Summary
Obesity and type 2 diabetes (T2D) are increasingly common worldwide. While these disorders have increased in prevalence over the past several decades, there has been a concomitant reduction in sleep duration. Short sleep duration has been associated with higher rates of obesity and T2D, and the causality of these associations and their directionality, continue to necessitate evaluation.
In this review we consider the evidence that sleep is an intrinsic factor in the development of obesity and chronic metabolic disorders, such as insulin resistance and T2D, while evaluating a potential bi-directional association. We consider the evidence that diet and meal composition, which are known to impact glycemic control, may have both chronic and acute impact upon sleep. Moreover, we consider that postprandial nocturnal metabolism and peripheral glycemia may affect sleep quality. We propose putative mechanisms whereby acute effects of nighttime glucose excursions may lead to increased sleep fragmentation. We conclude that dietary manipulations, particularly with respect to carbohydrate quality, may confer sleep benefits. Future research may seek to evaluate the effectiveness of synergistic nutrient strategies to promote sleep quality, with particular attention to carbohydrate quality, quantity, and availability as well as carbohydrate to protein ratio.
Keywords: Obesity, energy balance, diet, food intake, type 2 diabetes, glucose, orexin
1. Introduction
Obesity prevalence has been on the rise in the U.S. since the 1980’s, when its prevalence was ~15% to most recent rates of ~43% in the 2017–2018 National Health and Nutrition Examination Survey (NHANES) (1). Clearly the rapid increase in obesity observed since the 1980’s argues for environmental factors as drivers for this rise in prevalence, rather than change in genetic composition. Despite debates on the underlying instigators of the obesity epidemic, theories of obesity development agree that changes in the food environment promoting increases in energy intake have been instrumental in creating a positive energy balance necessary for changes in weight status (2, 3).
Multiple environmental factors influence the relation of individuals with their food environment. One’s psychosocial, economic, and cultural context influence food availability and choice. Environmental factors including endocrine disruptors, climate, and urban planning also impact the food environment. These factors interact with genetic and physiological risk factors to influence individual obesity risk (4). In turn, food insecurity and environmental factors have been exposed as determinants of insufficient sleep, poor sleep quality outcomes and sleep disorders (e.g see (5, 6)). The significance of socioeconomic status and food insecurity are undoubtably major components of ones relationship with food and the emotional and cognate processes that impact on eating behavior. The true weight of these important determinants is difficult to estimate and falls beyond the scope of the current review but interested readers are invited to refer to several excellent works (7–9). Here we propose that sleep is an integral behavior that may impact the risk of obesity and chronic disorders, such as type 2 diabetes (T2D), as it interacts at all levels of the causal pathway towards these adverse health conditions. Moreover, it is imperative to consider the potential bidirectionality of this relationship whereby glycemic regulation and insulin sensitivity impact sleep quality.
Average sleep duration in U.S. adults has declined since the mid-1980’s, when the mean age-adjusted sleep duration was 7.40 h/d, to 7.18 h/d in 2012 (10). During this time, the prevalence of adults reporting short sleep duration (≤6 h/d) increased from 22.3% in 1985 (10) to 32.9% in 2017 (11). Additionally, cross-sectional data from 413,417 individuals attained from the National Health Interview Survey from 2005–2018 showed that daily sleep duration had decreased −0.62 minutes annually over this time period (12). Interestingly, this trend appears to more pronounced by ethnicity, with this study demonstrating that Mexican Hispanic people experience the greatest decline. In another analysis of the same survey (2004–2018) the prevalence of short sleep duration was greatest in Black and Latino/Hispanic individuals and long sleep duration was persistently higher among Black individuals (13). These changes in population sleep duration and prevalence of insufficient sleep are concerning given their association with chronic health conditions. A 1-h loss in daily sleep duration has been reported to be linked to 4% greater prevalence of hypertension, 3% greater prevalence of diabetes, and 8% greater prevalence of obesity after adjusting for age, sex, employment, marital status, and year (12). Indeed, several meta-analyses report increased odds of developing obesity in short sleepers relative to adequate sleepers (14–16). The lowest risk of obesity has been noted in those sleeping 7–8 h/d (16). Similar findings have been noted for incidence of T2D. Meta-analyses of prospective cohort studies report increased risk of developing T2D in those reporting short sleep duration (17, 18) with lowest risk in those reporting 7–8 h of sleep per night (18). Reporting insomnia symptoms such as difficulty initiating sleep and difficulty maintaining sleep (17) or having an insomnia diagnosis (19) is also associated with increased incidence of T2D.
In addition to evidence from epidemiological studies, multiple intervention studies have supported a role of sleep as a causal factor in the development of obesity. A meta-analysis of 4 intervention studies reported a trend for greater weight gain in participants undergoing a sleep restriction intervention versus maintained adequate sleep with a standard mean difference of 0.68 (p-value=0.08) (20). A subsequent meta-analysis including 5 intervention studies reported no difference in body weight change between restricted and adequate sleep condition but the pooled standard mean difference was 0.44 kg (p-value= 0.13) (21). The lack of statistical significance for an effect of sleep restriction on weight in these meta-analyses could be due to the small number of studies included and the high degree of heterogeneity. Moreover, a recent study not included in those meta-analyses reported a net weight gain of 0.5 kg in participants undergoing sleep restriction compared to adequate sleep for 14 d (22). Subcutaneous and visceral fat accumulations, measured by dual energy x-ray absorptiometry, were also greater in the restricted versus adequate sleep condition. Therefore, overall, data are concordant in showing small increases in body weight due to sleep restriction.
Correspondingly, intervention studies also highlight a causal impact of insufficient sleep on greater energy intake. Using data from NHANES 2005–2010, Kant and Graubard noted that short sleepers (≤6 h of sleep/night) reported higher energy intakes from snacks and higher intakes of sugar than adequate sleepers (those reporting 7–8 h of sleep/night) (23). A meta-analysis of clinical intervention studies further supports a role of sleep duration on dietary intakes (24). Data from 15 clinical trials show that partial sleep restriction increases energy intakes with a mean difference of 204 kcal (95% confidence interval, 112–295 kcal) relative to the control sleep condition and leads to higher percent of energy intake (%En) from fat, protein, and/or carbohydrates. It has been estimated that a surplus of energy of as little as 100 kcal/d is sufficient to result in weight gain in most people (25).
Increased energy intake and weight gain are often accompanied by poorer glycemic regulation, insulin resistance and an increased risk of T2D. A meta-analysis of three intervention studies using intravenous glucose tolerance test found a reduced insulin sensitivity in participants undergoing sleep restriction relative to adequate sleep with no overall effect on beta-cell function (26). Similar findings were reported when data from 12 studies using an oral glucose tolerance test to assess insulin resistance were analyzed (26). Given the strong association between weight status, adiposity, and risk of T2D (27), those results, combined with findings of increased adiposity due to sleep restriction, further suggest a causal role of sleep in the development of T2D.
Models of obesity pathogenesis highlight high glycemic load (GL) or ultra-processed food-rich diets as being central to obesity development (Figure 1). In the energy balance model, authors posited that the availability of such foods triggers neuronal networks implicating food reward and promotion of appetite to stimulate food intake (2). In the carbohydrate-insulin model of obesity pathogenesis, the proposition is that a high GL diet upregulates adipose insulin sensitivity and fat storage which reduces availability of fuels and promotes energy intake (3). The goal of this narrative review is to expose sleep as central to both models of obesity pathogenesis and evaluate the literature related to the associations between sleep, obesity, and T2D from a glucose-regulatory angle and examine how dietary carbohydrates from whole foods contribute to these associations. Please note that we focus on carbohydrates consumed as part of normal dietary intake/ or meal intake and not as a form of dietary supplementation in this paper. We further propose a mechanism by which glycemic regulation and insulin resistance may impact sleep quality.
Figure 1.
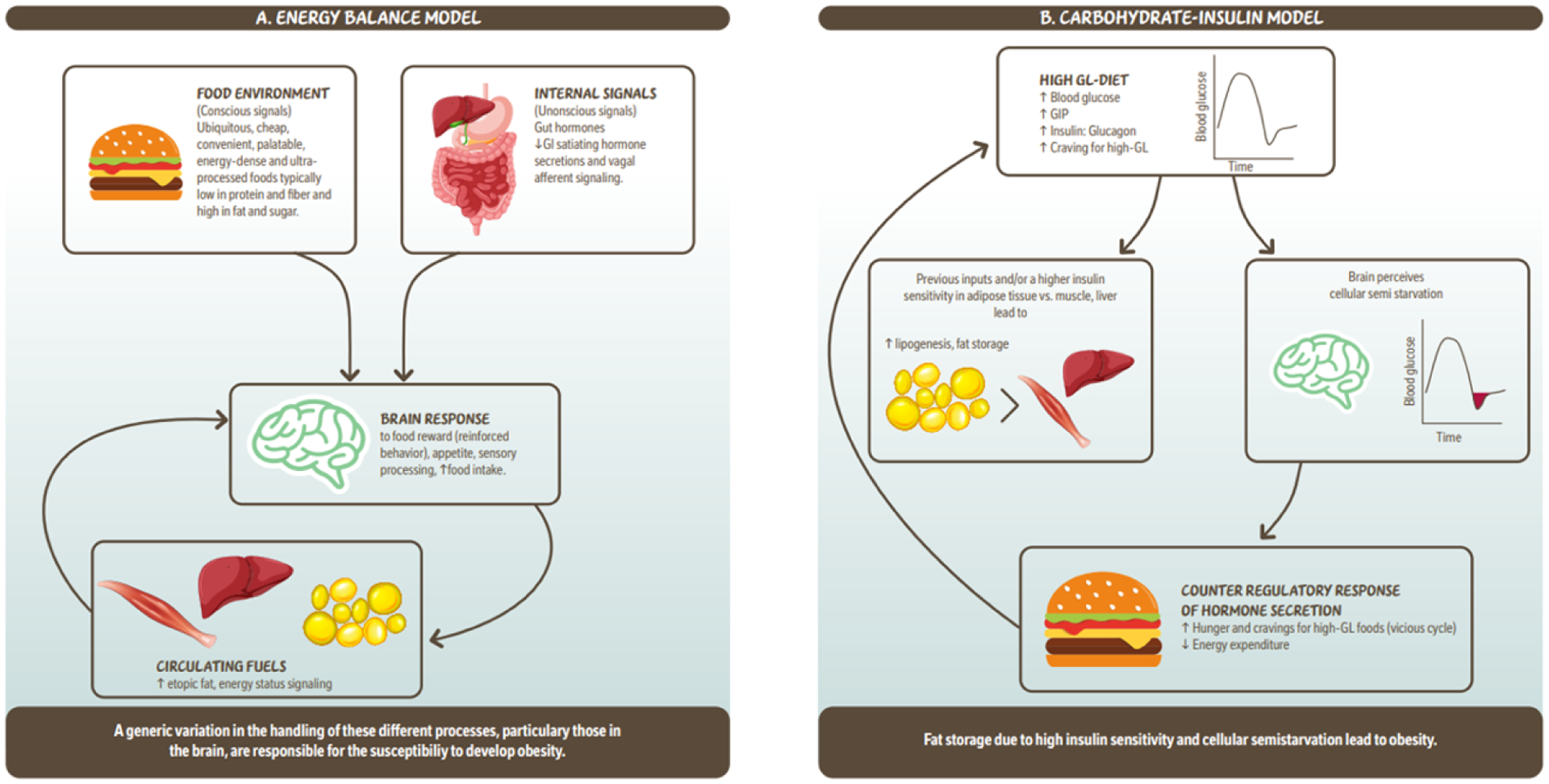
Simplified illustration of the ‘Energy balance model’ and the ‘Carbohydrate – insulin model’ of obesity. 1a. The energy balance model proposes that changes in the food environment as well as internal signals (hormones) driven by food intake, are cues to the brain which lead to increased food intake. These signals are integrated in the brain modulating behavour reinforcing of food reward, appetite which leads to more food intake and circulating fuels. Circulating fuels and signals (e.g. leptin) indicate the energy status of various organs and are sensed by the brain to control food intake. Overall this model proposes that a genetic variation in the handling of these different processes, particularly those in the brain, are responsible for the susceptibility to develop obesity. 1b. The carbohydrate- insulin model proposes that rapid absorption of glucose after consumption of a high-GL meal increases secretion of insulin to glucagon ratio, and elicits a glucose-dependent insulinotropic polypeptide (GIP)-dominant incretin response. These inputs and/or higher insulin sensitivity in adipose tissue vs. liver or muscle lead to increased lipogenesis and fat storage. The sharp insulin response and decline in blood glucose, possibly below baseline is interpreted in the brains as a state of “cellular semistarvation” and responds with a counter-regulatory hormone response and hunger and cravings for high-GL foods. Energy expenditure may also decline related to decreased fuel availability. Overall, this model proposes that fat storage due to high insulin sensitivity and cellular semistarvation lead to obesity. GI; gastrointestinal; GIP; glucose-dependent insulinotropic polypeptide; GL, glycemic load.
2. Impact of chronic dietary patterns and macronutrients on sleep quality: Epidemiological studies
2.1. Diets linked to inflammation and plant based diets
Epidemiological studies have evaluated dietary profiles linked to inflammation, such as the Dietary Inflammatory Index (DII®) which consists of a literature-derived score based on the potential of various foods to raise inflammatory markers such as C-reactive protein, interleukin-1ß, 6, and 10, and tumor necrosis factor-alpha. The DII dietary pattern is characterized by 45 different nutrients and food ingredients that have been shown to either increase or decrease markers of inflammation (28). Refined grains and simple carbohydrates are considered pro-inflammatory whereas fiber and n-3 fatty acids, for example, are associated with lower levels of inflammation (29). One study, conducted in Italian adults, showed that those scoring in the highest quartile of the DII had lower odds ratio (OR=0.49) for adequate sleep quality (as measured by the Pittsburg Sleep Quality Index (PSQI)) compared to those reporting consuming a lower inflammatory diet (30). Individuals with inadequate sleep quality, poor self-rated sleep quality, longer sleep onset latency (SOL), and lower sleep efficiency had higher scores on the DII than those reporting better sleep. The authors concluded that interventions to improve sleep quality should include a diet component that would lower chronic systemic inflammation (30). Data from the NHANES waves 2005–2008 and 2015–2018 also revealed that a higher DII was associated with higher risk of obstructive sleep apnea (OSA) (31) and pooled data from the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study showed an increased risk of developing OSA in participants reporting greater adherence to the Empirical Dietary Inflammatory Pattern (32).
Plant-based dietary patterns have also been studied in relation to sleep quality. One study examined the association of a plant-based dietary pattern with sleep and inflammation in 390 women, age 28–48 y, with overweight and obesity (33). Women reporting consuming a higher plant-based dietary pattern had higher intakes of carbohydrates and polyunsaturated fats but lower intakes of protein, fats, fiber and other micronutrients. When women were further categorized as having a healthy vs an unhealthy plant-based dietary pattern (as determined by a plant-based diet index from a food frequency questionnaire), women with a healthy plant-based dietary pattern had lower intakes of protein and saturated fats and higher intakes of fiber, monounsaturated fat and other micronutrients. The healthy plant-based dietary pattern was negatively associated, and the unhealthy pattern positively associated with C-reactive protein (a protein marker of inflammation produced by the liver). Furthermore, consumption of an unhealthy dietary pattern and C-reactive protein were both associated with poorer sleep quality on the PSQI. The authors concluded that consuming an unhealthy plant-based diet was associated with inflammation and poor sleep quality and suggested that healthy plant-based diets provide antioxidant compounds that may improve sleep quality.
Beyond those dietary patterns, a community study of males in South Australia noted that those consuming a dietary pattern characterized by high intakes of vegetables, fruits, and legumes had shorter SOL compared to those consuming a dietary pattern characterized by high intakes of processed meat, snacks, red meat and take away foods (34). Similarly, Katagiri and colleagues noted that Japanese female workers with higher intakes of vegetables had good sleep quality whereas those with higher intakes of confectionaries had poor sleep quality (35). And, high frequency of energy or sugar-sweetened beverage consumption was associated with worse sleep quality. In prospective analyses of the UK Women’s Cohort Study, various fruits and vegetables were inversely associated with sleep duration at 4-y follow-up (36). However, sleep duration was assessed from a single, unvalidated question asking how the participants spent their day on weekdays and weekends.
2.3. Mediterranean diet
The Mediterranean diet is another plant-rich diet characterized by high intake of fruits, vegetables, whole grains, and olive oil that are rich sources of polyphenolic compounds with anti-inflammatory and antioxidant properties (37). Higher adherence to a Mediterranean diet has been associated with better sleep quality, lower SOL, fewer sleep disturbances, and less daytime dysfunction in young college students (38). In older adults aged ≥60 y, greater adherence to a Mediterranean diet was associated with lower risk of large changes in sleep duration over a 2.8 y follow-up period and lower risk of reporting 2–3 indicators of poor sleep quality in cross-sectional analyses (39). Higher Mediterranean diet adherence was associated with lower risk of early morning awakening, sleep medication use, and poor overall sleep quality. Similarly, in a 1-y prospective evaluation of the association between Mediterranean diet adherence and sleep quality in women, Zuraikat et al. showed that a higher score on the alternate Mediterranean diet (Mediterranean diet adapted to US populations) was related to better overall sleep quality (measured by PSQI), higher sleep efficiency, and fewer sleep disturbances (40). Associations were similar for fruits and vegetables. In addition, higher intakes of legumes were associated with higher sleep efficiency and intakes of legumes and dark breads tended to be associated with better sleep quality. More recently, we further demonstrated that the amelioration of sleep quality via Mediterranean-type diet food components is an effect robust enough as to be detected cross-sectionally in a small cohort (n=34) of adequate sleepers (unpublished data). We observed that, consumption of a diet higher in fruits, vegetables, carbohydrates (quality and quantity not measured), fiber, and magnesium, (using 24-hour diet recall) were associated with less fragmented sleep measured by the Sleep Fragmentation Index (SFI). Using data from the Multi-Ethnic Study of Atherosclerosis (MESA), we also showed that a higher alternative Mediterranean diet score was associated with higher likelihood of sleeping 6–7 h/night vs <6 h/night (as measured by actigraphy) and with lower odds of insomnia symptoms (41). An increase in the diet score over ~10 y of follow-up was associated with lower likelihood of reporting <6 h of sleep/night (OR=0.77) in a fully-adjusted model (including exercise, total energy intake, BMI, hypertension, diabetes, depression, among others).
The Mediterranean diet is high in fiber and unsaturated fats and could be considered a low-glycemic index (GI) diet (42). Data from the Women’s Health Initiative revealed that higher dietary GI was associated with prevalent and incident insomnia (43) in fully-adjusted models. Specifically, higher intakes of added sugars, starch, and refined grains were associated with higher odds of developing insomnia. In a combined analysis of two population studies from Iran, consuming a higher GL diet was associated with higher odds of long sleep duration (>8 h/night) in fully-adjusted models (44). In that study, dietary GI was not related to sleep duration.
2.4. Time of dietary intake
While studies described above examined overall dietary patterns, Crispim and colleagues focused specifically on evening food intakes and their association with sleep (45). Fifty-two adults reported food intake using a 3-d food diary and underwent a sleep study with polysomnography. Negative associations between evening fat intakes and sleep efficiency and rapid-eye movement sleep were noted in men and women and positive associations with SOL and wake after sleep onset were observed in men. In women, higher fat intakes in the evening were associated with longer REM sleep latency, stage 2 sleep and wake after sleep onset, and lower sleep efficiency. Polysomnography was also employed in an intervention study by St-Onge et al. (46) in which participants consumed a controlled diet for 4 d followed by a self-selected diet for one day in an inpatient setting. The participants’ self-selected diet contained more total energy and saturated fat than the controlled diet. Participants fell asleep more quickly while consuming the controlled diet and they obtained more slow wave sleep (SWS) than after their self-selected diet. Nutrients contributing to differences in sleep stages when participants self-selected their diet included saturated fat, which was associated with less SWS; sugar and refined carbohydrates, which were associated with more arousals; and fiber, which was associated with more SWS. These findings suggest that the fat and carbohydrate quality of the diet may be important for sleep, although the study was not originally designed to test this question. Nonetheless, studies presented above suggest that various dietary patterns rich in specific foods and nutrients play a role in sleep quality, but clinical intervention studies are needed to effectively demonstrate causality.
3. Acute intake of macronutrients and sleep quality: Intervention studies
Dietary intervention studies testing the impact of various dietary macronutrient profiles on sleep have been performed (Table 1). Herein, we searched for intervention studies that compared the impact on sleep of dietary carbohydrate intakes. Studies that did not provide information on the macronutrient composition of the diet, or tested a specific food, were not included. We did not consider specific individual foods (for e.g. specific fruits associated with melatonin production like cherries) nor did we focus on specific ingredients intended to supplement ones diet by consumption of a pill, capsule, or liquid. In general, studies evaluated provide controlled diets, matched in energy content. Inevitably, altering the contribution of one macronutrient to the energy content of the diet requires a change in at least one other macronutrient. Here, we focus on studies that vary carbohydrate content in addition to fat or protein, keeping the alternate nutrient, either fat or protein, unchanged.
Table 1.
Clinical intervention studies of the impact of dietary carbohydrate content on sleep.
| Author | Sample characteristics | Study design* | Diet composition (%En) | Sleep outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CHO | Fat | Protein | Carbohydrate: Protein ratio | SWS | REM | SOL | WASO | Sleep quality | |||
| Phillips et al, 1975 (47) | Young Healthy Normal weight Sex: M (n=8) | 4 days Crossover Fixed diet | 80 | 10 | 10 | 8 | - | - | |||
| 57.5 | 42.5 | 10 | 5.8 | ↓ | ↑ | ||||||
| 13.3 | 76.6 | 10 | 1.3 | - | - | ||||||
| Lindseth et al., 2016 (50) | Young Healthy Normal weight/overweight Sex: M (n=32) F (n=4) | 4 days Crossover Controlled energytailored diet | 80 | 10 | 10 | 8 | ↓ | - | |||
| 50 | 35 | 15 | 3.3 | - | - | ||||||
| 40 | 15 | 45 | 0.9 | - | - | ||||||
| 25 | 65 | 10 | 2.5 | - | ↑ | ||||||
| Yajima et al., 2014 (49) | Young Healthy Normal weight Sex: M (n=10) | Dinner Crossover Controlled energytailored meals | 80 | 10 | 10 | 8 | ↓(1st cycle only) | ||||
| 12 | 10 | 10 | 1.2 | - | |||||||
| Porter et al., 1981 (48) | Young Healthy Normal weight Sex: M (n=6) | 3 days Crossover Fixed calorie bedtime snack | 72.8 | 22.7 | 4.5 | 16.2 | ↑ (1st half of night) | ||||
| 46.9 | 47.1 | 6 | 7.8 | ↑ | - | ||||||
| 0 | 0 | 0 | - | - | |||||||
| Zhou et al., 2016 (52) | Middle-aged Overweight/obesity Sex: M (n=3) F (n=11) | 4 weeks Crossover Energy-restricted diets Self-prepared diets | 65 | 25 | 10 | 6.5 | - | ||||
| 55 | 25 | 20 | 2.8 | ↑ | |||||||
| 45 | 25 | 30 | 1.5 | ||||||||
| Afaghi et al., 2008 (58) | Young Healthy Normal weight/overweight Sex: M (n=14) | 2 days Crossover Fixed diet | 60 | 25 | 15 | 4.0 | |||||
| 1 | 61 | 38 | 0.03 | ↑ | ↓ | ||||||
| Karl et al., 2015 (55) | Young Normal weight/overweight Physically fit Sex: M (n=32) F (n=7) | 10 days Crossover Energy-restricted diets Controlled diets | 59 | 31 | 10 | 5.9 | - | - | |||
| 51 | 30 | 19 | 2.7 | - | - | ||||||
| 40 | 30 | 30 | 1.3 | - | - | ||||||
| Lindseth et al., 2013 (56) | Young Healthy Normal weight/overweight Sex: M & F (n=44; sex distribution not available) | 4 days Crossover Controlled energytailored diet | 56 | 22 | 22 | 2.5 | ↓ | - | |||
| 50 | 35 | 15 | 3.3 | - | - | ||||||
| 22 | 56 | 22 | 1 | - | |||||||
| 22 | 22 | 56 | 0.4 | ↓ | |||||||
| Iacovides et al., 2019 (54) | Young Healthy Normal weight Sex: M (n=1) F (n=10) | 3 weeks Crossover Energy-tailored meal plans | 55 | 20 | 25 | 2.2 | - | ||||
| 15 | 60 | 25 | 1.7 | - | |||||||
| Saidi et al., 2022 (53) | Young Healthy Normal weight Sex: M (n=24) | 3 days Crossover Controlled energytailored diet | 51 | 11 | 38 | 5 | - | - | ↓ | - | |
| 42 | 20 | 38 | 2.3 | - | ↓ Delayed latency | - | - | ||||
| Kwan et al., 1986 (59) | Young Healthy Normal weight Sex: F (n=6) | 1 week Crossover Self-selected diets with meal plan | 50 | 38.7 | 13 | 3.8 | - | ||||
| 9.5 | 71.5 | 12.4 | 0.8 | Delayed latency | |||||||
| Watson et al., 2018 (57) | Young to older age Overweight/obesity Type 2 diabetes Sex: M (n=33) F (n=28) | 12 weeks weight loss + 12 weeks weight maintenance Parallel Self-selected diets with dietitian consultation | 48 | 22 | 20 | 2.4 | - | ||||
| 34 | 30 | 29 | 1.2 | - | |||||||
Arrows refer to direction of statistically significant difference from the diet with “—”. Grey cells indicate that the measure was not obtained.
Abbreviations: CHO, carbohydrates; F, females; M, males; REM, rapid-eye movement; SOL, sleep onset latency; SWS, slow-wave sleep; WASO, wake after sleep onset.
Fixed diet = non-individualized diets/meals; Energy-tailored = individualized diets/meals based on energy needs
A few studies have tested the impact of very high carbohydrate diets (>70%En) on sleep. In 1975, Philips and colleagues tested the influence of carbohydrates on sleep in young, healthy, normal weight males (47). In a randomized crossover design, participants consumed a balanced diet providing 57.5%En from carbohydrates representing 600g, 42.5%En from fat, and 10%En from protein for two days followed by a high-carbohydrate/low-fat (HC/LF) diet (80%En from carbohydrates and 10%En each from protein and fat) or a low-carbohydrate/high-fat (LC/HF) diet (13.3%En from carbohydrates, 76.6%En from fat and 10%En from protein) for two days. Study periods were separated by a two-week washout. Compared to the balanced and LC/HF diet, the HC/LF diet resulted in less SWS and more REM sleep, measured by electroencephalography.
Porter et al. also observed more REM sleep in healthy young males consuming a HC/LF bedtime snack (72.8%En from carbohydrates consisting of glucose and fried potatoes with 130g of available carbohydrate, 22.7%En from fat and 4.5%En from protein) for three days compared to a lower-carbohydrate/higher-fat snack (47%En each from carbohydrates [47g of available carbohydrate] and fat and 6%En from protein) and a non-caloric control snack (methylcellulose) (48). However, this was only statistically significant in the first half of the night with, according to the authors, full-night data approaching statistical significance (data were not presented in the paper). The lower carbohydrate/higher fat snack resulted in more SWS than the non-caloric control. In this study, snacks were consumed 45 min before bedtime in counterbalanced order. However, energy intakes were not identical and the HC/LF snack provided ~300 kcal more than the lower-carbohydrate/higher-fat snack, and ~700 kcal more than the non-caloric control. Consequently, it is unclear whether results are due to the macronutrient or the energy content of the snacks.
Yajima et al. also tested late-day carbohydrate intakes on sleep and observed differences in sleep architecture in the first half of the night (49). Healthy young males consumed isocaloric dinners differing in carbohydrate and fat content. There was less SWS in the first sleep cycle following a HC/LF dinner (80%En from carbohydrates consisting of boiled rice and bread (type, refined or whole-grain, was not specified), 10%En each from fat and protein) compared to a LC/HF dinner (12%En from carbohydrate, 78%En from fat mainly from dairy products and 10%En from protein). SWS was detected in the first three cycles of the night following the HC/LF dinner compared to two for the LC/HF. However, overall, total SWS did not differ between conditions. Despite the lack of difference in SWS over a full night’s sleep, these data may have implications for those restricting sleep by delaying bedtimes or advancing waketimes. If sleep is curtailed due to early awakening, a LC/HF dinner may be preferable to a HC/LF dinner in order obtain a full amount of SWS in fewer sleep cycles. On the other hand, if sleep is reduced by delaying bedtimes, then a HC/LF may be advisable to achieve more later-night SWS. The influence of timing of macronutrient intake on sleep deserves further attention.
One study evaluated perceived sleep quality following various dietary manipulations, including a HC/LF diet, for four days each in young healthy adults (n=32 males, 4 females) (50). Experimental diets provided 10%En from protein and 80, 40, or 25%En from carbohydrates with corresponding fat intakes of 10, 35, and 65%En. Carbohydrate quality was not specified. Wake times during sleep were shorter after participants consumed the highest carbohydrate diet compared to the other experimental diets and control (50%En from carbohydrates, 35%En from fat, and 15%En from protein). However, the lowest carbohydrate diet was accompanied by better self-reports of sleep quality compared to all other diets. Such findings highlight the need to use both objective and self-reported measures to fully capture the sleep experience of individuals.
Overall, these studies suggest that high dietary carbohydrate intakes, in the context of low overall protein intakes (~10%En or less), result in longer REM sleep (47, 48) but less SWS in the first sleep cycle, possibly spreading SWS over a longer portion of the night (49). This may have implications for one’s ability to dissipate sleep drive when carbohydrate intakes are very high (80%En), potentially more importantly when consumed in the evening meal. Carbohydrate quality was not reported in these studies and timing relative to sleep was also not usually presented. Afaghi and colleagues have shown that a high GI meal (768 kcal, 90.4%En from carbohydrates, 1.6%En from fat, and 8%En from protein) consisting of 600g steamed rice and 200g steamed vegetables in tomato puree, consumed 4 h before bedtime resulted in shorter SOL compared to a low GI meal of same caloric content and macronutrient distribution (51). Similarly, SOL was lower when the high GI meal was consumed 4 h compared to 1 h before bedtime, highlighting the importance of carbohydrate quality and timing of intake on sleep quality. However, all prior studies are limited by their inclusion of primarily young, healthy, normal weight males with no sleep problems. Moreover, studies with objective sleep measures had small sample sizes, ≤10 (47–49).
Other studies examined the impact of moderate carbohydrate intakes (40–65%En) on sleep at night. Zhou and colleagues compared three moderate carbohydrate diets, 65, 55, and 45%En, varying in protein and with fixed fat content (25%En) consumed as part of a weight loss program (52). The sources of carbohydrate were 20% grains, breads, and flours; and 25% other (i.e., vegetables, fruit, nuts, and beverages). Participants were middle-aged adults with overweight or obesity who consumed each diet consecutively for four weeks each, in random order. Participants rated their sleep quality as best when consuming the diet providing 55%En from carbohydrates and 20%En from protein. Increasing protein intakes at the expense of carbohydrates did not further improve sleep quality and there was no difference between the lower and higher carbohydrate diets (45 and 65%En from carbohydrates with 30 and 10%En from protein, respectively). On the other hand, Saidi et al. tested two controlled diets differing in carbohydrate and protein content and reported better sleep quality after three days of consuming a lower-protein/higher-carbohydrate diet (51%En from carbohydrates, 11%En from protein and 38% from fat; carbohydrate quality was not specified) compared to a higher-protein/lower-carbohydrate diet (42%En from carbohydrates, 20%En from protein and 38% from fat) (53). Change in SOL and sleep efficiency differed between diet conditions with worsening effect from the lower carbohydrate diet, which also increased REM onset latency and cortical arousals, measured using polysomnography, relative to the higher carbohydrate diet. Saliva melatonin levels, measured under dim light conditions, increased to a greater extent in the higher carbohydrate condition as well.
Four other studies tested diets with ~55%En from carbohydrates (54–57). In one study, young-to-middle-aged adults with no sleep problems consumed four different controlled diets for four days each, in random order, separated by two-week washout periods (56). Sleep was assessed by actigraphy and questionnaire. Diets provided 56%En from either protein, carbohydrates, or fat with the two remaining macronutrients providing each ~25%En. A control diet provided 50%En from carbohydrates, 35%En from fat, and 15%En from protein. Carbohydrate and fat quality were not specified. Compared to the control diet, SOL was shorter during the higher carbohydrate diet whereas fewer wake episodes during sleep were noted with the higher protein diet. Given the similar contribution of carbohydrates to the overall dietary profile between the higher carbohydrate and the control diet, it is unclear whether the benefit to sleep stemmed from consuming more carbohydrates or less fat. St-Onge et al. reported longer SOL when participants consumed a higher %En from saturated fat (46). Another possibility is that the higher protein content played a role in SOL. However, based on the data from Zhou et al. (52), this seems unlikely.
The other three studies did not note any differences in sleep quality, assessed by questionnaire, between dietary conditions (54, 55, 57). One study was conducted in adults with overweight or obesity and T2D, comparing diets providing ~48%En from carbohydrates, 22%En from fat, and 20%En from protein vs 34, 30, and 29%En from carbohydrates, fat, and protein, respectively, in the context of weight management (57). Carbohydrate quality was not specified whereas participants were counseled to consume <10%En from saturated fat. No differences were noted in ratings of sleep quality on the Leeds Sleep Evaluation Questionnaire. The other two studies enrolled healthy adults with normal weight and diets were provided for three weeks each (54, 55). Karl et al. varied carbohydrate (28 to 55%En; type was not specified) and protein (14 to 42%En) content with fat intakes fixed at 30%En (55) whereas Iacovides and colleagues fixed protein content at 25%En and varied carbohydrate and fat (55 vs 15%En from carbohydrates and 20 vs 60%En from fat) to induce nutritional ketosis (54). The lack of effect of nutritional ketosis on sleep observed by Iacovides and colleagues (54) differs from that of Afaghi and colleagues (58) who noted more SWS and less REM sleep with a two-day ketogenic diet providing < 1%En from carbohydrates, 61%En from fat, and 38%En from protein, relative to the control diet (60%En from carbohydrate, 25%En from fat, and 15%En from protein). However, arousal index was higher during non-REM sleep after a single ketogenic evening meal compared to control (58). This did not persist after two days on this diet. All participants were young, healthy males with normal weight and good sleep. The authors suggested that REM sleep is reduced on a ketogenic diet due to the higher metabolic demand for glucose by the brain during this sleep stage and that SWS could be increased to offset the fatigue experienced at onset of a very low carbohydrate diet. Whether extremes of carbohydrate and fat intakes are necessary to observe such effects remains to be replicated and tested.
One additional study was conducted in females only (n=6) to test the impact of a very low carbohydrate (<50 g/d; 9.5%En from carbohydrates, 71.5%En from fat, and 12.4%En from protein) compared to a typical diet (50%En from carbohydrates, 38.7%En from fat, 13%En from protein) on sleep (59). The test diet was consumed after the typical diet; both were followed for one week each. Total sleep time was 34 min shorter after the very low carbohydrate diet although this was not statistically significant. On the other hand, the authors noted significantly longer REM sleep latency and fewer REM sleep episodes with the very low carbohydrate diet compared to the typical diet. The authors suggested that brain tryptophan may have been reduced due to the very low carbohydrate intakes and also raised the possibility that findings were due to differences in fat intakes, which were necessary to maintain isocaloric intakes between diet conditions. These data support those of Crispim and colleagues (45) who noted a positive association between nocturnal fat intake and REM sleep latency in women. However, given the higher metabolic needs in REM sleep (60), and the brain’s reliance on carbohydrate as its major energy source, it is possible that reduced carbohydrate intakes limit REM sleep.
Studies to date show that very high carbohydrate intakes decrease wake during sleep (50) and may alter the distribution of SWS (49) and REM sleep (48) across sleep cycles in young, healthy males. Data on the impact of moderate intakes of carbohydrates on sleep are mixed with some finding decreased SWS and increased REM (47), decreased SOL (56), and improved (52) or unchanged (54, 55, 57) sleep quality. It is important to note that all studies but three (52, 54, 57) included only males or fewer than 20% of females and those three were the only ones conducted for >2 weeks. From these three studies, Zhou and colleagues found improvements in sleep quality ratings with a diet providing 55%En from carbohydrates and 20%En from protein compared to one providing 65%En from carbohydrates and 10%En from protein for four weeks (52). The other two studies did not show improvements in sleep quality over a three week (54) and 12-week (57) period with diets containing 48–55%En from carbohydrates.
Besides study duration, it may be that the carbohydrate to protein ratio is important when evaluating the impact of macronutrient intakes on sleep quality. Three studies with a carbohydrate to protein ratio of 8 showed less SWS overall (47) and in the first sleep cycle (49) and less wake during sleep (50) compared to diets with a lower ratio, whereas one found more SWS (48) compared to a non-caloric control. Studies with carbohydrate to protein ratios of 2.5–2.8 report shorter SOL (56) and improved self-reported sleep quality (50, 52) compared to higher (50, 52) and lower (50) ratios. However, three studies with carbohydrate to protein ratios of 2.2–2.7 (54, 55, 57) did not note differences among dietary conditions on sleep quality and one study noted improved sleep architecture with a carbohydrate to protein ratio of 5 vs 2.27 (53). Three of those were conducted in young, healthy adults (53–55) and one among patients with overweight or obesity and T2D (57).
Future studies should enroll adequate numbers of males and females, last longer than two weeks, and include individuals with poor sleep quality, who likely stand to benefit from a dietary intervention to improve sleep. Attention should also be paid to carbohydrate to protein ratio and macronutrient quality, such as the glycemic potential of carbohydrates, tryptophan content of proteins, and fatty acid saturation profile.
5. Mechanisms of macronutrient effects on sleep and interactions with energy metabolism
Carbohydrate, protein and fat may influence exert an impact on sleep parameters via differing but interacting mechanisms. Additionally, the means by which macronutrients act on the body to influence sleep may be impeded or facilitated by the metabolic status of the individual.
The role of protein on sleep has been largely attributed to the fact that the essential amino acid tryptophan (Trp) is the sole precursor for the biosynthesis of the neurotransmitter serotonin and, subsequently, the pineal hormone melatonin. Trp is transported into cells via the large neutral amino acid (LNAA) transporter where it competes with other LNAAs (valine, leucine, isoleucine, tyrosine, phenylalanine and methionine) for entry into the brain. Other LNAAs are naturally more abundant in whole foods than Trp (61), thus limiting access of Trp to the brain via competition to the carrier-binding site (62). The Trp/LNAA ratio may be modulated partially by the concurrent administration of carbohydrates, promoting insulin secretion, which in turn drives the uptake of LNAAs (excluding Trp) into muscle tissue (63). The resultant increase in Trp/LNAA ratio in plasma facilitates Trp access to the brain for serotonin and melatonin synthesis (64, 65). Conversion of Trp to melatonin is demonstrated by the rise in 6-hydroxymelatonin sulfate, a metabolite of melatonin, with Trp supplementation and decrease with its depletion (66). This effect mirrored by the consumption of a high GI meal compared with a low GI meal (51). It has also been suggested that dietary fat-mediated release of cholescystokinin may directly act on insulin secretion to promote the uptake of LNAAs into muscle tissue (67). Thus, carbohydrates and fats may mediate the effect of protein-derived Trp uptake and metabolism to serotonin and melatonin (67) (Figure 2).
Figure 2.
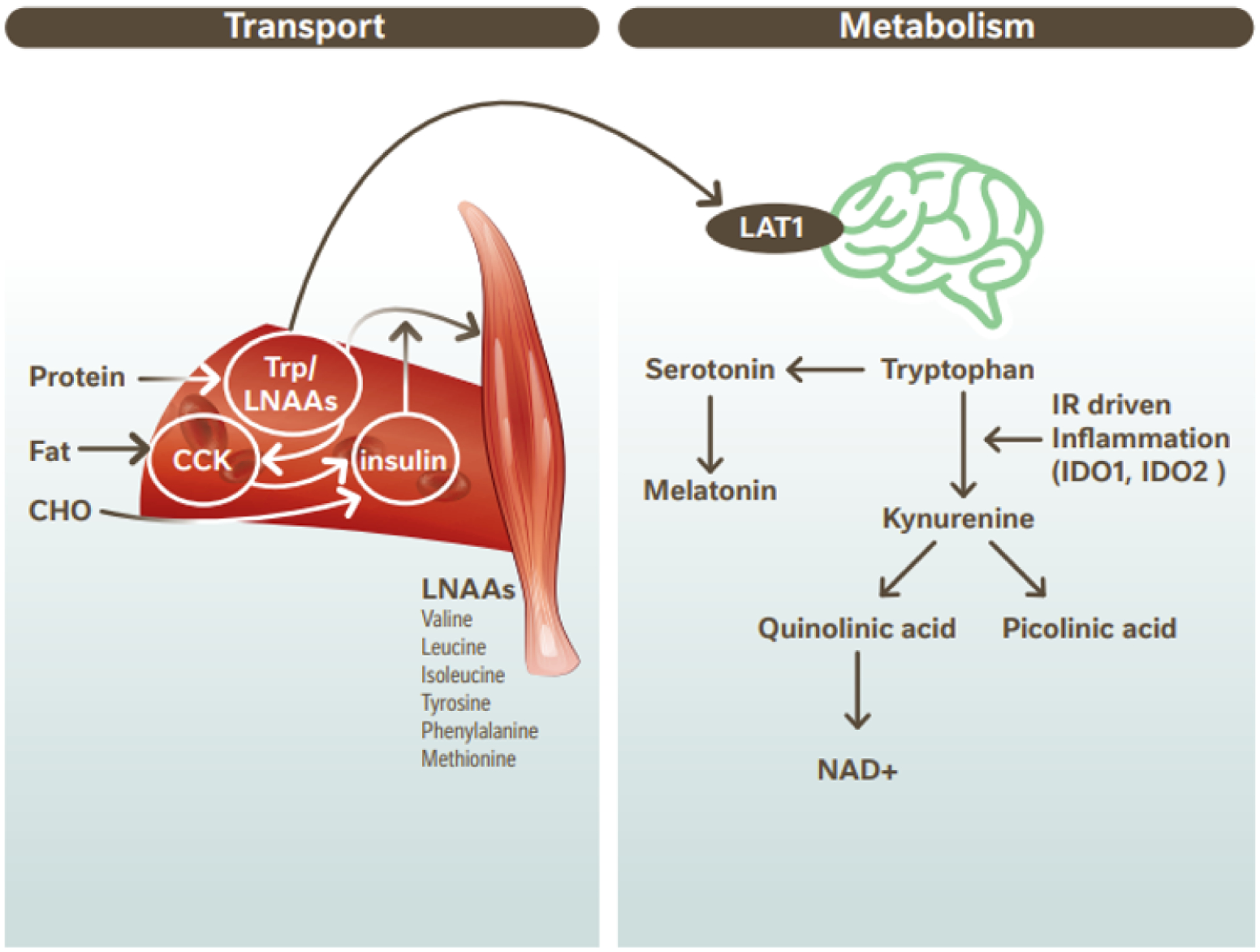
Transport and metabolism of dietary Trp in the CNS. Trp is derived from dietary protein along with other LNAAs. Transport of Trp/ LNAAs compete at Large Neutral Amino Acid Transporter (LAT1) sites. CHO ingestion leads to insulin secretion and dietary fat increases CCK which may also induce insulin secretion. Elevated insulin drives LNAA into muscle tissue excluding Trp. Trp transport into the brain is thus upregulated. In the brain Trp is metabolized to serotonin and melatonin or is metabolized via the kynurenine pathway. In individuals with obesity-induced inflammation leading to IR, the kynurenine pathway is upregulated. Diagram adapted from Cheon & Kim (67) CCK, cholecystokinin; CHO, carbohydrate; CNS, central nervous system; IDO, indolamine-2,3-dioxygenase; IR, insulin resistance; Trp; Tryptophan; LAT1, large neutral amino acid transporter 1; LNAA, large neutral amino acids.
It should be noted that for individuals with preexisting metabolic disorders, Trp supplementation may be less effective at elevating melatonin and serotonin. Trp is metabolized either to serotonin and melatonin or via the kynurenine pathway (see figure 2). Insulin resistance and metabolic syndrome are associated with chronic inflammation, pro-inflammatory cytokines and transcriptional induction of enzymes indoleamine 2,3-dioxygenase (68). These inflammatory molecules drive metabolism of Trp via the kynurenin pathway and both clinical and experimental data suggest that Trp metabolism is increased in T2D (69, 70). Consequently, it appears that metabolic disorders result in enhanced dominance of the Trp-kynurenin pathway with less Trp available for the production of serotonin and melatonin in these individuals.
Regardless of pre-existing metabolic disorders, a recent large prospective cohort study of 7,908 participants from the China Health and Nutrition Survey (1997–2011) demonstrated that dietary Trp was significantly associated with both decreased risk of T2D and positively associated with sleep duration (71), ultimately implicating the consumption of dietary sources of Trp as a prudent strategy to confer both metabolic and sleep benefits.
6. Glucose metabolism
6.1. Impact of glucose metabolism on sleep architecture
Given the observations that evening carbohydrate ingestion has the capacity to alter sleep architecture, it is relevant to consider the normal profile of glucose metabolism. The brain is the most metabolically active organ in the body, consuming ~20% of glucose (~5.6 mg glucose/100 g human brain tissue/ min) (72). As such, the brain is particularly vulnerable to disrupted energy supply hence the need for tight regulation of peripheral glucose. This preservation of glucose homeostasis to a daytime fasting range of 3.9–5.6 mmol/L is accomplished largely by the opposing and balanced actions of glucagon and insulin in response to glucose fluctuations. While we tolerate transient elevations beyond the fasting homeostatic range without immediate decrement e.g. following consumption of a meal where glucose values may peak up to 7.8 mmol/L in healthy adults (73), acute decrements in blood glucose below 3.0 mmol/L can have a profound impact. Nocturnal glycemia oscillations differ from daytime values in that they more closely over around 5.0 mmol/L (74) which may be due to differences in daytime and nightime systemic glucose turnover.
Boyle et al. (75) were the first to concurrently and continuously evaluate the relationship between brain glucose metabolism and systemic glucose turnover during sleep. Internal jugular and radial artery cannulation were used to determine cerebral blood flow and quantitate cross-brain glucose and oxygen differences before and every 3 h during sleep. Concurrent systemic glucose turnover was estimated by isotope dilution and sleep stage measured by continuous electroencephalogram (EEG). Energy metabolism during sleep was characterized by a decline in systemic glucose utilization driven by a reduction in brain glucose metabolism and brain activity compared to the awake state. Interestingly, this decline in brain glucose metabolism was independent of stage of brain EEG activity but was linked to duration of sleep. In a later study, Katayose et al (76) measured metabolic rate and fuel utilization during sleep using whole-body indirect calorimetry and found that energy expenditure decreased during the first half of night, mostly due to a reduction in fat oxidation from sleep onset, while carbohydrate oxidation was maintained in young, healthy males. Carbohydrate oxidation during REM was higher than during SWS. Bialasiewicz et al. (77) observed that during REM sleep, decreasing concentration of interstitial fluid glucose (which correlates closely blood glucose), as measured by continous glucose monitor (CGM), was observed in subjects with normal glucose tolerance. These authors suggest that the REM-related decline in glucose concentrations may be a risk factor for nighttime hypoglycemia. It is important to note that at night people tend to become more insulin resistant at night than in the daytime, particularly the as we age (78). Hence it is important to focus attention on carbohydrate quality and quantity particularly in evening meals.
Taken together it appears that peripheral blood glucose levels fall across the night and that REM sleep may be associated with greater central nervous system glucose utilization and corresponding lower systemic glucose. A more detailed account of nocturnal metabolism has been reported elsewhere (79). Previous studies have observed that glycemic variability (standard deviation and coefficient of variation) in adults with type 1 diabetes is associated with objectively measured sleep quality (80) but little is known about the existence of this effect in healthy populations.
6.2. Impact of nocturnal glycemic response on an evening meal
In the study described above by Boyle et al. (75), reduced rates of glucose turnover were shown at ~10 h following the last meal suggesting that a portion of systemic glucose production after a meal is caused by ongoing glucose absorption. However, little is known about the impact of the meal GL on the postprandial response through the night and the impact on acute glucose excursions. One study evaluated the effect of various late evening meal patterns on glycemic excursions in patients with T2D (81). In this randomized, cross-over study, 16 patients wore a CGM for five days and consumed identical test meals for three days. The test meal manipulations differed by administration times: entire dinner at i) 6 pm or ii) 9 pm; iii) divided dinner (vegetable and rice at 6 pm; vegetable and main dish at 9 pm). When dinner was consumed at 9 pm, incremental glucose area under the curve was higher through the night and resulted in a greater incremental glucose peak when compared to dinner consumed at 6 pm. Moreover, the mean amplitude of glycemic excursion when dinner was eaten at 9 pm tended to be higher than that following the 6 pm dinner. Importantly however, the divided dinner significantly reduced incremental area under the curve, incremental glucose peak and the mean amplitude of glucose excursions compared to the single meals.
Similarly, meal timing also appears to affect nocturnal metabolism, with dinner consumed closer to bedtime (at 10 pm) leading to a 4-h shift in the postprandial period and higher postpandrial glucose, delay in triglyceride peak, and lower postpandrial free fatty acids and dietary fatty acid oxidation than meals consumed earlier in the evening (at 6 pm) (82). This effect is likely, due in part, to the suprachiasmatic nucleus mediated circadian release of hormones that directly affect metabolism and glucose tolerance (for review see (83)). Taken together it appears that that late-night consumption of dinner may lead to postprandial hyperglycemia during the night, and that this hyperglycemia can be negated by either earlier meal consumption or divided intake of an evening meal (81).
While it remains unknown whether nocturnal glucose excursions correspond to nighttime awakenings or other sleep quality parameters, it has been observed that increased nighttime awakenings and fragmented sleep occur when the evening meal is consumed within 3 h of bedtime (84). It is conceivable that homeostatic processes relying on peripheral glucose-sensing interact or overlap with systems that are capable of triggering nighttime arousals.
7. Putative mechanisms by which nocturnal glucose regulation and insulin resistance may impact sleep quality
The mechanism/s of action delineating the role of GL and nocturnal glycemia on sleep remain to be fully elucidated. Plausibly, high glycemic response to an evening meal and compensatory hyperinsulinemia, may result in mild reactive hypoglycemia (85). Cryer et al. (86) note that critical glucose counterregulatory systems are activated at glycemic thresholds at which brain glucose uptake is first measurably reduced (~3.8 mmol/l), well above the thresholds for symptoms of hypoglycemia (~3.0 mmol/l) and neuroglycopenic cognitive dysfunction (~2.7 mmol/l). The secretion of autonomic counterregulatory hormones such as adrenaline, cortisol, glucagon, and growth hormone may initiate responses typical of elevation of these hormones: heart palpitations, tremor, cold sweats, anxiety, irritability, and hunger, which could plausibly produce arousals and reduce sleep quality. Indeed, hypoglycemic events have been shown to cause awakenings during sleep, compromising sleep efficiency even in healthy adults (87).
Additionally, we have previously proposed that a high-GI diet may increase the risk of insomnia through acute nighttime peripheral glucose excursions (spikes and troughs in nocturnal blood glucose) (88). This mechanism may implicate systems and sleep regulating molecules sensitive to peripheral glucose sensing. Here we propose that orexin and orexin receptor activity may, in part, account for the notion that nighttime peripheral blood glucose may perturb sleep duration and architecture.
Orexins A and B are neuropeptides generated from prepro-orexin (89). Orexin receptors, OX1R and OX2R, are expressed in the central nervous system and in the periphery. Orexin A binds to OX1R and OX2R, whereas orexin B selectively binds to OX2R, both with high affinity (90). Orexin-producing neurons are located in the lateral hypothalamic area, perifornical area, and posterior hypothalamus (91). Orexin is associated with the regulation of sleep/wake cycles as well as metabolic function (90, 91). Upon activation, orexin neurons strongly excite various brain nuclei with important roles in wakefulness, including the dopamine, norepinephrine, histamine and acetylcholine systems (92), stabilizing the wake-promoting system and reducing switching between wake and sleep. The mediation of sleep initiation by orexin has been demonstrated by the observation that orexin deficiency causes narcolepsy in humans and animals (93, 94). Additionally, it has been suggested that modulation of orexin signaling might also play a role in regulating sleep architecture via REM cycling (95).
Importantly, orexin neurons can directly sense nutritional status by responding to peripheral metabolic signals, such as glucose, leptin, and ghrelin (96–98). While the function of orexins in metabolic pathways are still to be completely understood, it is possible that hyperglycemic and hypoglycemic episodes exert effects on orexin output via glucose-sensing orexin neurons in the lateral hypothalamic area (99). Acutely, intraperitoneal injections of insulin result in low blood glucose levels (i.e. 1.99 mmol/L in rats) and greater expression of prepro-orexin mRNA and vice versa (90, 100). Elevated glucose concentrations (i.e. 5 mM extracellular glucose in mice brain slices) have been shown to block or silence the electrical activity of orexin neurons in vitro (98). Furthermore, glucose loading leading to hyperglycemia did not affect Hcrt neurons which are orexin-expressing neurons (100). Melanin-concentrating hormone (MCH) and orexin neurons in the lateral hypothalamus exert opposing effects on arousal and metabolism. Burdakov et al. reported that physiologically relevant concentrations of glucose dose-dependently enhance the electrical excitability of hypothalamic MCH neurons by inducing depolarization and increasing membrane resistance (98). Conversely, the same physiological shifts in glucose have the opposite effects on the electrical activity of orexin neurons.
Thus, we propose that glucose-sensing orexin neurons may represent a mechanism by which acute hypoglycemic episodes may trigger nighttime awakening via increased transcription of prepro-orexin and MCH silencing and orexin neuron excitation.
In chronic metabolic conditions, the actions of glucose on orexin neurons may suggest that chronically elevated fasting blood glucose could lead to longer sleep duration or greater daytime sleepiness. Researchers have indeed suggested that daytime sleepiness in obesity is not wholly explained by sleep apnea per se and is partly due to the contribution of diet and interacting endocrine systems (101). Indeed, the impact of chronic hyperglycemia and diabetes has been shown, in the rodent model of diabetes, to downregulate orexin gene expression (102).
Interestingly, orexin neurons behave differently in the presence of other energy-producing molecules and have been described as ‘conditional glucosensors’ with paradoxical regulation of carbohydrate-sensing in the presence of other intracellular fuels such as pyruvate and lactate (103). More specifically, it appears that orexin neurons are only responsive to glucose changes when the levels of other energy molecules are low. In insulin resistance and T2D, both glucose and lactate levels are elevated (104, 105). While high glucose would typically lead to orexin antagonism (neuronal silencing) and sleep promotion, elevated lactate, which precedes insulin resistance (105), may plausibly perturb the sensing of peripheral glucose. This action on orexin neurons may, in part, account for the observation that both short and long night-time sleep may be associated with elevated fasting glucose, with mediation by BMI and energy-producing molecules (106).
Serum orexin concentrations can be altered by improving insulin resistance and insulin sensitivity. Indeed, in a sample of patients with T2D undergoing anti-hyperglycemic treatment for over three months, insulin resistance was negatively correlated with orexin (107), but three-month treatment with antidiabetic drugs metformin and pioglitazone significantly improved insulin sensitivity and increased orexin concentrations by 26% and 14%, respectively. While sleep was not assessed in this study, it seems that interventions to improve insulin resistance, such as dietary modification, may help to ‘normalize’ orexin availability and translate into improved homeostatic regulation of daytime arousal/sleepiness and nighttime sleep outcomes.
8. Summary and conclusions
From the available literature, there is now a strong evidence from epidemiological studies and randomized controlled trials confirming the relationship between sleep duration and risk of obesity and T2D. Additionally, we have considered the possibility that nocturnal glycemia may itself act as a mediator of sleep quality. While this hypothesis remains to be substantiated, dietary manipulations, including low GI foods at evening meals and attention to the carbohydrate-to-protein ratio of the diet, may confer sleep benefits. It should be noted, however, that several studies failed to report carbohydrate type (i.e available vs total carbohydrates) and timing of intake. Thus, future research in this field should seek to better standardize the intervention and reporting of macronutrient manipulations. Future research may also seek to evaluate the effectiveness of synergistic nutrient strategies to promote sleep quality, with particular attention to carbohydrate quality and content. Mechanistic determinations of the involvement of the orexin system in glycemic excursions, and their repercussions on sleep quality, both objectively and subjectively measured, are warranted. From the perspective of public health awareness, the inclusion and recognition of the role of nutrition for sleep health should be promoted by both health practitioners and communicated more widely to the general public. Effort further needs to be made to understand the complex, and interconnected nature of socioeconomic factors underlying dietary and sleep behaviors. Food availability and socioeconomic influence both dietary and sleep quality. Untangling those issues is difficult in the context of population studies and requires controlled intervention studies to isolate diet as a driver of sleep health.
Research Agenda.
Dietary intervention studies must report macronutrient manipulations beyond intakes as a proportion of energy content and provide information on macronutrient quantity and quality.
Studies evaluating the impact of diet on sleep should include both objective and subjective measures of sleep to capture the full participant experience.
Mechanistic determinations are needed to evaluate the roles of carbohydrate and protein intakes on sleep.
Practice Points.
Sleep evaluations should take into consideration dietary quality, including amount and type of various macronutrients, and their proximity to bedtimes.
For patients with sleep difficulties and at risk of type 2 diabetes, providers should consider that postprandial nocturnal metabolism and peripheral glycemia may affect sleep quality.
Funding sources and conflict of interest:
MPSO is funded in part from R01HL142648 and R35HL155670 and has received consulting honorarium from Nestlé Institute of Health Sciences. Co-authors ACM, CD, KM, FPM, LO are or were employed at Nestlé Institute of Health Sciences at the time of drafting this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats 2020. [Google Scholar]
- 2.Hall KD, Farooqi IS, Friedman JM, Klein S, Loos RJF, Mangelsdorf DJ, O’Rahilly S, Ravussin E, Redman LM, Ryan DH, et al. The energy balance model of obesity: beyond calories in, calories out. The American journal of clinical nutrition 2022;115(5):1243–54. doi: 10.1093/ajcn/nqac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludwig DS, Aronne LJ, Astrup A, de Cabo R, Cantley LC, Friedman MI, Heymsfield SB, Johnson JD, King JC, Krauss RM, et al. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. The American journal of clinical nutrition 2021;114(6):1873–85. doi: 10.1093/ajcn/nqab270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fruhbeck G, Kiortsis DN, Catalan V. Precision medicine: diagnosis and management of obesity. Lancet Diabetes Endocrinol 2018;6(3):164–6. doi: 10.1016/S2213-8587(17)30312-1. [DOI] [PubMed] [Google Scholar]
- 5.Ding M, Keiley MK, Garza KB, Duffy PA, Zizza CA. Food insecurity is associated with poor sleep outcomes among US adults. J Nutr 2015;145(3):615–21. doi: 10.3945/jn.114.199919. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DA, Billings ME, Hale L. Environmental Determinants of Insufficient Sleep and Sleep Disorders: Implications for Population Health. Curr Epidemiol Rep 2018;5(2):61–9. doi: 10.1007/s40471-018-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Miller EE, Prather AA, Robinson WR, Avery CL, Yang YC, Haan MN, Aiello AE. US acculturation and poor sleep among an intergenerational cohort of adult Latinos in Sacramento, California. Sleep 2019;42(3). doi: 10.1093/sleep/zsy246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meza A, Altman E, Martinez S, Leung CW. “It’s a Feeling That One Is Not Worth Food”: A Qualitative Study Exploring the Psychosocial Experience and Academic Consequences of Food Insecurity Among College Students. Journal of the Academy of Nutrition and Dietetics 2019;119(10):1713–21 e1. doi: 10.1016/j.jand.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandner MA, Petrov ME, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. Journal of clinical sleep medicine: JCSM : official publication of the American Academy of Sleep Medicine 2013;9(9):897–905; A-D. doi: 10.5664/jcsm.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ES, Cunningham TJ, Croft JB. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep 2015;38(5):829–32. doi: 10.5665/sleep.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehan CM, Frochen SE, Walsemann KM, Ailshire JA. Are U.S. adults reporting less sleep?: Findings from sleep duration trends in the National Health Interview Survey, 2004–2017. Sleep 2019;42(2). doi: 10.1093/sleep/zsy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tubbs AS, Ghani SB, Valencia D, Jean-Louis G, Killgore WDS, Fernandez FX, Grandner MA. Racial/ethnic minorities have greater declines in sleep duration with higher risk of cardiometabolic disease: An analysis of the U.S. National Health Interview Survey. Sleep Epidemiology 2022;2:100022. [Google Scholar]
- 13.Caraballo C, Mahajan S, Valero-Elizondo J, Massey D, Lu Y, Roy B, Riley C, Annapureddy AR, Murugiah K, Elumn J, et al. Evaluation of Temporal Trends in Racial and Ethnic Disparities in Sleep Duration Among US Adults, 2004–2018. JAMA Netw Open 2022;5(4):e226385. doi: 10.1001/jamanetworkopen.2022.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacaro V, Ballesio A, Cerolini S, Vacca M, Poggiogalle E, Donini LM, Lucidi F, Lombardo C. Sleep duration and obesity in adulthood: An updated systematic review and meta-analysis. Obes Res Clin Pract 2020;14(4):301–9. doi: 10.1016/j.orcp.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Guimaraes KC, Silva CM, Latorraca COC, Oliveira RA, Crispim CA. Is self-reported short sleep duration associated with obesity? A systematic review and meta-analysis of cohort studies. Nutr Rev 2022;80(5):983–1000. doi: 10.1093/nutrit/nuab064. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, Zhang M, Hu D. Dose-response association between sleep duration and obesity risk: a systematic review and meta-analysis of prospective cohort studies. Sleep Breath 2019;23(4):1035–45. doi: 10.1007/s11325-019-01824-4. [DOI] [PubMed] [Google Scholar]
- 17.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes care 2010;33(2):414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes care 2015;38(3):529–37. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 19.Lin CL, Chien WC, Chung CH, Wu FL. Risk of type 2 diabetes in patients with insomnia: A population-based historical cohort study. Diabetes Metab Res Rev 2018;34(1). doi: 10.1002/dmrr.2930. [DOI] [PubMed] [Google Scholar]
- 20.Capers PL, Fobian AD, Kaiser KA, Borah R, Allison DB. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obesity reviews : an official journal of the International Association for the Study of Obesity 2015;16(9):771–82. doi: 10.1111/obr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Lu J, Jia P, Liu C, Cheng J. Experimental sleep restriction effect on adult body weight: a meta-analysis. Sleep Breath 2019;23(4):1341–50. doi: 10.1007/s11325-019-01828-0. [DOI] [PubMed] [Google Scholar]
- 22.Covassin N, Singh P, McCrady-Spitzer SK, St Louis EK, Calvin AD, Levine JA, Somers VK. Effects of Experimental Sleep Restriction on Energy Intake, Energy Expenditure, and Visceral Obesity. Journal of the American College of Cardiology 2022;79(13):1254–65. doi: 10.1016/j.jacc.2022.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. The American journal of clinical nutrition 2014;100(3):938–47. doi: 10.3945/ajcn.114.085191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenton S, Burrows TL, Skinner JA, Duncan MJ. The influence of sleep health on dietary intake: a systematic review and meta-analysis of intervention studies. J Hum Nutr Diet 2021;34(2):273–85. doi: 10.1111/jhn.12813. [DOI] [PubMed] [Google Scholar]
- 25.Hill JO, Wyatt HR, Peters JC. The importance of energy balance. European endocrinology 2013;9(2):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sondrup N, Termannsen AD, Eriksen JN, Hjorth MF, Faerch K, Klingenberg L, Quist JS. Effects of sleep manipulation on markers of insulin sensitivity: A systematic review and meta-analysis of randomized controlled trials. Sleep medicine reviews 2022;62:101594. doi: 10.1016/j.smrv.2022.101594. [DOI] [PubMed] [Google Scholar]
- 27.Jayedi A, Soltani S, Motlagh SZ, Emadi A, Shahinfar H, Moosavi H, Shab-Bidar S. Anthropometric and adiposity indicators and risk of type 2 diabetes: systematic review and dose-response meta-analysis of cohort studies. BMJ 2022;376:e067516. doi: 10.1136/bmj-2021-067516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17(8):1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hebert JR. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr 2009;139(12):2365–72. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godos J, Ferri R, Caraci F, Cosentino FII, Castellano S, Shivappa N, Hebert JR, Galvano F, Grosso G. Dietary Inflammatory Index and Sleep Quality in Southern Italian Adults. Nutrients 2019;11(6). doi: 10.3390/nu11061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melaku YA, Reynolds AC, Appleton S, Sweetman A, Shi Z, Vakulin A, Catcheside P, Eckert DJ, Adams R. High-quality and anti-inflammatory diets and a healthy lifestyle are associated with lower sleep apnea risk. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 2022;18(6):1667–79. doi: 10.5664/jcsm.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Tabung FK, Stampfer MJ, Redline S, Huang T. Overall diet quality and proinflammatory diet in relation to risk of obstructive apnea in three prospective US cohorts. The American journal of clinical nutrition 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pourreza S, Khademi Z, Mirzababaei A, Yekaninejad MS, Sadeghniiat-Haghighi K, Naghshi S, Mirzaei K. Association of plant-based diet index with inflammatory markers and sleep quality in overweight and obese female adults: A cross-sectional study. International journal of clinical practice 2021;75(9):e14429. doi: 10.1111/ijcp.14429. [DOI] [PubMed] [Google Scholar]
- 34.Cao Y, Taylor AW, Wittert G, Adams R, Shi Z. Dietary patterns and sleep parameters in a cohort of community dwelling Australian men. Asia Pac J Clin Nutr 2017;26(6):1158–69. doi: 10.6133/apjcn.122016.03. [DOI] [PubMed] [Google Scholar]
- 35.Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J Occup Health 2014;56(5):359–68. [DOI] [PubMed] [Google Scholar]
- 36.Noorwali E, Hardie L, Cade J. Fruit and Vegetable Consumption and Their Polyphenol Content Are Inversely Associated with Sleep Duration: Prospective Associations from the UK Women’s Cohort Study. Nutrients 2018;10(11). doi: 10.3390/nu10111803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro-Barquero S, Lamuela-Raventos RM, Domenech M, Estruch R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018;10(10). doi: 10.3390/nu10101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naja F, Hasan H, Khadem SH, Buanq MA, Al-Mulla HK, Aljassmi AK, Faris ME. Adherence to the Mediterranean Diet and Its Association With Sleep Quality and Chronotype Among Youth: A Cross-Sectional Study. Front Nutr 2021;8:805955. doi: 10.3389/fnut.2021.805955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campanini MZ, Guallar-Castillon P, Rodriguez-Artalejo F, Lopez-Garcia E. Mediterranean Diet and Changes in Sleep Duration and Indicators of Sleep Quality in Older Adults. Sleep 2017;40(3). doi: 10.1093/sleep/zsw083. [DOI] [PubMed] [Google Scholar]
- 40.Zuraikat FM, Makarem N, St-Onge MP, Xi H, Akkapeddi A, Aggarwal B. A Mediterranean Dietary Pattern Predicts Better Sleep Quality in US Women from the American Heart Association Go Red for Women Strategically Focused Research Network. Nutrients 2020;12(9). doi: 10.3390/nu12092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro-Diehl C, Wood AC, Redline S, Reid M, Johnson DA, Maras JE, Jacobs DR Jr., Shea S, Crawford A, St-Onge MP. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018;41(11). doi: 10.1093/sleep/zsy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estruch R, Salas-Salvado J. “Towards an even healthier Mediterranean diet”. Nutr Metab Cardiovasc Dis 2013;23(12):1163–6. doi: 10.1016/j.numecd.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Gangwisch JE, Hale L, St-Onge MP, Choi L, LeBlanc ES, Malaspina D, Opler MG, Shadyab AH, Shikany JM, Snetselaar L, et al. High glycemic index and glycemic load diets as risk factors for insomnia: analyses from the Women’s Health Initiative. The American journal of clinical nutrition 2020;111(2):429–39. doi: 10.1093/ajcn/nqz275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohammadi M, Nadjarzadeh A, Mirzaei M, Fallahzadeh H, Haghighatdoost F, Sakhaei R, Abolhosseini H, Salehi-Abargouei A. Dietary glycemic index and glycemic load in association with sleep duration: YaHS-TAMYZ and Shahedieh observational studies. Clin Nutr ESPEN 2021;46:471–6. doi: 10.1016/j.clnesp.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Crispim CA, Zimberg IZ, dos Reis BG, Diniz RM, Tufik S, de Mello MT. Relationship between food intake and sleep pattern in healthy individuals. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 2011;7(6):659–64. doi: 10.5664/jcsm.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St-Onge MP, Roberts A, Shechter A, Choudhury AR. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 2016;12(1):19–24. doi: 10.5664/jcsm.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips F, Chen CN, Crisp AH, Koval J, McGuinness B, Kalucy RS, Kalucy EC, Lacey JH. Isocaloric diet changes and electroencephalographic sleep. Lancet 1975;2(7938):723–5. doi: 10.1016/s0140-6736(75)90718-7. [DOI] [PubMed] [Google Scholar]
- 48.Porter JM, Horne JA. Bed-time food supplements and sleep: effects of different carbohydrate levels. Electroencephalogr Clin Neurophysiol 1981;51(4):426–33. doi: 10.1016/0013-4694(81)90106-1. [DOI] [PubMed] [Google Scholar]
- 49.Yajima K, Seya T, Iwayama K, Hibi M, Hari S, Nakashima Y, Ogata H, Omi N, Satoh M, Tokuyama K. Effects of nutrient composition of dinner on sleep architecture and energy metabolism during sleep. J Nutr Sci Vitaminol (Tokyo) 2014;60(2):114–21. doi: 10.3177/jnsv.60.114. [DOI] [PubMed] [Google Scholar]
- 50.Lindseth G, Murray A. Dietary Macronutrients and Sleep. West J Nurs Res 2016;38(8):938–58. doi: 10.1177/0193945916643712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Afaghi A, O’Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. The American journal of clinical nutrition 2007;85(2):426–30. doi: 10.1093/ajcn/85.2.426. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J, Kim JE, Armstrong CL, Chen N, Campbell WW. Higher-protein diets improve indexes of sleep in energy-restricted overweight and obese adults: results from 2 randomized controlled trials. The American journal of clinical nutrition 2016;103(3):766–74. doi: 10.3945/ajcn.115.124669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saidi O, Rochette E, Del Sordo G, Peyrel P, Salles J, Dore E, Merlin E, Walrand S, Duche P. Isocaloric diets with different protein-carbohydrate ratios: The effect on sleep, melatonin secretion and subsequent nutritional response in healthy young men. Nutrients 2022;14:5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iacovides S, Goble D, Paterson B, Meiring RM. Three consecutive weeks of nutritional ketosis has no effect on cognitive function, sleep, and mood compared with a high-carbohydrate, low-fat diet in healthy individuals: a randomized, crossover, controlled trial. The American journal of clinical nutrition 2019;110(2):349–57. doi: 10.1093/ajcn/nqz073. [DOI] [PubMed] [Google Scholar]
- 55.Karl JP, Thompson LA, Niro PJ, Margolis LM, McClung JP, Cao JJ, Whigham LD, Combs GF Jr., Young AJ, Lieberman HR, et al. Transient decrements in mood during energy deficit are independent of dietary protein-to-carbohydrate ratio. Physiology & behavior 2015;139:524–31. doi: 10.1016/j.physbeh.2014.11.068. [DOI] [PubMed] [Google Scholar]
- 56.Lindseth G, Lindseth P, Thompson M. Nutritional effects on sleep. West J Nurs Res 2013;35(4):497–513. doi: 10.1177/0193945911416379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson NA, Dyer KA, Buckley JD, Brinkworth GD, Coates AM, Parfitt G, Howe PRC, Noakes M, Murphy KJ. Comparison of two low-fat diets, differing in protein and carbohydrate, on psychological wellbeing in adults with obesity and type 2 diabetes: a randomised clinical trial. Nutrition journal 2018;17(1):62. doi: 10.1186/s12937-018-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afaghi A, O’Connor H, Chow CM. Acute effects of the very low carbohydrate diet on sleep indices. Nutr Neurosci 2008;11(4):146–54. doi: 10.1179/147683008X301540. [DOI] [PubMed] [Google Scholar]
- 59.Kwan RM, Thomas S, Mir MA. Effects of a low carbohydrate isoenergetic diet on sleep behavior and pulmonary functions in healthy female adult humans. J Nutr 1986;116(12):2393–402. doi: 10.1093/jn/116.12.2393. [DOI] [PubMed] [Google Scholar]
- 60.Bergel A, Deffieux T, Demene C, Tanter M, Cohen I. Local hippocampal fast gamma rhythms precede brain-wide hyperemic patterns during spontaneous rodent REM sleep. Nat Commun 2018;9(1):5364. doi: 10.1038/s41467-018-07752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. International Journal of Tryptophan Research 2009;2:IJTR. S2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pardridge WM. Brain metabolism: a perspective from the blood-brain barrier. Physiological reviews 1983;63(4):1481–535. [DOI] [PubMed] [Google Scholar]
- 63.Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Frontiers in endocrinology 2019:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. The American journal of clinical nutrition 2003;77(1):128–32. [DOI] [PubMed] [Google Scholar]
- 65.Fernstrom JD, Wurtman R. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science 1971;173(3992):149–52. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann RC, McDougle CJ, Schumacher M, Olcese J, Heninger GR, Price LH. Urinary 6-hydroxymelatonin sulfate as a measure of melatonin secretion during acute tryptophan depletion. Psychoneuroendocrinology 1993;18(8):567–78. [DOI] [PubMed] [Google Scholar]
- 67.Cheon J, Kim M. Comprehensive effects of various nutrients on sleep. Sleep and Biological Rhythms 2022:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oxenkrug GF. Metabolic syndrome, age‐associated neuroendocrine disorders, and dysregulation of tryptophan—kynurenine metabolism. Annals of the New York Academy of Sciences 2010;1199(1):1–14. [DOI] [PubMed] [Google Scholar]
- 69.Oxenkrug G Insulin resistance and dysregulation of tryptophan–kynurenine and kynurenine–nicotinamide adenine dinucleotide metabolic pathways. Molecular neurobiology 2013;48(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fierabracci V, Novelli M, Ciccarone A, Masiello P, Benzi L, Navalesi R, Bergamini E. Effects of tryptophan load on amino acid metabolism in type 1 diabetic patients. Diabetes & Metabolism 1996;22(1):51–6. [PubMed] [Google Scholar]
- 71.Wang W, Wang X, Liu L, Liu Z, Han T, Sun C, Yang X. Dietary tryptophan and the risk of obesity and type 2 diabetes: Total effect and mediation effect of sleep duration. Obesity 2022;30(2):515–23. [DOI] [PubMed] [Google Scholar]
- 72.Erbsloh F, Bernsmeier A, Hillesheim H. The glucose consumption of the brain & its dependence on the liver. Archiv fur Psychiatrie und Nervenkrankheiten, vereinigt mit Zeitschrift fur die gesamte Neurologie und Psychiatrie 1958;196(6):611–26. [DOI] [PubMed] [Google Scholar]
- 73.González-Rodríguez M, Pazos-Couselo M, García-López JM, Rodríguez-Segade S, Rodríguez-García J, Túñez-Bastida C, Gude F. Postprandial glycemic response in a non-diabetic adult population: the effect of nutrients is different between men and women. Nutrition & metabolism 2019;16(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Cauter E Polonsky KS, Scheen AJ Roles of circadian rhythmicity and sleep in human glucose regulation Endocr Rev 1997;18:716–38. [DOI] [PubMed] [Google Scholar]
- 75.Boyle PJ, Scott JC, Krentz AJ, Nagy RJ, Comstock E, Hoffman C. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. The Journal of clinical investigation 1994;93(2):529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katayose Y, Tasaki M, Ogata H, Nakata Y, Tokuyama K, Satoh M. Metabolic rate and fuel utilization during sleep assessed by whole-body indirect calorimetry. Metabolism 2009;58(7):920–6. [DOI] [PubMed] [Google Scholar]
- 77.Bialasiewicz P, Pawlowski M, Nowak D, Loba J, Czupryniak L. Decreasing concentration of interstitial glucose in REM sleep in subjects with normal glucose tolerance. Diabetic medicine 2009;26(4):339–44. [DOI] [PubMed] [Google Scholar]
- 78.Jamali R, Bachrach-Lindström M, Mohseni S. Continuous glucose monitoring system signals the occurrence of marked postprandial hyperglycemia in the elderly. Diabetes technology & therapeutics 2005;7(3):509–15. [DOI] [PubMed] [Google Scholar]
- 79.Mantantzis K, Campos V, Darimont C, Martin F-P. Effects of Dietary Carbohydrate Profile on Nocturnal Metabolism, Sleep, and Wellbeing: A Review. Frontiers in Public Health 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brandt R, Park M, Wroblewski K, Quinn L, Tasali E, Cinar A. Sleep quality and glycaemic variability in a real-life setting in adults with type 1 diabetes. Diabetologia 2021;64(10):2159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imai S, Kajiyama S, Hashimoto Y, Yamane C, Miyawaki T, Ozasa N, Tanaka M, Fukui M. Divided consumption of late-night-dinner improves glycemic excursions in patients with type 2 diabetes: A randomized cross-over clinical trial. diabetes research and clinical practice 2017;129:206–12. [DOI] [PubMed] [Google Scholar]
- 82.Gu C, Brereton N, Schweitzer A, Cotter M, Duan D, Børsheim E, Wolfe RR, Pham LV, Polotsky VY, Jun JC. Metabolic effects of late dinner in healthy volunteers—a randomized crossover clinical trial. The Journal of Clinical Endocrinology & Metabolism 2020;105(8):2789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol 2019;15(2):75–89. doi: 10.1038/s41574-018-0122-1. [DOI] [PubMed] [Google Scholar]
- 84.Chung N, Bin YS, Cistulli PA, Chow CM. Does the proximity of meals to bedtime influence the sleep of young adults? A cross-sectional survey of university students. International journal of environmental research and public health 2020;17(8):2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes care 2013;36(5):1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cryer PE. Glucose counterregulation: prevention and correction of hypoglycemia in humans. American Journal of Physiology-Endocrinology And Metabolism 1993;264(2):E149–E55. [DOI] [PubMed] [Google Scholar]
- 87.Gais S, Born J, Peters A, Schultes B, Heindl B, Fehm HL, Kern W. Hypoglycemia counterregulation during sleep. Sleep 2003;26(1):55–9. [DOI] [PubMed] [Google Scholar]
- 88.Venner A, Karnani MM, Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Orexin neurons as conditional glucosensors: paradoxical regulation of sugar sensing by intracellular fuels. J Physiol 2011;589(Pt 23):5701–8. doi: 10.1113/jphysiol.2011.217000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakurai T The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nature Reviews Neuroscience 2007;8(3):171–81. [DOI] [PubMed] [Google Scholar]
- 90.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998;92(4):573–85. [DOI] [PubMed] [Google Scholar]
- 91.Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends in pharmacological sciences 2011;32(8):451–62. [DOI] [PubMed] [Google Scholar]
- 92.Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. Journal of Neuroscience 2002;22(11):4568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999;98(4):437–51. [DOI] [PubMed] [Google Scholar]
- 94.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999;98(3):365–76. [DOI] [PubMed] [Google Scholar]
- 95.Toor B, Ray LB, Pozzobon A, Fogel SM. Sleep, Orexin and Cognition. The Orexin System Basic Science and Role in Sleep Pathology 2021;45:38–51. [DOI] [PubMed] [Google Scholar]
- 96.Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neuroscience letters 1999;264(1–3):101–4. [DOI] [PubMed] [Google Scholar]
- 97.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K-i, Sugiyama F, Goto K. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003;38(5):701–13. [DOI] [PubMed] [Google Scholar]
- 98.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. Journal of Neuroscience 2005;25(9):2429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsuneki H, Wada T, Sasaoka T. Role of orexin in the central regulation of glucose and energy homeostasis. Endocrine journal 2012:1201270672-. [DOI] [PubMed] [Google Scholar]
- 100.Griffond B, Risold P-Y, Jacquemard C, Colard C, Fellmann D. Insulin-induced hypoglycemia increases preprohypocretin (orexin) mRNA in the rat lateral hypothalamic area. Neuroscience letters 1999;262(2):77–80. [DOI] [PubMed] [Google Scholar]
- 101.Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea—a review. Sleep 2012;35(5):605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cai XJ, Lister CA, Buckingham RE, Pickavance L, Wilding J, Arch JR, Wilson S, Williams G. Down-regulation of orexin gene expression by severe obesity in the rats: studies in Zucker fatty and zucker diabetic fatty rats and effects of rosiglitazone. Molecular brain research 2000;77(1):131–7. [DOI] [PubMed] [Google Scholar]
- 103.Venner A, Karnani MM, Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Orexin neurons as conditional glucosensors: paradoxical regulation of sugar sensing by intracellular fuels. The Journal of physiology 2011;589(23):5701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krentz A, Singh B, Nattrass M. Impaired glucose tolerance is characterized by multiple abnormalities in the regulation of intermediary metabolism. Diabetic medicine 1991;8(9):848–54. [DOI] [PubMed] [Google Scholar]
- 105.Robertson D, Singh B, Nattrass M. Effect of obesity on circulating intermediary metabolite concentrations in the absence of impaired glucose tolerance. International journal of obesity 1991;15(10):635–45. [PubMed] [Google Scholar]
- 106.He J, Hong C, Zhang L, Wang Y, Fan Y, Guo P, Zhang B, Qi X, Chen S, Niu Y-j. Associations between night-time sleep duration and fasting glucose and ratio of triglyceride to high-density lipoprotein cholesterol among adults free of type 2 diabetes or without diagnosed type 2 diabetes: a multicentre, cross-sectional study in China. BMJ open 2022;12(7):e062239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zarifkar M, Noshad S, Shahriari M, Afarideh M, Khajeh E, Karimi Z, Ghajar A, Esteghamati A. Inverse association of peripheral orexin-A with insulin resistance in type 2 diabetes mellitus: a randomized clinical trial. The review of diabetic studies: RDS 2017;14(2–3):301. [DOI] [PMC free article] [PubMed] [Google Scholar]


