using either a short access (1-hour) SA paradigm or experimenter-administered drug delivery (Lepack et al., 2020), we chose to exclude these models from our current study. Following extended access cocaine SA (vs. saline non-reinforcement controls), we observed that while H3Q5dop levels remained unaltered in NAc immediately after cocaine exposures (AD0; Figure 1B, left), the mark was found to accumulate in this brain region following acute abstinence (AD1; Figure 1B, middle), a phenomenon that persisted to AD30, the height of ‘incubation’ of cocaine craving in this model (Figure 1B, right) (Grimm et al., 2001). In contrast, we did not detect changes in H3Q5dop levels in mPFC at any of the abstinence time points examined (Figure 1C). To next assess whether cocaine SA-induced accumulation of H3Q5dop in NAc was dependent on volitional cocaine consumption or was simply a reflection of the pharmacological properties of the drug, we assessed NAc H3Q5dop levels in cocaine vs. saline yoked controls, where animals received non-volitional drug administration with the same amount and frequency of infusions as the cocaine SA rats. Consistent with our previous results in VTA (Lepack et al., 2020), the mark remained unaffected in NAc following yoked cocaine administration at AD30 (Figure 1D), suggesting a plausible role for the mark in the context of an etiologically relevant drug addicted-like state. As expected, yoked cocaine administration did not result in alterations of H3Q5dop levels in mPFC (Figure 1E). Finally, chronic volitional exposures to a natural reinforcer (i.e., food) did not lead to changes in H3Q5dop expression in either brain region examined at AD30 (Figure 1F–G).
Attenuation of H3Q5dop in NAc, but not mPFC, inhibits cocaine-seeking
Considering the persistent nature of H3Q5dop accumulation observed in NAc following extended access cocaine SA, as well as our lab’s previous data implicating H3Q5dop in the regulation of cocaine-seeking behavior in VTA (Lepack et al., 2020), we next assessed possible roles for the mark in mediating relapse-like vulnerability in both NAc and mPFC. Following 10 days of extended access cocaine SA, rats were transduced intra-NAc or -mPFC (on AD1) with a lentivirus vector, (lenti-H3.3Q5A), expressing a dominant negative mutant version of the histone variant H3.3 harboring a point mutation of glutamine 5 to alanine – note that only H3.3, but not canonical H3.1 or H3.2 isoforms, can actively incorporate into neuronal chromatin (Maze et al., 2015). This H3.3Q5A histone mutant cannot be dopaminylated and thus competes with endogenous H3.3 for chromatin incorporation, leading to attenuation of the mark in vivo (Fulton et al., 2022; Lepack et al., 2020). Following 30 days of forced abstinence and viral transduction, animals were returned to the drug-paired context and cocaine-seeking behavior was assessed (Figure 2A). Consistent with our western blotting results in Figure 1, which indicated a brain region specific pattern of regulation for H3Q5dop by cocaine SA in NAc, but not in mPFC, we found that inhibition of H3Q5dop in NAc significantly reduced cocaine-seeking behavior compared to control animals transduced with a lentivirus vector expressing wildtype H3.3 (lenti-H3.3WT) (Figure 2B); note that previous data from our lab has demonstrated that transduction by lenti-H3.3WT in brain does not significantly impact gene expression vs. an empty vector control (Fulton et al., 2022; Lepack et al., 2020). Cocaine-seeking remained unchanged in mPFC following transduction by lenti-H3.3Q5A vs. lenti-H3.3WT (Figure 2C), suggesting that H3Q5dop does not contribute significantly to relapse-like vulnerability in this brain region.
Figure 2. Blocking H3Q5dop in NAc, but not mPFC, inhibits cocaine-seeking.
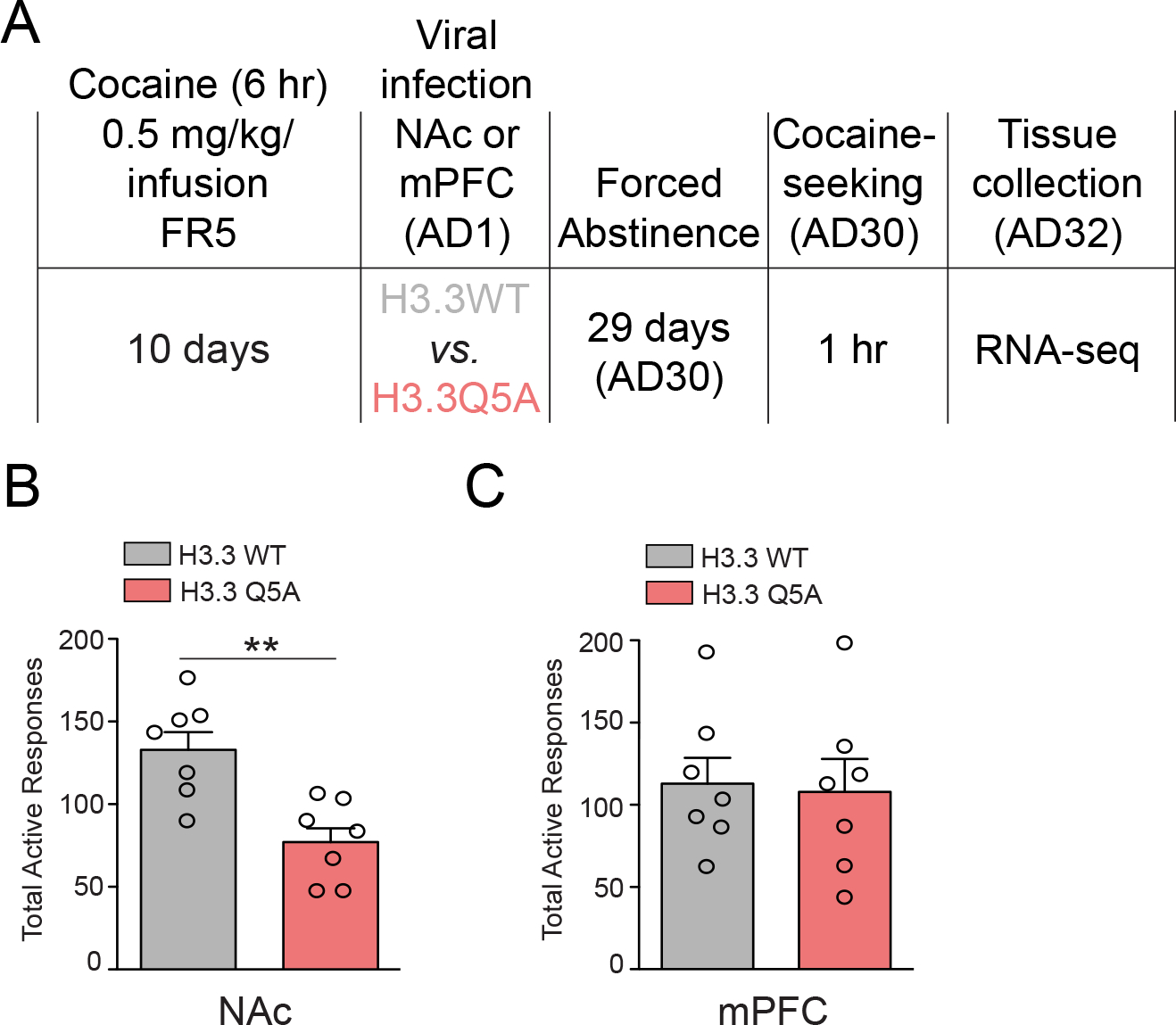
(A) Timeline of cocaine SA (extended access) drug-seeking experiments following viral transduction with either lenti-H3.3WT or lenti-H3.3Q5A viruses. Cocaine SA rats were infected intra- (B) NAc (n = 7/group) or (C) mPFC (n = 7/group) on AD1 with either lenti-H3.3WT or lenti-H3.3Q5A viruses, and following 30 days of abstinence were subjected to 1 hr of cocaine-seeking on AD30. Unpaired Student’s t-tests, **p<0.01. Data presented as average ± SEM.
Attenuation of H3Q5dop in NAc, but not mPFC, following cocaine SA regulates gene expression programs associated with drug-induced plasticity
Finally, given that H3Q5dop displays accumulation selectively in NAc vs. mPFC following cocaine SA, a phenomenon that contributes directly to cocaine-seeking behavior, we next wished to assess what impact, if any, the mark may have on gene expression programs within these brain structures following chronic cocaine exposures. As such, following prolonged abstinence from cocaine SA, virally transduced rats (intra-NAc vs. -mPFC; infected on AD1) used in cue-seeking experiments were euthanized 48 hr after behavioral assessments, and infected brain tissues were extracted for RNA-seq analysis. Mirroring the biochemical changes presented in Figure 1, and consistent with our behavioral results in Figure 2, we found that H3.3Q5A transduction in NAc resulted in the significant differential regulation of 112 genes at FDR<0.1 in comparison to lenti-H3.3WT transduced controls (Figure 3A). In contrast, only 9 significantly regulated genes were observed in mPFC – none of which overlapped with differentially expressed transcripts in NAc – when comparing the two viral groups following prolonged abstinence from cocaine SA. In NAc, as predicted based upon the generally permissive role that H3Q5serotonylation plays in brain (Farrelly et al., 2019; Lukasak et al., 2022; Zhao et al., 2021a; Zhao et al., 2021b), we found that attenuation of H3Q5dop resulted in a general trend toward downregulation of affected transcripts (Figure 3B), suggesting that blockade of the mark’s accumulation following cocaine SA may significantly reduce the expression of aberrantly activated loci induced by cocaine exposures. In line with this assumption, subsequent pathway analysis of these significantly regulated genes in NAc revealed numerous well-studied mediators of cocaine-seeking, such as opioid peptide precursors, dopamine metabolic processes and cellular Ca2+ homeostasis (Figure 3C) (Charbogne et al., 2014; Gutiérrez-Cuesta et al., 2014; Moeller et al., 2015; Simmons and Self, 2009), indicating that H3Q5dop accumulation in NAc, but not mPFC, during withdrawal from cocaine may function, at least in part, to induce specific gene expression programs that contribute to aberrant neural plasticity and relapse-related vulnerability.
Figure 3. Attenuation of H3Q5dop in NAc, but not mPFC, following cocaine SA regulates gene expression programs associated with drug-induced plasticity.
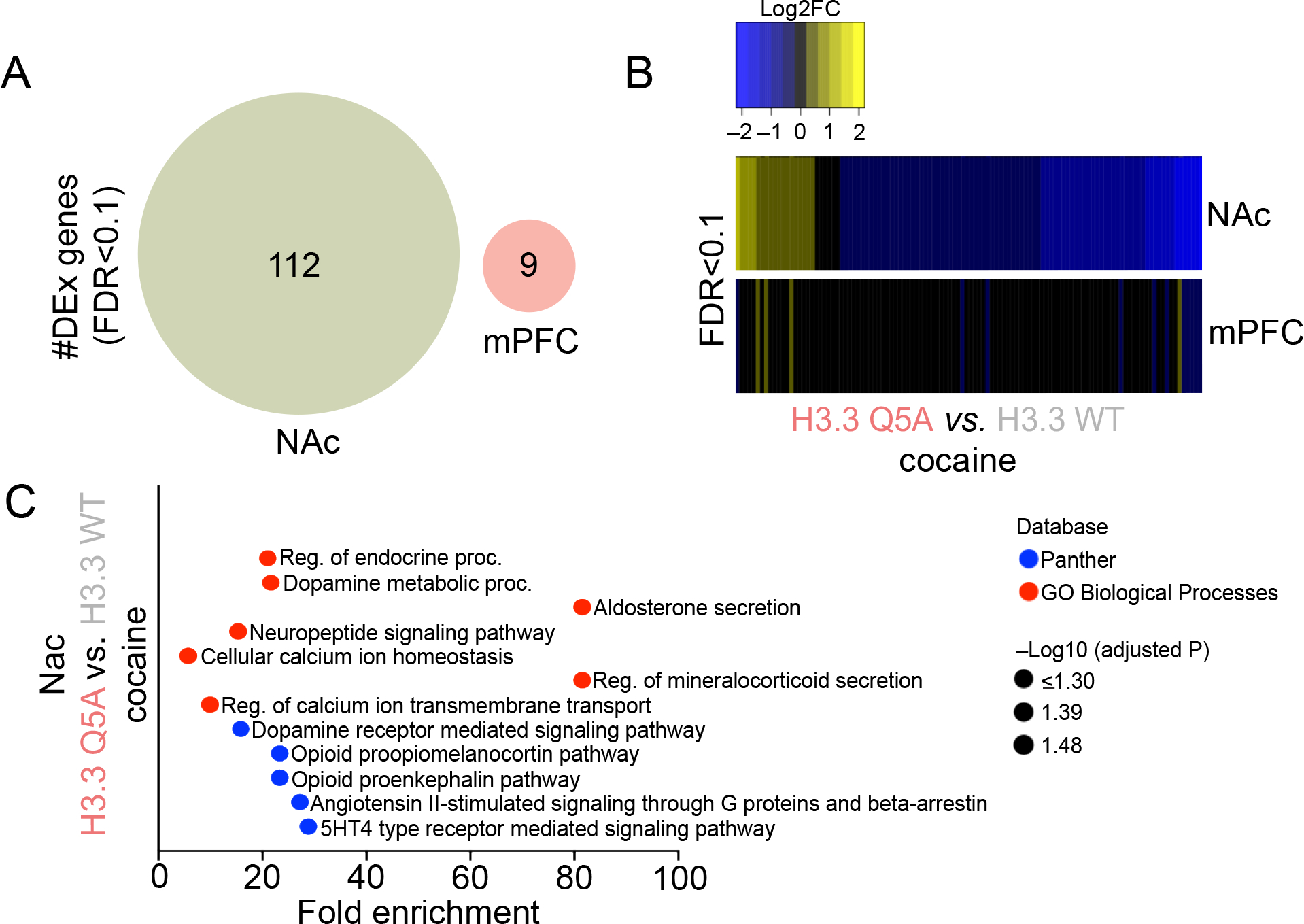
(A) Venn diagrams depicting the number and degree of overlap of significantly regulated genes (FDR<0.1; n = 6/viral group/brain region) in NAc and mPFC comparing H3.3Q5A vs. H3.3 WT transduced rats following extended access cocaine SA. (B) Heatmaps depicting the Log2 fold-change (FC) of significantly regulated transcripts in NAc and mPFC comparing H3.3Q5A vs. H3.3 WT transduced rats following extended access cocaine SA. (C) Pathway analysis (Panther and GO Biological Process) was performed to assess gene ontology enrichment for differentially expressed genes in NAc comparing H3.3Q5A vs. H3.3 WT transduced rats following extended access cocaine SA.
Discussion
In this manuscript, we provided evidence that histone H3 dopaminylation, beyond its role within dopamine synthesizing neurons of VTA (Fulton et al., 2022; Lepack et al., 2020), also functions as a critical post-synaptic signaling moiety in NAc involved in the precipitation of CUD relevant phenotypes. We demonstrated that H3Q5dop is regulated in a dynamic and region-specific manner following extended access cocaine SA, resulting in persistent accumulation of the mark in NAc, but not in mPFC. Additionally, these alterations were not observed following SA of a natural reinforcer or in yoked cocaine animals. We found that this aberrant accumulation of H3Q5dop in NAc plays a direct role in the precipitation of cocaine-seeking behavior during prolonged abstinence from cocaine SA, with blockade of H3Q5dop in NAc specifically leading to attenuation of relapse-like vulnerability in this model, similar to results previoulsy observed with blockade of H3Q5dop in VTA (Fulton et al., 2022; Lepack et al., 2020). The relative contribution, or lack thereof, of H3Q5dop to cocaine-related phenotypes within these mesocorticolimbic brain structures was further elucidated at the transcriptional level, where we found that blockade of H3Q5dop in cocaine SA rats elicited NAc-specific patterns of transcriptional regulation that implicate gene expression programs already known to be regulated by cocaine use. Importantly, this dominant negative approach did not affect cocaine-seeking behavior in mPFC, nor did manipulation of H3Q5dop in mPFC result in significant alterations in gene expression patterns within this brain region. Ultimately, these data highlight new brain region-specific roles for H3Q5dop in the precipitation of relapse-like vulnerability following chronic cocaine exposures.
Our analysis of the transcriptional pathways regulated by blockade of H3Q5dop accumulation in NAc following cocaine SA – with the vast majority of these genes displaying blunted responses following H3Q5dop attenuation – revealed prominent associations with known potentiators of cocaine-seeking behavior, including Ca2+ homeostasis, dopamine metabolic processes and endogenous opioid pathways, including proopiomelanocortin and proenkephalin related signaling processes (Charbogne et al., 2014; Gutiérrez-Cuesta et al., 2014; Moeller et al., 2015; Simmons and Self, 2009). Disruptions in Ca2+ signaling, for example, have been repeatedly implicated in the neuroplasticity changes that precipitate cocaine-seeking behavior — including increased expression of homomeric Ca2+-permeable AMPA receptors (CP-AMPARs) — that have been causally linked to relapse-associated vulnerability (Lee and Dong, 2011; Loweth et al., 2014; Wang et al., 2022; Wolf, 2016). Similarly, a large body of work has identified alterations in dopamine release dynamics following cocaine SA as a potential causative factor in drug-seeking behavior (Kalivas and Duffy, 1990; Parsons et al., 1991). Finally, endogenous opioid signaling has also been robustly implicated in cocaine-taking, -seeking and motivational responses for cocaine, suggesting that H3Q5dop accumulation during drug abstinence may regulate drug-seeking behavior in NAc similar to what was previously observed in VTA following exposures to cocaine and heroin (Fulton et al., 2022; Lepack et al., 2020).
It is important to note, however, that several significant questions remain with respect to H3Q5dop’s precise modes of regulation within non-dopaminergic regions of the brain. For example, it remains unclear within which post-synaptic cell-types H3Q5dop is actually expressed, and our lentiviral-based dominant negative strategy – while clearly demonstrating that blockade of H3Q5dop accumulation in NAc has functional consequences with respect to cocaine-seeking behavior – was not cell-type specific, as these viruses are capable of transducing both neurons and glia. While neurons are the most well-studied cell-type in CUD models, increasing evidence has begun to implicate glial cell-types (e.g., astrocytes) in mediating transcriptional and behavioral phenotypes associated with cocaine exposures (Savell et al., 2020; Wang et al., 2022). While H3Q5dop, like H3Q5ser (Farrelly et al., 2019), is expressed broadly throughout the brain (with H3Q5ser displaying expression in both neurons and glia of the dorsal raphe nucleus), canonical transporters for these monoamines (e.g., SLC6A4/SERT for serotonin and SLC6A3/DAT for DA) are generally believed to be restricted to monoamine synthesizing neurons, as well as select populations of glial cells (Aggarwal and Mortensen, 2017). With that being said, it is important to note that numerous other non-canonical transporters are expressed in brain – often more broadly in comparison to their respective canonical transporters – that carry affinity for monoamines (Duan and Wang, 2010). Additionally, even selective monoamine transporters, such as DAT, have been demonstrated to display promiscuity under circumstances in which alternative canonical transporters are inhibited (e.g., SERT uptake of dopamine) (Larsen et al., 2011). One such non-canonical transporter of potential interest is SLC22A3 (aka Organic Cation Transporter 3/OCT3), which represents a high capacity, low affinity transporter that displays affinities for nearly all monoamines assessed, is expressed broadly throughout the brain (in both neurons and glia) and decorates both plasma and nuclear membranes in the cells in which it is expressed (Gasser et al., 2017). SLC22A3 is expressed in NAc (Graf et al., 2013) and may therefore represent an intriguing target for future studies aimed at pharmacologically exploring the mechanisms of H3Q5dop establishment within non-DAergic cells of the brain. Another possible mechanism for DA uptake into non-DA synthesizing cells within the central nervous system may involve post-synaptic DA receptor internalization mechanisms, although this scenario seems less likely vs. active transport, as it remains unclear whether such processes would be sufficient to import enough DA from the synapse into post-synaptic cells to allow for monoaminylation PTMs to be deposited. While many possibilities exist, what remains clear is that these modifications are indeed present with non-DAergic regions of the brain, are likely influenced by DA release dynamics into these brain structures from DAergic neurons arising in VTA and appear to play critical roles in drug-induced plasticity, thus warranting future investigations into these processes.
While the findings outlined here with respect to H3 dopaminylation dynamics within non-DAergic regions of the brain in response to cocaine are certainly compelling, a much more profound question naturally arises from these data: is it possible that there are other protein substrates of this modification within non-DAergic neural cells, and if so, might these events (or their associated dynamics) also be functionally relevant to drug-induced phenotypes? We demonstrated that H3Q5dop not only exists in NAc, but is also involved in precipitating maladaptive behavioral and transcriptional responses to cocaine. However, the nucleus is notably a quite disparate structure in comparison to synaptic compartments in which dopamine is synthesized, released and engages directly with post-synaptic receptors and/or transporters. Furthermore, Transglutaminase 2 (TGM2), the “writer” enzyme for H3Q5dop, is a promiscuous enzyme expressed ubiquitously throughout the body and within the cell – with expression observed in nuclear, cytosolic and extracellular compartments – and is known to monoaminylate multiple target proteins other than H3 (Walther et al., 2003). As such, it seems highly plausible that many other substrates of this modification may exist within NAc (as well as in other regions receiving DAergic innervation) and they may also be subject to regulation by drugs of abuse. Future studies will be certainly required to fully elucidate these vast possibilities. What is becoming increasingly clear, however, is that protein dopaminylation (along with other protein monoaminylation events) may very well function as a major, yet largely uncharacterized, signaling pathway within brain that is divorced from DA’s canonical roles in receptor mediated signal transduction, and as a field, we may need to begin reconsidering traditional models that assume classical roles monoaminergic signaling as a precipitating factor in SUD.
Supplementary Material
Highlights.
Histone H3 dopaminylation (H3Q5dop), recently demonstrated to play important roles in ventral tegmental area (VTA) in the precipitation of drug-seeking behavior, is also expressed in non-dopaminergic brain regions receiving innervation from VTA
H3Q5dop accumulates in nucleus accumbens (NAc), but not medial prefrontal cortex (mPFC), during withdrawal from cocaine self-administration (SA)
H3Q5dop accumulation in NAc during cocaine abstinence contributes to cocaine-seeking behavior
H3Q5dop accumulation in NAc during cocaine abstinence contributes to drug-mediated alterations in gene expression that may precipitate relapse-like vulnerability
Acknowledgements
We would like to thank members of the Maze lab for critical readings of the manuscript. This work was partially supported by grants from the National Institutes of Health: DP1 DA042078 (I.M.), R01 MH116900 (I.M.), R01 DA056595 (I.M.), F31 DA055462 (A.F.S.), F31 DA045428 (A.L.L.), F31 MH124425 (S.L.F.), and F99 NS125774 (S.L.F.), as well as funds from the National Science Foundation (NSF GRFP; A.F.S.).
Footnotes
Competing interests
The Authors declare no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
The RNA-seq data generated in this study have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database under accession number GSE221177. We declare that the data supporting findings for this study are available within the article. Related data are available from the corresponding author upon reasonable request. No restrictions on data availability apply.
References
- World Drug Report 2021. In: Crime, U.N.O.o.D.a. (Ed.). United Nations publications. [Google Scholar]
- Diagnostic and statistical manual of mental disorders : DSM-5-TR, 5th edition, text revision. ed. American Psychiatric Association Publishing, Washington, DC. [Google Scholar]
- Aggarwal S, Mortensen OV, 2017. Overview of Monoamine Transporters. Current Protocols in Pharmacology 79, 12.16.11–12.16.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K, 2014. 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 76, 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A, 2013. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschatrettes E, Romieu P, Zwiller J, 2013. Cocaine self-administration by rats is inhibited by cyclic GMP-elevating agents: involvement of epigenetic markers. International Journal of Neuropsychopharmacology 16, 1587–1597. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu X-T, Malenka RC, White FJ, 2005. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. Journal of Neuroscience 25, 936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J, 2010. Selective Transport of Monoamine Neurotransmitters by Human Plasma Membrane Monoamine Transporter and Organic Cation Transporter 3. Journal of Pharmacology and Experimental Therapeutics 335, 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF, 2013. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behavioural pharmacology 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly LA, Thompson RE, Zhao S, Lepack AE, Lyu Y, Bhanu NV, Zhang B, Loh Y-HE, Ramakrishnan A, Vadodaria KC, Heard KJ, Erikson G, Nakadai T, Bastle RM, Lukasak BJ, Zebroski H, Alenina N, Bader M, Berton O, Roeder RG, Molina H, Gage FH, Shen L, Garcia BA, Li H, Muir TW, Maze I, 2019. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature 567, 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton SL, Mitra S, Lepack AE, Martin JA, Stewart AF, Converse J, Hochstetler M, Dietz DM, Maze I, 2022. Histone H3 dopaminylation in ventral tegmental area underlies heroin-induced transcriptional and behavioral plasticity in male rats. Neuropsychopharmacology, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Hurley MM, Chan J, Pickel VM, 2017. Organic cation transporter 3 (OCT3) is localized to intracellular and surface membranes in select glial and neuronal cells within the basolateral amygdaloid complex of both rats and mice. Brain Structure and Function 222, 1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robble MA, Vranjkovic O, Wheeler DS, Mantsch JR, Gasser PJ, 2013. Corticosterone Acts in the Nucleus Accumbens to Enhance Dopamine Signaling and Potentiate Reinstatement of Cocaine Seeking. The Journal of Neuroscience 33, 11800–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y, 2001. Incubation of cocaine craving after withdrawal. Nature 412, 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Cuesta J, Burokas A, Mancino S, Kummer S, Martín-García E, Maldonado R, 2014. Effects of genetic deletion of endogenous opioid system components on the reinstatement of cocaine-seeking behavior in mice. Neuropsychopharmacology 39, 2974–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, 1990. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse 5, 48–58. [DOI] [PubMed] [Google Scholar]
- Kampman KM, 2019. The treatment of cocaine use disorder. Science Advances 5, eaax1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A, 2016. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Research 44, W90–W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MB, Sonders MS, Mortensen OV, Larson GA, Zahniser NR, Amara SG, 2011. Dopamine Transport by the Serotonin Transporter: A Mechanistically Distinct Mode of Substrate Translocation. The Journal of Neuroscience 31, 6605–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Dong Y, 2011. Cocaine-induced metaplasticity in the nucleus accumbens: Silent synapse and beyond. Neuropharmacology 61, 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Werner CT, Stewart AF, Fulton SL, Zhong P, Farrelly LA, Smith ACW, Ramakrishnan A, Lyu Y, Bastle RM, Martin JA, Mitra S, O’Connor RM, Wang Z-J, Molina H, Turecki G, Shen L, Yan Z, Calipari ES, Dietz DM, Kenny PJ, Maze I, 2020. Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science 368, 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME, 2014. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology 76, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasak BJ, Mitchener MM, Kong L, Dul BE, Lazarus CD, Ramakrishnan A, Ni J, Shen L, Maze I, Muir TW, 2022. TGM2-mediated histone transglutamination is dictated by steric accessibility. Proceedings of the National Academy of Sciences 119, e2208672119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ, 2010. Essential Role of the Histone Methyltransferase G9a in Cocaine-Induced Plasticity. Science 327, 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Wenderski W, Noh K-M, Bagot Rosemary C., Tzavaras N, Purushothaman I, Elsässer Simon J., Guo Y, Ionete C, Hurd Yasmin L., Tamminga Carol A., Halene T, Farrelly L, Soshnev Alexey A., Wen D, Rafii S, Birtwistle Marc R., Akbarian S, Buchholz Bruce A., Blitzer Robert D., Nestler Eric J., Yuan Z-F, Garcia Benjamin A., Shen L, Molina H, Allis CD, 2015. Critical Role of Histone Turnover in Neuronal Transcription and Plasticity. Neuron 87, 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Beebe-Wang N, Schneider KE, Konova AB, Parvaz MA, Alia-Klein N, Hurd YL, Goldstein RZ, 2015. Effects of an opioid (proenkephalin) polymorphism on neural response to errors in health and cocaine use disorder. Behavioural brain research 293, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L, Smith A, Justice J Jr, 1991. Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse 9, 60–65. [DOI] [PubMed] [Google Scholar]
- Rogge GA, Wood MA, 2013. The Role of Histone Acetylation in Cocaine-Induced Neural Plasticity and Behavior. Neuropsychopharmacology 38, 94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha J-HJ, 2010. Cocaine-Induced Chromatin Remodeling Increases Brain-Derived Neurotrophic Factor Transcription in the Rat Medial Prefrontal Cortex, Which Alters the Reinforcing Efficacy of Cocaine. The Journal of Neuroscience 30, 11735–11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savell KE, Tuscher JJ, Zipperly ME, Duke CG, Phillips RA, Bauman AJ, Thukral S, Sultan FA, Goska NA, Ianov L, Day JJ, 2020. A dopamine-induced gene expression signature regulates neuronal function and cocaine response. Science Advances 6, eaba4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Self DW, 2009. Role of mu-and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology 34, 1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AF, Fulton SL, Maze I, 2020. Epigenetics of Drug Addiction. Cold Spring Harbor Perspectives in Medicine, a040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV, 2006. Repeated Cocaine Self-Administration Alters Processing of Cocaine-Related Information in Rat Prefrontal Cortex. The Journal of Neuroscience 26, 8004–8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suska A, Lee BR, Huang YH, Dong Y, Schlüter OM, 2013. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proceedings of the National Academy of Sciences 110, 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague CD, Nestler EJ, 2022. Key transcription factors mediating cocaine-induced plasticity in the nucleus accumbens. Molecular Psychiatry 27, 687–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Peter J-U, Winter S, Höltje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M, 2003. Serotonylation of Small GTPases Is a Signal Transduction Pathway that Triggers Platelet α-Granule Release. Cell 115, 851–862. [DOI] [PubMed] [Google Scholar]
- Wang J, Holt LM, Huang HH, Sesack SR, Nestler EJ, Dong Y, 2022. Astrocytes in cocaine addiction and beyond. Molecular Psychiatry 27, 652–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner CT, Mitra S, Martin JA, Stewart AF, Lepack AE, Ramakrishnan A, Gobira PH, Wang Z-J, Neve RL, Gancarz AM, Shen L, Maze I, Dietz DM, 2019. Ubiquitinproteasomal regulation of chromatin remodeler INO80 in the nucleus accumbens mediates persistent cocaine craving. Science Advances 5, eaay0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, 2016. Synaptic mechanisms underlying persistent cocaine craving. Nature reviews neuroscience 17, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, Lachmann A, Wojciechowicz ML, Kropiwnicki E, Jagodnik KM, Jeon M, Ma’ayan A, 2021. Gene Set Knowledge Discovery with Enrichr. Current Protocols 1, e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Chen W, Pan Y, Zhang Y, Sun H, Wang H, Yang F, Liu Y, Shen N, Zhang X, Mo X, Zang J, 2021a. Structural insights into the recognition of histone H3Q5 serotonylation by WDR5. Science Advances 7, eabf4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Chuh KN, Zhang B, Dul BE, Thompson RE, Farrelly LA, Liu X, Xu N, Xue Y, Roeder RG, 2021b. Histone H3Q5 serotonylation stabilizes H3K4 methylation and potentiates its readout. Proceedings of the National Academy of Sciences 118, e2016742118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data generated in this study have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database under accession number GSE221177. We declare that the data supporting findings for this study are available within the article. Related data are available from the corresponding author upon reasonable request. No restrictions on data availability apply.


