Abstract
Purinergic signaling is a key molecular pathway in the maintenance of bone health and regeneration. P1 receptor signaling, which is activated by extracellular adenosine, has emerged as a key metabolic pathway that regulates bone tissue formation, function, and homeostasis. Extracellular adenosine is mainly produced by ectonucleotidases, and alterations in the function of these enzymes or compromised adenosine generation can result in bone disorders, such as osteoporosis and impaired fracture healing. This mini review discusses the key role played by adenosine in bone health and how its alterations contribute to bone diseases, as well as potential therapeutic applications of exogenous adenosine to combat bone diseases like osteoporosis and injury.
Introduction
Bone is a dynamic tissue which continuously undergoes remodeling primarily through osteoblast-mediated bone formation and osteoclast-mediated bone resorption [1]. The close interplay between osteoblast and osteoclast activity ultimately determines the turnover, remodeling, and overall health of bone tissue. Disruption of this homeostasis due to alterations in molecular signaling, physiological aging, or injury can lead to various bone pathologies and compromised healing. Purinergic signaling has been identified as a key molecular pathway involved in bone remodeling and homeostasis [2]. Purinergic receptors can be categorized into either P2- or P1-type based on the activation ligand. P2 receptors are activated by nucleotides whereas P1 receptors are activated by the nucleoside adenosine. While P2 and P1 purinergic receptors are expressed by osteoblasts and osteoclasts, this review will focus on the role played by purinergic P1 receptors and adenosine signaling in bone tissue health and regeneration. For a more comprehensive review on P2 purinergic signaling and its effect on bone homeostasis, refer to other reviews [3].
Adenosine is a nucleoside that is produced mostly through enzymatic degradation of adenine nucleotides (ATP, ADP, and AMP). Typically, healthy physiological concentrations of adenosine are maintained between 20 and 300 nM [4]. Extracellular adenosine levels can be drastically increased in response to pathological conditions such as injury, inflammation, or factors that contribute to cellular stresses; where cells can produce over 10 times the basal level of adenosine, resulting in micromolar concentrations [5]. This effect is a result of a stress-induced increase in phosphatase activity along with activation of ecto-5’-nucleotidase (CD73) and ectonucleoside triphosphate diphosphohydrolase-1 (CD39) [6]. Specifically, upon injury or mechanical stress, intracellular ATP is released to the extracellular space through pannexin/connexin channels [7]. The extracellular ATP is hydrolyzed to ADP and AMP by CD39, and subsequently hydrolyzed to the adenosine metabolite via CD73. In addition, tissue nonspecific alkaline phosphatase (TNAP) has also been shown to contribute to the extracellular adenosine via degradation of ATP to adenosine [8]. Once in the extracellular space, adenosine functions through G-protein-coupled receptors, specifically binding to four distinct adenosine receptors – ADORA1, ADORA2A, ADORA2B, and ADORA3. Different concentrations of adenosine are needed to activate these receptors. At typical physiological adenosine concentration of 0.3–3 nM and 1–20 nM, ADORA1 and ADORA2A can be activated, respectively [9]. Contrarily, ADORA2B and ADORA3 require much higher levels of adenosine, with activation occurring at concentrations of 10 μM and 1 μM, respectively. Although a majority of adenosine is produced from nucleotide metabolism, alternative pathways also contribute to adenosine production. For instance, adenosine can be intracellularly generated from hydrolysis of S-adenosyl-homocysteine (SAH) in the methionine cycle, and subsequently transported to the extracellular space through equilibrative nucleoside transporters (ENT1/2) [4]. Interestingly, molecules other than adenosine, such as Netrin, can also enhance extracellular adenosine signaling. For example, Netrin-1 can activate ADORA2B and has been shown to promote revascularization during limb ischemia [10].
This mini review will discuss how alterations in adenosine signaling contribute to changes in bone health and repair outcomes. Although the significant role played by adenosine in bone homeostasis and its potential therapeutic impact has been widely recognized, severe side effects caused by systemic administration have limited its clinical use. This review will also discuss the technological advancements in the field to circumvent the challenges associated with adenosine delivery.
Adenosine and purinergic signaling in bone health and disease
Adenosine signaling has emerged as a key metabolic pathway that regulates bone tissue formation, function, and homeostasis. Bone tissue-specific cells (osteoblasts and osteoclasts) express all four types of adenosine receptors. Activation of ADORA1 has been shown to be critical for osteoclast differentiation by functioning through tumor necrosis factor receptor-associated factor 6 (TRAF6) and transforming growth factor-β-activated kinase 1 (TAK1) signaling [11]. In fact, ADORA1 knockout mice show significantly higher bone density than wild type mice, which is associated with the loss of ruffled borders of osteoclasts in the knockout mice [12]. ADORA2A activation counters the stimulation of ADORA1, in that it inhibits osteoclastogenesis and osteoclast function [13]. Similarly, ADORA2B activation has been shown to promote osteoblast differentiation and increased function, while also reducing osteoclast numbers [14,15]. ADORA3 has been investigated less compared to other adenosine receptors; however, studies have shown that ADORA3 activation can prevent osteoclast bone resorption [16]. Overall, adenosine receptor activation (or inhibition) is touted to have a major effect on bone homeostasis and bone tissue regeneration (Figure 1). Factors including changes in progenitor cell function, distorted inflammatory response, and reduced neo-vascularization have been implicated in impaired fracture healing [17,18]. Aside from bone-specific cells, adenosine signaling also influences various types of immune cells, both in innate and adaptive immunity. For example, monocytes, macrophages, neutrophils, and B cells express all four adenosine receptors and can be regulated by extracellular adenosine. An abundant source of extracellular adenosine can also be provided by immune cells such as neutrophils and regulatory T cells (Tregs). Adenosine also functions as an anti-inflammatory molecule. ADORA2A and ADORA2B activation have been shown to reduce macrophage production of pro-inflammatory mediators while increasing production of the anti-inflammatory cytokine IL-10 [19,20]. Additionally, adenosine has been shown to polarize macrophages from an M1-like to M2-like phenotype through ADORA2A and ADORA2B [21]. Adenosine, at micromolar concentrations, inhibits recruitment and activation of neutrophils and suppresses effector T cell function while promoting immunosuppressive Tregs [20]. Extracellular adenosine-mediated immunomodulation can also have a significant impact on bone tissue repair, as timely resolution of inflammation is necessary to achieve normal bone healing [22]. Angiogenesis and vascularization can be influenced by immune cells such as macrophages during bone tissue repair. Wang et al. demonstrated that administration of an ADORA2A antagonist altered macrophage secretome, which led to decreased angiogenesis and bone nonunion [23*]. Together these studies suggest that adenosine signaling influences multiple aspects of bone repair, and therefore, adenosine could serve as a potential therapeutic for bone healing.
Figure 1.
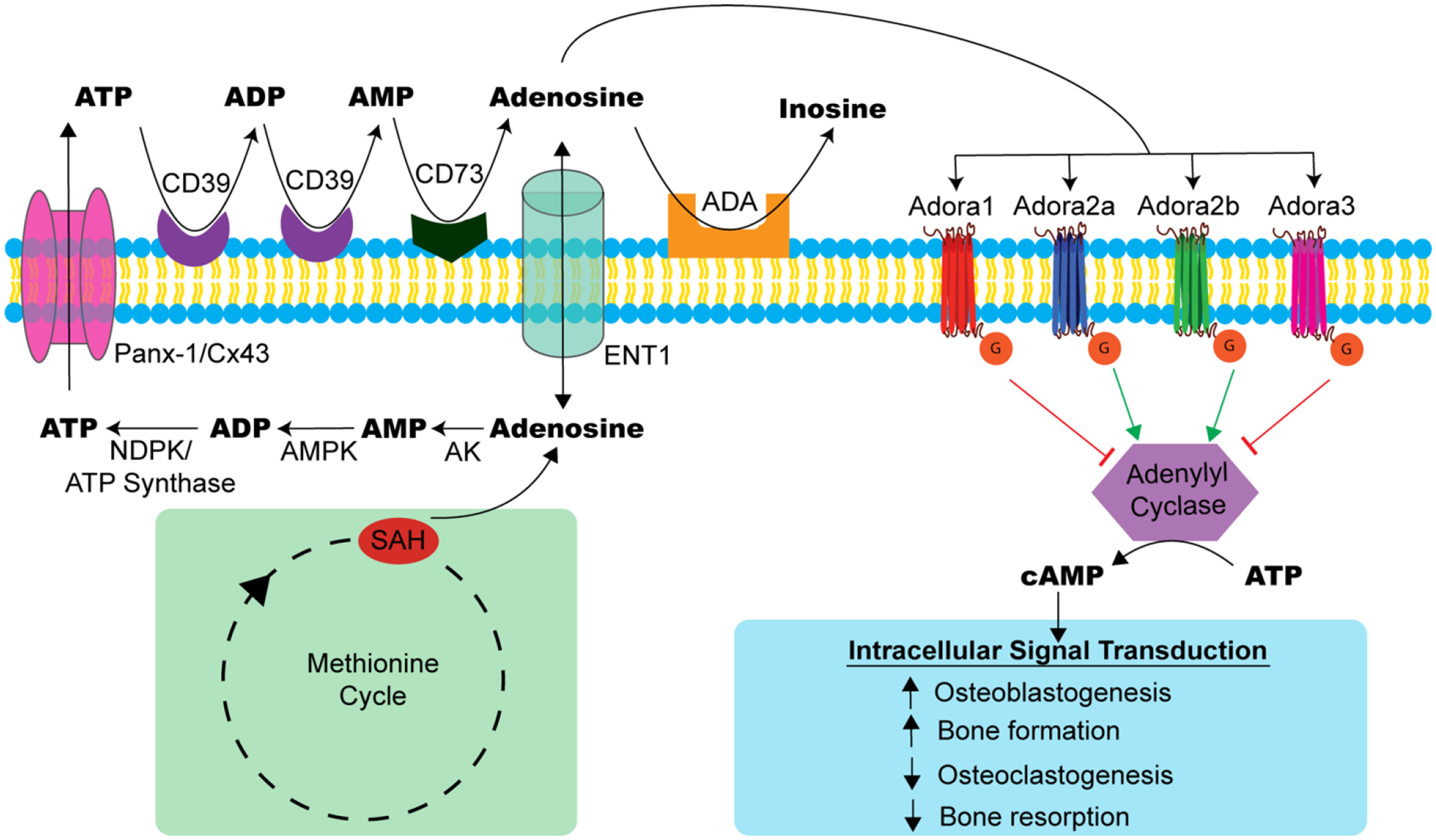
Extracellular adenosine generation and signaling in bone health. Extracellular adenosine can originate extracellularly or intracellularly. Most of the extracellular adenosine generation is mediated by ectonucleotidases (CD39 and CD73), which enzymatically degrade adenine nucleotides (ATP, ADP, AMP) to produce adenosine. Within the cell, adenosine can be generated from the methionine cycle where S-adenosyl-homocysteine (SAH) is hydrolyzed. Intracellular adenosine can be transported to the extracellular space through equilibrative nucleoside transporters (ENT1) or converted to ATP through phosphorylation by adenosine kinase (AK), AMP kinase (AMPK), and a combination of nucleoside diphosphate kinase (NDPK) and ATP synthase. The intracellular ATP can be released to the extracellular space through pannexin/connexin (Panx-1/Cx43) channels. Once in the extracellular space, ATP can be metabolized into adenosine via ectonucleotidases. Extracellular adenosine can activate four distinct adenosine receptors – ADORA1, ADORA2A, ADORA2B, and ADORA3. Following activation, these G-protein-coupled receptors can upregulate or inhibit adenylyl cyclase production of cyclic AMP (cAMP) and intracellular signal transduction. This ultimately affects bone cell activity and homeostasis. Adenosine deaminase (ADA) can irreversibly deamidate excess adenosine to inosine.
Multipotent stem cells such as bone marrow mesenchymal stem cells (MSCs) can differentiate to osteoblasts; MSCs can also differentiate into adipocytes and chondrocytes. We have shown that activation of ADORA2B promotes osteogenic differentiation of MSCs while inhibiting their differentiation into adipocytes [24]. Thus, adenosine-mediated osteogenic differentiation of mesenchymal progenitor cells could play a key role in promoting bone tissue regeneration. In fact, Adora2b knockout mice showed a reduction in MSC differentiation to osteoblasts as well as delayed/compromised fracture healing, demonstrating the role of adenosine signaling in bone tissue regeneration [25]. Similarly, Adora2a knockout mice have significantly decreased bone volume and trabecular number. Studies using CD73 knockout mice also show impaired osteoblast differentiation, osteopenia in young animals, and delayed bone regeneration in aged animals [26–28]. Consistent with these findings, a recent study by Kimura et al. showed endogenous CD73+ MSCs promote fracture repair and give rise to new cartilage and bone [29*].
Osteoporosis is a metabolic bone disorder characterized by a loss of bone mass. This disease is a result of an imbalance in bone homeostasis with enhanced osteoclastic activity leading to bone resorption. Recently, it has been shown that adenosine signaling is altered in osteoporosis, as CD39 and CD73 expression along with extracellular adenosine levels in the bone marrow are significantly decreased in ovariectomized animals [30]. Aside from changes in bone cell activity and function, increased inflammation and chronic inflammation have also been associated with osteoporosis. These characteristics were complemented by a study that showed animal intake of excessive caffeine, an adenosine antagonist, resulted in increased IL-1β and bone resorption in a periapical periodontitis model [31]. Contrarily, Llamas-Granda et al. have shown that use of molecules like dipyridamole, which augments the effects of extracellular adenosine, reduce bone loss in ovariectomized animals [32*]. Dipyridamole can inhibit equilibrative nucleoside transporter (ENT) uptake into the cell, and thereby increase the extracellular adenosine concentration. Similarly, ticagrelor can also increase extracellular adenosine concentration by functioning as an inhibitor of ENTs [33]. Together, these studies suggest the therapeutic potential of adenosine in combating osteoporosis and improving bone healing.
Harnessing adenosine signaling to promote bone tissue repair
Given that adenosine can contribute to various phases of bone homeostasis and bone regeneration, harnessing adenosine signaling provides a therapeutic opportunity to treat bone disorders. Leveraging adenosine signaling, either through the administration of adenosine or adenosine receptor agonists, could be an effective approach to address bone tissue regeneration; however, such an approach has remained elusive. One limitation is the extremely short half-life of adenosine, which is less than 10 seconds, and thus its reduced bioavailability [34]. Another complication is the severe side effects associated with systemic administration of adenosine, such as suppressed cardiac function, chest discomfort, and dyspnea [35]. A leading concept in drug delivery is utilizing a carrier to improve the delivery efficacy and biodistribution of the drug molecule. Studies have leveraged various drug carriers ranging from liposomes to polymer-based carriers to deliver adenosine [36,37*,38,39*,40*]. Adenosine can be incorporated into the biomaterial and its release can be designed to occur through one or a combination of mechanisms such as hydrolysis, tissue-specific enzymes, or diffusion-mediated release [40*,41,42]. The vicinal diol groups of adenosine offer a unique drug loading opportunity. Specifically, the diol groups can react with boronate groups leading to reversible boronate ester formation. The reversible covalent bond between the adenosine and boronate group allows for both loading and release of adenosine molecules [40*]. Recently, this reversible bond has been utilized to incorporate adenosine into a nanocarrier [37*]. The adenosine delivery units can be further modified to target delivery to the bone tissue. Bone targeting of nanocarriers functionalized with alendronate, which binds to hydroxyapatite minerals, and loaded with adenosine showed therapeutic efficacy in treating osteoporosis in a mouse model [37*,39*]. Since activated osteoclasts release hydrochloric acid and acidify the local niche, hydrogen ions which decrease the pH of the tissue can be used as a trigger for the release of adenosine [39*]. Unlike bisphosphonate-based drugs that only target osteoclast function, adenosine is an osteoanabolic molecule that promotes osteoblastogenesis while inhibiting excessive osteoclastogenesis . Consistent with these understandings, osteoporotic mice treated with adenosine showed new bone formation and bone qualities similar to healthy controls [37*,39*]. Recently, Larrañaga-Vera directly conjugated CGS21680, a selective ADORA2A agonist, to alendronate through a PEG-linker to treat osteoporotic pathology in a mouse model. Osteoporotic mice treated with the ADORA2A agonist exhibited new bone formation and reduced bone loss [43].
While bone disorders like osteoporosis require systemic administration, local delivery of adenosine might be adequate to treat bone injuries such as fractures. Zeng et al., devised a biomaterial approach to capitalize on the surge of extracellular adenosine released following fracture, where a macroporous hydrogel was designed to reversibly sequester the endogenous adenosine to increase both its bioavailability and local signaling [40*]. The results showed that the biomaterial-assisted localization of adenosine at the fracture site promoted vascularization and accelerated bone healing. In a following study, an injectable in situ curing scaffold that enables minimally invasive therapeutic administration, was designed to deliver endogenous adenosine to the fracture site [38]. Studies have also shown that local implantation of a fibrin gel containing an ADORA2A agonist improved bone healing in rat fractures [44]. The authors showed that local delivery of the ADORA2A agonist resulted in an altered inflammatory response of increased Tregs accompanied by decreased Th17 cells and IL-6. Similar to fracture healing studies, critical sized cranial defects treated using a biomaterial loaded with adenosine showed increased bone regeneration [45–47]. Purinergic signaling and increases in extracellular adenosine have also been used to treat several other localized musculoskeletal tissues such as rotator cuff tears, with a focus on increasing tendon-bone junction healing, as well as osteoarthritis, by reducing cartilage degeneration [36,48].
Concluding remarks
In summary, the recent studies discussed complement previous understandings of purinergic signaling in maintaining bone health and repair. These studies clearly demonstrate the key role played by extracellular adenosine, mostly generated by the “classical” adenosinergic pathway involving ectonucleotidases, in bone homeostasis and regeneration. Indeed, alterations in ectonucleotidase function and compromised extracellular adenosine production have been associated with bone metabolic diseases such as osteoporosis and compromised bone regeneration following injury. Bone regeneration is a complex, well-orchestrated physiological process involving multiple cell populations and cellular/molecular processes. Adenosine, which can act on multiple cell populations including immune cells, while simultaneously promoting osteogenesis and angiogenesis, represents a novel and effective therapeutic molecule. Towards this, various drug delivery strategies, both local and systemic, have begun to be explored. Biomaterial-based drug delivery systems, from macroscopic structures to nanocarriers, are not only effective in improving the safety but can also prolong the release kinetics and half-life of adenosine molecules. Although biomaterial-assisted delivery of adenosine is in the early stages of exploration, it has shown promising results in animal models of bone injuries and osteoporosis. Continued development of therapeutic strategies that enable exogenous adenosine delivery and/or activate the purinergic signaling pathway to promote bone health and combat skeletal disease, could potentially translate to clinical treatments.
Acknowledgement
This work is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number NIH R01 AR071552 and R01 AR079189, and the National Institute on Aging of the National Institutes of Health under Award Number NIH R01 AG074491. The authors would like to thank Anna Gilpin for her comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit author statement
Hunter Newman: Conceptualization, Writing- Original draft preparation, Writing- Reviewing and Editing. Shyni Varghese: Conceptualization, Supervision, Writing- Original draft preparation, Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Tanaka Y, Nakayamada S, Okada Y: Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy 2005, 4:325–328. [DOI] [PubMed] [Google Scholar]
- 2.Rumney RM, Wang N, Agrawal A, Gartland A: Purinergic signalling in bone. Front Endocrinol (Lausanne) 2012, 3:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orriss IR, Burnstock G, Arnett TR: Purinergic signalling and bone remodelling. Current Opinion in Pharmacology 2010, 10:322–330. [DOI] [PubMed] [Google Scholar]
- 4.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K: Pharmacology of Adenosine Receptors: The State of the Art. Physiological Reviews 2018, 98:1591–1625. [DOI] [PubMed] [Google Scholar]
- 5.Chen JF, Eltzschig HK, Fredholm BB: Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov 2013, 12:265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colgan SP, Eltzschig HK, Eckle T, Thompson LF: Physiological roles for ecto-5’-nucleotidase (CD73). Purinergic Signal 2006, 2:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikolajewicz N, Zimmermann EA, Willie BM, Komarova SV: Mechanically stimulated ATP release from murine bone cells is regulated by a balance of injury and repair. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin H, St Hilaire C, Huang Y, Yang D, Dmitrieva NI, Negro A, Schwartzbeck R, Liu Y, Yu Z, Walts A, et al. : Increased activity of TNAP compensates for reduced adenosine production and promotes ectopic calcification in the genetic disease ACDC. Sci Signal 2016, 9:ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazemzadeh-Narbat M, Annabi N, Tamayol A, Oklu R, Ghanem A, Khademhosseini A: Adenosine-associated delivery systems. J Drug Target 2015, 23:580–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke X, Liu C, Wang Y, Ma J, Mao X, Li Q: Netrin-1 promotes mesenchymal stem cell revascularization of limb ischaemia. Diab Vasc Dis Res 2016, 13:145–156. [DOI] [PubMed] [Google Scholar]
- 11.He W, Cronstein BN: Adenosine A1 receptor regulates osteoclast formation by altering TRAF6/TAK1 signaling. Purinergic Signal 2012, 8:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kara FM, Chitu V, Sloane J, Axelrod M, Fredholm BB, Stanley ER, Cronstein BN: Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2010, 24:2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mediero A, Perez-Aso M, Cronstein BN: Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFkappaB nuclear translocation. Br J Pharmacol 2013, 169:1372–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih YR, Hwang Y, Phadke A, Kang H, Hwang NS, Caro EJ, Nguyen S, Siu M, Theodorakis EA, Gianneschi NC, et al. : Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc Natl Acad Sci U S A 2014, 111:990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xaus J, Valledor AF, Cardo M, Marques L, Beleta J, Palacios JM, Celada A: Adenosine inhibits macrophage colony-stimulating factor-dependent proliferation of macrophages through the induction of p27kip-1 expression. J Immunol 1999, 163:4140–4149. [PubMed] [Google Scholar]
- 16.Rath-Wolfson L, Bar-Yehuda S, Madi L, Ochaion A, Cohen S, Zabutti A, Fishman P: IB-MECA, an A3 adenosine receptor agonist prevents bone resorption in rats with adjuvant induced arthritis. Clin Exp Rheumatol 2006, 24:400–406. [PubMed] [Google Scholar]
- 17.Clark D, Nakamura M, Miclau T, Marcucio R: Effects of Aging on Fracture Healing. Curr Osteoporos Rep 2017, 15:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stegen S, Carmeliet G: The skeletal vascular system - Breathing life into bone tissue. Bone 2018, 115:50–58. [DOI] [PubMed] [Google Scholar]
- 19.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM Jr., Gause WC, Leibovich SJ, et al. : Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J 2012, 26:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasko G, Cronstein B: Regulation of inflammation by adenosine. Front Immunol 2013, 4:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, Kepka-Lenhart D, SMM Jr., Gause WC, Leibovich SJ, et al. : Adenosine promotes alternative macrophage activation via A2A and A2B receptors. The FASEB Journal 2012, 26:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman H, Shih YV, Varghese S: Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials 2021, 277:121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Wang D, Wang J, Zhou J, Zheng X: The Role of Adenosine Receptor A2A in the Regulation of Macrophage Exosomes and Vascular Endothelial Cells During Bone Healing. J Inflamm Res 2021, 14:4001–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Macrophage exosomes and vascular endothelial cells are critical to bone healing. The authors demonstrated that administration of an ADORA2A antagonist depleted macrophage-secreted exosomes, which led to a decrease in angiogenesis and bone nonunion.
- 24.Kang H, Shih YR, Varghese S: Biomineralized matrices dominate soluble cues to direct osteogenic differentiation of human mesenchymal stem cells through adenosine signaling. Biomacromolecules 2015, 16:1050–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll SH, Wigner NA, Kulkarni N, Johnston-Cox H, Gerstenfeld LC, Ravid K: A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J Biol Chem 2012, 287:15718–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takedachi M, Oohara H, Smith BJ, Iyama M, Kobashi M, Maeda K, Long CL, Humphrey MB, Stoecker BJ, Toyosawa S, et al. : CD73-generated adenosine promotes osteoblast differentiation. J Cell Physiol 2012, 227:2622–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mediero A, Kara FM, Wilder T, Cronstein BN: Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol 2012, 180:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradaschia-Correa V, Josephson AM, Egol AJ, Mizrahi MM, Leclerc K, Huo J, Cronstein BN, Leucht P: Ecto-5’-nucleotidase (CD73) regulates bone formation and remodeling during intramembranous bone repair in aging mice. Tissue Cell 2017, 49:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Kimura K, Breitbach M, Schildberg FA, Hesse M, Fleischmann BK: Bone marrow CD73+ mesenchymal stem cells display increased stemness in vitro and promote fracture healing in vivo. Bone Reports 2021, 15:101133. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors used CD73-EGFP reporter mice to investigate the plasticity and osteogenic potential of CD73+ MSCs. Their results demonstrate CD73+ MSCs migrate into the fracture site and differentiate into cartilage and bone cells. This finding illustrates a prominent role of the CD73+ MSC subpopulation to promote fracture repair.
- 30.Shih YV, Liu M, Kwon SK, Iida M, Gong Y, Sangaj N, Varghese S: Dysregulation of ectonucleotidase-mediated extracellular adenosine during postmenopausal bone loss. Sci Adv 2019, 5:eaax1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dal-Fabbro R, Cosme-Silva L, Capalbo LC, Chaves-Neto AH, Ervolino E, Cintra LTA, Gomes-Filho JE: Excessive caffeine intake increases bone resorption associated with periapical periodontitis in rats. International Endodontic Journal 2021, 54:1861–1870. [DOI] [PubMed] [Google Scholar]
- 32*.Llamas-Granda P, Martin-Rodríguez L, Largo R, Herrero-Beaumont G, Mediero A: Tenofovir Modulates Semaphorin 4D Signaling and Regulates Bone Homeostasis, Which Can Be Counteracted by Dipyridamole and Adenosine A2A Receptor. International Journal of Molecular Sciences 2021, 22:11490. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrated that the antiviral drug, tenofovir, increases bone loss by activation of Sema4D/PlexinB1 signaling. By increasing local adenosine concentrations and activating ADORA2A signaling, the effect of tenofovir can be counteracted and bone loss mitigated.
- 33.Mediero A, Wilder T, Reddy VS, Cheng Q, Tovar N, Coelho PG, Witek L, Whatling C, Cronstein BN: Ticagrelor regulates osteoblast and osteoclast function and promotes bone formation in vivo via an adenosine-dependent mechanism. FASEB J 2016, 30:3887–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albrecht-Kupper BE, Leineweber K, Nell PG: Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signal 2012, 8:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rankin AC, Brooks R, Ruskin JN, McGovern BA: Adenosine and the treatment of supraventricular tachycardia. Am J Med 1992, 92:655–664. [DOI] [PubMed] [Google Scholar]
- 36.Corciulo C, Castro CM, Coughlin T, Jacob S, Li Z, Fenyö D, Rifkin DB, Kennedy OD, Cronstein BN: Intraarticular injection of liposomal adenosine reduces cartilage damage in established murine and rat models of osteoarthritis. Scientific Reports 2020, 10:13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Hoque J, Shih YV, Zeng Y, Newman H, Sangaj N, Arjunji N, Varghese S: Bone targeting nanocarrier-assisted delivery of adenosine to combat osteoporotic bone loss. Biomaterials 2021, 273:120819. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate the use of a bone-targeting nanocarrier for systemic administration of adenosine utilizing the bone-binding affinity of alendronate conjugation. Systemic administration of the bone-targeting nanocarrier loaded with adenosine increased localization of the drug-loaded carrier to the bone tissue and attenuated bone loss in ovariectomized (OVX) mice.
- 38.Hoque J, Zeng Y, Newman H, Gonzales G, Lee C, Varghese S: Microgel-Assisted Delivery of Adenosine to Accelerate Fracture Healing. ACS Biomaterials Science & Engineering 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Newman H, Hoque J, Shih Y-RV, Marushack G, Ko U, Gonzales G, Varghese S: pH-Sensitive nanocarrier assisted delivery of adenosine to treat osteoporotic bone loss. Biomaterials Science 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a pH-sensitive nanocarrier, the authors demonstrated that the acidic environment of active osteoclasts could serve as a potential trigger for the delivery of adenosine into the osteoporotic bone tissue. Systemic administration of the pH-sensitive nanocarrier containing adenosine attenuated bone loss in ovariectomized mice and showed comparable bone quality to that of healthy mice.
- 40*.Zeng Y, Shih Y-RV, Baht GS, Varghese S: In Vivo Sequestration of Innate Small Molecules to Promote Bone Healing. Advanced Materials 2020, 32:1906022. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrated for the first time that the local sequestration of the transient surge of adenosine following fracture results in accelerated healing by promoting both osteoblastogenesis and angiogenesis. A synthetic biomaterial patch containing boronate molecules was designed to sequester endogenous adenosine and establish an in situ stockpile of the therapeutic molecule.
- 41.Kar M, Vernon Shih YR, Velez DO, Cabrales P, Varghese S: Poly(ethylene glycol) hydrogels with cell cleavable groups for autonomous cell delivery. Biomaterials 2016, 77:186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng Y, Hoque J, Varghese S: Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomater 2019, 93:152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larrañaga-Vera A, Toti KS, Flatow JS, Haraczy AJ, Warnick E, Rao H, Gao ZG, Sussman SM, Mediero A, Leucht P, et al. : Novel alendronate-CGS21680 conjugate reduces bone resorption and induces new bone formation in post-menopausal osteoporosis and inflammatory osteolysis mouse models. Arthritis Res Ther 2022, 24:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng X, Wang D: The Adenosine A2A Receptor Agonist Accelerates Bone Healing and Adjusts Treg/Th17 Cell Balance through Interleukin 6. Biomed Res Int 2020, 2020:2603873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng X, Cheng G, Xing X, Yin C, Cheng Y, Zhou X, Jiang S, Tao F, Deng H, Li Z: Controlled release of adenosine from core-shell nanofibers to promote bone regeneration through STAT3 signaling pathway. Journal of Controlled Release 2020, 319:234–245. [DOI] [PubMed] [Google Scholar]
- 46.Verma NK, Kar AK, Singh A, Jagdale P, Satija NK, Ghosh D, Patnaik S: Control Release of Adenosine Potentiate Osteogenic Differentiation within a Bone Integrative EGCG-g-NOCC/Collagen Composite Scaffold toward Guided Bone Regeneration in a Critical-Sized Calvarial Defect. Biomacromolecules 2021, 22:3069–3083. [DOI] [PubMed] [Google Scholar]
- 47.Cheng X, Yin C, Deng Y, Li Z: Exogenous adenosine activates A2A adenosine receptor to inhibit RANKL-induced osteoclastogenesis via AP-1 pathway to facilitate bone repair. Molecular Biology Reports 2022, 49:2003–2014. [DOI] [PubMed] [Google Scholar]
- 48.Liao H, Yu H-P, Song W, Zhang G, Lu B, Zhu Y-J, Yu W, He Y: Amorphous calcium phosphate nanoparticles using adenosine triphosphate as an organic phosphorus source for promoting tendon–bone healing. Journal of Nanobiotechnology 2021, 19:270. [DOI] [PMC free article] [PubMed] [Google Scholar]


