Abstract
Rituximab is a chimeric anti-CD20 monoclonal antibody that targets CD20-expressing B-lymphocytes, has a well-defined efficacy and safety profile, and is broadly used to treat a wide array of diseases. In this review, we cover the mechanism of action of rituximab and focus on hypogammaglobulinemia and late-onset neutropenia—two immune effects secondary to rituximab—as well as subsequent infection. We review risk factors and highlight key considerations for immunological monitoring and clinical management of rituximab-induced secondary immune deficiencies. In patients treated with rituximab, monitoring for hypogammaglobulinemia and infections may help to identify the subset of patients at high risk for developing poor B-cell reconstitution, subsequent infections, and adverse complications. These patients may benefit from early interventions such as vaccination, antibacterial prophylaxis, and immunoglobulin replacement therapy. Systematic evaluation of immunoglobulin levels and peripheral B-cell counts by flow cytometry, both at baseline and periodically after therapy, are recommended for monitoring. Additionally, in those patients with prolonged hypogammaglobulinemia and increased infections following rituximab use, immunologic evaluation for inborn errors of immunity may be warranted in order to further risk stratify, increase monitoring, and assist in therapeutic decision making. As the immunologic effects of rituximab are further elucidated, personalized approaches to minimize the risk of adverse reactions while maximizing benefit will allow for improved care of patients with decreased morbidity and mortality.
Keywords: Rituximab, rituxan, anti-CD20, hypogammaglobulinemia, late-onset neutropenia, infection, secondary immune deficiency, humoral immune deficiency
Introduction
Rituximab is a chimeric anti-CD20 monoclonal antibody that targets B-lymphocytes expressing the CD20 surface antigen 1,2. Initially approved by the United States Food and Drug Administration in 1997, rituximab is used for a number of autoimmune conditions and B-cell malignancies, including rheumatoid arthritis, granulomatosis with polyangiitis, non-Hodgkin lymphoma, and chronic lymphocytic leukemia 3. Rituximab is increasingly used off-label for treatment of kidney, autoimmune rheumatologic, and neurological diseases 4–7. Rituximab therapy yields full B-cell depletion within 72 hours, a state of low or undetectable B-cell levels for 2–6 months, and a return to pretreatment B-cell levels within 12 months of rituximab treatment alone 8–10.
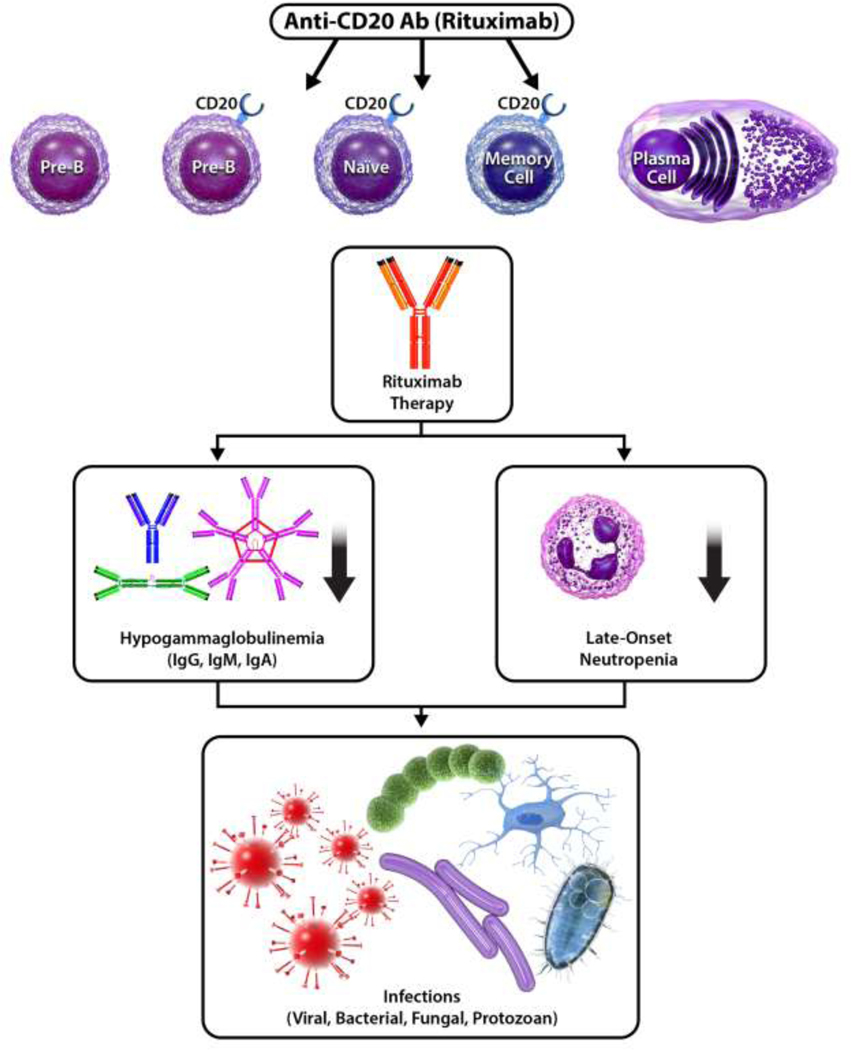
Although plasma cell and existing antibody levels are not altered, rituximab-induced B-cell depletion can affect humoral immune responses 8. As a result, secondary immune deficiency—an acquired decrease in immune cell count or function due to extrinsic, non-genetic factors such as a medication 11—can occur. Notably, in a subset of patients, rituximab has been associated with hypogammaglobulinemia and late-onset neutropenia, and these secondary immune deficiencies have subsequently been linked to increased risk of infection (Figure 1) 12–18. Several risk factors have been identified that predispose patients to the development of hypogammaglobulinemia, late-onset-neutropenia, and infection following rituximab therapy. In these patients, monitoring serum immunoglobulins and peripheral B-cell flow cytometry can help to risk stratify and mitigate morbidity and mortality.
Figure 1.
Secondary immune deficiencies following rituximab, such as hypogammaglobulinemia and late-onset neutropenia, subsequently lead to increased infection risk.
In this review, we begin with a summary of the mechanism of action of rituximab. Hypogammaglobulinemia and late-onset neutropenia, which are two notable secondary immune effects due to rituximab, and their risk factors are reviewed. We then cover infections following rituximab and risk factors thereof, which comprise a major consequence of rituximab-induced immune deficiency. Immunological monitoring and clinical management of rituximab secondary immune deficiencies are highlighted. Other considerations, such as unmasking of inborn errors of immunity, are briefly explored. We conclude with an eye towards future research.
Mechanism of Action of Rituximab
Rituximab is a chimeric monoclonal antibody (mAb) that targets the CD20 surface molecule, which is a B-cell marker expressed by the vast majority of pre-B-cells, mature B-lymphocytes in the periphery and bone marrow, and memory B-cells, but not by hematopoietic stem cells, pro-B-cells, or terminally-differentiated plasma cells 1,2. Rituximab consists of the variable region of murine anti-human CD20 fused to the Fc portion of human IgG1 kappa immunoglobulin 19. Structurally, rituximab binds to an epitope on the large extracellular loop of CD20, inducing the redistribution of CD20 into lipid raft microdomains 1,20–22. CD20 notably does not become internalized or shed from the cell membrane following mAb binding 23, and the close proximity of CD20’s extracellular epitopes to the cell membrane improves mAb effector function efficacy 24. Together, these features enable the sustained immunologic effect of rituximab 23.
Rituximab is thought to operate through several effector mechanisms. First, rituximab can cause direct signaling of apoptosis through caspase-dependent and caspase-independent pathways 19,25–31. Second, the antibody’s Fc portion can initiate complement-dependent cytotoxicity (CDC) and B-cell lysis via the assembly of a membrane attack complex through the classical complement pathway 32–34. Third, rituximab can cause antibody-dependent cellular cytotoxicity (ADCC) when its Fc region binds to the FcγRIIIa of natural killer (NK) cells, leading to the formation of a non-selective pore on the target B-cell membrane and target cell apoptosis through the actions of NK-released perforin and granzyme B, respectively 35–37. Fourth, antibody-dependent cellular phagocytosis (ADCP) can occur when macrophages recognize rituximab-coated target cells through various Fcγ receptors on the macrophage cell surface, leading to phagocytosis of the target cell 37. Synergistic and antagonistic interactions may exist between these different effector mechanisms 35,38–40.
Hypogammaglobulinemia After Rituximab
Hypogammaglobulinemia following rituximab has been reported in a subset of patients 41,42. Specifically, low IgG following rituximab has been reported in 3.5–40% of adult patients 13,43–45 and up to 27–50% of pediatric patients 46–50. Hypogammaglobulinemia after rituximab appears to vary based on the underlying indication for rituximab, with lower rates of hypogammaglobulinemia after rituximab in patients treated for autoimmune conditions and higher for patients with underlying malignancy 51. Additionally, both the prevalence 49,52 and severity 12 of hypogammaglobulinemia may increase with rituximab use. For example, in a cohort of 4,479 patients treated with rituximab, 19.3% of patients with normal IgG levels prior to rituximab developed hypogammaglobulinemia, 23.1% of patients with mild hypogammaglobulinemia pre-rituximab progressed to moderate or severe hypogammaglobulinemia, and 21.5% of those with moderate hypogammaglobulinemia pre-rituximab progressed to severe post-rituximab 12. While less often described in the literature, decreases in IgM and IgA have also been reported 49.
Risk Factors for Hypogammaglobulinemia After Rituximab
Numerous potential demographic, clinical, and pharmacologic risk factors have been associated with the development of hypogammaglobulinemia following rituximab (Table 1).
Table 1.
Potential risk factors for hypogammaglobulinemia after rituximab supported by multiple studies
Low immunoglobulin levels at baseline
In multiple adult and pediatric patient populations, lower serum IgG levels prior to the start of rituximab therapy have been found to be strongly predictive of hypogammaglobulinemia 12,49,51–57. Several studies have also found lower IgM levels at baseline or IgM deficiency to be predictive of low post-rituximab immunoglobulin levels 48,57–59. Additionally, in a smaller set of studies and particularly in the pediatric subset, lower IgA levels or IgA deficiency has been documented as a risk factor 48,49.
Longer rituximab treatment
The number of rituximab treatment cycles (i.e., longer-term rituximab treatment) has been shown to be a risk factor for hypogammaglobulinemia in numerous patient populations, including in many studies of rheumatoid arthritis patients 45,60–63. A clinical trial of more than 2,500 rheumatoid arthritis patients followed for over 5,000 person-years demonstrated that multiple rituximab infusions are one of the most important predictors of hypo-IgM: with each subsequent 6-month treatment cycle, the proportion of patients with low IgM increased, with an increase of ~10% of patients following the initial cycle and up to 40% of patients after five cycles 64,65. In lymphoma patients, rituximab given in more than 8 doses was associated with prolonged hypogammaglobulinemia longer than 6 months 66. Similarly, in a small cohort of 15 neuromyelitis optica patients, longer rituximab treatment was associated with hypogammaglobulinemia 67. In a pediatric population of roughly 400 patients with varying indications for rituximab use, a retrospective study found that the higher the number of doses of rituximab, the stronger the association with hypogammaglobulinemia 68. Taking the evidence together, a recent review has found that the use of more than one dose of rituximab and maintenance regimens of the antibody represent risk factors for hypogammaglobulinemia 51.
Glucocorticoids
Use of glucocorticoids has been associated with hypogammaglobulinemia, in particular moderate-to-high prednisone exposure at rituximab onset 69 and prednisolone use at 12 months following rituximab 53 in patients with autoimmune rheumatic diseases. Of note, hypogammaglobulinemia incidence was reported to be more pronounced in a subgroup of ANCA-associated vasculitis patients (45%) as compared to rheumatoid arthritis patients (22%) and connective tissue disorder patients (9.1%) 69. Further indirect evidence supporting glucocorticoid use as a risk factor for hypogammaglobulinemia can be found in studies of multiple sclerosis patients treated with rituximab, where IgG levels post-treatment remain stable when concomitant systemic glucocorticoids not used 64,70,71. In a pediatric cohort of rituximab-treated patients, glucocorticoid therapy in the month prior to rituximab use was a significant predictor of both new-onset hypogammaglobulinemia and infectious complications 50. In rare cases, dexamethasone has been implicated as a risk factor for hypogammaglobulinemia in immune thrombocytopenia patients treated with rituximab 72.
Other immunosuppressants
Use of immunosuppressive medications such as mycophenolate mofetil, when used in conjunction with rituximab, is associated with hypogammaglobulinemia 51,58,73. When the immunological mechanisms of these drugs are considered together, a two-pronged pathway emerges explaining how coadministration may lead to decreased levels of immunoglobulin and yield persistent hypogammaglobulinemia: naive, mature, and memory B-cells are depleted due to rituximab, while T cell and B-cell proliferation are inhibited due to the mycophenolate mofetil 74. Data on the risk of hypogammaglobulinemia with other immunosuppressants such as cyclophosphamide have been mixed, perhaps due to heterogeneity in patient populations assessed. Some studies have linked concomitant cyclophosphamide use 75,76, cumulative cyclophosphamide dose in patients with granulomatosis with polyangiitis 55, and prior cyclophosphamide exposure 53 with increased risk of IgG depletion post-rituximab. However, an association between cyclophosphamide and hypogammaglobulinemia was not replicated in studies of patients with autoimmune disorders such as lupus 52,77.
Chemotherapeutics
In patients with B-cell malignancies such as non-Hodgkin lymphoma, certain chemotherapeutic drugs have been identified as risk factors for low immunoglobulin levels post-rituximab in some studies, but the evidence is mixed. For instance, purine analogues 43,51,78 and fludarabine 66,78–80 have been identified as predictors of hypogammaglobulinemia in some studies. However, other studies have demonstrated no significant difference in hypogammaglobulinemia rates when rituximab and fludarabine are co-administered versus fludarabine regimens alone 78.
Disease-specific medical history
Disease- and context-specific medical history has been shown to be an important predictor of hypogammaglobulinemia. For example, in children with autoimmune cytopenias, the diagnosis of autoimmune hemolytic anemia or Evans syndrome is a risk factor for the development of hypogammaglobulinemia 48. In lymphoma patients with complicated nephrotic syndrome, a past history of steroid-resistant nephrotic syndrome is a risk factor 56. In pediatric patients with autoimmune diseases, the presence of autoimmune central nervous system disease is a risk factor for hypogammaglobulinemia 46. In patients with granulomatous polyangiitis, kidney involvement has been shown to be a risk factor 55. In lymphoma patients, there have been reported cases of hypogammaglobulinemia post-rituximab when patients were subjected to further cellular immunosuppression, such as organ transplantations 81–83, stem cell transplantations 84–86, and HIV infection 87. In particular, allogeneic stem cell transplants were complicated with low immunoglobulins more often than autologous transplantation 88.
Age
Age has been implicated as a risk factor for post-rituximab hypogammaglobulinemia, associated with the extremes of age. In adult patients, older age was found to be predictive of low IgG levels in numerous studies 12,54,55,63. However, in pediatric patients with autoimmune cytopenias, younger age at rituximab use was associated with low immunoglobulin post-therapy 48. In children with steroid-dependent nephrotic syndrome, a lower median age was associated with hypogammaglobulinemia in cohorts from Japan, France, and Italy 47,73,89.
Biological sex
The association between biological sex and hypogammaglobulinemia has been generally inconclusive. Some studies suggest that female sex is associated with decreased immunoglobulin levels 53, while other studies suggest that male sex is associated 55.
Late-Onset Neutropenia After Rituximab
Late-onset neutropenia has been an increasingly common following rituximab and is defined as low absolute neutrophil counts <1.5 × 109/L occurring 4 or more weeks after the last dose of rituximab, in the absence of pre-existing neutropenia, and without any other identifiable cause 90. Late-onset neutropenia has been reported to occur a median of 38 to 175 days following rituximab use 18,91,92. The prevalence of late-onset neutropenia ranges depending on underlying disease from 1.3 to 27% 17,93–96. In the majority of cases, this late-onset neutropenia is benign and resolves spontaneously, while in a subset of patients with neutropenia, subsequent infection develops. However, some studies argue that late-onset neutropenia does not contribute to infection risk as much as hypogammaglobulinemia 65,97,98.
Risk Factors for Late-Onset Neutropenia After Rituximab
Several risk factors for late-onset neutropenia have been identified, though there sometimes exists heterogeneity in the patient populations analyzed 99. Nevertheless, possible risk factors for late-onset neutropenia can be grouped into the following thematic categories: cancer stage and chemotherapeutics, receptor polymorphisms, immunological, and demographic (Table 2).
Table 2.
Potential risk factors for late-onset neutropenia after rituximab supported by multiple studies
| Risk Factor | References |
|---|---|
| Advanced stage of cancer | Choi et al. 2014; Nitta et al. 2007; Arai et al. 2015 |
| Exposure to chemotherapeutics (e.g., purine analogs) | Wolach et al. 2010; Nitta et al. 2007; Hirayama et al. 2009; Lemieux et al. 2004; Cattaneo et al. 2006; Cairoli et al. 2004 |
| IgG FcγIIIa receptor polymorphism (158V/V or 158V/F) | Weng et al. 2010; Tesfa and Palmblad 2011; Wolach et al. 2012; Li et al. 2010; Keane et al. 2012 |
| Autologous peripheral blood stem cell transplantation | Lemieux et al. 2004; Hirayama et al. 2009; Cairoli et al. 2004; Flinn et al. 2000 |
| Immunosuppressants (e.g., methotrexate) | Nitta et al. 2007; Arai et al. 2015; Castillo et al. 2019 |
| Older age | Moore 2016; Freifeld et al. 2011; Salmon et al. 2015; Choi et al. 2014; Arai et al. 2015 |
Cancer stage and chemotherapeutics
More advanced stages of cancer have been associated with late-onset neutropenia in B-cell lymphoma patient populations in several studies 100–102. For instance, in one particular retrospective cohort study, an advanced stage of cancer was associated with a 356% increase (HR CI: 1.02–12.7) in the risk of developing late-onset neutropenia 102. Similarly, higher Ann Arbor staging was associated with a 607% higher odds (OR CI: 1.47–25.13) of late-onset neutropenia in diffuse large B-cell lymphoma patients undergoing combination chemotherapy 100. Previous exposure to chemotherapeutics such as purine analogs is also a significant risk factor for late-onset neutropenia in several studies 18,96,101,103–105.
Receptor polymorphisms
Polymorphisms in the FCGR3A gene encoding the IgG FcγIIIa receptor—particularly the 158V/F and 158V/V variants—may correlate with increased risk of late-onset neutropenia post-rituximab 17,93,106. In one study of autologous stem cell transplant patients, each additional V allele tripled a patient’s risk of neutropenia 106. Results in B-cell lymphoma patients echoed these results, with one particular study highlighting that having the 158V/V or 158V/F polymorphism led to a 47% higher odds (OR CI: 1.21–1.78) of late-onset neutropenia as compared to 158F/F 107,108.
Immunological
Prior autologous peripheral blood stem cell transplantation was identified as a risk factor in several studies 103–105,109. Previous treatment with immunosuppressants such as methotrexate has been identified as a neutropenic risk factor in a set of studies 101,102,110. In contrast to hypogammaglobulinemia, there are fewer reports that support an association between longer rituximab treatment and late-onset neutropenia, and the evidence is mixed. While one study has reported that greater than four rituximab doses is a risk factor for late-onset neutropenia 110, other studies have found that more doses are not a significant risk factor 102. As it pertains to immunologic pathology, bone marrow involvement of diffuse large B-cell lymphoma was a risk factor for late-onset neutropenia in a single cohort of 181 patients 100.
Demographic
Several studies have shown that older age, defined as >60 years or >65 years, is a risk factor of late-onset neutropenia 98,100,102,111,112. However, two larger retrospective studies of combination chemotherapy with rituximab did not implicate old age as a factor of late-onset neutropenia 113,114. Female gender has also been implicated as a risk factor of late-onset neutropenia in a few studies 98,100. Age and gender both were not identified as risk factors in another study 110.
Infections After Rituximab
A number of viral, bacterial, fungal, and protozoal infections have been observed in patients treated with rituximab. Growing evidence suggests that rituximab predisposes to infections and can cause immunosuppression via hypogammaglobulinemia, late-onset neutropenia, and delayed onset cytopenia, particularly with maintenance therapy 115,116. Cohort studies have reported an increase in severe infections after rituximab use 12. Prevalence of post-rituximab severe infections has been estimated to be 15.4% in a large meta-analysis of ANCA-associated vasculitis patients (CI: 8.9%–23.3%) 117, and 23.7% in pediatric patients 49, with sinopulmonary infections as some of the most commonly reported particularly in patients with hypogammaglobulinemia 118,119. A pooled analysis of global clinical trial data reported that the overall serious infection rate was 3.94/100 patient-years in a population of 3,194 rheumatoid arthritis patients 65.
The association between infection and rituximab therapy appears to be dependent on the underlying disease process and cohort studied. For instance, recent systematic reviews and meta-analyses in patients with lymphoma have found that rituximab maintenance therapy was associated with significantly increased rates of infection and infection-related adverse events 115,120,121. However, in randomized control trials conducted with rituximab in rheumatoid arthritis patients, some studies have reported higher infection incidence 115,122,123 while others have not 124. Little data is available regarding infection in organ transplant recipients on rituximab, while evidence supports increased infection risk in renal transplant patients 115.
It remains challenging to determine if these infections are directly caused by rituximab-mediated processes such as low immunoglobulin levels or low neutrophil count, or whether they are facilitated by immune dysregulation inherent to the underlying disease pathologic process or concomitant immunomodulation therapy. Therefore, patients should be monitored for signs and symptoms of infection throughout the course of their rituximab regimens.
Acute viral infections
Several viral infections have been described in association with rituximab 125. These include enteroviral meningoencephalitis 126–129, parvovirus B19 130–133, cytomegalovirus 86,134,135, and West Nile virus 136,137. In context, enteroviral meningoencephalitis is a known complication in other B-cell immunodeficiencies, parvovirus B19 often co-presents with progressive bicytopenia or pure red cell aplasia in reported rituximab cases, and cytomegalovirus is highly uncommon except in cases of allogeneic transplant or concomitant HIV 125. Rare cases of viral infection with varicella zoster virus 138–140, herpes simplex virus 139, echovirus 134, BK virus 141,142, and influenza A virus 134,143 have been reported 115.
In one particular study that documented both bacterial and viral infections post-rituximab, the most common type of infection that manifested was upper respiratory infection and bacterial sinopulmonary infection, followed by pneumonia and cellulitis 43. A few cases of urinary tract infection, ocular infection, and respiratory syncytial virus were also observed in singular occurrences 43.
COVID-19
Large cohort studies and meta-analyses have demonstrated that rituximab therapy is associated with severe SARS-CoV-2 (COVID-19) outcomes 7,144–147. Several case reports echo these results, finding that COVID-19 outcomes are more likely to be fatal in addition to having longer, more relapsing, and more atypically-symptomatic clinical courses post-rituximab therapy 148,149. A study of multiple sclerosis and neuromyelitis optica spectrum disorder patients indicated that rituximab therapy was associated with a higher incidence of COVID-19 infection, a higher case fatality rate, and a higher frequency of serious illness 150.
Chronic viral infections
There is established evidence for increased viral infections with chronic pathology following rituximab 125. In particular, rituximab has been linked with reactivation of latent hepatitis B virus, with sequelae including acute hepatitis and fulminant liver failure, following chemotherapy in numerous cases of hematologic malignancies 125,151–155. Screening for latent hepatitis B infection is recommended prior to initiating rituximab 156. Additionally, screening for hepatitis B core antibody prior to initiation of immunoglobulin replacement therapy (IgRT) is recommended as hepatitis B core antibody can be transferred passively through IgRT 157. In patients on IgRT who require treatment with rituximab, presence of a positive hepatitis B core antibody may lead to concern for chronic latent hepatitis B that requires treatment with antivirals given the risk of reactivation of hepatitis B, which can potentially be fatal. Rituximab has also been associated with progressive multifocal leukoencephalopathy, caused by the JC polyomavirus, mainly in cases of hematologic malignancies but also in rheumatoid arthritis, immune cytopenia, and systemic lupus erythematosus 86,125,142,158.
Bacterial infections
Rituximab has been associated with acute infection from a variety of bacterial microbes. Multiple reports have documented infection with Haemophilus influenzae 13,116,159. Several cases have reported infection with Campylobacter fetus 160–162 and C. jejuni 163,164. Rare cases of Staphylococcus aureus, Streptococcus pneumoniae, shigella, and listeria have been reported 115,123,154. Severe infections with Mycobacterium kansasii, M. avium, and M. wolinskyi have been reported 165,166. The treatment setting may also contribute to infection risk. For instance, in patients with systemic autoimmune diseases, hospital-acquired infection may be an important cause of bacterial infections 167.
Fungal and protozoal infections
Fungal and protozoal infections have been reported rarely following rituximab. The risk for fungal and protozoal infections after rituximab is likely multifactorial due to factors including concomitant medications such as steroids or the underlying disease process. An additional potential mechanism that has been proposed is that given the effect of rituximab on B- cells, there may be a deleterious impact on the induction, maintenance, and activation of cell-mediated immunity due to impaired B- and T- cell cooperation 115,168,169. Fungal infections such as Pneumocystis jirovecii pneumonia have been described in several patients, though this has often been in combination with other immunosuppressive medications in the setting of hematological malignancies, autoimmune diseases, and organ transplantation 125,134,170–177. Other rare cases include aspergillosis 178,179 and osteomyelitis due to Cryptococcus neoformans var. grubii 180,181.
Infections with protozoan parasites have also been reported post-rituximab. A case-control study demonstrated that rituximab was associated with persistent babesiosis caused by Babesia microti 182. A report documented a case of cerebral toxoplasmosis caused by Toxoplasma gondii, with circulation of parasite DNA in the cerebrospinal fluid, presence of bradyzoites in the brain tissue, and existence of a strong temporal relationship between rituximab treatment and neurologic symptom onset 183. Acanthamoeba encephalitis was reported in a patient with lupus nephritis at one month post-rituximab 184.
Risk Factors for Infections After Rituximab
Many of the risk factors for post-rituximab hypogammaglobulinemia are similar to the risk factors for infection. Risk factors for infection can be conceptually grouped into the following categories: immunological, pharmacological, clinical comorbidities, and demographic (Table 3).
Table 3.
Potential risk factors for infection after rituximab supported by multiple studies
| Risk Factor | References |
|---|---|
| Low baseline serum IgG | Gottenberg et al. 2010; Heusele et al. 2014; Christou et al. 2017 |
| Number of rituximab treatment cycles | Kanbayashi et al. 2009; Christou et al. 2017; Varley & Winthrop 2021 |
| Low serum IgG or IgM post-therapy | Kanbayashi et al. 2009; Gottenberg et al. 2010; Christou et al. 2017; Labrosse et al. 2021 |
| Glucocorticoids and prednisone | Heusele et al. 2014; Guilpain et al. 2021; Stabler et al. 2021; Xavier et al. 2022 |
| Clinical comorbidities (cardiac insufficiency, chronic lung disease, diabetes, cancer, and extra-articular involvement in rheumatoid arthritis) | Gottenberg et al. 2010; Christou et al. 2017; Guilpain et al. 2021; Stabler et al. 2021; Xavier et al. 2022 |
| Older age | Christou et al. 2017; Kronbichler et al. 2018 |
Immunological
Low IgG levels at the initiation of the rituximab course 51,119,185 and increased number of rituximab courses 51,186,187 have been implicated as risk factors for infection in many studies. Similarly, pre-existing hypogammaglobulinemia has been linked with post-rituximab infection risk, suggesting that routine screening of patients to identify those individuals at higher infection risk or with baseline immune dysfunction may be beneficial 12. Low IgG or IgM levels post-rituximab therapy, in addition to the presence of low IgG levels for more than 6 months, have also been implicated as potential risk factors for infection in adult and pediatric patients 49,51,119,186.
Other immunological variables such as low count of CD4+ T helper lymphocytes at rituximab initiation, low baseline count of CD19+ B-lymphocytes, G-CSF administration following neutropenia, and low lymphocyte counts at nadir represent risk factors for infection 51,168,185,188. Notably, G-CSF administration may be a marker of the severity of neutropenia rather than an independent risk factor.
Pharmacological
Concomitant use of glucocorticoids and other immunosuppressants have been identified as risk factors of infection 168,185,189,190. Chemotherapy drugs have also been attributed as risk factors in some studies, though the results have been mixed. In one retrospective cohort study of autoimmune disease patients, it was found that previous cyclophosphamide treatment was a notable predictor of infection 190. However, in randomized control trials of lymphoma patients and in meta-analyses, the addition of rituximab to cyclophosphamide and fludarabine, as well as combination therapies such as FCM (fludarabine, cyclophosphamide, and mitoxantrone) and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), did not result in an increased incidence of infections 125,191–195.
Clinical comorbidities
In several patient populations, risk factors for infection include clinical comorbidities such as cardiac insufficiency, chronic lung disease, diabetes, cancer, and extra-articular involvement in rheumatoid arthritis 51,119,168,189,190. Certain risk factors, however, appear to be more specific to particular patient populations. A low creatinine clearance rate was found to be a risk factor for infection in patients with systemic autoimmune diseases in a single-center case control study 185. In patients with hematologic malignancies, HIV serostatus and type of malignancy were independently associated with infection in a two-year London cohort. A recent review suggests that smoking history and those who required a therapy switch are associated with infection risk in patients with underlying rheumatologic diseases 187. Lack of pneumococcal vaccination was found to be predictive of infection in a cohort of patients with autoimmune diseases 190.
Demographic
Older age has been identified as a risk factor for infection in numerous studies 51,196. In a single-center retrospective cohort of patients with autoimmune diseases, a higher Charlson comorbidity index and male gender was associated with higher infection risk 190.
Immunologic Monitoring and Clinical Management
Immunological monitoring can be used for surveillance and early identification in order to predict adverse outcomes such as hypogammaglobulinemia or infection both during and after the course of a patient’s rituximab therapy. Known immunological characteristics of B-cell depletion and B-cell reconstitution in rituximab patients can help guide immunological monitoring strategies. For example, the estimated duration of B-cell depletion ranges between 6 and 9 months in patients on rituximab therapy alone, and between 18 and 24 months in patients on chemotherapy in addition to rituximab 10. However, some studies have reported delayed recovery of B-cells, with a median of 23 months post-rituximab 41. The recovery of the CD27+ memory B-cell pool is slower than the recovery of non-memory B-cells, with CD27+ memory B-cells remaining significantly lower than that of controls (4.4% vs 31%) even after 1 year post-rituximab 197.
Serum immunoglobulin surveillance and peripheral B-cell flow cytometry
Measuring serum immunoglobulin levels and peripheral B-cell flow cytometry can aid in identifying pre-existing immune dysfunction as well as the development of immune dysfunction following rituximab. Pre-rituximab evaluation can identify baseline hypogammaglobulinemia, enabling preliminary risk stratification of patients from the start of rituximab initiation, and periodic monitoring following rituximab can aid with identification of secondary hypogammaglobulinemia or immune dysfunction that develops 12. In addition, peripheral B-cell flow cytometry can help identify pre-existing B-cell deficiencies as well as detect B-cell recovery, improving longitudinal monitoring of complications 12.
Neutropenia
Since opportunistic infections due to rituximab can be due to associated late-onset neutropenia, a complete blood count can be of clinical utility in monitoring neutrophil levels. However, neutropenia often develops asymptomatically and patients only present to the hospital when the infection is in advanced stages, so there are no widely accepted guidelines regarding timing and monitoring for neutropenia 198–200.
Clinical management
There are several key features in management of hypogammaglobulinemia and infection risk following rituximab, such as vaccination, consideration of antibacterial prophylaxis, and initiation of immunoglobulin replacement (IgRT) in specific cases. Maintaining routine age-appropriate vaccination is critical in infection prevention in patients receiving rituximab. Decreased vaccine responses following rituximab use have been described for polysaccharide, protein, and conjugated vaccines. Given this, vaccinations are recommended 5–12 months following the last course of rituximab and 4 weeks before the next if possible 201–205. Avoidance of live vaccines in patients on rituximab therapy is recommended as rituximab is considered high-level immunosuppression 204,206. Additionally, patients with persistent hypogammaglobulinemia and/or recurrent infections may be considered for antibacterial prophylaxis, and/or immunoglobulin replacement therapy to reduce the risk of infections 12.
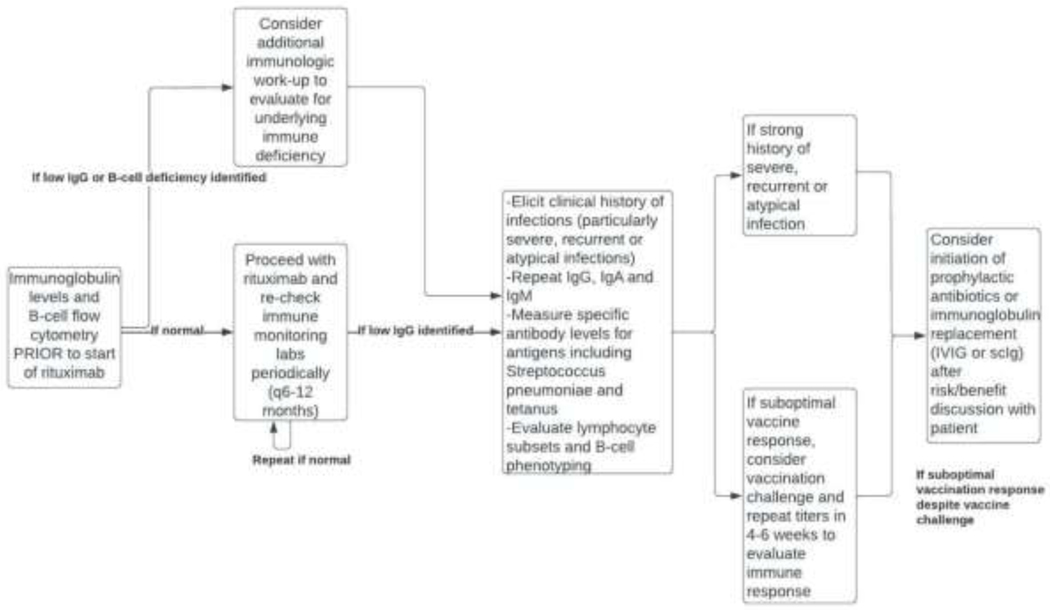
At our center, our approach is to extrapolate from the primary immune deficiency literature guidelines regarding evaluation of the function of antibodies using assessment of specific vaccine responses to determine if IgRT is warranted (Figure 2). This includes repeating the IgG level to assess for persistent hypogammaglobulinemia, as well as checking IgA and IgM. We then proceed with assessment of the Streptococcus pneumonia and tetanus IgG levels, with vaccination challenge if these are not adequately protective 207,208. We consider an adequately protective response to the pneumococcal polysaccharide vaccine to be at least 70% or more serotypes to be ≥1.3 mcg/mL in adults and 50% or more serotypes to be to be ≥1.3 mcg/mL in children ages 2–5 years 207,209,210. We consider an adequate protective level for tetanus vaccine to be 0.15 IU/mL 207,211. These specific antibody levels are repeated 4–6 weeks after vaccination to assess for immunologic response. Of note, vaccination challenge can be difficult to interpret in those patients without adequate B-cell recovery, so should be delayed to at least 5 months after the last course of rituximab if possible.
Figure 2.
Considerations for evaluation and management with rituximab.
If antibody levels are persistently low, we recommend shared decision making with the patient regarding initiation of IgRT (e.g., subcutaneous immunoglobulin or intravenous immunoglobulin) at a dose of 400–600 mg/kg/month as per the primary immune deficiency guidelines 208,212. For IgG levels <150 mg/dL, we initiate IgRT without additional functional evaluation 208. There is disease specific data for solid organ transplants and hematologic malignancies for consideration of IgRT for IgG levels <400 mg/dL 208. In those patients with severe, recurrent, or atypical infections, we may also consider initiation of IgRT after risk/benefit discussion with the patient. We additionally evaluate lymphocyte subset and B-cell phenotyping if B-cells are present. Baseline and serial monitoring of B-cell counts can be useful in identifying patients with failure of B-cell reconstitution as well as prognostication regarding recurrence or relapse of disease 79,213,214. Additionally, B-cell phenotyping can evaluate B-cell differentiation and recovery of switched memory B-cells 197, which may signal risk of relapse. We recheck immune monitoring labs periodically, including immunoglobulin levels and B-cell flow cytometry every 6 to 12 months to assess for immunologic impact and evidence of immune recovery (Figure 2).
Other Considerations in Rituximab Secondary Immune Deficiencies Unmasking of inborn errors of immunity
Rituximab has been associated with the unmasking of inborn errors of immunity and underlying immune deficiencies, particularly in pediatric patients 48,49. Determining whether the immune defect is a primary immune deficiency or secondary to rituximab can be challenging since patients with inborn errors of immunity may first present with autoimmune cytopenias, malignancy, or granulomatous disease 215–218 and be treated with rituximab. This can confound the diagnosis and make it difficult to ascertain if hypogammaglobulinemia and B-cell dysfunction is a primary defect (i.e., due to genetics) that is being unmasked by the use of rituximab or secondary (i.e., due to medication effect [rituximab] alone). This problem is amplified since the majority of patients do not undergo immunologic screening prior to starting rituximab 12.
However, evaluating pretreatment immunoglobulin levels and B-cell counts may help to identify patients with underlying immune deficiency or immune dysregulation. For example, one of the diagnostic criteria for common variable immunodeficiency (CVID) is low levels of IgG in combination with low IgA and/or IgM 219. There can be a significant delay in diagnosis of CVID and these patients may first present with, and be treated with rituximab for, autoimmune cytopenias 216. Evaluating pre-treatment immunoglobulin levels may thus be helpful in uncovering underlying inborn errors of immunity and aid in the early diagnosis of a humoral immunodeficiency. This, in turn, may help to reduce morbidity and mortality due to increased monitoring and consideration of immunoglobulin replacement. In those cases where an underlying inborn error of immunity is suspected, genetic testing may be helpful. Additionally, periodic monitoring post-rituximab can help to identify immune recovery.
Conclusion
Rituximab is a monoclonal antibody that targets CD20-expressing B-cells and used for a wide array of indications. While generally well-tolerated with excellent efficacy, prolonged hypogammaglobulinemia and late-onset neutropenia can occur following rituximab and can be clinically significant with an increased risk for infections. Given this, systematic serum surveillance of immunoglobulin levels and B-cell counts by flow cytometry, both at baseline and periodically after therapy, may be helpful tools for identifying those patients at high risk who may benefit from intervention with vaccination, antibacterial prophylaxis, or immunoglobulin replacement therapy. In those patients with prolonged hypogammaglobulinemia and increased infections following rituximab use, additional immunologic evaluation for underlying inborn errors of immunity may be warranted in order to further risk stratify, increase monitoring, and assist in therapeutic decision making. Additionally, in patients with infection following rituximab, a complete blood count can be of clinical utility in monitoring neutrophil levels. Notably, there have been an increasing number of B-cell targeted therapies developed including other CD20 targeting agents such as ofatumumab, ocrelizumab, and obinutuzumab, which are human/humanized anti-CD20 therapies, designed to minimize immunogenicity while enhancing binding affinity or increasing cytotoxicity 220–223. Given the expansion of B-cell targeted therapies, such as mAbs against CD19 224,225, CD22 226, and B-cell activating factor (BAFF) 227, BTK inhibitors 228, and chimeric antigen receptor (CAR) T-cell therapy targeting CD19 229,230 and B-cell maturation antigen (BCMA) 231, the data gleaned from the rituximab experience will be of significant utility in identifying potential immunologic impacts and risks for infection, morbidity, and mortality.
Future research to identify predictive clinical and laboratory biomarkers of persistent hypogammaglobulinemia and infectious risk will be of great utility. As the mechanisms of rituximab on the immune system are further elucidated, tailored approaches to minimize risk of adverse reactions while maximizing benefit will be critical for providing personalized care for patients to improve outcomes.
Funding Source:
TSA is supported by the National Institute of General Medical Sciences under grant number T32GM144273. SB is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under grant number K23AI163350. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations/Acronyms:
- mAb
monoclonal antibody
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IgA
immunoglobulin A
- Fc
fragment crystallizable region of antibody
- FcγRIIIa
Fc gamma receptor IIIa
- NK
natural killer cell
- CDC
complement-dependent cytotoxicity
- ADCC
antibody-dependent cellular cytotoxicity
- ADCP
antibody-dependent cellular phagocytosis
- G-CSF
granulocyte colony stimulating factor
- BAFF
B-cell activating factor
- BCMA
B-cell maturation antigen
- IgRT
immunoglobulin replacement therapy
- IVIG
intravenous immunoglobulin
- scIg
subcutaneous immunoglobulin
- OR
odds ratio
- HR
hazard ratio
- CI
95% confidence interval
Footnotes
Clinical Trial Registration: Not applicable.
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein C, Lammens A, Schäfer W, Georges G, Schwaiger M, Mössner E, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013;5(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavlasova G, Mraz M. The regulation and function of CD20: an ―“enigma” of B-cell biology and targeted therapy. Haematologica. 2020;105(6):1494–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grillo-López AJ, White CA, Dallaire BK, Varns CL, Shen CD, Wei A, et al. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr Pharm Biotechnol. 2000;1(1):1–9. [DOI] [PubMed] [Google Scholar]
- 4.Sinha R, Agrawal N, Xue Y, Chanchlani R, Pradhan S, Raina R, et al. Use of rituximab in paediatric nephrology. Arch Dis Child. 2021;106(11):1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margoni M, Preziosa P, Filippi M, Rocca MA. Anti-CD20 therapies for multiple sclerosis: current status and future perspectives. J Neurol. 2022;269(3):1316–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stathopoulos P, Dalakas MC. Evolution of Anti-B Cell Therapeutics in Autoimmune Neurological Diseases. Neurotherapeutics. 2022;19(3):691–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blincoe A, Labrosse R, Abraham RS. Acquired B-cell deficiency secondary to B-cell-depleting therapies. J Immunol Methods. 2022;511:113385. [DOI] [PubMed] [Google Scholar]
- 8.Furlan A, Forner G, Cipriani L, Vian E, Rigoli R, Gherlinzoni F, et al. COVID-19 in B Cell-Depleted Patients After Rituximab: A Diagnostic and Therapeutic Challenge. Front Immunol. 2021;12:763412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. [DOI] [PubMed] [Google Scholar]
- 10.Tolerability Kimby E. and safety of rituximab (MabThera®). Cancer Treat Rev. 2005;31(6):456–473. [DOI] [PubMed] [Google Scholar]
- 11.Ballow M, Sánchez-Ramón S, Walter JE. Secondary Immune Deficiency and Primary Immune Deficiency Crossovers: Hematological Malignancies and Autoimmune Diseases. Front Immunol. 2022;13:928062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barmettler S, Ong MS, Farmer JR, Choi H, Walter J. Association of Immunoglobulin Levels, Infectious Risk, and Mortality With Rituximab and Hypogammaglobulinemia. JAMA Netw Open. 2018;1(7):e184169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makatsori M, Kiani-Alikhan S, Manson AL, Verma N, Leandro M, Gurugama NP, et al. Hypogammaglobulinaemia after rituximab treatment-incidence and outcomes. QJM. 2014;107(10):821–828. [DOI] [PubMed] [Google Scholar]
- 14.Tallantyre EC, Whittam DH, Jolles S, Paling D, Constantinesecu C, Robertson NP, et al. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J Neurol. 2018;265(5):1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshayes S, Khellaf M, Zarour A, Layese R, Fain O, Terriou L, et al. Long-term safety and efficacy of rituximab in 248 adults with immune thrombocytopenia: Results at 5 years from the French prospective registry ITP-ritux. Am J Hematol. 2019;94(12):1314–1324. [DOI] [PubMed] [Google Scholar]
- 16.Md Yusof MY, Vital EM, McElvenny DM, Hensor EMA, Das S, Dass S, et al. Predicting Severe Infection and Effects of Hypogammaglobulinemia During Therapy With Rituximab in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 2019;71(11):1812–1823. [DOI] [PubMed] [Google Scholar]
- 17.Tesfa D, Palmblad J. Late-onset neutropenia following rituximab therapy: incidence, clinical features and possible mechanisms. Expert Rev Hematol. 2011;4(6):619–625. [DOI] [PubMed] [Google Scholar]
- 18.Wolach O, Bairey O, Lahav M. Late-onset neutropenia after rituximab treatment: case series and comprehensive review of the literature. Medicine. 2010;89(5):308–318. [DOI] [PubMed] [Google Scholar]
- 19.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22(47):7359–7368. [DOI] [PubMed] [Google Scholar]
- 20.Cragg MS, Morgan SM, Chan HTC, Morgan BP, Filatov AV, Johnson PWM, et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood. 2003;101(3):1045–1052. [DOI] [PubMed] [Google Scholar]
- 21.Deans JP, Robbins SM, Polyak MJ, Savage JA. Rapid redistribution of CD20 to a low density detergent-insoluble membrane compartment. J Biol Chem. 1998;273(1):344–348. [DOI] [PubMed] [Google Scholar]
- 22.Du J, Wang H, Zhong C, Peng B, Zhang M, Li B, et al. Structural Basis for Recognition of CD20 by Therapeutic Antibody Rituximab *. J Biol Chem. 2007;282(20):15073–15080. [DOI] [PubMed] [Google Scholar]
- 23.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44(16):3823–3837. [DOI] [PubMed] [Google Scholar]
- 24.Cleary KLS, Chan HTC, James S, Glennie MJ, Cragg MS. Antibody Distance from the Cell Membrane Regulates Antibody Effector Mechanisms. J Immunol. 2017;198(10):39994011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vega MI, Huerta-Yepaz S, Garban H, Jazirehi A, Emmanouilides C, Bonavida B. Rituximab inhibits p38 MAPK activity in 2F7 B NHL and decreases IL-10 transcription: pivotal role of p38 MAPK in drug resistance. Oncogene. 2004;23(20):3530–3540. [DOI] [PubMed] [Google Scholar]
- 26.Jazirehi AR, Huerta-Yepez S, Cheng G, Bonavida B. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-κB signaling pathway in non-Hodgkin’s lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosis. Cancer Res. 2005;65(1):264–276. [PubMed] [Google Scholar]
- 27.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin’s lymphoma B cells by Rituximab. Cancer Res. 2004;64(19):7117–7126. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki E, Umezawa K, Bonavida B. Rituximab inhibits the constitutively activated PI3K-Akt pathway in B-NHL cell lines: involvement in chemosensitization to drug-induced apoptosis. Oncogene. 2007;26(42):6184–6193. [DOI] [PubMed] [Google Scholar]
- 29.Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood, The Journal of the American Society of Hematology. 2002;99(3):1038–1043. [DOI] [PubMed] [Google Scholar]
- 30.Stanglmaier M, Reis S, Hallek M. Rituximab and alemtuzumab induce a nonclassic, caspase-independent apoptotic pathway in B-lymphoid cell lines and in chronic lymphocytic leukemia cells. Ann Hematol. 2004;83(10):634–645. [DOI] [PubMed] [Google Scholar]
- 31.Daniels I, Abulayha AM, Thomson BJ, Haynes AP. Caspase-independent killing of Burkitt lymphoma cell lines by rituximab. Apoptosis. 2006;11(6):1013–1023. [DOI] [PubMed] [Google Scholar]
- 32.Casan JML, Wong J, Northcott MJ, Opat S. Anti-CD20 monoclonal antibodies: reviewing a revolution. Hum Vaccin Immunother. 2018;14(12):2820–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall MJE, Stopforth RJ, Cragg MS. Therapeutic Antibodies: What Have We Learnt from Targeting CD20 and Where Are We Going? Front Immunol. 2017;8:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degn SE, Thiel S. Humoral pattern recognition and the complement system. Scand J Immunol. 2013;78(2):181–193. [DOI] [PubMed] [Google Scholar]
- 35.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47(2):115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Huang K, Liu L, Qu Y, Huang Y, Wu Y, et al. Effects of complement and serum IgG on rituximab-dependent natural killer cell-mediated cytotoxicity against Raji cells. Oncol Lett. 2019;17(1):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierpont TM, Limper CB, Richards KL. Past, Present, and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front Oncol. 2018;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishima Y, Terui Y, Mishima Y, Kuniyoshi R, Matsusaka S, Mikuniya M, et al. High reproducible ADCC analysis revealed a competitive relation between ADCC and CDC and differences between FcγRllla polymorphism. Int Immunol. 2012;24(8):477–483. [DOI] [PubMed] [Google Scholar]
- 39.Wang SY, Racila E, Taylor RP, Weiner GJ. NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111(3):1456–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beum PV, Lindorfer MA, Taylor RP. Within Peripheral Blood Mononuclear Cells, Antibody-Dependent Cellular Cytotoxicity of Rituximab-Opsonized Daudi cells Is Promoted by NK Cells and Inhibited by Monocytes due to Shaving1. J Immunol. 2008;181(4):2916–2924. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan B, Kopyltsova Y, Khokhar A, Lam F, Bonagura V. Rituximab and immune deficiency: case series and review of the literature. J Allergy Clin Immunol Pract. 2014;2(5):594–600. [DOI] [PubMed] [Google Scholar]
- 42.Barmettler S, Price C. Continuing IgG replacement therapy for hypogammaglobulinemia after rituximab—for how long? J Allergy Clin Immunol. 2015;136(5):1407–1409. [DOI] [PubMed] [Google Scholar]
- 43.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padoan R, Felicetti M, Gatto M, Polito P, Doria A, Schiavon F. Rituximab-associated hypogammaglobulinaemia in ANCA-associated vasculitis and connective tissue diseases: a longitudinal observational study. Clin Exp Rheumatol. 2020;38 Suppl 124(2):188–194. [PubMed] [Google Scholar]
- 45.Fierro A, Andres M, de la Torre-Aboki J, Vela-Casasempere P, Martínez-Vidal MP. AB0473 HYPOGAMMAGLOBULINEMIA AND INFECTIONS IN RHEUMATOLOGIC PATIENTS TREATED WITH RITUXIMAB. Ann Rheum Dis. 2019;78(Suppl 2):1700–1701. [Google Scholar]
- 46.Khojah AM, Miller ML, Klein-Gitelman MS, Curran ML, Hans V, Pachman LM, et al. Rituximab-associated Hypogammaglobulinemia in pediatric patients with autoimmune diseases. Pediatr Rheumatol Online J. 2019;17(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzuillo P, Guarino S, Esposito T, Di Sessa A, Orsini SI, Capalbo D, et al. Rituximab-induced IgG hypogammaglobulinemia in children with nephrotic syndrome and normal pre-treatment IgG values. World J Clin Cases. 2019;7(9):1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ottaviano G, Marinoni M, Graziani S, Sibson K, Barzaghi F, Bertolini P, et al. Rituximab Unveils Hypogammaglobulinemia and Immunodeficiency in Children with Autoimmune Cytopenia. J Allergy Clin Immunol Pract. 2020;8(1):273–282. [DOI] [PubMed] [Google Scholar]
- 49.Labrosse R, Barmettler S, Derfalvi B, Blincoe A, Cros G, Lacombe-Barrios J, et al. Rituximab-induced hypogammaglobulinemia and infection risk in pediatric patients. J Allergy Clin Immunol. 2021;148(2):523–532.e8. [DOI] [PubMed] [Google Scholar]
- 50.Ong MS, Rothman D, Barmettler S, Son MB, Lo M, Roberts J, et al. New-onset hypogammaglobulinaemia and infectious complications associated with rituximab use in childhood-onset rheumatic diseases. Rheumatology. 2022;61(4):1610–1620. [DOI] [PubMed] [Google Scholar]
- 51.Christou EAA, Giardino G, Worth A, Ladomenou F. Risk factors predisposing to the development of hypogammaglobulinemia and infections post-Rituximab. Int Rev Immunol. 2017;36(6):352–359. [DOI] [PubMed] [Google Scholar]
- 52.Roberts DM, Jones RB, Smith RM, Alberici F, Kumaratne DS, Burns S, et al. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun. 2015;57:60–65. [DOI] [PubMed] [Google Scholar]
- 53.Tieu J, Smith RM, Gopaluni S, Kumararatne DS, McClure M, Manson A, et al. Rituximab Associated Hypogammaglobulinemia in Autoimmune Disease. Front Immunol. 2021;12:671503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boleto G, Avouac J, Wipff J, Forien M, Dougados M, Roux C, et al. Predictors of hypogammaglobulinemia during rituximab maintenance therapy in rheumatoid arthritis: A 12-year longitudinal multi-center study. Semin Arthritis Rheum. 2018;48(2):149–154. [DOI] [PubMed] [Google Scholar]
- 55.Besada E, Koldingsnes W, Nossent JC. Serum immunoglobulin levels and risk factors for hypogammaglobulinaemia during long-term maintenance therapy with rituximab in patients with granulomatosis with polyangiitis. Rheumatology. 2014;53(10):1818–1824. [DOI] [PubMed] [Google Scholar]
- 56.Inoki Y, Kamei K, Nishi K, Sato M, Ogura M, Ishiguro A. Incidence and risk factors of rituximab-associated hypogammaglobulinemia in patients with complicated nephrotic syndrome. Pediatr Nephrol. 2022;37(5):1057–1066. [DOI] [PubMed] [Google Scholar]
- 57.Evangelatos G, Fragoulis GE, Klavdianou K, Moschopoulou M, Vassilopoulos D, Iliopoulos A. Hypogammaglobulinemia after rituximab for rheumatoid arthritis is not rare and is related with good response: 13 years real-life experience. Rheumatology. 2021;60(5):2375–2382. [DOI] [PubMed] [Google Scholar]
- 58.Reddy V, Martinez L, Isenberg DA, Leandro MJ, Cambridge G. Pragmatic treatment of patients with systemic lupus erythematosus with rituximab: Long-term effects on serum immunoglobulins. Arthritis Care Res. 2017;69(6):857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evangelatos G, Moschopoulou M, Iliopoulos A, Fragoulis GE. Hypogammaglobulinemia in rheumatoid arthritis patients treated with rituximab: should we switch biologics? Comment on the article by Fraenkel et al et al. Arthritis Rheumatol. 2022;74(1):174–175. [DOI] [PubMed] [Google Scholar]
- 60.De La Torre I, Leandro MJ, Valor L, Becerra E, Edwards JCW, Cambridge G. Total serum immunoglobulin levels in patients with RA after multiple B-cell depletion cycles based on rituximab: relationship with B-cell kinetics. Rheumatology. 2012;51(5):833–840. [DOI] [PubMed] [Google Scholar]
- 61.Einarsson JT, Evert M, Geborek P, Saxne T, Lundgren M, Kapetanovic MC. Rituximab in clinical practice: dosage, drug adherence, Ig levels, infections, and drug antibodies. Clin Rheumatol. 2017;36(12):2743–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keystone E, Fleischmann R, Emery P, Furst DE, van Vollenhoven R, Bathon J, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 2007;56(12):3896–3908. [DOI] [PubMed] [Google Scholar]
- 63.Van Vollenhoven RF, Emery P, Bingham CO, Keystone EC, Fleischmann R, Furst DE, et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol. 2010;37(3):558–567. [DOI] [PubMed] [Google Scholar]
- 64.Kridin K, Ahmed AR. Post-rituximab immunoglobulin M (IgM) hypogammaglobulinemia. Autoimmun Rev. 2020;19(3):102466. [DOI] [PubMed] [Google Scholar]
- 65.Van Vollenhoven RF, Emery P, Bingham CO, Keystone EC, Fleischmann RM, Furst DE, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. 2013;72(9):1496–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Filanovsky K, Miller EB, Sigler E. Incidence of profound hypogammaglobulinemia and infection rate in lymphoma patients following the combination of chemotherapy and rituximab. Recent patents on. Published online 2016. https://www.ingentaconnect.com/content/ben/pra/2016/00000011/00000002/art00008 [DOI] [PubMed]
- 67.Marcinnò A, Marnetto F, Valentino P, Martire S, Balbo A, Drago A, et al. Rituximab-induced hypogammaglobulinemia in patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newman EN, Israelsen RB, Williamson K, Hsieh EWY. Hypogammaglobulinemia after rituximab therapy in children. Ann Allergy Asthma Immunol. 2022;128(2):225–226. [DOI] [PubMed] [Google Scholar]
- 69.Wade SD, Kyttaris VC. Rituximab-associated hypogammaglobulinemia in autoimmune rheumatic diseases: a single-center retrospective cohort study. Rheumatol Int. 2021;41(6):1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-Cell Depletion with Rituximab in Relapsing–Remitting Multiple Sclerosis. N Engl J Med. 2008;358(7):676688. [DOI] [PubMed] [Google Scholar]
- 71.Bar-Or A, Calabresi PAJ, Arnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400. [DOI] [PubMed] [Google Scholar]
- 72.Levy R, Mahévas M, Galicier L, Boutboul D, Moroch J, Loustau V, et al. Profound symptomatic hypogammaglobulinemia: a rare late complication after rituximab treatment for immune thrombocytopenia. Report of 3 cases and systematic review of the literature. Autoimmun Rev. 2014;13(10):1055–1063. [DOI] [PubMed] [Google Scholar]
- 73.Fujinaga S, Tomii Y. Profound effect of post-rituximab mycophenolate mofetil administration for persistent hypogammaglobulinemia in young children with steroid-dependent nephrotic syndrome. Clin Exp Nephrol. 2020;24(4):386–387. [DOI] [PubMed] [Google Scholar]
- 74.Fujinaga S, Ozawa K, Sakuraya K, Yamada A, Shimizu T. Late-onset adverse events after a single dose of rituximab in children with complicated steroid-dependent nephrotic syndrome. Clin Nephrol. 2016;85(6):340–345. [DOI] [PubMed] [Google Scholar]
- 75.Kado R, Sanders G, McCune WJ. Suppression of normal immune responses after treatment with rituximab. Curr Opin Rheumatol. 2016;28(3):251–258. [DOI] [PubMed] [Google Scholar]
- 76.Venhoff N, Effelsberg NM, Salzer U, Warnatz K, Peter HH, Lebrecht D, et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS One. 2012;7(5):e37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 2010;62(8):2458–2466. [DOI] [PubMed] [Google Scholar]
- 78.De Angelis F, Tosti ME, Capria S, Russo E, D’Elia GM, Annechini G, et al. Risk of secondary hypogammaglobulinaemia after Rituximab and Fludarabine in indolent non-Hodgkin lymphomas: A retrospective cohort study. Leuk Res. 2015;39(12):1382–1388. [DOI] [PubMed] [Google Scholar]
- 79.Sacco KA, Abraham RS. Consequences of B-cell-depleting therapy: hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy. 2018;10(8):713–728. [DOI] [PubMed] [Google Scholar]
- 80.Cabanillas F, Liboy I, Pavia O, Rivera E. High incidence of non-neutropenic infections induced by rituximab plus fludarabine and associated with hypogammaglobulinemia: a frequently unrecognized and easily treatable complication. Ann Oncol. 2006;17(9):1424–1427. [DOI] [PubMed] [Google Scholar]
- 81.Verschuuren EAM, Stevens SJC, van Imhoff GW, Middeldorp JM, de Boer C, Koëter G, et al. Treatment of posttransplant lymphoproliferative disease with rituximab: the remission, the relapse, and the complication. Transplantation. 2002;73(1):100–104. [DOI] [PubMed] [Google Scholar]
- 82.Kuehnle I, Huls MH, Liu Z, Semmelmann M, Krance RA, Brenner MK, et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood. 2000;95(4):1502–1505. [PubMed] [Google Scholar]
- 83.Imashuku S, Teramura T, Morimoto A, Naya M, Kuroda H. Prolonged hypogammaglobulinemia following rituximab treatment for post transplant Epstein–Barr virus-associated lymphoproliferative disease. Bone Marrow Transplant. 2003;33(1):129–130. [DOI] [PubMed] [Google Scholar]
- 84.Shimoni A, Hardan I, Avigdor A, Yeshurun M, Raanani P, Ben-Bassat I, et al. Rituximab reduces relapse risk after allogeneic and autologous stem cell transplantation in patients with high-risk aggressive non-Hodgkin’s lymphoma. Br J Haematol. 2003;122(3):457–464. [DOI] [PubMed] [Google Scholar]
- 85.Brugger W, Hirsch J, Grünebach F, Repp R, Brossart P, Vogel W, et al. Rituximab consolidation after high-dose chemotherapy and autologous blood stem cell transplantation in follicular and mantle cell lymphoma: a prospective, multicenter phase II study. Ann Oncol. 2004;15(11):1691–1698. [DOI] [PubMed] [Google Scholar]
- 86.Goldberg SL, Pecora AL, Alter RS, Kroll MS, Rowley SD, Waintraub SE, et al. Unusual viral infections (progressive multifocal leukoencephalopathy and cytomegalovirus disease) after high-dose chemotherapy with autologous blood stem cell rescue and peritransplantation rituximab. Blood, The Journal of the American Society of Hematology. 2002;99(4):1486–1488. [DOI] [PubMed] [Google Scholar]
- 87.Miles SA, McGratten M. Persistent panhypogammaglobulinemia after CHOP-rituximab for HIV-related lymphoma. J Clin Oncol. 2005;23(1):247–248. [DOI] [PubMed] [Google Scholar]
- 88.Nishio M, Fujimoto K, Yamamoto S, Endo T, Sakai T, Obara M, et al. Hypogammaglobulinemia with a selective delayed recovery in memory B cells and an impaired isotype expression after rituximab administration as an adjuvant to autologous stem cell transplantation for non-Hodgkin lymphoma. Eur J Haematol. 2006;77(3):226–232. [DOI] [PubMed] [Google Scholar]
- 89.Parmentier C, Delbet JD, Decramer S, Boyer O, Hogan J, Ulinski T. Immunoglobulin serum levels in rituximab-treated patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2020;35(3):455–462. [DOI] [PubMed] [Google Scholar]
- 90.Cohen BA. Late-onset neutropenia following ocrelizumab therapy for multiple sclerosis. Neurology. 2019;92(9):435–436. [DOI] [PubMed] [Google Scholar]
- 91.Tesfa D, Ajeganova S, Hägglund H, Sander B, Fadeel B, Hafström I, et al. Late-onset neutropenia following rituximab therapy in rheumatic diseases: association with B lymphocyte depletion and infections. Arthritis Rheum. 2011;63(8):2209–2214. [DOI] [PubMed] [Google Scholar]
- 92.Dunleavy K, Tay K, Wilson WH. Rituximab-associated neutropenia. Semin Hematol. 2010;47(2):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolach O, Shpilberg O, Lahav M. Neutropenia after rituximab treatment: new insights on a late complication. Curr Opin Hematol. 2012;19(1):32–38. [DOI] [PubMed] [Google Scholar]
- 94.Shimony S, Bar-Sever E, Berger T, Itchaki G, Gurion R, Yeshurun M, et al. Late onset neutropenia after rituximab and obinutuzumab treatment - characteristics of a class-effect toxicity. Leuk Lymphoma. 2021;62(12):2921–2927. [DOI] [PubMed] [Google Scholar]
- 95.Zonozi R, Wallace ZS, Laliberte K, Huizenga NR, Rosenthal JM, Rhee EP, et al. Incidence, clinical features, and outcomes of late-onset neutropenia from rituximab for autoimmune disease. Arthritis rheumatol. 2021;73(2):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cattaneo C, Spedini P, Casari S, Re A, Tucci A, Borlenghi E, et al. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma. 2006;47(6):1013–1017. [DOI] [PubMed] [Google Scholar]
- 97.Besada E, Koldingsnes W, Nossent J. Characteristics of late onset neutropenia in rheumatologic patients treated with rituximab: a case review analysis from a single center. QJM. 2012;105(6):545–550. [DOI] [PubMed] [Google Scholar]
- 98.Salmon JH, Cacoub P, Combe B, Sibilia J, Pallot-Prades B, Fain O, et al. Late-onset neutropenia after treatment with rituximab for rheumatoid arthritis and other autoimmune diseases: data from the AutoImmunity and Rituximab registry. RMD Open. 2015;1(1):e000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reitblat T, Wechsler A, Reitblat O. Rituximab-related late-onset neutropenia in patients with rheumatic diseases: successful re-challenge of the treatment. Am J Case Rep. 2015;16:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi YW, Jeong SH, Ahn MS, Lee HW, Kang SY, Choi JH, et al. Patterns of neutropenia and risk factors for febrile neutropenia of diffuse large B-cell lymphoma patients treated with rituximab-CHOP. J Korean Med Sci. 2014;29(11):1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nitta E, Izutsu K, Sato T, Ota Y, Takeuchi K, Kamijo A, et al. A high incidence of late-onset neutropenia following rituximab-containing chemotherapy as a primary treatment of CD20-positive B-cell lymphoma: a single-institution study. Ann Oncol. 2007;18(2):364–369. [DOI] [PubMed] [Google Scholar]
- 102.Arai Y, Yamashita K, Mizugishi K, Nishikori M, Hishizawa M, Kondo T, et al. Risk factors for late-onset neutropenia after rituximab treatment of B-cell lymphoma. Hematology. 2015;20(4):196–202. [DOI] [PubMed] [Google Scholar]
- 103.Hirayama Y, Kohda K, Konuma Y, Hirata Y, Kuroda H, Fujimi Y, et al. Late onset neutropenia and immunoglobulin suppression of the patients with malignant lymphoma following autologous stem cell transplantation with rituximab. Intern Med. 2009;48(1):57–60. [DOI] [PubMed] [Google Scholar]
- 104.Lemieux B, Tartas S, Traulle C, Espinouse D, Thieblemont C, Bouafia F, et al. Rituximab-related late-onset neutropenia after autologous stem cell transplantation for aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;33(9):921–923. [DOI] [PubMed] [Google Scholar]
- 105.Cairoli R, Grillo G, Tedeschi A, D’Avanzo G, Marenco P, Morra E. High incidence of neutropenia in patients treated with rituximab after autologous stem cell transplantation. Haematologica. 2004;89(3):361–363. [PubMed] [Google Scholar]
- 106.Weng WK, Negrin RS, Lavori P, Horning SJ. Immunoglobulin G Fc receptor FcgammaRIIIa 158 V/F polymorphism correlates with rituximab-induced neutropenia after autologous transplantation in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li SC, Chen YC, Evens AM, Lee CC, Liao HF, Yu CC, et al. Rituximab-induced late-onset neutropenia in newly diagnosed B-cell lymphoma correlates with Fc receptor FcγRIIIa 158 (V/F) polymorphism. Am J Hematol. 2010;85(10):810–812. [DOI] [PubMed] [Google Scholar]
- 108.Keane C, Nourse JP, Crooks P. Homozygous FCGR3A‐158V alleles predispose to late onset neutropenia after CHOP‐R for diffuse large B‐cell lymphoma. Intern Med. Published online 2012. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1445-5994.2011.02587.x?casa_token=d1a0d8Bv6gcAAAAA:4jQYH9A5fr4BeWkpuJLmYx5rS3G-iSuFyRiVScO4BuFHUrOPUJzSPT4su1yWqr_ib-aWqnPYM4DAQuCL [DOI] [PubMed]
- 109.Flinn IW, O’Donnell PV, Goodrich A, Vogelsang G, Abrams R, Noga S, et al. Immunotherapy with rituximab during peripheral blood stem cell transplantation for non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2000;6(6):628–632. [DOI] [PubMed] [Google Scholar]
- 110.Malpica Castillo LE, Palmer S, Deal A, Chen SL, Zhu A, Moll S. Incidence and Time Course of Neutropenia in Patients Treated with Rituximab-Based Therapy for Non-Malignant Immune-Mediated Hematologic Diseases. Blood. 2019;134:390. [DOI] [PubMed] [Google Scholar]
- 111.Moore DC. Drug-Induced Neutropenia: A Focus on Rituximab-Induced Late-Onset Neutropenia. P T. 2016;41(12):765–768. [PMC free article] [PubMed] [Google Scholar]
- 112.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–e93. [DOI] [PubMed] [Google Scholar]
- 113.Lai GGY, Lim ST, Tao M, Chan A, Li H, Quek R. Late-onset neutropenia following RCHOP chemotherapy in diffuse large B-cell lymphoma. Am J Hematol. 2009;84(7):414417. [DOI] [PubMed] [Google Scholar]
- 114.Rozman S, Sonc M, Novakovic BJ. Late-onset neutropenia following primary treatment of diff use large B-cell lymphoma with rituximab-containing therapy. Leuk Lymphoma. 2012;53(10):1945–1948. [DOI] [PubMed] [Google Scholar]
- 115.Kelesidis T, Daikos G, Boumpas D, Tsiodras S. Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis. 2011;15(1):e2–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Diwakar L, Gorrie S, Richter A, Chapman O, Dhillon P, Al-Ghanmi F, et al. Does rituximab aggravate pre-existing hypogammaglobulinaemia? J Clin Pathol. 2010;63(3):275–277. [DOI] [PubMed] [Google Scholar]
- 117.Thery-Casari C, Euvrard R, Mainbourg S, Durupt S, Reynaud Q, Durieu I, et al. Severe infections in patients with anti-neutrophil cytoplasmic antibody-associated vasculitides receiving rituximab: A meta-analysis. Autoimmun Rev. 2020;19(5):102505. [DOI] [PubMed] [Google Scholar]
- 118.Winthrop KL, Saag K, Cascino MD, Pei J, John A, Jahreis A, et al. Long-Term Safety of Rituximab in Rheumatoid Arthritis: Analysis From the SUNSTONE Registry. Arthritis Care Res. 2018;71(8):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gottenberg JE, Ravaud P, Bardin T, Cacoub P, Cantagrel A, Combe B, et al. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum. 2010;62(9):2625–2632. [DOI] [PubMed] [Google Scholar]
- 120.Aksoy S, Dizdar O, Hayran M, Harputluoğlu H. Infectious complications of rituximab in patients with lymphoma during maintenance therapy: a systematic review and meta-analysis. Leuk Lymphoma. 2009;50(3):357–365. [DOI] [PubMed] [Google Scholar]
- 121.Vidal L, Gafter-Gvili A, Leibovici L, Shpilberg O. Rituximab as maintenance therapy for patients with follicular lymphoma. Cochrane Database Syst Rev. 2009;(2):CD006552. [DOI] [PubMed]
- 122.Edwards JCW, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–2581. [DOI] [PubMed] [Google Scholar]
- 123.Genovese MC, Breedveld FC, Emery P, Cohen S, Keystone E, Matteson EL, et al. Safety of biological therapies following rituximab treatment in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68(12):1894–1897. [DOI] [PubMed] [Google Scholar]
- 124.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010;47(2):187–198. [DOI] [PubMed] [Google Scholar]
- 126.Ganjoo KN, Raman R, Sobel RA, Pinto HA. Opportunistic enteroviral meningoencephalitis: an unusual treatable complication of rituximab therapy. Leuk Lymphoma. 2009;50(4):673–675. [DOI] [PubMed] [Google Scholar]
- 127.Kiani-Alikhan S, Skoulidis F, Barroso A, Nuovo G, Ushiro-Lumb I, Breuer J, et al. Enterovirus infection of neuronal cells post-Rituximab. Br J Haematol. 2009;146(3):333–335. [DOI] [PubMed] [Google Scholar]
- 128.Padate BP, Keidan J. Enteroviral meningoencephalitis in a patient with non-Hodgkin’s lymphoma treated previously with rituximab. Clin Lab Haematol. 2006;28(1):69–71. [DOI] [PubMed] [Google Scholar]
- 129.Quartier P, Tournilhac O, Archimbaud C, Lazaro L, Chaleteix C, Millet P, et al. Enteroviral meningoencephalitis after anti-CD20 (rituximab) treatment. Clin Infect Dis. 2003;36(3):e47–e49. [DOI] [PubMed] [Google Scholar]
- 130.Hartmann JT, Meisinger I, Kröber SM, Weisel K, Klingel K, Kanz L. Progressive bicytopenia due to persistent parvovirus B19 infection after immunochemotherapy with fludarabine/cyclophosphamide and rituximab for relapsed B cell lymphoma. Haematologica. 2006;91(12 Suppl):ECR49. [PubMed] [Google Scholar]
- 131.Isobe Y, Sugimoto K, Shiraki Y, Nishitani M, Koike K, Oshimi K. Successful high-titer immunoglobulin therapy for persistent parvovirus B19 infection in a lymphoma patient treated with rituximab-combined chemotherapy. Am J Hematol. 2004;77(4):370–373. [DOI] [PubMed] [Google Scholar]
- 132.Sharma VR, Fleming DR, Slone SP. Pure red cell aplasia due to parvovirus B19 in a patient treated with rituximab. Blood. 2000;96(3):1184–1186. [PubMed] [Google Scholar]
- 133.Song KW, Mollee P, Patterson B, Brien W, Crump M. Pure red cell aplasia due to parvovirus following treatment with CHOP and rituximab for B-cell lymphoma. Br J Haematol. 2002;119(1):125–127. [DOI] [PubMed] [Google Scholar]
- 134.Byrd JC, Peterson BL, Morrison VA, Park K, Jacobson R, Hoke E, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood, The Journal of the American Society of Hematology. 2003;101(1):6–14. [DOI] [PubMed] [Google Scholar]
- 135.Lee MY, Chiou TJ, Hsiao LT, Yang MH, Lin PC, Poh SB, et al. Rituximab therapy increased post-transplant cytomegalovirus complications in Non-Hodgkin’s lymphoma patients receiving autologous hematopoietic stem cell transplantation. Ann Hematol. 2008;87(4):285–289. [DOI] [PubMed] [Google Scholar]
- 136.Levi ME, Quan D, Ho JT, Kleinschmidt-Demasters BK, Tyler KL, Grazia TJ. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clin Transplant. 2010;24(2):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mawhorter SD, Sierk A, Staugaitis SM, Avery RK, Sobecks R, Prayson RA, et al. Fatal West Nile Virus Infection After Rituximab/Fludarabine–Induced Remission for Non-Hodgkin’s Lymphoma. Clinical Lymphoma and Myeloma. 2005;6(3):248–250. [DOI] [PubMed] [Google Scholar]
- 138.Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, et al. Rituximabrelated viral infections in lymphoma patients. Leuk Lymphoma. 2007;48(7):1307–1312. [DOI] [PubMed] [Google Scholar]
- 139.Del Poeta G, Del Principe MI, Buccisano F, Maurillo L, Capelli G, Luciano F, et al. Consolidation and maintenance immunotherapy with rituximab improve clinical outcome in patients with B-cell chronic lymphocytic leukemia. Cancer. 2008;112(1):119–128. [DOI] [PubMed] [Google Scholar]
- 140.Ghielmini M, Schmitz SFH, Cogliatti S, Bertoni F, Waltzer U, Fey MF, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol. 2005;23(4):705–711. [DOI] [PubMed] [Google Scholar]
- 141.Ferrari A, Luppi M, Marasca R, Potenza L, Morselli M, Volzone F, et al. BK virus infection and neurologic dysfunctions in a patient with lymphoma treated with chemotherapy and rituximab. Eur J Haematol. 2008;81(3):244–245. [DOI] [PubMed] [Google Scholar]
- 142.Matteucci P, Magni M, Di Nicola M, Carlo-Stella C, Uberti C, Gianni AM. Leukoencephalopathy and papovavirus infection after treatment with chemotherapy and anti-CD20 monoclonal antibody. Blood. 2002;100(3):1104–1105. [DOI] [PubMed] [Google Scholar]
- 143.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–2806. [DOI] [PubMed] [Google Scholar]
- 144.Avouac J, Drumez E, Hachulla E, Seror R, Georgin-Lavialle S, El Mahou S, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3(6):e419–e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schiavetti I, Ponzano M, Signori A, Bovis F, Carmisciano L, Sormani MP. Severe outcomes of COVID-19 among patients with multiple sclerosis under anti-CD-20 therapies: A systematic review and meta-analysis. Mult Scler Relat Disord. 2022;57:103358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sparks JA, Wallace ZS, Seet AM, Gianfrancesco MA, Izadi Z, Hyrich KL, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis. 2021;80(9):1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sparks JA, Wallace ZS, Robinson PC. Coronavirus disease 2019: update on coronavirus disease 2019 outcomes and vaccine efficacy in patients with immune-mediated inflammatory disease. Curr Opin Rheumatol. 2021;33(5):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tepasse PR, Hafezi W, Lutz M, Kühn J, Wilms C, Wiewrodt R, et al. Persisting SARS-CoV-2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol. 2020;190(2):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leipe J, Wilke EL, Ebert MP, Teufel A, Reindl W. Long, relapsing, and atypical symptomatic course of COVID-19 in a B-cell-depleted patient after rituximab. Semin Arthritis Rheum. 2020;50(5):1087–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Esmaeili S, Abbasi MH, Abolmaali M, Mojtahed M, Alavi SNR, Soleimani S, et al. Rituximab and risk of COVID-19 infection and its severity in patients with MS and NMOSD. BMC Neurol. 2021;21(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yeo W, Chan TC, Leung NWY, Lam WY, Mo FKF, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27(4):605–611. [DOI] [PubMed] [Google Scholar]
- 152.Targhetta C, Cabras MG, Mamusa AM, Mascia G, Angelucci E. Hepatitis B virus-related liver disease in isolated anti-hepatitis B-core positive lymphoma patients receiving chemo- or chemo-immune therapy. Haematologica. 2008;93(6):951–952. [DOI] [PubMed] [Google Scholar]
- 153.Zell JA, Yoon EJ, Ignatius Ou SH, Hoefs JC, Chang JC. Precore mutant hepatitis B reactivation after treatment with CHOP–rituximab. Anticancer Drugs. 2005;16(1):83. [DOI] [PubMed] [Google Scholar]
- 154.Ng HJ, Lim LC. Fulminant hepatitis B virus reactivation with concomitant listeriosis after fludarabine and rituximab therapy: case report. Ann Hematol. 2001;80(9):549–552. [DOI] [PubMed] [Google Scholar]
- 155.Law JK, Ho JK, Hoskins PJ, Erb SR, Steinbrecher UP, Yoshida EM. Fatal reactivation of hepatitis B post-chemotherapy for lymphoma in a hepatitis B surface antigen-negative, hepatitis B core antibody-positive patient: potential implications for future prophylaxis recommendations. Leuk Lymphoma. 2005;46(7):1085–1089. [DOI] [PubMed] [Google Scholar]
- 156.Mitka M. FDA: Increased HBV reactivation risk with ofatumumab or rituximab. JAMA. 2013;310(16):1664. [DOI] [PubMed] [Google Scholar]
- 157.Lu H, Lok AS, Warneke CL, Ahmed S, Torres HA, Martinez F, et al. Passive transfer of anti-HBc after intravenous immunoglobulin administration in patients with cancer: a retrospective chart review. Lancet Haematol. 2018;5(10):e474–e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood, The Journal of the American Society of Hematology. 2009;113(20):4834–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khalid S, Smith R, Yalakki L. SAT0112 EFFECT OF RITUXIMAB ON IMMUNOGLOBULIN LEVELS AND RISK OF ASSOCIATED INFECTION. Ann Rheum Dis. 2020;79(Suppl 1):989–990. [Google Scholar]
- 160.Meyer A, Theulin A, Chatelus E, Argemi X, Sordet C, Javier RM, et al. Campylobacter fetus infection in three rheumatoid arthritis patients treated with rituximab. Ann Rheum Dis. 2012;71(6):1094–1095. [DOI] [PubMed] [Google Scholar]
- 161.Nakazawa H, Nishina S, Sakai H, Ito T, Ishida F, Kitano K. Successful Empiric Therapy for Postsplenectomy Sepsis with Campylobacter fetus in an Abattoir Worker with Follicular Lymphoma. Intern Med. 2018;57(22):3329–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Russo I, Miotto S, Saponeri A, Alaibac M. Ultra-low dose rituximab for refractory pemghigus vulgaris: a pilot study. Expert Opin Biol Ther. 2020;20(6):673–678. [DOI] [PubMed] [Google Scholar]
- 163.Hartman J, Westerman M, Wagenaar JFP. Two-sided femoral Campylobacter jejuni osteomyelitis in a patient with acquired hypogammaglobulinemia: a case report. BMC Infect Dis. 2020;20(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gharamti AA, Moukalled N, Taher A, Kanafani ZA. Recurrent Campylobacter Bacteremia as the First Manifestation of Hypogammaglobulinemia: a Case Report and Literature Review. Infect Chemother. 2020;52(3):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lutt JR, Pisculli ML, Weinblatt ME, Deodhar A, Winthrop KL. Severe nontuberculous mycobacterial infection in 2 patients receiving rituximab for refractory myositis. J Rheumatol. 2008;35(8):1683–1685. [PubMed] [Google Scholar]
- 166.Chen YC, Jou R, Huang WL, Huang ST, Liu KC, Lay CJ, et al. Bacteremia caused by Mycobacterium wolinskyi. Emerg Infect Dis. 2008;14(11):1818–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nixon A, Ogden L, Woywodt A, Dhaygude A. Infectious complications of rituximab therapy in renal disease. Clin Kidney J. 2017;10(4):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stabler S, Giovannelli J, Launay D, Cotteau-Leroy A, Heusele M, Lefèvre G, et al. Serious Infectious Events and Immunoglobulin Replacement Therapy in Patients With Autoimmune Disease Receiving Rituximab: A Retrospective Cohort Study. Clin Infect Dis. 2021;72(5):727–737. [DOI] [PubMed] [Google Scholar]
- 169.van der Kolk LE, Baars JW, Prins MH, van Oers MHJ. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100(6):2257–2259. [PubMed] [Google Scholar]
- 170.Ennishi D, Terui Y, Yokoyama M, Mishima Y, Takahashi S, Takeuchi K, et al. Increased incidence of interstitial pneumonia by CHOP combined with rituximab. Int J Hematol. 2008;87(4):393–397. [DOI] [PubMed] [Google Scholar]
- 171.Katsuya H, Suzumiya J, Sasaki H, Ishitsuka K, Shibata T, Takamatsu Y, et al. Addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy has a high risk of developing interstitial pneumonia in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2009;50(11):1818–1823. [DOI] [PubMed] [Google Scholar]
- 172.Brusamolino E, Rusconi C, Montalbetti L, Gargantini L, Uziel L, Pinotti G, et al. Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: a phase II study of feasibility and toxicity. Haematologica. 2006;91(4):496–502. [PubMed] [Google Scholar]
- 173.Kolstad A, Holte H, Fosså A, Lauritzsen GF, Gaustad P, Torfoss D. Pneumocystis jirovecii pneumonia in B-cell lymphoma patients treated with the rituximab-CHOEP-14 regimen. Haematologica. 2007;92(1):139–140. [DOI] [PubMed] [Google Scholar]
- 174.Venhuizen AC, Hustinx WNM, van Houte AJ, Veth G, van der Griend R. Three cases of Pneumocystis jirovecii pneumonia (PCP) during first-line treatment with rituximab in combination with CHOP-14 for aggressive B-cell non-Hodgkin’s lymphoma. Eur J Haematol. 2008;80(3):275–276. [DOI] [PubMed] [Google Scholar]
- 175.Kumar D, Gourishankar S, Mueller T, Cockfield S, Weinkauf J, Vethanayagam D, et al. Pneumocystis jirovecii pneumonia after rituximab therapy for antibody-mediated rejection in a renal transplant recipient. Transpl Infect Dis. 2009;11(2):167–170. [DOI] [PubMed] [Google Scholar]
- 176.Shelton E, Yong M, Cohney S. Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology. 2009;14(7):696–699. [DOI] [PubMed] [Google Scholar]
- 177.Teichmann LL, Woenckhaus M, Vogel C, Salzberger B, Schölmerich J, Fleck M. Fatal Pneumocystis pneumonia following rituximab administration for rheumatoid arthritis. Rheumatology. 2008;47(8):1256–1257. [DOI] [PubMed] [Google Scholar]
- 178.Sem M, Molberg Ø, Lund MB, Gran JT. Rituximab treatment of the anti-synthetase syndrome—a retrospective case series. Rheumatology. 2009;48(8):968–971. [DOI] [PubMed] [Google Scholar]
- 179.Fianchi L, Rossi E, Murri R, De Stefano V, Pagano L, Leone G. Severe infectious complications in a patient treated with rituximab for idiopathic thrombocytopenic purpura. Ann Hematol. 2007;86(3):225–226. [DOI] [PubMed] [Google Scholar]
- 180.Lunardon L, Tsai KJ, Propert KJ, Fett N, Stanley JR, Werth VP, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148(9):1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ettahar N, Legout L, Ajana F, Patoz P, Massongo M, Rose C, et al. Cryptococcose osseuse chez une patiente porteuse d’une leucémie lymphocytique traitée par fludarabine-cyclophosphamide-rituximab. J Mycol Med. 2013;23(1):57–63. [DOI] [PubMed] [Google Scholar]
- 182.Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46(3):370–376. [DOI] [PubMed] [Google Scholar]
- 183.Safa G, Darrieux L. Cerebral toxoplasmosis after rituximab therapy. JAMA Intern Med. 2013;173(10):924–926. [DOI] [PubMed] [Google Scholar]
- 184.Alkhunaizi AM, Dawamneh MF, Banda RW, Daabil RA, Al-Tawfiq JA, Akkad SA, et al. Acanthamoeba encephalitis in a patient with systemic lupus treated with rituximab. Diagn Microbiol Infect Dis. 2013;75(2):192–194. [DOI] [PubMed] [Google Scholar]
- 185.Heusele M, Clerson P, Guery B, Lambert M, Launay D, Lefevre G, et al. Risk factors for severe bacterial infections in patients with systemic autoimmune diseases receiving rituximab. Clin Rheumatol. 2014;33(6):799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kanbayashi Y, Nomura K, Fujimoto Y, Yamashita M, Ohshiro M, Okamoto K, et al. Risk factors for infection in haematology patients treated with rituximab. Eur J Haematol. 2009;82(1):26–30. [DOI] [PubMed] [Google Scholar]
- 187.Varley CD, Winthrop KL. Long-Term Safety of Rituximab (Risks of Viral and Opportunistic Infections). Curr Rheumatol Rep. 2021;23(9):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lanini S, Molloy AC, Prentice AG, Ippolito G, Kibbler CC. Infections in patients taking Rituximab for hematologic malignancies: two-year cohort study. BMC Infect Dis. 2013;13:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guilpain P, Le Bihan C, Foulongne V, Taourel P, Pansu N, Maria ATJ, et al. Rituximab for granulomatosis with polyangiitis in the pandemic of covid-19: lessons from a case with severe pneumonia. Ann Rheum Dis. 2021;80(1):e10. [DOI] [PubMed] [Google Scholar]
- 190.Xavier B, Lafaurie M, Treiner E, Walter O, Pugnet G, Martin-Blondel G, et al. POS1178 PRESCRIBING RITUXIMAB IN PATIENTS WITH AUTO-IMMUNE DISEASES AND ACQUIRED HYPOGAMMAGLOBULINEMIA: DESCRIPTION OF THE RISK OF SEVERE INFECTION IN 121 PATIENTS BEFORE THE SARS-Cov2 ERA. Ann Rheum Dis. 2022;81(Suppl 1):917–917. [Google Scholar]
- 191.Forstpointner R, Dreyling M, Repp R, Hermann S, Hänel A, Metzner B, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(10):3064–3071. [DOI] [PubMed] [Google Scholar]
- 192.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. [DOI] [PubMed] [Google Scholar]
- 193.Eve HE, Linch D, Qian W, Ross M, Seymour JF, Smith P, et al. Toxicity of fludarabine and cyclophosphamide with or without rituximab as initial therapy for patients with previously untreated mantle cell lymphoma: results of a randomised phase II study. Leuk Lymphoma. 2009;50(2):211–215. [DOI] [PubMed] [Google Scholar]
- 194.Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99(9):706–714. [DOI] [PubMed] [Google Scholar]
- 195.Rafailidis PI, Kakisi OK, Vardakas K, Falagas ME. Infectious complications of monoclonal antibodies used in cancer therapy: a systematic review of the evidence from randomized controlled trials. Cancer. 2007;109(11):2182–2189. [DOI] [PubMed] [Google Scholar]
- 196.Kronbichler A, Kerschbaum J, Gopaluni S, Tieu J, Alberici F, Jones RB, et al. Trimethoprim-sulfamethoxazole prophylaxis prevents severe/life-threatening infections following rituximab in antineutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis. 2018;77(10):1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122(2):139–145. [DOI] [PubMed] [Google Scholar]
- 198.Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116(23):5555–5563. [DOI] [PubMed] [Google Scholar]
- 199.Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit Rev Oncol Hematol. 2014;90(3):190–199. [DOI] [PubMed] [Google Scholar]
- 200.Inan H, Kingsley JL, Ozen MO, Tekin HC, Hoerner CR, Imae Y, et al. Monitoring Neutropenia for Cancer Patients at the Point of Care. Small Methods. 2017;1(9). doi: 10.1002/smtd.201700193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Buch MH, Smolen JS, Betteridge N, Breedveld FC, Burmester G, Dörner T, et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(6):909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Van Assen S, Agmon-Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70(3):414–422. [DOI] [PubMed] [Google Scholar]
- 203.Papp KA, Haraoui B, Kumar D, Marshall JK, Bissonnette R, Bitton A, et al. Vaccination Guidelines for Patients With Immune-Mediated Disorders on Immunosuppressive Therapies. J Cutan Med Surg. 2019;23(1):50–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tanrıöver MD, Akar S, Türkçapar N, Karadağ Ö, Ertenli İ, Kiraz S. Vaccination recommendations for adult patients with rheumatic diseases. Eur J Rheumatol Inflamm. 2016;3(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Centers for Disease Control and Prevention. ACIP general best practice guidelines for immunization. Published online 2021.
- 206.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309–318. [DOI] [PubMed] [Google Scholar]
- 207.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, De La Morena M, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 Suppl):S1–S24. [DOI] [PubMed] [Google Scholar]
- 208.Otani IM, Lehman HK, Jongco AM, Tsao LR, Azar AE, Tarrant TK, et al. Practical guidance for the diagnosis and management of secondary hypogammaglobulinemia: A Work Group Report of the AAAAI Primary Immunodeficiency and Altered Immune Response Committees. J Allergy Clin Immunol. 2022;149(5):1525–1560. [DOI] [PubMed] [Google Scholar]
- 209.Paris K, Sorensen RU. Assessment and clinical interpretation of polysaccharide antibody responses. Ann Allergy Asthma Immunol. 2007;99(5):462–464. [DOI] [PubMed] [Google Scholar]
- 210.Butler JC. Epidemiology of pneumococcal serotypes and conjugate vaccine formulations. Microb Drug Resist. 1997;3(2):125–129. [DOI] [PubMed] [Google Scholar]
- 211.Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332(12):761–766. [DOI] [PubMed] [Google Scholar]
- 212.Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol. 2017;139(3, Supplement):S1–S46. [DOI] [PubMed] [Google Scholar]
- 213.Becerra E, De La Torre I, Leandro MJ, Cambridge G. B cell phenotypes in patients with rheumatoid arthritis relapsing after rituximab: expression of B cell-activating factor-binding receptors on B cell subsets. Clin Exp Immunol. 2017;190(3):372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Marasco E, Aquilani A, Cascioli S, Moneta GM, Caiello I, Farroni C, et al. Switched memory B cells are increased in oligoarticular and polyarticular juvenile idiopathic arthritis and their change over time is related to response to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2018;70(4):606–615. [DOI] [PubMed] [Google Scholar]
- 215.Schiavo E, Martini B, Attardi E, Consonni F, Ciullini Mannurita S, Coniglio ML, et al. Autoimmune Cytopenias and Dysregulated Immunophenotype Act as Warning Signs of Inborn Errors of Immunity: Results From a Prospective Study. Front Immunol. 2021;12:790455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cunningham-Rundles C. Autoimmune manifestations in common variable immunodeficiency. J Clin Immunol. 2008;28 Suppl 1(Suppl 1):S42–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mayor PC, Eng KH, Singel KL, Abrams SI, Odunsi K, Moysich KB, et al. Cancer in primary immunodeficiency diseases: Cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol. 2018;141(3):1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ardeniz O, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol. 2009;133(2):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Florou D, Katsara M, Feehan J, Dardiotis E, Apostolopoulos V. Anti-CD20 Agents for Multiple Sclerosis: Spotlight on Ocrelizumab and Ofatumumab. Brain Sci. 2020;10(10). doi: 10.3390/brainsci10100758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Freeman CL, Sehn LH. A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol. 2018;182(1):29–45. [DOI] [PubMed] [Google Scholar]
- 222.Lin TS . Ofatumumab: a novel monoclonal anti-CD20 antibody. Pharmgenomics Pers Med. 2010;3:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies. Adv Ther. 2017;34(2):324–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Davis JA, Shockley A, Glode AE. Newly approved anti-CD19 monoclonal antibodies for the treatment of relapsed or refractory diffuse large B-cell lymphoma. J Oncol Pharm Pract. 2022;28(3):686–690. [DOI] [PubMed] [Google Scholar]
- 225.Rensel M, Zabeti A, Mealy MA, Cimbora D, She D, Drappa J, et al. Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin4-immunoglobulin G-seropositive participants taking inebilizumab for ≥4 years in the NMOmentum trial. Mult Scler. 2022;28(6):925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dörner T, Shock A, Goldenberg DM, Lipsky PE. The mechanistic impact of CD22 engagement with epratuzumab on B cell function: Implications for the treatment of systemic lupus erythematosus. Autoimmun Rev. 2015;14(12):1079–1086. [DOI] [PubMed] [Google Scholar]
- 227.Zhang Y, Tian J, Xiao F, Zheng L, Zhu X, Wu L, et al. B cell-activating factor and its targeted therapy in autoimmune diseases. Cytokine Growth Factor Rev. 2022;64:57–70. [DOI] [PubMed] [Google Scholar]
- 228.Estupiñán HY, Berglöf A, Zain R, Smith CIE. Comparative Analysis of BTK Inhibitors and Mechanisms Underlying Adverse Effects. Front Cell Dev Biol. 2021;9:630942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 231.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]