Abstract
Increase in stress-related disorders in women begins post-puberty and persists throughout the lifespan. To characterize sex differences in stress response in early adulthood, we used functional magnetic resonance imaging while participants underwent a stress task in conjunction with serum cortisol levels and questionnaires assessing anxiety and mood. Forty-two healthy subjects aged 18–25 years participated (21M, 21F). Interaction of stress and sex in brain activation and connectivity were examined. Results demonstrated significant sex differences in brain activity with women exhibiting increased activation in regions that inhibit arousal compared to men during the stress paradigm. Women had increased connectivity among stress circuitry regions and default mode network, whereas men had increased connectivity between stress and cognitive control regions. In a subset of subjects (13F, 17M), we obtained gamma-aminobutyric acid (GABA) magnetic resonance spectroscopy in rostral anterior cingulate cortex (rostral ACC) and dorsolateral prefrotal cortex (dlPFC) and conducted exploratory analyses to relate GABA measurements with sex differences in brain activation and connectivity. Prefrontal GABA levels were negatively associated with inferior temporal gyrus activation in men and women and with ventromedial prefrontal cortex activation in men. Despite sex differences in neural response, we found similar subjective ratings of anxiety and mood, cortisol levels, and GABA levels between sexes, suggesting sex differences in brain activity result in similar behavioral responses among the sexes. These results help establish sex differences in healthy brain activity from which we can better understand sex differences underlying stress-associated illnesses.
Keywords: Sex differences, GABA MRS, functional MRI, MIST, MAST
1. Introduction
Stress affects quality of life and risk for many chronic diseases (1). Acute and long-term consequences of stress differ between sexes and at different points across the lifespan (2). In response to stress, men exhibit an increased physiological response with higher levels of cortisol release compared to women whereas women report higher subjective feelings of stress, negative affect, and anxiety than men (3–7). Stress-related mood and anxiety disorders are more prevalent in women beginning post-puberty (8–10). To understand the impact of sex on susceptibility to disease, it is important to understand sex differences in response to stress in the healthy brain. Our prior studies using a mild stress task, in which subjects passively viewed pictures with negative or neutral valence and arousal, demonstrated sex differences in circuitry that regulate response to stress in healthy adults and the role of sex steroid hormones (10, 11). Here, we tested whether these sex differences were evident in young adults while undergoing a more potent stress task combining physiological and psychosocial acute stressors.
The stress response involves activation of limbic brain circuitry and the hypothalamic-pituitary-adrenal (HPA) axis. Primary regions in stress response circuitry include hypothalamus, hippocampus (HIPP), amygdala, anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), and brainstem regions, including raphe nucleus and locus coeruleus (12, 13). Preclinical and clinical studies have demonstrated these brain regions develop differently in male and female brains, beginning prenatally, and function differently across the lifespan (14–19). Several sexually dimorphic brain regions are part of the default mode network, or DMN, a network of brain regions that includes mPFC, posterior cingulate cortex (PCC), angular gyrus (AG), and HIPP that are active when the brain is not focused on a task (20, 21). Research indicates that stress can activate the DMN which may interfere with the ability to focus on a task (22, 23). A recent study examined sex differences in DMN connectivity during rest in a large cohort of healthy adults aged 36–100 and found increased connectivity in women in bilateral parahippocampal gyri compared to men in subjects in their 30s. For those in their 40s and 50s, women had increased connectivity in posterior nodes (PCC, AG) compared to men. Men in their 60s and 80s had increased connectivity between anterior nodes (mPFC) compared to women (24). To our knowledge, there has not been a study to examine sex differences in DMN connectivity in healthy adults during stress.
Gamma aminobutyric acid (GABA), the primary inhibitory neurotransmitter, is critical to understanding regulation of response to stress (25, 26). In rodents, reduced GABA signaling in utero impacts sex-dependent development of HPA circuitry, with long-term consequences for response to stress and anxiety and mood-related behaviors in adult animals (27–29). Previous studies in humans using magnetic resonance spectroscopy (MRS) to measure GABA levels in ACC have shown associations between GABA and ACC activation during emotional perception and judgment tasks. Higher GABA levels were associated with greater deactivation in mPFC and ACC while viewing negative but not positive or neutral stimuli (26, 30). A recent study in males found that GABA in dorsal ACC was positively associated with amygdalar and hippocampal activation and negatively associated with ACC activation while viewing positive and negative valence pictures (31). In our study, in a subset of subjects, MRS was conducted in rostral ACC and dorsolateral prefrontal cortex (dlPFC), frontal regions that inhibit arousal from negative stress. We hypothesized that GABA would be associated with sex differences in stress response circuitry specifically in prefrontal regions involved in inhibitory control of arousal (i.e. mPFC, ACC), more likely in women than men.
Neuroimaging studies from our lab and others have demonstrated the role of adrenal and gonadal hormones on sex differences in stress response circuitry in adulthood (10, 11, 32–36). Steroid hormones interact with GABAergic mechanisms to regulate stress response. Androgens, estrogens, and their metabolites modulate response of corticotropin-releasing factor (CRF) neurons through receptor mechanisms in paraventricular nucleus of hypothalamus (PVN), the key relay station of the HPA axis (37). Testosterone inhibits CRF release in PVN through activation of GABAergic projections to PVN (15). Estradiol can affect CRF release directly through estrogen receptors in the PVN or indirectly through upstream regions, such as the amygdala, which projects to the bed nucleus of the stria terminalis and stimulates GABAergic projections to PVN (38).
The goal of this study was to characterize sex differences in response to physiological and psychological stress in early adulthood. In earlier studies using a mild stress reactivity paradigm, we demonstrated sex differences in stress response when women were in mid-late follicular menstrual cycle phase (driven by 17β estradiol), whereas there were no significant sex differences when women were in early follicular phase or in subjective feelings of stress when women were in either phase (10, 11). We hypothesized that young healthy men and women, who were tested during the early follicular menstrual cycle phase, would show similar levels of activation and connectivity in stress circuitry at baseline, but that with exposure to acute physiological and psychological stress through the Maastricht Acute Stress Test (MAST), young women would have a lower threshold for activation of arousal and inhibitory regions compared to men. During the second part of the task, participants performed the difficult version of the Montreal Imaging Stress Task (MIST) in which they were exposed to psychosocial stress while being cognitively challenged. We hypothesized again that women compared with men would exhibit less ability to regulate response to negative stress and thus less ability to activate inhibitory regions and connections with subcortical arousal regions.
Further, we extended this work by examining associations of brain activity and connectivity with GABA levels in a subset of subjects. We hypothesized that higher GABA levels in prefrontal regions would attenuate neural and physiological responses to negative stress and that this relationship would be stronger in women than men.
2. Methods
2.1. Participants
Forty-two healthy individuals aged 18–25 years (21M:21F) were recruited from the community to undergo functional magnetic resonance imaging (fMRI) during a stress paradigm. All participants provided written informed consent to a protocol approved by MGB Human Research Committee. Participants were interviewed by a master’s level clinician with three decades of experience in psychiatric diagnosis using Structured Clinical Interview for DSM-5 (39) to ensure absence of psychiatric disorders. Subjects did not have any medical or neurological disorder (assessed via medical history questionnaire), were not taking medications at the time of the study, and were right-handed. Female participants were tested during early follicular menstrual cycle phase. Sessions took place in the afternoon to minimize diurnal cortisol variability.
2.2. Task Description
Subjects underwent fMRI while performing a sustained stress protocol combining the MAST (40) and MIST (41, 42). The session included four MIST blocks of 3.5 minutes each, with completion of 10-minute MAST between first and second blocks. First, participants performed the baseline control condition in scanner, consisting of easy arithmetic calculations without time pressure and with performance feedback. Then, subjects were removed from the scanner for the MAST, which combines physical pain (submergence of one hand in ice-cold water, 2–4°C), a cognitive challenge (counting backward from four-digit number in steps of 17), social evaluation (two experimenters who make the subject start over if they make a mistake), and uncontrollability (duration of trials vary in length between 45 and 90 seconds, with duration changing from trial to trial without the subject knowing in advance how long each trial would last) (40, 43). Subjects return to the scanner for a second block of easy MIST (postMAST1), followed by a block of the difficult version of the MIST (postMAST2). In the difficult MIST, variable time pressure was imposed on each arithmetic problem, problems maximized difficulty in relation to subject’s ability, and a mock performance bar showed participants’ performance in relation to an alleged average of prior participants. After the third block, one experimenter informed the subject that their performance was suboptimal and they must try harder for their data to be useable. Following negative feedback, participants underwent difficult MIST again (postMAST3). The study was designed so that each condition (easy or difficult) MIST was presented twice with an intervening acute stressor (MAST and negative feedback, respectively). (At study conclusion, participants were debriefed about the study design and purpose.) Figure 1 depicts the task/session flow.
Figure 1.
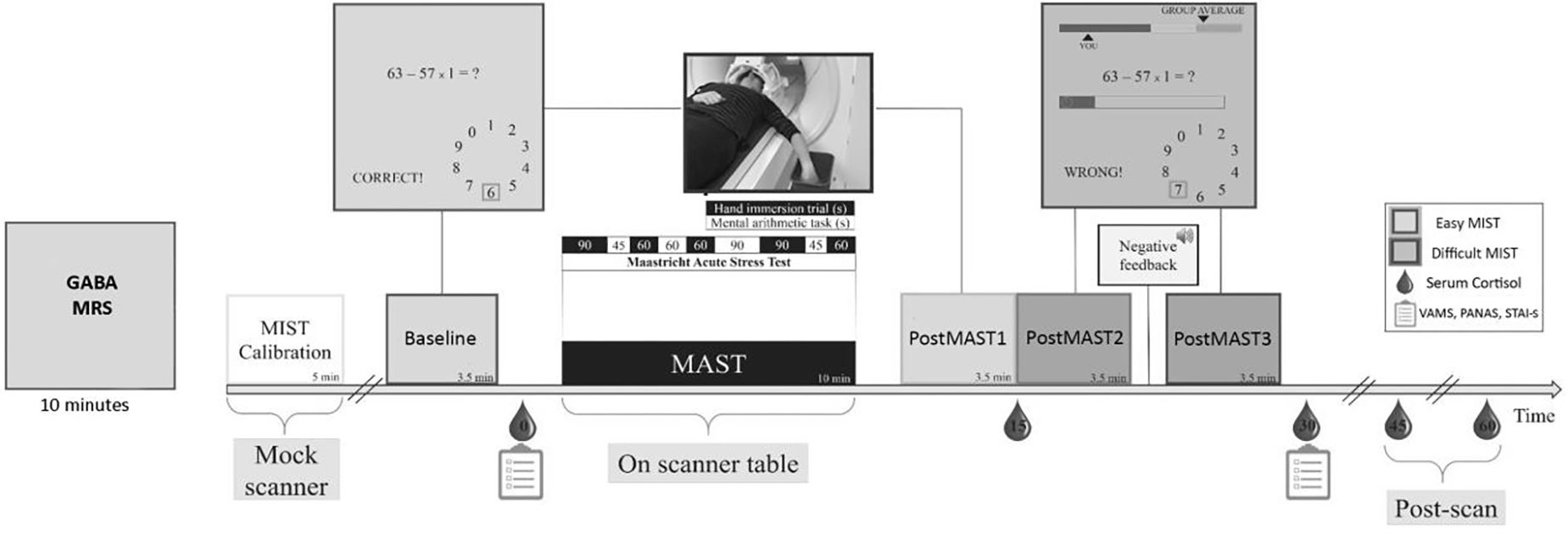
Schematic showing the phases of stress task with timing of blood draws and questionnaire administration. The task was a hybrid of the MAST and the MIST.
Subjects completed questionnaires before and after scanning assessing anxiety (State-Trait Anxiety Inventory, STAI) (44) and mood (Positive and Negative Affect Schedule, PANAS (45), and Visual Analogue Mood Scale, VAMS (46)). The effect of stress on self-reported anxiety and mood were examined using paired t-tests, comparing overall means before and after stress exposure. Sex differences in effect of stress were examined using independent-samples t-tests.
2.3. MRI Data and Physiology
MRI data were collected on a 3T Siemens MAGNETOM Prisma scanner equipped with a 64-channel head coil at the McLean Imaging Center. Functional MRI data were acquired using a gradient echo T2*-weighted echo planar imaging sequence with the following parameters: repetition time (TR)=2000 ms; echo time (TE) =30 ms; field of view=204 mm; voxel dimension=1.5×1.5×1.5; 84 interleaved slices with a multiband acceleration factor of 3. Structural data were acquired with a T1-weighted magnetization-prepared rapid acquisition having gradient multi-echo (MPRAGE) imaging sequences with the following acquisition parameters: repetition time (TR)=2530 ms; echo times (TE)=1.69, 3.55, 5.41 and 7.27 ms; field of view=256 mm; voxel dimenions=1.0×1.0×1.0 mm3; 176 slices.
Blood oxygen-level dependent (BOLD) signal change images were motion-corrected, realigned, normalized to the MNI152 brain template in a non-linear, volume-based method, spatially smoothed with a 6 mm FWHM Gaussian filter and re-sampled to 3 mm isotropic. Outliers in global mean image time series (threshold: 3.5 SDs from mean) and movement (threshold: 0.7 mm, measured as scan-to-scan movement, separately for translation and rotation) were detected using an artifact detection toolbox (ART; RRID: SCR_005994, https://www.nitrc.org/projects/artifact_detect) and entered as nuisance regressors in the first-level, single-subject general linear model (GLM). Data were band-pass filtered with a 420-second high pass filter to ensure that all relevant data were included in the long blocks. Masks excluding voxels outside the brain were applied to ensure that voxels in regions with high interparticipant variability in signal dropout were not arbitrarily excluded.
Outputs from first-level, single-subject analyses were submitted to second-level full factorial analyses examining the interaction of sex and condition. BOLD activation was examined using three separate ANOVAs that contrasted each stressful condition (postMAST1, postMAST2, and postMAST3) with the baseline control condition and sex (male vs. female). Each stressful condition was compared to baseline, given that there was no expectation of a linear or higher order change in activation or connectivity across conditions. Condition × Sex interactions were designated as primary outcomes of interest and examined at the whole brain level. Results were considered significant if they met whole-brain, cluster-level threshold of pFWE<0.05 and peak level threshold of pUNC<0.001. Mean beta weights within significant clusters were extracted for each participant using REX toolbox for post-hoc analyses in SPSS (IBM Corp. Released 2020. IBM SPSS Statistics, Version 27.0. Armonk, NY: IBM Corp.).
Functional connectivity was examined in CONN (47) using seed-to-voxel analyses with seeds in right and left amygdala and hippocampus, mPFC, ACC, and hypothalamus. All masks were created by the Core Morphology Group at Martinos Center for Biomedical Imaging at Massachusetts General Hospital. To address aims, the interaction of sex and condition was explored using F-tests. Results were considered significant if they met whole-brain, cluster-level threshold of pFWE<0.05 and peak level threshold of pUNC<0.001.
2.4. Serological Data Acquisition and Analysis
The study visit began in early afternoon for all participants to insure consistency of timing of serologic acquisition. Trained technicians inserted an intravenous line and obtained a baseline blood draw to evaluate HPA axis hormones. Blood was collected at five subsequent timepoints – in-scanner baseline prior to task (T0) and 15 minutes (T15), 30 minutes (T30), 45 minutes (T45), 60 minutes (T60) and 90 minutes (T90) after the task began. Serum cortisol was measured by immunoassay (Quest Diagnostics), with an assay sensitivity of 0.5–75.0 micrograms(μg)/deciliter (dL), a dynamic range of 4.0–22.0 μg/dL (morning) and 3.0–17.0 (afternoon), and intra-assay variation coefficient of 0.996. Area under the curve with respect to increase over in-scanner baseline (AUCi) was calculated using the following (48):
Analyses with cortisol were conducted on a subset of subjects who had all or most cortisol measurements. Of the original sample of 42 subjects (21 women, 21 men), seven women and one man were excluded from only the cortisol analyses given inability for blood draw, and two additional women excluded because their cortisol levels were outliers (>±2 SD from the mean for women). Therefore, the final sample for cortisol analyses included 12 women and 20 men. There were significantly more women than men excluded from the cortisol analyses but the excluded subjects did not differ from the rest of the sample in terms of age , years of education , race , or income . For subjects who were missing cortisol at one or two timepoints (three subjects were missing one timepoint; one subject was missing two timepoints), we calculated AUCi based on acquired measurements and time between samples.
The effect of the task on cortisol was examined in the overall sample using a one-sample t-test, comparing overall mean AUCi to 0. Sex differences in task effects on cortisol were examined using independent-samples t-test.
2.5. GABA MRS Acquisition and Processing
T1-weighted, high resolution structural images were used to place a voxel in the rostral ACC (17.5 ml; 35×20×25mm3) and left dlPFC (18.75 ml; 25×30×25mm3) for MRS data collection (by CZ). Methodology for GABA MRS acquisition had to change during the study due to death of the MR physicist. Data could not be merged given methodologic differences, thus a subset of participants (13 F, 17 M) is presented here. As a result, findings are considered exploratory. Proton GABA+ (macromolecular-contaminated) measurement employed a MEshcher-GArwood Point RESolved Spectroscopy (MEGA-PRESS) sequence obtained from the University of Minnesota with the acquisition frequency sitting at 3.0ppm and frequency-selective editing pulses, each with a duration of 17ms alternatively at 1.9 ppm (on) and 7.5 ppm (off) interleaved with the average (49–52). Total scanning time was 10 minutes. MEGA-PRESS is an established MRS acquisition protocol for GABA detection that has demonstrated superior GABA test-retest reliability compared with other sequences (53). The magnetic field homogeneity within the prescribed voxel was adjusted using a vendor-provided 3D shimming routine with additional water suppression optimization. GABA+ concentrations were calculated as ratios of GABA+/water and were small volume corrected for percentage of tissue types in the voxels. Given that our hypotheses were specific to GABA, no other metabolite measurements were analyzed in relation to brain activation and connectivity. For additional details on the MRS protocol, see our prior publication (53). See Figure 2 for an image showing voxel placement and the MRS spectrum.
Figure 2.
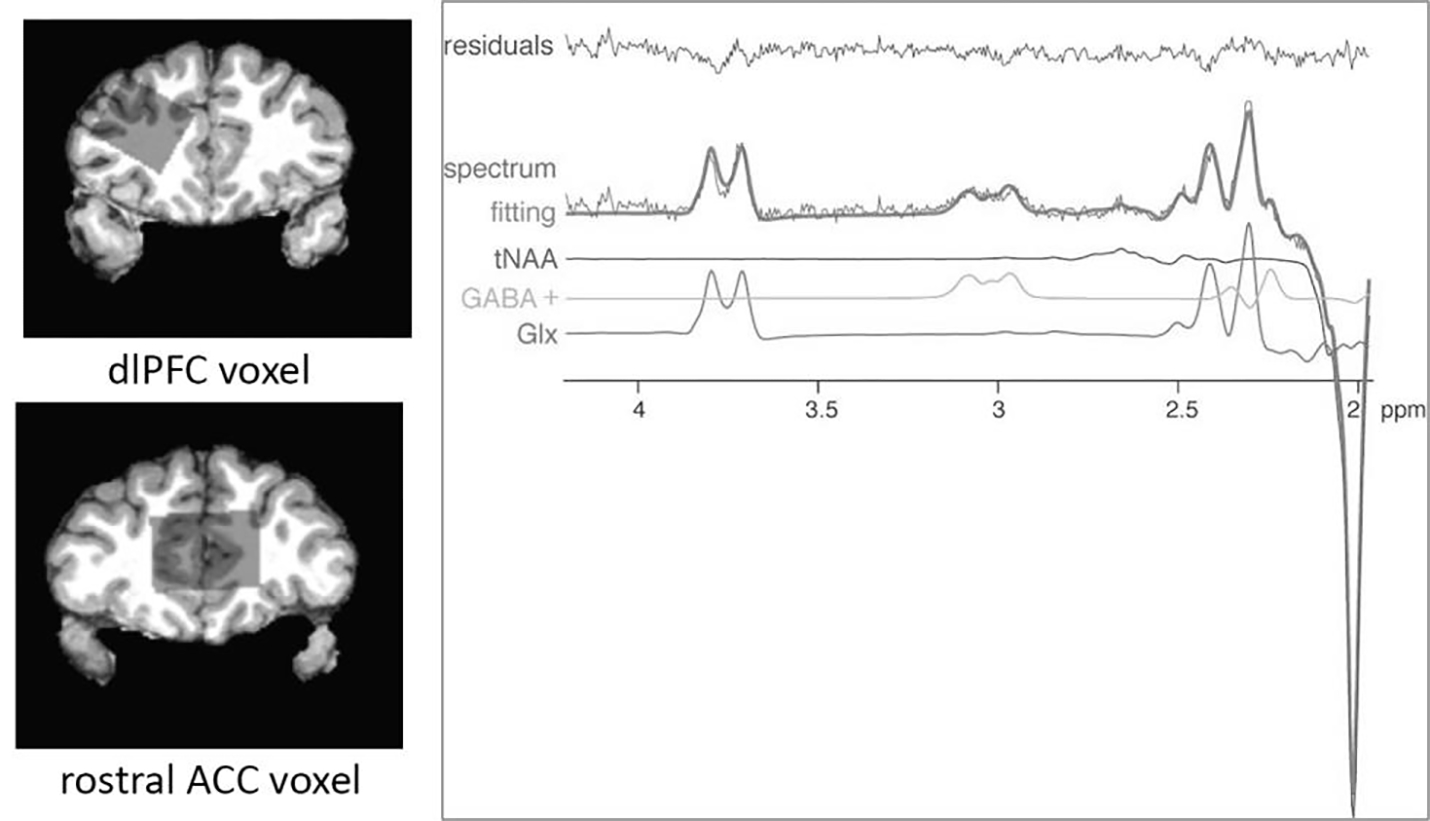
GABA+-edited (difference) spectrum showing metabolite fitting lines as estimated with LCModel, depicting the GABA+-edited spectrum, fitting line, total N-acetyl aspartate (tNAA), GABA+, glutamate+glutamine (Glx), and residuals. Based on hypotheses specific to GABA, no other metabolites were analyzed in this study.
Spectra were visually assessed by MR physicists (XC and FD) for severe baseline distortion. Spearman’s bivariate correlation analyses were conducted between GABA+ dlPFC and rostral ACC concentrations and significant fMRI results. Non-parametric tests were used with GABA+ analyses due to non-symmetrical distribution of the data.
Multiple comparisons were corrected using the B-H procedure with a false discovery rate (FDR) of 5%. We used this method and threshold for correction to avoid missing any significant associations while balancing true and false positives.
3. Results
3.1. Sample Characteristics
See Table 1 for demographics of participants. They were young (average age 21 years), well educated (average 15 years), and approximately 40% people of color (see Table 1). There were no significant differences between the sexes in terms of age, education, income level, or ethnicity/race.
Table 1.
Subject Demographics
| Group | Age (years) | Years of Education | People of color (POC) | White/Eur. American | Income < 50,000 | Income > 50,000 |
|---|---|---|---|---|---|---|
| Males (n = 21) | 21.43 (± 0.11) | 15.00 (± 0.11, | 9 (4 2.9%) | 12 (57.1%) | 11 (52.4%) | 10 (47.6%) |
| Females (n = 21) | 21.86 (± 0.09) | 15.26 (± 0.12) | 8 (38.1%) | 13 (61.9%) | 8 (38.1%) | 13 (61.9%) |
| t- and p- values | t(40)=0.13, p=0.78 | t(40)=0.34, p=073 | t(40)=−0.30, p=0.77 | t(40)=0.30, p=0.77 | t(40)=0.48, p=0.64 | t(40)=−0.48, p=0.64 |
3.2. Physiological & Emotional Response
Using AUCi as a measure of cortisol response, a one sample t-test revealed significant cortisol increase in response to stress (AUCi: t(31)=3.28, p=0.002), with no significant differences between sexes in independent-samples t-test (AUCi: F(1,30=0.71, p=0.41).
According to subjective feelings of mood and anxiety, participants endorsed increased anxiety, decreased positive affect, and increased negative affect post- compared to pre- task (STAI: t(38)=−.48, p<0.001, Positive PANAS: t(37)=−3.10, p=0.004; Negative PANAS: t(37)=−2.84, p=0.007), which did not differ by sex (STAI: t(37)=0.03, p=0.98; Positive PANAS: t(36)=−0.39, p=0.70; Negative PANAS: t(36)=−0.26, p=0.79). Questionnaires were completed by all participants, except for one female and two males due to practical time constraints.
3.3. Brain Activation Responses
3.3.1. Baseline vs. Post-MAST1
At whole brain level, BOLD results showed significant Condition × Sex interactions in the ventromedial PFC (vmPFC) (F(1,80)=17.79, pFWE=0.002) and left inferior temporal gyrus (ITG) and hippocampus (F(1,80)=17.22, pFWE= 0.0005) (see Table 2, Figure 3). Paired-samples t-test showed both sexes exhibited differences in vmPFC activation but in opposite directions. Men exhibited decreased (t(20)=−3.29, p=0.004) and women increased (t(20)=3.05, p=0.006) vmPFC activation during postMAST1 compared to baseline. Relative to men, women had increased BOLD activation during the easy MIST at baseline (t(40)=2.26, p=0.03, d=0.71 95% CI [0.04–0.64]) and postMAST1 (t(40)=3.3, p=0.002; d=0.88 95% CI [−0.79—0.19]) in vmPFC. A similar pattern was found in left ITG and hippocampus with men having decreased and women increased activation during postMAST1 compared to baseline (men: t(20)=−2.82, p=0.01; women: t(20)=4.05, p=0.001). Relative to men, women had significantly greater BOLD signal in left ITG and hippocampus in response to easy MIST at baseline (t(40)=4.57, p<0.001, d=1.42 95% CI [0.26–0.67]) ) and postMAST1 (t(40)=−2.16, p=0.04, d=0.67 95% CI [−0.62—0.02]) ).
Table 2.
Functional Connectivity Results
| Contrast | Seed egion | Cluster region | Cluster size | pFWE (cluster) | T(40) | Peak coordinates |
|---|---|---|---|---|---|---|
| PostMASTl > Baseline, M > W | mPFC | R dlPFC | 332 voxels | 0.01 | 4.58 | 36 28 28 |
| L hipp | R temporal pole | 269 voxels | 0.02 | 4.38 | 22 0 –12 | |
| R amygdala | L dlPFC | 358 voxels | 0.004 | 4.03 | −58 12 22 | |
| W > M | hypothalamus | R SMG | 253 voxels | 0.04 | 4.51 | −50 –48 32 |
| L amygdala | R lat occ ctx | 354 voxels | 0.004 | 6.59 | 50 –64 -10 | |
| mPFC | PCC | 531 | <0.001 | 5.35 | −4 –24 42 | |
| voxels | ||||||
| precuneus | 446 voxels | 0.03 | 4.49 | 18 –52 72 | ||
| R hipp | R cerebellum | 530 voxels | 0.0002 | 4.76 | 16 –74 46 | |
| R amygdala | L precuneus | 457 voxels | <0.001 | 4.90 | −4 50 12 | |
| R ang gyrus | 307 voxels | 0.01 | 4.99 | 42 –60 40 | ||
| mPFC | 267 voxels | 0.03 | 4.90 | −18 68 4 | ||
| R MTG | 344 voxels | 0.006 | 4.99 | 62 –2 -22 | ||
| ACC | PCC | 389 voxels | 0.0)4 | 4.50 | −6 –54 72 | |
| PostMAST2 > Baseline M > W | R amygdala | L dlPFC | 278 voxels | 0.0 2 | 4.38 | 50 –34 22 |
| L caudate | 2/0 voxels | 0.02 | 3.97 | −8 8 10 | ||
| W > M | ACC | R cerebellum | 55i voxels |
0.0002 | 4.25 | 34 –74 -52 |
| R post/prec ntral gyi-s |
554 voxels | 0.0002 | 4.78 | 16 –18 78 | ||
| L post/precentral gyrus | 356 voxels | 0.006 | 4.52 | −26 –28 76 | ||
| PostMAST3 > Baseline M > W | R hipp | L dlPFC | 299 voxels | 0.01 | 4.03 | −46 6 20 |
ACC=anterior cingulate cortex, dlPFC=dorsolateral prefrontal cortex, HIPP=hippocampus, Lat occ ctx=lateral occipital cortex, L=left, M=men, mPFC=medial prefrontal cortex, MTG=middle temporal gyrus, Occ pole=occipital pole, PCC=posterior cingulate cortex, R=right, SMG=supramarginal gyrus, W=women
Figure 3.
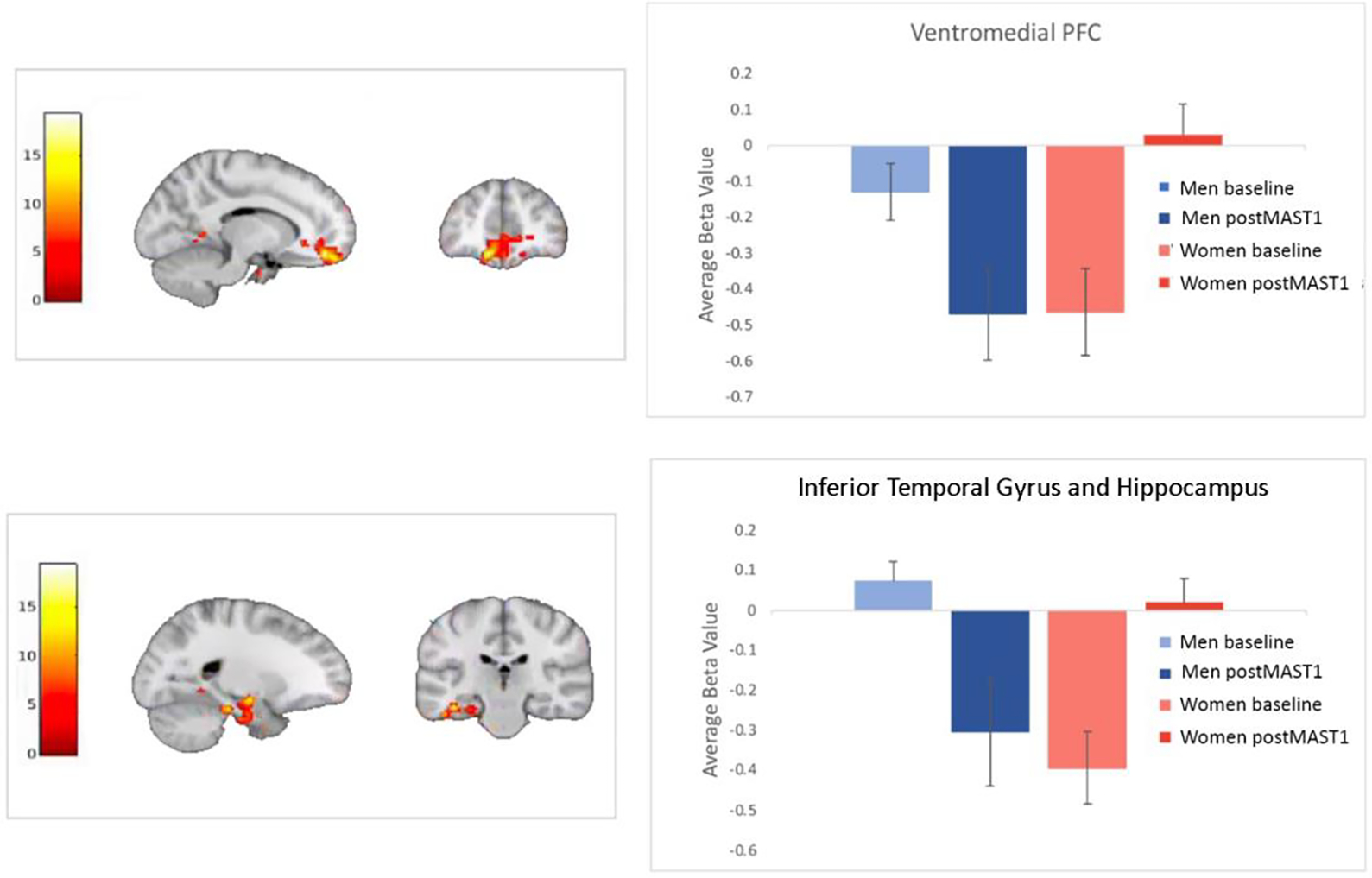
Beta values showed that, in relation to the baseline condition, men showed deactivation and women increased activation in vmPFC and ITG during postMAST1.
In functional connectivity analyses, men compared to women showed increased connectivity between vmPFC and right dlPFC (k=332 voxels, t(40)=4.58, pFWE=0.01, d=1.43, 95% CI [0.11–0.3]) and right amygdala and left dlPFC (k=358 voxels, t(40)=4.03, pFWE=0.004, d=1.91 95% CI [0.11–0.23]) during postMAST1 compared to baseline (see Table 2, Figure 4). Relative to men, women had increased functional connectivity between right amygdala and precuneus (k=457 voxels, t(40)=3.90, pFWE<0.001, d=1.87 95% CI [−0.25-−0.12] ), right MTG (k=344 voxels, t(40)=4.13, pFWE=0.006, d=1.83 95% CI [−0.24—0.12), and right angular gyrus (AG) (k=307 voxels, t(40)=4.99, pFWE=0.01, d=1.75 95% CI [−0.26—0.12]), between vmPFC and posterior cingulate cortex (PCC, k=531 voxels, t(40)=5.35, pFWE<0.001, d=2.67 95% CI [−0.27-−0.13]) and precuneus (k=446 voxels, t(40)=4.49, pFWE=0.03, d=7.92 95% CI [−0.24-−0.11]), and between ACC and PCC (k=389 voxels, t(40)=4.50, pFWE=0.004, d=1.90 95% CI [−0.24—0.12]).
Figure 4.
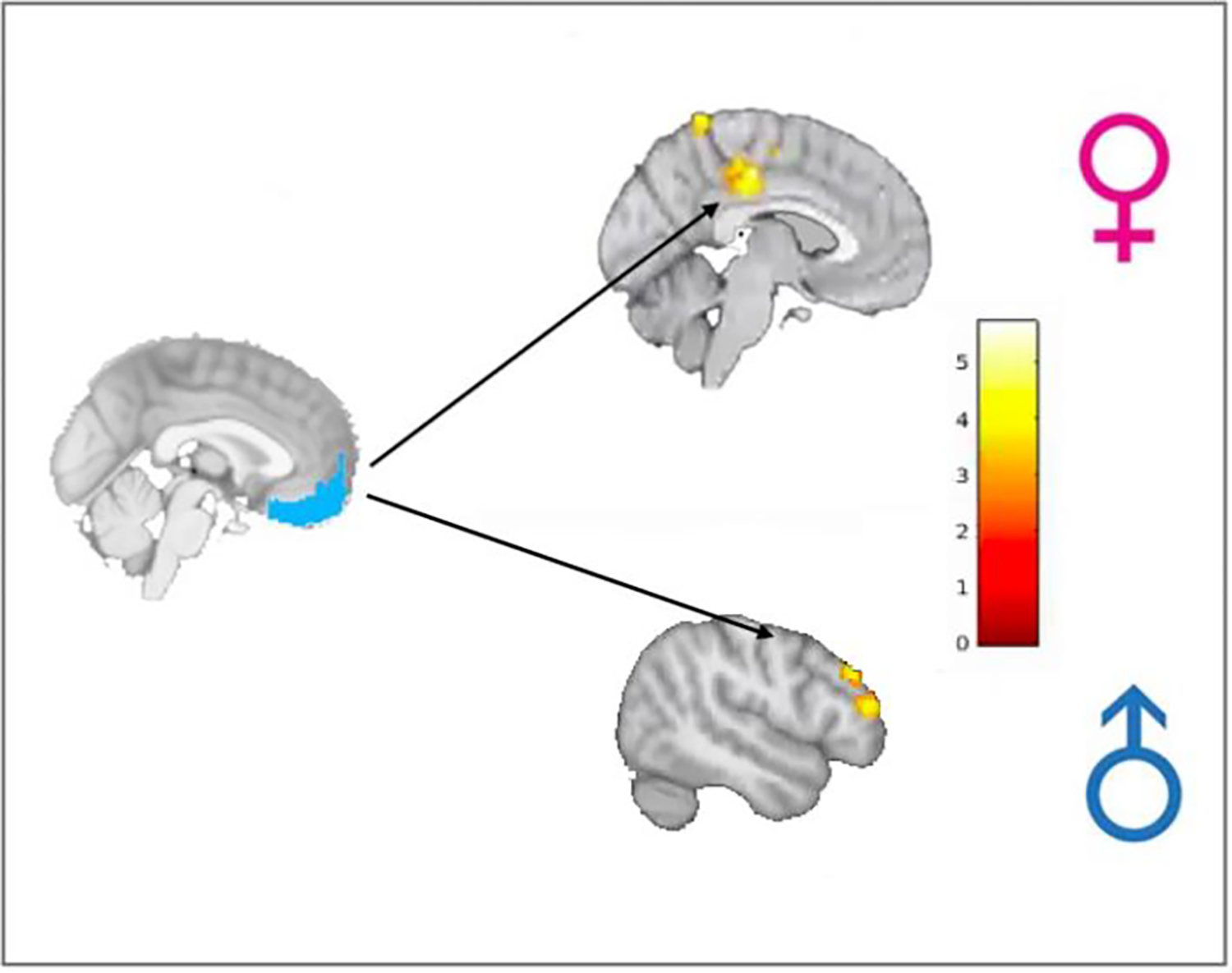
Functional connectivity results indicated connectivity from vmPFC was stronger to posterior regions including posterior cingulate cortex (PCC) in women compared to men and to dlPFC, a cognitive control region, in men compared to women during postMAST1 compared to the preMAST baseline.
3.3.2. Baseline vs. PostMAST2
Differences in BOLD signal in the interaction of condition and sex were found in left fusiform gyrus (F(1,80)=20.15, pFWE=0.0001) and left ITG and hippocampus (F(1,80)=17.78, pFWE=0.02) when preMAST baseline was compared to postMAST2 (see Table 2). Paired-samples t-tests revealed that women had increased activation in fusiform gyrus (t(20)=4.52, p<0.001) and left ITG and hippocampus (t(20)=4.05, p=0.03, d=), whereas men had decreased activation in both regions (left ITG: t(20)=−4.05, p=0.001 and fusiform gyrus: t(20)=−1.99, p=0.06 during postMAST2 compared to baseline. Relative to men, women had significantly greater activation in fusiform gyrus at baseline (t(40)=4.17, p<0.001; d=1.29, 95% CI [0.23–0.66]) and post-MAST2 (t(40)=2.39, p=0.02; d=0.74 95% CI [−0.92—0.08]) and left ITG at post-MAST2 (t(40)=4.35, p<0.001; d=1.29 95% CI [−0.84—0.31]) but not baseline (t(40)=1.45, p=0.16).
Compared to women, men had increased connectivity between right amygdala and left dlPFC (k=278 voxels, t(40)=4.38, pFWE=0.02, d=1.66 95% CI [0.11–0.24]) and left caudate (k=270 voxels, t(40)=3.97, pFWE=0.02, d=2.1 95% CI [0.12–0.23]) during postMAST2 compared to baseline (see Table 2).
3.3.3. Baseline vs. Post-MAST3
A significant Condition × Sex interaction in BOLD signal was found in right precuneus (F(1,80)=13.51, pFWE<0.0001) and bilateral cerebellum (F(1,80)=13.69, pFWE<0.00001) when post-MAST3 was compared to baseline (see Table 2). Men exhibited increased activation in precuneus (t(20)=3.01, p=0.007) and cerebellum (t(20)=3.29, p=0.004), while women showed decreased activation in precuneus (t(20)=−2.24, p=0.04) and no difference in cerebellum (t(20)=1.53, p=0.14) during post-MAST3 compared to baseline. Relative to women, men had increased BOLD signal in precuneus at baseline (t(40)=2.12, p=0.04; d=0.58 95% CI [−0.42—0.01]) and during post-MAST3 (t(40)=2.34, p=0.03; d=0.72 95% CI [0.10–1.41) and in cerebellum at baseline (t(40)=2.0, p=0.05; d=0.61 95% CI [−0.53–0.003]a).
Relative to women, men exhibited increased connectivity between right hippocampus and left dlPFC (k=299 voxels, t(40)=4.03, pFWE=0.01, d=1.28 95% CI [0.11–0.33]) during postMAST3 compared to baseline (see Table 2).
Taken together, sex differences in BOLD signal activity indicated that women exhibited increased activation in regions that inhibit arousal (vmPFC) following exposure to acute physiological and psychological stress. During the cognitively demanding portion of the MIST, after given negative feedback on performance, sex differences in inhibitory regions were attenuated and men had increased activation in posterior regions (precuneus, cerebellum) compared to women. Functional connectivity analyses demonstrated increased connectivity in women among regions of stress circuitry and default mode network (DMN; i.e., precuneus, PCC), whereas men had increased connectivity to regions associated with cognitive control following exposure to acute physiological and psychological stress.
3.5. GABA MRS
GABA MRS values in dlPFC and rostral ACC were measured in a subset of participants (women: n=13; men: n=17) and did not differ significantly between sexes (dlPFC: F(1,28)=−0.17, p=0.87; rostral ACC: F(1,28)=0.75, p=0.46). In an exploratory analysis, correlation analyses examined relationships between beta values extracted from regions showing significant interactions in BOLD activation between men and women and GABA values (dlPFC, rostral ACC). In men, significant clusters in left MTG at baseline (R=−0.62, p=0.004; R=−0.61, p=0.005) and postMAST2 (R=−0.65, p=0.003) and vmPFC at postMAST1 (R=−0.59, p=0.007) were negatively correlated with GABA levels in dlPFC. See Figure 5. In women, rostral ACC GABA was negatively associated with left ITG activation at postMAST1 (R=−0.61, p=0.01) and positively correlated with left fusiform activation at baseline (R=0.59, p=0.01)..
Figure 5.
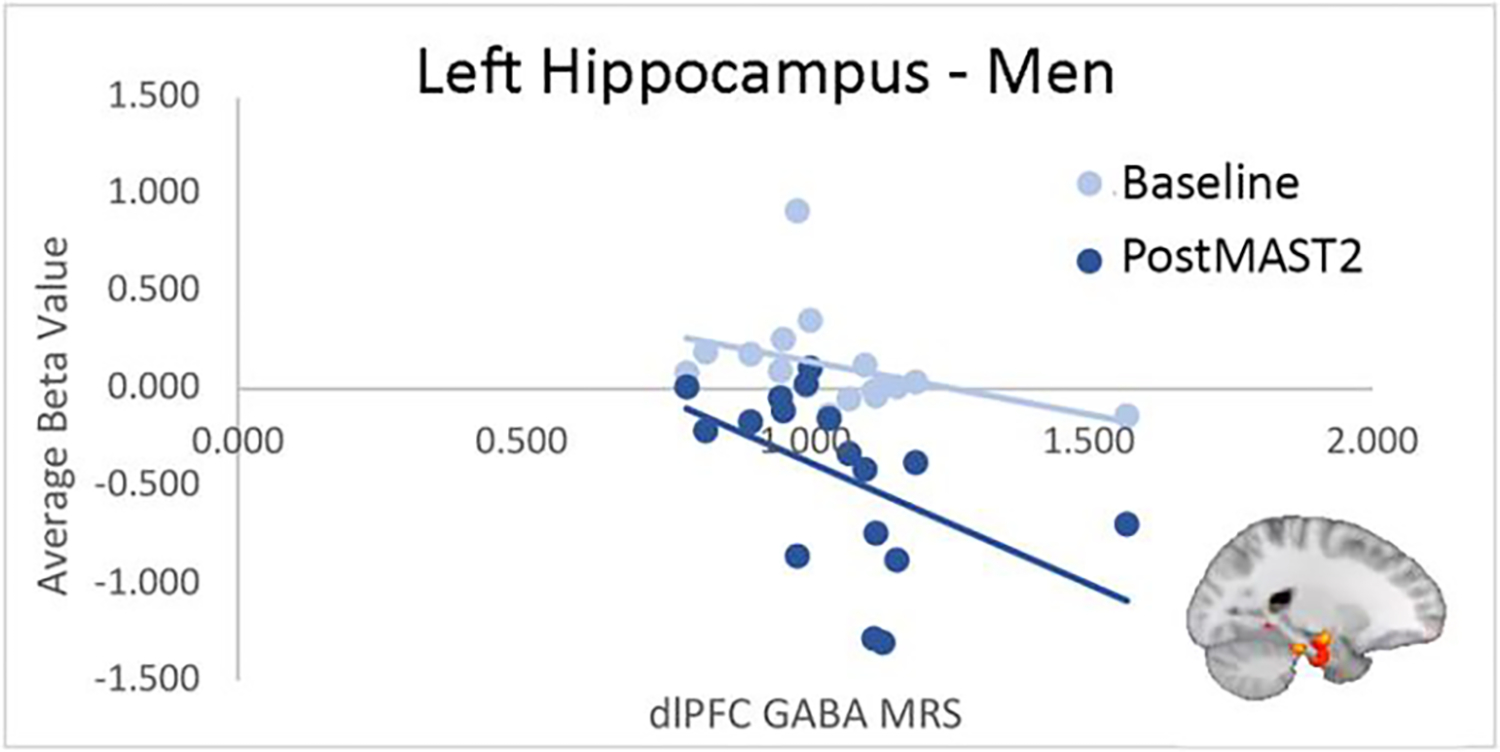
Men showed a negative correlation between GABA levels in the dlPFC and activation in the ITG and hippocampus during postMAST2. Decreased activation in the ITG and hippocampus during the MIST has previously been shown in men but this is the first indication that this is associated with GABA in the PFC (75, 76).
No correlational analyses examining relationships between GABA and functional connectivity results survived B-H correction.
4. Discussion
Taken together, sex differences in brain activation and connectivity indicated that women responded to the stress task through downstream activation of limbic regions potentially orchestrated by GABA in the rostral ACC, a functional hub connecting emotion and cognitive control (54). In contrast, men responded to stress through increased connectivity to cognitive control regions and maintained deactivation of DMN regions, which correlated with GABA levels in dlPFC, the prefrontal nexus of cognitive control (55, 56). Despite differences in brain activation and connectivity, men and women had similar subjective feelings of increased anxiety and decreased mood and comparable physiological responses to the stress protocol.
We previously showed significant sex differences in activation of brain regions during a mild visual stress reactivity paradigm and comparable subjective feelings of stress between sexes in early midlife adults (11, 32, 33). Extended here, following exposure to acute, unpredictable physiological and psychological stress, young adult women, compared with men, showed increased vmPFC activation, a region that inhibits arousal and is associated with aversive control (57–59). Men showed vmPFC deactivation during baseline and significantly greater deactivation following exposure to the MAST whereas women significantly deactivated vmPFC at baseline but increased activation following MAST exposure (see Figure 3). Due to its network of connections from disparate brain regions, the vmPFC is considered a site where emotional and cognitive processes bind (60, 61) and is involved in inhibitory control of emotional reactivity (62, 63). We suggest that exposure to the MAST, with its physiological stress, cognitive challenge, and psychosocial pressure, impacted women to a greater degree than men causing increased activation in the vmPFC to maintain control over emotional reactivity during the task. In men, GABA levels in prefrontal cortex were negatively associated with activation in the vmPFC potentially allowing for attenuation of vmPFC activity.
In addition, women showed increased connectivity between stress circuitry regions (amygdala and ACC), DMN regions (precuneus, AG, MTG, and PCC) and vmPFC (included in DMN and stress circuitry) compared to men. DMN is associated with internally focused thought, including autobiographical memory, self-directed planning, and mind-wandering (20, 64). Failure to deactivate DMN during a task is associated with negative performance, and other studies have indictated that stress can attenuate deactivation of DMN regions (22). In a study examining task-induced deactivations, subjects undergoing a stress paradigm deactivated DMN regions significantly less than non-stressed subjects and had increased functional connectivity within DMN (23), similar to the women in our study, suggesting a normative response to stress. Previous studies examining sex differences in DMN connectivity have shown increased within-network connectivity (mPFC-PCC, precuneus-HIPP, PCC-HIPP) in women compared to men in older adults at rest (65, 66). Interestingly, an earlier neuroimaging study using one of the same arithmetic tasks used in our study (counting backwards by 17 from a 4 digit number) found increased and prolonged activation of ACC and PCC that correlated with both indices of perceived stress and salivary cortisol in women but not in men (67).
In men, GABA in dlPFC negatively correlated with activation in vmPFC, indicating those with higher GABA levels in prefrontal cognitive control regions were better able to sustain deactivation of this DMN region under stress while performing a cognitive task. These findings potentially indicate that GABA is involved in stress-related connectivity in men and women but is sex- and region-specific. However, due to the small sample of subjects included in the GABA analyses, these findings can only be considered exploratory and require confirmation with larger sample sizes.
A neurocognitive network for stress regulation has recently been proposed that consists of amygdala projections to frontal regions including mPFC, dlPFC, and ACC (68). In a study examining this network in relation to a social exclusion stressor (Cyberball) and MIST, women had increased amygdala to mPFC connectivity following Cyberball, but not MIST. They also found a correlation between increased left amygdala-mPFC connectivity and decreased positive affect following the MIST in both sexes (69). Our results support the possibility of an amygdala-prefrontal network involved in stress regulation but in our study a sex difference in projections from the amygdala emerged with men showing increased connectivity from amygdala to dlPFC and women from amygdala to mPFC.
Healthy men showed increased functional connectivity between stress circuitry (mPFC, hippocampus) and cognitive control regions (right and left dlPFC). In previous studies, connectivity between hippocampus and dlPFC was correlated with better performance on arithmetic as stress increased in men indicating a potential compensatory mechanism during achievement stress (70, 71). In a recent study using the ScanSTRESS task, which also combines achievement and psychosocial stressors, men had increased activation in prefrontal regions associated with cognitive control, including ACC and dlPFC (72). In addition, activation in hippocampus and parahippocampal gyrus had a positive correlation with subjective feelings of stress in men but a negative correlation with subjective feelings of stress in women. Perhaps the increased connectivity to cognitive control regions enables men to continue to perform the task during increased feelings of stress.
The only region in which men increased activation compared to women was the precuneus while performing the difficult MIST after receiving negative feedback on performance. Precuneus is involved in cognitive tasks, including arithmetic, and social cognitive tasks that require mentalizing or self-referential thinking (73). Increased precuneus activation was also reported in a prior study using MIST in healthy young men compared to men with cannabis addiction (74). Our results fit with these findings, suggesting adaptive recruitment of precuneus to regulate stress associated with heightened cognitive load.
An earlier neuroimaging study using the MAST found increased activation in the right anterior PFC and right angular gyrus in men compared to women and in left OFC, dorsal ACC, left insula and left MTG in women compared to men (67). Our results replicate what they found in women except for the increased activation in left insula. We did not find significantly increased activation in right anterior PFC or right angular gyrus as they did in men, potentially, in part, due to differences in analytic strategies, perfusion vs. BOLD activations, and/or the fact that all women in their study were on oral contraceptives. In our previous study menstrual cycle phase had a significant impact on sex differences in activation of PFC (i.e., were significant only when the women were in midcycle) (11).
Despite men and women in this sample having comparable subjective feelings of mood and anxiety, they activated different brain regions suggesting different strategies in response to stress. The majority of sex differences in BOLD activation were revealed immediately after the MAST, with women having increased activation and men decreased activation in the same regions, suggesting sex differences in the deployment of neural nodes to regulate stress.
Limitations of this study include a small sample size when testing for interaction effects in BOLD and functional connectivity analyses. In addition, GABA analyses were limited to two regions in prefrontal cortex, due to practical concerns, including limited duration of scan time and difficulty in accessing GABA signal in subcortical regions. Finally, while this was a study of sex differences, we did not have a large enough sample size to address the spectrum of differences that exists when taking into account variations in hormones, social and cultural influences of gender, and gender identity.
In summary, we examined sex differences in stress response in a healthy population during early adulthood using fMRI, steroid hormone physiology (cortisol), and MRS GABA. We found that young men and women responded differently to physiological and psychosocial stress exposure during a cognitive task. Immediately following an acute stress exposure, results replicated what we demonstrated in healthy adults in early midlife during a mild stress reactivity task (6). Here, we further found that women had increased connectivity between stress regions and DMN regions, a finding suggesting greater impact of stress on task performance in women than men. Under higher cognitive demand, men and women had fewer differences in activation of stress circuitry, possibly indicating that women had a lower threshold for the need to regulate arousal than men.
Despite these significant differences between young men and women, we found similar subjective ratings of anxiety and mood, cortisol levels, and GABA levels. Thus, importantly, sex differences in brain activation did not result in sex differences in affective or cortisol responses to the stressor, indicating sex differences in brain activations can lead to similar behavioral and physiological outcomes. This notion replicated findings in our previous study of healthy early midlife adults in response to a mild negative stress (6). Finally, sex differences in stress response circuitry activation in the healthy brain may be associated with differential vulnerabilities to anxiety and mood disorders that begin to emerge post-puberty, the same period of time assessed in this study. Thus, our future work will compare sex differences in the healthy brain with sex differences in major depression in response to stress.
Highlights.
Brain activity and connectivity differs in young men and women under stress
Task performance and affective response during stress did not differ between sexes
However, under stress, women activated limbic, and in men cognitive control regions.
GABA in prefrontal cortex negatively associated with the brain activity by sex
Acknowledgments
The MEGA-PRESS sequence was developed by Edward J. Auerbach and Małgorzata Marjańska and provided by the University of Minnesota under a C2P agreement. We would like to thank David Olson MD for assistance with blood collection protocol and Courtney Miller for assistance with blood draws. We would also like to thank Madeline (Lynn) Alexander, Ph.D., Laurie A. Scott, A.M., and Harlyn Aizley, Ed.M. for clinical interviews to establish study eligibility, and Monica Landi, M.S.W. for her help establishing diagnostic reliability.
Funding and Disclosures
This project was supported by R01MH108602 (DAP/JMG multi-PIs) from the National Institute of Mental Health. In addition, with regard to analyses and writing, JEC, LH and JMG were, in part, supported by ORWH-NIMH U54 MH118919 (JMG/Tobet, multi-PIs) and NIA R01AG057505 (JMG, PI). DAP was partially supported by R37 MH068376. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. JMG is on the scientific advisory board and has an equity interest in Cala Health, a neuromodulation device company, but there is no conflict of interest with this study. The relationship is managed by the MassGeneral Brigham Office of Industry Interactions. Over the past 3 years, Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (formerly BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka, Sunovion, and Takeda; he has received honoraria from the Psychonomic Society (for editorial work) and Alkermes; he has received research funding from the Brain and Behavior Research Foundation, the Dana Foundation, Millennium Pharmaceuticals, and National Institute of Mental Health (NIMH); he has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics, and Neuroscience Software. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. MM has served on the advisory board of Ipsen and Abbvie and served as a consultant for Abbvie and Sanofi, but no conflicts of interest with this work. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. The other authors have no financial disclosures.
Footnotes
Declaration of Competing Interest
Dr. Goldstein is on the scientific advisory board and has an equity interest in Cala Health, a neuromodulation device company, but there is no conflict of interest with this study. The relationship is managed by the Mass General Brigham Office of Industry Interactions. Over the past 3 years, Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (formerly BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka, Sunovion, and Takeda; he has received honoraria from the Psychonomic Society (for editorial work) and Alkermes; he has received research funding from the Brain and Behavior Research Foundation, the Dana Foundation, Millennium Pharmaceuticals, and National Institute of Mental Health (NIMH); he has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics, and Neuroscience Software. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. Dr. Misra has served on the advisory board of Ipsen and Abbvie and served as a consultant for Abbvie and Sanofi, but no conflicts of interest with this work. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. The other authors have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
The data that support the findings of this study will be openly available in the National Data Archive of the National Institute of Mental Health at https://nda.nih.gov/edit_collection.html?id=2485, reference number 2485.
References
- 1.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. (2012): Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 109:5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale TL, Epperson CN (2015): Sex differences and stress across the lifespan. Nat Neurosci. 18:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu JJW, Ein N, Peck K, Huang V, Pruessner JC, Vickers K (2017): Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology. 82:26–37. [DOI] [PubMed] [Google Scholar]
- 4.Kirschbaum C, Wust S, Hellhammer D (1992): Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 54:648–657. [DOI] [PubMed] [Google Scholar]
- 5.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH (1999): Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 61:154–162. [DOI] [PubMed] [Google Scholar]
- 6.Childs E, Dlugos A, De Wit H (2010): Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 47:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL (2008): Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psychiatry. 39:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zahn-Waxler C, Shirtcliff EA, Marceau K (2008): Disorders of childhood and adolescence: gender and psychopathology. Annu Rev Clin Psychol. 4:275–303. [DOI] [PubMed] [Google Scholar]
- 9.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A (2003): Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 60:837–844. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs EG, Holsen LM, Lancaster K, Makris N, Whitfield-Gabrieli S, Remington A, et al. (2015): 17beta-estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology. 40:566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N (2010): Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 30:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS (2007): Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 87:873–904. [DOI] [PubMed] [Google Scholar]
- 13.McEwen BS (2017): Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress; Thousand Oaks: ). 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heck AL, Handa RJ (2019): Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 44:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel N, Bale TL (2008): Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology. 149:6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA (1994): Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 28:464–476. [DOI] [PubMed] [Google Scholar]
- 17.Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. (2010): Early life programming and neurodevelopmental disorders. Biol Psychiatry. 68:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein JM (2018): Impact of Prenatal Stress on Offspring Psychopathology and Comorbidity With General Medicine Later in Life. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein JM, Cohen JE, Mareckova K, Holsen L, Whitfield-Gabrieli S, Gilman SE, et al. (2021): Impact of prenatal maternal cytokine exposure on sex differences in brain circuitry regulating stress in offspring 45 years later. Proc Natl Acad Sci U S A. 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckner RL, Andrews-Hanna JR, Schacter DL (2008): The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 1124:1–38. [DOI] [PubMed] [Google Scholar]
- 21.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional-anatomic fractionation of the brain’s default network. Neuron. 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soares JM, Marques P, Magalhaes R, Santos NC, Sousa N (2017): The association between stress and mood across the adult lifespan on default mode network. Brain Struct Funct. 222:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, et al. (2013): Stress Impact on Resting State Brain Networks. PLoS One. 8:e66500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ficek-Tani B, Horien C, Ju S, Xu W, Li N, Lacadie C, et al. (2022): Sex differences in default mode network connectivity in healthy aging adults. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman JP, Tasker JG, Ziegler DR, Cullinan WE (2002): Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 71:457–468. [DOI] [PubMed] [Google Scholar]
- 26.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. (2007): GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 10:1515–1517. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JM, Hale T, Foster SL, Tobet SA, Handa RJ (2018): Sex differences in major depression and comorbidity of cardiometabolic disorders: impact of prenatal stress and immune exposures. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClellan KM, Stratton MS, Tobet SA (2010): Roles for gamma-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus. J Comp Neurol. 518:2710–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stratton MS, Staros M, Budefeld T, Searcy BT, Nash C, Eitel C, et al. (2014): Embryonic GABA(B) receptor blockade alters cell migration, adult hypothalamic structure, and anxiety- and depression-like behaviors sex specifically in mice. PLoS One. 9:e106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Fan X, Hu Y, Zuo C, Whitfield-Gabrieli S, Holt D, et al. (2019): Regional GABA Concentrations Modulate Inter-network Resting-state Functional Connectivity. Cereb Cortex. 29:1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levar N, van Leeuwen JMC, Denys D, van Wingen GA (2017): Divergent influences of anterior cingulate cortex GABA concentrations on the emotion circuitry. Neuroimage. 158:136–144. [DOI] [PubMed] [Google Scholar]
- 32.Mareckova K, Holsen L, Admon R, Whitfield-Gabrieli S, Seidman LJ, Buka SL, et al. (2017): Neural - hormonal responses to negative affective stimuli: Impact of dysphoric mood and sex. J Affect Disord. 222:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mareckova K, Holsen L, Admon R, Whitfield-Gabrieli S, Goldstein J (2015): Neural and Hormonal Responses to Negative Affective Stimuli: Impact of Sex and Depressed Mood. Neuropsychopharmacology. 40:S176–S177. [Google Scholar]
- 34.Holsen LM, Lancaster K, Klibanski A, Whitfield-Gabrieli S, Cherkerzian S, Buka S, et al. (2013): HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience. 250:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreano JM, Cahill L (2010): Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 53:1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albert K, Pruessner J, Newhouse P (2015): Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 59:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulrich-Lai YM, Herman JP (2009): Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. (2003): Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 24:151–180. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Williams JBW, Karg RS, Spitzer RL (2015): Structured Clinical Interview for DSM-5- Research Version (SCID-F for DSM-5, Research Version; SCID-5-RV). Arlington, VA, American Psychiatric Association. [Google Scholar]
- 40.Smeets T, Cornelisse S, Quaedflieg CW, Meyer T, Jelicic M, Merckelbach H (2012): Introducing the Maastricht Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology. 37:1998–2008. [DOI] [PubMed] [Google Scholar]
- 41.Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD (2011): Microglia and memory: modulation by early-life infection. J Neurosci. 31:15511–15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC (2005): The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. 30:319–325. [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, et al. (2018): Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol Med. 48:1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983): Manual for the State-Trait Anxiety Inventory Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- 45.Watson D, Clark LA, Tellegen A (1988): Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. Journal of Personality and Social Psychology. 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 46.Killgore WD (1999): The visual analogue mood scale: can a single-item scale accurately classify depressive mood state? Psychol Rep. 85:1238–1243. [DOI] [PubMed] [Google Scholar]
- 47.Whitfield-Gabrieli S, Nieto-Castanon A (2012): Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2:125–141. [DOI] [PubMed] [Google Scholar]
- 48.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH (2003): Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 28:916–931. [DOI] [PubMed] [Google Scholar]
- 49.Marjanska M, Lehericy S, Valabregue R, Popa T, Worbe Y, Russo M, et al. (2013): Brain dynamic neurochemical changes in dystonic patients: a magnetic resonance spectroscopy study. Mov Disord. 28:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R (1998): Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11:266–272. [DOI] [PubMed] [Google Scholar]
- 51.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, et al. (2014): Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 86:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremblay S, Beaule V, Proulx S, Lafleur LP, Doyon J, Marjanska M, et al. (2014): The use of magnetic resonance spectroscopy as a tool for the measurement of bi-hemispheric transcranial electric stimulation effects on primary motor cortex metabolism. J Vis Exp.e51631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duda JM, Moser AD, Zuo CS, Du F, Chen X, Perlo S, et al. (2021): Repeatability and reliability of GABA measurements with magnetic resonance spectroscopy in healthy young adults. Magn Reson Med. 85:2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang W, Jbabdi S, Zhu Z, Cottaar M, Grisot G, Lehman JF, et al. (2019): A connectional hub in the rostral anterior cingulate cortex links areas of emotion and cognitive control. Elife. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24:167–202. [DOI] [PubMed] [Google Scholar]
- 56.Braver TS, Paxton JL, Locke HS, Barch DM (2009): Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 106:7351–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerr DL, McLaren DG, Mathy RM, Nitschke JB (2012): Controllability modulates the anticipatory response in the human ventromedial prefrontal cortex. Front Psychol. 3:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cremers H, Keedy S, Coccaro E (2021): The development of an fMRI protocol to investigate vmPFC network functioning underlying the generalization of behavioral control. Psychiatry Res Neuroimaging. 307:111197. [DOI] [PubMed] [Google Scholar]
- 59.Wade-Bohleber LM, Haugg A, Huber S, Ernst J, Grimm S, Recher D, et al. (2021): Anticipating control over aversive stimuli is mediated by the medial prefrontal cortex: An fMRI study with healthy adults. Hum Brain Mapp. 42:4327–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roy M, Shohamy D, Wager TD (2012): Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pessoa L (2010): Emergent processes in cognitive-emotional interactions. Dialogues Clin Neurosci. 12:433–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW (2003): The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 41:1474–1483. [DOI] [PubMed] [Google Scholar]
- 63.Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P (2009): Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Soc Cogn Affect Neurosci. 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mak LE, Minuzzi L, MacQueen G, Hall G, Kennedy SH, Milev R (2017): The Default Mode Network in Healthy Individuals: A Systematic Review and Meta-Analysis. Brain Connect. 7:25–33. [DOI] [PubMed] [Google Scholar]
- 65.Cavedo E, Chiesa PA, Houot M, Ferretti MT, Grothe MJ, Teipel SJ, et al. (2018): Sex differences in functional and molecular neuroimaging biomarkers of Alzheimer’s disease in cognitively normal older adults with subjective memory complaints. Alzheimers Dement. 14:1204–1215. [DOI] [PubMed] [Google Scholar]
- 66.Bluhm RL, Osuch EA, Lanius RA, Boksman K, Neufeld RW, Theberge J, et al. (2008): Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 19:887–891. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, et al. (2007): Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Raedt R, Hooley JM (2016): The role of expectancy and proactive control in stress regulation: A neurocognitive framework for regulation expectation. Clin Psychol Rev. 45:45–55. [DOI] [PubMed] [Google Scholar]
- 69.Burger Z, Muller VI, Hoffstaedter F, Habel U, Gur RC, Windischberger C, et al. (2023): Stressor-Specific Sex Differences in Amygdala-Frontal Cortex Networks. J Clin Med. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, Neuper C (2009): To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 47:604–608. [DOI] [PubMed] [Google Scholar]
- 71.Grabner RH, Reishofer G, Koschutnig K, Ebner F (2011): Brain correlates of mathematical competence in processing mathematical representations. Front Hum Neurosci. 5:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuhn L, Noack H, Wagels L, Prothmann A, Schulik A, Aydin E, et al. (2023): Sex-dependent multimodal response profiles to psychosocial stress. Cereb Cortex. 33:583–596. [DOI] [PubMed] [Google Scholar]
- 73.Cavanna AE, Trimble MR (2006): The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 129:564–583. [DOI] [PubMed] [Google Scholar]
- 74.Zhao W, Zimmermann K, Zhou X, Zhou F, Fu M, Dernbach C, et al. (2020): Impaired cognitive performance under psychosocial stress in cannabis-dependent men is associated with attenuated precuneus activity. J Psychiatry Neurosci. 45:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dedovic K, D’Aguiar C, Pruessner JC (2009): What stress does to your brain: a review of neuroimaging studies. Can J Psychiatry. 54:6–15. [DOI] [PubMed] [Google Scholar]
- 76.Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. (2008): Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 63:234–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study will be openly available in the National Data Archive of the National Institute of Mental Health at https://nda.nih.gov/edit_collection.html?id=2485, reference number 2485.


